Abstract
Binaural pitch fusion is the fusion of dichotically presented tones that evoke different pitches between the ears. In normal-hearing (NH) listeners, the frequency range over which binaural pitch fusion occurs is usually <0.2 octaves. Recently, broad fusion ranges of 1–4 octaves were demonstrated in bimodal cochlear implant users. In the current study, it was hypothesized that hearing aid (HA) users would also exhibit broad fusion. Fusion ranges were measured in both NH and hearing-impaired (HI) listeners with hearing losses ranging from mild-moderate to severe-profound, and relationships of fusion range with demographic factors and with diplacusis were examined. Fusion ranges of NH and HI listeners averaged 0.17 ± 0.13 octaves and 1.7 ± 1.5 octaves, respectively. In HI listeners, fusion ranges were positively correlated with a principal component measure of the covarying factors of young age, early age of hearing loss onset, and long durations of hearing loss and HA use, but not with hearing threshold, amplification level, or diplacusis. In NH listeners, no correlations were observed with age, hearing threshold, or diplacusis. The association of broad fusion with early onset, long duration of hearing loss suggests a possible role of long-term experience with hearing loss and amplification in the development of broad fusion.
I. INTRODUCTION
In normal-hearing (NH) listeners, the two ears provide essentially matched spectral information for a given signal, allowing integration of “multiple looks” to reduce uncertainty about the signal spectrum and average out independent noise to the two ears; this is similar to the uncertainty reduction provided by two eyes or multisensory inputs (Ernst and Banks, 2002; Hillis et al., 2002). Diplacusis, a phenomenon in which a single tone frequency evokes different pitches for each ear, is often very small in NH listeners (e.g., Burns, 1982; Ogura et al., 2003).
In contrast, hearing-impaired (HI) listeners often have significant diplacusis, (e.g., Albers and Wilson, 1968; Burns and Turner, 1986). Diplacusis may be due to a shift in neural tuning that occurs with hearing impairment (Robertson and Johnstone, 1979; Liberman, 1984) or to the presence of dead regions that create gaps in tonotopic organization (Florentine and Houtsma, 1983; Turner et al., 1983; Huss and Moore, 2005). Diplacusis is, however, measured using sequentially presented tones, and some data suggest that diplacusis is not typically perceived during simultaneous presentation due to the width of binaural pitch fusion (Thurlow and Bernstein, 1957; Odenthal, 1963; van den Brink et al., 1976). Binaural pitch fusion is the fusion of dichotically presented tones that evoke different pitches between the ears, and the binaural fusion range is the frequency range of tones in one ear over which binaural pitch fusion occurs with a tone in the other ear. If the pitch difference due to diplacusis is within the binaural fusion range, the diplacusis will not be perceived when stimuli to each ear are perceived simultaneously. van den Brink et al. (1976) demonstrated that the offsets of the centers of the binaural fusion ranges were correlated with diplacusis in NH listeners, and suggested that fusion ranges adjust to prevent the perception of any interaural pitch differences due to diplacusis.
In an analogous population, previous studies have shown that bimodal cochlear implant (CI) users who wear a CI in one ear and a hearing aid (HA) in the other exhibit fusion of pitches that differ by as much as 3–4 octaves between ears, and that the width of these broad fusion ranges are correlated with large interaural spectral mismatches introduced by the CI programming (the CI equivalent of diplacusis; Reiss et al., 2014b). This broad pitch fusion is not easily explained by a weak pitch percept, as most of the subjects exhibited sharp pitch match functions for each electrode to the acoustic ear during sequential, non-simultaneous presentation, and generally reported a tonal rather than noise-like percept; broad, shallow pitch match functions are associated with a noise-like percept (Reiss et al., 2014a).
In addition, in this population, broad fusion led to the averaging of different pitches between the ears for fused stimulus pairs (Reiss et al., 2014b); this phenomenon has also been observed in NH listeners on a smaller scale (Thurlow and Bernstein, 1957; van den Brink et al., 1976). Such broad fusion and averaging may lead to interference for speech perception. For example, if there is a large amount of diplacusis between the ears, such as that often introduced by CI programming (Reiss et al., 2014a), spectral information will differ greatly between the ears. If broad fusion and averaging are also present, spectral information will be averaged across the ears, and smeared or distorted. A recent study showed that both HA users and bimodal CI listeners with broad fusion can experience such interference in vowel perception with binaural stimulation, i.e., individuals with broad fusion exhibited shallower vowel discrimination slopes with two ears compared to the better ear alone, and this phenomenon was associated with the presence of both large diplacusis and pitch averaging in the formant frequency regions of those vowels (Reiss et al., 2016). Thus, while pitch differences between ears may not be perceived due to broad fusion, the associated pitch averaging may lead to detrimental, broad averaging of spectral information between the ears in HI listeners.
Based on the 2014 study, it was hypothesized that HI listeners who use HAs would also exhibit broad binaural fusion ranges similar to bimodal CI listeners, and that the width of the fusion range would be correlated with the degree of diplacusis. In the current study, binaural pitch fusion ranges were measured in NH and HI listeners, and correlated with interaural pitch differences across subjects. Fusion ranges in HI listeners were also correlated with various subject factors, as well as factors specific to the reference frequencies (in addition to diplacusis) to determine associations of these factors with broad fusion. Subject factors included age, average hearing thresholds, average HA amplification levels, duration of hearing loss, duration of HA use, daily hours of HA use, and musical experience. Frequency-specific factors included reference frequency hearing threshold and HA amplification level in addition to diplacusis. Fusion ranges in NH listeners were correlated with a subset of these factors as applicable.
II. METHODS
A. Subjects
These studies were conducted according to the guidelines for the protection of human subjects as set forth by the Institutional Review Board (IRB) of Oregon Health and Sciences University (OHSU), and the methods employed were approved by that IRB. Ten adult subjects with normal hearing (7 females, 3 males) and 16 adult subjects with mild-moderate to severe-profound hearing loss (8 females, 8 males) participated in this study. All subjects were screened for normal cognitive function using the Mini Mental Status Examination with a minimum score of 25 out of 30 required to qualify (MMSE; Folstein et al., 1975; Souza et al., 2007). Tympanometry was also conducted for all subjects to verify normal middle ear function.
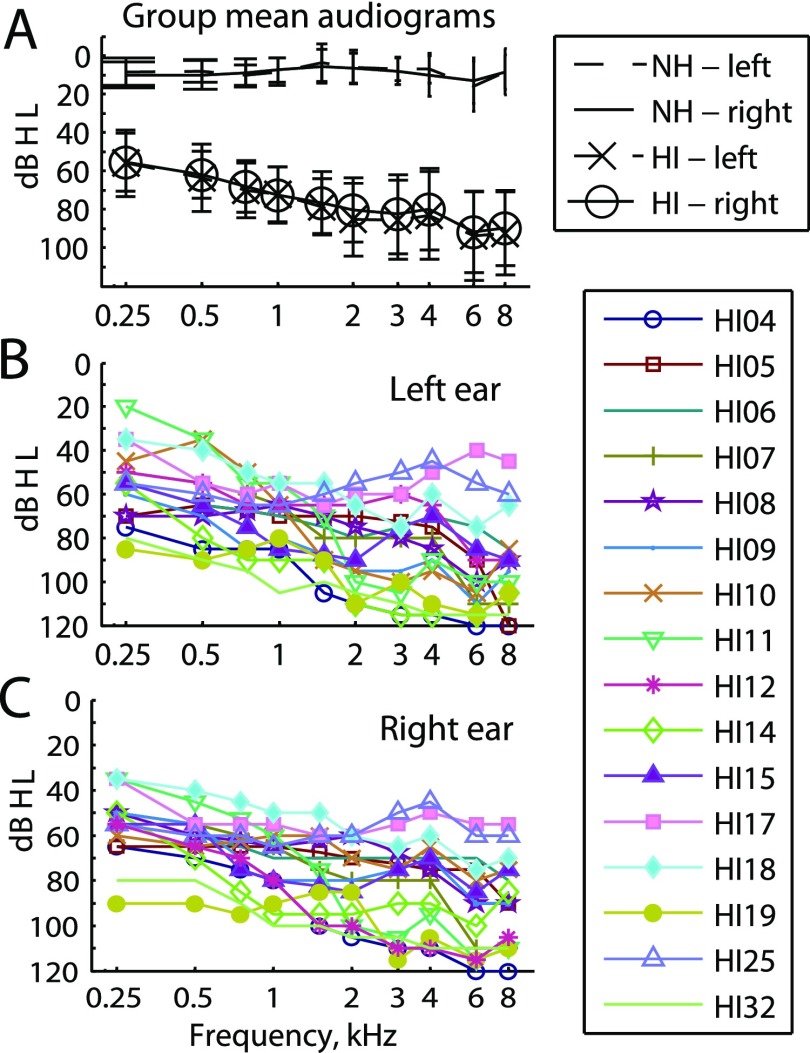
The ten NH subjects ranged in age from 22 to 72 years of age [mean and standard deviation (std) = 35.1 ± 16.2 yr]. The subject ages were 72, 25, 28, 24, 28, 22, 22, 42, 36, and 52 years old for subjects NH58, NH65, NH68, NH69, NH72, NH74, NH75, NH78, NH83, and NH84, respectively. All NH subjects were screened for audiometric thresholds within normal limits [thresholds ≤25 dB hearing level (dB HL) from 250 to 4000 Hz; all testing was conducted with tones below 4000 Hz]. Group average audiograms are shown in Fig. 1(A).
FIG. 1.
(Color online) Audiograms for the subjects in this study. (A) Group averaged audiograms for both NH and HI subjects. Error bars show standard deviations. (B) Individual audiograms for the left ears of HI subjects. (C) Individual audiograms for the right ears of HI subjects.
The HI subjects' demographic data, including age, gender, duration of moderate-severe or worse hearing loss, duration of HA use, daily hours of HA use, and HA model(s) are shown in Table I. The subject ages ranged from 31 to 80 yr (mean and std = 59.7 ± 17.3), duration of hearing loss ranged from 2 to 46 yr (mean and std = 22.6 ± 13.7), and duration of HA use ranged from 0.25 to 46 yr (mean and std = 21.9 ± 13.7). Audiograms are shown for left and right ears for HI listeners in Figs. 1(B) and 1(C), and group averages are shown for comparison with NH thresholds in Fig. 1(A). Fourteen of the 16 HI subjects were bilateral HA users, although of these, 2 wore the HAs for less than 3 h a day and 1 had only 4 mo of experience with the HAs. In order to measure the amount of amplification provided by the HAs at each frequency, real ear aided measurements were obtained in the ear using an Audioscan Axiom (Ontario, Canada).
TABLE I.
Demographic information for HI listeners: age, gender, duration of hearing loss, duration and daily hours of HA use, HA model, and IPD threshold. HL, hearing loss; n/a, not applicable; and “;” denotes different numbers for left and right ears if the ears are different.
| Subject ID | Age (years) | Gender | Duration of HL (years) | Duration of HA use (years) | Daily HA use (h/day) | HA model | IPD threshold (Hz) |
|---|---|---|---|---|---|---|---|
| (ear if only one worn) | |||||||
| HI04 | 68 | M | 35 | 33; 35 | 12 | ReSound RITE | 188 |
| HI05 | 61 | M | 25 | 25 | 17 | Oticon Alta Pro Power BTE | 957 |
| HI06 | 70 | M | 25 | 23 | 16 | Phonak Naida sp | 918 |
| HI07 | 80 | F | 10 | 10 | 16 | Phonak Certena M | 188 |
| HI08 | 75 | F | 21 | 15; 12 | 2 | Siemens Pure RITE | 188 |
| HI09 | 33 | M | 32 | 32 | 17 | Oticon Epoq PxW BTE | 801 |
| HI10 | 67 | M | 3 | 0.25 | 16 | Costco General Hearing | 188 |
| HI11 | 66 | F | 35 | 35; 28 | 17 | Resond AL562 | 753 |
| HI12 | 78 | M | 15 | 20; 15 | 20 | Phonak Naida Q50 SP | 188 |
| HI14 | 46 | M | 46 | 46 | 9 | Phonak Naida III UP | 188 |
| HI15 | 32 | F | 32 | 29 | 14 | Phonak Naida S | 897 |
| HI17 | 63 | F | 2 | 0; 4 | n/a; 0 | Oticon Nera (R) | 380 |
| HI18 | 79 | M | 10 | 4 | 14; 0 | Widex Flash FL-m | 576 |
| HI19 | 31 | F | 5 | 27 | 15 | Phonak Solana 10 Petite | 188 |
| HI25 | 65 | F | 27 | 26 | 15 | Phonak Versata P | 188 |
| HI32 | 41 | F | 39 | 39 | 15 | Siemens Nitro | 188 |
In addition, subjects were asked about musical experience, specifically how many years of experience with playing a musical instrument or with singing, and what instruments. All NH subjects except NH69 had at least one year of musical experience. Subjects HI04, HI06, HI11, HI15, HI17, HI18, HI19, HI25, and HI32 all had at least one year of experience with a musical instrument, with HI11 having the most experience at 58 yr with multiple instruments. The remaining HI subjects had no musical experience.
B. Stimuli and procedures
All experiments were conducted in a double-walled, sound attenuated booth. Signals were generated at a sampling rate of 44.1 kHz with MATLAB (version R2010b, Mathworks, Natick, MA), processed through an ESI Juli sound card (Leonberg, Germany), Tucker-Davis Technologies PA5 digital attenuator and HB7 headphone buffer (Alachua, FL), and presented over Sennheiser HD-25 headphones (Wedemark, Germany). Each headphone's frequency response was equalized using calibration measurements obtained with a Brüel and Kjær sound level meter (Nærum, Denmark) with a 1 in. microphone in an artificial ear.
All stimuli consisted of sinusoidal pure tones with 10-ms raised-cosine onset/offset ramps. Prior to all experiments, loudness balancing was conducted using a method of adjustment. First, 300-ms tones at 0.125, 0.175, 0.25, 0.375, 0.5, 0.625, 0.75, 0.875, 1, 2, 3, and 4 kHz were initialized to “medium loud and comfortable” levels corresponding to a “6” (“most comfortable)” on a visual loudness scale ranging from “0” (“no sound”) to “10” (“too loud”). Loudness was then adjusted for each frequency to be equally loud to a tone in the left ear at 500 Hz during sequential presentation within or across the ears, based on subject feedback. The frequencies and order of presentation were randomized to minimize the effect of biases such as time-order error and overestimation of the loudness for high-frequency tones (Florentine et al., 2011). Adjustments were repeated until equal loudness was achieved with all comparison sequences within and across ears. In HI subjects, tone frequencies that could not be presented loud enough to be considered a medium loud and comfortable level, due to the limited range of residual hearing, were excluded. Interpolation (on a dB scale) was then used to determine appropriate levels for all tone frequencies used in testing. This loudness balancing procedure was performed to minimize use of level-difference cues and maximize focus on pitch differences as the decision criteria.
Moreover, upper limit frequencies for detection of interaural phase differences (IPDs) were measured by using a rapid, adaptive IPD test (Grose and Mamo, 2010). This test was conducted in order to determine the highest frequencies at which binaural fine structure cues could be perceived, in the form of binaural beats, and thus potentially interfere with the subject's ability to judge fusion. A three-interval, three-alternative forced choice procedure was used, in which three binaural tone stimuli were presented at the test frequency, with 800 ms duration. For two of the intervals, stimuli were in phase between ears over the whole duration. For the third interval, the phase was inverted to be out of phase between ears every 200 ms, such that listeners sensitive to phase cues at that frequency would effectively hear a binaural beat. The interval of the phase-inverted stimulus was randomized among three intervals. A 5 Hz amplitude envelope modulation was used to mask IPD transitions. Subjects were asked to pick the interval that was different. The starting test frequency was selected from the middle of the audible frequency range, and was increased or decreased according to a three-up, one-down procedure that converged on the 79% correct point on the psychometric function. The threshold track consisted of a total of ten reversals, initially in one-octave steps for two reversals and then in 1/4-octave steps. The IPD threshold was computed as the average of the last eight reversals, and the subject's IPD was determined as the average IPD threshold over two repeats of the test. Average IPD thresholds for each HI subject are shown in the last column of Table I; the lower limit of 188 Hz corresponds to the lowest frequency at which IPD sensitivity was tested. IPD thresholds for all NH subjects except for NH58 (188 Hz) and NH78 (326 Hz) exceeded 1 kHz. Testing was conducted only with reference frequencies above this upper limit of detectability for IPDs in both NH and HI listeners.
After completion of loudness balancing and IPD tests, stimuli were presented binaurally in two procedures: interaural pitch matching and dichotic fusion range measurements. Reference tone frequencies for one ear, designated the reference ear, were selected from relatively low frequencies of 0.25, 0.5, 1, 1.2, 1.4, and 1.6 kHz to allow comparison between NH and HI subjects with limited high frequency hearing. The lowest reference frequency that could be used for each subject was determined by the IPD threshold. The highest reference frequency that could be used in each HI subject was determined by the upper frequency limit of the loudness balanced frequency range. Comparison tone frequencies presented to the contralateral ear were varied in steps ranging from 1/64 to 1/4 octaves within the loudness balanced frequency range, with step size determined by preliminary pitch matching or fusion range measurements. The reference ear was chosen randomly for NH subjects and for HI subjects with bilaterally symmetric upper frequency limits; for HI subjects with asymmetric upper frequency limits, the reference ear was chosen to be the ear with the smaller loudness balanced frequency range to maximize the range of comparison tone frequencies that could be tested.
1. Interaural pitch matching procedure
A two-interval, two-alternative constant-stimulus procedure was used to obtain pitch matches. One interval contained a reference tone delivered to the reference ear. The other interval contained a comparison tone delivered to the contralateral, comparison ear. All tones were 500 ms in duration separated by a 500-ms interstimulus interval, with interval order randomized. The reference tone was held constant in frequency while the comparison tone was varied in frequency. In each trial, the subject was asked to indicate which interval had the higher perceived pitch.
Stimulus frequency was varied in pseudorandom sequence across trials to reduce context effects on pitch judgments (Reiss et al., 2007; Reiss et al., 2012). Pitch matches were then computed as the 50% point on the psychometric function generated from the average of the responses at each tone frequency. The range of pitch-matched frequencies were selected as the 25% and 75% points in the function; while these points are arbitrary, such measurements still give an indication of the pitch-matched range that can be compared with the fusion range results.
In addition, for a pitch match to be considered valid, the subject was required to “bracket” the pitch for that reference tone. In other words, for HI listeners, if the pitch of the reference tone was too high-pitched for the subject to rank any audible acoustic tone frequencies in the comparison ear as higher in pitch 100% of the time (due to the limited high-frequency residual hearing), that pitch match was conservatively recorded as “out of range.” At least two repetitions were collected for each pitch match measurement.
2. Dichotic fusion range measurements
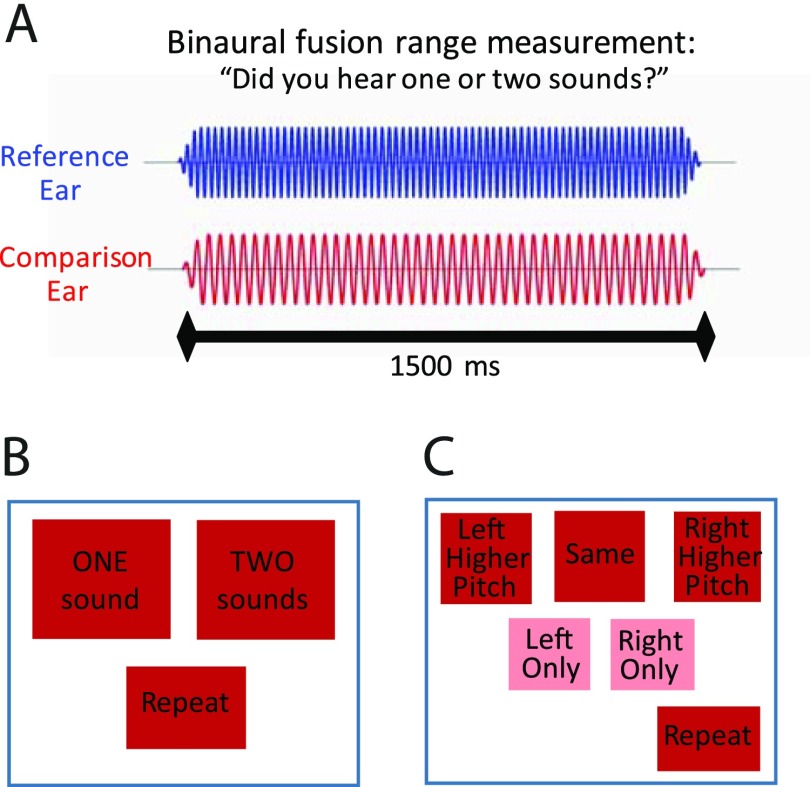
Generally, in order to measure fusion, in each trial a reference tone was presented to the reference ear simultaneously with a dichotic comparison tone in the contralateral ear, with both stimuli of 1500-ms duration, as shown in Fig. 2(A). The subject was asked to indicate whether a single fused tone or two different tones were heard. The reference tone was held constant in frequency across trials, while the comparison tone frequency varied in frequency in a pseudorandom sequence to find the fusion range, or range of contralateral tone frequencies that fused with the reference tone.
FIG. 2.
(Color online) Schematics illustrating the binaural fusion range measurement procedure. (A) Stimuli consisted of dichotic pure tones presented simultaneously to the reference and contralateral ears. (B) Screenshot of the touchscreen choices for the two-alternative forced choice (2AFC) version of the task. (C) Screenshot of the touchscreen choices for the 5AFC version of the task.
More specifically, two different tasks were used to measure an individual's fusion range for each reference frequency. One task was a two-alternative forced choice (2AFC) task, with the touchscreen choices shown in Fig. 2(B). In this task, subjects were only required to indicate whether they heard a single sound (“one sound”; fused) or different sounds in each ear (“two sounds”; not fused). At least one repetition was obtained for this task.
The other task was a five-alternative forced choice (5AFC) task, with the touchscreen choices shown in Fig. 2(C). This task also required subjects to indicate whether they heard a single sound or different sounds in each ear (Reiss et al., 2014b). However, if they heard different sounds, they were also asked to determine which ear had the higher pitch (“left higher” or “right higher”). In addition, if they heard a single sound, they had the option of indicating whether they heard that single sound in both ears (“same”), the left ear (“left only”), or the right ear (“right only”); these responses were all considered fused based on previous observations that lateralized sounds generally have properties consistent with fusion (Reiss et al., 2014b). At least two repetitions were obtained for this task.
For both of these tasks, contralateral tone frequencies were varied from trial to trial in a pseudorandom (Latin-square like) sequence. For NH listeners, finer sampling of the frequency range of around the reference frequency was used, typically 1/64 or 1/32 octave steps. For HI listeners, the frequency range was typically sampled in 1/8 or 1/4 octave steps. A “repeat” button was also provided for both tasks to allow subjects to listen to the stimuli again if needed. All subjects were provided with a practice training run with feedback to help instruct and confirm appropriate usage of the buttons.
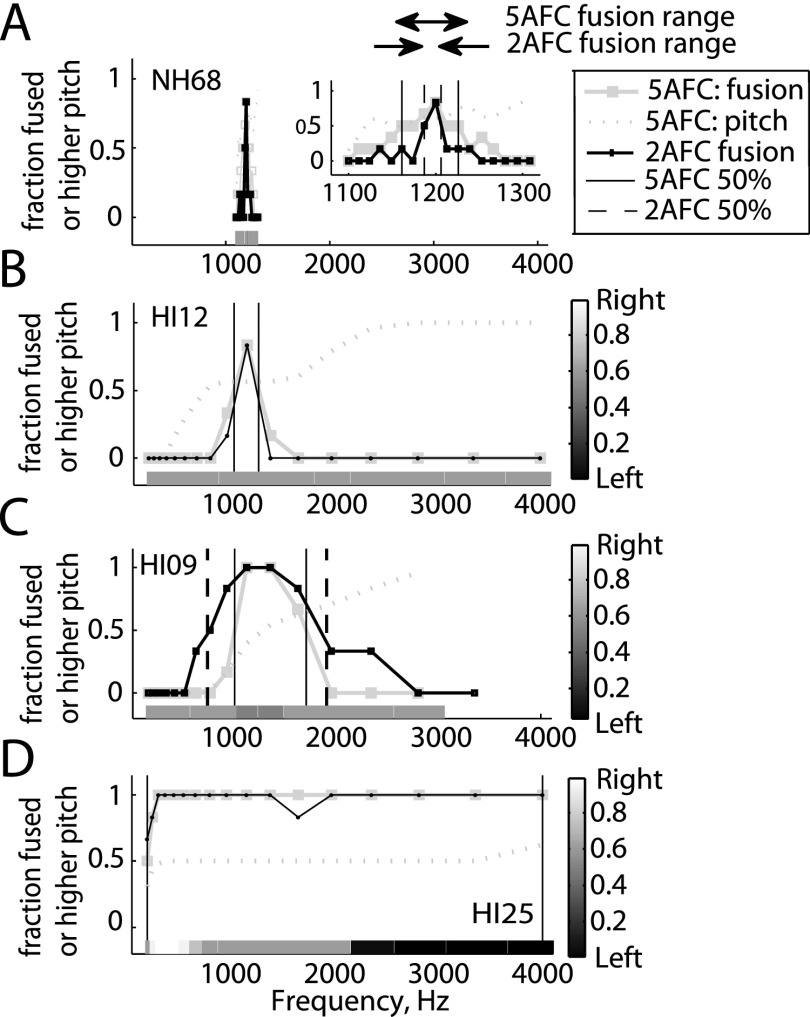
For analysis, values were assigned to generate a fusion function as follows: Responses corresponding to fused sounds were assigned a value of 1 (“one sound” in the 2AFC task or “same,” “left only,” and “right only” in the 5AFC task). Responses corresponding to non-fused sounds were assigned a value of 0 (“two sounds” in the 2AFC task, or “left higher,” or “right higher” in the 5AFC task). Values were averaged over all trials to generate a fusion function; examples are shown in Fig. 3. Figure 3(A) shows a NH subject's fusion functions for the 2AFC (small black squares) and 5AFC (large gray squares) tasks. Figures 3(B)–3(D) show the 2AFC and 5AFC fusion functions for three different HI subjects. The fusion range was defined as the range where averaged fusion values were above 0.5 (range between vertical lines in Fig. 3).
FIG. 3.
Example fusion range functions for a representative NH listener (A) and three HI listeners (B)–(D), for a reference tone of 1200 Hz. Fusion functions obtained using two different tasks, a 2AFC task and a 5AFC task are shown. Grayscale bars below the functions indicate reported lateralization in the 5AFC task. Gray dotted lines indicate how often the pair tone was heard as higher in pitch in the 5AFC task when two sounds were heard. For the NH listener, the inset shows the fusion functions at a smaller frequency scale. The fusion range is the frequency range between the vertical lines indicating the 50% limits for each task, indicated by the horizontal arrows in (A).
For both pitch match and fusion range measurements, reference frequency was randomized across runs. Each run typically took between 8 and 15 min to complete, depending on the subject and the frequency range of residual hearing. Total testing, including informed consent, questionnaires, audiograms, loudness balancing, IPD testing, pitch match, and fusion range measurements, was completed in 4–6 h for NH subjects and 5–7 h for HI subjects. Testing was usually divided into 2–3 shorter visits of 2–4 h each, and ample breaks were provided to minimize test fatigue.
C. Statistical analyses
The association between fusion range and subject- or frequency-specific characteristics was assessed by correlation analysis, either crude or partial correlation, and was done separately for the HI and NH cohorts. Frequency-specific factors were limited to measurements taken at the most common frequency for each cohort. Key demographic characteristics for HI subjects (e.g., age, age of hearing loss onset, duration of hearing loss, duration of HA use, and daily hours of HA use) were anticipated to be highly correlated. Principal component analysis (PCA) was therefore used to reduce this set of five correlated variables into a smaller number of uncorrelated components. The components were used to estimate partial correlation coefficients for fusion and subject- or frequency-specific characteristics while controlling for combined effects of demographic factors. Diagnostic checks (half-normal plots and q-q plots, both with simulated envelopes) were used to check for outliers or other violations that might affect inference. If problems were discovered, then Spearman's non-parametric correlation coefficient was used to quantify the association.
III. RESULTS
A. Binaural pitch fusion in HI and NH listeners
Example fusion functions are shown in Fig. 3. Each panel shows fusion functions for a single subject for both the 5AFC (large gray circles) and 2AFC (small black squares) tasks. Each fusion function shows the fraction of trials that a reference tone at 1200 Hz was fused with a contralateral pair tone, as a function of pair tone frequency. Generally, the fusion functions showed a single peak over which fusion occurred, centered on the reference frequency. Solid and dotted vertical lines indicate the lower and upper 50% boundaries for each fusion function in the 5AFC task and the 2AFC task, respectively. The fusion range is then defined as the frequency range between these lines [horizontal arrows in Fig. 3(A)], and represents the frequency range over which fusion occurred more than 50% of the time.
The examples illustrate that HI listeners can have broader fusion functions [Figs. 3(B)–3(D)] and thus broader fusion ranges than NH listeners [Fig. 3(A)]. The fusion ranges for the NH listener were between 1.19 and 1.20 kHz (0.01 octaves) and 1.16 and 1.23 kHz (0.08 octaves) for the 2AFC and 5AFC tasks, respectively. The three HI listeners all had broader fusion ranges, although there was a great deal of variability across subjects, with HI12 representing subjects with sharper fusion on the order of 0.29 octaves, HI09 representing intermediate fusion on the order of 1.2–1.3 octaves, and HI25 representing subjects with broad fusion over the entire frequency range on the order of 4.0 octaves. Note that the fusion range measurements could differ for the two different tasks, though not in a consistent direction. These differences may indicate small shifts in decision boundaries with task, especially for NH listeners who experienced a gradual rather than sharp transition zone from fusion to no fusion.
Also shown in Fig. 3 are the additional lateralization functions from the 5AFC task. Occasionally, when sounds were fused, subjects were only able to hear the sound in one ear (even if the sounds were loudness balanced when presented sequentially). In those cases, subjects chose the ear that the sound was perceived in, providing additional data on lateralization, expressed as the fraction of trials in which a fused sound was lateralized to the right (shaded bars at bottom of each panel; see grayscale bar at right for shading map, with black indicating lateralization to the left and white indicating lateralization to the right). Significant lateralization can be seen in Fig. 3(D) for subject HI25, who had broad fusion, such that fused sounds were lateralized to the right (the reference ear) for low pair tone frequencies, centered for intermediate pair tone frequencies, and lateralized to the left (the pair ear) for high pair tone frequencies. In other words, this subject lateralized fused dichotic tone pairs to the higher frequency ear. Such lateralization provides additional support for a fused percept. Note also the lateralization to the right ear for high pair frequencies for both subjects HI07 and HI10, for which the left ear was the reference, meaning that sounds were again lateralized to the higher frequency ear (see Figs. S1 and S3 available in the supplemental material1).
Across-ear pitch comparisons were also recorded when two sounds were heard, and shown in Fig. 3 as generally monotonically increasing functions (gray dotted lines) that indicate the percent of time that the comparison tone was higher in pitch than the reference tone. It can be seen that NH and HI subjects with narrower fusion had regions of ambiguity around the fusion range, and were not always able to distinguish which ear had the higher pitch in this region, even though two distinct sounds were heard and not fused [Fig. 3(A), inset; Fig. 3(B)]. In contrast, subjects with intermediate fusion were easily able to determine the ear with higher pitch right outside of the fusion range [Fig. 3(C)]. Subjects with broad fusion always heard one sound, so did not have the opportunity to choose which sound was higher in pitch in this test [Fig. 3(D)].
It should be noted that in a few of the HI subjects, multiple fusion peaks were seen. Subject HI07 had fusion functions with two peaks for all reference frequencies, with one sharp peak around the reference frequency and a second, broad peak at higher frequencies above 1400 Hz (see Fig. S1 available in the supplemental material1). The sharp peak centered at the reference was the one recorded; as the reference frequency increased, the two peaks gradually merged into a single broad peak of fusion, although the first peak could sometimes be resolved at higher resolution (bottom row of Fig. S1 available in the supplemental material1). A similar double peak was also seen for subject HI19 (see bottom row of Fig. S2 available in the supplemental material1), HI15 (for 1000 Hz and 1200 Hz, which accounts for discrepancies in fusion ranges measured for 5AFC and 2AFC tasks at those frequencies, when valley between peaks hovers around 0.5), and HI18 (for 1600 Hz reference frequency; not shown).
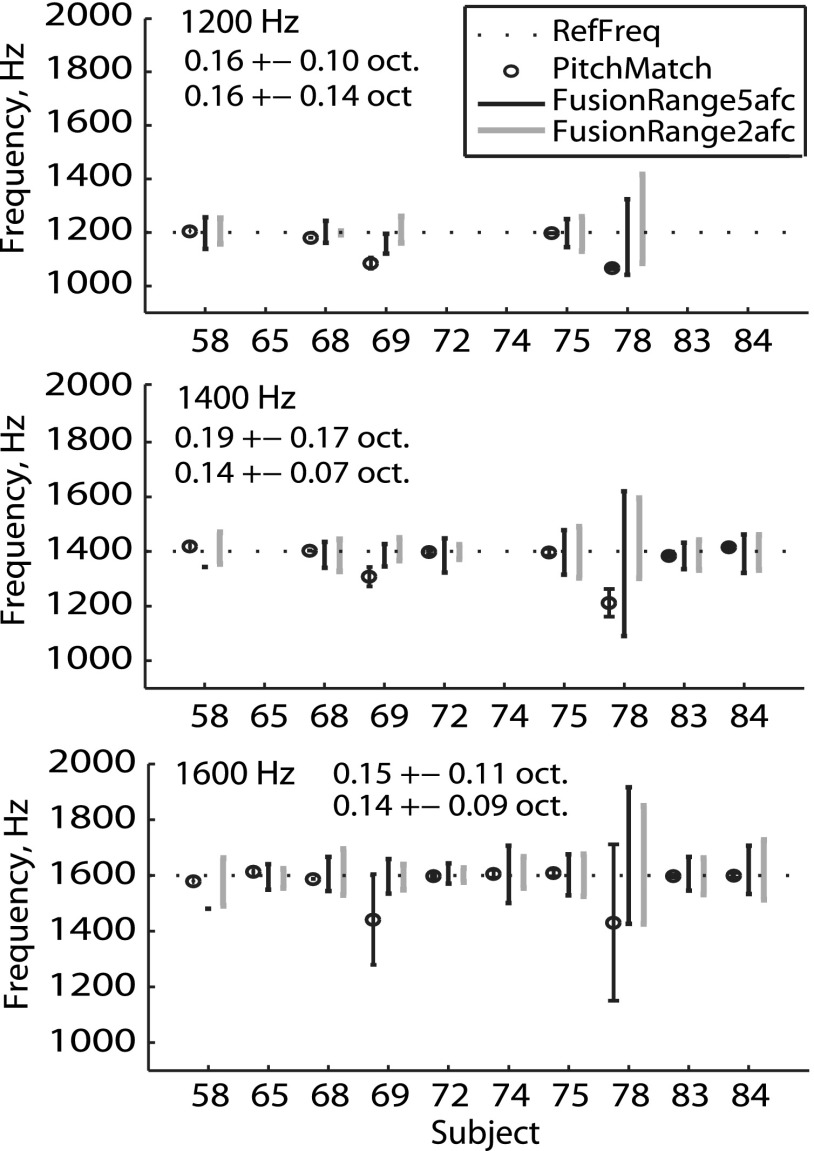
Figure 4 shows summary fusion ranges (vertical black and gray lines) and interaural pitch matches (open circles) for all NH listeners. Each panel shows results for a different reference frequency. Minimal differences in fusion ranges were evident between the 2AFC (gray lines) and 5AFC (black lines) methods, or across reference frequency. Interaural pitch matches also showed minimal diplacusis, which would appear as deviations of the pitch match (open circles) from the corresponding reference frequency (horizontal dotted line). The subject with the broadest fusion was a middle-aged subject, NH78 (42 years of age), but generally there were no consistent associations of fusion ranges with age; the two oldest subjects, NH58 and NH84 (72 and 52 years of age), had comparable fusion ranges to the two youngest subjects, NH74 and NH75 (both 22 years of age).
FIG. 4.
Individual interaural pitch matches and binaural fusion ranges for all NH listeners. Each panel shows summary results for a different reference frequency (indicated at top left of panel). The group means and standard deviations of fusion ranges for the 5AFC and 2AFC tasks, respectively, are shown immediately below or adjacent to the reference frequency. Results for the pitch matches, 2AFC, and 5AFC fusion tasks are shown slightly offset from each other, in that order, on the x axis for clarity.
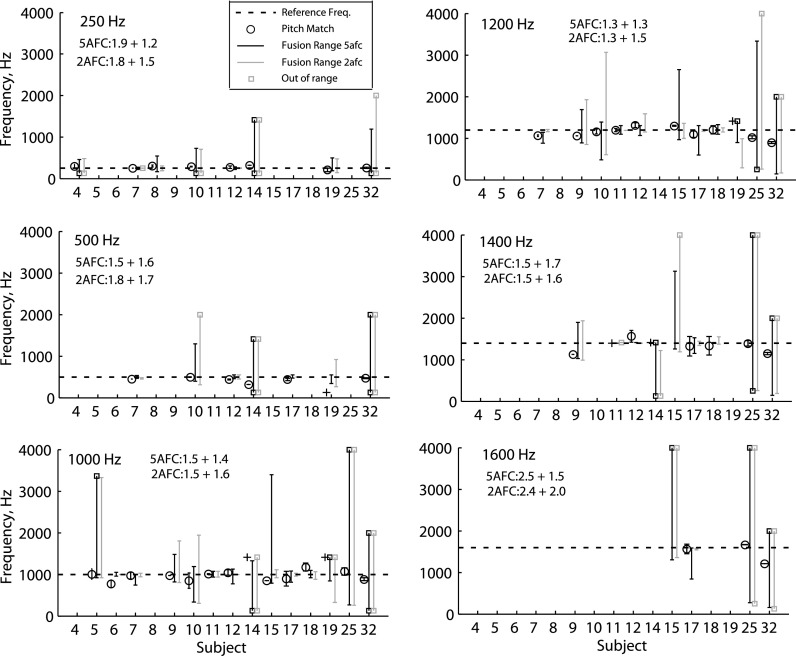
Figure 5 shows the summary fusion ranges and interaural pitch matches for all HI listeners. Again, each panel shows results for a different reference frequency, and most HI listeners were tested on a subset of frequencies due to higher IPD detection frequency limits or lower residual hearing frequency limits. More variation is seen in the fusion ranges and pitch matches across frequency and across the HI subjects than the NH subjects. Larger interaural pitch differences are seen, such as for subject HI09 at 1400 Hz. Fusion ranges can be small for some frequencies and large for other frequencies within a subject such as for subject HI10, which had narrower fusion at 250 Hz and broader fusion at higher frequencies. Subjects HI05, HI09, HI10, HI14, HI15, HI25, and HI32 had broader fusion than seen in NH listeners. Subjects HI14, HI15, HI25, and HI32 had particularly broad fusion on the scale of 3–4 octaves. Also note that the fusion ranges are much wider than the 25%–75% range of the interaural pitch matches, indicating that frequency discrimination of two dichotic tones presented simultaneously is much worse than discrimination of tones presented sequentially.
FIG. 5.
Individual interaural pitch matches and binaural fusion ranges for all HI listeners. Plotted as in Fig. 4. Plus (+) symbols indicate when the pitch matches were “out of range,” and low or high values indicate that the pitch match was below the lowest frequency tested or above that subject's highest comfortably audible frequency, respectively. Open squares at the top or bottom of fusion range lines indicate when the upper or lower limit of fusion could not be measured, respectively, due to limitations in the residual hearing frequency range or because fusion occurred up to the lower or upper limit of the frequency range tested.
Larger differences were seen for a few HI subjects between the 5AFC and 2AFC tasks than in NH listeners. For instance, subject HI19 had slightly broader fusion functions with the 2AFC task than the 5AFC task (see Fig. S2 available in the supplemental material1). Subject HI10, on the other hand, had more jagged fusion functions with the 5AFC task than the 2AFC task, suggesting more variability with the 5AFC task. A closer look at the lateralization data for this subject shows highly inconsistent “lateralization” responses in the 5AFC task, indicating likely confusion of the “left higher” buttons with the “left only” buttons and the “right higher” buttons with the “right only” buttons in that task, which would erroneously indicate fusion instead of two sounds heard (or vice versa) and contribute to the variability in the fusion function (see Fig. S3 available in the supplemental material1).
Comparison of Fig. 5 with Fig. 1 illustrates the lack of association of broad fusion with the overall degree of hearing loss in either ear. HI17 and HI25 have similar audiometric thresholds as seen in Fig. 1, but very different fusion range widths. Further, subjects with more overall hearing loss can have narrower fusion such as HI19, which has more hearing loss at all frequencies than HI25 (Fig. 1).
Similarly, when looking at age in Table I and fusion ranges in Fig. 5, there is no apparent relationship of fusion range to age in HI listeners. HI09, at 33 years old, showed a fusion range intermediate between the older subjects in Fig. 5, with broader fusion than HI07 at 80 years old but narrower fusion than HI10 or HI25 at 67 and 65 years old, respectively.
The HI subjects had group mean fusion ranges of 1.7 ± 1.5 octaves for the 5AFC task and 1.7 ± 1.6 octaves for the 2AFC task when averaged across reference frequencies; note the large standard deviation reflects the wide variation in fusion ranges, which ranged from as small as 0.03 octaves (when re-measured at higher resolutions than 1/4 or 1/8 octave steps for HI subjects with narrow fusion; otherwise 0) to as large as 4 octaves. In contrast, NH subjects as a group had narrower average fusion ranges of 0.17 ± 0.13 octaves for the 5AFC task and 0.14 ± 0.09 octaves for the 2AFC task when averaged across reference frequencies, ranging from 0.015 to no larger than 0.6 octaves. However, as noted, there were several cases of narrow fusion in HI subjects of similar scale as NH listeners.
When population distributions of fusion ranges were compared between HI and NH listeners for reference frequencies of 1200 and 1400 Hz (the frequencies for which sufficient data was available for both groups), a significant difference in the group distributions was seen at 1200 Hz for the 5AFC task (p = 0.010, Kolmogorov-Smirnov test), but not quite significant with the 2AFC task (p = 0.070). Differences were not significant at 1400 Hz with either task (p = 0.093 and p = 0.093, respectively), due to the within-group variability and smaller number of subjects that could be tested at 1400 Hz in the HI group.
B. Factors associated with broad fusion ranges
Correlation analyses were performed for fusion results obtained using the 5AFC and 2AFC fusion range measurement procedures against various demographic and reference frequency specific parameters.
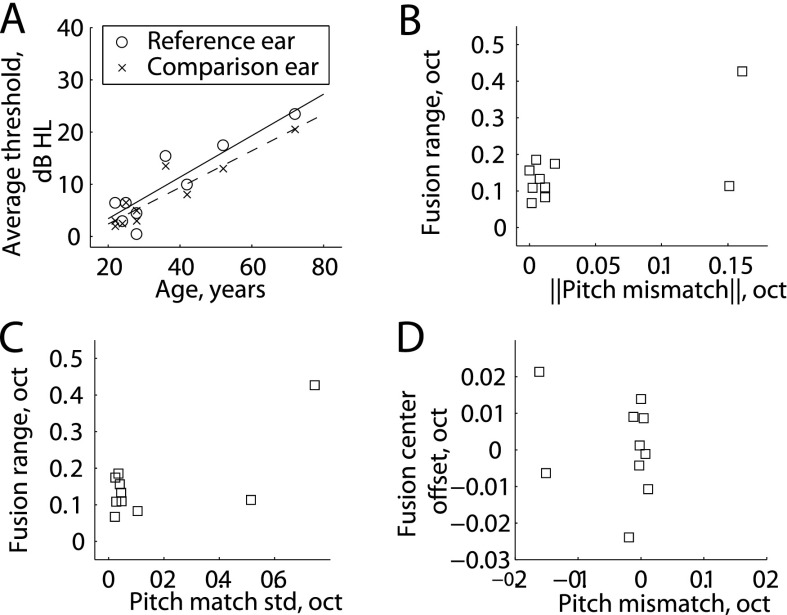
For NH listeners, subject-specific correlations are shown in Table II. No correlations were seen between average fusion ranges (obtained using either method and averaged over all frequencies tested), and any demographic factors (age and average threshold in either ear; Pearson correlation test). Not surprisingly, though, a highly significant correlation was seen between the demographic factors of age and average thresholds in the reference and contralateral ear (r = 0.89 and 0.93; p < 0.001, Pearson correlation), even though NH listeners all had thresholds in the normal range [Fig. 6(A)]. Frequency-specific correlation results are also shown in Table II. For the reference frequency with the most data in NH listeners, 1600 Hz, fusion ranges obtained using either method were not significantly correlated with the absolute value of the pitch mismatch [diplacusis; Fig. 6(B)] or with the average pitch match bandwidth [25%–75% range; Fig. 6(C)], though there is a potential trend due to one listener outside of the tightly clustered range of values for NH listeners; the Spearman correlation was used here because of influence exerted by the one atypical listener. Fusion center offsets were also not correlated with the pitch mismatch for individual reference frequencies [Fig. 6(D)]. No correlations were seen between fusion ranges and audiometric thresholds at the reference frequencies, similar to the averaged data.
TABLE II.
Table of correlations of fusion results obtained with the 5AFC and 2AFC tests with various subject- and frequency-specific variables for NH listeners. Frequency-specific variables are represented by data at 1600 Hz. “*” indicates significant p-values (p < 0.05). || || denote absolute values.
| Correlation results: NH subjects | 5AFC task | 2AFC task | ||
|---|---|---|---|---|
| r | p | r | p | |
| Subject-specific factors | ||||
| Age | 0.31 | 0.389 | 0.42 | 0.232 |
| Average threshold | 0.22 | 0.547 | 0.29 | 0.417 |
| Frequency-specific factors | ||||
| Reference threshold | 0.18 | 0.612 | 0.28 | 0.436 |
| ||Pitch mismatch||a | 0.37 | 0.296 | 0.26 | 0.470 |
| Pitch bandwidtha | 0.24 | 0.514 | 0.22 | 0.537 |
| Fusion center versus pitch mismatcha | −0.20 | 0.584 | −0.14 | 0.707 |
Spearman test used instead of Pearson correlation test. Correlations are with fusion ranges unless indicated otherwise.
FIG. 6.
Scatterplots of results in NH listeners. (A) Thresholds versus age showed significant correlations (p < 0.001). (B), (C). Fusion range results obtained using the 5AFC method at the 1600 Hz reference frequency versus absolute pitch mismatch (B), bandwidth of the pitch match (C). (D) Fusion center offset versus absolute pitch mismatch.
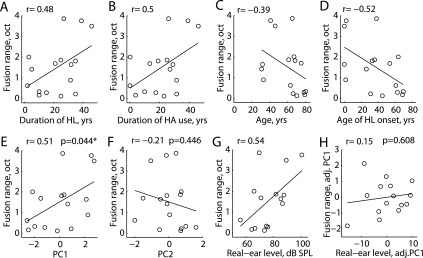
In HI listeners, certain demographic characteristics (age at onset of hearing loss, age, duration of severe-profound hearing loss, duration of HA use, and daily hours of HA use) were found to be significantly inter-correlated (p < 0.05; Pearson's correlation; r-values shown in the top of Table III), leading to multiple correlations with fusion range for the first four variables [r = 0.48, 0.50, −0.39, and −0.52, respectively; Figs. 7(A)–7(D)]. Little correlation was seen with daily hours of HA use (r = 0.02). Hence, attempts to find associations between fusion range and any of these factors would be clouded by these correlations. To reduce collinearity among these demographic characteristics, PCA was applied to the standardized (mean centered then scaled by standard deviation) variables. The first two uncorrelated principal components accounted for 84.7% of the variance. The correlation of each of the five characteristics with each component is shown at the bottom of Table III. The first principal component (PC1) is essentially a weighted average of standardized hearing loss duration and HA use duration contrasted against a weighted average of age and age of onset of hearing loss, such that persons with high positive scores on PC1 tend to have above average durations of hearing loss and HA use while having below average age and age of onset of hearing loss, and vice versa. The second principal component (PC2) is driven almost entirely by and is positively correlated with daily hours of HA use.
TABLE III.
Inter-correlations and principal components analysis of subject-specific variables. AgeOnset, age of onset of hearing loss; Age, age at time of testing; HLDur, duration of hearing loss; HADur, duration of HA use; HAhours, daily hours of HA use.
| AgeOnset | Age | HLDur | HADur | HAhours | |
|---|---|---|---|---|---|
| Intercorrelations between subject variables | |||||
| AgeOnset | 1.000 | 0.866 | −0.775 | −0.882 | −0.207 |
| Age | 1.000 | −0.354 | −0.636 | −0.154 | |
| HLDur | 1.000 | 0.845 | 0.192 | ||
| HADur | 1.000 | 0.363 | |||
| HAhours | 1.000 | ||||
| Correlation of each component with PC1 and PC2 | |||||
| PC1 | −0.975 | −0.784 | 0.831 | 0.954 | 0.361 |
| PC2 | 0.180 | 0.244 | −0.027 | 0.061 | 0.917 |
FIG. 7.
Scatterplots of average fusion ranges obtained using the 5AFC procedure versus various subject-specific parameters, including first principal component (PC1) and second principal component (PC2), in HI listeners. (A) Fusion range versus duration of hearing loss. (B) Fusion range versus duration of HA use. (C) Fusion range versus subject age. (D) Fusion range versus age of onset of hearing loss. (E) Fusion range versus PC1, a weighted sum mainly of factors in (A)–(D). (F) Fusion range versus PC2, weighted mainly by daily hours of HA use. (G) Fusion range versus average real-ear levels in the reference ear. (H) Fusion range versus average real-ear levels in the reference ear, adjusted for PC1. “*” indicates significant p-values (p < 0.05); p-values are only shown for adjusted, not crude, correlation analyses in (E), (F), and (H).
These two uncorrelated principal components were then used as surrogates for the full set of five inter-correlated variables in subsequent analyses; Table IV shows the results of adjusted analyses based on the principal components. Average 5AFC fusion range values were significantly correlated with PC1 [r = 0.51, p = 0.044; Fig. 7(E)] but not with PC2 [r = −0.21, p = 0.446; Fig. 7(F)]. The average 2AFC fusion range was only marginally correlated with PC1 (r = 0.46, p = 0.072) and not with PC2 (r = −0.03, p = 0.90; not shown). Associations between 5AFC and 2AFC fusion ranges and other characteristics (e.g., average hearing threshold, average real-ear level) were adjusted for influences of demographic characteristics, i.e., partial correlations were conducted after adjusting for effects of PC1. No correlations were observed between average hearing thresholds in the reference ear and 5AFC and 2AFC fusion ranges. While crude (unadjusted) correlations between average real-ear levels in the reference ear and 5AFC and 2AFC fusion ranges were significant or marginally significant [r = 0.54, p = 0.04 and r = 0.50, p = 0.056, respectively; Fig. 7(G)], average real-ear levels were also correlated with PC1 (r = 0.79, p < 0.001). When adjusted for PC1, correlations with real-ear levels were no longer significant [Table IV; Fig. 7(H)]. No correlations were observed with real-ear levels relative to NAL-NL2 (National Acoustics Laboratory, Australia) targets either (Table IV). Similarly, no significant correlations were seen with thresholds, real-ear levels, or real-ear levels relative to targets in the comparison ear after adjusting for PC1.
TABLE IV.
Table of correlations of fusion results obtained with the 5AFC and 2AFC tests with various subject- and frequency-specific variables for HI listeners. Subject-specific variables are incorporated into independent principal components PC1 and PC2, described in Table III. Frequency-specific variables are represented by data at 1000 Hz. “*” indicates significant p-values (p < 0.05). Correlations are with fusion ranges unless indicated otherwise.
| Correlation results: HI subjects | 5AFC task | 2AFC task | ||
|---|---|---|---|---|
| r | p | r | p | |
| Subject-specific factors | ||||
| PC1 | 0.51 | 0.044* | 0.46 | 0.072 |
| PC2 | −0.21 | 0.446 | −0.03 | 0.898 |
| PC1, adjusted for PC2 | 0.52 | 0.047* | 0.46 | 0.084 |
| Average threshold, adjusted for PC1 | 0.02 | 0.936 | 0.17 | 0.555 |
| Average real-ear level, adjusted for PC1 | 0.15 | 0.608 | 0.20 | 0.504 |
| Average real-ear level difference from NAL-2 target, adjusted for PC1 | 0.11 | 0.710 | −0.0002 | 0.9995 |
| Frequency-specific factors | ||||
| Reference threshold shift, adjusted for PC1 | 0.24 | 0.426 | 0.32 | 0.294 |
| Reference real-ear level, adjusted for PC1 | −0.04 | 0.905 | 0.11 | 0.742 |
| ||Pitch mismatch||, adjusted for PC1 | 0.08 | 0.811 | 0.10 | 0.983 |
| Pitch bandwidth, adjusted for PC1 | 0.19 | 0.569 | −0.18 | 0.597 |
| Fusion center versus pitch mismatch, adjusted for PC1 | 0.24 | 0.484 | 0.30 | 0.374 |
Similarly, no correlations with frequency-specific factors such as threshold or diplacusis at the reference frequency were seen once adjusted for subject-specific factors (age, age of hearing loss onset, duration of hearing loss, duration of HA use, and hourly HA use) via PC1 (Table IV). Fusion range was again weakly correlated with threshold and real-ear levels at the reference frequency in crude correlation analyses, but no correlation remained once subject-specific factors were controlled. Similar to NH listeners, no correlations were observed between fusion range and the absolute pitch mismatch or pitch bandwidth, or between fusion center offset and pitch mismatch.
When subdivided based on musical experience (at least one year of experience versus no experience), no significant differences in fusion ranges were seen between those with and without musical experience (p > 0.05, Wilcoxon rank-sum test).
IV. DISCUSSION
A. Binaural pitch fusion in HI and NH listeners
This is the first study to systematically investigate binaural pitch fusion in HI listeners who use HAs. The findings of this study show that, as hypothesized, HI listeners exhibit broad binaural pitch fusion ranges with an average of 1.7 ± 1.5 octaves with the 5AFC task, compared to NH listeners who have narrow fusion ranges with an average of 0.17 ± 0.13 octaves with the same task. The fusion ranges seen in NH listeners in this study are similar in scale to those reported previously, on the order of 3%–15% of the reference frequency, equivalent to 0.04–0.2 octaves (Thurlow and Bernstein, 1957; Odenthal, 1963; van den Brink et al., 1976). In contrast, the fusion ranges of some HI listeners were as large as 4 octaves, though it should be noted that other HI listeners had narrow fusion ranges similar to the scale of NH listeners.
Broad fusion ranges indicate an inability to separate different tones that differ greatly in frequency between ears under simultaneous, but not sequential, presentation. The interaural pitch-matching data show that these same subjects could clearly discriminate small pitch differences between ears under sequential presentation. Similarly, HI listeners with broad fusion all have narrow within-ear frequency discrimination limens on the order of 0.05–0.38 octaves (Oh and Reiss, 2016). Thus, broad binaural pitch fusion in HI listeners is not explained by poor sequential frequency discrimination.
One possibility is that these HI listeners have overlap in the patterns of activation over frequency, i.e., that broad frequency tuning and overlap in the activation patterns between the ears permits sequential but not simultaneous frequency discrimination. Further studies to measure psychophysical tuning curves are needed to determine whether this is the case.
Alternatively, binaural pitch fusion may be a more central auditory process governed by rules underlying auditory object formation. Auditory object formation, or auditory streaming, is determined by several cues, including similar pitch, harmonicity, co-modulation, common onset, and common location (for a review, see Bregman, 2004). If the normal presentation and thus the statistical relationships of these cues are altered peripherally by hearing loss, different rules of auditory object formation may be learned. For example, in the case of broad fusion, repeated exposure to two sounds with differing pitch but common co-modulation and common onset in the two ears might eventually lead a listener to reduce the weighting of pitch differences when deciding if a binaural stimulus is one or two objects.
The association with early onset of hearing loss suggests that rules of auditory object formation may be learned neurally via temporal correlation detection mechanisms such as STDP (spike-timing dependent plasticity) and hardwired during development; in the visual system, there are intriguing parallels with a disorder called amblyopia, the loss of binocular visual acuity, depth perception, and contrast sensitivity even in the presence of normal monocular acuity. Amblyopia is a central visual processing disorder resulting from abnormal visual experience during childhood development, and is similarly associated with deficits in visual object formation and segregation (for a review, see Levi et al., 2015). This alternative interpretation is supported by the additional finding of pitch averaging between ears when the dichotic stimuli are fused in HI listeners (Oh and Reiss, 2016) and bimodal CI users (Reiss et al., 2014b), similar to averaging of color and texture for fused dichoptic stimuli in vision (Hillis et al., 2002; Anstis and Rogers, 2012); such averaging may similarly explain the binocular loss of acuity and contrast in amblyopia.
B. Comparison of tasks used to measure fusion
Two different tasks were used to measure binaural fusion ranges: a 2AFC task and a more complex 5AFC task. Both tasks yielded similar measurements for most subjects in this study. The 5AFC task provides more data that help verify that subjects understand the task and that they are truly fusing the sounds. However, the 5AFC task was reported informally to be more difficult by some subjects, with potential for confusion of the “left only” and “left higher” buttons, and the “right only” and “right higher” buttons; this was evident in one subject with variable fusion and lateralization results. As there were no clear advantages of using the 5AFC task, the 2AFC task may be better suited to measurements of binaural pitch fusion in a broad subject population with varied cognitive and attentional abilities.
Interestingly, lateralization was observed with the 5AFC task in the presence of large interaural frequency differences in some HI subjects with broad fusion. The three subjects that showed lateralization all lateralized to the ear with the higher pitch, consistent with the 80% rate of lateralization to the higher pitch ear reported for fused dichotic stimuli in both bilateral CI users and NH listeners listening to CI simulations (Kan et al., 2013). The reason for lateralization to the higher frequency is not clear, but has been speculated to be the result of greater numbers of neurons being recruited for high frequencies than low frequencies (Kan et al., 2013), such that differences in loudness and thus interaural loudness differences (ILDs; a potential cue for sound localization) are created.
It is also possible that small ILDs were present due to inaccuracies in the loudness balancing procedure, and may have influenced fusion as well as led to lateralization. In particular, small changes in level can lead to larger changes in loudness and thus ILDs for HI listeners than NH listeners. Presently, it is unknown how ILDs influence fusion range. Perceived sound source location in auditory object formation due to ILDs may promote broader fusion; more research is needed to study the effects of ILDs and other sound localization cues on fusion.
C. Factors associated with broader fusion ranges
In NH listeners, fusion ranges were not correlated with subject factors of age or hearing thresholds, or with frequency-specific factors including diplacusis, pitch match bandwidth, and the offset of the center of the fusion range. The lack of correlation of the fusion center offset with diplacusis differs from previous findings in NH listeners (van den Brink et al., 1976).
Similar to NH listeners, no correlations were observed between fusion range and the absolute diplacusis or pitch match bandwidth, or between the offset of the center of the fusion range from the reference frequency (fusion center offset) and diplacusis in HI listeners. It is important to note that the diplacusis in the NH and HI listeners in the current study was relatively small on the order of 0.1–0.2 octaves or 7%–15%, due to the generally symmetric rather than asymmetric losses between ears, and consistent with the smaller amounts of 1%–3% diplacusis in previous studies of symmetric losses (Burns and Turner, 1986) compared to 10%–100% diplacusis with asymmetric losses (e.g., Schubert, 1957; Gaeth and Norris, 1965; Turner et al., 1983; Colin et al., 2016). Future studies in NH listeners with more diplacusis and in HI listeners with asymmetric losses and greater diplacusis may indicate stronger correlations of fusion range or fusion center offset with diplacusis.
Surprisingly, though, fusion ranges in HI listeners were found to be correlated with demographic factors, specifically with a principal component measure (PC1) of the covarying subject factors of age, age of hearing loss onset, duration of hearing loss, and duration of HA use. Note that PC1 positively weights hearing loss duration and HA use duration and negatively weights overall age and age of onset of hearing loss. Thus, the positive correlation of fusion range with PC1 means that broad fusion ranges are positively associated with long durations of hearing loss and HA use, and negatively associated with age and age of onset of hearing loss. In other words, younger individuals with early onset of hearing loss and long durations of hearing loss and HA use are most likely to have broad fusion. After adjusting for this aggregate PC1 measure, fusion ranges were not correlated with any other factors, including hearing thresholds, real-ear levels in the HAs, and real-ear level differences from target. Future studies with more subjects with greater independence of these demographic variables (duration of hearing loss, duration of HA use, age, and age of onset of hearing loss) are needed to determine the most important factors and possibly non-linear interactions, such as a greater effect of a certain duration of hearing loss at a younger age than at an older age.
One possibility considered was whether general age-related central changes could lead to broader fusion in NH as well as HI listeners. Certainly, several studies have shown upregulation of excitatory neurotransmitters (and reduction of inhibitory neurotransmitters) in the central auditory system in response to aging as well as peripheral age-related hearing loss (for a review, see Caspary et al., 2008), which cumulatively could broaden central neural tuning and fusion. However, the lack of correlation of fusion range with age in NH listeners, and the negative rather than positive association within PC1 in HI listeners, indicates that older age is unlikely to contribute to broad fusion in either population. This negative trend of fusion range with age combined with the positive trend with duration of hearing loss (and HA use) within PC1 is surprising, since duration of hearing loss and age typically vary in the same, not opposite, direction. This emphasizes the more likely role of early age of onset of hearing loss, which in this case varies in the same direction with duration of hearing loss within PC1.
The positive trend with duration of hearing loss and HA use within PC1 suggests another alternative mechanism for broad fusion in HI listeners: greater amplification and recruitment combined with experience may slowly, over time, lead to maladaptive plasticity of auditory pathways. While slightly broadened fusion may be a beneficial adaptation in minimizing the perception of diplacusis due to slight differences in pitch perception between the ears, as well as for sound localization in the presence of small place-mismatches (Blanks et al., 2008), extremely broad fusion on the order of octaves may be detrimental for speech perception and considered maladaptive. As demonstrated previously, broad fusion across mismatched frequencies often leads to averaging of spectral information between ears (Reiss et al., 2014b; Reiss et al., 2016) and can worsen vowel discrimination (Reiss et al., 2016). Broad fusion may also lead to an inability to separate voices in a room full of talkers, i.e., the “cocktail party effect,” if voices of different pitches and thus their speech are fused together, leading to various forms of speech fusion (Cutting, 1976). In addition, this study in HI listeners who use HAs, as well as several other studies in NH, bimodal CI, and bilateral CI listeners have shown that broad fusion of mismatched frequencies often leads to spurious lateralization in the absence of interaural time or intensity differences (Goupell et al., 2013; Reiss et al., 2014b; Kan et al., 2013).
Amplification may lead to greater spread of excitation along the basilar membrane, increase interaural temporal envelope correlation of energy across a broad frequency range, and over a long time period, slowly increase the synaptic weighting of binaural integration (via STDP) across a broader frequency range than normal. Alternatively, early onset of hearing loss and the resulting broad tuning with amplification may deprive the developing auditory system of finely tuned guiding cues for refinement of binaural auditory pathways. These implications of experience with amplification extend to bimodal CI users, many of whom experience broad fusion (Reiss et al., 2014a), which may have been inherited from long durations of hearing loss and HA use prior to implantation. Certainly, long durations of hearing loss prior to implantation have been shown to correlate with poorer speech perception outcomes in CI users, previously attributed to loss of surviving nerve for stimulation (e.g., Rubinstein et al., 1999); however, these findings suggest that changes in central auditory processing may also be a factor.
More data from more subjects are needed in order to clearly separate the contributions of each of these factors, and narrow down potential mechanisms in the development of broad fusion. Once the mechanisms are determined, treatments can be developed to sharpen fusion and reduce speech perception interference from abnormally broad fusion. Depending on the mechanism, potential treatments include brain retraining programs, new HA programming strategies, or even cochlear implantation.
V. CONCLUSIONS
The binaural pitch fusion ranges of HI listeners were on average larger than in NH listeners, and more variable. Some HI listeners had fusion ranges as large as 4 octaves, while others had narrow fusion similar to NH listeners. Fusion ranges were correlated with a principal component measure of the covarying factors of age, age of hearing loss onset, duration of hearing loss, and duration of HA use, but not with hearing threshold, amplification level, or diplacusis in HI listeners. No correlations were observed with age, hearing threshold, or diplacusis in NH listeners. The association of broad fusion with long durations of hearing loss and HA use in particular suggests that long-term experience with hearing loss and HAs may potentially have a role in the development of broad fusion.
ACKNOWLEDGMENTS
This research was supported by Grant Nos. R01 DC013307, P30 DC010755, and P30 DC005983 from the National Institutes of Deafness and Communication Disorders, National Institutes of Health and BDP grant (UL1TR000128 [OHSU CTSA]). We thank Erick Gallun for helpful comments on the manuscript.
Portions of this work were presented in “Binaural pitch fusion is broader in hearing-impaired listeners than normal-hearing listeners,” 2015 Meeting of the Association for Research in Otolaryngology, Baltimore, MD, USA, February 2015, and “Abnormal binaural spectral integration in hearing aid and cochlear implant users,” 2014 Meeting of the Association for Research in Otolaryngology, San Diego, CA, USA, February 2014.
Footnotes
See supplementary material at http://dx.doi.org/10.1121/1.4978009E-JASMAN-141-029703 for Figs. S1, S2, and S3, which are plots of fusion functions for various reference frequencies for subjects HI07, HI19, and HI10, respectively. Plotted as in Fig. 3, but in color. Lateralization is indicated by colored bars below plots, with red indicating lateralization to the right and blue indicating lateralization to the left.
References
- 1. Albers, G. D. , and Wilson, W. H. (1968). “ Diplacusis. I. Historical review,” Arch. Otolaryngol. 87(6), 601–603. 10.1001/archotol.1968.00760060603009 [DOI] [PubMed] [Google Scholar]
- 2. Anstis. S., and Rogers, B. (2012). “ Binocular fusion of luminance, color, motion, and flicker—Two eyes are worse than one,” Vision Res. 53, 47–53. 10.1016/j.visres.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 3. Blanks, D. A. , Buss, E. , Grose, J. H. , Fitzpatrick, D. C. , and Hall, J. W. (2008). “ Interaural time discrimination of envelopes carried on high frequency tones as a function of level and interaural carrier mismatch,” Ear Hear. 29, 674–683. 10.1097/AUD.0b013e3181775e03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bregman, A. S. (2004). “ Auditory scene analysis,” in International Encyclopedia of the Social and Behavioral Sciences, edited by Smelzer N. J. and Baltes P. B. ( Pergamon, Amsterdam: ), pp. 940–942. [Google Scholar]
- 50. Burns, E. M. (1982). “ Pure-tone pitch anomalies. I. Pitch-intensity effects and diplacusis in normal ears,” J. Acoust. Soc. Am. 72, 1394–1402. 10.1121/1.388445 [DOI] [PubMed] [Google Scholar]
- 5. Burns, E. M. , and Turner, C. (1986). “ Pure-tone pitch anomalies. II. Pitch-intensity effects and diplacusis in impaired ears,” J. Acoust. Soc. Am. 79, 1530–1540. 10.1121/1.393679 [DOI] [PubMed] [Google Scholar]
- 6. Caspary, D. M. , Ling, L. , Turner, J. G. , and Hughes, L. F. (2008). “ Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system,” J. Exp. Biol. 211(Pt. 11), 1781–1791. 10.1242/jeb.013581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colin, D. , Micheyl, C. , Girod, A. , Truy, E. , and Gallego, S. (2016). “ Binaural diplacusis and its relationship with hearing-threshold asymmetry,” PLoS One 11(8), e0159975. 10.1371/journal.pone.0159975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cutting, J. E. (1976). “ Auditory and linguistic process in speech perception: Inferences from six fusions in dichotic listening,” Psychol. Rev. 83, 114–140. 10.1037/0033-295X.83.2.114 [DOI] [PubMed] [Google Scholar]
- 10. Ernst, M. O. , and Banks, M. S. (2002). “ Humans integrate visual and haptic information in a statistically optimal fashion,” Nature 415(24), 429–433. 10.1038/415429a [DOI] [PubMed] [Google Scholar]
- 11. Florentine, M. , and Houtsma, A. J. M. (1983). “ Tuning curves and pitch matches in a listener with a unilateral, low-frequency hearing loss,” J. Acoust. Soc. Am. 73, 961–965. 10.1121/1.389021 [DOI] [PubMed] [Google Scholar]
- 12. Florentine, M. , Popper, A. N. , and Fay, R. R. (2011). Loudness ( Springer, New York: ), Chap. 2, pp. 17–56. [Google Scholar]
- 13. Folstein, M. F. , Folstein, S. E. , and McHugh, P. R. (1975). “ Mini-mental state: A practical method for grading the cognitive state of patients for the clinician,” J. Psychiatr. Res. 12, 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 14. Gaeth, J. H. , and Norris, T. W. (1965). “ Diplacusis in unilateral high-frequency hearing losses,” J. Speech Hear. Res. 8, 63–75. 10.1044/jshr.0801.63 [DOI] [PubMed] [Google Scholar]
- 15. Goupell, M. J. , Stoelb, C. , Kan, A. , and Litovsky, R. Y. (2013). “ Effect of mismatched place-of-stimulation on the salience of binaural cues in conditions that simulate bilateral cochlear-implant listening,” J. Acoust. Soc. Am. 133, 2272–2287. 10.1121/1.4792936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grose, J. H. , and Mamo, S. K. (2010). “ Processing of temporal fine structure as a function of age,” Ear Hear. 31(6), 755–760. 10.1097/AUD.0b013e3181e627e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hillis, J. M. , Ernst, M. O. , Banks, M. S. , and Landy, M. S. (2002). “ Combining sensory information: Mandatory fusion within, but not between, senses,” Science 298(5598), 1627–1630. 10.1126/science.1075396 [DOI] [PubMed] [Google Scholar]
- 18. Huss, M. , and Moore, B. C. J. (2005). “ Dead regions and pitch perception,” J. Acoust. Soc. Am. 117, 3841–3852. 10.1121/1.1920167 [DOI] [PubMed] [Google Scholar]
- 19. Kan, A. , Stoelb, C. , Litovsky, R. Y. , and Goupell, M. J. (2013). “ Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear implant listeners,” J. Acoust. Soc. Am. 134(4), 2923–2936. 10.1121/1.4820889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Levi, D. M. , Knill, D. C. , and Bavelier, D. (2015). “ Stereopsis and amblyopia: A mini-review,” Vision Res. 114, 17–30. 10.1016/j.visres.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liberman, M. C. (1984). “ Single-neuron labeling and chronic cochlear pathology. I. Threshold shift and characteristic-frequency shift,” Hear. Res. 16, 33–41. 10.1016/0378-5955(84)90023-6 [DOI] [PubMed] [Google Scholar]
- 22. Odenthal, D. W. (1963). “ Perception and neural representation of simultaneous dichotic pure tone stimuli,” Acta Physiol. Pharmacol: Neerl. 12, 453–496. [PubMed] [Google Scholar]
- 23. Ogura, M. , Kawase, T. , Kobayashi, T. , and Suzuki, Y. (2003). “ Modified binaural pitch-matching test for the assessment of diplacusis,” Int. J. Audiol. 42, 297–302. 10.3109/14992020309101321 [DOI] [PubMed] [Google Scholar]
- 24. Oh, Y. , and Reiss, L. A. (2016). “ Toward a systematic analysis of binaural pitch averaging trends in hearing impaired listeners,” in Abstracts of the 39th Annual Midwinter Meeting of the Association for Research in Ototlaryngology, p. 265. [Google Scholar]
- 25. Reiss, L. A. , Eggleston, J. L. , Walker, E. P. , and Oh, Y. (2016). “ Two ears are not always better than one: Mandatory vowel fusion across spectrally mismatched ears in hearing-impaired listeners,” J. Assoc. Res. Otolaryngol. 17, 341–356. 10.1007/s10162-016-0570-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reiss, L. A. J. , Ito, R. A. , Eggleston, J. L. , Liao, S. , Becker, J. J. , Lakin, C. E. , Warren, F. M. , and McMenomey, S. O. (2014a). “ Pitch adaptation patterns in bimodal cochlear implant users: Over-time and after experience,” Ear. Hear. 36(2), e23–e34. 10.1097/AUD.0000000000000114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reiss, L. A. J. , Ito, R. A. , Eggleston, J. L. , and Wozny, D. R. (2014b). “ Abnormal binaural spectral integration in cochlear implant users,” J. Assoc. Res. Otolaryngol. 15(2), 235–248. 10.1007/s10162-013-0434-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reiss, L. A. , Perreau, A. E. , and Turner, C. W. (2012). “ Effects of lower frequency-to-electrode allocations on speech and pitch perception with the Hybrid short-electrode cochlear implant,” Audiol. Neurotol. 17(6), 357–372. 10.1159/000341165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reiss, L. A. J. , Turner, C. W. , Erenberg, S. R. , and Gantz, B. J. (2007). “ Changes in pitch with a cochlear implant over time,” J. Assoc. Res. Otolaryngol. 8(2), 241–57. 10.1007/s10162-007-0077-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robertson, D. , and Johnsone, B. M. (1979). “ Aberrant tonotopic organization in the inner ear damaged by Kanamycin,” J. Acoust. Soc. Am. 66, 466–469. 10.1121/1.383097 [DOI] [PubMed] [Google Scholar]
- 31. Rubinstein, J. T. , Parkinson, W. S. , Tyler, R. S. , and Gantz, B. J. (1999). “ Residual speech recognition and cochlear implant performance: Effects of implantation criteria,” Am. J. Otology 20, 445–452. [PubMed] [Google Scholar]
- 32. Schubert, K. (1957). “ On pathological pitch perception,” in Translations of the Beltone Institute for Hearing Research, edited by Lightfoot C. ( Beltone Institute for Hearing Research, Chicago: ), Vol. 5, pp. 1–12. [Google Scholar]
- 33. Souza, P. E. , Boike, K. T. , Witherall, K. , and Tremblay, K. (2007). “ Prediction of speech recognition from audibility in older listeners with hearing loss: Effects of age, amplification, and background noise,” J. Am. Acad. Audiol. 18, 54–65. 10.3766/jaaa.18.1.5 [DOI] [PubMed] [Google Scholar]
- 34. Thurlow, W. R. , and Bernstein, S. (1957). “ Simultaneous two-tone pitch discrimination,” J. Acoust. Soc. Am. 29, 515–519. 10.1121/1.1908946 [DOI] [Google Scholar]
- 35. Turner, C. W. , Burns, E. M. , and Nelson, D. A. (1983). “ Pure tone pitch perception and low-frequency hearing loss,” J. Acoust. Soc. Am. 73, 966–975. 10.1121/1.389022 [DOI] [PubMed] [Google Scholar]
- 36. van den Brink, G. , Sintnicolaas, K. , and van Stam, W. S. (1976). “ Dichotic pitch fusion,” J. Acoust. Soc. Am. 59(6), 1471–1476. 10.1121/1.380989 [DOI] [PubMed] [Google Scholar]