Abstract
Bendamustine has shown a favorable safety profile when included in chemotherapy regimens for several types of lymphoma, including chronic lymphocytic leukemia (CLL). This study investigated the long-term effect of adding bendamustine to a conditioning regimen on survival, rate of engraftment, immune recovery, and graft-versus-host disease (GVHD) after allogeneic stem cell transplantation (alloSCT) in CLL patients. These outcomes were compared to the FCR (fludarabine, cyclophosphamide, rituximab) conditioning regimen. We reviewed the data for 89 CLL patients treated on three trials at our institution. Twenty-six (29%) patients received BFR (bendamustine, fludarabine, rituximab) and 63 (71%) received FCR. Patient characteristics were similar in both groups. Ten (38%) BFR- vs. only two (3%) FCR-treated patients did not experience severe neutropenia (P = < 0.001). The 3-year overall survival estimates for the BFR and FCR groups were 82% and 51% (P = 0.03) and the 3-year progression-free survival estimates were 63% and 27% (P = 0.001). The 2-year treatment-related mortality was 8% and 23% and the incidence of grade 3 or 4 GVHD was 4% and 10%, respectively. This study is the first to report that addition of bendamustine to alloSCT conditioning for CLL patients is associated with improved survival and lower mortality, myelosuppression, and GVHD.
INTRODUCTION
The outlook for patients with chronic lymphocytic leukemia (CLL) undergoing allogeneic stem cell transplantation (alloSCT) has improved undoubtedly with the use of less toxic non-myeloablative transplant-conditioning regimens.1–4 However, a relapse rate of 40–50%, an acute graft-versus-host disease (GVHD) incidence of 30%–40%, and the advent of novel less toxic agents for treating CLL by conventional methods have made alloSCT a less-favored option.5
Among the novel less-toxic agents for conventional treatment of CLL, bendamustine is of interest owing to its reported favorable efficacy, safety, and survival profiles in regimens for several types of lymphoma.6 The efficacy and safety profiles of bendamustine were evident from the results of a randomized trial comparing bendamustine plus rituximab (BR) with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP): patients with BR-treated indolent and mantle cell lymphomas had higher survival rates with fewer toxicities.7 Similarly, results from a phase 3 trial comparing BR with conventional fludarabine, cyclophosphamide and rituximab in CLL found the BR regimen to be better tolerated in the elderly,8 making it the preferred choice in this patient population.9
We recently reported favorable results of bendamustine (130 mg/m2/day for 3 days) coupled with a fixed-dose schedule of fludarabine and rituximab (BFR) as a non-myeloablative alloSCT conditioning regimen with proven safety and efficacy.10 However, the small number of CLL patients and the short follow-up time in our prior study precluded defining the durability of remissions and the impact of treatment on overall survival (OS) rate. Herein, we report longer-term outcomes with BFR allogeneic conditioning in CLL patients. We compared results, safety, engraftment, GVHD, survival and immune reconstitution of BFR with those of the previously developed FCR (fludarabine, cyclophosphamide, rituximab) conditioning regimen in CLL patients.
METHODS
Study design and eligibility
We reviewed the database and electronic records for 89 adult CLL patients treated on three investigator-initiated trials at The University of Texas MD Anderson Cancer Center (Houston, TX, USA). These trials have been completed and previously published.1,10,11 Twenty-six (29%) patients received BFR and 63 (71%) received FCR. The trials included a BFR trial10 (ClinicalTrials.gov number, NCT00880815) that was recently reported (15 patients); another trial11 (ClinicalTrials.gov number, NCT00899431; June 2009–June 2013) of FCR (25 patients) that within the same trial was later changed to BFR (11 patients); and an earlier (January 2000–January 2009), third trial that included 38 FCR patients treated with alloSCT.1 The study analysis was approved by the center’s institutional review board.
The eligibility criteria were similar for all three trials. These criteria included age 18 to 70 years and a diagnosis of resistant or relapsed CD20+ CLL that had failed the best conventional treatments available. The other inclusion criteria have been previously described1,10 and included an Eastern Cooperative Oncology Group performance status score of 0–2 and adequate liver function (bilirubin and liver enzyme concentrations up to 3 times the upper limit of normal), renal function (creatinine < 1.6 mg/dL), cardiac function (ejection fraction higher than 40%), and pulmonary function (higher than 40% of predictive value). In addition, patients were required to have a 6/6 human leukocyte antigen-compatible sibling donor or human leukocyte antigen-A, -B, -C, and -DRB1 identical unrelated donor if no sibling donors were available, according to our department’s Standard Practice Guidelines. The exclusion criteria included active central nervous system involvement with disease, prior refractoriness or hypersensitivity to bendamustine (for those enrolled on the bendamustine-containing regimens), a prior alloSCT, pregnancy, breastfeeding, or known infection with human immunodeficiency virus, human T-lymphotropic virus, or hepatitis B or C virus. Additional exclusion criteria were the concurrent presence of other malignancies (with the exception of cutaneous squamous cell or basal cell carcinoma), uncontrolled infection, stroke or myocardial infarction within 6 months of study entry, and the use of other investigational drugs.
Procedures
The BFR regimen consisted of bendamustine, 130 mg/m2 infused intravenously (IV) over 60 minutes daily on days −5 to − 3 prior to transplantation, replacing cyclophosphamide in the FCR regimen. The dose and schedule of fludarabine (30 mg/m2 IV daily for 3 days, −-5 to −3 days prior to alloSCT) and rituximab (375 mg/m2 IV on day −13 and 1000 mg/m2 on days −6, +1, +8) were similar in both regimens. AlloSCT was performed on day 0. GVHD prophylaxis consisted of tacrolimus, 0.015 to 0.03 mg/kg starting on day −2, and methotrexate, 5 mg/m2 on days 1, 3, and 6 in all patients.1,10,11 Patients who received a transplant from a matched unrelated donors (MUD) received an additional dose of methotrexate at 5 mg/m2 on day 11. Rabbit antithymocyte globulin (rATG) (1 mg/kg IV on days −2 and −1 before alloSCT) was given to all patients receiving a MUD transplant, and since 2009, to FCR patients who received a sibling donor. As rATG was not available in earlier years of trials, FCR patients who underwent transplants from a MUD between years 2000–2008, received 15 mg/kg instead lymphocyte immune globulin (equine) IV on days −5 to −3 before transplantation, as described previously.1
Post-SCT responses in CLL patients were scored according to the recommendations of the National Cancer Institute-Sponsored Working Group.12 Disease extent was assessed in all patients by computed tomography scans of the chest, abdomen, and pelvis. Patients were evaluated 1, 3, 6, and 12 months after alloSCT; then every 6 months for up to 5 years; and yearly thereafter. Patients received supportive care with antibiotics, antifungals, antivirals, and immunizations after alloSCT as per institutional guidelines.
To assess immune function and quantify lymphocyte subsets, the distribution of peripheral blood lymphocytes was examined after 2009 in CLL patients with available samples at alloSCT and 1,3 and 6 months after completing treatment. Analysis was performed by flow cytometry with monoclonal antibodies specific for CD3, CD4, CD8, CD19 (all from BD Biosciences, San Jose, CA).
Statistical analysis
For analysis of the data from the three trials, we obtained data for all study-eligible patients from protocol and patients’ electronic records. Patient characteristics were compared using chi square and Fisher exact tests for categorical variables and the Mann Whitney test for continuous variables. Actuarial OS and progression-free survival (PFS) rates were estimated starting on the day of transplant using the method of Kaplan-Meier. Patients who had persistent detectable disease were considered treatment failures even without disease progression. Cox models were fit to assess the association between OS, the year of transplant and the use of ATG. The incidence of acute and chronic GVHD was estimated using the cumulative incidence method considering progression of the malignancy or death before GVHD as competing risks. Statistical significance was determined at the .05 level. Statistical analyses were performed using Stata 11.0 software (StataCorp, College Station, TX).
RESULTS
Patient age, sex distribution, proportion of patients with β2-microglobulin ≥3 mg/L at study entry, and disease status (sensitive vs. refractory) prior to alloSCT were similar for the BFR and FCR groups (Table 1). Patients were extensively treated and their prior treatment regimens are shown in Table 2. Routine fluorescence in situ hybridization for CLL cases at our center was initiated in 2004,1 and therefore, 33 FCR patients were tested and 17p deletion was detected in 8 (24%); a similar proportion of patients with 17p deletion, 7/26 (27%), was observed in the BFR-treated group. However, more patients received their transplants from unrelated donors in the BFR group than in the FCR group (54% vs. 32%, P = 0.05).
Table 1.
Characteristics of chronic lymphocytic leukemia patients according to treatment regimen
| Patient/Disease Characteristic | Regimen BFR | Regimen FCR | P Value |
|---|---|---|---|
| No. Patients | 26 | 63 | |
| Median age, years (range) | 58 (49–72) | 57 (35–72) | 0.2 |
| Male sex, no. (%) | 18(69) | 50(79) | 0.5 |
| Disease status at transplantation, no. (%) | 0.4 | ||
| Complete response | 2(8) | 6(10) | |
| Partial response | 14(54) | 27(43) | |
| Refractory | 10(38) | 30(48) | |
| b-2 microglobulin ≥ 4 mg/L, no. (%) | 8(32) | 27(43) | 0.3 |
| IGHV unmutated, no. (%) | 19/21(90) | 22/24(92) | 0.3 |
| 17p deletion present, no. (%) | 7(27) | 8/33(24) | 0.8 |
| Complex Karyotype | 4(15) | 7(11) | 0.4 |
| Median prior lines of therapy, (range) | 3(1–6) | 3(1–8) | 0.6 |
| Donor type, no. (%) | .05 | ||
| - Related | 12(46) | 43(68) | |
| - Unrelated | 14(54) | 20(32) | |
| Cell type, no. (%) | 0.4 | ||
| - Blood | 24(92) | 55(87) | |
| - Marrow | 2(8) | 8(13) | |
| Sex-mismatched, no. (%) | 16(62) | 33(52) | 0.7 |
| CMV+ (D and/or R) | 18(69) | 47(75) | 0.5 |
Abbreviations: BFR, bendamustine, fludarabine, rituximab; CMV, cytomegalovirus; D, R, donor, recipient; FCR, fludarabine, cyclophosphamide, rituximab; IGHV, immunoglobulin variable region heavy chain gene;
Table 2.
Previous treatments of study patients according to treatment regimen
| Types of prior therapies | Regimen BFR (% patients) | Regimen FCR (% patients) |
|---|---|---|
|
| ||
| Alkylating agent | 92% | 89% |
| Purine analog | 84% | 100% |
| No. Regimen with rituximab | ||
| 1 | 92% | 100% |
| 2 | 84% | 66% |
| 3 or more | 40% | 21% |
| Bendamustine | 56% | 5% |
| Ofatumumab | 8% | 5% |
| Lenalidomide | 12% | 7% |
| BTK inhibitor | 8% | 2% |
| Bcl-2 inhibitor | 8% | 0% |
Abbreviations: BTK, Bruton’s tyrosine kinase; BFR, bendamustine, fludarabine, rituximab; FCR, fludarabine, cyclophosphamide, rituximab
Most patients (92% in BFR vs. 87% in FCR) received unfractionated peripheral blood as the source of stem cells. The median number of CD34-positive cells infused in the BFR and FCR groups was 5.6 (range: 0.35, 19) ×106/kg and 4.9 (range: 2,15) ×106/kg, respectively (P = 0.3). Ten (38%) BFR and 2 (3%) FCR patients did not experience an absolute neutrophil count (ANC) ≤ 0.5 × 109/L (P = < 0.001) post alloSCT and 21 (81%) BFR and 39 (63%) FCR patients, did not require platelet transfusions (P = 0.08). This difference was observed in both siblings (33% and 2%, respectively) and unrelated transplants (43% and 5%, respectively).
All patients who underwent alloSCT, whether from sibling or unrelated donors, experienced donor cell engraftment. By day 90 +/− 10 days after SCT, the median values of donor T-cells in the BFR and FCR groups were 98% (range: 47,100) and 93%, range (12, 100), respectively (P = 0.4).
Clinical response
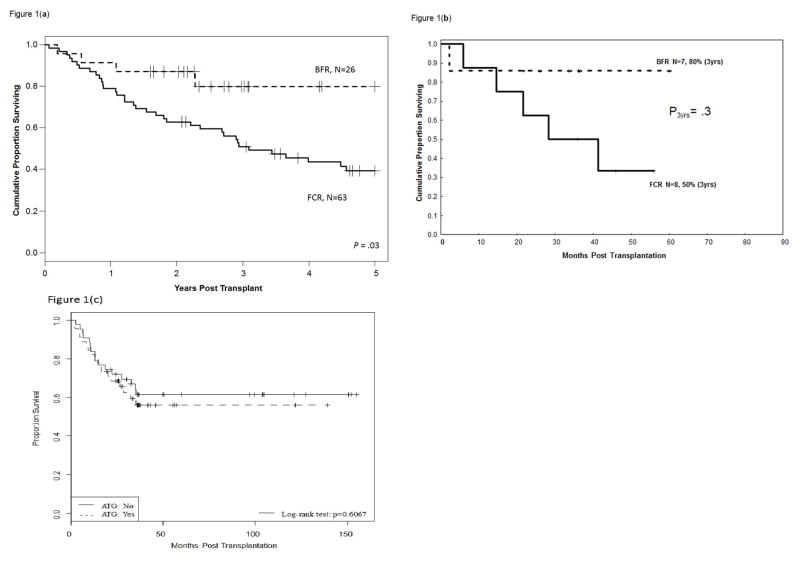
The median follow-up durations for BFR- and FCR-treated patients were 29 months (range: 19, 60 months) and 104 months (range: 34,195 months), respectively. The estimated 3-year OS rates were 82% (95% confidence interval [CI]: 56, 93) and 51% (95% CI: 38, 62), respectively [hazard ratio (HR) = 0.3; (P = 0.03) (Figure 1a). The 3-year PFS rates were 63% (95% CI: 38, 80) and 27% (95% CI: 17, 38), respectively (HR = 0.3; P = 0.001).
Figure 1.
Figure 1a. Improved overall survival rate in CLL patients after non-myeloablative allogeneic transplantation with bendamustine, fludarabine, and rituximab conditioning (upper curve) vs. findings with fludarabine, cyclophosphamide, and rituximab conditioning (lower curve).
Figure 1b. Overall survival rate in patients with CLL associated with 17p deletion after non-myeloablative allogeneic transplantation with bendamustine, fludarabine, and rituximab conditioning (upper curve) vs. findings with fludarabine, cyclophosphamide, and rituximab conditioning (lower curve).
Figure 1c. Overall survival rate in patients with CLL after non-myeloablative allogeneic transplantation according to the use of ATG.
The majority (90% and 92%, respectively) of patients studied in both BFR and FCR groups had unmutated immunoglobulin variable region heavy chain gene. Improvements in 3-year OS and PFS rates after BFR were observed in all categories of patients and transplant characteristics that are of importance in alloSCT for CLL (Table 3). The 3-year OS rates for FCR patients who received a sibling or unrelated donor transplants were 60% and 30%, respectively, compared to 71% and 93% in the BFR groups. The 3-year OS rates in CLL patients with 17p deletion were 86% (95% CI: 32, 77) in the BFR group (n=7) and 50% in the FCR group (n=8) (Figure 1b). The 3-year PFS rates for the same patients were 69% and 37%, respectively, for the BRF and FCR groups.
Table 3.
3-year survival outcomes by treatments and selected factors
| Selected factors | Treatment BFR (N=26) | Treatment FCR (N=63) | P Value |
|---|---|---|---|
| MUD transplant | |||
| OS | 93% | 30% | 0.02 |
| PFS | 81% | 25% | 0.005 |
| Age >50 years | |||
| OS | 79% | 50% | 0.05 |
| PFS | 69% | 37% | 0.008 |
| 17p deletion+ | |||
| OS | 86% | 50% | 0.3 |
| PFS | 69% | 37% | 0.2 |
| 17p deletion-, (n) | |||
| OS | 79% | 35% | 0.02 |
| PFS | 62% | 20% | 0.003 |
| Prior therapies ≥3, | |||
| OS | 70% | 43% | 0.1 |
| PFS | 53% | 21% | 0.01 |
Abbreviations: BFR, bendamustine, fludarabine, rituximab; FCR, fludarabine, cyclophosphamide, rituximab; MUD, matched unrelated donor; OS, overall survival; PFS, progression-free survival
In order to adjust for differences in the conditioning regimen, years of transplantation, the use of ATG (Figure 1c) and OS, uni- and multivariate analysis that included these parameters were undertaken (Tables 4, 5). Only the conditioning regimen was found to be of importance with improved survival in patients who received BFR.
Table 4.
Univariate analysis for overall survival
| Parameter | level | Total # of pts | # of pts died | P-value | HR (95%CI) |
|---|---|---|---|---|---|
| Regimen | FCR | 63 | 30 | 0.02 | 3.47 (1.22, 9.85) |
| BFR | 26 | 4 | ref | ||
| ATG | No | 43 | 16 | 0.61 | 0.84 (0.43, 1.65) |
| Yes | 46 | 18 | ref | ||
| Year of transplant group | <2009 | 36 | 15 | 0.80 | 1.09 (0.55, 2.16) |
| ≥ 2009 | 53 | 19 | ref | ||
| Year of transplant (continuous) | 89 | 34 | 0.49 | 0.97 (0.89, 1.06) |
Abbreviations: Pts, patients; FCR, fludarabine, cyclophosphamide, rituximab; BFR, bendamustine, fludarabine, rituximab; ATG, anitithymocyte globulin.
Table 5.
Multivariate analysis for overall survival
| Parameter | level | P-value | HR (95%CI) |
|---|---|---|---|
| Regimen | FCR | 0.013 | 5.44(1.33, 14.85) |
| BFR | ref | ||
| ATG | No | 0.57 | 1.48(0.38, 5.73) |
| Yes | ref | ||
| Year of transplantgroup | <2009 | 0.27 | 0.44(0.10, 1.87) |
| ≥2009 | ref | ||
| Year of transplant (continuous) | 0.66 | 1.07(0.91, 1.25) |
Abbreviations: FCR, fludarabine, cyclophosphamide, rituximab; BFR, bendamustine, fludarabine, rituximab; ATG, antithymocyte globulin.
Immune reconstitution
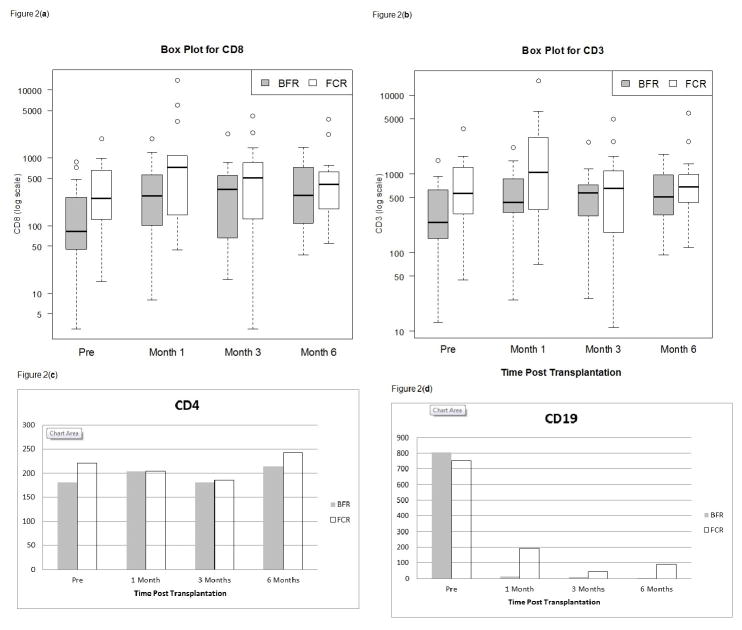
Figure 2 shows the kinetics of immune cell reconstitution. CD3+ (Figure 2a), CD3+/CD8+ T cells (Figure 2b), and the CD3+/CD4+ cell counts (Figure 2c) remained similar in the BFR (n=20) and FCR (n=19) groups throughout the period of observation at 1, 3 and 6 months post alloSCT. For example, the median CD3+ cell counts observed at 3 months was 579/μL(range: 26,2523)/μL and 656/μL (range: 11,4999)/μL in the BFR and FCR groups, respectively (Figure 2a; P = 0.6). The median CD3+/CD8+ T cells observed at 3 months was 356/μL (range: 16, 2261) and 510/μL (range: 3,4184)/μL, respectively (P = 0.6) and the median levels of CD3+/CD4+ cells counts were 180.5 (range: 6,450) and 185 (range:8,809) at 3 in the BFR and FCR groups, respectively (Figure 2c; P = 0.9). B cell (CD19+) counts rapidly decreased after alloSCT and remained near zero at 3 and 6 months in both groups (Figure 2d) as we described previously when high-dose rituximab is incorporated in the conditioning regimens.13
Figure 2.
Figure 2a. CD3 T cell recovery in CLL patients who received non-myeloablative allogeneic transplantation with bendamustine, fludarabine, and rituximab (BFR) conditioning compared with findings in patients who received fludarabine, cyclophosphamide (FCR), and rituximab at 1, 3 and 6 months post transplantation.
Figure 2b. CD8 T cell recovery in CLL patients who received non-myeloablative allogeneic transplantation with bendamustine, fludarabine, and rituximab (BFR) conditioning compared with findings in patients who received fludarabine, cyclophosphamide (FCR), and rituximab at 1, 3 and 6 months post transplantation.
Figure 2c. CD4 T cell recovery in CLL patients who received non-myeloablative allogeneic transplantation with bendamustine, fludarabine, and rituximab conditioning, is similar to findings in patients who received fludarabine, cyclophosphamide, and rituximab at 1, 3 and 6 months post transplantation.
Figure 2d. CD19 cells remain severely depleted at 1, 3 and 6 months, in CLL patients who received non-myeloablative allogeneic transplantation with high-dose rituximab contained in both bendamustine, fludarabine, rituximab and fludarabine, cyclophosphamide, rituximab conditionings.
GVHD and toxicity
Patients had at least 19 months of follow-up after transplantation and were therefore evaluable for GVHD. The incidence rates of grades 2–4 acute GVHD in the BFR and FCR groups were 23% and 40%, respectively (HR = 0.5; P = 0.2), and the incidence rates of grades 3–4 acute GVHD in the BFR and FCR groups were 4% and 10%, respectively (HR = 0.4; P = 0.1). The 3-year cumulative incidence rates of extensive chronic GVHD were 45% and 58%, respectively, in the BFR and FCR groups, (HR = 0.4; P = 0.01).
The treatment-related mortality (TRM) rates at 1 and 2 years for the BFR group were both 8%, compared with 16% and 23% TRM rates at 1 and 2 years, respectively, for the FCR group (P = 0.09). When taking into account the hematopoietic stem cell transplant-comorbidity index (HSCT-CI),2 2-year TRM rates in low-risk patients with an HSTC-CI of zero were 0% and 19% in the BFR and FCR groups, respectively. In higher risk patients with HSC-CI of ≥ 3, TRM rates at 2 years in the BFR and FCR groups were 14% and 28%, respectively.
Thirty-nine (62%) FCR-treated CLL patients died. Disease progression was the main cause of death (n=19, 49%). Five (13%) deaths were related to infection [3 of cytomegalovirus (CMV), 1 of Pneumocystis jiroveci pneumonia, and 1 of viral pneumonia], and eight deaths (21%) to infection in the context of chronic (n=7) or acute GVHD (n=1). Two (11%) patients died of a secondary malignancy, two (11%) of intracerebral accident, and three (8%) of unknown causes. Three (12%) BFR-treated CLL patients died: one of acute GVHD, one of chronic GVHD, and one of bacteremia.
CMV reactivation occurred in 17 (27%) FCR patients and was the cause of death in three patients as described above. Of the nine (35%) BFR patients who reactivated CMV none died of the disease.
DISCUSSION AND CONCLUSIONS
In patients with relapsed/refractory CLL undergoing alloSCT, the addition of bendamustine to fludarabine plus rituximab conditioning resulted in 3-year OS and PFS rates of 82% and 63%, respectively. These rates were significantly improved compared with earlier trials using the FCR regimen, including survival rates in trial NCT00899431 utilizing FCR (n=25) initially and later changed to BFR (n=11) within the trial period.11 The safety profile of BFR was favorable, with less myelosuppression (38% of BFR vs. 2% of FCR patients did not have an absolute neutrophil count ≤ 0.5 × 109/L). Furthermore, with the use of BFR, there was a trend toward lower frequencies of grade 2–4 acute GVHD (23% vs. 40%) and extensive chronic GVHD (45% vs.58%) than for FCR-treated patients despite the higher proportion of BFR-treated patients receiving a transplant from an unrelated donor.
This improvement in survival with BFR was observed even in patients with unmutated immunoglobulin variable region heavy chain gene (IGHV) and 17p deletion. In CLL patients, deletion 17p and unmutated IGHV are strongly associated with adverse outcomes and resistance to chemotherapy-based treatment.14 When rituximab at conventional doses was combined with bendamustine at 90 mg/m2 daily × 2 and without alloSCT, none of eight patients treated achieved a complete response, and only three patients achieved a partial response, with a 7.9-month median PFS.15 In this current study, the 3-year PFS rates of 17p deletion CLL treated with alloSCT was 69% with the BFR regimen, much improved from the 37% rate observed with the FCR-based regimen. All alive patients are in remission. The difference between the two groups did not reach statistical significance owing to the small sample. It is unclear whether the higher dose of bendamustine used in our trial conferred an additional benefit to the known GVT effect in these patients.
Comorbidity is the major driver of therapeutic decisions in a significant proportion of patients with hematologic malignancies, especially CLL. The HSCT-CI has been reported in several studies to be an important predictor of TRM in CLL patients undergoing alloSCT.2 We found the reduction of TRM in BFR-treated patients to be less than that in FCR-treated patients among low-risk (0% vs. 19%, respectively) and high-risk (14% vs. 28%, respectively) CLL patients with HSCT-CI of ≥3, elucidating the favorable observations in elderly and comorbid CLL patients treated with bendamustine at conventional doses.8,9
Therapy with BR at conventional doses has been reported to be associated with lymphocytopenia.7,8,16–19 Specifically, a significant decrease in CD4+ and CD8+ T-lymphocyte counts was observed for up to six months after the end of BR therapy although the impact of lymphopenia on the rate of infections has been controversial. Some studies found no increase of atypical infections, 7,8,17,18 whereas others noted conflicting data, 16,19 particularly with regard to cytomegalovirus infections.19
In this study, we analyzed immune recovery in CLL patients receiving alloSCT conditioning after changing only one drug in our historical FCR therapy (cyclophosphamide to bendamustine), without changing the GVHD prophylaxis type, duration, or pattern of tapering. We found that patients who received BFR or FCR conditioning maintained similar levels of CD3+, CD3+/CD8+, CD3+/CD4+ T as well as B cells (CD19+) levels 1, 3 and 6 months after alloSCT and that the rates of CMV infection were similar.
In conclusion, this study is the first to suggest that conditioning in alloSCT for CLL affects outcomes, with less severe neutropenia, a trend toward less GVHD, and improved survival results with BFR than when conditioned with FCR. Prospective randomized trials are warranted.
Acknowledgments
Supported by the NIH/NCI under award number P30CA016672
We thank Diane Hackett and Christopher Graber from the Department of Scientific Publications at MD Anderson Cancer Center for editing this manuscript.
Footnotes
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTION
Conception and design: IFK, RLB Jr., AMG
Collection and assembly of data: IFK, DS, CL, RLB Jr.
Data analysis and interpretation: IFK, DS, EJ, BIS, FT, GA, PA, SA, BO, SOC, DM, AO, KP, URP, CL, TMK, AF, JAB, JLJ, LJM, RLB Jr., AMG
Manuscript writing: All Authors.
Final approval of manuscript: All Authors.
References
- 1.Khouri IF, Bassett R, Poindexter N, O’Brien S, Bueso-Ramos CE, Hsu Y, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: long-term follow-up, prognostic factors and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer. 2011;117:4779–4788. doi: 10.1002/cncr.26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorror ML, Storer BE, Sandmaier BM, Maria M, Shizuru J, Maziarz R, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26:4912–4920. doi: 10.1200/JCO.2007.15.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown JR, Kim HT, Armand P, et al. Long term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: prognostic model to predict outcome. Leukemia. 2013;27:362–369. doi: 10.1038/leu.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dreger P, Schnaiter A, Zenz T, Böttcher S, Rossi M, Paschka P, et al. TP53, SF3B1, and NOTCH1 mutations and outcome of allotransplantation for chronic lymphocytic leukemia: six-year follow-up of the GCLLSG CLL3X trial. Blood. 2013;121:3284–3288. doi: 10.1182/blood-2012-11-469627. [DOI] [PubMed] [Google Scholar]
- 5.Jain N, O’Brien S. Initial treatment of CLL. integrating biology and functional status. Blood. 2015;126:463–470. doi: 10.1182/blood-2015-04-585067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheson BD, Rummel MJ. Bendamustine: rebirth of an old drug. J Clin Oncol. 2009;27:1492–1501. doi: 10.1200/JCO.2008.18.7252. [DOI] [PubMed] [Google Scholar]
- 7.Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicenter, randomized, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 8.Burchardt A, Barth J, Rummel MJ, et al. Immunochemotherapy with bendamustine-rituximab (BR) as induction therapy for indolent lymphomas results in a severe lymphopenia with low CD4 amd CD8 counts without an increase in atypical infections. First results of the infectious disease (ID) project of a prospective, randomized, multicentre study [abstract] Hematol Oncol. 2013;31(Suppl 1):032a. [Google Scholar]
- 9.Laurenti L, Innocenti I, Autore F, Vannata B, Efremov DG, Ciolli S, et al. Bendamustine in combination with rituximab for elderly patients with previously untreated b-cell chronic lymphocytic leukemia: a retrospective analysis of real-life practice in Italian hematology departments. Leukemia Res. 2015;39:1066–1070. doi: 10.1016/j.leukres.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Khouri IF, Wei W, Korbling M, Turturro F, Ahmed S, Alousi A, et al. BFR (bendamustine, fludarabine, and rituximab) allogeneic conditioning for chronic lymphocytic leukemia/lymphoma: reduced myelosuppression and GVHD. Blood. 2014;124:2306–2312. doi: 10.1182/blood-2014-07-587519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khouri I, Gulbis A, Ledesma C, Korbling M, Turturro F, Bueso-Ramos C, et al. Feasibility of lenalidomide maintenance after nonmyeloablative allogeneic transplantation (NMAT) in chronic lymphocytic leukemia [abstract] Hematologica. 2015;100(Suppl 1):P716. [Google Scholar]
- 12.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2012;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, et al. Long term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127:208–215. doi: 10.1182/blood-2015-06-651125. [DOI] [PubMed] [Google Scholar]
- 15.Fischer K, Cramer P, Busch R, Böttcher S, Bahlo J, Schubert J, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30:3209–3216. doi: 10.1200/JCO.2011.39.2688. [DOI] [PubMed] [Google Scholar]
- 16.Flinn IW, van der Jagt R, Kahl BS, Wood B, Hawkins TE, Macdonald D, et al. Randomized trial of bendamustine-rituximab or R-CHOP/RCVP in first-line treatment advanced indolent NHL or MCL: the BRIGHT study. Blood. 2014;123:2944–2952. doi: 10.1182/blood-2013-11-531327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz RG, Izquierdo-Gil A, Muñoz A, Roldan-Galiacho V, Rabasa P, Panizo C. Lymphocyte recovery is impaired in patients with chronic lymphocytic leukemia and indolent non-Hodgkin lymphomas treated with bendamustine plus rituximab. Ann Hematol. 2014;93:1879–1887. doi: 10.1007/s00277-014-2135-8. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Okamoto M, Ando M, Kakumae Y, Okamoto A, Inaguma Y, et al. Influence of rituximab plus bendamustine chemotherapy on the immune system in patients with refractory or relapsed follicular lymphoma and mantle cell lymphoma. Leuk Lymphoma. 2015;56:1123–1125. doi: 10.3109/10428194.2014.921298. [DOI] [PubMed] [Google Scholar]
- 19.Hosoda T, Yokoyama A, Yoneda M, Yamamoto R, Ohashi K, Kagoo T, et al. Bendamustine can severely impair T cell immunity against cytomegalovirus. Leuk Lymphoma. 2013;54:1327–1328. doi: 10.3109/10428194.2012.739285. [DOI] [PubMed] [Google Scholar]