Abstract
Differences in formant frequencies and fundamental frequencies (F0) are important cues for segregating and identifying two simultaneous vowels. This study assessed age- and hearing-loss-related changes in the use of these cues for recognition of one or both vowels in a pair and determined differences related to vowel identity and specific vowel pairings. Younger adults with normal hearing, older adults with normal hearing, and older adults with hearing loss listened to different-vowel and identical-vowel pairs that varied in F0 differences. Identification of both vowels as a function of F0 difference revealed that increased age affects the use of F0 and formant difference cues for different-vowel pairs. Hearing loss further reduced the use of these cues, which was not attributable to lower vowel sensation levels. High scores for one vowel in the pair and no effect of F0 differences suggested that F0 cues are important only for identifying both vowels. In contrast to mean scores, widely varying differences in effects of F0 cues, age, and hearing loss were observed for particular vowels and vowel pairings. These variations in identification of vowel pairs were not explained by acoustical models based on the location and level of formants within the two vowels.
I. INTRODUCTION
Younger adults with normal hearing (YNH) have a remarkable ability to understand a single talker in the presence of competing talkers. Differences in talkers' fundamental frequencies (F0) and other speech characteristics are important cues for successful segregation of the target and competing speech (e.g., Cherry, 1953; Brokx and Nooteboom, 1982; Bregman, 1990). Listeners' ability to identify two vowels presented concurrently demonstrates the extent to which differences in F0 and vowel formants (or other speech characteristics) contribute to segregation and identification of multiple talkers. For example, identification of two simultaneously presented vowels by YNH subjects improves by ∼15%–30% as the F0 difference between the two vowels increases (from 0 to 3 Hz) and then asymptotes at larger F0 differences (6–12 Hz) (e.g., Zwicker, 1984; Assmann and Summerfield, 1990; Culling and Darwin, 1993, 1994; Chintanpalli and Heinz, 2013; see review by Micheyl and Oxenham, 2010). From identification of concurrent vowels, it is also clear that cues other than F0 differences, such as formant frequency differences, are contributing to vowel segregation, because vowel identification scores are well above chance even when the two vowels have identical F0s (e.g., Chintanpalli and Heinz, 2013; Chintanpalli et al., 2014).
The improvement in identification of both vowels for YNH subjects is thought to be largely dependent on improvement in F0 segregation (i.e., segregating two simultaneous vowels using F0 differences); this improvement primarily occurs with F0 differences that are less than 6 Hz. Culling and Darwin (1994) suggested beating or temporal envelope cues as an alternative cue for identification at non-zero F0 differences, which might arise from harmonic interactions between vowels. However, de Cheveigné (1999) varied the temporal envelope cue of each vowel by modifying the phase of the harmonics and found no change in vowel identification. Assmann and Paschall (1998) found that listeners can successfully adjust the F0 of a harmonic tone complex to match the pitch of a concurrent vowel only when the F0 difference is at least 26 Hz. Larsen et al. (2008) found that the phase locking of auditory-nerve (AN) fibers provides sufficient information to correctly determine both F0s of concurrent harmonic tone complexes with F0 differences of 7 and 22 Hz. However, the studies by Assmann and Paschall (1998) and Larsen et al. (2008) included F0 identification but not F0-based segregation for concurrent vowels. Nevertheless, these results support the hypothesis that F0 differences between vowels serve as the primary cue for identification of concurrent vowels, at least for YNH subjects.
Recognition of a target talker in the presence of one or more competing talkers declines for older adults with normal and impaired hearing (e.g., Lee and Humes, 2012; Helfer and Freyman, 2008). Similarly, adults of all ages with hearing loss have poorer concurrent vowel identification than listeners with normal hearing, even when identification of single vowels is equivalent to that of YNH subjects. In addition, younger and older adults with hearing loss show less improvement in vowel identification with increasing F0 differences between the two vowels (e.g., Arehart et al., 1997, 2005; Summers and Leek, 1998) than YNH subjects. Only limited information is available on effects of age (without hearing loss) and results are inconsistent (Snyder and Alain, 2005; Vongpaisal and Pichora-Fuller, 2007; Arehart et al., 2011). Whereas results of these studies showed reduced concurrent vowel identification for older compared to younger adults, two studies also showed a reduced F0 benefit (Vongpaisal and Pichora-Fuller, 2007; Arehart et al., 2011), while the other showed an equivalent F0 benefit (Snyder and Alain, 2005).
Several changes in anatomical structures and physiological function in the cochlea and AN fibers that have been attributed to increased age and hearing loss may partially explain poorer concurrent vowel identification. For example, the overall number of AN fibers is reduced with age, especially medium- and low-spontaneous-rate (MSR and LSR) AN fibers (e.g., Makary et al., 2011; Schmiedt et al., 1996, 2002). The amplitude of the compound action potential is smaller in older than younger gerbils, which may relate to reduced phase locking of AN fibers in older gerbils (Hellstrom and Schmiedt, 1990). With regard to physiological and functional changes related to cochlear hearing loss, several changes occur: (1) cochlear nonlinearities (e.g., frequency selectivity, compression, suppression) are reduced (e.g., Liberman and Dodds, 1984a; Ruggero et al., 1997; Miller et al., 1997), (2) phase locking of AN fibers to vowel formants is reduced (e.g., Miller et al., 1997), (3) AN fibers' thresholds are higher, which can result in reduced audibility to formants (e.g., Liberman and Dodds, 1984a), and (4) the number of high-spontaneous-rate (HSR) fibers is reduced, resulting in an increase in the proportion of MSR and LSR fibers remaining (e.g., Liberman and Dodds, 1984b; Heinz and Young, 2004).
These results demonstrate that differential changes in the auditory periphery are associated with increased age and hearing loss, notwithstanding any additional central-auditory and cognitive effects (including memory, attention, speed of processing, and ability to inhibit irrelevant information) that may interact with changes in the auditory periphery. Unfortunately, experiments to date with human subjects that were designed to explore mechanisms that underlie reduced concurrent vowel recognition in older adults with normal and impaired hearing did not include equivalent comparison groups. In some cases, groups included YNH and adults of all ages with hearing loss (Arehart et al., 1997, 2005). In another case, groups included middle-to-older age adults with normal hearing and older adults with hearing loss (Summers and Leek, 1998). These group comparisons make the currently available results inadequate to disentangle potentially confounding factors and independently assess effects of age and hearing loss on concurrent vowel identification.
Thus, the goal of the current experiment was to assess the effects of age and hearing loss on the ability to utilize F0 and formant difference cues to identify two simultaneous vowels. YNH, older adults with normal hearing (ONH), and older adults with hearing loss (OHI) listened to concurrent vowel pairs that varied in F0 differences between the two vowels. This design provided the means to assess effects of age (without hearing loss) by comparing vowel identification for YNH and ONH subjects; similarly, effects of hearing loss (controlling for age) was assessed by comparing vowel identification for ONH and OHI subjects. Use of F0 difference cues was quantified by measuring concurrent vowel identification as a function of F0 differences between the vowels in the pair. Use of formant difference cues was quantified by comparing scores for specific vowel pairs that varied in first and second formant frequencies (F1 and F2). Identical-vowel pairs were also included to provide conditions in which F0 differences varied between the vowel pair, but formant frequencies for the vowel pair were the same.
II. METHODS
A. Subjects
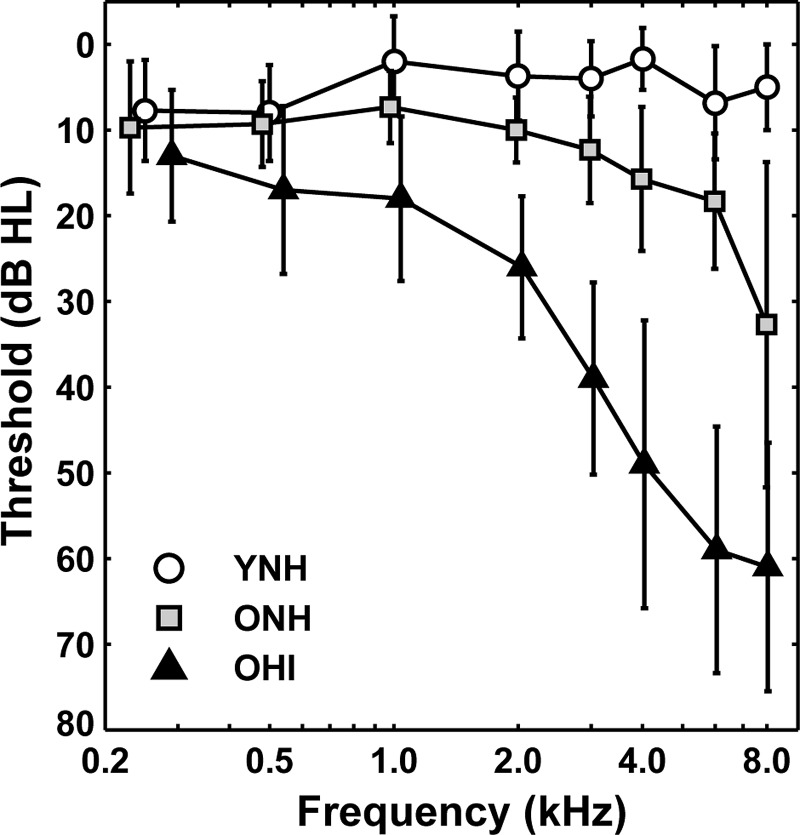
A total of 45 adults participated in this experiment, 15 adults in each of three subject groups: YNH (mean age = 24.7 yrs; range 21–29 yrs; five males), ONH (mean age = 66.5 yrs; range 60–81 yrs; two males), and OHI (mean age = 72.5 yrs; range 62–81 yrs; seven males). Inclusion criterion for auditory thresholds were as follows: (1) YNH subjects had audiometric thresholds ≤25 dB hearing level (HL) between 0.25 and 8.0 kHz, (2) ONH subjects had audiometric thresholds ≤25 dB HL between 0.25 and 3.0 kHz; ≤30 dB HL at 4 kHz; ≤35 dB HL at 6 kHz; and ≤40 dB HL at 8 kHz, and (3) OHI subjects had mild-to-moderate sloping sensorineural hearing loss with thresholds ≤60 dB HL between 0.25 and 3 kHz. All subjects were required to have normal immittance. Figure 1 shows means and standard deviations of audiometric thresholds (in dB HL) for the test ears of subjects in the three groups. For most subjects, the test ear was the right ear; the number of subjects for whom the test ear was the left ear for YNH, ONH, and OHI subjects was 1, 6, and 8, respectively. Pearson correlation coefficients of age and pure-tone average (PTA, average thresholds at 0.5, 1.0, and 2.0 kHz), similar to the frequency range of F1 and F2 of the five vowels, were not statistically significant within the ONH and OHI subject groups and for both groups combined. Prior to participation, subjects provided informed consent, in accordance with the Institutional Review Board of the Medical University of South Carolina. Subjects were paid for their participation, which required four sessions (approximately 2 h per session).
FIG. 1.
Mean pure-tone thresholds (in dB HL) for YNH (open circles), ONH (gray squares), and OHI (black triangles). Error bars indicate ±1 standard deviation. Note that the x axis is shifted slightly for the ONH and OHI groups for clarity.
B. Stimuli and apparatus
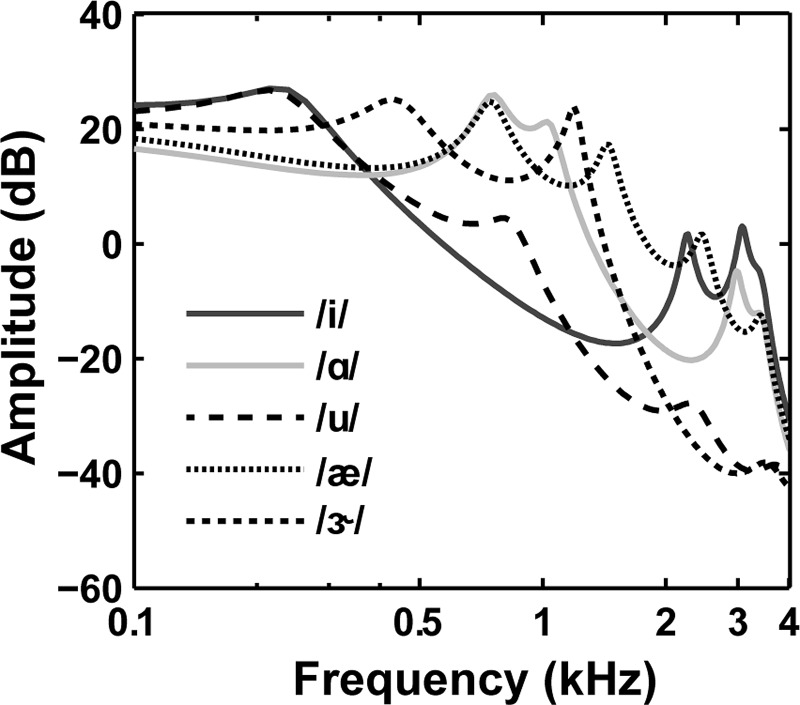
Five vowels (/i/, /ɑ/, /u/, /æ/, /ɝ/) were generated using a Matlab implementation of a cascade formant synthesizer (Klatt, 1980). Vowel duration was 400 ms, including 15-ms raised-cosine rise and fall ramps. Each vowel is characterized by fundamental frequency (F0) and formant frequencies. The harmonic structure of the vowel is provided by its F0 whereas the local maxima correspond to the formant frequencies. Table I includes the formant frequencies and bandwidths for each vowel, which were the same as those used in earlier studies of concurrent vowel identification (e.g., Summers and Leek, 1998; Chintanpalli et al., 2014). Figure 2 shows the envelope spectrum for each vowel, computed using linear predictive coding. The frequencies and levels of each formant (or local maxima in Fig. 2) differed for each vowel.
TABLE I.
Formant frequencies (in Hz) for five vowels. Values in parentheses in the first column are bandwidths (in Hz) for each formant.
| Vowel | /i/ | /ɑ/ | /u/ | /æ/ | /ɝ/ |
|---|---|---|---|---|---|
| “beet” | “father” | “food” | “bat” | “bird” | |
| F1 (90) | 250 | 750 | 250 | 750 | 450 |
| F2 (110) | 2250 | 1050 | 850 | 1450 | 1150 |
| F3 (170) | 3050 | 2950 | 2250 | 2450 | 1250 |
| F4 (250) | 3350 | 3350 | 3350 | 3350 | 3350 |
| F5 (300) | 3850 | 3850 | 3850 | 3850 | 3850 |
FIG. 2.
Envelope spectrum for each of the five vowels, computed using linear predictive coding. The local maxima correspond to the formant frequencies of each vowel.
Subjects listened to pairs of vowels presented simultaneously. The vowels in each pair were either identical (e.g., /ɑ/ and /ɑ/) or different (e.g., /ɑ/ and /u/). F0 difference (in Hz) between the vowels in each pair was varied; F0 for one vowel was always 100 Hz whereas F0 of the other vowel was set to 100, 101.5, 103, 106, 112, or 126 Hz. These conditions correspond to F0 differences of 0, 1.5, 3, 6, 12, and 26 Hz, and are equivalent to 0, 0.25, 0.5, 1, 2, and 4 semitones, respectively (e.g., Assmann and Summerfield, 1990, 1994; Chintanpalli and Heinz, 2013).
There were 25 vowel pairs for each non-zero F0 difference condition. This included five identical-vowel pairs + ten different-vowel pairs (F0 for one vowel was 100 Hz and F0 for the other was selected from 101.5, 103, 106, 112, or 126 Hz) + the same ten different-vowel pairs with the order of F0 of the two vowels reversed (e.g., 101.5 and 100 Hz). To maintain equivalent numbers of vowel pairs when the F0 difference was 0 Hz, this condition also included 25 vowel pairs (five identical-vowel pairs + ten different-vowel pairs presented twice, where F0 was 100 Hz for both vowels in the pair).
Individual vowels were presented at 65 dB sound pressure level (SPL) for subjects with normal hearing (YNH and ONH). To minimize possible confounding effects of reduced audibility due to hearing loss, OHI subjects listened to vowels presented at 85 dB SPL. Although each vowel was presented at an equal root-mean-square level (65 or 85 dB SPL), the levels of each formant differed across vowels (see Fig. 2). The overall level of the vowel pair was ∼3 dB higher than the level of the individual vowels.
To confirm that the presentation levels provided sufficient audibility for all subjects, sensation levels for F1 and F2 for each vowel were computed for each subject, using Reference Equivalent Threshold Sound Pressure Levels published in ANSI S3.6 (2010) for the Sennheiser HDA 200 headphones (Sennheiser Electronic Corp., Old Lyme, CT). Thresholds selected for calculation of sensation levels were those measured at a frequency or a range of frequencies closest to the vowel formant frequency. Table II shows the sensation levels (dB SL) for F1 and F2 for the “worst case” subject in each group at each frequency (i.e., the subject with the poorest threshold at each frequency), with vowel presentation levels of 65 dB SPL for YNH and ONH subjects and 85 dB SPL for OHI subjects. The results of this analysis verified that vowel formant levels were higher than thresholds of all subjects in each group.
TABLE II.
“Worst-case” sensational levels (dB SL) for F1 and F2 for each of the five vowels. These values indicate the difference in dB between the highest pure-tone threshold(s) nearest the vowel formant frequency and the formant level.
| Formant (vowel), formant frequency | YNH | ONH | OHI |
|---|---|---|---|
| F1 (/i/) = 250 Hz F1 (/u/) = 250 Hz | 27 | 22 | 42 |
| F1 (/ɝ/) = 450 Hz | 34 | 34 | 34 |
| F1 (/ɑ/) = 750 Hz F1 (/æ/) = 750 Hz | 41 | 41 | 41 |
| F2 (/u/) = 850 Hz | 18 | 18 | 18 |
| F2 (/ɑ/) = 1050 Hz | 39 | 39 | 39 |
| F2 (/ɝ/) = 1150 Hz | 40 | 40 | 40 |
| F2 (/æ/) = 1450 Hz | 36 | 36 | 34 |
| F2 (/i/) = 2250 Hz | 20 | 20 | 15 |
Pairs of vowels were converted from digital to analog form using a Tucker-Davis Technologies (TDT) RX6 array processor (Tucker-Davis Technologies, Alachua, FL) (sampling frequency = 48.8 kHz) and passed through separate programmable attenuators (TDT PA4) and a mixer (TDT SM3). The vowel pair was passed through a headphone buffer (TDT HB6) and delivered to the subject's test ear through one of a pair of Sennheiser HDA 200 headphones. The right ear was selected as the test ear if the thresholds for both ears were similar; otherwise, the ear that had better average thresholds between 0.25 and 4 kHz was selected as the test ear.
C. Procedures
As subjects had no previous experience with the speech materials or tasks in this experiment, and the listening task is known to be difficult, a reference sheet of orthographic examples of each vowel (“beet,” “father,” “food,” “bat,” “bird”) was provided as part of the familiarization procedure and was available to subjects throughout the experiment. Each subject was tested in the following three phases: screening, two stages of practice, and test. During the screening phase, subjects listened to 30 single vowels with six different F0s (five vowels × six F0 differences); order of F0 was randomized. These 30 single vowels were repeated 5 times for a total of 150 single vowels per block (five vowels × six F0 differences × five repetitions). Subjects responded by selecting the vowel from a row of five vowel symbols (/i/, /ɑ/, /u/, /æ/, /ɝ/) displayed on a touch-screen monitor. Correct-answer feedback was provided for the screening phase only. Subjects were required to achieve ≥90% correct identification for single vowels to proceed further in the experiment. Each subject was given a maximum of three blocks to achieve the criterion score. Most subjects whose single vowel identification scores were ≥90% achieved this criterion after their first block of trials. Exceptions were one ONH subject who required three blocks, one OHI subject who required two blocks, and two OHI subjects who required three blocks. In addition, one ONH subject and eight OHI1 subjects did not meet the ≥90% criterion for single vowel identification after three blocks and, therefore, did not continue further in the experiment. The 15 subjects in each group continued to the two practice stages only after meeting the criterion for the screening phase.
During the first practice stage, each of the 25 vowel pairs with a 26-Hz F0 difference was presented in a random order. Subjects were instructed to identify both vowels in each pair. Two rows of five vowel symbols were displayed on a touch-screen monitor and subjects responded by selecting one vowel from each row; the order of selection was ignored and no feedback was provided. This procedure was then repeated for the 25 vowel pairs with a 0-Hz F0 difference. In the second stage of practice, 25 vowel pairs were presented 4 times in random order at each of six F0 differences (6, 12, 26, 3, 1, 0 Hz, in this order), for a total of 600 vowel pairs per block (25 vowel pairs × four repetitions × six F0 differences). Thus, 600 response trials were contained in the second stage of practice. The test phase was similar to the second stage of practice except four repetitions of the block were included, and F0 differences within each block were randomized. Overall, 2400 response trials were included during the test phase (600 × 4) and no feedback was provided.
For each subject group, vowel identification as a function of F0 difference was computed as follows: (1) mean scores for both vowels correct, calculated from 20 different-vowel pairs and 5 identical-vowel pairs, (2) mean scores for one of the vowels correct calculated from 20 different-vowel and 5 identical-vowel pairs, (3) mean scores of different-vowel pairs that were incorrectly identified as identical-vowel pairs, (4) mean scores for both vowels correct calculated from a specific vowel pair, and (5) mean scores for each vowel correct calculated from a specific vowel pair. All scores were transformed to rationalized arcsine units (rau) to stabilize the variance across conditions (Studebaker, 1985). Effects of F0 difference, block, and subject group on vowel identification scores were assessed with repeated-measures analyses of variance (ANOVA) using the Statistical Package for the Social Sciences software (Version 22). Post hoc comparisons were adjusted using Bonferroni corrections.
III. RESULTS
As a first step in the analysis, identification scores for all vowel pairs [item (1) above] for each of the four blocks were calculated to assess significant changes in scores that may be attributed to procedural learning. A three-way, repeated measures ANOVA, with F0 difference (0, 1, 3, 6, 12, 26 Hz) and block (four blocks) as repeated measures, and subject group (YNH, ONH, OHI) as a grouping factor, was performed on the identification scores (in rau). Main effects of subject group [F(2,42) = 48.57, p = 0.00], F0 difference [F(5,210) = 97.29, p = 0.00], and block [F(3,126) = 9.24, p = 0.00] were significant. Interactions between subject group and block [F(6,126) = 1.35, p = 0.240] and between F0 difference and block [F(15,630) = 1.45, p = 0.12] were not significant. A significant three-way interaction was found [F(30,630) = 1.49, p = 0.047]. Post hoc comparisons, using Bonferroni corrections, showed no significant differences in scores between the third and fourth blocks (p = 0.21); however, significant differences in scores were observed between the first and third blocks (p = 0.002), the first and fourth blocks (p = 0.004), the second and third blocks (p = 0.00), and the second and fourth blocks (p = 0.00). Thus, identification scores from the third and fourth blocks were averaged; these scores were included in figures and used in subsequent data analyses to assess effects of age and hearing loss on concurrent vowel identification, using two-way repeated measures ANOVAs, with F0 difference as a repeated measure and subject group as a grouping factor.
A. Effects of F0 differences on concurrent vowel identification
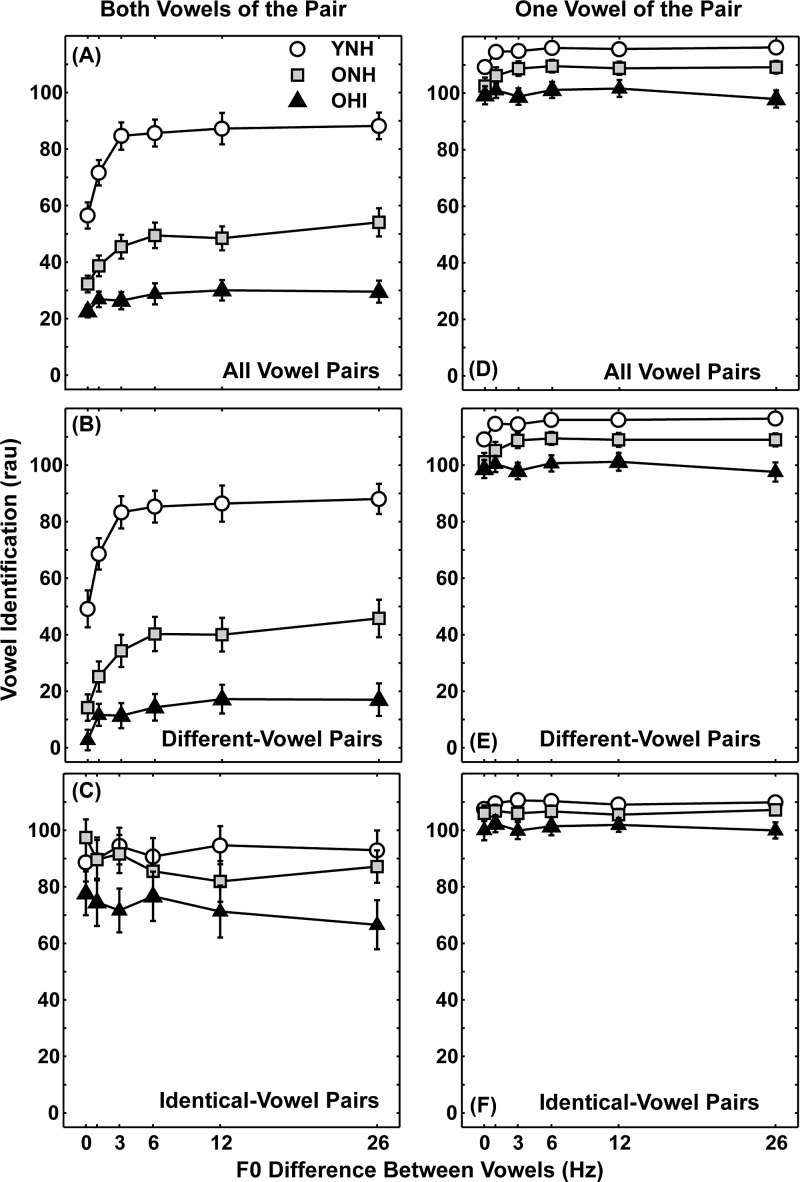
Figures 3(A)–3(C) show identification scores as a function of F0 difference for the three subject groups for all 25 vowel pairs [panel (A)], 20 different-vowel pairs [panel (B)], and 5 identical-vowel pairs [panel (C)]. For all three groups, identification of two vowels improves as the F0 difference between the two vowels increased [Fig. 3(A)]. However, overall vowel identification and the extent to which vowel identification improves with F0 difference (“F0 benefit”) varied with subject group. These general patterns are similar to those in Fig. 3(B) for different-vowel pairs, which would be expected as the complete set of vowel pairs [25 pairs; Fig. 3(A)] were primarily different-vowel pairs [20 pairs; Fig. 3(B)]. In contrast, quite different patterns were observed for identical-vowel pairs [5 pairs; Fig. 3(C)], most notably the relatively small or no change in identification with increasing F0 difference and the higher and generally similar scores for the three subject groups. Therefore, statistical analyses were conducted separately for scores for 20 different-vowel pairs [Fig. 3(B)] and scores for 5 identical-vowel pairs [Fig. 3(C)].
FIG. 3.
Mean vowel identification scores (in rau) as a function of F0 difference for YNH subjects (open circles), ONH subjects (gray squares), and OHI subjects (black triangles). Left panels [(A), (B), (C)] display identification scores for both vowels in the pair and right panels [(D), (E), (F)] display identification scores for one vowel in the pair. [(A) and (D)] Identification scores for all 25 vowel pairs. [(B) and (E)] Identification scores for 20 different-vowel pairs. [(C) and (F)] Identification scores for five identical-vowel pairs. Error bars indicate ±1 Standard Error of the Mean (SEM). Note that scores for all vowel pairs are not the average of scores for different-vowel and identical-vowel pairs due to differences in the numbers of vowel pairs.
ANOVA results for identification scores for different-vowel pairs revealed significant main effects of subject group [F(2,42) = 41.09, p = 0.000], F0 difference [F(5,210) = 81.13, p = 0.000], and an interaction between subject group and F0 difference [F(10,210) = 7.22, p = 0.000]. Post hoc comparisons of F0 differences for each subject group (using Bonferroni corrections) focused on significant changes in scores for adjacent F0 differences. For YNH subjects, scores improved significantly from 0 to 1 Hz to 3 Hz (p < 0.001), but no further improvement was observed at larger F0 differences. For ONH subjects, scores significantly improved with each F0 difference increment from 0 Hz through 26 Hz (p ≤ 0.003), except no significant improvement was observed between 6 and 12 Hz. In contrast, scores for OHI subjects did not change significantly as a function of F0 difference.
Comparing scores for different-vowel pairs for F0 differences of 0 and 26 Hz [Fig. 3(B)], the mean F0 benefit was 38.9 rau for YNH subjects and 31.5 rau for ONH subjects. The F0 benefit for these two normal-hearing groups was not significantly different and both were significantly larger than the F0 benefit of 14.2 rau for OHI subjects [YNH vs OHI: t(28) = 4.21, p = 0.000; ONH vs OHI: t(28) = 2.49, p = 0.019]. These patterns of identification scores and F0 benefit are generally consistent with previous studies of concurrent vowels for these three groups (e.g., Chintanpalli and Heinz, 2013; Snyder and Alain, 2005; Vongpaisal and Pichora-Fuller, 2007; Arehart et al., 1997).
For identical-vowel pairs [Fig. 3(C)], main effects of subject group and F0 difference were not significant, but a significant interaction of subject group and F0 difference was observed [F(10,210) = 3.17, p = 0.001]. Post hoc comparisons of scores at an adjacent F0 difference revealed no statistically significant changes in scores. Note that, for identical-vowel pairs, some changes in score with increasing F0 difference were decreases in scores, rather than the improvements seen for different-vowel pairs. In contrast to F0 benefit for different-vowel pairs, F0 benefit (0 Hz vs 26 Hz) for identical-vowel pairs was much smaller for YNH subjects (4.2 rau) and negative for the two older groups (−10.3 rau for ONH subjects and −11.1 rau for OHI subjects). The F0 benefit for the two older groups was not significantly different and both were significantly smaller than the F0 benefit for the YNH group [YNH vs ONH: t(28) = 2.32, p = 0.028; YNH vs OHI: t(28) = 2.17, p = 0.039].
Smaller (or negative) F0 benefits, higher overall scores, and equivalent scores across subject groups demonstrate that F0 difference cues are relatively ineffective for segregating identical-vowel pairs. Moreover, F0 differences between vowels can be detrimental to concurrent vowel identification for older adults for vowel pairs with the same formants (consistent with Vongpaisal and Pichora-Fuller, 2007 for ONH subjects). These results provide additional evidence that F0 difference cues are beneficial only for vowel pairs that also have different formants.
B. Effects of age on concurrent vowel identification
Effects of age (without effects of hearing loss) can be assessed by comparing identification scores of concurrent vowels for YNH and ONH subjects. For different-vowel pairs [Fig. 3(B)], post hoc comparisons revealed that identification scores for ONH subjects were significantly poorer at each F0 difference than scores for YNH subjects (p = 0.000). In contrast, post hoc comparisons for identical-vowel pairs [Fig. 3(C)] revealed no significant differences in identification scores for YNH and ONH subjects.
C. Effects of hearing loss on concurrent vowel identification
Effects of hearing loss (controlling for age) can be assessed by comparing identification scores of concurrent vowels for ONH and OHI subjects. For different-vowel pairs [Fig. 3(B)], post hoc comparisons revealed that identification scores for OHI subjects were significantly poorer than scores for ONH subjects at the four largest F0 differences (3, 6, 12, 26 Hz, p ≤ 0.007). In contrast, post hoc comparisons for identical-vowel pairs [Fig. 3(C)] revealed no significant differences in identification scores for ONH and OHI subjects. To further assess individual differences related to subject age and hearing loss on vowel identification, Pearson correlation coefficients were computed between age, PTA, identification scores, and F0 benefit for each group, but none of these correlation coefficients was statistically significant. Relatively weak correlations could not be attributed to narrow ranges of ages and PTAs, as ages ranged from 60 to 81 and 62 to 81 yrs for the two older groups, and PTAs ranged from 6.7 to 31.7 dB HL (average of 0.5, 1.0, and 2.0 kHz). The inability of subject age to serve as a predictor of vowel identification is not consistent with results of Summers and Leek (1998), who reported strong correlations of age and scores.
D. Effects of F0 difference on identification of one vowel in the pair
Figures 3(D)–3(F) show scores for the three subject groups for one vowel correct as a function of F0 difference, including results for all vowel pairs [panel (D)], different-vowel pairs [panel (E)], and identical-vowel pairs [panel (F)]. For all vowel pairs [Fig. 3(D)], identification scores for one vowel were very high (means ≥ 97 rau) with smaller changes in scores with increasing F0 difference. For different-vowel pairs [Fig. 3(E)] and identical-vowel pairs [Fig. 3(F)], separate two-way repeated measures ANOVAs were performed on identification scores for one vowel (in rau), with F0 difference as a repeated measure and subject group as a grouping factor. For different-vowel pairs, ANOVA results for identification scores revealed significant main effects of subject group [F(2,42) = 11.29, p = 0.000], F0 difference [F(5,210) = 8.55, p = 0.000], and the interaction between subject group and F0 difference [F(10,210) = 2.26, p = 0.016]. However, none of the changes in scores between adjacent F0 differences reached statistical significance for any subject group. Analyzing the interaction another way, scores for YNH subjects were significantly higher than for OHI subjects at all F0 differences except 0 Hz (all p = 0.000). However, scores for YNH and ONH subjects and scores for ONH and OHI subjects were not significantly different at any F0 difference.
For identical-vowel pairs [Fig. 3(F)], a main effect of subject group was significant [F(2,42) = 4.30, p < 0.020]; post hoc comparisons attributed this main effect to significantly higher scores for the YNH group than the OHI group (p < 0.018). The main effect of F0 difference and the interaction of subject group and F0 difference were not statistically significant.
E. Effects of F0 difference on incorrect identification of different-vowel pairs as identical-vowel pairs
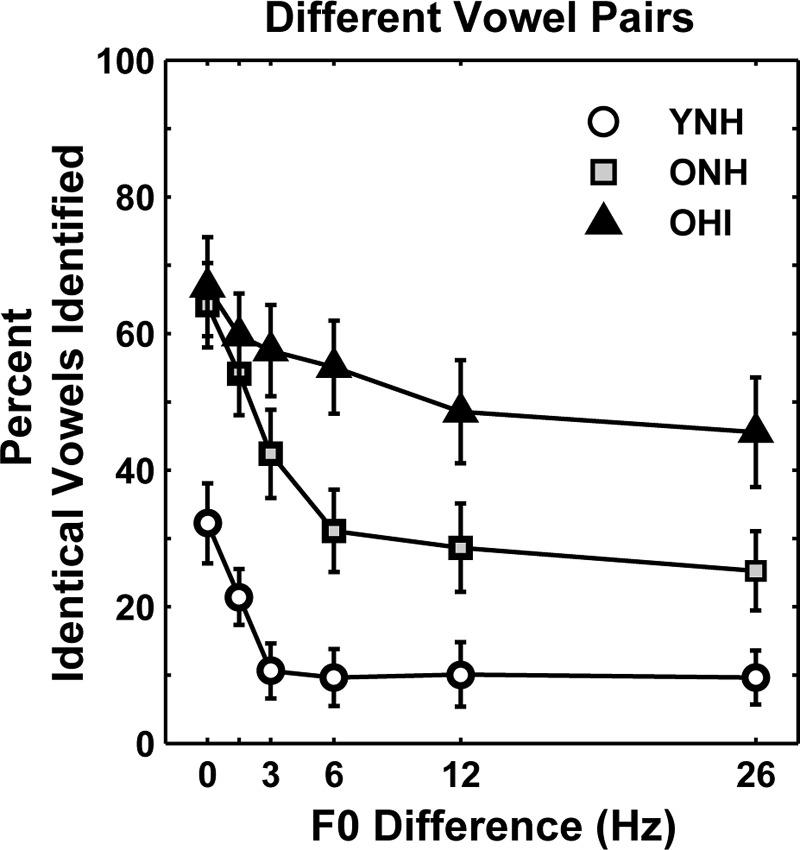
Figure 4 shows the mean percentage of different-vowel pairs that were incorrectly identified as identical-vowel pairs as a function of F0 difference for each subject group. For all subject groups, the highest percentage was for 0-Hz F0 differences; percentages decreased as F0 difference increased. Percentages of different-vowel pairs identified as identical-vowel pairs were highest for OHI subjects, lowest for YNH subjects, and intermediate for ONH subjects (except for different-vowel pairs with 0-Hz F0 differences where ONH and OHI were equivalent). These general patterns were also observed for individual different-vowel pairs and for different vowel identities. In order to take into account group differences in the absolute number of errors, the proportion of incorrect responses to different-vowel pairs that were different-vowel or identical-vowel pairs was calculated (not shown). The proportion of responses that were identical-vowel pairs was always higher than different-vowel pairs but became more evenly split with increasing F0 difference.
FIG. 4.
Mean percentage of different-vowel pairs that were incorrectly identified as identical-vowel pairs as a function of F0 difference for YNH subjects (open circles), ONH subjects (gray squares), and OHI subjects (black triangles). Error bars indicate ±1 SEM.
F. Variations in identification of both vowels in the pair
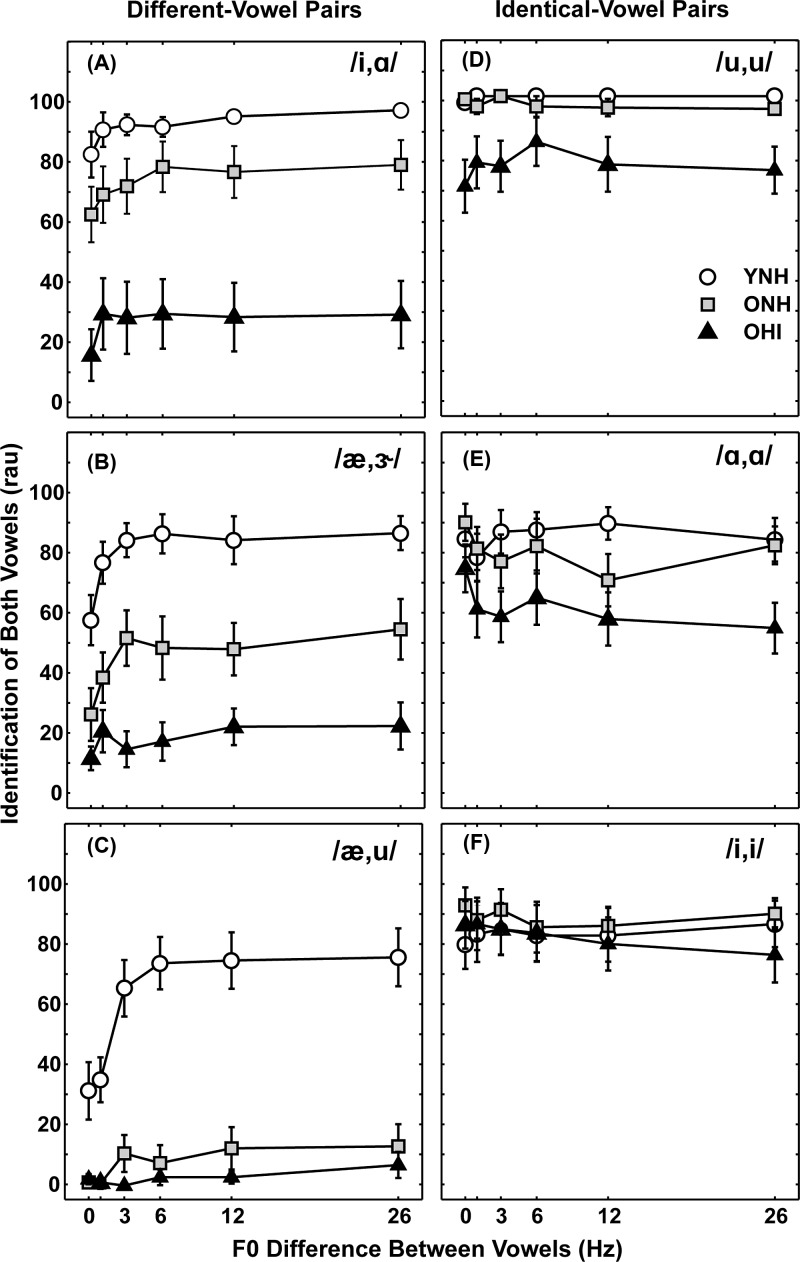
For each different-vowel pair, formant frequency differences vary depending on the particular vowel pairing (see Table I). Therefore, rather than averaging across vowel pairs as shown in Fig. 3, it was of interest to determine both-vowel identification scores for specific vowel pairings and the role of F0 difference cues. Figure 5 displays scores for identification of both vowels as a function of F0 difference for three different-vowel pairs (left column) and three identical-vowel pairs (right column). These figures illustrate the wide range of scores obtained as a function of F0 difference and among the three subject groups, even for identical-vowel pairs. Although only a sub-set of vowel pairs are included, these differences are representative of the variation observed for the 20 different-vowel pairs and the five identical-vowel pairs. Among the most striking disparities across vowel pair is seen for the ONH group for the different-vowel pairs, where scores were either similar to YNH subjects [Fig. 5(A)], intermediate [Fig. 5(B)], or similar to OHI subjects [Fig. 5(C)]. Although scores for identical-vowel pairs were higher, substantial variations in performance across vowel identity, F0 difference, and subject group were observed [Figs. 5(D)–5(F)].
FIG. 5.
Mean identification scores (in rau) for both vowels in individual vowel pairs as a function of F0 difference for YNH subjects (open circles), ONH subjects (gray squares), and OHI subjects (black triangles). The left column [panels (A)–(C)] includes different-vowel pairs and the right column [panels (D)–(F)] includes identical-vowel pairs. Error bars indicate ±1 SEM. Vowel pairs are identified in each panel.
G. Variations in identification of one vowel in the pair
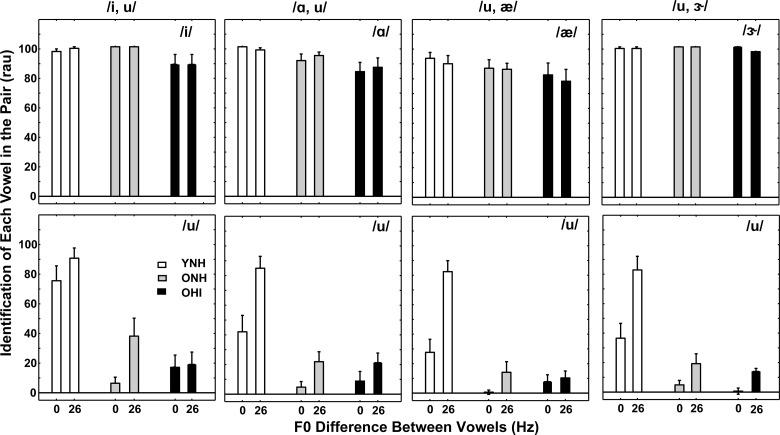
Given the variation in identification of both vowels in the pair, as shown in Fig. 5, it was likely that large differences would also be apparent in identification of each vowel in the pair. Analysis of identification of each vowel in the pair can determine the extent to which one vowel is consistently identified correctly more often than its pair (Arehart et al., 2005; Fogerty et al., 2012; de Cheveigné et al., 1997; McKeown, 1992; McKeown and Patterson, 1995; Zwicker 1984; Hedrick and Madix, 2009; Lentz and Marsh, 2006; Chintanpalli and Heinz, 2013). Figure 6 shows identification scores (in rau) of each vowel in four vowel pairs that included /u/. Mean scores are shown for the three subject groups for the smallest and largest F0 differences (left bar: 0 Hz; right bar: 26 Hz). For all groups, scores for vowels paired with /u/ were relatively high for both 0- and 26-Hz F0 differences. That is, when paired with /u/, F0 difference cues were not required for near perfect performance. These results are consistent with those of Chintanpalli and Heinz (2013) for YNH subjects, but had not yet been observed for ONH and OHI subjects. Scores for /u/ were poorer (especially for ONH and OHI subjects where scores were near chance); identification of /u/ within the pair benefited substantially from F0 differences for YNH subjects but minimally for OHI subjects.
FIG. 6.
Mean identification scores (in rau) for each vowel in four different-vowel pairs that included /u/ for the smallest (0 Hz) and largest (26 Hz) F0 differences. Scores for YNH subjects are the left two bars (white), scores for ONH subjects are the middle two bars (gray), and scores for OHI subjects are the right two bars (black). The vowel pair is displayed at the top of each column and the individual vowel of the pair is labeled within each panel. Error bars indicate ±1 SEM.
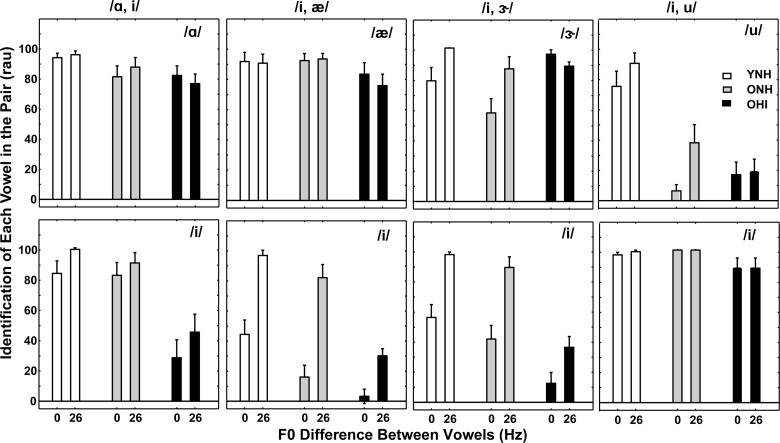
A different pattern of results is shown in Fig. 7, which includes identification scores (in rau) for each vowel in four vowel pairs that included /i/. Mean scores are shown for the three subject groups for the smallest and largest F0 differences (to provide a complete set, vowel pair /i, u/ is repeated from Fig. 6). When /i/ is one of the vowels in the pair, identification scores of the other vowels were generally higher than scores for /i/, and little benefit of F0 differences was noted. Scores for /i/ were somewhat poorer for the 0-Hz F0 difference and some benefit of increasing F0 difference was noted for all groups.
FIG. 7.
Mean identification scores (in rau) for each vowel in four different-vowel pairs that included /i/ for the smallest (0 Hz) and largest (26 Hz) F0 differences. Scores for YNH subjects are the left two bars (white), scores for ONH subjects are the middle two bars (gray), and scores for OHI subjects are the right two bars (black). The vowel pair is displayed at the top of each column and the individual vowel of the pair is labeled within each panel. Error bars indicate ±1 SEM. To provide a complete set, vowel pair /i, u/ is repeated from Fig. 6.
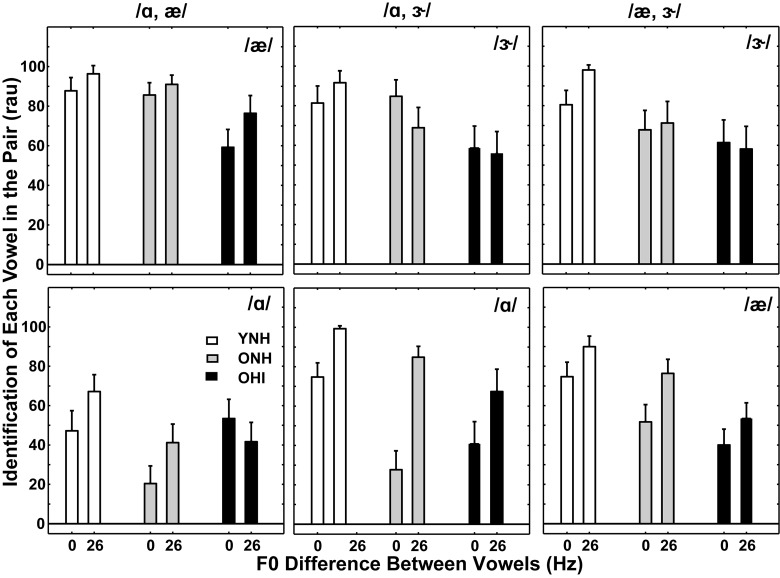
A final set of examples of the effect of vowel pair is shown in Fig. 8, which includes identification scores (in rau) for each vowel in three vowel pairs for the three subject groups for the smallest and largest F0 differences. The results shown in Figs. 3(B) and 3(E) represent mean identification scores and changes in scores with increasing F0 differences for different-vowel pairs (with highest scores and largest F0 benefit for YNH subjects, lowest scores and smallest F0 benefit for OHI subjects, and intermediate results with ONH subjects). However, substantial deviations from these mean results were apparent for identification of one vowel in the pair (Figs. 6–8) and for identification of two vowels within specific vowel pairings (Fig. 5). Similar variations in identification scores across groups were observed for all vowel pairs, even when F0 order in the pair was reversed.
FIG. 8.
Mean identification scores (in rau) for each vowel for three additional different-vowel pairs (shown at the top of each panel) for the smallest (0 Hz) and largest (26 Hz) F0 differences. Scores for YNH subjects are the left two bars (white), scores for ONH subjects are the middle two bars (gray), and scores for OHI subjects are the right two bars (black). The individual vowel of the pair is labeled within each panel. Error bars indicate ±1 SEM.
H. Sources of variation in concurrent vowel identification
Possible sources of variations in scores among vowels and vowel pairs include (1) differences in locations of the vowel formant frequencies between the two vowels of the pair, (2) level differences of the vowel formant frequencies between the two vowels of the pair, and (3) interactions of these effects with age-related and hearing-loss-related changes in perception. Concerning the role of formant frequency locations for the two vowels, F0 benefit for identification of different-vowel pairs has been shown previously to be predicted by formant spacings between F1 and F2 of the two vowels in the pair (e.g., Chintanpalli and Heinz, 2013). More specifically, a larger F0 benefit for YNH subjects was observed for identification of vowel pairs whose formant frequencies were closely spaced, whereas a smaller F0 benefit was observed for identification of vowel pairs whose formant frequencies were distinctly spaced (see Chintanpalli and Heinz, 2013; Fig. 5, p. 2992). To determine the extent to which this pattern was replicated in the current study for YNH subjects and extended to ONH and OHI subjects, we calculated the correlation between the smallest formant distance (in octaves) between the two vowels in the pair and their F0 benefit for the three subject groups. We hypothesized that distinct formant spacings may be even more critical for OHI subjects, due to their impaired frequency selectivity, broader auditory filters, and greater susceptibility to upward spread of masking. Using the same formant distance metric as reported in Chintanpalli and Heinz (2013), a statistically significant, but weak, negative correlation was observed for YNH subjects in the current experiment, which accounted for only 21% of the variance in F0 benefit (p = 0.0424). Moreover, for ONH and OHI subjects, the F0 benefit and formant distance between the two vowels of the pair were not significantly correlated (R2 = 1% and 0% for ONH and OHI, respectively, ns).
A similar approach was taken to incorporate formant levels for the five vowels and formant level differences between vowels in the pair and determine their relationship to F0 benefit. First, recall that vowel presentation levels for the three subjects groups (65 dB SPL for YNH and ONH and 85 dB SPL for OHI) made it unlikely that reduced sensation levels of vowel formants (see Table II) contributed significantly to poorer scores (and a smaller F0 benefit) for ONH and OHI subjects as compared to YNH subjects. Second, a larger F0 benefit would be expected for identification of vowel pairs with smaller formant level differences, whereas a smaller F0 benefit would be expected for identification of vowel pairs with larger formant level differences. Expected group differences were hypothesized to relate to formant audibility and spread-of-masking effects. However, no significant correlations were observed between formant level differences and F0 benefit for YNH, ONH, or OHI subjects (R2 = 17%, 18%, and 11%, respectively, ns). Thus, acoustically based models of vowel formant differences (formant frequency and level) could not reliably predict the large variations in identification of specific pairs of concurrent vowels, the use of F0 difference cues for specific vowel pairs, and differences in identification and F0 benefit among subject groups of different ages and magnitudes of hearing loss.
IV. DISCUSSION
A. Effect of F0 differences on concurrent vowel identification
Identification of both vowels in different-vowel pairs was poorer across F0 differences for older than younger subjects with normal hearing (YNH vs ONH). These results suggest that increased age affects the use of F0 and formant difference cues for vowel pairs that have formant or other spectral differences. Differences in scores between YNH and ONH subjects were smallest for the condition in which only formant difference cues were available [F0 difference = 0 Hz, Fig. 3(B)]. Larger differences in scores with non-zero F0 differences suggest that F0 difference cues are more degraded due to age than formant difference cues (consistent with Vongpaisal and Pichora-Fuller, 2007). Also consistent with this assumption is that no significant differences in scores for YNH and ONH subjects were observed for identical-vowel pairs at any F0 difference [Fig. 3(C)].
Subjects with hearing loss had the lowest mean scores across F0 difference and the smallest F0 benefit. These results suggest that hearing loss degrades the use of F0 and formant difference cues for identification of vowel pairs that have formant (or other spectral) differences. Additionally, larger differences in scores at larger F0 difference conditions [Fig. 3(B)] suggest that OHI subjects had greater difficulty using F0 difference cues than formant difference cues. These new findings were not available in previous reports on concurrent vowel identification, as most studies compared vowel identification for YNH to that of OHI (e.g., Arehart et al., 1997; Summers and Leek, 1998). A lack of significant associations of magnitude of hearing loss and vowel identification is consistent with results of Arehart (1998), who found that introduction of high-frequency amplification did not improve concurrent vowel identification in hearing-impaired listeners. Both results further suggest that poorer performance for hearing-impaired individuals within these studies cannot be attributed to reduced vowel sensation levels.
Identification scores for one of the vowels in the pair were high (≥97 rau) for both identical-vowel and different-vowel vowel pairs [Figs. 3(E) and 3(F)]. In addition, post hoc comparisons revealed that scores did not differ significantly as a function of F0 difference for any of the three subject groups. These results suggest that formant cues are sufficient for correct identification of one vowel in the pair (ignoring the ability to identify the other vowel). When considering identification of both vowels in the pair, it is clear that scores improve as a function of F0 difference for all three groups [Figs. 3(B) and 3(C)]. These results suggest that F0 difference cues are important for identifying both vowels in the pair (consistent with Chintanpalli and Heinz, 2013 for YNH subjects and Snyder and Alain, 2005 for ONH subjects).
The higher percentage of different-vowel pairs with a non-zero F0 difference that were incorrectly identified as identical-vowel pairs (Fig. 4) indicates that ONH and OHI subjects had greater difficulty utilizing F0 difference cues to segregate different-vowel pairs. Moreover, the higher percentage of different-vowel pairs with a 0-Hz F0 difference that were incorrectly identified as identical-vowel pairs (Fig. 4) further suggests that these subjects had difficulty utilizing formant differences for segregation. These results are consistent with the findings obtained from the correct identification of different-vowel pairs across F0 difference in terms of the availability of F0 difference and formant difference cues for each group [see Fig. 3(B)].
The overall identification scores for each vowel in the pair (Figs. 6–8), the benefit of F0 difference (0 Hz vs 26 Hz), and the effect of hearing loss and age (differences between YNH, ONH, OHI groups), vary considerably with vowel identity and the particular pairings of the vowels, perhaps related to their formant (or other spectral) differences. The older subjects show a strong bias against responding to either /u/ (Fig. 6) or /i/ (Fig. 7, only for OHI). Apart from the other vowels (Table I and Fig. 1), these two vowels have a lower frequency F1 and a higher frequency F2 (/i/) or lower level F2 (/u/). These are also the vowels for which F0 discrimination has been shown to be poorer for listeners with normal hearing (Summers and Leek, 1998). Thus, OHI subjects may perceive different-vowel pairs containing /i/ or /u/ as single vowels; similarly, ONH subjects may perceive different-vowel pairs containing /u/ as single vowels.
B. Plausible physiological mechanisms underlying age-related and hearing-loss-related differences in concurrent vowel identification
Given that simple acoustically based models were unsuccessful in explaining concurrent vowel identification at the level of individual vowel pairs, models based more directly on specific physiological mechanisms may be more appropriate. Degradation in F0 and formant difference cues for older subjects could be attributed to a reduction in phase locking of AN fibers to F0s and vowel formants due to an age-related loss of MSR and LSR fibers (e.g., Schmiedt et al., 1996, 2002). These cues are even more degraded with hearing loss and could be attributed to poorer phase locking to F0s and vowel formants resulting from reduced frequency selectivity and sensitivity of each fiber (e.g., Liberman and Dodds, 1984a; Ruggero et al., 1997) and loss of HSR fibers (e.g., Liberman and Dodds, 1984b; Heinz and Young, 2004).
Along with phase locking (or temporal) cues, subjects may be utilizing rate-place cues for vowel identification. For younger subjects with normal hearing, although HSR fibers may be saturated at 65 dB SPL, responses of MSR and LSR fibers (i.e., fibers with higher thresholds and larger dynamic ranges) might provide these cues. However, with increasing age, these cues may be less effective due to additional loss of MSR and LSR fibers. For subjects with hearing loss, utilization of rate-place cues may be limited by a reduction in cochlear nonlinearities (e.g., frequency selectivity, compression, suppression) and a reduction in the sensitivity of each fiber.
There are several physiologically based computational models that attempted to capture the effect of F0 differences on identification of concurrent vowels, as typically observed in YNH (Assmann and Summerfield, 1990; Culling and Darwin, 1993; Meddis and Hewitt, 1992; de Cheveigné, 1997; Chintanpalli and Heinz, 2013). Note that the neural cancellation model of de Cheveigné (1997) is appropriate for cases in which the vowels in the pair are presented at different levels. Among other existing models, only Meddis and Hewitt (1992) and Chintanpalli and Heinz (2013) were successful in capturing the improvement in vowel identification as a function of F0 difference. These modeling studies suggest that, as F0 differences increase, the strength of phase locking of AN fibers to F0s of both vowels improves and results in better vowel segregation. This improvement in segregation could enhance the phase locking representation of AN fibers to vowel formants and thereby result in better identification of both vowels. A similar F0-based modeling framework, with several key modifications, could be adopted to predict changes in mean identification scores as a function of F0 difference for older adults with normal and impaired hearing. These modifications are based on the known anatomic and physiologic changes in the cochlea and AN fibers due to increased age and hearing loss (see Sec. I). Based on current findings, it will be critical to advance beyond predictions of trends that show increases in mean vowel identification with increasing F0 differences across all vowel pairs, and focus on models that explain large differences in identification of specific vowel pairings and interactions of these effects with age and hearing loss.
Vowel identification by older adults with normal or impaired hearing could be affected by age-related declines in phase locking at various stages of the auditory system (Hellstrom and Schmiedt, 1990; Backoff and Caspary, 1994; Raza et al., 1994; Caspary et al., 1995; Clinard et al., 2010; Clinard and Cotter, 2015). It is also possible that age- and hearing-loss-related differences in concurrent vowel identification may be attributed to declines in speech recognition (e.g., Bidelman et al., 2014) and certain cognitive factors, including age-related changes in memory, attention, and speed of processing. For example, segregating and comparing individual F0 and formant components from two simultaneous vowels may require that characteristics of the two vowels are stored in memory longer than is required for perception of individual sounds or concurrent sounds that vary along more dimensions, such as speech and broadband noise. This hypothesis is supported by evidence of longer reaction times by ONH in selecting the second of two vowels in the pair, especially for smaller F0 differences (Snyder and Alain, 2005). Of course, these cognitive effects are not independent of age-related changes in the auditory periphery (cochlea and auditory nerve), which result in reduced detection of low-level signals (such as lower level formants) and delivery of degraded signal representations for processing by aging central auditory pathways and cortex. Peripheral, central, and cognitive declines impose increased demands on an aging brain with already limited resources.
V. SUMMARY AND CONCLUSIONS
The results led to several general conclusions, which are summarized as follows:
-
(1)
Identification of both vowels in different-vowel pairs was significantly poorer for older than younger adults with normal hearing, suggesting that increased age affects the use of F0 and formant or other spectral difference cues. Hearing loss further reduced the benefit of these cues, as identification of both vowels was significantly poorer for older adults with hearing loss than older adults with normal hearing.
-
(2)
The contribution of F0 difference cues to vowel identification also differed with age and hearing loss. For younger adults with normal hearing, identification of both vowels in different-vowel pairs improved with very small increases in F0 difference between the two vowels and then remained constant with larger F0 differences. For older adults with normal hearing, vowel identification continued to improve with larger F0 differences. For older adults with hearing loss, concurrent vowel identification did not improve significantly with increasing F0 differences.
-
(3)
F0 benefit for younger and older adults with normal hearing was equivalent and significantly larger than F0 benefit for older adults with hearing loss. These results provide additional evidence that hearing loss degrades the use of F0 and formant difference cues for identification of vowel pairs that have formant or other spectral differences. Poor correlations of vowel identification and magnitude of hearing loss support the assumption that reduced vowel identification by hearing-impaired individuals was not attributable to lower vowel sensation levels.
-
(4)
Identification scores for one vowel (rather than both vowels) were high for all vowel pairs and showed reduced effects of age and hearing loss (except younger normal-hearing subjects outperforming older hearing-impaired subjects). Identification of one vowel also remained constant with increasing F0 difference, suggesting that F0 cues are primarily important for identifying both vowels.
-
(5)
Two- and one-vowel identification for each of the 25 vowel pairings (rather than averages across vowel pairs) revealed very large ranges of scores, with widely varying differences across vowel identity and particular vowel pairings. Thus, although mean scores across vowels and vowel pairs can reveal general trends related to effects of F0 differences, age, and hearing loss, substantial and non-systematic deviations from mean results were apparent.
-
(6)
Acoustically based models of vowel formant differences in frequency and level could not explain the large variations in identification of specific individual or pairs of vowels, use of F0 difference cues for specific vowel pairs, and differences in identification and F0 benefit for individuals varying in age and hearing loss. Additional research is needed using physiologically appropriate models that capture changes in processing of F0 and formant differences for specific vowels and vowel pairings, as well as effects of increasing age and hearing loss.
ACKNOWLEDGMENTS
This work was supported (in part) by research Grant Nos. R01 DC000184 and P50 DC000422 from NIH/NIDCD and by the South Carolina Clinical and Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCRR Grant No. UL1 RR029882. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant No. C06 RR14516 from the National Center for Research Resources, National Institutes of Health. We would like to thank Tyler W. Eisenhart for his help in data collection.
Footnotes
Previous studies (Summers and Leek, 1998; Arehart et al.,1997, 2005) suggest that hearing impaired listeners have to undergo extensive practice on single vowels to reach a 90% criterion score. Furthermore, HI subjects from Summers and Leek (1998) did not meet the criterion score unless changes were made in their stimulus set. In the current study, the protocol for the screening phase was restricted to a maximum of three blocks to achieve the 90% criterion score and hence this constraint could have contributed to a higher rejection rate for our OHI subjects. Additionally, higher vowel levels (≥90 dB SPL) used in previous studies may have affected identification scores for HI listeners, even for single vowels.
References
- 1.ANSI (2010). ANSI S3.6-2010, American National Standard Specification for Audiometers ( American National Standards Institute, Inc., New York: ). [Google Scholar]
- 2. Arehart, K. H. (1998). “ Effects of high-frequency amplification of double-vowel identification in listeners with hearing loss,” J. Acoust. Soc. Am. 104, 1733–1736. 10.1121/1.423619 [DOI] [PubMed] [Google Scholar]
- 3. Arehart, K. H. , King, C. A. , and McLean-Mudgett, K. S. (1997). “ Role of fundamental frequency differences in the perceptual separation of competing vowel sounds by listeners with normal hearing and listeners with hearing loss,” J. Speech Lang. Hear. Res. 40, 1434–1444. 10.1044/jslhr.4006.1434 [DOI] [PubMed] [Google Scholar]
- 4. Arehart, K. H. , Rossi-Katz, J. , and Swensson-Prutsman, J. (2005). “ Double-vowel perception in listeners with cochlear hearing loss: Differences in fundamental frequency, ear of presentation, and relative amplitude,” J. Speech Lang. Hear. Res. 48, 236–252. 10.1044/1092-4388(2005/017) [DOI] [PubMed] [Google Scholar]
- 5. Arehart, K. H. , Souza, P. E. , Muralimanohar, R. K. , and Miller, C. W. (2011). “ Effects of age on concurrent vowel perception in acoustic and simulated electroacoustic hearing,” J. Speech Lang. Hear. Res. 54, 190–201. 10.1044/1092-4388(2010/09-0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Assmann, P. F. , and Paschall, D. D. (1998). “ Pitches of concurrent vowels,” J. Acoust. Soc. Am. 103, 1150–1160. 10.1121/1.421249 [DOI] [PubMed] [Google Scholar]
- 7. Assmann, P. F. , and Summerfield, Q. (1990). “ Modeling the perception of concurrent vowels: Vowels with different fundamental frequencies,” J. Acoust. Soc. Am. 88, 680–697. 10.1121/1.399772 [DOI] [PubMed] [Google Scholar]
- 8. Assmann, P. F. , and Summerfield, Q. (1994). “ The contribution of waveform interactions to the perception of concurrent vowels,” J. Acoust. Soc. Am. 95, 471–484. 10.1121/1.408342 [DOI] [PubMed] [Google Scholar]
- 9. Backoff, P. M. , and Caspary, D. M. (1994). “ Age-related changes in auditory brainstem responses in Fischer 344 rats: Effects of rate and intensity,” Hear. Res. 73, 163–172. 10.1016/0378-5955(94)90231-3 [DOI] [PubMed] [Google Scholar]
- 10. Bidelman, G. M. , Villafuerte, J. W. , Moreno, S. , and Alain, C. (2014). “ Age-related changes in the subcortical-cortical encoding and categorical perception of speech,” Neurobiol. Aging 35, 2526–2540. 10.1016/j.neurobiolaging.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 11. Bregman, A. S. (1990). Auditory Scene Analysis: The Perceptual Organization of Sound ( MIT Press, Cambridge, MA: ), pp. 1–792. [Google Scholar]
- 12. Brokx, J. P. L. , and Nooteboom, S. G. (1982). “ Intonation and the perceptual separation of simultaneous voices,” J. Phon. 10, 23–36. [Google Scholar]
- 13. Caspary, D. M. , Milbrandt, J. C. , and Helfert, R. H. (1995). “ Central auditory aging: GABA changes in the inferior colliculus,” Exp. Gerentol. 30, 349–360. 10.1016/0531-5565(94)00052-5 [DOI] [PubMed] [Google Scholar]
- 14. Cherry, E. C. (1953). “ Some experiments on the recognition of speech, with one and witih two ears,” J. Acoust. Soc. Am. 25, 975–979. 10.1121/1.1907229 [DOI] [Google Scholar]
- 15. Chintanpalli, A. , Ahlstrom, J. B. , and Dubno, J. R. (2014). “ Computational model predictions of cues for concurrent vowel identification,” J. Assoc. Res. Otolaryngol. 15, 823–837. 10.1007/s10162-014-0475-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chintanpalli, A. , and Heinz, M. G. (2013). “ The use of confusion patterns to evaluate the neural basis for concurrent vowel identification,” J. Acoust. Soc. Am. 134, 2988–3000. 10.1121/1.4820888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clinard, C. G. , and Cotter, C. M. (2015). “ Neural representation of dynamic frequency is degraded in older adults,” Hear. Res. 323, 91–98. 10.1016/j.heares.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 18. Clinard, C. G. , Tremblay, K. L. , and Krishnan, A. R. (2010). “ Aging alters the perception and physiological representation of frequency: Evidence from human frequency-following response recordings,” Hear. Res. 264, 48–55. 10.1016/j.heares.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Culling, J. F. , and Darwin, C. J. (1993). “ Perceptual separation of simultaneous vowels: Within and across-formant grouping by F0,” J. Acoust. Soc. Am. 93, 3454–3467. 10.1121/1.405675 [DOI] [PubMed] [Google Scholar]
- 20. Culling, J. F. , and Darwin, C. J. (1994). “ Perceptual and computational separation of simultaneous vowels: Cues arising from low-frequency beating,” J. Acoust. Soc. Am. 95, 1559–1569. 10.1121/1.408543 [DOI] [PubMed] [Google Scholar]
- 21. de Cheveigné, A. (1997). “ Concurrent vowel identification III: A neural model of harmonic interference cancellation,” J. Acoust. Soc. Am. 101, 2857–2865. 10.1121/1.419480 [DOI] [Google Scholar]
- 22. de Cheveigné, A. (1999). “ Waveform interactions and the segregation of concurrent vowels,” J. Acoust. Soc. Am. 106, 2959–2972. 10.1121/1.428115 [DOI] [PubMed] [Google Scholar]
- 23. de Cheveigné, A. , Kawahara, H. , Tsuzaki, M. , and Aikawa, K. (1997). “ Concurrent vowel identification. I: Effects of relative amplitude and F0 difference,” J. Acoust. Soc. Am. 101, 2839–2847. 10.1121/1.418517 [DOI] [Google Scholar]
- 24. Fogerty, D. , Kewley-Port, D. , and Humes, L. E. (2012). “ Asynchronous vowel-pair identification across the adult lifespan for monaural and dichotic presentations,” J. Speech Lang. Hear. Res. 55, 487–499. 10.1044/1092-4388(2011/11-0102) [DOI] [PubMed] [Google Scholar]
- 25. Hedrick, M. S. , and Madix, S. G. (2009). “ Effect of vowel identity and onset asynchrony on concurrent vowel identification,” J. Speech Lang. Hear. Res. 52, 696–705. 10.1044/1092-4388(2008/07-0094) [DOI] [PubMed] [Google Scholar]
- 26. Heinz, M. G. , and Young, E. D. (2004). “ Response growth with sound level in auditory-nerve fibers after noise-induced hearing loss,” J. Neurophysiol. 91, 784–795. 10.1152/jn.00776.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Helfer, K. S. , and Freyman, R. L. (2008). “ Aging and speech-on-speech masking,” Ear Hear. 29, 87–98. 10.1097/AUD.0b013e31815d638b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hellstrom, L. I. , and Schmiedt, R. A. (1990). “ Compound action potential input/output functions in young and quiet-raised gerbils,” Hear. Res. 50, 163–174. 10.1016/0378-5955(90)90042-N [DOI] [PubMed] [Google Scholar]
- 29. Klatt, D. H. (1980). “ Software for a cascade/parallel formant synthesizer,” J. Acoust. Soc. Am. 67, 971–995. 10.1121/1.383940 [DOI] [Google Scholar]
- 30. Larsen, E. , Cedolin, L. , and Delgutte, B. (2008). “ Pitch representations in the auditory nerve: Two concurrent complex tones,” J. Neurophysiol. 100, 1301–1319. 10.1152/jn.01361.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee, J. H. , and Humes, L. E. (2012). “ Effect of fundamental-frequency and sentence-onset differences on speech-identification performance of young and older adults in a competing-talker background,” J. Acoust. Soc. Am. 132, 1700–1717. 10.1121/1.4740482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lentz, J. J. , and Marsh, S. L. (2006). “ The effect of hearing loss on identification of asynchronous double vowels,” J. Speech Lang. Hear. Res. 49, 1354–1367. 10.1044/1092-4388(2006/097) [DOI] [PubMed] [Google Scholar]
- 33. Liberman, M. C. , and Dodds, L. W. (1984a). “ Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves,” Hear. Res. 16, 55–74. 10.1016/0378-5955(84)90025-X [DOI] [PubMed] [Google Scholar]
- 34. Liberman, M. C. , and Dodds, L. W. (1984b). “ Single-neuron labeling and chronic cochlear pathology. II. Stereocilia damage and alterations of spontaneous discharge rates,” Hear. Res. 16, 43–53. 10.1016/0378-5955(84)90024-8 [DOI] [PubMed] [Google Scholar]
- 35. Makary, C. A. , Shin, J. , Kujawa, S. G. , Liberman, M. C. , and Merchant, S. N. (2011). “ Age-related primary cochlear neuronal degeneration in human temporal bones,” J. Assoc. Res. Otolaryngol. 12, 711–717. 10.1007/s10162-011-0283-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McKeown, J. D. (1992). “ Perception of concurrent vowels: The effect of varying their relative level,” Speech Commun. 11, 1–13. 10.1016/0167-6393(92)90059-G [DOI] [Google Scholar]
- 37. McKeown, J. D. , and Patterson, R. D. (1995). “ The time course of auditory segregation: Concurrent vowels that vary in duration,” J. Acoust. Soc. Am. 98, 1866–1877. 10.1121/1.413373 [DOI] [PubMed] [Google Scholar]
- 38. Meddis, R. , and Hewitt, M. J. (1992). “ Modeling the identification of concurrent vowels with different fundamental frequencies,” J. Acoust. Soc. Am. 91, 233–245. 10.1121/1.402767 [DOI] [PubMed] [Google Scholar]
- 39. Micheyl, C. , and Oxenham, A. J. (2010). “ Pitch, harmonicity and concurrent sound segregation: Psychoacoustical and neurophysiological findings,” Hear. Res. 266, 36–51. 10.1016/j.heares.2009.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller, R. L. , Schilling, J. R. , Franck, K. R. , and Young, E. D. (1997). “ Effects of acoustic trauma on the representation of the vowel / ɛ / in cat auditory nerve fibers,” J. Acoust. Soc. Am. 101, 3602–3616. 10.1121/1.418321 [DOI] [Google Scholar]
- 41. Raza, A. , Milbrandt, J. C. , Arneric, S. P. , and Caspary, D. M. (1994). “ Age-related changes in brainstem auditory neurotransmitters: Measure of GABA and acetylcholine function,” Hear. Res. 77, 221–230. 10.1016/0378-5955(94)90270-4 [DOI] [PubMed] [Google Scholar]
- 42. Ruggero, M. A. , Rich, N. C. , Recio, A. , Narayan, S. S. , and Robles, L. (1997). “ Basilar-membrane responses to tones at the base of the chinchilla cochlea,” J. Acoust. Soc. Am. 101, 2151–2163. 10.1121/1.418265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmiedt, R. A. , Lang, H. , Okamura, H. O. , and Schulte, B. A. (2002). “ Effects of furosemide chronically applied to the round window: A model of metabolic presbyacusis,” J. Neurosci. 22, 9643–9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schmiedt, R. A. , Mills, J. H. , and Boettcher, F. A. (1996). “ Age-related loss of activity of auditory-nerve fibers,” J. Neurophysiol. 76, 2799–2803. [DOI] [PubMed] [Google Scholar]
- 46. Snyder, J. S. , and Alain, C. (2005). “ Age-related changes in neural activity associated with concurrent vowel identification,” Cognit. Brain Res. 24, 492–499. 10.1016/j.cogbrainres.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 47. Studebaker, G. A. (1985). “ A ‘rationalized’ arcsine transform,” J. Speech Hear. Res. 28, 455–462. 10.1044/jshr.2803.455 [DOI] [PubMed] [Google Scholar]
- 48. Summers, V. , and Leek, M. R. (1998). “ F0 processing and the separation of competing speech signals by listeners with normal hearing and with hearing loss,” J. Speech Lang. Hear. Res. 41, 1294–1306. 10.1044/jslhr.4106.1294 [DOI] [PubMed] [Google Scholar]
- 49. Vongpaisal, T. , and Pichora-Fuller, M. K. (2007). “ Effect of age on F0 difference limen and concurrent vowel identification,” J. Speech Lang. Hear. Res. 50, 1139–1156. 10.1044/1092-4388(2007/079) [DOI] [PubMed] [Google Scholar]
- 50. Zwicker, U. T. (1984). “ Auditory recognition of diotic and dichotic vowel pairs,” Speech Commun. 3, 265–277. 10.1016/0167-6393(84)90023-2 [DOI] [Google Scholar]