Abstract
OBJECTIVES
We assessed the safety, effectiveness and haemodynamic performance of a new bovine stented aortic valve bioprosthesis (Avalus™).
METHODS
The PERIGON Pivotal Trial is a prospective, non-randomized, multicentre study. Subjects had symptomatic moderate or severe aortic stenosis or chronic, severe aortic regurgitation. Death, valve-related adverse events (AEs), functional recovery and haemodynamic performance were assessed at discharge, 3–6 months and 1 year. The primary analysis compared ‘late’ (>30 days post-implant) linearized rates of valve-related thromboembolism, thrombosis, all and major haemorrhage, all and major paravalvular leak (PVL) and endocarditis after implantation with objective performance criteria (OPC) for AEs, in accordance with EN ISO 5840:2009. We hypothesized that the upper 95% confidence bounds of the true linearized AE rates would be ≥ 2 × OPC; rejection of the null hypothesis would demonstrate that these rates were below acceptable rates. The analysis was required to include at least 150 patients followed to 1 year and 400 valve-years. Kaplan–Meier survival analysis was also performed.
RESULTS
Total number of valve-years was 459.5 (n = 686). Linearized rates were <2 × OPC for death and valve-related thromboembolism, valve thrombosis, all and major PVL, and endocarditis, but ≥2 × OPC for all and major haemorrhage. Survival at 1 year (n = 270) was 96.4%. Patients showed good functional recovery, and haemodynamic performance was within expected range.
CONCLUSIONS
This analysis demonstrated a good safety profile and clinical effectiveness of the Avalus valve except for bleeding rates. The linearized rates of all and major haemorrhage may be related to long-term anticoagulation for non-valvular indications and the length of follow-up of this cohort.
Trial registration
Keywords: Aortic valve replacement , Aortic stenosis , Bioprosthetic valves , Bovine pericardial valves , Avalus valve , Aortic valve haemodynamics
INTRODUCTION
Surgical aortic valve replacement (AVR) is the second most frequently performed cardiac operation, with nearly 50 000 procedures performed in the USA in 2015 and probably an equal amount in Europe [1]. In the population aged >65 years, approximately 1 in every 1000 people will undergo AVR in the Western world [2]. Although transcatheter alternatives have been developed for high-risk patients, most patients still are at low risk and are candidates for a surgical procedure. At the end of the 20th century, about half of the replacements were mechanical prostheses; nowadays, however, >80% of replacements are biological prostheses [3]. With the ageing of the population, this will undoubtedly increase over time.
The ideal valve substitute does not exist, and each prosthetic valve has inherent limitations. Biocompatibility and the absence of the need for anticoagulation are the most important advantages of biological prostheses compared with mechanical valves, while limited durability is one of their shortcomings. Other issues related to valve prostheses are their implantability, pressure gradients, regurgitation and thrombogenicity. These characteristics drive the development of new prostheses with better performance. The Avalus™ valve (Medtronic, Minneapolis, MN, USA) is a new pericardial bioprosthesis designed for easy implantability, a low gradient across the valve and improved durability. In this report, we present the first results from a multicentre trial investigating the safety, effectiveness and haemodynamic performance of this new prosthesis in a cohort of patients with aortic valve disease and an indication for surgical AVR.
MATERIALS AND METHODS
Study design
The PERIcardial SurGical AOrtic Valve ReplacemeNt (PERIGON) Pivotal Trial is a prospective, non-randomized, international, multicentre trial to evaluate the safety and effectiveness of the Avalus aortic valve bioprosthesis. The study was designed according to the recommendations of the International Organization for Standardization (ISO) standard for cardiac valve prostheses (EN ISO 5840:2009) [4] and the US Food and Drug Administration (FDA) heart valve guidance (2010) [5]. The trial is being conducted at 36 sites in Europe, Canada and the USA.
This trial was designed and conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. The institutional review board of each centre approved the protocol, and written informed consent was obtained from all patients. All serious adverse events (AEs) and deaths were adjudicated by an independent clinical events committee. All study echocardiograms were analysed by an independent core laboratory (MedStar Health Research Institute, Washington, DC, USA). Safety oversight was provided by an independent data and safety monitoring board. This trial is registered at www.clinicaltrials.gov, NCT02088554.
Device and procedure
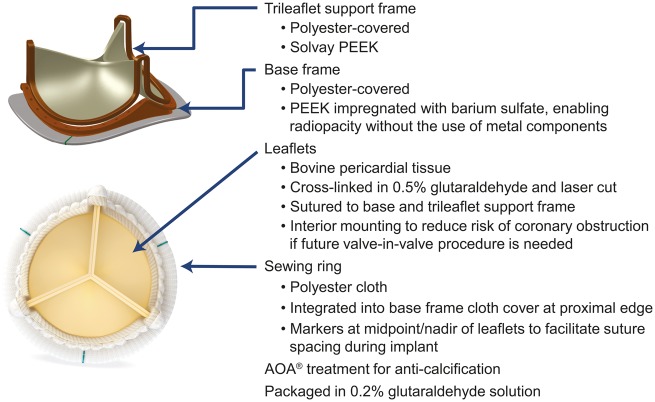
The Avalus aortic valve bioprosthesis is a bovine, stented, pericardial tissue valve that has a supra-annular design and a lower profile height than current bovine pericardial valves. Design details are provided in Fig. 1. A disposable valve holder is attached to the outflow of the valve to facilitate implantation. The valves sizes available for this study were 17, 19, 21, 23, 25, 27 and 29.
Figure 1.
Photograph and schematic of the Avalus bovine pericardial aortic bioprosthesis. AOA, alpha-amino oleic acid [6]; PEEK, polyether ether ketone.
The implant technique for the Avalus bioprosthesis is similar to that for other stented aortic bioprosthetic valves. Surgeons were allowed to use their preferred implant technique and cardioplegic and cardiopulmonary bypass strategies. Appropriate postoperative anticoagulation therapy was left to the discretion of the physician. Current practice is too disparate and the guidelines inconclusive on the most appropriate postoperative anticoagulation regimen to justify a prespecified protocol. Our goal was to reflect the real world of surgical AVR as much as possible.
Study population
Patients with moderate or severe aortic stenosis or aortic regurgitation and a clinical indication for replacement of their native or prosthetic aortic valve with a bioprosthesis were eligible. Concomitant procedures were allowed but early in the study were limited to left atrial appendage ligation, coronary artery bypass graft, patent foramen ovale closure, ascending aortic aneurysm or dissection repair not requiring circulatory arrest or resection of a subaortic membrane not requiring myectomy. Patients found intraoperatively to require other procedures were exited from the study and treated with a commercial valve. Supplementary Material, Table S1, lists exclusion criteria. Patients who met all inclusion criteria, no exclusion criteria, and provided written informed consent were considered enrolled into the trial.
Baseline, perioperative and follow-up evaluations
Baseline evaluation included collection of demographic data, medical history and use of relevant medications, particularly antiplatelet and anticoagulant medications; physical examination; assessment of New York Heart Association (NYHA) functional status and Society of Thoracic Surgeons (STS) score; 12-lead electrocardiography; haematology and chemistry tests, including serum creatinine; and transthoracic echocardiography (TTE). Baseline evaluations were required to be completed within 45 days before the scheduled implant procedure except for TTE, which could be completed within 90 days before implant.
Perioperative evaluation included collection of additional required procedures or interventions, valve data, device failure or malfunction, perioperative TTE, AEs or device deficiency and medication use.
Patients who received the study valve were scheduled for follow-up at hospital discharge (up to 30 days), 3 to 6 months and 1 year. These visits included assessment of NYHA classification, 12-lead electrocardiography, haematology and chemistry data, TTE, medications and AEs or device deficiency. After the 1-year visit, follow-up will continue annually through 5 years. Telephone follow-up calls will be made to assess vital status, medications and AEs or device deficiency at 18 and 30 months.
Study end-points
Safety end-points were the incidence of valve-related death and AEs over time, as defined by Akins et al. [7]. Valve-related AEs included thromboembolism, thrombosis, all and major haemorrhage, all and major paravalvular leak (PVL) and endocarditis. The primary analysis compared linearized valve-related AE rates after valve implantation with the objective performance criteria (OPC) defined by the ISO and FDA for commercially available tissue valves [4, 5] (Supplementary Material, Table S2). The ISO and FDA guidance requires that new or modified bioprostheses perform as well as or better than the OPC for valve-related AEs. Linearized rates of late AEs were determined because the risk of an AE is assumed to remain constant over time after valve implantation. We hypothesized that the upper 95% confidence bounds of the true linearized valve-related AE rates for the study valve would be ≥2 × OPC. Secondary safety end-points included the incidence of reinterventions and explants over time.
Effectiveness end-points were NYHA functional classification and haemodynamic performance, including effective orifice area (EOA) indexed EOA (EOAi), and mean and peak aortic pressure gradients. Prosthesis–patient mismatch (PPM) was defined as follows: none/mild, EOAi > 0.85 cm2/m2; moderate, EOAi >0.65–≤0.85 cm2/m2; and severe, EOAi ≤ 0.65 cm2/m2 [8].
Statistical analysis
The EN ISO 5840:2009 standards require that analyses be based on (i) a minimum of 150 patients followed up for 1 year and (ii) at least 400 valve-years of follow-up [4]. These standards also specify that all implants are to be included in the analysis. These requirements are based on the method of Grunkemeier et al. [9].
Descriptive statistics are used to report clinical characteristics and echocardiographic data. For categorical data, the number and percentage of patients in the category are presented. For continuous data, the mean ± standard deviation are presented. Early AE rates (those occurring ≤30 days post-implant) were calculated as the number of early events/total number of subjects, expressed as a percentage. Linearized rates of late AEs were calculated as the total number of late events (those occurring >30 days post-implant) divided by the total follow-up time (the sum of accumulated late postoperative valve-years), expressed as a percentage. For AEs with OPC available, the linearized rates and their associated 1-sided upper 95% confidence bounds were compared with 2× the OPC rate. We hypothesized that the upper 95% confidence bounds of the true linearized valve-related AE rates for the study valve would be ≥2 times greater than the OPC. If the upper 95% confidence limit for a complication rate is <2× the OPC rate, the null hypothesis can be rejected at a 1-sided significance level of 0.05 [5, 9]. Kaplan–Meier survival analyses were also performed.
RESULTS
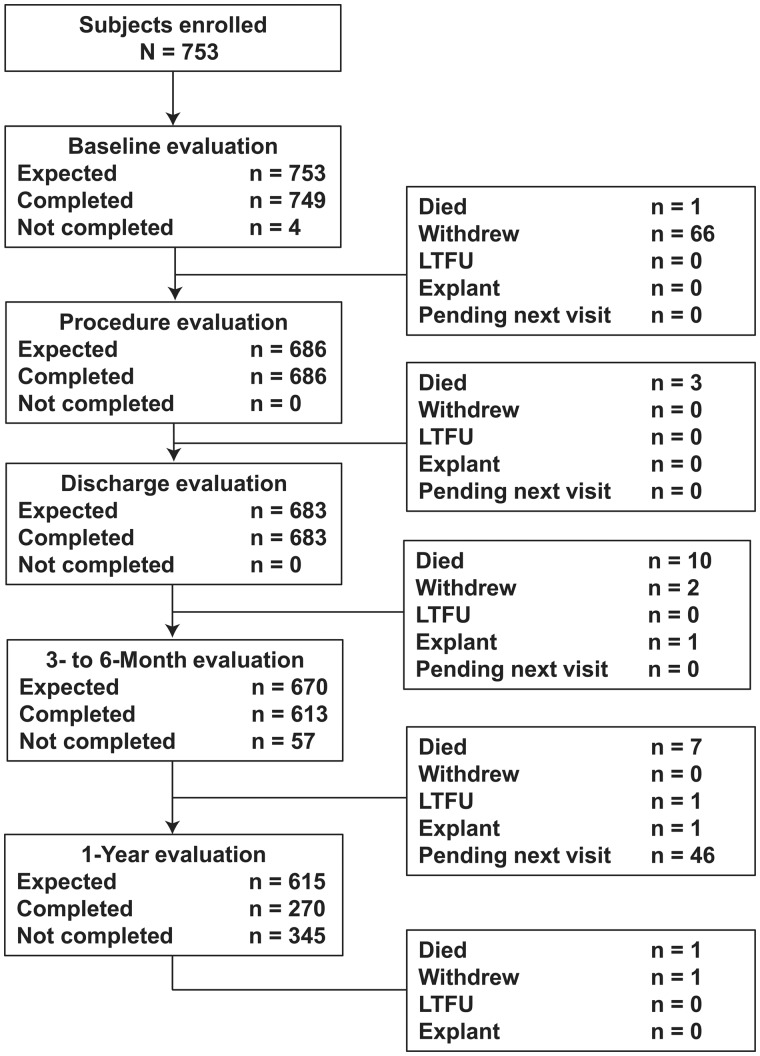
From May 2014 through January 2016, 753 subjects were enrolled into the PERIGON Pivotal Trial. Among the enrolled patients, 1 died and 66 withdrew before implantation (Supplementary Material, Table S3, lists reasons for withdrawal). This analysis includes 459.5 valve-years of follow-up with 686 subjects having received an implant and 270 subjects having completed 1 year of follow-up, thereby meeting the requirements of the ISO guidance. The total number of late (>30 days) valve-years was 405.3 (n = 683). Figure 2 shows patient disposition from enrollment through 1 year. One hundred twenty patients were enrolled before the inclusion and exclusion criteria were updated.
Figure 2.
Patient disposition from screening through 1-year follow-up evaluation.
Table 1 presents baseline patient characteristics. The study population consisted of typical patients referred for AVR with common comorbid conditions. STS criteria [10] indicated 627 (91.4%) patients were at low risk of mortality (predicted risk of mortality [STS PROM] <4%), 55 (8.0%) were at intermediate risk (STS PROM 4–8%) and 4 (0.6%) were at high risk (STS PROM >8%). Nearly 80% had received no previous coronary intervention, and only 2 (0.3%) had undergone prior AVR.
Table 1.
| Characteristic | Patients (n = 686) |
|---|---|
| Age (years) | 70.5 ± 9.2 |
| Male | 506 (73.8%) |
| Body surface area (m2) | 2.0 ± 0.2 |
| NYHA Class | |
| I | 76 (11.1%) |
| II | 324 (47.2%) |
| III | 273 (39.8%) |
| IV | 13 (1.9%) |
| STS mortality risk (%) | 2.1 ± 1.4 |
| STS morbidity/mortality risk (%) | 15.0 ± 6.1 |
| Comorbid conditions | |
| Coronary artery disease | 282 (41.1%) |
| Congestive heart failure | 150 (21.9%) |
| Hypertension | 525 (76.5%) |
| Angina | 276 (40.2%) |
| Dyslipidaemia | 400 (58.3%) |
| Smoking | 315 (45.9%) |
| Current smoking | 51 (7.4%) |
| Left ventricular hypertrophy | 273 (39.8%) |
| Diabetes | 178 (25.9%) |
| Endocarditis | 2 (0.3%) |
| Rhythm on ECG | |
| Sinus rhythm | 548 (80.1%) |
| Pacing | 14 (2.0%) |
| Atrial fibrillation | 32 (4.7%) |
| Other | 90 (13.2%) |
| Previous coronary interventions | |
| Coronary artery bypass graft | 20 (2.9%) |
| Percutaneous coronary intervention | 90 (13.1%) |
| Implanted cardiac device | 20 (2.9%) |
| Percutaneous valvuloplasty | 1 (0.1%) |
| Previous aortic valve implant | 6 (0.9%) |
| Previous open-heart surgeries | |
| 1 | 27 (3.9%) |
| 2+ | 1 (0.1%) |
Values are mean ± SD or n (%).
Supplementary Material, Table S5, contains additional baseline characteristics.
Procedural details
Table 2 provides procedural information, including indications for AVR and surgical approaches used. Among the 686 implanted valves, 210 (30.6%) were placed with simple interrupted sutures, 369 (53.8%) with pledgeted mattress sutures, 39 (5.7%) with a continuous running technique, 42 (6.1%) with everted mattress sutures, 316 (46.1%) with non-everted mattress sutures, 2 (0.3%) with a figure of eight suture, 72 (10.5%) with Cor-Knot fasteners and 17 (2.5%) with another technique. The valve was placed in an intra-annular position in 111 patients (16.2%) and the supra-annular position in 555 (80.9%). Mean total aortic cross-clamp time was 77.6 ± 30.8 min; for isolated AVR (n = 338), it was 65.4 ± 23.1 min; and for combined procedures (n = 348), 89.5 ± 32.8 min. Supplementary Material, Fig. S1, shows valve size distribution.
Table 2.
Procedural details
| Procedural information | Subjects (n = 686) |
|---|---|
| Primary indication for AVR | |
| Aortic stenosis | 587 (85.6%) |
| Aortic regurgitation | 37 (5.4%) |
| Mixed | 59 (8.6%) |
| Failed prosthesis | 3 (0.4%) |
| Surgical approach | |
| Median sternotomy | 540 (78.7%) |
| Hemisternotomy | 96 (14.0%) |
| Right thoracotomy | 38 (5.5%) |
| Other | 12 (1.7%) |
| Combined procedures | |
| None | 338 (49.3%) |
| Coronary artery bypass graft | 225 (32.8%) |
| Left atrial appendage closure | 49 (7.1%) |
| Patent foramen ovale closure | 4 (0.6%) |
| Ascending aortic aneurysm not requiring circulatory arrest | 41 (6.0%) |
| Myectomy | 21 (3.1%) |
| Ascending aorta replacement | 20 (2.9%) |
| Annular enlargement | 3/125 (2.4%) |
| Aortotomy enlargement | 17/126 (13.5%) |
| Resection of subaortic membrane not requiring myectomy | 12 (1.7%) |
| Maze procedure | 12 (1.7%) |
| Dissection repair not requiring circulatory arrest | 1 (0.1%) |
| Aortic arch replacement | 2 (0.3%) |
| Mitral valve replacement (unplanned) | 1 (0.1%) |
| Root replacement | 1 (0.1%) |
| Other | 22 (3.2%) |
| Total bypass time (min) | 102.6 ± 39.3 |
| Isolated AVR | 88.8 ± 30.3 |
| AVR + combined procedures | 116.1 ± 42.2 |
Values are mean ± SD or n (%).
Clinical events
Figure 3 compares the late linearized rates and upper 95% confidence limits for valve-related AEs to OPC. As shown, the upper 95% confidence limits of the late linearized AE rates were below 2× OPC for all events except all and major haemorrhage. Details about the bleeding events are provided in the Supplementary Material.
Figure 3.
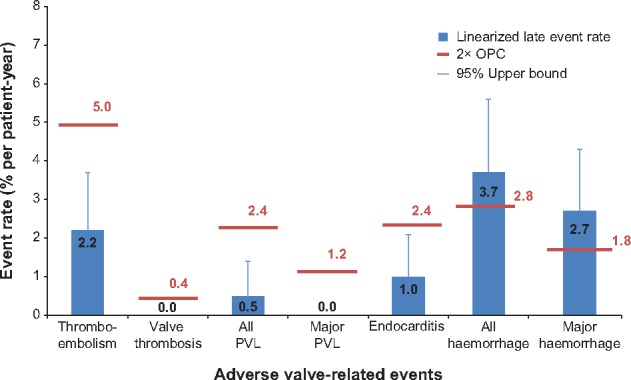
Valve-related late adverse events (>30 days to 1 year) compared with 2× objective performance criteria (OPC). Error bars indicate 95% upper bound of late linearized rate for each event.
There were 21 deaths (7 up to 30 days and 14 >30 days post-implant); 2 were valve related and 7 were cardiac deaths. The Kaplan–Meier estimates of freedom from all-cause, cardiac and valve-related mortality at 1 year were 96.4% (95% CI 94.1–97.8), 98.9% (95% CI 97.4–99.5) and 99.6% (95% CI 97.4–99.9), respectively. No valve thrombosis, haemolysis or structural valve deterioration was observed throughout the study period. The early mortality rate was 1.0% (n = 7) overall, 0.7% (n = 5) among low-risk patients, 0.3% (n = 2) among intermediate-risk patients, and 0.0% (n = 0) among high-risk patients. Supplementary Material, Table S4, shows additional early safety end-points. As shown, the rates of endocarditis, reintervention and explant were all very low. Table 3 provides medication use at each office follow-up visit. The Supplementary Material contains additional information about anticoagulation.
Table 3.
Antiplatelet and anticoagulant use at baseline and follow-up visits
| Visit | Antiplatelet | Anticoagulant | Antiplatelet + anticoagulant | No medication |
|---|---|---|---|---|
| Baseline (n = 686) | 386 (56.3%) | 43 (6.3%) | 30 (4.4%) | 227 (33.1%) |
| Discharge (n = 683) | 373 (54.6%) | 71 (10.4%) | 230 (33.7%) | 9 (1.3%) |
| 3-6 months (n = 612) | 411 (67.2%) | 54 (8.8%) | 97 (15.8%) | 50 (8.2%) |
| 1 year (n = 270) | 195 (72.2%) | 25 (9.3%) | 26 (9.6%) | 24 (8.9%) |
Effectiveness
Figure 4 illustrates NYHA functional classification at baseline, 3–6 months and 1 year. Nearly three-fourths of patients improved 1 or 2 classes from baseline to 1 year, whereas 1.5% worsened by 1 class over the same period.
Figure 4.
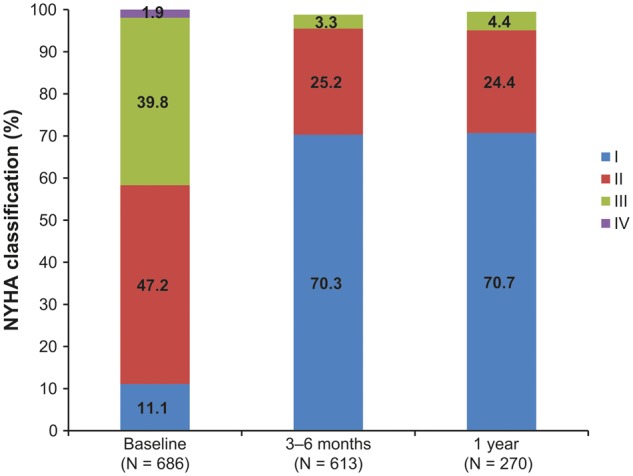
New York Heart Association (NYHA) classification from baseline through 1-year follow-up evaluation.
Haemodynamic performance
Figure 5 illustrates the mean aortic gradient and EOA from baseline through the 1-year evaluation, showing improvement in these parameters after AVR. Peak aortic gradient was 67.9 ± 25.3 at baseline, 24.2 ± 8.6 at discharge/30 days, 22.3 ± 7.7 at 3–6 months and 23.3 ± 7.7 at 1 year. Mean EOAi for all valves was 0.44 ± 0.25 at baseline, 0.80 ± 0.19 at discharge/30 days, 0.80 ± 0.18 at 3–6 months and 0.76 ± 0.16 at 1 year. The proportion of subjects with no, moderate and severe PPM at 1 year was 24.4%, 50.0% and 25.6%, respectively.
Figure 5.
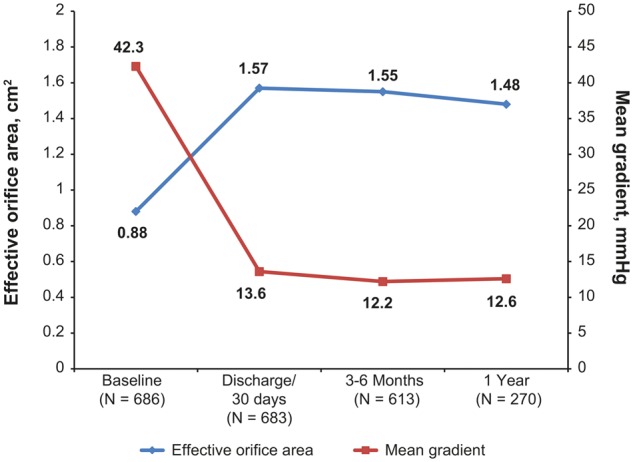
Haemodynamic performance from baseline through 1-year follow-up evaluation.
Regurgitation
PVL was classified as none or trace in >94% of patients at discharge/30 days, 3–6 months and 1 year. Mild PVL was observed in 1.8%, 3.0% and 1.2% of patients at the same time points, respectively. Moderate PVL occurred in 0.5% of patients at 3–6 months. There were no cases of severe PVL.
Transvalvular regurgitation (TVR) was classified as none or trace in ≥93% of patients at the 3 evaluations. Mild TVR was observed in 3.3% of patients at discharge/30 days, 4.5% at 3–6 months and 4.7% at 1 year. One patient (0.2%) had moderate TVR at the 3- to 6-month visit, and none was found to have severe TVR.
DISCUSSION
In this first analysis of the PERIGON Pivotal Trial, we found low rates of early and late mortality and valve-related AEs and improvement in NYHA functional status and haemodynamic performance.
The mortality rates in this study compare favourably with those reported for other bioprostheses. We observed an early mortality rate of 1.0%, compared with 30-day mortality rates of 1.8% reported for the Trifecta valve [11] and 2.0% and 0% reported for the Magna Ease and Trifecta valves, respectively [12]. Our rate of freedom from all-cause mortality at 1 year (96.4%) was similar to the rates reported for the Trifecta (95.8% and 96.0%) and Magna Ease (90.6%) valves [11, 12]. In the Nordic Aortic Valve Intervention (NORDIC) Trial, an all-comers randomized study comparing surgical and transcatheter AVR, the 1-year all-cause mortality rate in the surgical AVR group was 7.5% (Kaplan–Meier estimate) [13].
Both early event rates and late linearized rates for other valve-related safety outcomes compared favourably with results reported for the Trifecta valve [11]. In both studies, there were no early or late cases of valve thrombosis, major PVL, structural valve deterioration or haemolysis. Late linearized rates were similar for all other valve-related AEs, including thromboembolism (Avalus and Trifecta, respectively: 2.2% and 1.9% per valve-year), PVL (0.5% and 0% per valve-year), endocarditis (1.0% and 1.1% per valve-year), non-structural valve dysfunction (0.5 and 0.1% per valve-year), reintervention (0.5% and 0.6% per valve-year) and explant (0.5% and 0.6% per valve-year).
The primary results for bleeding were somewhat unexpected, though also reported by others on biological valves [11]. There were 15 late all-haemorrhage events (n = 13), 11 of which were major (n = 9). These events yielded late linearized rates of 3.7% per valve-year and 2.7% per valve-year, respectively; with upper bounds of 5.6% and 4.3%, both exceeded the 2× OPC rates of 2.8% and 1.8% per valve-year. The current analysis includes 1-year data for only 270 of 686 (39%) implanted patients, so it is likely the linearized rates calculated for haemorrhage are inflated owing to the proportion of patients still receiving prophylactic anticoagulant administration following implantation. As explained in Methods, linearization assumes a relatively constant rate of events over time. This is clearly not true for bleeding, as these events are much more frequent in the early months of follow-up (3–6 months postoperatively) than in the later months after implant. Linearization, or extrapolation from these early months to 1 year, yields a rate that is likely higher than would be the case if the entire follow-up of 1 year would have been captured. In addition, two-thirds of the patients were taking an antiplatelet or anticoagulant agent, or both, at baseline for non-valvular indications. Eleven of the 15 all-haemorrhage events occurred in patients taking warfarin (Coumadin®) or other anticoagulants, such as dabigatran (Pradaxa®), clopidogrel (Plavix®) or heparin. In patients taking chronic anticoagulation for comorbid conditions such as atrial fibrillation, the presence of a bioprosthesis does not directly affect the risk of an anticoagulant-related haemorrhage event. Rather, the risk of anticoagulant-related bleeding is more likely attributable to the management of the anticoagulation regimen for non-valvular indications. In fact, only 1 bleeding event was directly related to anticoagulation taken to prevent early thrombosis after implantation (see Supplementary Material). As with other tissue bioprostheses, the instructions for use of the Avalus valve recommend anticoagulation during the initial healing stages following implant. Thus, the risk of anticoagulant-related haemorrhage is increased during this time. However, the use of anticoagulation in this study decreased over time (Table 3), but 11 of the 15 late haemorrhage events occurred during the early postoperative months, between 31 days and 6 months. These findings, along with the wide variability of anticoagulation usage, suggest that bleeding events might not be a very useful performance criterion for bioprostheses considering the current OPC definition of a ‘late’ event (>30 days post-implant). However, because this is an ISO requirement, it was kept in the analysis.
NYHA functional classification was improved in almost all patients receiving the Avalus valve with only 4.4% of patients in Class III and none in Class IV at 1 year, comparable to other commercially available valves [11] and proving the effectiveness in treating aortic valve disease. The mean pressure gradient for all valve sizes in our cohort was 13.6 ± 4.8 mmHg at discharge/30 days, 12.2 ± 4.2 mmHg at 3–6 months and 12.6 ± 4.3 mmHg at 1 year. In recent studies of other valves, mean gradients ranged from 9.1 ± 3.9 to 14.0 ± 5.0 mmHg at discharge, 8.9 ± 3.9 to 11.4 ± 3.1 at 6 months and 9.4 ± 4.3 to 12.9 ± 3.8 at 1 year [12, 14]. EOAs in this cohort were 1.6 ± 0.4 cm2 at discharge/30 days, 1.6 ± 0.4 cm2 at 3–6 months and 1.5 ± 0.4 cm2 at 1 year, which compare favourably with EOA values ranging from 1.5 ± 0.3 to 1.8 ± 0.4 cm2 at discharge, 1.6 ± 0.5 to 2.1 ± 0.5 cm2 at 6 months, and 1.4 ± 2.4 cm2 and 1.7 ± 0.4 at 1 year for other valves [11, 12, 14]. These differences are clinically insignificant, and all these contemporary pericardial valves seem to perform well. The proportion of subjects with moderate or severe PPM in our study was 50.0% and 24.4%, respectively, at 1 year. Severe PPM has been reported in 20.7% to 33.9% of patients undergoing surgical AVR in clinical trials comparing surgical and transcatheter AVR [15–17]. The meaning of severe PPM has been questioned [18] and should be interpreted with caution.
Limitations
Due to the design of the study, the 1-year results could be reported on only 270 patients. This analysis was designed to meet the requirements of EN ISO 5840:2009 [4], which required at least 150 patients followed to 1 year and at least 400 valve-years. However, because the PERIGON Pivotal Trial was powered to meet the requirement of the US FDA [5], a minimum of 800 valve-years is needed to accurately detect the smallest acceptable haemorrhage event rate (1.2%) with a power of 0.80; even more valve-years will be required for major haemorrhage. In addition, the majority of patients in this cohort had not completed their 1-year visit and were in the early postoperative period. It is likely that many of those patients were still receiving anticoagulation and thus had a higher risk of bleeding events. Furthermore, the exclusion criteria became more restrictive after the first 120 patients were enrolled, as did the allowable combined procedures, which may have influenced early bleeding events.
Another limitation is that we did not include a control arm. A true control group is difficult to define as all commercial valves have their own benefits and limitations; there is no ‘gold standard’ for AVR studies. We therefore elected to compare this valve to OPC as many new valves have been tested against the same standards.
This article describes safety and efficacy of a new bioprosthesis. The most important information for these types of prosthesis, however, is durability. Although several design features have been incorporated in this new valve to increase its durability, only longer term follow-up studies can demonstrate this promise.
CONCLUSIONS
This analysis demonstrated a good safety profile and haemodynamic performance of the Avalus valve except for bleeding rates. The upper 95% confidence bounds of the late linearized rates were <2× OPC for death and valve-related thromboembolism, thrombosis, all and major PVL and endocarditis. The late linearized rates of all and major haemorrhage were >2× OPC but appear to be related to long-term anticoagulation for non-valvular indications and the length of follow-up in this cohort.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Supplementary Material
ACKNOWLEDGEMENTS
Ryan Palmer and Aimee Weber provided study management, and Julie Linick, ELS, CMPP, provided editorial support (all of Medtronic).
Funding
This work was supported by Medtronic.
Conflict of interest: Robert J.M. Klautz reports unrestricted research grants from Edwards, Medtronic and Admedus for his institutional department and travel expenses and presenter reimbursements from several industries. Vinayak Bapat is a consultant for Medtronic and Boston Scientific and reports receiving speaker fees from Medtronic, Edwards Lifesciences, Boston Scientific, and LivaNova Michael Moront is a consultant for Medtronic, Edwards Lifesciences, LSI Solutions and Terumo. Cathy Zeng is an employee of Medtronic. Joseph Sabik receives research support from Edwards Lifesciences (local principal investigator for Intuity Trial) and has served on advisory boards for Medtronic and LivaNova. Rüdiger Lange reports lecture fees, royalties and serving on an advisory board for Medtronic; lecture fees and serving on an advisory board for LivaNova; and lecture fees, shares and serving on an advisory board for Highlife. The other authors have no conflicts of interest to declare.
REFERENCES
- 1. Society of Thoracic Surgeons, Duke Clinical Research Institute. STS Adult Cardiac Surgery Database: Executive Summary: 10 Years: Period Ending 12/31/15. Chicago, IL: Society of Thoracic Surgeons, 2016, 1–6. http://www.sts.org/sites/default/files/documents/2016Harvest1_ExecutiveSummary.pdf (16 September 2016, date last accessed).
- 2. Barreto-Filho JA, Wang Y, Dodson JA, Desai MM, Sugeng L, Geirsson A. et al. Trends in aortic valve replacement for elderly patients in the United States, 1999-2011. JAMA 2013;310:2078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown JM, O'Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS.. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82–90. [DOI] [PubMed] [Google Scholar]
- 4. Cardiovascular Implants : Cardiac Valve Prostheses. Standard EN ISO 5840:2009. Geneva, Switzerland: International Organization for Standardization. http://shop.bsigroup.com/ProductDetail/?pid=000000000030205036 (16 September 2016, date last accessed).
- 5. Draft Guidance for Industry and FDA Staff : Heart Valves—Investigational Device Exemption (IDE) and Premarket Approval (PMA) Applications Silver Spring, Maryland: US Food and Drug Administration. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm193096.htm (16 September 2016, date last accessed).
- 6. Walther T, Falk V, Autschbach R, Diegeler A, Rauch T, Weigl C. et al. Comparison of different anticalcification treatments for stentless bioprostheses. Ann Thorac Surg 1998;66:S249–54. [DOI] [PubMed] [Google Scholar]
- 7. Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL. et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Eur J Cardiothorac Surg 2008;33:523–8. [DOI] [PubMed] [Google Scholar]
- 8. Dumesnil JG, Pibarot P.. Prosthesis-patient mismatch: an update. Curr Cardiol Rep 2011;13:250–7. [DOI] [PubMed] [Google Scholar]
- 9. Grunkemeier GL, Johnson DM, Naftel DC.. Sample size requirements for evaluating heart valves with constant risk events. J Heart Valve Dis 1994;3:53–8. [PubMed] [Google Scholar]
- 10. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA. et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
- 11. Bavaria JE, Desai ND, Cheung A, Petracek MR, Groh MA, Borger MA. et al. The St Jude Medical Trifecta aortic pericardial valve: results from a global, multicenter, prospective clinical study. J Thorac Cardiovasc Surg 2014;147:590–7. [DOI] [PubMed] [Google Scholar]
- 12. Fiegl K, Deutsch MA, Rondak IC, Lange R, Guenzinger R.. Matched comparison of two different biological prostheses for complete supra-annular aortic valve replacement. Thorac Cardiovasc Surg 2015;63:459–66. [DOI] [PubMed] [Google Scholar]
- 13. Thyregod HG, Steinbruchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P. et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol 2015;65:2184–94. [DOI] [PubMed] [Google Scholar]
- 14. Wendt D, Thielmann M, Plicht B, Aßmann J, Price V, Neuh㴳er M. et al. The new St Jude Trifecta versus Carpentier-Edwards Perimount Magna and Magna Ease Aortic bioproshesis: is there a hemodynamic superiority? J Thorac Cardiovasc Surg 2014;147:1553–60. [DOI] [PubMed] [Google Scholar]
- 15. Zorn GL 3rd, Little SH, Tadros P, Deeb GM, Gleason TG, Heiser J. et al. Prosthesis-patient mismatch in high-risk patients with severe aortic stenosis: a randomized trial of a self-expanding prosthesis. J Thorac Cardiovasc Surg 2016;151:1014–22, 1023.e1–3. [DOI] [PubMed] [Google Scholar]
- 16. Pibarot P, Weissman NJ, Stewart WJ, Hahn RT, Lindman BR, McAndrew T. et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort-A analysis. J Am Coll Cardiol 2014;64:1323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thyregod HG, Steinbruchel DA, Ihlemann N, Ngo TA, Nissen H, Kjeldsen BJ. et al. No clinical effect of prosthesis-patient mismatch after transcatheter versus surgical aortic valve replacement in intermediate- and low-risk patients with severe aortic valve stenosis at mid-term follow-up: an analysis from the NOTION trial. Eur J Cardiothorac Surg 2016;50:721–8. [DOI] [PubMed] [Google Scholar]
- 18. Jamieson WR, Ye J, Higgins J, Cheung A, Fradet GJ, Skarsgard P. et al. Effect of prosthesis-patient mismatch on long-term survival with aortic valve replacement: assessment to 15 years. Ann Thorac Surg 2010;89:51–8; discussion 59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.