Abstract
Successful speech communication often requires selective attention to a target stream amidst competing sounds, as well as the ability to switch attention among multiple interlocutors. However, auditory attention switching negatively affects both target detection accuracy and reaction time, suggesting that attention switches carry a cognitive cost. Pupillometry is one method of assessing mental effort or cognitive load. Two experiments were conducted to determine whether the effort associated with attention switches is detectable in the pupillary response. In both experiments, pupil dilation, target detection sensitivity, and reaction time were measured; the task required listeners to either maintain or switch attention between two concurrent speech streams. Secondary manipulations explored whether switch-related effort would increase when auditory streaming was harder. In experiment 1, spatially distinct stimuli were degraded by simulating reverberation (compromising across-time streaming cues), and target-masker talker gender match was also varied. In experiment 2, diotic streams separable by talker voice quality and pitch were degraded by noise vocoding, and the time alloted for mid-trial attention switching was varied. All trial manipulations had some effect on target detection sensitivity and/or reaction time; however, only the attention-switching manipulation affected the pupillary response: greater dilation was observed in trials requiring switching attention between talkers.
I. INTRODUCTION
The ability to selectively attend to a target speech stream in the presence of competing sounds is required to communicate in everyday listening environments. Evidence suggests that listener attention influences auditory stream formation;1 for listeners with peripheral hearing deficits, changes in the encoding of stimuli often result in impaired stream selection and consequent difficulty communicating in noisy environments.2 In many situations (e.g., a debate around the dinner table), it is also necessary to rapidly switch attention among multiple interlocutors—in other words, listeners must be able to continuously update what counts as foreground in their auditory scene, in order to keep up with a lively conversation.
Prior results show that when cueing listeners in a target detection task to either maintain attention to one stream or switch attention to another stream mid-trial, switching attention both reduced accuracy and led to longer response latency even on targets prior to the attentional switch.3 This suggests that the act of preparing or remembering to switch imposes some degree of mental effort or cognitive load that can compromise the success of the listening task. Given that listeners are aware of linguistic cues to conversational turn-taking,4 the pre-planning of attention switches (and associated hypothesized load) may be part of ordinary listening behavior in everyday conditions, not just an artifact of laboratory experimentation.
Pupillometry, the tracking of pupil diameter, has been used for over five decades to measure cognitive load in a variety of task types.5,6 Pupil dilation is an involuntary, time-locked, physiological response that is present from infancy in humans and other animal species. In general, as the cognitive demands of a task increase, pupil dilation of up to about 5–6 mm can be observed up to 1 s after onset of relevant stimuli.5–7 While this task-evoked pupillary response is slow (∼1 Hz), recent results show that it is possible to track attention and cognitive processes with higher temporal resolution (∼10 Hz) with deconvolution of the pupillary response.8,9
Prior work has shown that the pupillary response co-varies with differences in memory demands,10 sentence complexity,11 lexical frequency of isolated written words,12 or difficulty of mathematical operations.13 In the auditory domain, larger pupil dilations have been reported in response to decreased speech intelligibility due to background noise,14 speech maskers versus fluctuating noise maskers,15 and severity of spectral degradation of spoken sentences.16 The pupillary response has also emerged as a measure of listening effort, which has been defined as “the mental exertion required to attend to, and understand, an auditory message,”17 or, more broadly, as “the deliberate allocation of mental resources to overcome obstacles in goal pursuit when carrying out a task” involving listening.18 In this guise, pupillometry has been used in several studies to investigate the effects of age and hearing loss on listening effort.16,19,20
Recent evidence suggests that the pupillary response is also sensitive to auditory attention. Dividing attention between two auditory streams is known to negatively affect performance in psychoacoustic tasks;21,22 greater pupil dilation and later peak pupil-size latency have also been reported for tasks in which listeners must divide their attention between both speech streams present in the stimulus instead of attending only one of the two,22 or when the expected location or talker of a speech stream were unknown as opposed to predictable.23
However, it is unknown whether the greater pupil dilation in divided attention tasks is due to the demands of processing more information or the effort of switching attention back and forth between streams (or both). The present study was designed to test whether auditory attention switches in a strictly selective attention task would elicit mental effort that was detectable using pupillometry. Both experiments involve selective attention to one of two auditory streams (spoken alphabet letters) and a pre-trial cue indicating (1) which stream to attend to and (2) whether to maintain attention on that stream throughout the trial, or switch attention to the other stream at a designated mid-trial gap. In this way, there is no need or advantage for listeners to try to attend both streams throughout the trial, so any increase in pupil dilation seen in the switch attention trials should index the effort due to attention switching, rather than effort due to processing two streams' worth of information. On the assumption that the divided attention results of Koelewijn and colleagues22 were at least partially due to listeners switching back and forth between streams, we predicted greater pupil dilation on trials that required attention switching.
Additionally, the two experiments include manipulations of the stimuli designed to compromise auditory streaming, and thereby make the task of maintaining or switching attention more difficult. We thus expected that the pupillary response would be larger in trials with more degraded stimuli, trials where target and masker streams were harder to distinguish, or trials where the time allocated for switching between streams was shorter. Secondarily, these manipulations provide a test of whether the kind of pupillary response seen in previous studies that required semantic processing of meaningful sentences might also be seen in a simpler, closed-set target detection task. Based on findings showing that harder pitch discrimination trials elicit larger dilations than easier trials,24 and based on findings from Winn and colleagues that differences in dilation to sentences with different degrees of spectral degradation occurred during sentential stimuli as well as in the post-stimulus delay and response period,16 we expected that the stimulus degradations in and of themselves might also yield larger dilations (in addition to any effect the degradations might have on auditory stream selection).
II. EXPERIMENT 1
Experiment 1 involved target detection in one of two spatially separated speech streams. In addition to the maintain- versus switch-attention manipulation, there was a stimulus manipulation previously shown25 to cause variation in task performance: degradation of binaural cues to talker location (implemented as presence/absence of simulated reverberation). Reduced task performance and greater pupil dilation were predicted for the reverberant condition. This manipulation was incorporated into the pre-trial cue (i.e., on reverberant trials, the cue was also reverberant). Additionally, the voice of the competing talker was varied (either the same male voice as the target talker or a female voice); this manipulation was not signaled in the pre-trial cue. The same-voice condition was expected to degrade the separability of the talkers26 and therefore decrease task performance and increase pupil dilation.
A. Methods
1. Participants
Sixteen adults (ten female, aged 21–35 yr, mean 25.1 yr) participated in experiment 1. All participants had normal audiometric thresholds [20 dB hearing level (HL) or better at octave frequencies from 250 Hz to 8 kHz], were compensated at an hourly rate, and gave informed consent to participate as overseen by the University of Washington Institutional Review Board.
2. Stimuli
Stimuli comprised spoken English alphabet letters from the ISOLET v1.3 corpus27 from one female and one male talker. Mean fundamental frequencies of the unprocessed recordings were 103 Hz (male talker) and 193 Hz (female talker). Letter durations ranged from 351 to 478 ms, and were silence-padded to a uniform duration of 500 ms, root-mean-square (RMS) normalized, and windowed at the edges with a 5 ms cosine-squared envelope. Two streams of four letters each were generated for each trial, with a gap of 600 ms between the second and third letters of each stream. The letters “A” and “B” were used only in the pre-trial cues (described in Sec. II A 3); the target letter was “O” and letters “IJKMQRUXY” were non-target items. To allow unambiguous attribution of button presses, the letter “O” was always separated from another “O” (in either stream) by at least 1 s; thus there were between zero and two “O” tokens per trial. The position of “O” tokens in the letter sequence was balanced across trials and conditions, with ∼40% of all “O” tokens occurring in the third letter slot (just after the switch gap, since that slot is most likely to be affected by attention switches), and ∼20% in each of the other three timing slots.
Reverberation was implemented using binaural room impulse responses (BRIRs) recorded by Shinn-Cunningham and colleagues.28 Briefly, an “anechoic” condition was created by processing the stimuli with BRIRs truncated to include only the direct impulse response and exclude reverberant energy, while stimuli for the “reverberant” condition were processed with the full BRIRs. In both conditions, the BRIRs recorded at ±45° for each stream were used, simulating a separation of 90° azimuth between target and masker streams.
3. Procedure
All procedures were performed in a sound-treated booth; illumination was provided only by the LCD monitor that presented instructions and fixation points. Auditory stimuli were delivered via a TDT RP2 real-time processor (Tucker Davis Technologies, Alachula, FL) to Etymotic ER-2 insert earphones at 65 dB sound pressure level (SPL). A white-noise masker with π-interaural-phase was played continuously during experimental blocks at a level of 45 dB SPL, yielding a stimulus-to-noise ratio of 20 dB. The additional noise was included to provide masking of environmental sounds (e.g., friction between subject clothing and earphone tubes) and to provide consistency with follow-up neuroimaging experiments (required due to the acoustic conditions in the neuroimaging suite).
Pupil size was measured continuously during each block of trials at a 1000 Hz sampling frequency using an EyeLink1000 infra-red eye tracker (SR Research, Kanata, ON, Canada). Participants' heads were stabilized by a chin rest and forehead bar, fixing their eyes at a distance of 50 cm from the EyeLink camera. Target detection accuracy and response time were also recorded for comparison with pupillometry data and the results of past studies.
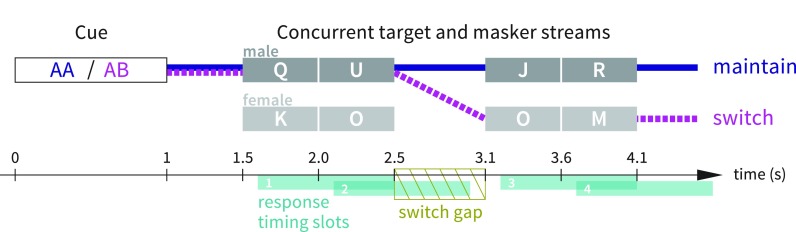
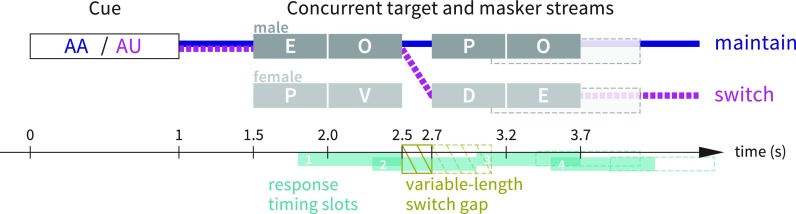
Participants were instructed to fixate on a white dot centered on a black screen and maintain this gaze throughout test blocks. Each trial began with a 1 s auditory cue (spoken letters “AA” or “AB”); the cue was always in a male voice, and its spatial location prompted the listener to attend first to the male talker at that location. The letters spoken in the cue indicated whether to maintain attention to the cue talker's location throughout the trial (“AA” cue) or to switch attention to the talker at the other spatial location at the mid-trial gap (“AB” cue). The cue was followed by 0.5 s of silence, followed by the main portion of the trial: two concurrent four-letter streams with simulated spatial separation and varying talker gender (either the same male voice in both streams or one male and one female voice), with a 600 ms gap between the second and third letters. The task was to respond by button press to the letter “O” spoken by the target talker while ignoring “O” tokens spoken by the competing talker (Fig. 1).
FIG. 1.
(Color online) Illustration of maintain and switch trial types in experiment 1. In the depicted switch trial (heavy dashed line), listeners would hear cue “AB” in a male voice, attend to the male voice (“QU”) for the first half of the trial, switch to the female voice (“OM”) for the second half of the trial, and respond once (to the “O” occurring at 3.1–3.6 s). In the depicted maintain trial (heavy solid line), listeners would hear cue “AA” in a male voice, maintain attention to the male voice (“QUJR”) throughout the trial, and not respond at all. In the depicted trials, a button press anytime during timing slot 2 would be counted as response to the “O” at 2–2.5 s, which is a “foil” in both trial types illustrated; a button press during slot 3 would be counted as response to the “O” at 3.1–3.6 s (which is considered a target in the switch-attention trial and a foil in the maintain-attention trial), and button presses at any other time would be counted as non-foil false alarms. Note that “O” tokens never occurred in immediately adjacent timing slots (unless separated by the switch gap) so response attribution to targets or foils was unambiguous.
Before starting the experimental task, participants heard two blocks of ten trials for familiarization with anechoic and reverberant speech (one with a single talker, one with two simultaneous talkers). Next, listeners did three training blocks of ten trials each (one block of “maintain” trials, one block of “switch” trials, and one block of randomly mixed maintain and switch trials). Training blocks were repeated until participants achieved ≥50% of trials correct on the homogenous blocks and ≥40% of trials correct on the mixed block. During testing, the three experimental conditions (maintain/switch, anechoic/reverberant speech, and male-male versus male-female talker combinations) were counterbalanced, intermixed within each block, and presented in 10 blocks of 32 trials each for a total of 320 trials.
4. Behavioral analysis
Listener responses were labeled as “hits” if the button press occurred between 100 and 1000 ms after the onset of “O” stimuli in the target stream. Responses at any other time during the trial were considered “false alarms.” False alarm responses occurring between 100 and 1000 ms following the onset of “O” stimuli in the masker stream were additionally labeled as “responses to foils” to aid in assessing failures to selectively attend to the target stream. As illustrated in Fig. 1, the response windows for adjacent letters partially overlap in time; responses that occurred during these overlap periods were attributed to an “O” stimulus if possible (e.g., given the trial depicted in Fig. 1, a button press at 3.8 s was assumed to be in response to the “O” at 3.1–3.6 s, and not to the “M”). If no “O” tokens had occurred in that period of time, the response was coded as a false alarm for the purpose of calculating sensitivity, but no reaction time was computed (in other words, only responses to targets and foils were considered in the reaction time analyses).
Listener sensitivity and reaction time were analyzed with (generalized) linear mixed-effects regression models. A model for listener sensitivity was constructed to predict probability of button press at each timing slot (four timing slots per trial; see Fig. 1) from the interaction among the fixed-effect predictors specifying trial parameters (maintain/switch, anechoic/reverberant, and talker gender match/mismatch) and an indicator variable encoding whether a target, foil, or neither was present in the timing slot. A random intercept was also estimated for each listener. An inverse probit link function was used to transform button press probabilities (bounded between 0 and 1) into unbounded continuous values suitable for linear modeling. This model has the convenient advantage that coefficient estimates are interpretable as differences in bias and sensitivity on a scale resulting from the various experimental manipulations.29–31 Full model specifications are given in Eqs. (1) and (3) of the supplementary material;39 the general form of this model is given here in Eq. (1), where Φ−1 is the inverse probit link function, Pr(Y = 1) is the probability of button press, X is the design matrix of trial parameters and indicator variables, and β is the vector of parameter coefficients to be estimated:
| (1) |
Reaction time was analyzed using linear mixed-effects regression (i.e., with identity link function), but was otherwise analyzed similarly to listener sensitivity. Significance of predictors in the reaction time model was computed via F-tests using the Kenward-Roger approximation for degrees of freedom; significance in the sensitivity model was determined by likelihood ratio tests between models with and without the predictor of interest (as the Kenward-Roger approximation has not been demonstrated to work with non-normally-distributed response variables, i.e., when modeling probabilities). See Secs. III A and III B and Tables I–III of the supplementary material39 for full details.
5. Analysis of pupil diameter
Recordings of pupil diameter for each trial were epoched from −0.5 to 6 s, with 0 s defined as the onset of the pre-trial cue. Periods where eye blinks were detected by the EyeLink software were linearly interpolated from 25 ms before blink onset to 100 ms after blink offset. Epochs were normalized by subtracting the mean pupil size between −0.5 and 0 s on each trial, and dividing by the standard deviation of pupil size across all trials (to allow pooling across subjects). Normalized pupil size data were then deconvolved with a pupil impulse response kernel.8,9 Briefly, the pupil response kernel represents the stereotypical time course of a pupillary response to an isolated stimulus, modeled as an Erlang gamma function with empirically determined parameters tmax (latency of response maximum) and n (Erlang shape parameter).7 The parameters used here were tmax = 0.512 s and n = 10.1, following previous literature.7,9
Fourier analysis of the subject-level mean pupil size data and the deconvolution kernel indicated virtually no energy at frequencies above 3 Hz, so for computational efficiency the deconvolution was realized as a best-fit linear sum of kernels spaced at 100 ms intervals (similar to downsampling both signal and kernel to 10 Hz prior to deconvolution), as implemented in the pyeparse software.32 After deconvolution, the resulting time series can be thought of as an indicator of mental effort that is time-aligned to the stimulus (i.e., the response latency of the pupil has been effectively removed). Statistical comparison of deconvolved pupil dilation time series (i.e., “effort” in Figs. 4 and 8) was performed using a non-parametric cluster-level one-sample t-test on the within-subject differences in deconvolved pupil size between experimental conditions (clustering across time only),33 as implemented in mne-python.34
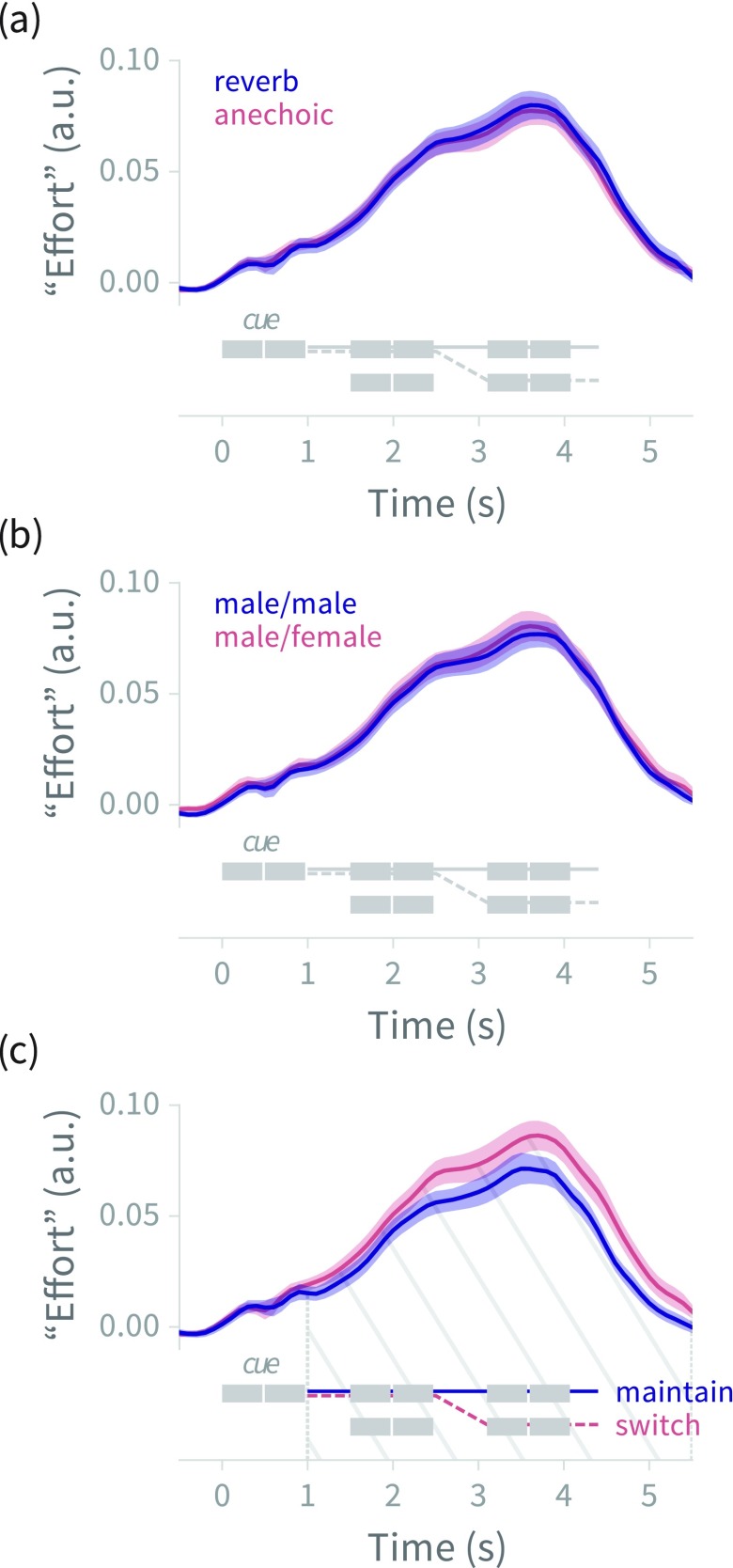
FIG. 4.
(Color online) Deconvolved pupil size (mean ± 1 standard error across subjects) for (a) reverberant versus anechoic trials, (b) talker gender-match versus -mismatch trials, and (c) maintain- versus switch-attention trials, with trial schematics showing the time course of stimulus events (compare to Fig. 1). Hatched region shows temporal span of statistically significant differences between time series. The onset of statistically significant divergence (vertical dotted line) of the maintain/switch conditions is in close agreement with the end of the cue. a.u. = arbitrary units (see Sec. II A 5 for explanation of effort).
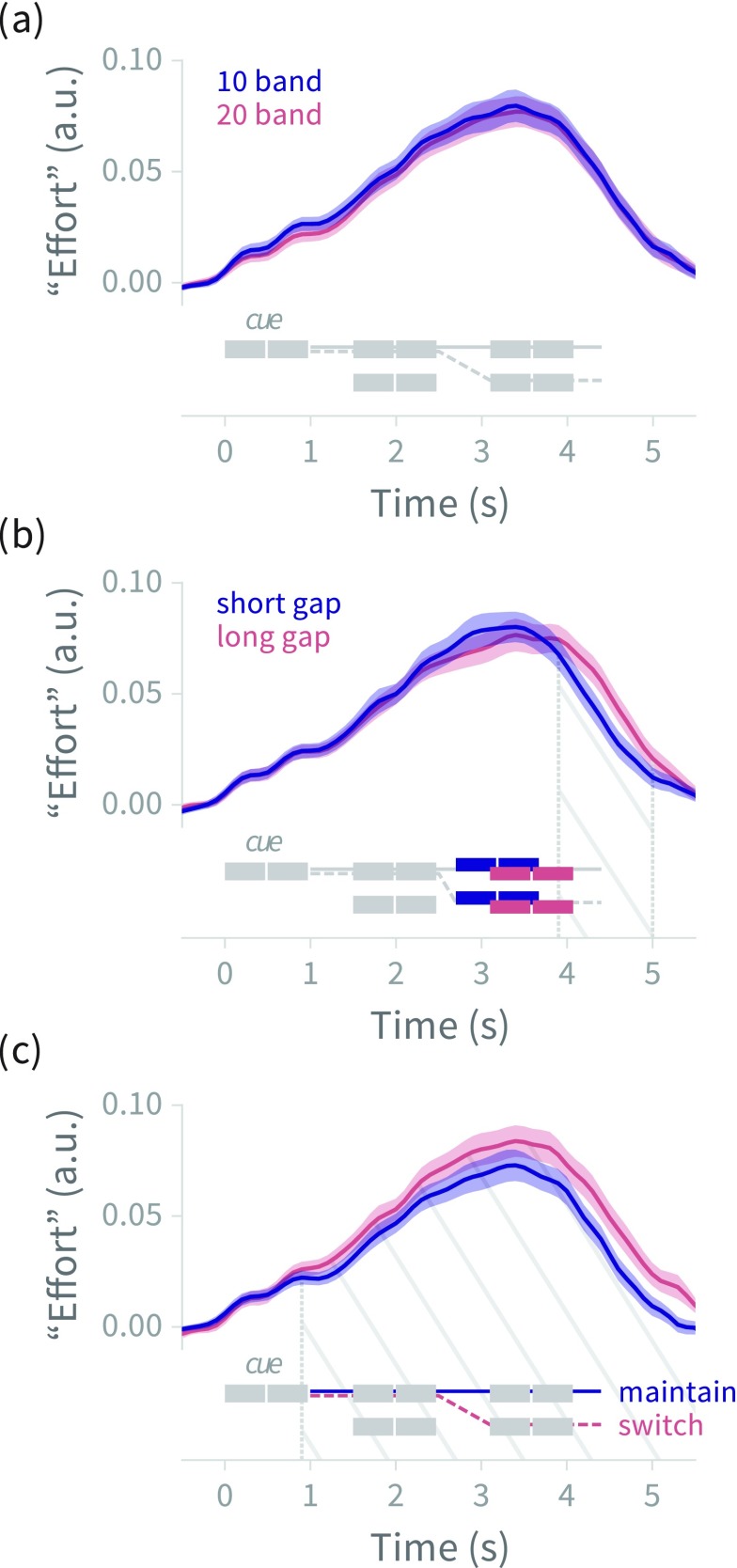
FIG. 8.
(Color online) Deconvolved pupil size (mean ±1 standard error across subjects) for (a) 10- versus 20-band vocoded stimuli, (b) 200 versus 600 ms mid-trial switch gap durations, and (c) maintain- versus switch-attention trials, with trial schematics showing the time course of stimulus events (compare to Fig. 5). Hatched region shows temporal span of statistically significant differences between time series. The late-trial divergence in (b) is attributable to the delay of stimulus presentation in the long-gap condition; the onset of divergence in (c) aligns with the end of the cue, as in experiment 1 [see Fig. 4(c)]. a.u. = arbitrary units (see Sec. II A 5 for explanation of effort).
B. Results
1. Sensitivity
Over all trials, sensitivity () ranged across subjects from 1.7 to 4.2 (first quartile 1.9, median 2.4, third quartile 3.0). Box-and-swarm plots displaying quartile and individual differences in values between experimental conditions are shown in Fig. 2. Note that is an aggregate measure of sensitivity that does not distinguish between responses to foil items versus other types of false alarms; however, the statistical model does separately estimate significant differences between experimental conditions for both target response rate and foil response rate, and also estimates a bias term for each condition that captures non-foil false alarm response rates.
FIG. 2.
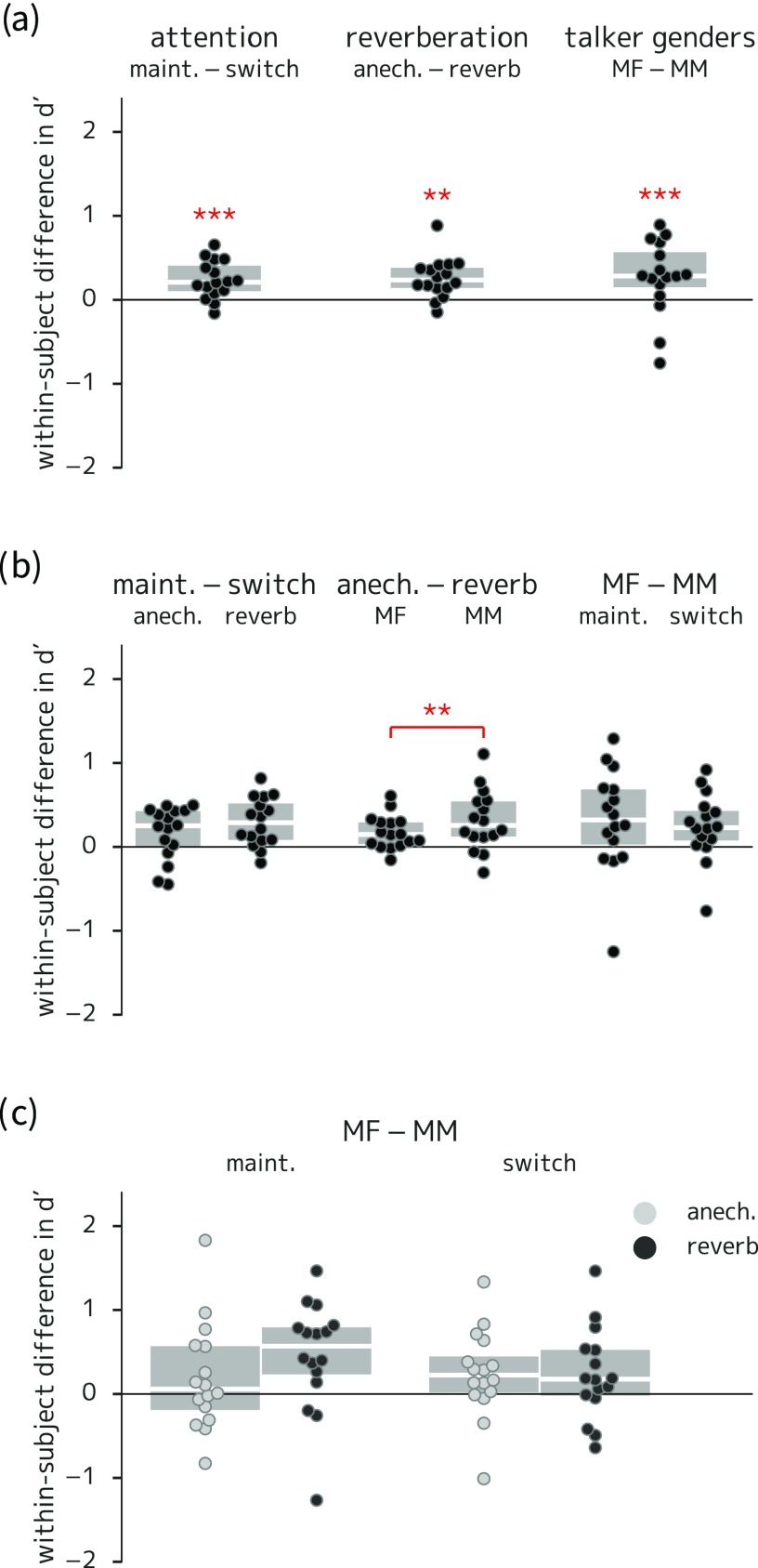
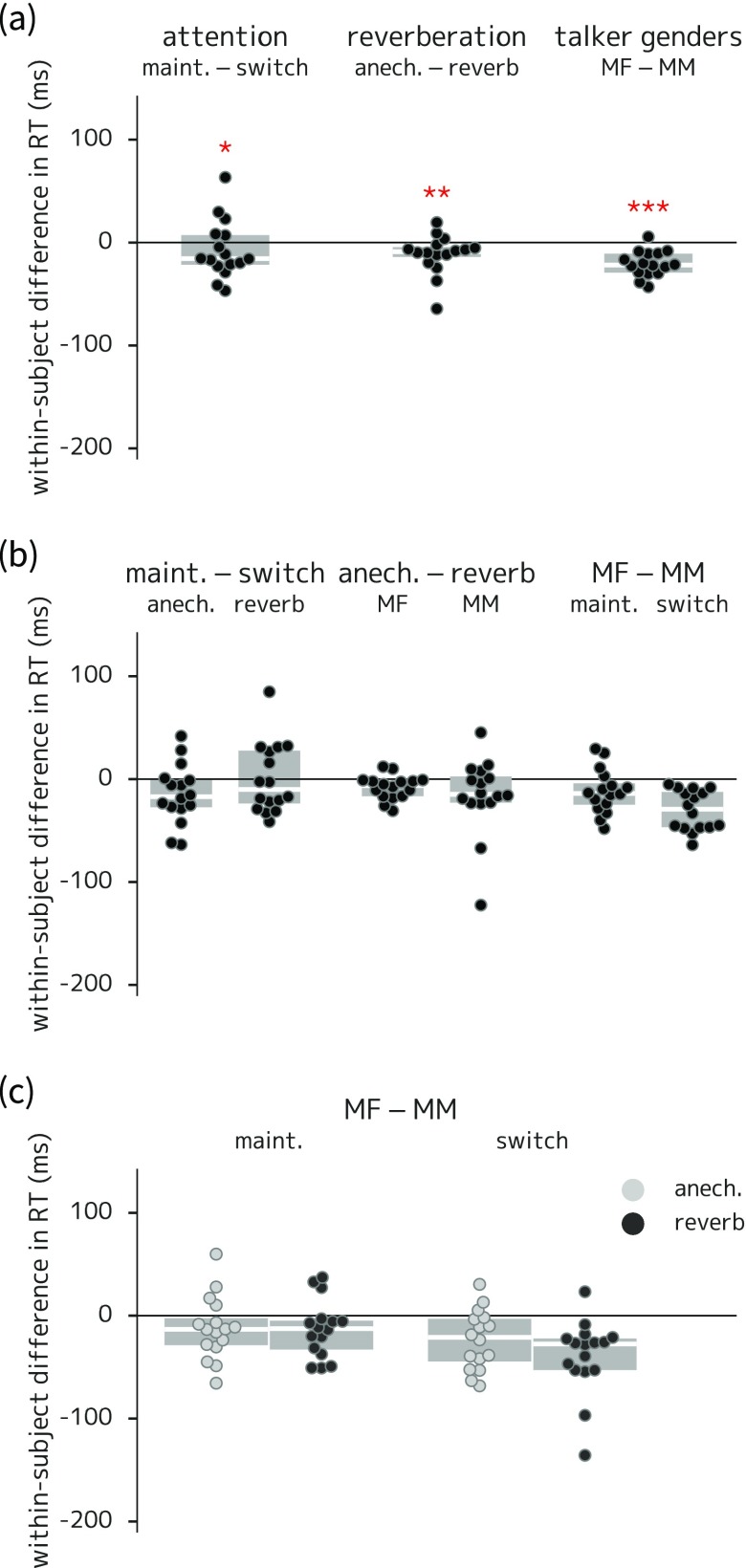
(Color online) Box-and-swarm plots of between-condition differences in listener sensitivity for experiment 1. Boxes show first and third quartiles and median values; individual data points correspond to each listener; asterisks indicate comparisons with corresponding coefficients in the statistical model that were significantly different from zero. (a) Main effects of attention (higher sensitivity in maintain than switch trials), reverberation (higher sensitivity in anechoic than reverberant trials), and talker gender (mis)match (higher sensitivity in trials with different-gendered target and masker talkers). (b) Two-way interactions; the difference between anechoic and reverberant trials was significantly larger in the gender-match (MM) than in the gender-mismatch (MF) condition. (c) Three-way interaction (no statistically significant differences). ** = p < 0.01; *** = p < 0.001.
The model indicated significant main effects for all three trial type manipulations, as seen in Fig. 2(a), with effect sizes around 0.2–0.3 on a scale. Model results indicate that the attentional manipulation led to more responses to both targets (Wald z = 5.23, p < 0.001) and foils (Wald z = 2.82, p = 0.005) in maintain- versus switch-attention trials, though the net effect was an increase in in the maintain attention condition for nearly all listeners. The model also showed a significant difference in response bias in the attentional contrast (Wald z = −2.57, p = 0.01), with responses more likely in the switch- than the maintain-attention condition. In fact, there were slightly fewer total button presses in the switch-attention trials, but there were more non-foil false alarm responses in those trials. This suggests that the bias term is in fact capturing a difference in non-foil false alarm responses (i.e., presses that are not captured by terms in the model equation encoding responses to targets and foils).
Regarding reverberation, listeners were better at detecting targets in the anechoic trials (Wald z = 3.08, p = 0.002), but there was no significant difference in response to foils between anechoic and reverberant trials. Regarding talker gender (mis)match, the model indicated both better target detection (Wald z = 2.43, p = 0.015) and fewer responses to foils (Wald z = −2.31, p = 0.021) when the target and masker talkers were different genders. The model also indicated a two-way interaction for target detection between reverberation and talker gender (Wald z = −2.09, p = 0.036); this can be seen in Fig. 2(b): the difference between anechoic and reverberant trials was smaller when the target and masker talkers were of different genders. The three-way interaction among attention, reverberation, and talker gender was not significant.
To address the concern that listeners might have attempted to monitor both streams, and especially that they might do so differently in maintain- versus switch-attention trials, the rate of listener response to foil items was examined separately for each timing slot. Foil response rates ranged from 1% to 4% for slots 1 and 2 (before the switch gap), and from 9% to 15% for slots 3 and 4 (after the switch gap), but showed no statistically reliable difference between maintain- and switch-attention trials for any of the four slots (see Sec. III D 1 of the supplementary material39 for details).
2. Reaction time
Over all correct responses, median reaction time for each subject ranged from 434 ms to 692 ms after the onset of the target letter. Box-and-swarm plots showing quartile and individual differences in reaction time values between experimental conditions are shown in Fig. 3. The statistical model indicated significant main effects of attentional condition, reverberation, and talker gender mismatch. Faster response times were seen for targets in maintain-attention trials [9 ms faster on average, F(1, 5868.1) = 4.45, p = 0.035], anechoic trials [13 ms faster, F(1, 5868.1) = 9.35, p = 0.002], and trials with mismatched talker gender [25 ms faster, F(1, 5868.2) = 35.74, p < 0.001]. The model showed no significant interactions in reaction time among these trial parameters.
FIG. 3.
(Color online) Box-and-swarm plots of between-condition differences in reaction time for experiment 1. Boxes show first and third quartiles and median values; individual data points correspond to each listener; asterisks indicate comparisons with corresponding coefficients in the statistical model that were significantly different from zero. (a) Main effects of attention (faster reaction time in maintain than switch trials), reverberation (faster reaction time in anechoic than reverberant trials), and talker gender (mis)match (faster reaction time in trials with trials with different-gendered target and masker talkers). (b) Two-way interactions (no statistically significant differences). (c) Three-way interaction (no statistically significant difference). * = p < 0.05; ** = p < 0.01; *** = p < 0.001; MM = matching talker genders; MF = mismatched talker genders.
Post hoc analysis of reaction time by response slot showed no significant differences for the reverberation contrast. For the talker gender (mis)match contrast and the maintain- versus switch-attention contrasts, there were significant differences only in slot 3 (see Sec. II D 2 of the supplementary material39 for details). This is consistent with a view that the act of attention switching creates a lag or slow-down in auditory perception.3
3. Pupillometry
Mean deconvolved pupil diameter as a function of time for the three stimulus manipulations (reverberant/anechoic trials, talker gender match/mismatch trials, and maintain/switch attention trials) are shown in Fig. 4. Only the attentional manipulation shows a significant difference between conditions, with “switch attention” trials showing greater pupillary response than “maintain attention” trials in the time range from 1.0 to 5.5 (tcrit = 2.13, p < 0.001; see Sec. III C and Table IV of the supplementary material39 for full statistical details). The time courses diverge as soon as listeners have heard the cue, and the response remains significantly higher in the switch-attention condition throughout the remainder of the trial.
C. Discussion
The models of listener sensitivity and reaction time showed main effects in the expected directions for all three manipulations: put simply, listener sensitivity was better and responses were faster when the talkers had different voices, when there was no reverberation, and when mid-trial switching of attention was not required. The difference between anechoic and reverberant trials was smaller in trials where the talkers had different voices, suggesting that the advantage of anechoic conditions and the advantage due to talker voice differences are not strictly additive. A possible explanation for this finding is that either talker voice difference or anechoic conditions are sufficient to support auditory source separation and streaming,25,26 but the presence of both conditions cannot overcome difficulty arising from other aspects of the task. Conversely, one might say that both segregating two talkers with the same voice and segregating two talkers in highly reverberant conditions are hard tasks, which when combined make for a task even more difficult than would be expected if the manipulations were additive (i.e., reverberation hurt performance more when both talkers were male).
Unlike listener sensitivity and reaction time, the pupillary response differed only in response to the attentional manipulation. Interestingly, the difference in pupillary response was seen across the entire trial, whereas the reaction time difference for the maintain-versus-switch contrast was restricted to slot 3 (the immediately post-switch time slot). The fact that patterns of pupillary response do not recapitulate patterns of listener behavior would make sense if, for normal hearing listeners, reverberation and talker gender mismatch are not severe enough degradations to cause sufficient extra mental effort or cognitive load to be observable in the pupil (in other words, the pupillary response may reflect the same processes as the behavioral signal, but may not be as sensitive). However, the magnitude of the effect size in is roughly equal for all three trial parameters [see Fig. 2(a)]; if behavioral effect size reflects degree of effort or load, then the explanation that pupillometry is just “not sensitive enough” seems unlikely. Another possibility is that the elevated pupil response is simply due to a higher number of button presses in the switch trials: motor planning and execution are known to cause pupillary dilations.35 However, as mentioned in Sec. II B 1, the total number of button presses is in fact higher in the maintain-attention condition. A third possibility is that the pupil dilation only reflects certain kinds of effort or load, and that stimulus degradations that mainly affect listener ability to form and select auditory streams are not reflected in the pupillary response, whereas differences in listener attentional state, such as preparing for a mid-trial attention switch, are reflected by the pupil. Experiment 2 tests this latter explanation by repeating the maintain/switch manipulation while increasing stimulus degradation to further impair formation and selection of auditory streams.
III. EXPERIMENT 2
Since no effect of talker gender on pupil dilation was seen in experiment 1, in experiment 2 the target and masker talkers were always of opposite gender, and their status as initial target or masker was counterbalanced across trials. Since no effect of reverberation on pupillary response was seen in experiment 1, experiment 2 also removed the simulated spatial separation of talkers and involved a more severe cued stimulus degradation known to cause variation in task demand: spectral degradation implemented as variation in number of noise-vocoder channels, 10 or 20. Based on results from Winn and colleagues showing increased dilation for low versus high numbers of vocoder channels with full-sentence stimuli,16 greater pupil dilation was expected here in the (more difficult, lower-intelligibility) ten-channel condition. As in experiment 1, a pre-trial cue indicated whether to maintain or switch attention between talkers at the mid-trial gap; here, the cue also indicated whether spectral degradation was mild or severe (i.e., the cue underwent the same noise vocoding procedure as the main portion of the trial).
Additionally, in experiment 2 the duration of the mid-trial temporal gap provided for attention switching was varied (either 200 ms or 600 ms). Behavioral and neuroimaging research suggest that the time course of attention switching in the auditory domain is around 300–400 ms;3,36 accordingly, we expected the short gap trials to be challenging and thus predicted greater pupil dilation in short-gap trials (though only in the post-gap portion of the trial). The duration of the gap was not predictable from the pre-trial cue.
A. Methods
1. Participants
Sixteen adults (eight female, aged 19–35 yr, mean 25.5 yr) participated in experiment 2. All participants had normal audiometric thresholds (20 dB HL or better at octave frequencies from 250 Hz to 8 kHz), were compensated at an hourly rate, and gave informed consent to participate as overseen by the University of Washington Institutional Review Board.
2. Stimuli
Stimuli were based on spoken English alphabet letters from the ISOLET v1.3 corpus27 from the same female and male talkers used in experiment 1, with the same stimulus preprocessing steps (padding, amplitude normalization, and edge windowing). Two streams of four letters each were generated for each trial with a gap of either 200 or 600 ms between the second and third letters of each stream. The letters “A” and “U” were used only in the pre-trial cues (described below); the target letter was “O” and letters “DEGPV” were non-target items. The cue and non-target letters differed from those used in experiment 1 in order to maintain discriminability of cue, target, and non-target letters even under the most degraded (ten-channel vocoder) condition. Specifically, the letters were chosen so that the vowel nuclei differed between the cue, target, and non-target letters: representations of the vowel nuclei in the International Phonetic Alphabet are /e/ and /u/ (cues “A” and “U”), /o/ (target “O”), and /i/ (non-target letters “DEGPV”).
Spectral degradation was implemented following a conventional noise vocoding strategy.37 The stimuli were fourth-order Butterworth bandpass filtered into 10 or 20 spectral bands of equal equivalent rectangular bandwidths.38 This filterbank ranged from 200 to 8000 Hz (low cutoff of lowest filter to high cutoff of highest filter). Each band was then half-wave rectified and filtered with a 160 Hz low-pass fourth-order Butterworth filter to extract the amplitude envelope. The resulting envelopes were used to modulate corresponding noise bands (created from white noise filtered with the same filterbank used to extract the speech bands). These modulated noise bands were then summed, and presented diotically at 65 dB SPL. As in experiment 1, a simultaneous white-noise masker was also presented (see Sec. II A 3).
3. Procedure
Participants were instructed to fixate on a white dot centered on a black screen and maintain such gaze throughout test blocks. Each trial began with a 1 s auditory cue (spoken letters “AA” or “AU”); the cue talker's gender indicated whether to attend first to the male or female voice, and additionally indicated whether to maintain attention to that talker throughout the trial (“AA” cue) or to switch attention to the other talker at the mid-trial gap (“AU” cue). The cue was followed by 0.5 s of silence, followed by the main portion of the trial: two concurrent, diotic four-letter streams (one male voice, one female voice), with a variable-duration gap between the second and third letters. The task was to respond by button press to the letter “O” spoken by the target talker (Fig. 5). To allow unambiguous attribution of button presses, the letter “O” was always separated from another “O” (in either stream) by at least 1 s, and its position in the letter sequence was balanced across trials and conditions. Distribution of targets and foils across timing slots was equivalent to experiment 1.
FIG. 5.
(Color online) Illustration of maintain and switch trial types in experiment 2. The short-gap version is depicted; timing of long-gap trial elements (where different) are shown with faint dashed lines. In the depicted switch trial (heavy dashed line), listeners would hear cue “AU” in a male voice, attend to the male voice (“EO”) for the first half of the trial and the female voice (“DE”) for the second half of the trial, and respond once (to the “O” occurring at 2–2.5 s). In the depicted maintain trial (heavy solid line), listeners would hear cue “AA” in a male voice, attend to the male voice (“EOPO”) throughout the trial, and respond twice (once for each “O”).
Before starting the experimental task, participants heard two blocks of ten trials for familiarization with noise-vocoded speech (one with a single talker, one with the two simultaneous talkers). Next, they did three training blocks of ten trials each (one block of maintain trials, one block of switch trials, and one block of randomly mixed maintain and switch trials). Training blocks were repeated until participants achieved ≥50% of trials correct on the homogenous blocks and ≥40% of trials correct on the mixed block. During testing, the three experimental conditions (maintain/switch, 10/20 channel vocoder, and 200/600 ms gap duration) were counterbalanced, intermixed within each block, and presented in 10 blocks of 32 trials each for a total of 320 trials.
4. Behavioral analysis
As in experiment 1, listener responses were labeled as hits if the button press occurred within a defined temporal response window after the onset of “O” stimuli in the target stream, and all other responses were considered “false alarms.” However, unlike experiment 1, the designated response window for targets and foil items ran from 300 to 1000 ms after the onset of “O” stimuli (in experiment 1 the window ranged from 100 to 1000 ms). This change resulted from a design oversight, in which the placement of target or foil items in both of slots 2 and 3 (on either side of the switch gap) yielded a period of overlap of the response windows for slots 2 and 3 in the short gap trials, in which presses could not be unambiguously attributed. However, in experiment 1 (where response times as fast as 100 ms were allowed) the fastest response time across all subjects was 296 ms and was the sole instance of a sub-300 ms response. Therefore, raising the lower bound on the response time window to 300 ms for experiment 2 is unlikely to have disqualified any legitimate responses (especially given the more severe signal degradation, which is likely to increase response times relative to experiment 1), and eliminates the overlap between response slots 2 and 3 on short-gap trials.
Statistical modeling of sensitivity used the same approach as was employed in experiment 1: predicting probability of button press in each timing slot based on fixed-effect predictors (maintain/switch, 10 – or 20-channel vocoder, and short/long mid-trial gap duration), a target/foil/neither indicator variable, and a subject-level random intercept. Statistical modeling of response time also mirrored experiment 1 in omitting the indicator variable and considering only responses to targets and foils. See Secs. IV A and IV B and Tables VI–VIII of the supplementary material39 for full details.
5. Analysis of pupil diameter
Analysis of pupil diameter was carried out as in experiment 1: trials epoched from −0.5 to 6 s, linear interpolation of eye blinks, per-trial baseline subtraction, and per-subject division by standard deviation of pupil size. Deconvolution and statistical analysis of normalized pupil size data were also carried out identically to experiment 1.
B. Results
1. Sensitivity
Over all trials, sensitivity () ranged across subjects from 1.4 to 4.2 (first quartile 1.8, median 2.2, third quartile 2.7). Box-and-swarm plots displaying quartile and individual differences in values between experimental conditions are shown in Fig. 6. Again, note that is an aggregate measure of sensitivity that does not distinguish between responses to foil items versus other types of false alarms, but the statistical model does estimate separate coefficients for target response rate, foil response rate, and a bias term capturing non-foil false alarm responses. The model indicated significant main effects for all three trial type manipulations, as seen in Fig. 6(a). Specifically, model results indicate no significant difference in target detection between maintain- and switch-attention trials (Wald z = 1.07, p = 0.284), but did show fewer responses to foils in maintain-attention trials (Wald z = −2.54, p = 0.011; estimated effect size 0.15 ); a corresponding increase in in the maintain attention condition is seen for nearly all listeners in Fig. 6(a), left column. Regarding spectral degradation, listeners were better at detecting targets in 20-channel trials (Wald z = 4.09, p < 0.001; estimated effect size 0.19 ), but there was no significant difference in response to foils for the spectral degradation manipulation (Wald z = 0.69, p = 0.489). For the switch gap length manipulation, the model indicated much lower response to target items (Wald z = −7.51, p < 0.001; estimated effect size 0.35 ) and much greater response to foil items (Wald z = 9.24, p < 0.001; estimated effect size 0.56 ) in the long gap trials.
FIG. 6.
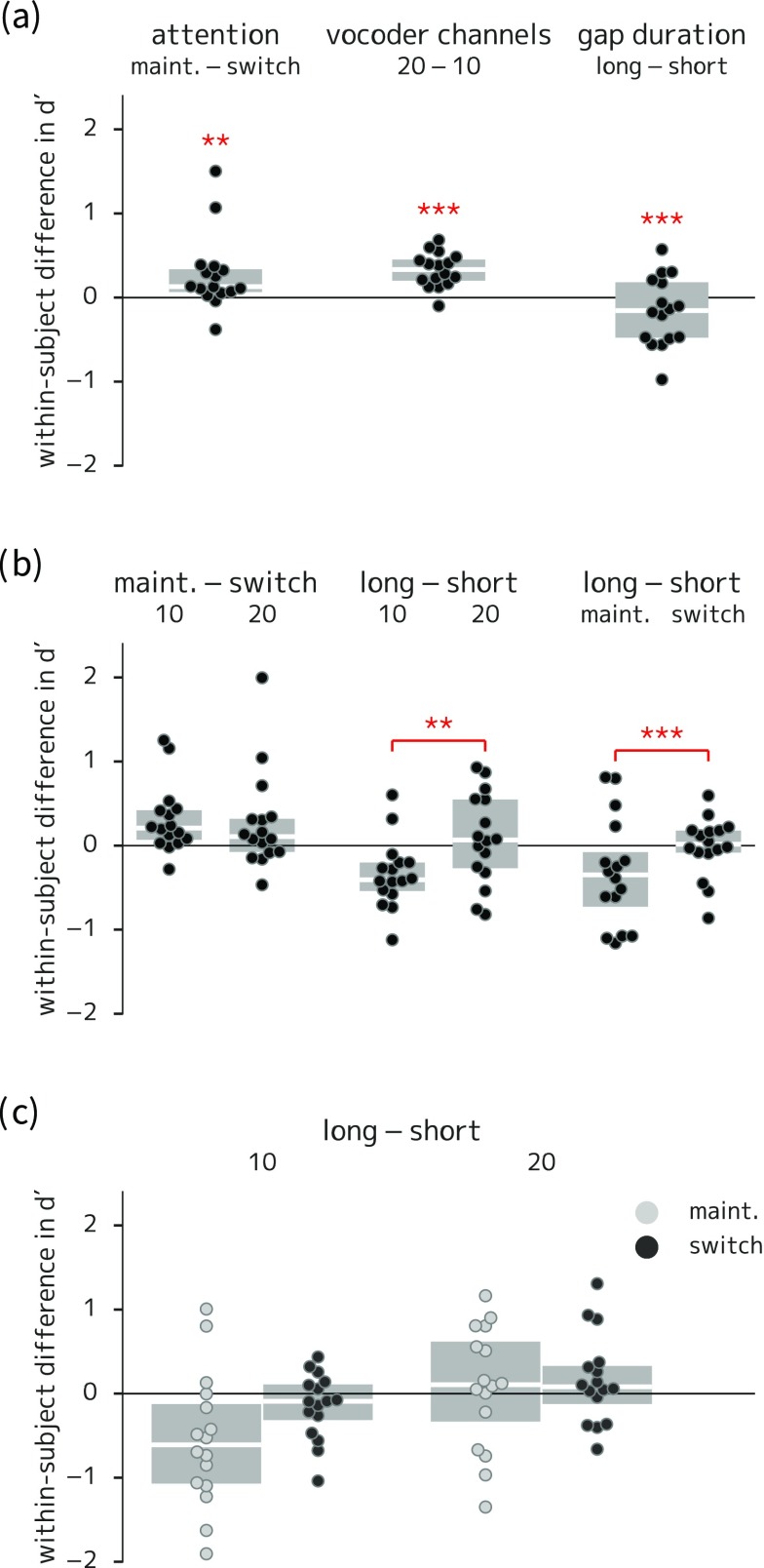
(Color online) Box-and-swarm plots of between-condition differences in listener sensitivity for experiment 2. Boxes show first and third quartiles and median values; individual data points correspond to each listener; asterisks indicate comparisons with corresponding coefficients in the statistical model that were significantly different from zero. (a) Main effects of attention (higher sensitivity in maintain than switch trials), spectral degradation (higher sensitivity in 20-channel than 10-channel vocoded trials), and switch gap duration (higher sensitivity in trials with a short gap). (b) Two-way interactions: the difference between long- and short-gap trials was greater (more negative) in the ten-channel-vocoded trials and in the maintain-attention trials. (c) Three-way interaction (not significant). * = p < 0.05; ** = p < 0.01; *** = p < 0.001.
The model also showed two-way interactions between gap duration and spectral degradation [lower sensitivity in ten-channel long-gap trials; Fig. 6(b), middle column], and between gap duration and the attentional manipulation [lower sensitivity in maintain-attention long-gap trials; Fig. 6(b), right column]. The interaction between gap duration and the attentional manipulation showed increased responses to foil items in maintain-attention long-gap trials (Wald z = 2.98, p = 0.003). The terms modeling interaction between gap duration and spectral degradation were not significantly different from zero at the p < 0.05 level when targets and foils are modeled separately (Wald z = 1.66, p = 0.097 for targets; Wald z = −1.92, p = 0.055 for foils), but the exclusion of these terms from the model did significantly decrease model fit according to a likelihood ratio test [χ2(2) = 11.38, p = 0.003].
Post hoc analysis of target detection accuracy showed no significant differences by slot when correcting for multiple comparisons, but the trend suggested that the two-way interaction between gap duration and spectral degradation was driven by the first time slot, while the two-way interaction between gap duration and attentional condition was predominantly driven by the last time slot (paired t-tests by slot on logit-transformed hit rates all p > 0.04; Bonferroni-corrected significance level 0.00625).
2. Reaction time
Over all correct responses, median reaction time for each subject ranged from 493 ms to 689 ms after the onset of the target letter. Box-and-swarm plots showing quartile and individual differences in reaction time values between experimental conditions are shown in Fig. 7. The statistical model indicated a significant main effect of spectral degradation and switch gap length. Faster response times were seen for targets in trials processed with 20-channel vocoding [35 ms faster on average, F(1, 4605.0) = 21.79, p < 0.001], and trials with a long switch gap [66 ms faster, F(1, 4606.9)= 77.52, p < 0.001]. The model also showed a significant interaction between spectral degradation and switch gap length [44 ms faster with 20-channel vocoding and long gaps, F(1, 4604.4) = 8.57, p = 0.003].
FIG. 7.
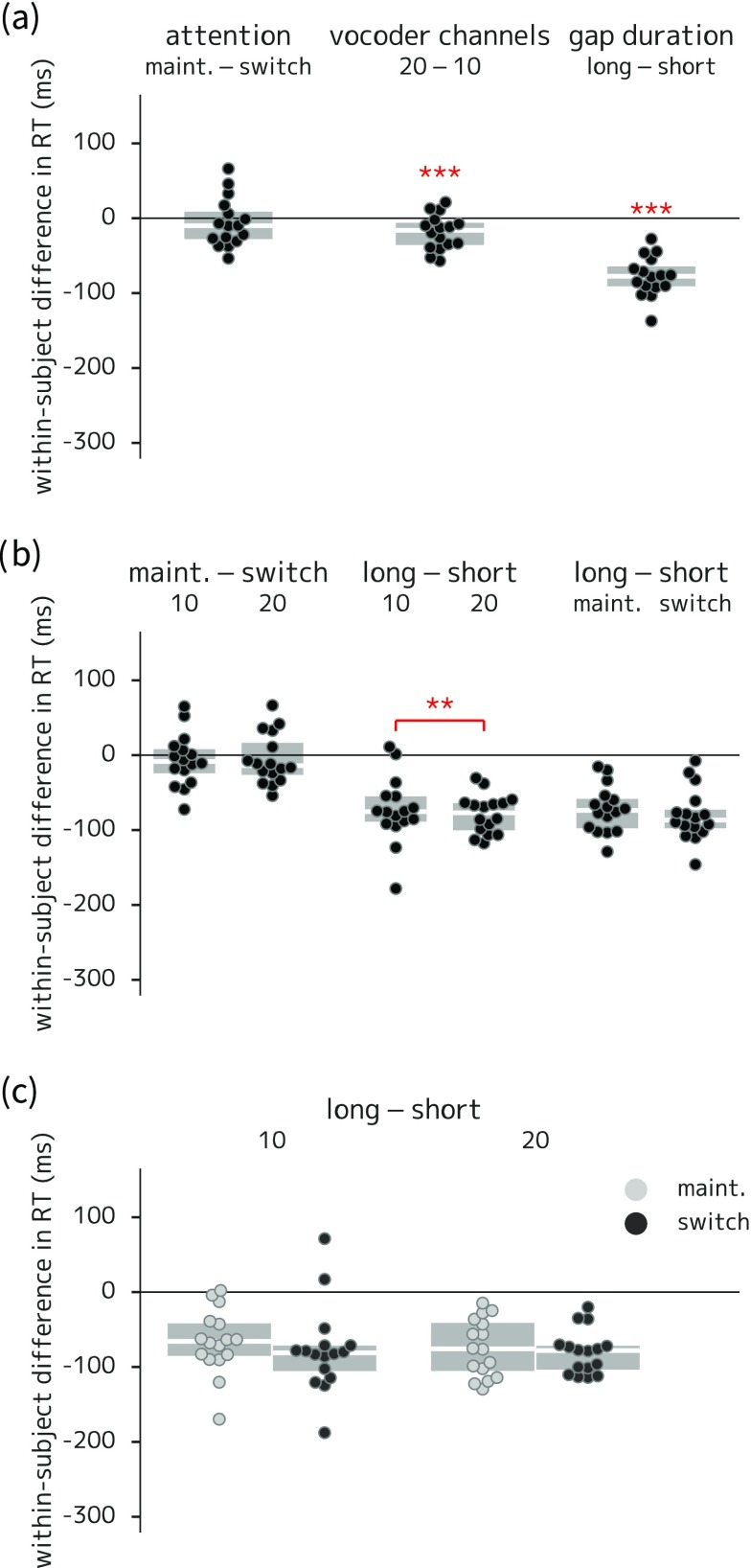
(Color online) Box-and-swarm plots of between-condition differences in reaction time for experiment 2. Boxes show first and third quartiles and median values; individual data points correspond to each listener; asterisks indicate comparisons with corresponding coefficients in the statistical model that were significantly different from zero. (a) Main effects of attention, spectral degradation, and gap duration (faster response time in trials with 20-channel vocoding, and in long-gap trials). (b) Two-way interactions (larger difference in reaction times between long- and short-gap trials in the 10- versus the 20-channel condition). (c) Three-way interaction (no statistically significant difference). *** = p < 0.001.
As in experiment 1, post hoc tests of reaction time difference between maintain- and switch-attention trials by slot showed a significant difference localized to slot 3 (the immediately post-gap slot), with faster reaction times in maintain-attention trials (28 ms faster on average). For the spectral degradation contrast, a significant difference was seen only in slot 1, with faster reaction times in the 20-channel trials (68 ms faster on average); this pattern of results could arise if listener adaptation to the level of degradation was incomplete when the trial started, but was in place by the end of slot 1. For the gap length manipulation, significantly faster reaction times were seen in the long-gap trials for slot 3 (155 ms faster on average) and slot 4 (135 ms faster on average) and significantly slower reaction times in the long-gap trials for slot 1 (261 ms slower on average). The faster reaction times in the long-gap trials in slots 3 and 4 are expected given that listeners had additional time to process the first half of the trial and/or prepare for the second half in the long-gap condition. However, the difference in reaction time in slot 1 is unexpected and inexplicable given that the gap length manipulation was uncued. See Sec. IV D 1 of the supplementary material39 for details.
3. Pupillometry
Mean deconvolved pupil diameter as a function of time for the three stimulus manipulations (10/20 vocoder channels, gap duration, and maintain/switch attention trials) is shown in Fig. 8. Similar to experiment 1, the attentional manipulation shows a significant difference between conditions with switch-attention trials showing greater pupillary response than maintain-attention trials in the time range from 0.9 to 5.6 s (tcrit = 2.13, p < 0.001); in experiment 1, the significant difference spanned 1.0–5.5 s. Also as in experiment 1, the time courses diverge as soon as listeners have heard the cue, and the response remains higher in the switch-attention condition throughout the rest of the trial. There is also a significant difference in the time course of the pupillary response between long- and short-gap trials in the time range 3.9–5.0 s (tcrit = 2.13, p < 0.01), with the signals diverging around the onset of the mid-trial gap (though only differing statistically in the final ∼1 s of the trial). See Sec. IV C and Table IX of the supplementary material39 for full details.
C. Discussion
The model of listener sensitivity for experiment 2 showed main effects of the spectral degradation and attentional manipulations in the expected directions (based on past literature16,22 and the results of experiment 1): listener sensitivity was better when there were more vocoder channels (better spectral resolution) and when mid-trial switching of attention was not required. However, the results of the gap duration manipulation were unexpected; based on past findings that auditory attention switches take between 300 and 400 ms,3,36 we hypothesized that a gap duration of 200 ms would cause listeners to fail to detect targets in the immediate post-gap position (i.e., timing slot 3). We did see slower reaction time in the short-gap trials, but sensitivity was actually better in the short-gap trials than in the long-gap ones for most listeners [Fig. 6(a), right column]. However, according to the statistical model this effect appears to be restricted to the ten-channel and maintain-attention trials [see Fig. 6(b), middle and right columns, and Fig. 6(c), left column]. Interestingly, the model coefficient estimates indicated that the interactions were more strongly driven by a difference in responses to foil items, not targets.
A possible explanation for the elevated response to foils in the long-gap condition is that the long-gap condition interfered with auditory streaming, the ten-channel condition also interfered with streaming, and when both conditions occurred simultaneously there was a strong effect on listener ability to group the pre- and post-gap letters into a single stream (i.e., to preserve stream identity across the gap). Using minimally processed stimuli (monotonized, but without intentional degradation), Larson and Lee showed a similar “drop off” in performance in their maintain-attention trials when the gap duration reached 800 ms;3 perhaps the spectral degradation in our stimuli decreased listeners' tolerance for gaps in the stream, causing performance to drop off at shorter (600 ms) gap lengths. However, this explanation still does not account for the finding that the ten-channel plus long-gap difficulty seems to occur only in the maintain-attention trials. One might speculate that the act of switching attention at the mid-trial gap effectively “fills in” the gap, making the temporal disconnect between pre- and post-gap letters less noticeable, and thereby preserving attended stream identity across a longer gap duration than would be possible if attention were maintained on a single source. In other words, if listeners must conceive of the “stream of interest” as a source that undergoes a change in voice quality partway through the trial, the additional mental effort required to make the switch might result in more accurate post-gap stream selection, whereas the putatively less effortful task of maintaining attention to a consistent source could lead to less accurate post-gap stream selection when stream formation is already difficult (due to strong spectral degradation) and stream interruptions are long. Further study of the temporal dynamics of auditory attention switching is needed to clarify how listeners' intended behavior affects stream stability across temporal caesuras of varying lengths, and how this process interacts with signal degradation or quality.
If this speculation is correct—that signal degradation reduces listener tolerance of gaps in auditory stream formation and preservation—then this finding may have important implications for listeners experiencing both hearing loss and cognitive decline. Specifically, poor signal quality due to degradation of the auditory periphery could lead to greater difficulty in stream preservation across long gaps, but cognitive decline may make rapid switching difficult. In other words, the cognitive abilities of older listeners might require longer pauses to switch attention among multiple interlocutors, but the longer pauses may in fact make it harder to preserve focus in the face of degraded auditory input.
It is also interesting that the post hoc analyses suggested possibly different temporal loci for the effects of different stimulus manipulations (i.e., affecting pre- versus post-gap time slots). This might indicate that differences in the strength of sensory memory traces of the stimuli played a role. However, it is important to note that we attempted to include time slot as an additional (interacting) term in the statistical model, but those more complex models were non-convergent; therefore, we hesitate to draw any strong conclusions from the post hoc t-tests.
Regarding the pupillary response, we again saw a difference between maintain- and switch-attention trials, with the divergence beginning as soon as listeners heard the attentional cue. We also saw a significant difference in the pupillary response to long- versus short-gap trials, though the difference appears to be a post-gap delay in the long-gap trials (mirroring the stimulus time course), rather than a vertical shift indicating increased effort. Contrary to our hypothesis, there was no apparent effect of spectral degradation on the pupillary response.
IV. GENERAL DISCUSSION
The main goal of these experiments was to see whether the pupillary response would reflect the mental effort of switching attention between talkers who were spatially separated (experiment 1), or talkers separable only by talker voice quality and pitch (experiment 2). The overall finding was that attention switching is clearly reflected in the pupillary signal as an increase in dilation that begins either as soon as listeners are aware that a switch will be required, or perhaps as soon as they begin planning the switch; since we did not manipulate the latency between the cue and the onset of the switch gap these two possibilities cannot be disambiguated.
A secondary goal of these experiments was to reproduce past findings regarding the pupillary response to degraded sentential stimuli, but using a simpler stimulus paradigm (spoken letter sequences) and (in experiment 1) relatively mild stimulus degradations like reverberation. In fact, we failed to see any effect of stimulus degradation in the pupillary response, neither when degrading the temporal cues for spatial separation through simulated reverberation, nor with more severe degradation of the signal's spectral resolution through noise vocoding (experiment 2). We believe the key difference lies in our choice of stimuli: detecting a target letter in a sequence of spoken letters is not the same kind of task as computing the meaning of a well-formed sentence, and our results suggest that simply detecting targets among a small set of possible stimulus tokens does not engage the same neural circuits or invoke the same kind of mental effort or cognitive load that is responsible for pupillary dilations seen in the sentence comprehension tasks of Zekveld and colleagues [showing greater dilation to sentences with lower signal-to-noise ratios (SNRs)]14,19 or Winn and colleagues (showing greater dilation to sentences with more severe spectral degradation).16 Taking those findings together with the results of the present study, one might say that signal degradation itself was not the proximal cause of pupil dilation in those sentence comprehension experiments; rather, it was the additional cogitation or effort needed to construct a coherent linguistic meaning from degraded speech that led to the pupillary responses they observed.
Notably, Winn and colleagues showed a sustained pupillary response in cases where listeners failed to answer correctly, suggesting that continued deliberation about how to respond may be reflected by pupil size. Similarly, Kuchinsky and colleagues20 showed greater pupillary response in word-identification tasks involving lower SNRs when lexical competitors were present among response choices; their results show a sustained elevation in the time course of the pupillary response in the harder conditions (as well as a parallel increase in reaction time). Both sets of findings suggest that the pupillary response reflects effort exerted by the listener, as do the sustained large dilations seen in Koelewijn and colleagues' divided attention trials (where listeners heard two talkers presented dichotically and had to report both sentences).23
The present study, on the other hand, shows that for an experimental manipulation to elicit a larger pupillary response than other tasks, it is not enough that the task simply be made harder. Rather, there is an important distinction between a task being harder and a listener trying harder; or what, in the terms of a recent consensus paper from a workshop on hearing impairment and cognitive energy, might be described as the difference between “demands” and “motivation.”18 In this light, we can understand why our stimulus manipulations yielded no change in pupillary response: our task required rapid-response target identification, in which listeners had little opportunity to ponder a distorted or partial percept nor could they later reconstruct whether a target had been present based on surrounding context. Thus, the listener has no recourse by which to overcome the increased task demands, and consequently there should be no difference in motivation, no difference in effort, and no difference in the pupillary response. In contrast, our behavioral “maintain/switch” manipulation did provide an opportunity for the listener to exert effort (in the form of a well-timed mid-trial attention switch) to achieve task success, and the difference in pupillary responses between maintain- and switch-attention trials reflects this difference.
ACKNOWLEDGMENTS
Portions of this work were supported by National Institutes of Health (NIH) Grant Nos. R01-DC013260 to A.K.C.L., F32-DC012456 to E.L., T32-DC000018 to the University of Washington, and NIH LRP awards to E.L. and D.R.M. The authors are grateful to Susan McLaughlin and two anonymous reviewers for helpful suggestions on an earlier draft of this paper, and to Maria Chait for suggesting certain useful post hoc analyses.
Portions of the research described here were previously presented at the 37th Annual MidWinter Meeting of the Association for Research in Otolaryngology and are published in Ref. 9.
References
- 1. Shamma S. A., Elhilali M., and Micheyl C., “ Temporal coherence and attention in auditory scene analysis,” Trends Neurosci. 34(3), 114–123 (2011). 10.1016/j.tins.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shinn-Cunningham B. G. and Best V., “ Selective attention in normal and impaired hearing,” Trends Amplif. 12(4), 283–299 (2008). 10.1177/1084713808325306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larson E. D. and Lee A. K. C., “ Influence of preparation time and pitch separation in switching of auditory attention between streams,” J. Acoust. Soc. Am. 134(2), EL165–EL171 (2013). 10.1121/1.4812439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Ruiter J.-P., Mitterer H., and Enfield N. J., “ Projecting the end of a speaker's turn: A cognitive cornerstone of conversation,” Language 82(3), 515–535 (2006). 10.1353/lan.2006.0130 [DOI] [Google Scholar]
- 5. Kahneman D. and Beatty J., “ Pupil diameter and load on memory,” Science 154(3756), 1583–1585 (1966). 10.1126/science.154.3756.1583 [DOI] [PubMed] [Google Scholar]
- 6. Beatty J., “ Task-evoked pupillary responses, processing load, and the structure of processing resources,” Psychol. Bull. 91(2), 276–292 (1982). 10.1037/0033-2909.91.2.276 [DOI] [PubMed] [Google Scholar]
- 7. Hoeks B. and Levelt W. J. M., “ Pupillary dilation as a measure of attention: A quantitative system analysis,” Behav. Res. Meth. Ins. C 25(1), 16–26 (1993). 10.3758/BF03204445 [DOI] [Google Scholar]
- 8. Wierda S. M., van Rijn H., Taatgen N. A., and Martens S., “ Pupil dilation deconvolution reveals the dynamics of attention at high temporal resolution,” Proc. Natl. Acad. Sci. U.S.A. 109(22), 8456–8460 (2012). 10.1073/pnas.1201858109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCloy D., Larson E., Lau B., and Lee A. K. C., “ Temporal alignment of pupillary response with stimulus events via deconvolution,” J. Acoust. Soc. Am. 139(3), EL57–EL62 (2016). 10.1121/1.4943787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor J. S., “ Pupillary response to auditory versus visual mental loading: A pilot study using super 8-mm photography,” Percept. Mot. Skills 52(2), 425–426 (1981). 10.2466/pms.1981.52.2.425 [DOI] [PubMed] [Google Scholar]
- 11. Ahern S. and Beatty J., “ Physiological evidence that demand for processing capacity varies with intelligence,” in Intelligence and Learning, edited by Friedman M. P., Das J. P., and O'Connor N. ( Springer, Boston, 1981), NATO Conference Series No. 14, pp. 121–128. [Google Scholar]
- 12. Papesh M. H. and Goldinger S. D., “ Pupil-BLAH-metry: Cognitive effort in speech planning reflected by pupil dilation,” Atten. Percept. Psychophys. 74(4), 754–765 (2012). 10.3758/s13414-011-0263-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hess E. H. and Polt J. M., “ Pupil size in relation to mental activity during simple problem-solving,” Science 143(3611), 1190–1192 (1964). 10.1126/science.143.3611.1190 [DOI] [PubMed] [Google Scholar]
- 14. Zekveld A. A., Kramer S. E., and Festen J. M., “ Pupil response as an indication of effortful listening: The influence of sentence intelligibility,” Ear Hear. 31(4), 480–490 (2010). 10.1097/AUD.0b013e3181d4f251 [DOI] [PubMed] [Google Scholar]
- 15. Koelewijn T., Zekveld A. A., Festen J. M., Rönnberg J., and Kramer S. E., “ Processing load induced by informational masking is related to linguistic abilities,” Int. J. Otolaryngol. 2012, 865731. 10.1155/2012/865731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Winn M. B., Edwards J. R., and Litovsky R. Y., “ The impact of auditory spectral resolution on listening effort revealed by pupil dilation,” Ear Hear. 36(4), e153–e165 (2015). 10.1097/AUD.0000000000000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGarrigle R., Munro K. J., Dawes P., Stewart A. J., Moore D. R., Barry J. G., and Amitay S., “ Listening effort and fatigue: What exactly are we measuring? A British Society of Audiology Cognition in Hearing Special Interest Group ‘white paper,’ ” Int. J. Audiol. 53(7), 433–445 (2014). 10.3109/14992027.2014.890296 [DOI] [PubMed] [Google Scholar]
- 18. Pichora-Fuller M. K., Kramer S. E., Eckert M. A., Edwards B., Hornsby B. W., Humes L. E., Lemke U., Lunner T., Matthen M., Mackersie C. L., Naylor G., Phillips N. A., Richter M., Rudner M., Sommers M. S., Tremblay K. L., and Wingfield A., “ Hearing impairment and cognitive energy: The framework for understanding effortful listening (FUEL),” Ear Hear. 37, 5S–27S (2016). 10.1097/AUD.0000000000000312 [DOI] [PubMed] [Google Scholar]
- 19. Zekveld A. A., Kramer S. E., and Festen J. M., “ Cognitive load during speech perception in noise: The influence of age, hearing loss, and cognition on the pupil response,” Ear Hear. 32(4), 498–510 (2011). 10.1097/AUD.0b013e31820512bb [DOI] [PubMed] [Google Scholar]
- 20. Kuchinsky S. E., Ahlstrom J. B., Vaden K. I., Cute S. L., Humes L. E., Dubno J. R., and Eckert M. A., “ Pupil size varies with word listening and response selection difficulty in older adults with hearing loss,” Psychophysiology 50(1), 23–34 (2013). 10.1111/j.1469-8986.2012.01477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Best V., Gallun F. J., Mason C. R., G. D. Kidd, Jr. , and Shinn-Cunningham B. G., “ The impact of noise and hearing loss on the processing of simultaneous sentences,” Ear Hear. 31(2), 213–220 (2010). 10.1097/AUD.0b013e3181c34ba6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koelewijn T., Shinn-Cunningham B. G., Zekveld A. A., and Kramer S. E., “ The pupil response is sensitive to divided attention during speech processing,” Hear. Res. 312, 114–120 (2014). 10.1016/j.heares.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koelewijn T., de Kluiver H., Shinn-Cunningham B. G., Zekveld A. A., and Kramer S. E., “ The pupil response reveals increased listening effort when it is difficult to focus attention,” Hear. Res. 323, 81–90 (2015). 10.1016/j.heares.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kahneman D. and Beatty J., “ Pupillary responses in a pitch-discrimination task,” Percept. Psychophys. 2(3), 101–105 (1967). 10.3758/BF03210302 [DOI] [Google Scholar]
- 25. Nábělek A. K. and Robinson P. K., “ Monaural and binaural speech perception in reverberation for listeners of various ages,” J. Acoust. Soc. Am. 71(5), 1242–1248 (1982). 10.1121/1.387773 [DOI] [PubMed] [Google Scholar]
- 26. Brungart D. S., “ Informational and energetic masking effects in the perception of two simultaneous talkers,” J. Acoust. Soc. Am. 109(3), 1101–1109 (2001). 10.1121/1.1345696 [DOI] [PubMed] [Google Scholar]
- 27. Cole R. A., Muthusamy Y., and Fanty M., “ The ISOLET spoken letter database,” Technical Report 90-004, Oregon Graduate Institute, Hillsboro, OR (1990), paper 205.
- 28. Shinn-Cunningham B. G., Kopco N., and Martin T. J., “ Localizing nearby sound sources in a classroom: Binaural room impulse responses,” J. Acoust. Soc. Am. 117(5), 3100–3115 (2005). 10.1121/1.1872572 [DOI] [PubMed] [Google Scholar]
- 29. DeCarlo L. T., “ Signal detection theory and generalized linear models,” Psychol. Methods 3(2), 186–205 (1998). 10.1037/1082-989X.3.2.186 [DOI] [Google Scholar]
- 30. Sheu C.-F., Lee Y.-S., and Shih P.-Y., “ Analyzing recognition performance with sparse data,” Behav. Res. Meth. 40(3), 722–727 (2008). 10.3758/BRM.40.3.722 [DOI] [PubMed] [Google Scholar]
- 31. McCloy D. R. and Lee A. K. C., “ Auditory attention strategy depends on target linguistic properties and spatial configuration,” J. Acoust. Soc. Am. 138(1), 97–114 (2015). 10.1121/1.4922328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larson E. D. and Engemann D. A., “pyeparse (version 0.1.0),” http://dx.doi.org/10.5281/zenodo.14566.
- 33. Maris E. and Oostenveld R., “ Nonparametric statistical testing of EEG- and MEG-data,” J. Neurosci. Meth. 164(1), 177–190 (2007). 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- 34. Gramfort A., Luessi M., Larson E. D., Engemann D. A., Strohmeier D., Brodbeck C., Goj R., Jas M., Brooks T., Parkkonen L., and Hämäläinen M. S., “ MEG and EEG data analysis with MNE-Python,” Front. Neurosci. 7, 267 (2013). 10.3389/fnins.2013.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hupé J.-M., Lamirel C., and Lorenceau J., “ Pupil dynamics during bistable motion perception,” J. Vision 9(7), 10 (2009). 10.1167/9.7.10 [DOI] [PubMed] [Google Scholar]
- 36. Larson E. D. and Lee A. K. C., “ The cortical dynamics underlying effective switching of auditory spatial attention,” NeuroImage 64, 365–370 (2013). 10.1016/j.neuroimage.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shannon R. V., Zeng F.-G., Kamath V., Wygonski J., and Ekelid M., “ Speech recognition with primarily temporal cues,” Science 270(5234), 303–304 (1995). 10.1126/science.270.5234.303 [DOI] [PubMed] [Google Scholar]
- 38. Moore B. C. J. and Glasberg B. R., “ Formulae describing frequency selectivity as a function of frequency and level, and their use in calculating excitation patterns,” Hear. Res. 28(2-3), 209–225 (1987). 10.1016/0378-5955(87)90050-5 [DOI] [PubMed] [Google Scholar]
- 39.See supplementary material at http://dx.doi.org/10.1121/1.4979340 E-JASMAN-141-014704 for details of statistical model specifications and results, post-hoc analyses, and location of data/code repository.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1121/1.4979340 E-JASMAN-141-014704 for details of statistical model specifications and results, post-hoc analyses, and location of data/code repository.