Abstract
Context
Observational data support a role for vitamin D in type 2 diabetes, but evidence from trials is inconclusive.
Objective
To evaluate the effect of vitamin D supplementation on β-cell function and hemoglobin A1c (HbA1c) in patients with well-controlled type 2 diabetes.
Design
Double-blind, randomized, placebo-controlled clinical trial.
Setting
Tufts Medical Center, Boston, MA; VA Medical Center, Cincinnati, OH.
Participants
A total of 127 patients (mean age, 60 years) with stable (HbA1c ≤7.5%) diabetes managed with lifestyle only or lifestyle plus metformin.
Intervention
Subjects were given 4000 units of vitamin D3 (cholecalciferol) daily or placebo for 48 weeks.
Main Outcome Measure
Insulin secretion rate (ISR) was estimated from peripheral plasma C-peptide levels after a 3-hour 75-g oral glucose tolerance test done at baseline and week 24. Changes in HbA1c were assessed at 16, 24, 36, and 48 weeks.
Results
Baseline mean plasma 25-hydroxyvitamin D [25(OH)D] concentration was 26.6 ng/mL, mean HbA1c was 6.6%, and 78% of patients were on metformin. At week 24, mean 25(OH)D changed by 20.5 and −1.6 ng/mL in the vitamin D and placebo groups, respectively (P < 0.001). The vitamin D and placebo groups did not differ in change in ISR or HbA1c. Among patients treated with lifestyle only (n = 28), vitamin D supplementation reduced HbA1c compared with placebo (−0.1% vs 0.3%, respectively; P = 0.034) at week 24. This result was not observed at the other time points and could be due to chance.
Conclusion
Vitamin D3 at 4000 IU/d did not change ISR or HbA1c in patients with well-controlled type 2 diabetes on metformin not selected for vitamin D deficiency.
Keywords: beta cell function, clinical trial, diabetes mellitus, insulin sensitivity, vitamin D
In adults with stable type 2 diabetes, there was no effect of 4000 IU/d of vitamin D for 24 weeks on β-cell function and HbA1c. HbA1c was reduced in patients not on diabetes pharmacotherapy.
Although type 2 diabetes–specific pharmacotherapy is well studied and efficacious, it is associated with poor long-term adherence, potential side effects, and high costs. Therefore, identification of alternative therapeutic options that can be applied at the public health level are needed to decrease diabetes-related burdens and costs.
Suboptimal vitamin D status has emerged as a potential contributor to the pathophysiology of type 2 diabetes, with several lines of evidence supporting a role for vitamin D in pancreatic β-cell function and insulin sensitivity [1, 2]. Several trials have examined the effect of vitamin D supplementation (with or without calcium) on glycemia and insulin sensitivity in patients with type 2 diabetes; results are summarized in recent meta-analyses [3–7]. George et al. [3] performed a meta-analysis of trials among patients with type 2 diabetes or impaired glucose tolerance and noted a significant reduction of fasting glucose (~6 mg/dL) and improvement in insulin sensitivity in patients given vitamin D supplementation vs placebo. A meta-analysis by Seida et al. [4] reported nonstatistically significant improvements in hemoglobin A1c (HbA1c), fasting glucose, and insulin sensitivity. In 2017, three meta-analyses of trials using vitamin D in patients with type 2 diabetes reported concordant results [5–7]. In the first study, Wu et al. [5] found that vitamin D supplementation reduced HbA1c significantly (24 trials) and reduced fasting glucose nonsignificantly (18 trials). Among patients with baseline 25-hydroxyvitamin D [25(OH)D] <20 ng/mL, reductions in HbA1c and fasting glucose were significant. In the second study, Mirhosseini et al. [6] reported statistically significant reductions in HbA1c, fasting glucose, and insulin resistance (assessed by Homeostatic Model Assessment of Insulin Resistance) after vitamin D supplementation compared with placebo in an analysis of 24 trials. Finally, Krul-Poel et al. [7] reported no statistically significant improvements in HbA1c and fasting glucose favoring vitamin D. Although the summary results from these meta-analyses support a beneficial effect of vitamin D supplementation on glycemia and insulin sensitivity in patients with type 2 diabetes, the available trials were short term and of varied quality, had small sample sizes, and had a varied mode of vitamin D administration; therefore, definitive conclusions cannot be drawn from individual trials and meta-analyses. Only two small, short-term trials (n = 24 patients, 16 weeks [8]; n = 37 patients, 12 weeks [9]) have been conducted in the United States; therefore, the effect of vitamin D supplementation among American adults with type 2 diabetes is not known.
The aim of this study was to evaluate the effectiveness of oral daily vitamin D supplementation on pancreatic β-cell function and glycemia and its safety in US adults with well-controlled type 2 diabetes.
1. Materials and Methods
A. Overview of Study Design
The Vitamin D for Established Type 2 Diabetes Mellitus (DDM2) study was a two-site (Tufts Medical Center, Boston, MA, and Veterans Affairs Medical Center, Cincinnati, OH), parallel-group, double-blind, randomized, placebo-controlled trial conducted between March 2013 (first patient enrolled) and July 2015 (final data collection) to examine the effect of vitamin D supplementation on pancreatic β-cell function (primary outcome) and HbA1c (secondary outcome) in US adults with stable, well-controlled type 2 diabetes. The study was approved by the Institutional Review Board of Tufts Medical Center and University of Cincinnati, and all participants provided written informed consent.
B. Setting and Participants
Patients were recruited via a search of electronic medical records. Eligible patients were 25 to 75 years of age, had a body mass index (BMI) of 23 to 42 kg/m2, had type 2 diabetes defined by being treated with a stable dose of metformin monotherapy or meeting laboratory criteria for diabetes at screening [fasting glucose ≥126 mg/dL, HbA1c ≥6.5%, or glucose ≥200 mg/dL 2 hours after a 75-g oral glucose load (2hPG)], and had stable diabetes defined by HbA1c ≤7.5% without any anticipated change in diabetes therapy in the next 24 weeks. Exclusion criteria included use of any diabetes pharmacotherapy other than metformin; history of type 1 diabetes or secondary diabetes (e.g., cystic fibrosis); and recent history of hyperparathyroidism, nephrolithiasis, or hypercalcemia. Vitamin D status, defined by blood 25(OH)D concentration at baseline, was not an inclusion criterion because (1) the definition of “optimal” vitamin D status is controversial, and no consensus exists on an optimal blood 25(OH)D level [10–14]; (2) 25(OH)D concentration varies by season and race [10]; (3) the study was designed to be as inclusive as possible to ensure that results are generalizable to clinical practice; and (4) screening with blood 25(OH)D would have been cumbersome and expensive.
C. Intervention
After baseline measurements, patients were randomly assigned in a 1:1 ratio to receive either one pill of 4000 IU of vitamin D3 (cholecalciferol) or placebo daily for 48 weeks. The dose of 4000 IU daily was selected to provide an appropriate balance of safety (consistent with the upper safe limit suggested by the Institute of Medicine [10]) and efficacy in terms of obtaining a substantial difference in blood 25(OH)D concentration between the active and placebo groups. Randomization was computer generated in random blocks of four or eight and stratified by BMI (<30 or ≥30 kg/m2), race [white or non-white (e.g., American Indian, Asian, Pacific Islander, Black)], and diabetes therapy (lifestyle only or lifestyle plus metformin). Patients and investigators were blinded to treatment assignment. For the first 24 weeks, patients’ physicians were asked not to change diabetes therapy whenever possible. For the last 24 weeks, changes in pharmacotherapy were allowed consistent with good clinical practice. Vitamin D3 and matching placebos were manufactured by Tishcon Corporation (Salisbury, MD). Quality control analyses were performed on each lot shipped from the manufacturing plant to ensure that vitamin D pills contained the stated amount. The first lot contained 4400 IU and the second lot 4384 IU per pill; both amounts are within specifications (3600 to 4800 IU). Placebo pills did not contain vitamin D. Adherence was assessed by pill count as the percentage of pills taken in relation to the number that should have been taken. A food frequency questionnaire was completed at baseline [15, 16].
D. Follow-up and Measurements
Patients were seen at 16, 24, 36, and 48 weeks, and blood was obtained after an 8-hour overnight fast. Patients held their study pill (vitamin D or placebo), metformin, and any other medications until after testing was completed. At baseline and week 24, a 75-g oral glucose tolerance test (OGTT) was done, and blood for glucose, insulin, and C-peptide was collected at 0, 15, 30, 60, 90, 120, 150, and 180 minutes after ingestion of the glucose load. Screening and safety laboratories and HbA1c were analyzed on the same day at each site’s clinical laboratory. Blood for other outcomes was processed and stored at −80°C until analyses, which were done by the central laboratory (Tufts Medical Center or Duke University) in pairs (before/after intervention) in the same analytical run to reduce systematic error and interassay variability. HbA1c was measured at baseline and at week 16, 24, 36, and 48 using high-performance liquid chromatography (Tosoh G8; Tosoh Bioscience, South San Francisco, CA; coefficient of variation 0.78 to 1.89). Total 25(OH)D was measured by liquid chromatography-mass spectrometry, which was certified through the National Institute of Standards and Technology vitamin D quality assurance program. Glucose was measured on a Beckman DxC600 clinical analyzer (Beckman, Brea, CA; coefficient of variation <1%). Insulin and C-peptide were measured by enzyme-linked immunosorbent assay (Alpco, Salem, NH; coefficient of variation <5%).
E. Outcomes
The primary endpoint was change from baseline to 24 weeks in insulin secretion rate (ISR), estimated from glucose and C-peptide from the OGTT by deconvolution analysis using a two-compartment model with standard parameters for C-peptide kinetics [17, 18]. Additional indices included change in β-cell function by the Insulin Secretion-Sensitivity Index-2 (ISSI-2) [19], which is equivalent to the disposition index for β-cell function, and change in insulin sensitivity by the Matsuda insulin sensitivity index (ISI-M), calculated as [10,000/sqrt (G0 × I0 × G120 × I120)], where 10,000 is a constant, G0 and G120 represent the plasma glucose concentrations at time 0 and 120 minutes, I0 and I120 are the plasma insulin concentrations at time 0 and 120 minutes, and sqrt is the mathematical function to calculate the square root [20]. ISSI-2 is defined as the incremental area under the curve (i.e., above baseline) for insulin (AUCins) to the incremental area under the curve for glucose (AUCglu) multiplied by the ISI-M [(AUCins/AUCglu)ISI-M] [21, 22]. Other prespecified analyses included change in HbA1c, change in glycemia, variability of response to vitamin D supplementation in subgroups defined by the following baseline characteristics: race (white vs non-white), 25(OH)D concentration (<20 vs ≥20 ng/mL), diabetes therapy (lifestyle only vs lifestyle plus metformin), effect of vitamin D supplementation on plasma 25(OH)D concentration, and safety and tolerability of vitamin D supplementation. Change in glycemia was a dichotomous (“decrease” or “no change”) composite outcome to assess concomitant reductions in the related outcomes of HbA1c and diabetes medication use. Patients who had a “decrease” in the diabetes medications or a reduction of ≥0.4 HbA1c units from baseline without increasing medications were defined as having achieved the composite outcome.
F. Statistical Methods
Analyses were by intention-to-treat, defined as all participants in their randomly assigned treatment group and including all available measurements irrespective of adherence to assigned treatment. The difference between treatment groups in the primary endpoint (i.e., change from baseline to 24 weeks in ISR) was assessed using linear regression. Analyses adjusted for the stratification variables [(BMI <30 or ≥30 kg/m2), race (white vs non-white), and diabetes therapy (lifestyle only vs lifestyle plus metformin)] and baseline value of the outcome variable.Between-group differences for the change in HbA1c were determined using a mixed model approach to account for within-participant correlation across the four time points. A linear interaction term between treatment assignment and time from baseline visit, included as a covariate, was used to assess if the change trajectories in HbA1c levels differed significantly between randomization groups. Secondary analyses explored potential nonlinear changes over the 48 weeks of follow-up. The likelihood of achieving the “glycemia” composite outcome was assessed with χ2 tests. In sample size calculations for the primary endpoint at 6 months, we assumed a between-group difference at week 24 in “change in total ISR” of 65 pmol/min/m2, a standard deviation of the difference in ISR of 104 (which represents a difference of ~30% improvement) [21–23], and a 90% participant retention rate. The required sample size assuming a two-sided 5% type 1 error rate and 90% power was 124 participants. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
2. Results
A. Participants and Baseline Characteristics
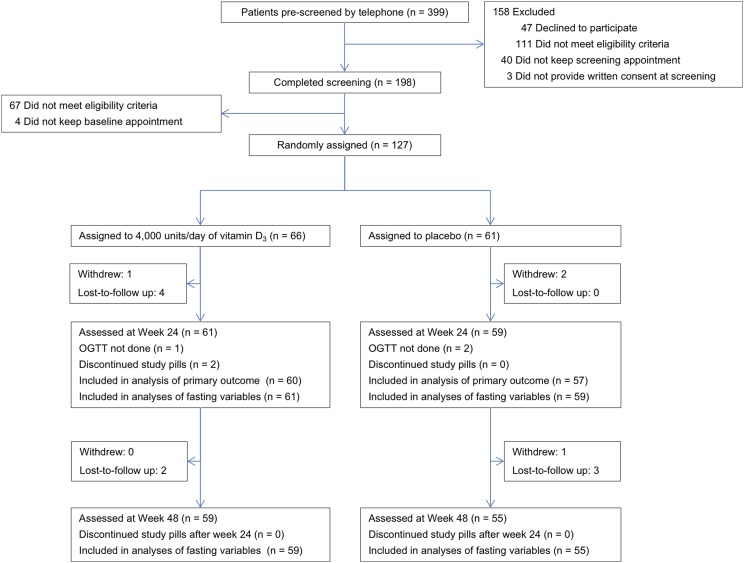
From February 2013 through July 2014, a total of 399 patients were prescreened by telephone, and 198 completed in-person screening (Fig. 1). Of these, 127 met eligibility criteria and were randomly assigned to vitamin D3 (n = 66) or placebo (n = 61). Patient characteristics at baseline were balanced between the two groups, especially for the key factors BMI, race, and diabetes therapy (Table 1). Consistent with a population with type 2 diabetes, the mean age was 60.2 years, and mean BMI was 30.9 kg/m2. Diabetes was well controlled (mean HbA1c, 6.6%), and most patients (78%) were on metformin. Thirty percent were women, and 62% were white. Mean plasma 25(OH)D concentration was 26.6 ng/mL. Twenty-six percent of patients (n = 33) had a 25(OH)D concentration <20 ng/mL. Mean self-reported vitamin D intake was 399 IU/d.
Figure 1.
Flow of participants. All four patients who withdrew from the study did so for personal reasons. Three patients returned for the week 24 visit but did not complete the OGTT for technical reasons. These patients are not included in the analysis of OGTT-based indices but are included in the HbA1c analysis.
Table 1.
Baseline Characteristics
| Characteristics | Total (n = 127) | Vitamin D (n = 66) | Placebo (n = 61) |
|---|---|---|---|
| Demographics | |||
| Age, y | 60.2 (8.4) | 60.1 (8.4) | 60.3 (8.5) |
| Female, n (%) | 38 (29.9) | 17 (25.8) | 21 (34.4) |
| Race, n (%)a | |||
| White | 79 (62.2) | 41 (62.1) | 38 (62.3) |
| Black or African-American | 38 (29.9) | 17 (25.8) | 21 (34.4) |
| Asian | 6 (4.7) | 4 (6.1) | 2 (3.3) |
| Other | 4 (3.1) | 4 (6.1) | 0 (0) |
| Hispanic or Latino ethnicity, n (%)a | 3 (2.4) | 2 (3.0) | 1 (1.6) |
| Season at study entry, n (%) | |||
| January–March | 33 (26.0) | 15 (22.7) | 18 (29.5) |
| April–June | 45 (35.4) | 27 (40.9) | 18 (29.5) |
| July–September | 32 (25.2) | 17 (25.8) | 15 (24.6) |
| October–December | 17 (13.4) | 7 (10.6) | 10 (16.4) |
| Self-reported vitamin D intake, units/db | 399 (343) | 407 (381) | 391 (301) |
| Self-reported calcium intake, mg/db | 931 (513) | 1006 (597) | 852 (394) |
| Smoking status, n (%) | |||
| Never smoked | 48 (37.8) | 22 (33.3) | 26 (42.6) |
| Currently smoking | 14 (11.0) | 8 (12.1) | 6 (9.8) |
| Formerly smoked | 64 (50.4) | 35 (53.0) | 29 (47.5) |
| Prefer not to answer | 1 (0.8) | 1 (1.5) | 0 (0) |
| Clinical characteristics | |||
| Weight, kg | 92.0 (15.5) | 91.2 (15.9) | 92.9 (15.1) |
| BMI, kg/m2 | 30.9 (3.8) | 30.7 (3.9) | 31.2 (3.8) |
| Metformin use, n (%) | 99 (78.0) | 51 (77.3) | 48 (78.7) |
| Laboratory | |||
| Serum calcium, mg/dL | 9.5 (0.4) | 9.5 (0.4) | 9.4 (0.3) |
| Vitamin D status | |||
| Plasma 25(OH)D, ng/mL | 26.6 (11.1) | 25.8 (10.3) | 27.5 (12.0) |
| Plasma 25(OH)D <20 ng/mL, n (%) | 33 (26.0) | 19 (28.8) | 14 (23.0) |
| Plasma 25(OH)D <30 ng/mL, n (%) | 80 (63.0) | 42 (63.6) | 38 (62.3) |
| HbA1c, % | 6.6 (0.5) | 6.6 (0.6) | 6.5 (0.5) |
Values are means (standard deviation) unless otherwise specified. Percentages may not total 100 because of rounding. To convert 25(OH)D from ng/mL to mmol/L, multiply by 2.456; to convert vitamin D intake from international units to μg, divide by 40.
Race and ethnicities were self-reported and followed National Institutes of Health guidelines. Participants could check multiple categories. Asian includes “Chinese” (n = 3) and “Other Asian” (n = 3). Other includes “Native Hawaiian or Other Pacific Islander” (n = 1) and “Other” (n = 3).
Data are derived from the food frequency questionnaire.
B. Retention and Adherence to Study Pills
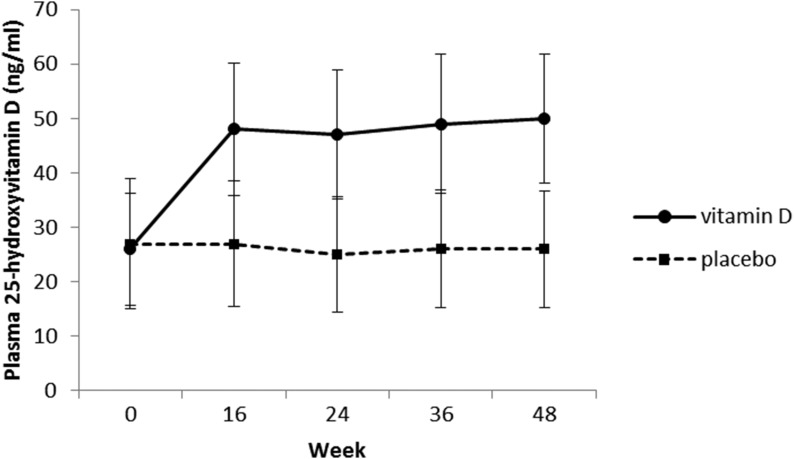
Of the 127 patients, 94% (n = 120) and 90% (n = 114) returned for the week 24 and week 48 visits, respectively (Fig. 1). Mean adherence to study pills was 94% for vitamin D3 and 93% for placebo. At week 24, the plasma 25(OH)D concentration was 47.1 ng/mL (an increase of 20.5 ng/mL from baseline) in the vitamin D3 group vs 25.5 ng/mL (a decrease of 1.6 ng/mL from baseline) in the placebo group (P < 0.001 for vitamin D3 vs placebo) (Fig. 2). Plasma 25(OH)D concentration remained constant at week 48.
Figure 2.
Plasma 25(OH)D concentration during the study. *P < 0.001 for vitamin D vs placebo.
C. Change in OGTT-Based Indices at Week 24
After supplementation with vitamin D, there was no difference in the change from baseline to 24 weeks in fasting or total ISR between the two groups (Table 2). There was also no difference in the ISI-M between vitamin D and placebo. The ISSI-2 increased in vitamin D vs placebo-treated patients, but the difference was not statistically significant (0.149 for vitamin D vs 0.023 for placebo; P = 0.118). Changes in AUCglu and AUCins were not different between the two groups. There was also no difference in glycemia and C-peptide between vitamin D and placebo (Supplemental Fig. 1).
Table 2.
Effects of Vitamin D Supplementation on OGTT Indices at 24 Weeks
|
Change from Baseline to 24 weeks
|
P Value | |||
|---|---|---|---|---|
| N used | Vitamin D (n = 61) | Placebo (n = 59) | ||
| ISR fasting, pmol/min/m2 | 120 | −0.700 ± 5.237 | −1.684 ± 5.326 | 0.896 |
| ISR total, pmol/min/m2 | 118 | 622.88 ± 1786.0 | 778.41 ± 1816.6 | 0.952 |
| ISI-Matsuda | 118 | 0.111 ± 0.264 | −0.108 ± 0.269 | 0.562 |
| ISSI-2 | 118 | 0.149 ± 0.056 | 0.023 ± 0.057 | 0.118 |
| AUCglu | 119 | 3.357 ± 883.27 | −130.7 ± 890.75 | 0.915 |
| AUCins | 119 | −772.5 ± 424.61 | −890.3 ± 428.19 | 0.845 |
Values are mean ± standard error of the mean after adjustment for stratified variables [(BMI <30 or ≥30 kg/m2), race (white vs non-white), diabetes therapy (metformin or lifestyle)], and baseline value of the outcome variable.
After excluding four participants who changed diabetes medications during the first 24 weeks, results did not change (not shown). In subgroup analyses by diabetes therapy (lifestyle only vs lifestyle plus metformin), 25(OH)D concentration at baseline (<20 vs ≥20 ng/mL), or race (white vs nonwhite), there was no significant effect of vitamin D supplementation on OGTT-based indices compared with placebo (results not shown).
D. Change in Glycemia at Week 24 and Week 48
During follow-up, HbA1c concentration increased in both the vitamin D and placebo groups without any difference between the two treatment groups (Table 3). More patients treated with vitamin D experienced improvement in the “glycemia” composite outcome at 24 weeks (14.8% vs 10.2% for vitamin D vs placebo, respectively) and 48 weeks (10.2% vs 5.4%), but these differences were not statistically significant.
Table 3.
Effect of Vitamin D Supplementation on Glycemia
| N Used in Analyses |
Change From Baseline
|
P Value | ||
|---|---|---|---|---|
| Vitamin D (n = 61) | Placebo (n = 59) | |||
| HbA1c, % | 0.882a | |||
| Wk 16 | 117 | 0.1 ± 0.06 | 0.1 ± 0.06 | 0.749b |
| Wk 24 | 120 | 0.1 ± 0.06 | 0.1 ± 0.06 | 0.699b |
| Wk 36 | 114 | 0.2 ± 0.07 | 0.1 ± 0.07 | 0.581b |
| Wk 48 | 114 | 0.2 ± 0.06 | 0.2 ± 0.07 | 0.866b |
| Change in glycemia from baseline to wk 24, % (n/N)c | 120 | |||
| Decreased | 14.8 (9/61) | 10.2 (6/59) | 0.448 | |
| Increased or no change | 85.2 (52/61) | 89.8 (53/59) | ||
| Change in glycemia from baseline to wk 48, % (n/N) | 114 | |||
| Decreased | 10.2 (6/59) | 5.4 (3/55) | 0.351 | |
| Increased or no change | 89.8 (53/59) | 94.6 (52/55) | ||
Values are mean ± standard error of the mean after adjustment for stratified variables [(BMI <30 or ≥30 kg/m2), race (white vs non-white), diabetes therapy (metformin or lifestyle)] and baseline value of the outcome variable.
P value for the overall difference over time from linear mixed model.
P values for the difference at each time point.
“Change in glycemia” is a composite outcome prespecified as follows: “decreased,” if the intensity of diabetes medication was lowered or HbA1c decreased by ≥0.4% during the follow-up period; “Increased or no change.”
After excluding four participants who changed diabetes medications by week 24 and 11 participants who changed diabetes medications at any point during the trial, HbA1c results did not change (results not shown). Among patients treated with lifestyle only (n = 28), vitamin D supplementation reduced HbA1c compared with placebo (−0.1 vs 0.3%, respectively; P = 0.034) at week 24 (Table 4); however, there was no difference at the other time points. More patients treated with vitamin D also experienced improvement in the “glycemia” composite outcome (28.6% vs 0% for vitamin D vs placebo, respectively); however, the differences were not statistically significant. In subgroup analyses by 25(OH)D concentration at baseline (<20 vs ≥20 ng/mL) or race (white vs non-white), there was no significant effect of vitamin D supplementation on change in HbA1c compared with placebo (results not shown).
Table 4.
Effect of Vitamin D Supplementation on Glycemia Among Patients Treated With Lifestyle Only (i.e., Not on Metformin)
|
Change From Baseline
|
P Value | |||
|---|---|---|---|---|
| N Used in Analyses | Vitamin D (n = 14) | Placebo (n = 13) | ||
| Hba1c, % | 0.515a | |||
| Wk 16 | 27 | 0.1 ± 0.14 | 0.1 ± 0.14 | 0.929b |
| Wk 24 | 27 | −0.1 ± 0.12 | 0.3 ± 0.12 | 0.034b |
| Wk 36 | 27 | −0.0 ± 0.13 | 0.1 ± 0.13 | 0.450b |
| Wk 48 | 27 | 0.0 ± 0.12 | 0.0 ± 0.12 | 0.954b |
| Change in glycemia from baseline to wk 24, % (n/N)c | 27 | 0.098 | ||
| Decrease | 28.6 (4/14) | 0(0/13) | ||
| Increase or no change | 71.4 (10/14) | 100 (13/13) | ||
| Change in glycemia from baseline to wk 48, % (n/N) | 27 | 0.222 | ||
| Decrease | 21.4 (3/14) | 0 (0/13) | ||
| Increase or no change | 78.6 (11/14) | 100 (13/13) | ||
Values are mean ± standard error of the mean after adjustment for stratified variables [BMI <30 or ≥30 kg/m2, race (white vs non-white), diabetes therapy (metformin or lifestyle)], and baseline value of the outcome variable.
P value for the overall difference over time from linear mixed model.
P values for the difference at each time point.
“Change in glycemia” is a composite outcome prespecified as follows: “decreased,” if the intensity of diabetes medication was lowered or HbA1c declined by ≥0.4% during the follow-up period; “Increased or no change.”
E. Safety
The supplements were well tolerated. Two patients discontinued study pills, one due to an adverse event (nephrolithiasis, found incidentally on imaging) and one due to personal choice. Both patients returned for their follow-up visits. There were no cases of hypercalcemia (defined as higher than the upper limit of the normal for the local laboratory). Adverse event rates were similar in the two groups [0.62 adverse events per patient-year (35 adverse events during 55.9 patient-years of follow-up) in vitamin D vs 0.85 adverse events per patient-year (45 adverse events during 52.6 patient-years of follow-up)] in placebo.
3. Discussion
In this double-blind, placebo-controlled, randomized clinical trial, the effect of oral daily vitamin D3 supplementation was investigated in patients with well-controlled type 2 diabetes not selected for vitamin D deficiency. Despite a substantial increase in plasma 25(OH)D concentration, there were no differences in OGTT-based insulin secretion or insulin sensitivity indices after 24 weeks of vitamin D supplementation compared with placebo. We did not find a significant effect of vitamin D supplementation on HbA1c after 48 weeks. In a subgroup of patients treated with lifestyle only, there was a reduction of the HbA1c in the vitamin D group compared with placebo at week 24, which was not seen at other time points.
A. Studies (Observational or Trials) With Similar OGTT Indices
Observational studies support an association between blood 25(OH)D concentration and OGTT-based indices of β-cell function (assessed by ISSI-2) [24–27] and insulin sensitivity (assessed by Matsuda Index) [28, 29]. Although the summary results from meta-analyses of trials in patients with type 2 diabetes suggest small benefits of vitamin D supplementation in glycemia and insulin sensitivity in patients with type 2 diabetes [5–7], the available trials are short-term and of varied quality, have small sample sizes, and have varied modes of vitamin D administration; therefore, firm conclusions cannot be drawn. In the largest trial to date in 266 patients with type 2 diabetes in Netherlands, there was no difference in HbA1c or insulin resistance by Homeostatic Model Assessment of Insulin Resistance [30]. However, in patients with baseline 25(OH)D <12 ng/mL, there was a significant reduction in HbA1c with vitamin D supplementation. This trial involved a well-controlled patient population (baseline HbA1c, 6.8%) and gave high intermittent doses of vitamin D (50,000 IU/mo; equivalent to 1600 units/d), which may not be physiologic [31].
Only two trials based in the United States have tested the effect of vitamin D supplementation in patients with type 2 diabetes. Both studies are small and short-term. Soric et al. [9] randomized 37 patients with type 2 diabetes (baseline HbA1c, 8.6%) to 2000 IU/d vitamin D3 or 500 mg/d vitamin C for 12 weeks and reported a nonstatistically significant change in HbA1c favoring vitamin D (−0.4% vs 0.1%). Patel et al. [8] randomized 32 patients with type 2 diabetes (HbA1c, 6.7%) to 1200 or 400 IU/d of vitamin D3 and found no change in HbA1c or insulin resistance quantitative insulin sensitivity check index between the two groups in 24 patients with complete data.
B. Why Negative Results
There are several possible explanations as to why vitamin D supplementation did not improve glycemic control of insulin secretion in our cohort besides the possibility that vitamin D may not be relevant to glucose metabolism. The baseline 25(OH)D concentration of the cohort was ∼27 ng/mL, which may indicate an adequate vitamin D status for the metabolic outcomes assessed in DDM2, as suggested by a recent meta-analysis that reported a significant reduction in HbA1c and fasting glucose after vitamin D supplementation only among patients with baseline 25(OH)D <20 ng/mL. Therefore, the study’s results might have been different if the patients had lower baseline 25(OH)D concentrations. In subgroup analyses, we found no difference among patients with 25(OH)D <20 ng/mL at baseline; however, there were too few individuals with these levels (n = 33) to provide adequate statistical power to evaluate this possibility. Likewise, vitamin D may have no detectable effect in persons with well-controlled diabetes. We specifically recruited patients with good glycemic control to minimize possible interference by multiple antidiabetic drugs or the need to change diabetes pharmacotherapy during the study. As a result, the DDM2 cohort had excellent control of diabetes (mean HbA1c, 6.6%), which may have made it difficult to detect an effect of vitamin D. Additionally, most patients (78%) were on metformin, which may have masked a small effect of vitamin D supplementation on outcomes because there was essentially no change in the placebo group at week 24 (HbA1c rose by only 0.1%). In contrast, in the subgroup of patients managed with lifestyle only, there was a reduction in HbA1c at 24 weeks (−0.1 for vitamin D vs 0.3% for placebo). This result was not observed at the other time points and could be due to chance because of multiple comparisons. These results indicate that any effect of vitamin D supplementation is expected to be relatively small and would be most noticeable among patients with early diabetes not requiring pharmacotherapy. Finally, our power calculations may have been too optimistic, and it is possible that a larger sample size and longer follow-up would be needed to observe an effect.
C. Assessment of Safety and Tolerability of 4000 IU/d of Vitamin D3
A potential concern with long-term vitamin D supplementation is the development of hypercalcemia, hypercalciuria, and kidney stones. A recent meta-analysis of trials showed that vitamin D supplementation increased the risk of hypercalcemia and hypercalciuria in a non–dose-related fashion but did not increase risk of nephrolithiasis [32]. The dosage of 4000 IU/d is the upper limit for toxicity by the Institute of Medicine [10]; however, the safety of this daily dosage has not been tested in a long-term trial. Exposure to 4000 IU/d of vitamin D3 for 48 weeks was safe in the DDM2 trial: there were no cases of hypercalcemia and only one case of nephrolithiasis, which was detected incidentally. No patient withdrew for safety reasons.
D. Strengths and Limitations
Given the multiple factors than can influence glycemia, the DDM2 trial carefully monitored and adjusted for potential confounders between active and placebo groups (e.g., concurrent diabetes medications). The study has additional strengths, including its randomized, double-blind, placebo-controlled study design; the use of a high-dose (4000 IU) daily vitamin D supplementation; a baseline 25(OH)D that is representative of the US adult population [33]; and excellent retention of participants and adherence to study medications. The study also has some limitations. Because one clinical site recruited patients from a Veterans Administration medical center, the cohort had relatively few women (30%). Additionally, selection of a population that is deplete with vitamin D at baseline might be necessary to see an effect of vitamin D supplementation, especially in a well-controlled cohort that progresses slowly.
4. Conclusion
Daily administration of 4000 IU vitamin D3 compared with placebo did not improve HbA1c or OGTT-based indices of β-cell function or insulin secretion in the largest US-based trial among patients with well-controlled, stable type 2 diabetes not selected for vitamin D deficiency. A reduction in HbA1c was observed at week 24 among patients treated with lifestyle only; however, this result could be due to chance.
Supplementary Material
Acknowledgments
We thank the DDM2 trial participants for their outstanding dedication and commitment to the study, the staff at the Tufts Medical Center Clinical Translational Research Center (a unit of the Tufts CTSI) and the Clinical Research Unit at the Cincinnati VA Medical Center (a unit of the University of Cincinnati CCTST) for assistance with study participants, the Tufts Medical Center Clinical Laboratory, and Dr. Radha Krishna at the Duke Molecular Physiology Institute for assay measurements.
Financial Support: This work was supported by National Institute of Diabetes and Digestive and Kidney Disease Research Grant R01DK76092 (to A.G.P.); by National Center for Research Resources Grants UL1RR025752 (to Tufts Medical Center) and UL1TR001425 (to University of Cincinnati); and by funds from the US Department of Agriculture Cooperative Agreement No. 58-1950-04-014401 from the USDA Agricultural Research Service (to B.D.H.).
Clinical Trial Information: ClinicalTrials.gov no. NCT01736865 (registered 29 November 2012).
Author Contributions: A.G.P., D.D., and B.D.H. designed the study. E.A. and J.N. analyzed the data. E.A. and A.G.P. wrote the manuscript draft. All authors contributed to the writing and critical review of the manuscript. E.A. and A.G.P. are the guarantors of this work and, as such, had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- AUCglu
area under the curve glucose
- AUCins
area under the curve insulin
- BMI
body mass index
- DDM2
Vitamin D for Established Type 2 Diabetes Mellitus
- HbA1c
hemoglobin A1c
- ISI-M
Matsuda insulin sensitivity index
- ISR
insulin secretion rate
- ISSI-2
Insulin Secretion-Sensitivity Index-2
- OGTT
oral glucose tolerance test.
References and Notes
- 1. Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2014;43(1):205–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leung PS. The potential protective action of vitamin D in hepatic insulin resistance and pancreatic islet dysfunction in type 2 diabetes mellitus. Nutrients. 2016;8(3):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. 2012;29(8):e142–e150. [DOI] [PubMed] [Google Scholar]
- 4. Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA. Clinical review: effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(10):3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu C, Qiu S, Zhu X, Li L. Vitamin D supplementation and glycemic control in type 2 diabetes patients: A systematic review and meta-analysis. Metabolism. 2017;73:67–76. [DOI] [PubMed] [Google Scholar]
- 6. Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. The effect of improved serum 25-hydroxyvitamin D status on glycemic control in diabetic patients: a meta-analysis. J Clin Endocrinol Metab. 2017;102(9):3097–3110. [DOI] [PubMed] [Google Scholar]
- 7. Krul-Poel YH, Ter Wee MM, Lips P, Simsek S. Management of endocrine disease: the effect of vitamin D supplementation on glycaemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Eur J Endocrinol. 2017;176(1):R1–R14. [DOI] [PubMed] [Google Scholar]
- 8. Patel P, Poretsky L, Liao E. Lack of effect of subtherapeutic vitamin D treatment on glycemic and lipid parameters in Type 2 diabetes: a pilot prospective randomized trial. J Diabetes. 2010;2(1):36–40. [DOI] [PubMed] [Google Scholar]
- 9. Soric MM, Renner ET, Smith SR. Effect of daily vitamin D supplementation on HbA1c in patients with uncontrolled type 2 diabetes mellitus: a pilot study. J Diabetes. 2012;4(1):104–105. [DOI] [PubMed] [Google Scholar]
- 10. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Institute of Medicine Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 11. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. [DOI] [PubMed] [Google Scholar]
- 12. Engelman CD. Vitamin D recommendations: the saga continues. J Clin Endocrinol Metab. 2011;96(10):3065–3066. [DOI] [PubMed] [Google Scholar]
- 13. Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Manson JE, Mayne ST, Ross AC, Shapses SA, Taylor CL. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97(4):1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97(4):1153–1158. [DOI] [PubMed] [Google Scholar]
- 15. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126, discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 16. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–796. [DOI] [PubMed] [Google Scholar]
- 17. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41(3):368–377. [DOI] [PubMed] [Google Scholar]
- 18. Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90(1):493–500. [DOI] [PubMed] [Google Scholar]
- 19. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA; San Antonio metabolism study . Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47(1):31–39. [DOI] [PubMed] [Google Scholar]
- 20. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 21. Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med. 2009;26(12):1198–1203. [DOI] [PubMed] [Google Scholar]
- 22. Defronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Kitabchi AE, Mudaliar S, Ratner RE, Stentz FB, Musi N, Reaven PD, Gastaldelli A; ACT NOW Study . Prediction of diabetes based on baseline metabolic characteristics in individuals at high risk. Diabetes Care. 2013;36(11):3607–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mari A, Scherbaum WA, Nilsson PM, Lalanne G, Schweizer A, Dunning BE, Jauffret S, Foley JE. Characterization of the influence of vildagliptin on model-assessed -cell function in patients with type 2 diabetes and mild hyperglycemia. J Clin Endocrinol Metab. 2008;93(1):103–109. [DOI] [PubMed] [Google Scholar]
- 24. Kayaniyil S, Vieth R, Retnakaran R, Knight JA, Qi Y, Gerstein HC, Perkins BA, Harris SB, Zinman B, Hanley AJ. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes [published correction appears in Diabetes Care. 2011;34(1):247]. Diabetes Care. 2010;33(6):1379–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kayaniyil S, Retnakaran R, Harris SB, Vieth R, Knight JA, Gerstein HC, Perkins BA, Zinman B, Hanley AJ. Prospective associations of vitamin D with β-cell function and glycemia: the PROspective Metabolism and ISlet cell Evaluation (PROMISE) cohort study. Diabetes. 2011;60(11):2947–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lacroix M, Battista MC, Doyon M, Houde G, Ménard J, Ardilouze JL, Hivert MF, Perron P. Lower vitamin D levels at first trimester are associated with higher risk of developing gestational diabetes mellitus. Acta Diabetol. 2014;51(4):609–616. [DOI] [PubMed] [Google Scholar]
- 27. Mansuri S, Badawi A, Kayaniyil S, Cole DE, Harris SB, Mamakeesick M, Maguire J, Zinman B, Connelly PW, Hanley AJ. Associations of circulating 25(OH)D with cardiometabolic disorders underlying type 2 diabetes mellitus in an Aboriginal Canadian community. Diabetes Res Clin Pract. 2015;109(2):440–449. [DOI] [PubMed] [Google Scholar]
- 28. Ashraf A, Alvarez J, Saenz K, Gower B, McCormick K, Franklin F. Threshold for effects of vitamin D deficiency on glucose metabolism in obese female African-American adolescents. J Clin Endocrinol Metab. 2009;94(9):3200–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao Y, Wu X, Fu Q, Li Y, Yang T, Tang W. The relationship between serum 25-hydroxy vitamin D and insulin sensitivity and beta-cell function in newly diagnosed type 2 diabetes. J Diabetes Res. 2015;2015:636891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krul-Poel YH, Westra S, ten Boekel E, ter Wee MM, van Schoor NM, van Wijland H, Stam F, Lips PT, Simsek S. Effect of vitamin D supplementation on glycemic control in patients with type 2 diabetes (SUNNY Trial): a randomized placebo-controlled trial. Diabetes Care. 2015;38(8):1420–1426. [DOI] [PubMed] [Google Scholar]
- 31. Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135(2):317–322. [DOI] [PubMed] [Google Scholar]
- 32. Malihi Z, Wu Z, Stewart AW, Lawes CM, Scragg R. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104(4):1039–1051. [DOI] [PubMed] [Google Scholar]
- 33. Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, Yetley EA, Chaudhary-Webb M, Maw KL, Pfeiffer CM, Johnson CL. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016;104(2):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.