Abstract
Cerebrovascular disease (CVD) and amyloid burden are the most frequent pathologies in subjects with cognitive impairment. However, the relationship between CVD, amyloid burden, and cognition are largely unknown. We aimed to evaluate whether CVD (lacunes, white matter hyperintensities, and microbleeds) and amyloid burden (Pittsburgh compound B [PiB] retention ratio) contribute to cognitive impairment independently or interactively. We recruited 136 patients with subcortical vascular cognitive impairment who underwent magnetic resonance imaging, PiB–positron emission tomography, and neuropsychological testing. The number of lacunes was associated with memory, frontal dysfunctions, and disease severity. The volume of white matter hyperintensities and the PiB retention ratio were associated only with memory dysfunction. There was no direct correlation between CVD markers and PiB retention ratio except that the number of lacunes was negatively correlated with the PiB retention ratio. In addition, there were no interactive effects of CVD and PiB retention ratio on cognition. Our findings suggest that CVD and amyloid burden contribute independently and not interactively to specific patterns of cognitive dysfunction in patients with subcortical vascular cognitive impairment.
Keywords: Cerebrovascular disease, Amyloid, Cognition, White matter hyperintensity, Lacune, Microbleed, Pittsburgh compound B
1. Introduction
Subcortical vascular cognitive impairment (SVCI) includes vascular dementia and mild cognitive impairment (MCI) of the subcortical type (Kim et al., 2011). SVCI is characterized by extensive cerebrovascular disease (CVD) including lacunes, white matter hyperintensities (WMHs), or microbleeds (MBs) (Roman et al., 2002). A recent study using Pittsburgh compound B (PiB)–positron emission tomography (PET), which detects the amyloid burden (Klunk et al., 2004), demonstrated that approximately 30% of clinically diagnosed subcortical vascular dementia patients had a significant amyloid burden (Lee et al., 2011).
Several studies have evaluated the relationships between CVD markers, especially with regard to frontal-subcortical circuit and cognitive impairment in patients with SVCI (Carey et al., 2008; Schmidt et al., 2005; Seo et al., 2007; Werring et al., 2004; Wright et al., 2008). However, these studies did not control for the confounding effects of amyloid deposition. More importantly, although most patients with cognitive impairments might have each CVD marker and an amyloid burden, there have been no studies to determine which imaging markers are the best predictors of the specific domains of cognition.
Cardiovascular risk factors including hypertension and diabetes mellitus are known to be important risk factors for Alzheimer’s disease (AD) and SVCI patients with dementia (SVaD; Jellinger, 2013). Preclinical studies have also suggested that there are correlations between CVD and Alzheimer’s pathologies (Hiltunen et al., 2009; Jablonski et al., 2011) and they have interactive effects on cognition (Choi et al., 2011; Jeon et al., 2011). However, the results are inconsistent among human studies (Chui et al., 2006; Hedden et al., 2012a; Rutten-Jacobs et al., 2011; Schneider et al., 2004).
In this study, we aimed to determine whether CVD markers (WMH, lacunes, and MB) and amyloid burden (PiB retention ratio) are independently associated with cognitive impairment. We also determined whether CVD markers correlated with the PiB retention ratio. Furthermore, we tested hypotheses concerning the possible interactive effects of CVD and amyloid beta burden on cognitive impairment.
2. Methods
2.1. Participants
We consecutively recruited 190 patients with SVCI at Samsung Medical Center from September 2008 to August 2011. Patients with SVCI met the following criteria: (1) the patient or his or her caregiver reported a subjective cognitive complaint; (2) neuropsychological testing revealed an objective cognitive decline in any domain including attention, language, visuospatial, memory, or frontal function below the 16th percentile; and (3) the patient had a subcortical vascular feature associated with both a focal neurologic symptom or sign and significant ischemia on magnetic resonance imaging (MRI) scan. SVCI patients were classified as having either MCI (svMCI) or dementia (SVaD) according to impairment in Activities of Daily Living (ADL; Seoul Instrumental ADL score less than 7) (Lawton and Brody, 1969). All SVCI patients had significant ischemia on their MRI scans, defined as a cap or band ≥10 mm and a deep white matter lesion of ≥25 mm based on a modification of Fazekas ischemia criteria (Supplementary Fig. 1). We excluded patients with high-signal abnormalities on MRI scan because of radiation injury, multiple sclerosis, vasculitis, or leukodystrophy, using their clinical history and other information such as blood test results, if necessary.
All patients completed a 3-step diagnostic process, as described previously (Lee et al., 2011). Briefly, patients first completed a medical interview with medical history and history of cognitive, behavioral, and functional impairments and underwent neurologic examinations, including the Mini-Mental State Examination and Clinical Dementia Rating–Sum of Boxes (CDR-SOB). An experienced neurologist performed both assessments. Second, the neuropsychology team performed a number of neuropsychological tests and conducted a clinical interview for cognitive, behavioral, and functional impairments using semi-structured questionnaires. Additionally, scales such as the Neuropsychiatric Inventory and ADL scales were completed. The ADL scale was a modified version of Lawton Instrumental ADL. Based on these measures, neuropsychologists classified patients into categories representing cognitively healthy individuals, those with subjective memory impairment, MCI, and dementia. Third, patients were diagnosed using appropriate diagnostic tests (neuropsychological reports, blood tests, and MRI scans). Blood tests included a complete blood count, blood chemistry, vitamin B12 and folate levels, syphilis serology, thyroid function tests, and apolipoprotein E (APOE) genotyping.
We defined vascular risk factors based on clinical history, physical examination (blood pressure), and laboratory tests (glucose level, hemoglobin A1c, and lipid tests). A history of stroke was considered present when the patient had a clinical history and possible corresponding stroke lesions on MRI scan.
Among the 190 patients with SVCI, 54 patients were excluded because they or their caregivers chose not to participate in the study. Therefore, 136 patients with SVCI who consisted of 66 svMCI patients and 70 SVaD patients were included. Of which, 45 SVaD patients have been previously described with regard to their clinical characteristics and [11C] PiB-PET findings (Lee et al., 2011). Results of a comparison between included and excluded patients are listed in Supplementary Table 1. We obtained written consent from each patient, and the Institutional Review Board of Samsung Medical Center approved the study protocol.
2.2. Neuropsychological testing
All patients underwent neuropsychological testing using the Seoul Neuropsychological Screening Battery (Ahn et al., 2010, 2011). This screening contains quantitative tests which allow evaluation of attention (digit span [forward and backward]), language (Korean version of the Boston Naming Test; Kim and Na, 1999), visuospatial function (Rey-Osterrieth Complex Figure Test [RCFT]), visual memory (RCFT; immediate and 20-minute delayed recall and recognition), verbal memory (Seoul Verbal Learning Test [SVLT; 3 learning-free recall trials of 12 words, a 20-minute delayed recall trial for these 12 items, and a recognition test]), frontal function (phonemic and semantic Controlled Oral Word Association Test (COWAT), a Stroop Test [word and color reading of 112 items during a 2-minute period]), Mini-Mental State Examination, and CDR-SOB.
2.3. MRI techniques
T2, T1, 3-D fluid attenuated inversion recovery (FLAIR), T2* gradient-echo (GRE), and 3-D T1 Turbo Field Echo magnetic resonance images were acquired using the same 3.0 T MRI scanner (Philips 3.0T Achieva). 3-D FLAIR magnetic resonance images were acquired in the axial plane with the following parameters: axial slice thickness, 2 mm; no gap; repetition time, 11000.0 ms; echo time,125.0 ms; flip angle, 90°; and matrix size, 512 × 512 pixels. T2* GRE magnetic resonance images were obtained using the following parameters: axial slice thickness, 5.0 mm; interslice thickness, 2 mm; repetition time, 669 ms; echo time 16 ms; flip angle, 18°; and matrix size, 560 × 560 pixels.
2.4. Assessment of lacunes and MB on MRI
Lacunes were defined as small lesions (≤15 mm and ≥3 mm in diameter) with low signal on T1-weighted images, high signal on T2-weighted images, and a perilesional halo on 80 axial slices of FLAIR images. MBs were defined as ≤10 mm in diameter using criteria proposed by Greenberg et al. (2009) on 20 axial slices of T2* GRE–magnetic resonance images. The number of lacunes and MBs were counted in 4-lobar white matter (frontal, parietal, temporal, and occipital), thalamus, basal ganglia, and infratentorial regions. Lacunes or MBs in the thalamus and basal ganglia were incorporated into the frontal region because the thalamus and basal ganglia are part of the frontal-subcortical circuit.
Two experienced neurologists, who were blinded to other patient data, reviewed the number and location of lacunes and MBs. The rate of agreement between the 2 neurologists was 83.0% for lacunes and 92.3% for MBs, and consensus was reached in all cases of discrepancy.
2.5. Measurement of regional WMH volume
We quantified WMH volume (in mL) on FLAIR images using an automated method as previously described (Kim et al., 2013). Extracted WMHs were localized and quantified according to the brain lobes (frontal, parietal, temporal, and occipital) using application of prelabeled 3-D probabilistic anatomical atlases using a nonlinear registration-based technique. Unfortunately, we could not analyze the volume of WMH in each lobe in 2 of 136 patients because of technical problems. Eventually, the WMHs in the thalamus and basal ganglia were incorporated into the frontal region.
2.6. [11C] PiB-PET analysis
All patients completed the [11C] PiB-PET scan at Samsung Medical Center or Asan Medical Center. All subjects completed the same type of PET scan with a Discovery STe PET/CT scanner (GE Medical Systems, Milwaukee, WI, USA). The detailed radiochemistry profiles and scanning protocol were described in a previous study (Lee et al., 2011).
PiB-PET images were coregistered to individual magnetic resonance images, which were normalized to a T1-weighted MRI template. Using these parameters, MRI-coregistered PiB-PET images were normalized to the MRI template. The quantitative regional values of PiB retention on the spatially normalized PiB images were obtained using an automated volume of interest (VOI) analysis using the automated anatomical labeling atlas. Data processing was performed using SPM, version 2, (SPM2) under Matlab 6.5 (Mathworks, Natick, MA, USA). To measure PiB retention, we used the cerebral cortical region–to–cerebellum uptake ratio, which is identical to the standardized uptake value ratios (SUVRs). The cerebellum was used as a reference region because the cerebellar cortex shows low PiB retention (Lee et al., 2011). We selected 28 cortical VOIs each from the left and right hemispheres using the automated anatomical labeling atlas. The cerebral cortical VOIs chosen for this study consisted of bilateral frontal (superior and middle frontal gyri, medial part of the superior frontal gyrus, opercular part of the inferior frontal gyrus, triangular part of the inferior frontal gyrus, supplementary motor area, orbital part of the superior, middle, and inferior orbital frontal gyri, rectus, and olfactory cortex), posterior cingulate gyri, parietal (superior and inferior parietal, supramarginal and angular gyri, and precuneus), lateral temporal (superior, middle, and inferior temporal gyri and heschl gyrus), and occipital (superior, middle, and inferior occipital gyri, cuneus, calcarine fissure, and lingual and fusiform gyri). Regional cerebral cortical SUVRs were calculated by dividing each cortical VOI’s SUV by the mean SUV of the cerebellar cortex (cerebellum crus 1 and crus 2). The global PiB uptake ratio was calculated from the volume-weighted average SUVR of 28 bilateral cerebral cortical VOIs (Lee et al., 2011). We defined the PiB uptake ratio as a continuous variable.
2.7. Statistical analysis
The relationships between the number of lacunes and MBs and WMH volume were evaluated using multiple linear regression analysis after adjusting for possible confounders including age, sex, hypertension, and APOE genotype. Those imaging markers were transformed to log scale.
To explore the association of each imaging markers (WMH, lacunes, MB, or global cortical PiB retention ratio) with neuropsychological results, the generalized linear model with a negative binomial distribution was used because of the skewed distribution of the neuropsychological test results. We used age, sex, education, existence of hypertension, APOE genotypes, WMH, lacunes, MB, and global cortical PiB retention ratio as independent variables.
To evaluate the regional specificity of imaging variables (lacunes, WMH, and PiB retention ratio), imaging variables for the frontal, temporal, or parietal regions were used as predictors in this model instead of imaging variables for the entire brain. The imaging variables in occipital regions were not included in the analyses because the occipital region has little influence on the results of neuropsychological test results.
Interaction terms (the number of lacunes or WMH by PiB retention ratio) were added to the model to evaluate the potential interactive effects of the number of lacunes or WMH volume and PiB retention ratio on cognitive impairments.
The relationships between CVD markers and PiB retention ratio were evaluated using multiple linear regression analysis after adjusting for possible confounders including age, sex, hypertension, and APOE genotype. We calculated the effect size using Fisher z transformation of r. We defined statistical significance as a corrected false discovery rate (FDR) of p < 0.05. Statistical analyses were conducted with SAS software, version 9.1, (SAS Institute Inc, Cary, NC, USA).
3. Results
3.1. Patient characteristics
Of the 136 patients with SVCI, 123 (90.4%) had lacunes (median, 7; interquartile range, 2–16), and 90 (66.2%) had MBs (median, 2; interquartile range, 0–8). The mean WMH volume was 38.5 ± 17.1 mL. The mean global cortical PiB retention ratio was 1.5 ± 0.4 (range, 0.96–2.85). According to the PiB cutoff value of 1.5 (PiB retention ratio of 2 standard deviations greater than the normal population; Lee et al., 2011), 45 (33.1%) patients had a “high” PiB burden and 91 (66.9%) had CVD in the absence of a high amyloid burden. Detailed demographic characteristics, imaging markers, and neuropsychological test results are presented in Table 1.
Table 1.
Demographic, clinical, MRI, and neuropsychological characteristics of the SVCI patients
| Characteristic | Total subjects (n = 136) |
|---|---|
| Demographic characteristic | |
| Age, ya | 73.8 ± 6.8 |
| Sex, male:female | 53:77 |
| Education, y | 9 ± 5.3 |
| MCI:dementia, n | 66:70 |
| Cardiovascular risk factor, n (%) | |
| Hypertension | 104 (76.5) |
| Diabetes mellitus | 34 (25) |
| Hyperlipidemia | 47 (34.6) |
| Heart disease | 25 (18.4) |
| Stroke | 33 (24.3) |
| APOE, n (percentage of subjects examined)b | |
| ε2ε3 | 17 (12.8) |
| ε3ε3 | 80 (60.2) |
| ε3ε4 | 32 (24.1) |
| ε4ε4 | 4 (3.0) |
| MRI marker | |
| WMH, mL | 38.6 ± 17.1 |
| Frontal/parietal/temporal/ occipital (n = 134) | 24.3 ± 10.5/10.1 ± 6.0/3.7 ± 1.9/0.4 ± 0.5 |
| Lacunes, total, n | 12.0 ± 13.9 |
| Frontal/parietal/temporal/occipital | 9.4 ± 11.0/1.5 ± 3.8/0.3 ± 1.0/ 0.3 ± 1.0 |
| Microbleeds, total, n | 8.0 ± 15.4 |
| Frontal/parietal/temporal/ occipital | 3.7 ± 6.9/0.9 ± 2.3/1.5 ± 3.5/0.7 ± 1.9 |
| PiB-PET | |
| Global cortical PiB retention ratio | 1.5 ± 0.4 |
| Frontal/parietal/temporal/ cingulate | 1.5 ± 0.5/1.4 ± 0.5 /1.5 ± 0.4 /1.7 ± 0.5 |
| Neuropsychological testing | |
| Attention | |
| Digit span: forward (8) | 5.0 ± 1.2 |
| Digit span: backward (8) | 2.9 ± 1.2 |
| Language and related disorder | |
| K-BNT (60) | 34.7 ± 11.6 |
| Calculation (12) | 9.4 ± 3.2 |
| Praxis (5) | 3.7 ± 1.5 |
| Visuospatial function | |
| RCFT (36) | 23.5 ± 10 |
| Memory | |
| SVLT immediate recall (36) | 13.6 ± 5.7 |
| SVLT delayed recall (12) | 2.7 ± 2.8 |
| SVLT recognition (24) | 17.9 ± 3.3 |
| RCFT immediate recall (36) | 7 ± 6.2 |
| RCFT delayed recall (36) | 6.4 ± 6 |
| RCFT recognition (24) | 17.6 ± 3.2 |
| Frontal/executive function | |
| COWAT, animal | 9.4 ± 4.1 |
| COWAT, supermarket | 9.5 ± 5.6 |
| COWAT, phonemic | 11.7 ± 8.6 |
| Stroop test: letter reading (112) | 91.6 ± 32.6 |
| Stroop test: color reading (112) | 43.1 ± 30.1 |
| Mini-mental status examination | 23.3 ± 4.9 |
| CDR, Sum of Boxes | 3.8 ± 3.7 |
Highest possible score on each neuropsychological test is indicated in parentheses. Key: CDR, Clinical Dementia Rating; COWAT, Controlled Oral Word Association Test; K-BNT, Korean version of the Boston Naming Test; MCI, mild cognitive impairment; MRI, magnetic resonance imaging; PET, positron emission tomography; PiB, Pittsburgh compound B; RCFT, Rey-Osterrieth Complex Figure Test; SVCI, subcortical vascular cognitive impairment; SVLT, Seoul Verbal Learning Test; WMH, white matter hyperintensity.
Continuous variables are presented as mean ± SD.
APOE genotyping was only performed in 133 patients because 3 patients refused the test.
3.2. Relationships between CVD markers
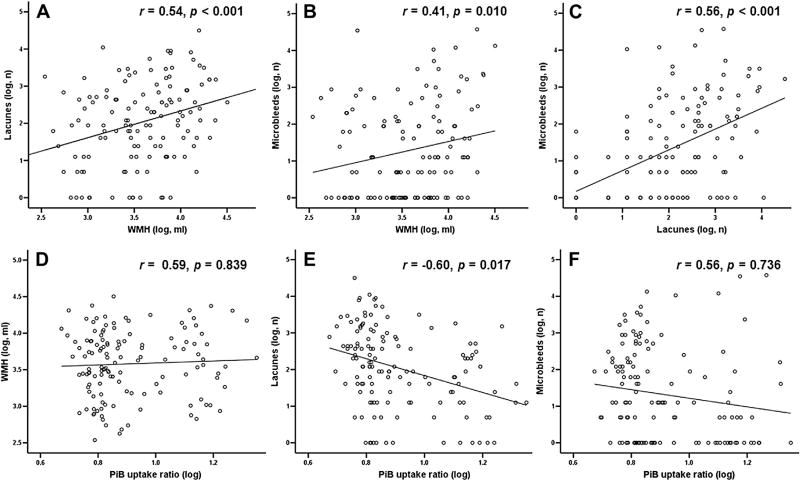
As shown in Fig. 1, there were significant correlations between lacunes, WMHs, and MBs (lacune and WMH: effect size [r] = 0.54, p < 0.001, Fig.1A; MB and WMH: r = 0.41, p = 0.010, Fig. 1B; MB and lacune: r = 0.56, p < 0.001, Fig.1C; in which the first variable of each combination was the dependent variable).
Fig. 1.
Relationships among WMH, lacunes, microbleeds, and PiB retention ratio. Multiple linear regression models were adjusted for age, sex, hypertension, and APOE genotype. There were significant correlations between CVD magnetic resonance imaging markers (WMH, lacunes, and microbleeds; A–C) and no positive correlation between CVD markers and global cortical PiB retention ratio (D–F). Independent variables are located on the x-axis, and dependent variables on the y-axis. Abbreviations: CVD, cerebrovascular disease; PiB, Pittsburgh compound B; r, effect sizes (calculated with Fisher z transformation); WMH, white matter hyperintensities.
3.3. Effects of CVD markers or PiB retention on cognitive impairment
The number of lacunes was associated with verbal memory (immediate and delayed recall of SVLT), frontal/executive function (semantic [animal and supermarket] COWAT and Stroop color reading), and CDR-SOB (Table 2). WMH volume was independently associated with visual memory (RCFT delayed recall). There was no association between the number of MBs and any of the neuropsychological test results. The PiB retention ratio was associated with visual memory (delayed recall of SVLT and RCFT).
Table 2.
Multivariate analyses of the number of lacunes, PiB retention ratio, and neuropsychological results
| Neuropsychological test (dependent variable) |
Imaging marker (independent variable) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lacune | WMH | Microbleed | PiB retention ratio | |||||
|
|
|
|
|
|||||
| B (SE) | p value | B (SE) | p value | B (SE) | p value | B (SE) | p value | |
| Attention | ||||||||
| Digit span: forward | −0.002 (0.004) | 0.657 | −0.003 (0.003) | 0.230 | 0.001 (0.003) | 0.746 | 0.098 (0.108) | 0.366 |
| Digit span: backward | −0.008 (0.005) | 0.129 | −0.003 (0.003) | 0.376 | −0.004 (0.004) | 0.383 | 0.157 (0.14) | 0.261 |
| Language | ||||||||
| K-BNT | −0.005 (0.002) | 0.040 | −0.003 (0.002) | 0.070 | 0.001 (0.002) | 0.875 | −0.167 (0.079) | 0.033 |
| Visuospatial function | ||||||||
| RCFT | 0.001 (0.004) | 0.914 | −0.006 (0.003) | 0.042 | −0.005 (0.002) | 0.031 | −0.078 (0.138) | 0.571 |
| Verbal memory | ||||||||
| SVLT immediate recall | −0.008 (0.002) | 0.001a | −0.001 (0.002) | 0.443 | 0.001 (0.002) | 0.615 | −0.171 (0.076) | 0.025 |
| SVLT delayed recall | −0.033 (0.009) | <0.001a | 0.002 (0.006) | 0.759 | −0.008 (0.008) | 0.313 | −0.879 (0.294) | 0.003a |
| SVLT recognition | −0.004 (0.002) | 0.029 | −0.001 (0.001) | 0.437 | 0.002 (0.002) | 0.108 | −0.14 (0.06) | 0.020 |
| Visual memory | ||||||||
| RCFT immediate recall | −0.007 (0.01) | 0.446 | −0.007 (0.009) | 0.451 | 0.007 (0.012) | 0.536 | −0.877 (0.377) | 0.020 |
| RCFT delayed recall | −0.004 (0.014) | 0.786 | −0.027 (0.009) | 0.003a | 0.004 (0.009) | 0.624 | −1.643 (0.491) | 0.001a |
| RCFT recognition | −0.004 (0.002) | 0.025 | −0.001 (0.001) | 0.491 | 0.002 (0.002) | 0.175 | −0.058 (0.059) | 0.329 |
| Frontal/executive function | ||||||||
| COWAT, animal | −0.011 (0.003) | 0.001a | −0.001 (0.002) | 0.596 | 0.003 (0.003) | 0.237 | −0.248 (0.101) | 0.014 |
| COWAT, supermarket | −0.013 (0.005) | <0.001a | −0.003 (0.003) | 0.359 | 0.001 (0.004) | 0.839 | −0.123 (0.139) | 0.376 |
| COWAT, phonemic | −0.013 (0.007) | 0.047 | −0.009 (0.005) | 0.069 | 0 (0.005) | 0.957 | −0.065 (0.202) | 0.749 |
| Stroop test: letter reading | −0.004 (0.004) | 0.413 | −0.005 (0.003) | 0.123 | 0.002 (0.004) | 0.520 | −0.042 (0.141) | 0.766 |
| Stroop test: color reading | −0.023 (0.007) | 0.002a | −0.011 (0.006) | 0.050 | 0.002 (0.006) | 0.720 | −0.178 (0.234) | 0.446 |
| CDR, Sum of Boxes | 0.027 (0.009) | 0.003a | 0.017 (0.007) | 0.008 | −0.004 (0.008) | 0.625 | 0.172 (0.255) | 0.500 |
Key: B, unstandardized regression coefficient; CDR, Clinical Dementia Rating; COWAT, Controlled Oral Word Association Test; K-BNT, Korean version of the Boston Naming Test; PiB, Pittsburgh compound B; RCFT, Rey-Osterrieth Complex Figure Test; SE, standard error; SVLT, Seoul Verbal Learning Test; WMH, white matter hyperintensity.
False discovery rate–corrected p value of <0.05. Generalized linear model with a negative binomial distribution after adding all imaging markers simultaneously as independent variables and adjustment for age, sex, education, existence of hypertension, and APOE genotype.
Most of the lacunes (82.7%) and white matter lesions (63.1%) were located in the frontal region (Table 1). Lacunes in the frontal region were associated with verbal memory (immediate and delayed recall of SVLT; p < 0.001 and p = 0.001, respectively, before FDR correction and p = 0.002 and 0.015, respectively, after FDR correction), frontal/executive function (semantic [animal and supermarket] COWAT and Stroop color reading; p = 0.001, 0.001, and 0.004, respectively, before FDR correction and p = 0.012, 0.019, and 0.031, respectively, after FDR correction), and CDR-SOB (p = 0.002 before FDR correction and p = 0.019 after FDR correction), and the presence of WMH in the frontal region was associated with visual memory (RCFT delayed recall; p < 0.001 before FDR correction and p = 0.013 after FDR correction; Supplementary Table 2). However, lacunes and WMH in other regions were not associated with any of the neuropsychological test results. PiB retention ratios for each region were associated with verbal and visual memory (delayed recall of SVLT and RCFT; [frontal] p = 0.007 and 0.003, respectively, before FDR correction and p = 0.047 and 0.031, respectively, after FDR correction; [parietal] p < 0.001 and 0.001 before FDR correction and p = 0.010 and 0.0004 after FDR correction; [temporal] p = 0.005 and 0.002 before FDR correction and p = 0.047 and 0.032 after FDR correction). In addition, the PiB retention ratio in the parietal region was additionally associated with frontal/executive function (semantic [animal] COWAT; p = 0.006 before FDR correction and p = 0.044 after FDR correction).
3.4. Relationships between CVD markers and PiB retention ratio
There was no positive association between any CVD marker and the PiB retention ratio. However, the number of lacunes was negatively correlated with PiB retention ratio (Fig. 1E; r = −0.60, p = 0.017, in which the number of lacunes was the dependent variable).
3.5. Interaction effects of CVD markers and PiB retention on cognition
No significant interactions were found for any of the neuropsychological tests (Supplementary Table 3).
4. Discussion
This study yielded two primary findings. First, CVD markers (WMH and lacunes) especially in the frontal region and amyloid burden were independently associated with specific cognitive impairments. Lacunes were related to memory and frontal executive dysfunctions and disease severity. WMH and PiB retention ratio affected only memory dysfunction. Second, there were no positive correlations between CVD and amyloid burden and no interactive effects on cognition. Taken together, these findings suggested that CVD markers (lacunes and WMH) and amyloid burden independently and not interactively contribute to cognitive impairments in specific domains.
Our first major finding was that CVD, particularly in the frontal region, independently affected cognition, irrespective of amyloid beta burden. Lacunes in the frontal region were associated with frontal dysfunction, which is known to be 1 of the characteristic symptoms of SVCI. However, there were no relationships between WMH and frontal dysfunction. Our findings are consistent with previous studies suggesting that the lacunes probably play a greater role in frontal dysfunction than the WMH (Jokinen et al., 2011; Marchant et al., 2013; Mungas et al., 2005). The differential effects between these 2 CVD markers might be because of differences in the nature of their pathogenesis. That is, lacunes result from complete obstruction of small arteries and therefore mainly reflect ischemia. However, stenosis or hypoperfusion of medullary arterioles leads to WMH. WMHs also result from other causes including demyelination or secondary degeneration because of AD or amyloid angiopathy (Goodkin et al., 1998; Gurol et al., 2013; Lee et al., 2009).
Lacunes and WMH also affected memory function in the present study. To our knowledge, few studies have evaluated the relationship between these 2 markers and memory function (Au et al., 2006; Benisty et al., 2009). Moreover, previous studies did not control for the possible effects of amyloid pathology. Our findings might be consistent with studies involving patients with CVD proven in autopsy (Reed et al., 2007), in which patients with vascular dementia had equal impairments of verbal and nonverbal memory and frontal function. The association of lacunes and WMH with memory dysfunction has not been fully clarified. However, our findings that the presence of lacunes and WMH, especially in the frontal region, was more strongly associated with poor performance on memory recall tests than that on recognition tests suggest that memory dysfunction related to these markers may be because of disrupted prefrontal-subcortical circuits that result in retrieval defects (Cummings, 1993).
In the present study, there was no association between MB and cognition after adjustment for PiB retention, which was not consistent with the results of our previous study (Seo et al., 2007). This discrepancy may be related to the fact that we did not exclude Cerebral amyloid angiopathy (CAA) in the previous study. It is also possible that the reduced frequency (84.9% vs. 69.3%) and burden (13 vs. 2) of MB compared with the previous study might have influenced the discrepancy between the previous and present results.
We also found that the amyloid burden independently contributed to memory function regardless of CVD markers. Our findings were not consistent with those of previous studies showing that CVD, not amyloid burden, is associated with cognitive impairment (Marchant et al., 2012, 2013). However, a meta-analysis based on cognitively normal subjects showed that amyloid burden was associated with episodic memory (Hedden et al., 2013). Moreover, a recent study showed that CVD and amyloid burden influence distinct cognitive domains (Hedden et al., 2012a).
Our second major finding was that there were no positive correlations between CVD markers and amyloid burden. This result is inconsistent with previous studies showing that patients with AD had a greater WMH burden than cognitively normal subjects (Brun and Englund, 1986) and increasing changes in WMH were greater in patients with AD than those in healthy control subjects (Carmichael et al., 2010). However, our findings are in line with the recent amyloid imaging studies showing that WMH volumes were not associated with amyloid burdens (Hedden et al., 2012a, 2012b; Marchant et al., 2012, 2013). Moreover, a study showed that WMH negatively correlated with amyloid burden (Provenzano et al., 2013). Along with this finding, our finding of a negative correlation between the number of lacunes and PiB retention ratio could be interpreted with previous pathologic studies showing that AD patients with CVD showed lower densities of plaques and tau pathology than those without CVD for a given level of cognitive deficit (Nagy et al., 1997; Snowdon et al., 1997; Zekry et al., 2002). However, more recent studies suggested that AD pathologies might be associated with coronary risk score (Reed et al., 2012) or WMH in patients with CAA (Gurol et al., 2013). Therefore, further studies focused on this issue are needed in patients with other forms of CVD or in specific populations.
Finally, we found no interactive effects of CVD and amyloid burden on cognition, which is consistent with other human studies (Hedden et al., 2012a; Marchant et al., 2012, 2013; Provenzano et al., 2013). A previous pathology study reported that cerebral infarcts independently contribute to the likelihood of dementia but do not interact with AD pathology (Schneider et al., 2004). Recent PiB-PET studies also revealed that the effects of CVD and PiB retention do not interact with regard to cognition (Hedden et al., 2012a; Marchant et al., 2012, 2013; Provenzano et al., 2013). Our findings that CVD markers (especially number of lacunes and WMH volume) and PiB retention independently affected memory dysfunction and that their effects were additive are also consistent with a pathology study that showed the additive effects of AD pathology and CVD on cognition (Chui et al., 2006).
The strengths of our study are its prospective design, standardized PiB-PET and MRI protocol, and standardized phenotyping of cognitive impairment. However, there are limitations to this study. First, the patients in this study had a high degree of CVD defined according to diagnostic criteria, which may limit the generalizability of our data to other populations. Because CVD and PiB retention were related to cognitive impairment, it is possible that in a population of patients with a lower burden of CVD, the effect of amyloid burden would have been stronger than that found in the present study. Second, use of the location and volume of lacunes and voxel-based lesion-symptom mapping of WMH rather than just volume or number would be more relevant for predicting the clinical impact of CVD (Duering et al., 2011). Therefore, further studies are needed to evaluate the location and volume of lacunes and voxel-based lesion symptom mapping in WMH. Third, we did not take into consideration other pathologies such as cortical microinfarct, tau pathology, or hippocampal sclerosis because we did not perform a pathologic study. Fourth, we did not correct the PiB retention ratio for atrophy, which might have affected the negative correlation between the number of lacunes and PiB retention ratio. Finally, because of the large number of tests and relatively small sample size in our study, the statistical power might be relatively low, and consequently, we may not have identified existing associations between several imaging variables and neuropsychological tests results. Therefore, large population-based longitudinal studies are needed to investigate the effects of CVD and amyloid on cognition throughout the population.
Nevertheless, this study demonstrates that CVD is associated with memory and frontal dysfunctions regardless of amyloid burdens. Furthermore, the amyloid burden in patients with SVCI did not correlate with CVD and independently contributed to memory dysfunction regardless of CVD.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Korean Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (number A120798, A102065 and A070001), Korean Science and Engineering Foundation (KOSEF) NRL program grant funded by the Korean government (MEST; 2011-0028333), Samsung Medical Center Clinical Research Development Program grant (CRL-108011 and CRS 110-14-1), and Converging Research Center Program through the Ministry of Education, Science and Technology (2010K001054).
Footnotes
Disclosure statement
The authors have no actual or potential conflicts of interest. The Institutional Review Board of Samsung Medical Center approved the study protocol.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neurobiolaging.2013.06.026.
References
- Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, Na DL. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J. Korean Med. Sci. 2010;25:1071–1076. doi: 10.3346/jkms.2010.25.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn HJ, Seo SW, Chin J, Suh MK, Lee BH, Kim ST, Im K, Lee JM, Lee JH, Heilman KM, Na DL. The cortical neuroanatomy of neuropsychological deficits in mild cognitive impairment and Alzheimer’s disease: a surface-based morphometric analysis. Neuropsychologia. 2011;49:3931–3945. doi: 10.1016/j.neuropsychologia.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, D’Agostino RB, DeCarli C. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch. Neurol. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- Benisty S, Gouw AA, Porcher R, Madureira S, Hernandez K, Poggesi A, van der Flier WM, Van Straaten EC, Verdelho A, Ferro J, Pantoni L, Inzitari D, Barkhof F, Fazekas F, Chabriat H. Location of lacunar infarcts correlates with cognition in a sample of non-disabled subjects with age-related white-matter changes: the LADIS study. J. Neurol. Neurosurg. Psychiatry. 2009;80:478–483. doi: 10.1136/jnnp.2008.160440. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann. Neurol. 1986;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- Carey CL, Kramer JH, Josephson SA, Mungas D, Reed BR, Schuff N, Weiner MW, Chui HC. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke. 2008;39:397–402. doi: 10.1161/STROKEAHA.107.491795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR, Jr, Weiner M, DeCarli C Alzheimer’s Disease Neuroimaging Initiative. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch. Neurol. 2010;67:1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BR, Lee SR, Han JS, Woo SK, Kim KM, Choi DH, Kwon KJ, Han SH, Shin CY, Lee J, Chung CS, Kim HY. Synergistic memory impairment through the interaction of chronic cerebral hypoperfusion and amlyloid toxicity in a rat model. Stroke. 2011;42:2595–2604. doi: 10.1161/STROKEAHA.111.620179. [DOI] [PubMed] [Google Scholar]
- Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, Jagust WJ, Mungas D, Reed BR, Kramer JH, Decarli CC, Weiner MW, Vinters HV. Cognitive impact of subcortical vascular and Alzheimer’s disease pathology. Ann. Neurol. 2006;60:677–687. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch. Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Duering M, Zieren N, Herve D, Jouvent E, Reyes S, Peters N, Pachai C, Opherk C, Chabriat H, Dichgans M. Strategic role of frontal white matter tracts in vascular cognitive impairment: a voxel-based lesion-symptom mapping study in CADASIL. Brain. 2011;134:2366–2375. doi: 10.1093/brain/awr169. [DOI] [PubMed] [Google Scholar]
- Goodkin DE, Rooney WD, Sloan R, Bacchetti P, Gee L, Vermathen M, Waubant E, Abundo M, Majumdar S, Nelson S, Weiner MW. A serial study of new MS lesions and the white matter from which they arise. Neurology. 1998;51:1689–1697. doi: 10.1212/wnl.51.6.1689. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurol ME, Viswanathan A, Gidicsin C, Hedden T, Martinez-Ramirez S, Dumas A, Vashkevich A, Ayres AM, Auriel E, van Etten E, Becker A, Carmasin J, Schwab K, Rosand J, Johnson KA, Greenberg SM. Cerebral amyloid angiopathy burden associated with leukoaraiosis: a positron emission tomography/magnetic resonance imaging study. Ann. Neurol. 2013;73:529–536. doi: 10.1002/ana.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Buckner RL, Johnson KA, Sperling RA, Rentz DM. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J. Neurosci. 2012a;32:16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Shire EH, Sperling RA, Johnson KA, Buckner RL. Failure to modulate attentional control in advanced aging linked to white matter pathology. Cereb. Cortex. 2012b;22:1038–1051. doi: 10.1093/cercor/bhr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen M, Makinen P, Peraniemi S, Sivenius J, van Groen T, Soininen H, Jolkkonen J. Focal cerebral ischemia in rats alters APP processing and expression of Abeta peptide degrading enzymes in the thalamus. Neurobiol. Dis. 2009;35:103–113. doi: 10.1016/j.nbd.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Jablonski M, Maciejewski R, Januszewski S, Ulamek M, Pluta R. One year follow up in ischemic brain injury and the role of Alzheimer factors. Physiol. Res. 2011;60(suppl 1):S113–S119. doi: 10.33549/physiolres.932186. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci. 2013;5:17. doi: 10.3389/fnagi.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S, Yoon U, Park J-S, Seo SW, Kim J-H, Kim ST, Kim SI, Na DL, Lee J-M. Fully automated pipeline for quantification and localization of white matter hyperintensity in brain magnetic resonance image. Int. J. Imag. Syst. Tech. 2011;21:193–200. [Google Scholar]
- Jokinen H, Gouw AA, Madureira S, Ylikoski R, van Straaten EC, van der Flier WM, Barkhof F, Scheltens P, Fazekas F, Schmidt R, Verdelho A, Ferro JM, Pantoni L, Inzitari D, Erkinjuntti T. Incident lacunes influence cognitive decline: the LADIS study. Neurology. 2011;76:1872–1878. doi: 10.1212/WNL.0b013e31821d752f. [DOI] [PubMed] [Google Scholar]
- Kim H, Na DL. Normative data on the Korean version of the Boston Naming Test. J. Clin. Exp. Neuropsychol. 1999;21:127–133. doi: 10.1076/jcen.21.1.127.942. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kang SJ, Kim C, Kim GH, Jeon S, Lee JM, Oh SJ, Kim JS, Choe YS, Lee KH, Noh Y, Cho H, Yoon CW, Chin J, Cummings JL, Lee JH, Na DL, Seo SW. The effects of small vessel disease and amyloid burden on neuropsychiatric symptoms: a study among patients with subcortical vascular cognitive impairments. Neurobiology of Aging. 2013;34:1913–1920. doi: 10.1016/j.neurobiolaging.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Kim SH, Park JS, Ahn HJ, Seo SW, Lee JM, Kim ST, Han SH, Na DL. Voxel-based analysis of diffusion tensor imaging in patients with subcortical vascular cognitive impairment: correlates with cognitive and motor deficits. J. Neuroimaging. 2011;21:317–324. doi: 10.1111/j.1552-6569.2010.00527.x. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Martinez O, Ortega M, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, DeCarli C. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology. 2009;73:1722–1728. doi: 10.1212/WNL.0b013e3181c33afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim SH, Kim GH, Seo SW, Park HK, Oh SJ, Kim JS, Cheong HK, Na DL. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology. 2011;77:18–25. doi: 10.1212/WNL.0b013e318221acee. [DOI] [PubMed] [Google Scholar]
- Marchant NL, Reed BR, DeCarli CS, Madison CM, Weiner MW, Chui HC, Jagust WJ. Cerebrovascular disease, beta-amyloid, and cognition in aging. Neurobiol. Aging. 2012;33:1006.e1025–1006.e1036. doi: 10.1016/j.neurobiolaging.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NL, Reed BR, Sanossian N, Madison CM, Kriger S, Dhada R, Mack WJ, Decarli C, Weiner MW, Mungas DM, Chui HC, Jagust WJ. The aging brain and cognition: contribution of vascular injury and abeta to mild cognitive dysfunction. JAMA Neurol. 2013;70:488–495. doi: 10.1001/2013.jamaneurol.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, Mack WJ, Kramer JH, Weiner MW, Schuff N, Chui HC. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald B, Joachim C, Litchfield S, Barnetson L, Smith AD. The effects of additional pathology on the cognitive deficit in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1997;56:165–170. doi: 10.1097/00005072-199702000-00007. [DOI] [PubMed] [Google Scholar]
- Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, Guzman VA, Meier IB, Zimmerman ME, Brickman AM Alzheimer’s Disease Neuroimaging Initiative. White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013;70:455–461. doi: 10.1001/jamaneurol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Marchant NL, Jagust WJ, DeCarli CC, Mack W, Chui HC. Coronary risk correlates with cerebral amyloid deposition. Neurobiology of Aging. 2012;33:1979–1987. doi: 10.1016/j.neurobiolaging.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Mungas DM, Kramer JH, Ellis W, Vinters HV, Zarow C, Jagust WJ, Chui HC. Profiles of neuropsychological impairment in autopsy-defined Alzheimer’s disease and cerebrovascular disease. Brain. 2007;130:731–739. doi: 10.1093/brain/awl385. [DOI] [PubMed] [Google Scholar]
- Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- Rutten-Jacobs LC, de Leeuw FE, Geurts-van Bon L, Gordinou de Gouberville MC, Schepens-Franke AN, Dederen PJ, Spliet WG, Wesseling P, Kiliaan AJ. White matter lesions are not related to beta-amyloid deposition in an autopsy-based study. Curr. Gerontol. Geriatr. Res. 2011;2011:826862. doi: 10.1155/2011/826862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Ropele S, Enzinger C, Petrovic K, Smith S, Schmidt H, Matthews PM, Fazekas F. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian stroke prevention study. Ann. Neurol. 2005;58:610–616. doi: 10.1002/ana.20630. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- Seo SW, Hwa Lee B, Kim EJ, Chin J, Sun Cho Y, Na DL, Yoon U. Clinical significance of microbleeds in subcortical vascular dementia. Stroke. 2007;38:1949–1951. doi: 10.1161/STROKEAHA.106.477315. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- Werring D, Frazer D, Coward L, Losseff N, Watt H, Cipolotti L, Brown M, Jager H. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127:2265. doi: 10.1093/brain/awh253. [DOI] [PubMed] [Google Scholar]
- Wright CB, Festa JR, Paik MC, Schmiedigen A, Brown TR, Yoshita M, DeCarli C, Sacco R, Stern Y. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39:800–805. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekry D, Duyckaerts C, Moulias R, Belmin J, Geoffre C, Herrmann F, Hauw JJ. Degenerative and vascular lesions of the brain have synergistic effects in dementia of the elderly. Acta Neuropathol. 2002;103:481–487. doi: 10.1007/s00401-001-0493-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.