Summary
Seminal replication of multiple human herpesviruses was common in this study’s HIV primary infection cohort. Understanding the complex viral milieu in semen is important for HIV transmission, but might also play a role in HIV pathogenesis and disease progression.
Keywords: human herpesviruses, CMV, early HIV infection, genital secretion
Abstract
Background.
Multiple viruses coinfect the male genital tract, influencing each other’s replication and perhaps affecting human immunodeficiency virus (HIV) pathogenesis and disease progression.
Methods.
This study included 453 longitudinal seminal samples from 195 HIV-infected men from the San Diego Primary Infection Resource Consortium and 67 seminal samples from HIV-negative healthy controls. Seminal HIV RNA and DNA from 7 human herpesviruses (HHVs) were measured by real-time polymerase chain reaction. Longitudinal shedding rates were determined by Kaplan-Meier survival analysis. Predictors of viral shedding were determined using backwards selection in a multivariable generalized estimating equation model.
Results.
HIV-infected participants presented significantly increased rates of seminal HHV shedding compared with HIV-uninfected controls. Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) were the most commonly detected HHV in semen of HIV-infected participants. Persistent shedding was more common for CMV and EBV when compared to other HHVs. With exception of HHV-7, HHV shedding was not significantly influenced by HIV RNA levels, CD4+ cell counts, or antiretroviral therapy. Presence of CMV, EBV, and herpes simplex virus (HSV) were independent predictors of genital HIV RNA shedding after adjusting for plasma HIV RNA and longitudinal measurements.
Conclusions.
Seminal replication of multiple HHVs is common in our HIV primary infection cohort. Genital replication of CMV and EBV was the most common and was significantly associated with seminal HIV RNA shedding. Prevalence of HSV shedding was lower and mostly intermittent, but its association with seminal HIV RNA was the strongest. Understanding the complex viral milieu in semen is important for HIV transmission but might also play a role in HIV pathogenesis and disease progression.
The male genital tract is an important milieu for human immunodeficiency virus (HIV) replication [1–3]. Even when HIV-infected men are suppressed with antiretroviral therapy (ART), they can still shed HIV RNA [4] and transmit it to their partners [5, 6]. This is likely a result of viral compartmentalization [7], poor drug penetration [4, 8], coinfection with other sexually transmitted infections [9, 10] or genital inflammation [11, 12]. Similarly, human herpesviruses (HHVs) replicate in the genital tract, and have been linked to HIV shedding and sexual transmission of HIV [13, 14]. Moreover, subclinical replication of HHV (and cytomegalovirus in particular [15]) has been associated with increased systemic T-cell activation, proliferation, and exhaustion [16–18], greater HIV DNA reservoirs [17, 19], HIV disease progression [20, 21], and possibly chronic inflammation and “premature” aging in HIV-infected persons [22, 23]. Several studies have shown that HIV coinfection provides a synergistic effect for the replication of HHV, especially in the genital tract, where HHV shedding commonly fluctuates [24, 25]. Both cytomegalovirus (CMV) and Epstein-Barr virus (EBV) are reactivated as a result of changes to the cytokine network caused by HIV coinfection [26] and as a consequence of HIV-induced immune dysfunction [18]. In general, HIV-infected individuals have higher rates of HHV shedding compared with HIV-uninfected individuals [25], with varying effects depending upon the particular HHV type and cohort characteristics [27–29].
We previously described the rates of genital HHV replication and their association with genital levels of HIV RNA in a cross-sectional cohort of treated HIV-infected persons [30] and longitudinally during untreated HIV infection [31]. However, no study has described the longitudinal dynamics of genital HHV shedding during treated and untreated early HIV infection and what factors might impact the shedding of these viruses.
MATERIALS AND METHODS
Participants, Samples, and Clinical Laboratory Tests
Paired semen and blood samples were collected longitudinally from 195 HIV-infected men who were prospectively followed as part of the San Diego Primary Infection Resource Consortium (PIRC) between 1997 and 2014. The estimated dates of infection was established using a series of well-defined stepwise rules that characterize stages of infection based on serologic and virologic criteria, as described in [32] (and summarized in Supplementary Table 1). Routine study visits included regular lymphocyte counts and plasma viral loads. Semen was collected and processed as previously described [16, 33].
We also collected a single seminal sample from a similar cohort of 67 “at risk” HIV-uninfected control participants.
The studies were conducted with appropriate written subject consent and were approved by the Human Research Protections Program at the University of California, San Diego.
Quantification of HIV RNA and HHV DNA in Seminal Plasma
HIV RNA levels were measured in seminal plasma by first concentrating HIV RNA from 500 μL of seminal plasma with high-speed centrifugation (23500g at 4°C for 1 hour) after 1:1 dilution with phosphate-buffered saline [16]. Concentrated RNA was extracted using the High Pure Viral RNA Kit (Roche) and HIV complementary cDNA was generated using the SuperScript III First-Strand Synthesis Kit (Invitrogen) with specific primer mf302 [34]. HIV RNA in seminal plasma was quantified by real-time polymerase chain reaction (PCR) in an ABI 7900HT thermocycler (Applied Biosystems) [16, 35]. HIV RNA quantification standards were obtained from the Division of AIDS (DAIDS) Virology Quality Assurance (VQA) program [36]. Similarly, levels of 7 herpesviruses (CMV, EBV, herpes simplex virus types 1/2 [HSV-1/2], and human herpesvirus types 6, 7, and 8 [HHV-6, HHV-7, HHV-8]) were measured in DNA extracted from seminal plasma by real-time PCR [37]. Varicella zoster virus was not included as it is typically not detected in seminal plasma.
Statistical Analysis
Statistical analysis was performed using SAS software (version 9.4). Viral loads were transformed to logarithmic scales. Continuous variables were compared and expressed as median with interquartile range (IQR). Seminal HIV and HHV shedding were dichotomized based upon presence of any detectable virus. Proportions were compared using Fisher exact test or χ2 test as appropriate. Shedding rates of individual virus types were determined over the course of 1 year (ie, 365 days) by survival analysis using Kaplan-Meier estimates. Comparisons between groups were done by log-rank test. Factors associated with genital viral shedding were analyzed in generalized estimating equation (GEE) models using a subset of participants with the following characteristics: available visits that had a viral load within 7 days, visits that were at least 7 days apart, and no more than 6 visits per subject. The GEE approximated relative risk through a modified Poisson model with an autoregressive correlation matrix that adjusted for multiple visits [38]. Because of the exploratory/descriptive character of this analysis, results were not adjusted for multiple comparisons. Participants with ≥2 visits (with HHV detectable in at least 1 seminal sample) separated by >30 days were further classified based upon shedding pattern of each HHV. Specifically, shedding was classified as “persistent” if all longitudinal time points remained positive. Otherwise, shedding was classified as “intermittent.”
RESULTS
HIV-Infected Study Participants and Baseline Samples
A total of 453 seminal samples from 195 HIV-infected men were analyzed. All participants were men who have sex with men (MSM) diagnosed with acute and early HIV infection, prospectively enrolled in the PIRC, and willing to provide semen samples (Table 1). The first time point (baseline) was collected within a median estimated duration of infection of 90 (IQR, 75–158) days. At baseline, the median age was 34 (IQR, 27–40) years, median HIV RNA levels in blood plasma were 4.64 (IQR, 3.74–5.21) log10 copies/mL, and median CD4+ count was 532 (IQR, 412–710) cells/µL. At the time of the first semen collection, 33 of the 195 individuals (17%) were already on ART and 15 (7%) had achieved suppressed plasma HIV RNA. Overall, seminal HIV RNA was detectable in 146 of 195 samples (75%) with a median seminal viral load of 3.2 (IQR, 2.1–3.8) log10 copies/mL. HHV shedding of any type in semen was present in 122 participants at baseline (62.6%), specifically: 7 (3.6%) HSV-1/2; 92 (47.2%) CMV; 56 (28.7%) EBV; 6 (3.1%) HHV-6; 18 (9.2%) HHV-7; and 13 (6.7%) HHV-8.
Table 1.
HIV-Infected Cohort Characteristics and Seminal Viral Shedding at Baseline
| Baseline Characteristic | No. (%) |
|---|---|
| Participants | 195 |
| Age, y, median (IQR) (n = 195) | 34 (27–40) |
| Race/ethnicity (n = 192) | |
| White | 113 (58.9) |
| Hispanic | 46 (24.0) |
| Other | 33 (17.1) |
| Time since infection, d, median (IQR) (n = 176) | 90 (75–158) |
| Participants with detectable plasma viral load | 174 (90.2) |
| HIV RNA log10 copies, median (IQR) | 4.64 (3.74–5.21) |
| CD4+ count, cells/µL, median (IQR) | 532 (412–710) |
| CD4%, median (IQR) | 28.0 (22.0–35.0) |
| CD8+ count, cells/µL, median (IQR) | 890 (657–1234) |
| CD8%, median (IQR) | 48.0 (40.0–55.0) |
| Baseline seminal viral shedding | |
| Detectable HIV RNA in semen | 146 (74.9) |
| HIV in semen, copies/mL, median (IQR) | 552 (117–4659) |
| Any herpesvirusa | 122 (62.6) |
| HSV (n = 193) | 7 (3.7) |
| CMV (n = 195) | 92 (47.2) |
| EBV (n = 194) | 56 (28.9) |
| HHV-6 (n = 192) | 6 (3.1) |
| HHV-7 (n = 192) | 18 (9.2) |
| HHV-8 (n = 195) | 13 (6.7) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, human herpesvirus; HIV, human immunodeficiency virus; HSV, herpes simplex virus 1/2; IQR, interquartile range.
Herpesvirus 1–8 excluding varicella.
HIV-Uninfected Healthy Controls
A total of 67 HIV-uninfected healthy controls were enrolled as part of this study. Median age was 36 (IQR 35–41) years, 87% were MSM, and 21% were on tenofovir/emtricitabine for preexposure prophylaxis within the previous month of the sample collection. HHV shedding of any type in semen was present in 25 participants (37.3%), specifically: 1 (1.5%) HSV-1/2; 13 (19.4%) CMV; 9 (13.4%) EBV; 1 (1.5%) HHV-6; 6 (9.0%) HHV-7; and 1 (1.5%) HHV-8. Compared with HIV-infected individuals measured on their first visit, the HIV-uninfected controls were significantly less likely to present any HHV shedding (37.3% vs 62.6%; P < .001), CMV shedding (19.4% vs 47.2%; P < .0001), or EBV shedding (13.4% vs 28.7%; P < .01). Frequency of HHV-8 shedding was also lower (1.5% vs 6.7%; P = .13) but did not reach statistical significance. There was no significant difference for shedding of HSV-1 or -2 (1.5% vs 3.6%; P > .2), HHV-6 (1.5% vs 3.1%; P > .2), or HHV-7 (9% vs 9.2%; P > .2).
Longitudinal Patterns of Seminal Herpesviruses and HIV Shedding
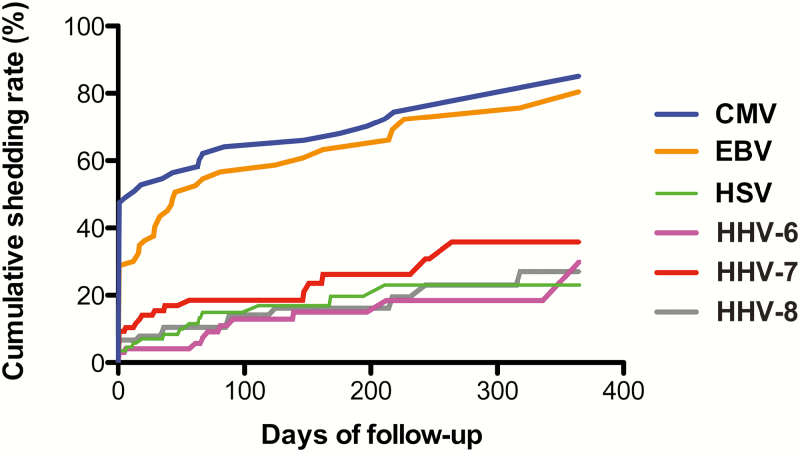
Half of HIV-infected participants (97 of 195) had 1 or more longitudinal visits with a median of 3 (IQR, 2–4) seminal samples provided over a median follow-up of 111 (IQR, 36–230) days. There was no significant difference by race/ethnicity or age between participants who had follow-up visits and those who did not (all P > .05). Using Kaplan-Meier methods in the full cohort of HIV-infected men (treated and untreated), we determined the time to HHV shedding and the overall cumulative shedding rates (ie, the percentage with any shedding) of HHV in semen over an observed period of 365 days from baseline. Based on this analysis, 94.3% of early HIV–infected individuals presented detectable DNA for at least 1 HHV in at least 1 sampled time point over a period of 365 days. This included 86% with CMV DNA shedding, 71% with EBV DNA shedding, 23% with HSV shedding (type 1 or 2), 30% with HHV-6 shedding, 36% with HHV-7 shedding, and 27% with HHV-8 shedding (Figure 1).
Figure 1.
Kaplan-Meier analysis of time to herpesvirus shedding (n=195), evaluated over the course of 365 days from baseline. This figure represents the cumulative shedding rate for each human herpesvirus using a survival curve to indicate the probability of viral shedding over 1 year. For example, although at any given time point only about half of men are shedding cytomegalovirus (CMV) in semen, over the period of a year almost all men will eventually shed CMV. Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, human herpesvirus; HSV, herpes simplex virus 1/2.
To determine if factors such as age, ART status, HIV RNA levels, and CD4+ T-cell counts affected HHV shedding, we generated GEE models (Table 2). When considering all HHVs as a group, there were no statistically significant factors associated with presence of seminal shedding. However, subanalysis of each HHV revealed some distinctions. Older age was associated with a decreased shedding rate for EBV and HHV-7 (with 27% and 73% reduction, respectively, in shedding rates per decade of age). Interestingly, longer time from estimated HIV infection was significantly associated with a decrease in the shedding rate of HSV (types 1 and 2) and an increase in the shedding rates of HHV-6 and EBV (all P < .05). Notably, there was no statistically significant effect of ART use or HIV suppression on any HHV shedding as a group. When looking at each HHV individually, HHV-7 presented a significant 74% reduction (relative risk [RR], 0.26 [95% confidence interval {CI}, .09–.70]) in genital shedding in the presence of ART, but this did not seem to be related to viral suppression or CD4+ recovery, as these factors (ie, HIV RNA levels or CD4+ T-cell count) were not directly associated with presence or absence of HHV-7. HSV-1/2, EBV, and HHV-8 also presented reduced shedding rates in the presence of ART use, but these were not statistically significant. ART usage had no effect on CMV and HHV-6 shedding. Overall, CMV and HHV-8 shedding were not modified by any factors we measured. For those HHV with >1 significant modifying factor, we performed multivariate analysis and all factors remained significantly associated with viral shedding in the multivariable models.
Table 2.
Factors Associated With Human Herpesvirus Sheddinga as Determined by Generalized Estimating Equation Modeling
| Factors Associated With Shedding | Any HHVb | HSV | EBV | CMV | ||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | |
| Age (10-y increments) | 0.91 (.81–1.02) | .09 | 0.76 (.37–1.60) | .48 | 0.73 (.59–.93) | .01 | 0.93 (.78–1.10) | .38 |
| White non-Hispanic | 0.97 (.81–1.17) | .76 | 1.02 (.26–3.96) | .98 | 1.03 (.69–1.54) | .87 | 0.99 (.74–1.32) | .94 |
| Time since HIV infection, mo | 1.00 (1.00–1.01) | .18 | 0.87 (.77–.98) | .02 | 1.01 (1.00–1.01) | .03 | 1.00 (1.00–1.01) | .39 |
| ART use | 0.95 (.79–1.15) | .61 | 0.63 (.17–2.34) | .49 | 0.84 (.56–1.27) | .41 | 1.04 (.82–1.31) | .77 |
| HIV RNA log10 copies | 0.98 (.92–1.04) | .5 | 1.24 (.88–1.75) | .21 | 0.97 (.85–1.09) | .6 | 0.99 (.90–1.08) | .76 |
| HIV RNA <50 copies | 0.98 (.79–1.20) | .81 | 0.33 (.07–1.54) | .16 | 0.83 (.49–1.42) | .5 | 1.02 (.73–1.41) | .92 |
| CD4+ cells/µL (100-cell increase) | 0.97 (.94–1.01) | .12 | 0.97 (.85–1.10) | .63 | 0.95 (.89–1.02) | .17 | 0.98 (.93–1.03) | .39 |
| CD8+ cells/µL (100-cell increase) | 0.99 (.98–1.01) | .44 | 0.99 (.92–1.08) | .88 | 0.99 (.96–1.02) | .55 | 1.00 (.99–1.02) | .57 |
| HHV-6 | HHV-7 | HHV-8 | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | |
| Age (10-y increments) | 1.04 (.62–1.78) | .86 | 0.37 (.23–.60) | <.01 | 0.91 (.51–1.61) | .74 |
| White non-Hispanic | 1.41 (.42–4.74) | .58 | 0.53 (.25–1.12) | .1 | 1.21 (.43–3.44) | .72 |
| Time since HIV infection, mo | 1.02 (1.00–1.03) | .04 | 0.99 (.96–1.02) | .38 | 1.00 (.98–1.01) | .49 |
| ART use | 1.00 (.41–2.44) | 1 | 0.26 (.09–.70) | <.01 | 0.71 (.29–1.76) | .46 |
| HIV RNA log10 copies | 0.87 (.61–1.25) | .46 | 0.96 (.76–1.21) | .72 | 0.79 (.63–1.00) | .05 |
| HIV RNA <50 copies | 1.29 (.32–5.18) | .72 | 0.44 (.13–1.58) | .21 | 1.76 (.73–4.24) | .2 |
| CD4+ cells/µL (100-cell increase) | 0.89 (.74–1.07) | .22 | 0.96 (.86–1.07) | .63 | 1.09 (.94–1.28) | .26 |
| CD8+ cells/µL (100-cell increase) | 0.89 (.77–1.03) | .11 | 0.96 (.90–1.02) | .2 | 0.99 (.93–1.05) | .67 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, human herpesvirus; HIV, human immunodeficiency virus; HSV, herpes simplex virus 1/2; RR, relative risk.
Defined as detectable shedding during any time point.
Herpes 1–8 excluding varicella.
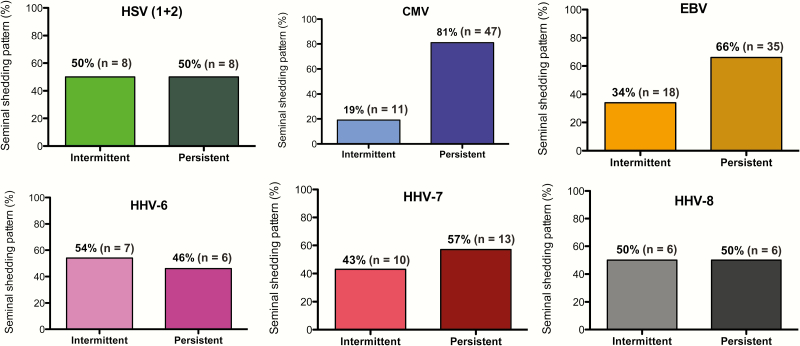
In participants with at least 1 follow-up visit, we investigated the shedding pattern for each HHV virus (“intermittent” vs “persistent”). As an example, of the 16 men who ever shed HSV-1/2 and with at least 2 time points, 8 men shed virus intermittently (50%) while 8 men presented HSV-1/2 at all their time points (ie, persistent shedding pattern). Similar shedding pattern rates were observed for HHV-6 (54%), HHV-7 (43%), and HHV-8 (50%). In comparison, persistent shedding was found more frequently for CMV and EBV, which had intermittent shedding proportions of 19% and 34%, respectively (Figure 2).
Figure 2.
Shedding patterns of human herpesviruses, evaluated for participants with at least 1 follow-up visit with at least 30 days between visits. Shedding was classified as intermittent if shedding for a particular herpesvirus was undetectable during 1 or more time points. Otherwise, shedding was classified as persistent. Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, human herpesvirus; HSV, herpes simplex virus 1/2.
Associations Between HHV and HIV RNA Semen Shedding
Last, we used univariate and multivariable GEE modeling to determine associations between seminal shedding of HIV RNA and seminal shedding of the various types of HHV (Table 3). In univariate analysis, higher HIV RNA levels, no ART use, shorter time since infection, lower CD4+ T-cell count, higher CD8+ T-cell count, and presence of CMV, EBV, HHV-6, and HSV shedding were all significantly associated with presence of seminal HIV RNA shedding (all P < .05). In multivariate analysis, only plasma HIV RNA levels and shedding of CMV, EBV, and HSV remained independently associated with seminal HIV RNA shedding. In particular, for every log10 viral load increase in the blood plasma, there was an increase in seminal plasma shedding (adjusted RR, 1.22 [95% CI, 1.14–1.32]). Shedding of HSV had an adjusted RR of 1.30 (95% CI, 1.09–1.54), compared with 1.16 (95% CI, 1.01–1.33) for CMV and 1.17 (95% CI, 1.03–1.33) for EBV. Repeating the model only with visits where HIV was not suppressed did not significantly change this finding (viral load adjusted RR [ARR], 1.18 [95% CI, 1.09–1.28]; CMV ARR, 1.16 [95% CI, 1.02–1.31]; EBV ARR, 1.17 [95% CI, 1.03–1.32]; and HSV 1/2 ARR, 1.18 [95% CI, 1.01–1.38]). Unfortunately, the sample size was too small to perform a separate model for suppressed people, but it is interesting to note that a third of time points with suppressed HIV RNA in plasma during ART had simultaneous detectable HIV RNA in semen during the first year of infection.
Table 3.
Factors Associated With HIV Shedding as Determined by Generalized Estimating Equation Modelinga
| Factor | Visits With HIV Shedding (% of Visits) | Univariate | Univariate | Multivariate | Multivariate |
|---|---|---|---|---|---|
| RR (95% CI) | P Value | Adjusted RR (95% CI) | P Value | ||
| Age (10-y increments) | 0.97 (.90–1.05) | .49 | |||
| White non-Hispanic | 154 (70.0) | 1.08 (.91–1.28) | .37 | ||
| Other | 84 (69.4) | 0.97 (.90–1.05) | .49 | ||
| ART use | 24 (53.3) | 0.75 (.61–.94) | .01 | ||
| Time since infection, mo | 218 (72.7) | 0.99 (.98–1.00) | .03 | ||
| HIV RNA log10 copies | 1.21 (1.13–1.31) | <.01 | 1.22 (1.14–1.32) | <.01 | |
| CD4+ count, cells/µL | 0.95 (.91–.98) | <.01 | |||
| CD8+ count, cells/µL | 1.01 (1.00–1.02) | .05 | |||
| Herpesvirus | |||||
| HSV 1 or 2 | 1.34 (1.16–1.56) | <.01 | 1.30 (1.09–1.54) | <.01 | |
| Any | 19 (95.0) | ||||
| None | 220 (69.4) | ||||
| CMV | 1.16 (1.01–1.34) | .04 | 1.16 (1.01–1.33) | .03 | |
| Any | 130 (74.3) | ||||
| None | 112 (65.9) | ||||
| EBV | 1.21 (1.06–1.39) | <.01 | 1.17 (1.03–1.33) | .02 | |
| Any | 91 (79.1) | ||||
| None | 151 (65.9) | ||||
| HHV-6 | 1.26 (1.06–1.48) | <.01 | |||
| Any | 13 (72.2) | ||||
| None | 221 (69.5) | ||||
| HHV-7 | 1.14 (.96–1.37) | .14 | |||
| Any | 28 (80.0) | ||||
| None | 210 (69.1) | ||||
| HHV-8 | 0.94 (.67–1.31) | .72 | |||
| Any | 15 (71.4) | ||||
| None | 227 (70.1) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HSV, herpes simplex virus 1/2; HHV, human herpesvirus; HIV, human immunodeficiency virus; RR, relative risk.
Obtained using data from a subset of participants as described in the Methods (participants: n = 176; total visits: n = 330).
DISCUSSION
As part of this study, we used the longitudinal data of 195 HIV-infected men with 453 longitudinal seminal samples followed from primary infection to examine factors associated with viral shedding over a period of 48 weeks of follow-up. As previously reported, shedding of HHV was more common in HIV-infected individuals compared with uninfected healthy controls, despite early HIV infection and high CD4+ T-cell counts [39]. Specifically, we observed that CMV and EBV are highly prevalent during the first 48 weeks of HIV infection, followed by HHV-7, HHV-8, HSV, and HHV-6 [8, 40]. Interestingly, the shedding rates of most HHVs did not seem to be affected by ART status, HIV RNA levels, or CD4+ T-cell counts. A notable exception was HHV-7, which was significantly reduced by ART, but this effect did not correlate with HIV RNA level of CD4+ count, suggesting a possible direct drug effect on the virus. For HSV-1/2 and HHV-8, the small sample size may have impaired our ability to detect a more modest effect of ART on shedding. Younger age was a positive factor in shedding for EBV and HHV-7, and early HIV infection was associated with higher shedding rates in HSV-1/2 and HHV-6.
We also determined the shedding patterns of these HHVs (“persistent shedding” vs “intermittent shedding”) on a subset of participants with ≥2 longitudinal seminal samples. As shown in Figure 2, HSV, HHV-6, HHV-7, and HHV-8 were somewhat evenly distributed between intermittent and persistent shedding patterns. On the contrary, notably more participants were identified as persistent shedders for both CMV (81%) and EBV (66%). Various factors could result in such differences, including differences in HHV pathogenesis, replication dynamics, collection methods of genital secretion, and other technical issues [14, 25]. This observation might have important clinical consequences as coinfections with HHV (and CMV in particular) have been associated with immunosenescence, inflammation, and aging, particularly during HIV infection but also with various non-AIDS-related complications [22, 23, 41]. Furthermore, HIV-infected individuals can present very high levels of CMV-specific T cells (up to 50% of CD4+ T cells and even higher for CD8+ T cells), which might be a consequence of persistent exposure to CMV antigens, and are associated with cardiovascular complications [16, 17, 23, 42].
We have also shown that shedding of HHV may be associated with comorbidities such as acquisition of syphilis and persistence of human papillomavirus infections [43, 44]. Understanding the pattern and frequency of subclinical viral replication is important to inform future studies of anti-HHV therapy (in particular CMV) and to determine which patient population might particularly benefit from such interventions.
Several studies described associations between various HHVs and genital HIV shedding, with potential implications for HIV transmission. As expected, our study found an association between HIV RNA in blood and seminal plasma [11, 35]. We further confirmed associations between HIV RNA shedding and CMV, EBV, and HSV-1/2. Notably, HSV was less frequent and mostly intermittent (compared with CMV and EBV) but when it was present, its association with HIV shedding was very strong. This is consistent with several studies showing that HSV-2 increases the risk of both HIV acquisition and transmission [9]. Interestingly, one-third of early HIV–infected participants on ART and with suppressed HIV RNA in blood plasma presented detectable HIV RNA in semen. This is higher than what we found in a cohort of chronically HIV-infected people on long-term ART [45] and consistent with the fact that HIV RNA in semen might take longer to suppress than in blood.
This study has several limitations. Because this is an observational cohort study with varied numbers of visits and time between visits, we relied on estimates of shedding rates over time using Kaplan-Meier methods (ie, there was censoring of many who did not reach 1 year of follow-up). Also, not all assessments were completed at the same visit; for example, viral loads and ART use were not always assessed at sampling time points and certain data had to be excluded from the analyses. Also, HHV serologies were not available for this cohort (except for EBV and CMV) and shedding rates are therefore reported at a population level rather than stratified by serological status. It is also not clear if HHV replication in the male genital tract is a surrogate for lower-level replication systemically or just a consequence of localized genital viral shedding. As part of this study, we did not measure levels of HHV DNA in blood or any other compartment, but this should be pursued in future studies. Similarly, the clinical significance of genital viral shedding is unclear. For example, the presence of HHV-8 shedding might predict an increased risk for Kaposi sarcoma if participants remained untreated for their HIV, but this is purely speculative and a connection between subclinical HHV-8 genital shedding and Kaposi sarcoma is currently unknown. Finally, we only measured levels of HIV and 7 HHVs as part of this study, but various other viruses are replicating in semen at high levels (eg, Zika) and might interact directly or indirectly through alteration in the microenvironment and should be investigated as part of future clinical trials.
Finally, we only had a very limited number of participants who started ART and continued to provide samples throughout the study, which limited our power to perform a subanalysis of the intraperson change in HHV before and after ART initiation.
In summary, we collected seminal samples from a large group of early HIV–infected people on and off ART, and we characterized the longitudinal detection of HHV shedding. We found differences of shedding patterns by HHV type and variation in factors that might affect shedding. Most HHV shedding does not appear to be significantly modified by ART use, CD4+ count, or HIV RNA load, although HHV-7 does seem to be largely curtailed by ART and there is some trend for both HSV-1/2 and HHV-8 to also be reduced. Considering the abundance of HHV in HIV-infected men, there remains a need for further understanding of the pathogenesis of HHV in HIV and whether treatment of HHV might be beneficial in managing HIV.
Supplementary Material
Notes
Acknowledgments. We are grateful to all of the participants in the California Collaborative Treatment Group, the Center for AIDS Research Genomic, Translational Virology, and Flow Cytometry Cores. We acknowledge all the nurses, and Christy Anderson for her very helpful discussion. HIV RNA quantification standard was obtained through the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, DAIDS, National Institute of Allergy and Infectious Diseases. Primer and probe for quantification of HHVs as well as the plasmids and quantification standards were kindly provided by Fred Lakeman.
Author contributions. S. G. participated in the study design, performed the laboratory experiments, participated in the data analyses for this study, and wrote the primary version of the manuscript; S. R. M. participated in the study design and performed all the analysis and primary version of the manuscript; M. Z. participated in the data analyses and wrote the primary version of the manuscript; M. V. V. performed the laboratory experiments; D. M. S. and S. J. L. enrolled participants, participated in study design, and revised the manuscript. All authors read and approved the final manuscript.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported primarily by a grant from the NIH, University of California, San Francisco–Gladstone Institute of Virology and Immunology Center for AIDS Research (grant number P30-AI027763; Creative and Novel Ideas in HIV Research), California HIV Research Program Idea award to Sara Gianella, by the department of Veterans Affairs, the James B. Pendleton Charitable Trust and additional grants from the NIH (grant numbers AI100665, MH100974, MH097520, DA034978, AI007384, AI027763, AI106039, AI43638, AI074621, AI036214, MH101012, UL1TR000100, and AI068636-09).
Potential conflicts of interest. D. M. S. has received grant support from ViiV Pharmaceuticals and consultant fees from Gen-Probe and Testing Talent Services. All other authors report no potential conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mestecky J, Alexander RC, Wei Q, Moldoveanu Z. Methods for evaluation of humoral immune responses in human genital tract secretions. Am J Reprod Immunol 2011; 65:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaudhary S, Noel RJ, Rodríguez N, et al. Correlation between CD4 T cell counts and virus compartmentalization in genital and systemic compartments of HIV-infected females. Virology 2011; 417:320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Politch JA, Marathe J, Anderson DJ. Characteristics and quantities of HIV host cells in human genital tract secretions. J Infect Dis 2014; 210:S609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor S, Davies S. Antiretroviral drug concentrations in the male and female genital tract: implications for the sexual transmission of HIV. Curr Opin HIV AIDS 2010; 5:335–43. [DOI] [PubMed] [Google Scholar]
- 5. Lu W, Zeng G, Luo J, et al. HIV transmission risk among serodiscordant couples: a retrospective study of former plasma donors in Henan, China. J Acquir Immune Defic Syndr 2010; 55:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stürmer M, Doerr HW, Berger A, Gute P. Is transmission of HIV-1 in non-viraemic serodiscordant couples possible? Antivir Ther 2008; 13:729–32. [PubMed] [Google Scholar]
- 7. Craigo JK, Gupta P. HIV-1 in genital compartments: vexing viral reservoirs. Curr Opin HIV AIDS 2006; 1:97–102. [DOI] [PubMed] [Google Scholar]
- 8. Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther 2011; 16:1149–67. [DOI] [PubMed] [Google Scholar]
- 9. Lisco A, Vanpouille C, Margolis L. Coinfecting viruses as determinants of HIV disease. Curr HIV/AIDS Rep 2009; 6:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rotchford K, Strum AW, Wilkinson D. Effect of coinfection with STDs and of STD treatment on HIV shedding in genital-tract secretions: systematic review and data synthesis. Sex Transm Dis 2000; 27:243–8. [DOI] [PubMed] [Google Scholar]
- 11. Politch JA, Mayer KH, Welles SL, et al. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS 2012; 26:1535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson JA, Ping LH, Dibben O, et al. ; Center for HIV/AIDS Vaccine Immunology HIV-1 populations in semen arise through multiple mechanisms. PLoS Pathog 2010; 6:e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gianella S, Morris SR, Vargas MV, et al. Role of seminal shedding of herpesviruses in HIV type 1 transmission. J Infect Dis 2013; 207:257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gianella S, Massanella M, Wertheim JO, Smith DM. The sordid affair between human herpesvirus and HIV. J Infect Dis 2015; 212:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weinberg A, Spritzler J, Nokta M, et al. ; ACTG CMV Task Force In vitro cell-mediated immune responses of human immunodeficiency virus-infected and -uninfected individuals to whole cytomegalovirus antigens and their subunits. Clin Vaccine Immunol 2008; 15:1398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gianella S, Strain MC, Rought SE, et al. Associations between virologic and immunologic dynamics in blood and in the male genital tract. J Virol 2012; 86:1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gianella S, Massanella M, Richman DD, et al. ; California Collaborative Treatment Group 592 Team Cytomegalovirus replication in semen is associated with higher levels of proviral HIV DNA and CD4+ T cell activation during antiretroviral treatment. J Virol 2014; 88:7818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dan JM, Massanella M, Smith DM, et al. Effect of cytomegalovirus and HIV transcription on CD57 and PD-1 T cell expression during suppressive ART. J Acquir Immune Defic Syndr 2016; 72:133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gianella S, Anderson CM, Var SR, et al. Replication of human herpesviruses is associated with higher HIV DNA levels during antiretroviral therapy started at early phases of HIV infection. J Virol 2016; 90:3944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deayton JR, Prof Sabin CA, Johnson MA, Emery VC, Wilson P, Griffiths PD. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet 2004; 363:2116–21. [DOI] [PubMed] [Google Scholar]
- 21. Smith DM, Nakazawa M, Freeman ML, et al. Asymptomatic CMV replication during early human immunodeficiency virus (HIV) infection is associated with lower CD4/CD8 ratio during HIV treatment. Clin Infect Dis 2016; 63:1517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freeman ML, Lederman MM, Gianella S. Partners in crime: the role of CMV in immune dysregulation and clinical outcome during HIV infection. Curr HIV/AIDS Rep 2016; 13:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Effros RB. The silent war of CMV in aging and HIV infection. Mech Ageing Dev 2015; 158:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaspersen MD, Larsen PB, Kofod-Olsen E, Fedder J, Bonde J, Höllsberg P. Human herpesvirus-6A/B binds to spermatozoa acrosome and is the most prevalent herpesvirus in semen from sperm donors. PLoS One 2012; 7:e48810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaspersen MD, Höllsberg P. Seminal shedding of human herpesviruses. Virol J 2013; 10:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lisco A, Munawwar A, Introini A, et al. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis 2012; 205:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gianella S, Redd AD, Grabowski MK, et al. Vaginal cytomegalovirus shedding before and after initiation of antiretroviral therapy in Rakai, Uganda. J Infect Dis 2015; 212:899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Low AJ, Nagot N, Weiss HA, et al. ; Yerelon Study Group Herpes simplex virus type-2 cervicovaginal shedding among women living with HIV-1 and receiving antiretroviral therapy in Burkina Faso: an 8-year longitudinal study. J Infect Dis 2016; 213:731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martró E, Esteve A, Schulz TF, et al. ; Euro-Shaks Study Group Risk factors for human herpesvirus 8 infection and AIDS-associated Kaposi’s sarcoma among men who have sex with men in a European multicentre study. Int J Cancer 2007; 120:1129–35. [DOI] [PubMed] [Google Scholar]
- 30. Gianella S, Anderson CM, Vargas MV, et al. Cytomegalovirus DNA in semen and blood is associated with higher levels of proviral HIV DNA. J Infect Dis 2013; 207:898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gianella S, Morris SR, Anderson C, et al. Herpes viruses and HIV-1 drug resistance mutations influence the virologic and immunologic milieu of the male genital tract. AIDS 2013; 27:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368:218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Butler DM, Delport W, Kosakovsky Pond SL, et al. The origins of sexually transmitted HIV among men who have sex with men. Sci Transl Med 2010; 2:18re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Althaus CF, Gianella S, Rieder P, et al. Rational design of HIV-1 fluorescent hydrolysis probes considering phylogenetic variation and probe performance. J Virol Methods 2010; 165:151–60. [DOI] [PubMed] [Google Scholar]
- 35. Gianella S, Morris SR, Anderson C, et al. Herpesviruses and HIV-1 drug resistance mutations influence the virologic and immunologic milieu of the male genital tract. AIDS 2013; 27:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yen-Lieberman B, Brambilla D, Jackson B, et al. Evaluation of a quality assurance program for quantitation of human immunodeficiency virus type 1 RNA in plasma by the AIDS Clinical Trials Group virology laboratories. J Clin Microbiol 1996; 34:2695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gianella S, Strain MC, Rought SE, et al. Associations between virologic and immunologic dynamics in blood and in the male genital tract. J Virol 2012; 86:1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 39. Gianella S, Strain MC, Rought SE, et al. Associations between virologic and immunologic dynamics in blood and in the male genital tract. J Virol 2012; 86:1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gianella S, Morris SR, Anderson C, et al. Herpes viruses and HIV-1 drug resistance mutations influence the virologic and immunologic milieu of the male genital tract. AIDS 2013; 27:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barrett L, Fowke KR, Grant MD. Cytomegalovirus, aging, and HIV: a perfect storm. AIDS Rev 2012; 14:159–67. [PubMed] [Google Scholar]
- 42. Savva GM, Pachnio A, Kaul B, et al. ; Medical Research Council Cognitive Function and Ageing Study Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell 2013; 12:381–7. [DOI] [PubMed] [Google Scholar]
- 43. Gianella S, Smith DM, Daar E, et al. Genital cytomegalovirus replication predicts syphilis acquisition among HIV-1 infected men who have sex with men. PLoS One 2015; 10:e0130410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gianella S, Ginocchio CC, Daar ES, Dube MP, Morris SR. Genital Epstein Barr virus is associated with higher prevalence and persistence of anal human papillomavirus in HIV-infected men on antiretroviral therapy. BMC Infect Dis 2016; 16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gianella S, Smith DM, Vargas MV, et al. ; CCTG 592 Team Shedding of HIV and human herpesviruses in the semen of effectively treated HIV-1-infected men who have sex with men. Clin Infect Dis 2013; 57:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.