Abstract
Background
Some of the most widely recognized coral reef fishes are clownfish or anemonefish, members of the family Pomacentridae (subfamily: Amphiprioninae). They are popular aquarium species due to their bright colours, adaptability to captivity, and fascinating behavior. Their breeding biology (sequential hermaphrodites) and symbiotic mutualism with sea anemones have attracted much scientific interest. Moreover, there are some curious geographic-based phenotypes that warrant investigation. Leveraging on the advancement in Nanopore long read technology, we report the first hybrid assembly of the clown anemonefish (Amphiprion ocellaris) genome utilizing Illumina and Nanopore reads, further demonstrating the substantial impact of modest long read sequencing data sets on improving genome assembly statistics.
Results
We generated 43 Gb of short Illumina reads and 9 Gb of long Nanopore reads, representing approximate genome coverage of 54× and 11×, respectively, based on the range of estimated k-mer-predicted genome sizes of between 791 and 967 Mbp. The final assembled genome is contained in 6404 scaffolds with an accumulated length of 880 Mb (96.3% BUSCO-calculated genome completeness). Compared with the Illumina-only assembly, the hybrid approach generated 94% fewer scaffolds with an 18-fold increase in N50 length (401 kb) and increased the genome completeness by an additional 16%. A total of 27 240 high-quality protein-coding genes were predicted from the clown anemonefish, 26 211 (96%) of which were annotated functionally with information from either sequence homology or protein signature searches.
Conclusions
We present the first genome of any anemonefish and demonstrate the value of low coverage (∼11×) long Nanopore read sequencing in improving both genome assembly contiguity and completeness. The near-complete assembly of the A. ocellaris genome will be an invaluable molecular resource for supporting a range of genetic, genomic, and phylogenetic studies specifically for clownfish and more generally for other related fish species of the family Pomacentridae.
Keywords: clownfish, long reads, genome, transcriptome, hybrid assembly
Data Description
The clown anemonefish, Amphiprion ocellaris (Fig. 1, NCBI Taxon ID: 80 972, Fish Base ID:6509), is a well-known tropical marine fish species among the nonscientific community especially following the Pixar film Finding Nemo and its sequel Finding Dory [1]. The visual appeal of A. ocellaris due to its bright coloration and behaviour and ease of husbandry have maintained a strong global demand for this species in the marine aquarium trade, driving a fine balance between positive environmental awareness and sustainable ornamental use [1, 2]. Further, given high survival rates and ability to complete their life cycle in captivity, captive-breeding programs to partially sustain their global trade have been successful [3]. For the scientific community, A. ocellaris or anemonefishes in general are actively studied due to their intriguing reproductive strategy, i.e., sequential hermaphroditism [4–7] and mutualistic relationships with sea anemones [8–12]. Phenotypic body colour variation based on host-anemone use and geography also pose additional questions regarding adaptive genetic variation [13].
Figure 1:

The clown anemonefish (Amphiprion ocellaris). Photo by Michael P. Hammer.
In recent years, concurrent with the advent of long read sequencing technologies [14], several studies have explored combining short but accurate Illumina reads with long but less accurate Nanopore/PacBio reads to obtain genome assemblies that are usually more contiguous with higher completeness than assemblies based on Illumina-only reads [15–19]. To further contribute to the evaluation of long read technology in fish genomics [15], we sequenced the whole genome of A. ocellaris using Oxford Nanopore and Illumina technologies and demonstrate that hybrid assembly of long and short reads greatly improved the quality of genome assembly.
Whole-genome sequencing
Tissues for genome assembly and as reference material were sourced from the collection of the Museum and Art Gallery of the Northern Territory (NTM). The samples used for DNA extraction and subsequent whole-genome sequencing were from freshly vouchered captive bred A. ocellaris specimens, representing a unique black and white colour phenotype found only in the Darwin Harbour region, Australia (NTM A3764, A4496, A4497).
Genomic DNA was extracted from multiple fin clip and muscle samples using the E.Z.N.A. Tissue DNA Kit (Omega Bio-tek, Norcross, GA, USA). For Illumina library prep, approximately 1 μg of gDNA from isolate A3764 was sheared to 300 bp using a Covaris Focused-Ultrasonicator (Covaris, Woburn, MA, USA) and subsequently processed using the TruSeq DNA Sample Prep Kit (Illumina, San Diego, CA, USA) according to the manufacturer's instructions. Paired-end sequencing was performed on a single lane of HiSeq 2000 (Illumina, San Diego, CA, USA) located at the Malaysian Genomics Resource Centre Berhad. Two additional libraries were constructed from specimen NTM A3764, and both libraries were sequenced on the MiSeq (2 × 300 bp setting), located at the Monash University Malaysia Genomics Facility.
To generate Oxford Nanopore long reads, approximately 5 μg of gDNA was extracted from isolates NTM A4496 and A4497, size-selected (8–30 kb) with a BluePippin (Sage Science, Beverly, MA, USA), and processed using the Ligation Sequencing 1D Kit (Oxford Nanopore, Oxford, UK) according to the manufacturer's instructions. Three libraries were prepared and sequenced on 3 different R9.4 flowcells using the MinION portable DNA sequencer (Oxford Nanopore, Oxford, UK) for 48 hours.
Sequence read processing
Raw Illumina short reads were adapter-trimmed with Trimmomatic v.0.36 (ILLUMINACLIP:2:30:10, MINLEN:100; Trimmomatic, RRID:SCR_011848) [20], followed by a screening for vectors and contaminants, using Kraken v.0.10.5 (Kraken, RRID:SCR_005484) [21] based on the MiniKraken DB. Kraken-unclassified reads, i.e., nonmicrobial/viral origin, were aligned to the complete mitogenome of NTM A3764 (see the Mitogenome Assembly section) to exclude sequences of organellar origin. This results in a total of 42.35 Gb of “clean” short reads. Nanopore reads were base-called from their raw FAST5 files using the Oxford Nanopore proprietary base-caller, Albacore, version 2.0.1. Applying a minimum length cutoff of 500 bp, this study produced a total of 8.95 Gbp in 895 672 Nanopore reads (N50: 12.7 kb). Sequencing statistics are available in Supplementary Table 1.
Genome size estimation
K-mer counting with the “clean” Illumina reads was performed with Jellyfish v.2.2.6 (Jellyfish, RRID:SCR_005491) [22], generating k-mer frequency distributions of 17-, 21-, and 25-mers. These histograms were processed by GenomeScope [23], which estimated a genome size of 791 to 794 Mbp with approximately 80% of unique content and a heterozygosity level of 0.6% (Supplementary Fig. 1). Given that we had previously excluded adapters as well as sequences from contaminant or organellar sources, the max kmer coverage filter was not applied (max kmer coverage: -1). A separate estimation performed by BBMap [24] estimated a haploid genome size of 967 Mbp. The genome sizes estimated from both approaches are within the range of sizes listed for other Amphiprion species (792 Mb–1.2 Gb) as reported on the Animal Genome Size Database [25].
Hybrid genome assembly
Short reads used for assemblies described in this study were only trimmed for adapters, but not for quality. Both short-read-only and hybrid de novo assemblies were performed with the Maryland Super-Read Celera Assembler v.3.2.2 (MaSuRCA, RRID:SCR_010691) [26]. During hybrid assembly, errors were encountered in the fragment correction step of the Celera Assembler (CA; Celera assembler, RRID:SCR_010750). To overcome this, given that the CA assembler is no longer maintained, we disabled the frgcorr step based on one of the developer's recommendations, and the hybrid assembly was subsequently improved with 10 iterations of Pilon v.1.22 (Pilon, RRID:SCR_014731) [27], using short reads to correct bases, fix misassemblies, and fill assembly gaps. To assess the completeness of the genome, Benchmarking Universal Single-Copy Orthologs v.3.0.2 (BUSCO, RRID:SCR_015008) [28] was used to locate the presence or absence of the Actinopterygii-specific set of 4584 single-copy orthologs (OrthoDB v9).
The short-read-only and hybrid assemblies yielded total assembly sizes of 851 Mb and 880 Mb, respectively. Statistics for assemblies for each Pilon iteration are available in Supplementary Table 2. Inclusion of Nanopore long reads for a hybrid assembly representing approximately ×11 genome coverage led to a 94% decrease in the number of scaffolds (>500 bp) from 106 526 to 6404 scaffolds and an 18-fold increase in the scaffold N50 length from 21 802 bp to 401 715 bp (Table 1). In addition, the genome completeness was also substantially improved in the hybrid assembly, with BUSCO detecting complete sequences of 96.3% (4417/4584) of single-copy orthologs in the Actinopterygii-specific dataset.
Table 1:
Genome and transcriptome statistics of the clownfish (Amphiprion ocellaris) genome
| Illumina (≥500 bp) | Illumina + Nanopore (≥500 bp) | |
|---|---|---|
| Genome assembly | ||
| Contig statistics | ||
| Number of contigs | 133 997 | 7810 |
| Total contig size, bp | 851 389 851 | 880 159 068 |
| Contig N50 size, bp | 15 458 | 323 678 |
| Longest contig, bp | 204 209 | 2051 878 |
| Scaffold statistics | ||
| Number of scaffolds | 106 526 | 6404 |
| Total scaffold size, bp | 852 602 726 | 880 704 246 |
| Scaffold N50 size, bp | 21 802 | 401 715 |
| Longest scaffold, bp | 227 111 | 3111 502 |
| GC/AT/N, % | 39.6/60.2/0.14 | 39.4/60.5/0.06 |
| BUSCO genome completeness | ||
| Complete | 3691 (80.5%) | 4417 (96.3%) |
| Complete and single copy | 3600 (78.5%) | 4269 (93.1%) |
| Complete and duplicated | 91 (2.0%) | 148 (3.2%) |
| Fragmented | 534 (11.6%) | 63 (1.4%) |
| Missing | 359 (7.9%) | 104 (2.3%) |
| Transcriptome assembly | ||
| Number of contigs | 25 364 | |
| Total length, bp | 68 405 796 | |
| Contig N50 size, bp | 3670 | |
| BUSCO completeness | ||
| Complete | 4253 (92.8%) | |
| Complete and single-copy | 4128 (90.1%) | |
| Complete and duplicated | 125 (2.7%) | |
| Fragmented | 127 (2.8%) | |
| Missing | 204 (4.4%) | |
| Genome annotation | ||
| Number of protein-coding genes | 27 420 | |
| Number of functionally annotated proteins | 26 211 | |
| Mean protein length | 514 aa | |
| Longest protein | 29 084 aa (titin protein) | |
| Average number (length) of exon per gene | 9 (355 bp) | |
| Average number (length) of intron per gene | 8 (1532 bp) |
Transcriptome sequencing and assembly
Total RNA extraction from RNAshield-preserved whole-body and muscle tissues of isolate A4496 used Quick-RNA MicroPrep (Zymo Research Corpt, Irvine, CA, USA) according to the manufacturer's protocols. After assessing total RNA intactness on the Tapestation2100 (Agilent), mRNA was enriched using NEBNext Poly(A) mRNA Magnetic Isolation Kit (NEB, Ipwich, MA, USA) and processed with NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, Ipwich, MA, USA). Libraries from both whole-body and muscle tissues were sequenced on a fraction of MiSeq V3 flowcell (1 × 150 bp). Single-end reads from both libraries in addition to 2 publicly available A. ocellaris transcriptome sequencing data (SRR5253145 and SRR5253146, Bioproject ID: PRJNA374650) were individually assembled using Scallop v0.10.2 [29] based on HiSat2 [30] alignment of RNA-sequencing reads to the newly generated A. ocellaris genome. The transcriptome assemblies were subsequently merged using the tr2aacds pipeline from the EvidentialGene [31] package and similarly assessed for completeness using BUSCO, version 3 [28]. The final nonredundant transcriptome assembly, which was subsequently used to annotate the A. ocellaris genome, contains 25 264 contigs/isotigs (putative transcripts) with an accumulated length of 68.4 Mb and BUSCO-calculated completeness of 92.8% (Table 1).
Genome annotation
Protein-coding genes were predicted with the MAKER v.2.31.9 genome annotation pipeline (MAKER, RRID:SCR_005309) [32]. A total of 3 passes were run with MAKER2; the first pass was based on hints from the assembled transcripts as RNA-seq evidence (est2genome) and protein sequences from 11 fish species downloaded from Ensembl (Ensembl, RRID:SCR_002344) [33] (protein2genome), whereas the second and third passes included gene models trained from the first (and then second) passes with ab initio gene predictors SNAP (SNAP, RRID:SCR_002127) [34] and Augustus (Augustus: Gene Prediction, RRID:SCR_008417) [35]. In the final set of genes predicted, sequences with annotation edit distance (AED) values of less than 0.5 were retained. A small AED value suggests a lesser degree of difference between the predicted protein and the evidence used in the prediction (i.e., fish proteins, transcripts). This resulted in a final set of 27 240 protein-coding genes with an average AED of 0.14 (Table 1). A BUSCO analysis on the completeness of the predicted protein dataset detected the presence of 4259 (92.9%) single-copy orthologs from the Actinopterygii-specific dataset.
Further, to infer the putative function of these predicted proteins, NCBI’s blastp v.2.6.0 (-evalue 1e-10, -seg yes, -soft_masking true, -lcase_masking; BLASTP, RRID:SCR_001010) [36] was used to find homology to existing vertebrate sequences in the nonredundant (NR) database. Applying a hit fraction filter to include only hits with ≥70% target length fraction, the remaining unannotated sequences were subsequently aligned to all sequences in the NR database. With this method, 20 107 proteins (74%) were annotated with a putative function based on homology. Additionally, InterProScan v.5.26.65 (InterProScan, RRID:SCR_005829) [37] was used to examine protein domains, signatures, and motifs present in the predicted protein sequences. This analysis detected domains, signatures, or motifs for 26 211 proteins (96%). Overall, 96% of the predicted clownfish protein-coding genes were functionally annotated with information from at least 1 of the 2 approaches.
Mitogenome recovery via genome skimming
Genome skimming [38, 39] was performed on 3 additional A. ocellaris individuals from known localities (Supplementary Table 3). Mitogenome assembly was performed with MITObim, version 1.9 (MITObim, RRID:SCR_015056) [40], using the complete mitogenome of A. ocellaris (GenBank: NC009065.1) as the bait for read mapping. The assembled mitogenomes were subsequently annotated with MitoAnnotator [41]. Consistent with the original broodstock collection from northern Australia, the captive-bred black and white A. ocellaris NTM A3764 exhibits strikingly high whole-mitogenome nucleotide identity (99.98%) to sample NTM A3708 as a wild collection from Darwin Harbour, Australia. In addition, the overall high pair-wise nucleotide identity (>98%) of NTM A3764 to newly generated and publicly available A. ocellaris whole mitogenomes further supports its morphological identification as A. ocellaris (Supplementary Table 3).
Identification of the cyp19a1a gene associated with sexual differentiation
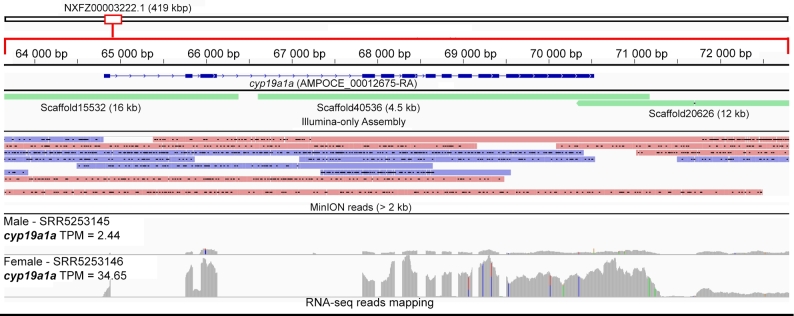
The validated cyp19a1a enzyme of Danio rerio (Uniprot: O42145) was used as the query (E-value = 1e-10) for blastp search against the predicted A. ocellaris proteins. The top blast hit, AMPOCE_00 012675-RA (71.5% protein identity to O42145), was searched (tblastn) against the NCBI TSA database (Taxon: Amphirion) and showed strikingly high protein identity (99%) to a translated RNA transcript from Amphiprion bicinctus (c183337_g1_i2: GDCV01327693) [5]. The cyp19a1a gene codes for a steroidogenic enzyme that converts androgens into estrogens [42] were recently shown to be instrumental during sex change in Amphiprion bicinctus, as evidenced by significant correlation and differential expression of this gene between males and mature females [5]. We also observed a similar profile based on mapping of RNA reads from the publicly available male and female transcriptomes of A. ocellaris to the cyp19a1a gene region as visualized using the Integrative Genomics Viewer (Fig. 2) [43]. The A. ocellaris cyp19a1a gene is located on a 419-kb scaffold and is spanned by multiple Minimap2-aligned Nanopore reads [44]. It is noteworthy that in the Illumina-only assembly, this gene is fragmented and located on 3 relatively short scaffolds (Fig. 2).
Figure 2:
Mapping of MinION long reads, Illumina-assembled scaffolds, and RNA-sequencing reads of male and female A. ocellaris to the genomic region containing the cyp19a1a gene. Transcripts per million (TPM) values were calculated using Kallisto, version 0.43.1 [46].
Conclusion
We present the first clownfish genome co-assembled with high-coverage Illumina short reads and low-coverage (∼11×) Nanopore long reads. Hybrid assembly of Illumina and Nanopore reads is one of the new features of the MaSuRCA assembler, version 3.2.2, which works by constructing long and accurate mega-reads from the combination of long and short read data. Although this is a relatively computationally intensive strategy with long run times, we observed substantial improvement in the genome statistics when compared with Illumina-only assembly. As Nanopore technology becomes more mature, it is likely that future de novo genome assembly will shift toward high-coverage long read–only assembly, followed by multiple iterations of genome polishing using Illumina reads.
Availability of supporting data
Data supporting the results of this article are available in the GigaDB repository [45]. Raw Illumina and Nanopore reads generated in this study are available in the Sequence Read Archive (SRP123679), whereas the Whole Genome Shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession NXFZ00000000, both under BioProject PRJNA407816.
Abbreviations
bp: base pair; CDS: coding sequence; Gb: giga base; kb: kilo base; Mb: mega base; SRA: Sequence read archive; TE: transposable elements; TSA: transcriptome shotgun assembly.
Additional files
Additional file 1: Figure S1: Genome profiling of A. ocellaris based on Illumina short reads.
Additional file 1: Table S1: Summary of raw reads generated from genome and transcriptome sequencing.
Additional file 1: Table S2: Assembly details after each pilon iteration.
Additional file 1: Table S3: Mitogenome similarity of Amphiprion ocellaris between the target sample (NTM A3764) and other isolates with known locality; body-colour phenotype is marked where known.
Competing interests
The authors declare that they have no competing interests.
Supplementary Material
04 Dec 2017 Reviewed
06 Dec 2017 Reviewed
Funding
This study was funded by the Monash University Malaysia Tropical and Biology Multidisciplinary Platform.
References
- 1. Militz TA, Foale S. The “Nemo Effect”: perception and reality of Finding Nemo's impact on marine aquarium fisheries. Nat Biotechnol 2017;18(3):525–7. [Google Scholar]
- 2. Madduppa HH, von Juterzenka K, Syakir M et al. Socio-economy of marine ornamental fishery and its impact on the population structure of the clown anemonefish Amphiprion ocellaris and its host anemones in Spermonde Archipelago, Indonesia. Ocean Coast Manag 2014;100(Supplement C):41–50. [Google Scholar]
- 3. Hall H, Warmolts D. The role of public aquariums in the conservation and sustainability of the marine ornamentals trade. In: Cato JC, Brown CL, eds. Marine Ornamental Species Ames, Iowa, USA: Blackwell Publishing Company; 2008:305–24. [Google Scholar]
- 4. Madhu R, Madhu K, Retheesh T. Life history pathways in false clown Amphiprion ocellaris Cuvier, 1830: a journey from egg to adult under captive condition. J Marine Biol Assoc India 2012;54(1):77–90. [Google Scholar]
- 5. Casas L, Saborido-Rey F, Ryu T et al. Sex change in clownfish: molecular insights from transcriptome analysis. Sci Rep 2016;6:35461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buston P. Social hierarchies: size and growth modification in clownfish. Nature 2003;424(6945):145–6. [DOI] [PubMed] [Google Scholar]
- 7. Kobayashi Y, Horiguchi R, Miura S et al. Sex- and tissue-specific expression of P450 aromatase (cyp19a1a) in the yellowtail clownfish, Amphiprion clarkii. Comp Biochem Physiol A Mol Integr Physiol 2010;155(2):237–44. [DOI] [PubMed] [Google Scholar]
- 8. Davenport D, Norris KS. Observations on the symbiosis of the sea anemone Stoichactis and the pomacentrid fish, Amphiprion percula. Biol Bull 1958;115(3):397–410. [Google Scholar]
- 9. Arvedlund M, Nielsen LE. Do the anemonefish Amphiprion ocellaris (Pisces: Pomacentridae) imprint themselves to their host sea anemone Heteractis magnifica (Anthozoa: Actinidae)? Ethology 1996;102(2):197–211. [Google Scholar]
- 10. Mariscal RN. An experimental analysis of the protection of Amphiprion xanthurus Cuvier & Valenciennes and some other anemone fishes from sea anemones. J Exp Marine Biol Ecol 1970;4(2):134–49. [Google Scholar]
- 11. Hattori A. Coexistence of two anemonefishes, Amphiprion clarkii and A. perideraion, which utilize the same host sea anemone. Environ Biol Fish 1995;42(4):345–53. [Google Scholar]
- 12. Schmiege PF, D’Aloia CC, Buston PM. Anemonefish personalities influence the strength of mutualistic interactions with host sea anemones. Marine Biol 2017;164(1):24. [Google Scholar]
- 13. Allen GR. Damselfishes of the World. Melle, Germany: Mergus Publishers; 1991. [Google Scholar]
- 14. Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics 2016;107(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Austin CM, Tan MH, Harrisson KA et al. De novo genome assembly and annotation of Australia's largest freshwater fish, the Murray cod (Maccullochella peelii), from Illumina and Nanopore sequencing read. Gigascience 2017;6(8):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gan HM, Lee YP, Austin CM. Nanopore long-read guided complete genome assembly of Hydrogenophaga intermedia, and genomic insights into 4-aminobenzenesulfonate, p-aminobenzoic acid and hydrogen metabolism in the genus Hydrogenophaga. Front Microbiol 2017;8:1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zimin AV, Puiu D, Hall R et al. The first near-complete assembly of the hexaploid bread wheat genome, Triticum aestivum. Gigascience 2017;6(11):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zimin AV, Stevens KA, Crepeau MW et al. An improved assembly of the loblolly pine mega-genome using long-read single-molecule sequencing. Gigascience 2017;6(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zimin AV, Puiu D, Luo M-C et al. Hybrid assembly of the large and highly repetitive genome of Aegilops tauschii, a progenitor of bread wheat, with the mega-reads algorithm. Genome Res 2017;27(5):787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014;30(15):2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 2014;15(3):R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marçais G, Kingsford C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 2011;27(6):764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vurture GW, Sedlazeck FJ, Nattestad M et al. GenomeScope: fast reference-free genome profiling from short reads. Bioinformatics 2017;33(14):2202–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bushnell B. BBMap Short Read Aligner. Berkeley, CA: University of California; 2016. http://sourceforgenet/projects/bbmap (15 June 2017, date last accessed). [Google Scholar]
- 25. http://www.genomesize.com (15 June 2017, date last accessed). [Google Scholar]
- 26. Zimin AV, Marçais G, Puiu D et al. The MaSuRCA genome assembler. Bioinformatics 2013;29(21):2669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walker BJ, Abeel T, Shea T et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 2014;9(11):e112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simão FA, Waterhouse RM, Ioannidis P et al. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015;31(19):3210–2. [DOI] [PubMed] [Google Scholar]
- 29. Shao M, Kingsford C. Accurate assembly of transcripts through phase-preserving graph decomposition. Nat Biotechnol 2017; doi:10.1038/nbt.4020. https://www.nature.com/articles/nbt.4020#supplementary-information (15 June 2017, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 2015;12:357 https://www.nature.com/articles/nmeth.3317#supplementary-information (15 June 2017, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilber D. Gene-omes built from mRNA seq not genome DNA. F1000Res 2016;5(1695):1. [Google Scholar]
- 32. Holt C, Yandell M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinformatics 2011;12(1):491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hubbard T, Barker D, Birney E et al. The Ensembl genome database project. Nucleic Acids Res 2002;30(1):38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Korf I. Gene finding in novel genomes. BMC Bioinformatics 2004;5(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stanke M, Steinkamp R, Waack S et al. AUGUSTUS: a web server for gene finding in eukaryotes. Nucleic Acids Res 2004;32(suppl–2):W309–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boratyn GM, Camacho C, Cooper PS et al. BLAST: a more efficient report with usability improvements. Nucleic Acids Res 2013;41(W1):W29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zdobnov EM, Apweiler R. InterProScan–an integration platform for the signature-recognition methods in InterPro. Bioinformatics 2001;17(9):847–8. [DOI] [PubMed] [Google Scholar]
- 38. Gan HM, Schultz MB, Austin CM. Integrated shotgun sequencing and bioinformatics pipeline allows ultra-fast mitogenome recovery and confirms substantial gene rearrangements in Australian freshwater crayfishes. BMC Evol Biol 2014;14(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grandjean F, Tan MH, Gan HM et al. Rapid recovery of nuclear and mitochondrial genes by genome skimming from Northern Hemisphere freshwater crayfish. Zool Scripta 2017; doi:10.1111/zsc.12247. [Google Scholar]
- 40. Hahn C, Bachmann L, Chevreux B. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads—a baiting and iterative mapping approach. Nucleic Acids Res 2013;41(13):e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iwasaki W, Fukunaga T, Isagozawa R et al. MitoFish and MitoAnnotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol Biol Evol 2013;30(11):2531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kallivretaki E, Eggen R, Neuhauss S et al. Aromatase in zebrafish: a potential target for endocrine disrupting chemicals. Mar Environ Res 2006;62(90):7. [DOI] [PubMed] [Google Scholar]
- 43. Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinformatics 2013;14(2):178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li H. Minimap2: fast pairwise alignment for long nucletide sequences. arXiv 2017. https://arxiv.org/abs/1708.01492 (15 June 2017, date last accessed). [Google Scholar]
- 45. Tan MH, Austin CM, Hammer MP et al. Supporting data for “Finding Nemo: hybrid assembly with oxford nanopore and illumina reads greatly improves the clownfish (Amphiprion ocellaris) genome assembly.” GigaScience Database 2017. http://dx.doi.org/10.5524/100397 (15 June 2017, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bray NL, Pimentel H, Melsted P et al. Near-optimal probabilistic RNA-seq quantification. Nat Biotech 2016;34(5):525–7. http://www.nature.com/nbt/journal/v34/n5/abs/nbt.3519.html#supplementary-information (15 June 2017, date last accessed). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
04 Dec 2017 Reviewed
06 Dec 2017 Reviewed