Abstract
Objective
Determine if autoantibody titer magnitude and variability predict glucose abnormalities in subjects at risk for type 1 diabetes.
Research Designs and Methods
Demographic information, longitudinal autoantibody titers, and oral glucose tolerance test data were obtained from the TrialNet Pathway to Prevention study. Subjects (first and second degree relatives of individuals with type 1 diabetes) with at least 2 diabetes autoantibodies were selected for analysis. Autoantibody titer means were calculated for each subject for the duration of study participation and the relationship between titer tertiles and glucose value tertiles from oral glucose tolerance tests (normal, impaired and diabetes) was assessed with a proportional odds ordinal regression model. A matched pairs analysis was used to examine the relationship between changes in individual autoantibody titers and 120 minute glucose values. Titer variability was quantified using cumulative titer standard deviations.
Results
We studied 778 subjects recruited in the TrialNet Pathway to Prevention study between 2006–014. Increased cumulative mean titer values for both ICA512 and GAD65 (estimated increase in proportional odds = 1.61, 95% CI = 1.39, 1.87, p < 1×10−9 and 1.17, 95% CI = 1.03, 1.32, p = 0.016 respectively) were associated with peak 120-minute glucose values. While fluctuating titer levels were observed in some subjects, no significant relationship between titer standard deviation and glucose values was observed.
Conclusion
ICA512 autoantibody titers associate with progressive abnormalities in glucose metabolism in subjects at risk for type 1 diabetes. Fluctuations in autoantibody titers do not correlate with lower rates of progression to clinical disease.
Keywords: type 1 diabetes, autoantibodies, ICA512, GAD65
Introduction
Type 1 diabetes is a prototypical autoimmune disease with an asymptomatic prodrome characterized by the development of autoantibodies against islet antigens and the progressive loss of β-cell function (1). There are five autoantibodies associated with type 1 diabetes: islet cell cytoplasmic autoantibodies (ICA), insulin autoantibodies (IAA), islet cell cytoplasmic 512 autoantibodies (ICA512), glutamic acid decarboxylase antibodies (GAD65), and zinc transporter 8 antibodies (ZnT8). Multiple longitudinal studies have confirmed that increasing numbers of circulating autoantibodies are a strong predictor of progression to diabetes (2–4). Studies have been less consistent, however, when evaluating the impact of the levels or titers of individual autoantibodies on progression to clinical disease.
Early data on the first autoantibody assay, islet cell antigen (ICA), showed that quantity of autoantibody (i.e. titer), not just the presence of autoantibody, predicted development of type 1 diabetes (5). With the development of additional autoantibody assays such as GAD65 and ICA512, analyses of large prevention cohorts like the Diabetes Prevention Trial (DPT-1) demonstrated that titers fluctuate in the at-risk population (6). In the DPT-1, at-risk subjects who were ICA+ were enrolled to receive either oral insulin or parenteral insulin. In those that progressed to diabetes, it was observed that baseline GAD65 titers decreased while IA2 (now referred to as ICA512) titers increased at the time of diagnosis (6). This was true even when subjects were positive for both GAD65 and IA2 at baseline. A comparable analysis of non-progressors was not reported. Additional analysis of DPT-1 subjects with abnormal first phase insulin release (FPIR) demonstrated that higher titers of ICA512 and IAA correlated negatively, albeit weakly, with FPIR while titers of GAD65 did not (7).
A cross-sectional study by Bonifacio and Ziegler on a German cohort of first-degree relatives also demonstrated that autoantibody titers correlated with diabetes progression over 10 years, similar to the DPT-1. They observed that those with the higher titers of insulin autoantibodies (IAA) and IA2 were more likely to develop type 1 diabetes but GAD65 titer was not predictive (8). However, in the longitudinal DAISY study, subjects with higher autoantibody titers were more likely to remain positive but titer elevation did not seem to contribute to progression of disease (9).
Type 1 Diabetes TrialNet is an NIH sponsored clinical trial consortium that studies first and second degree relatives of individuals with type 1 diabetes. It collects longitudinal data with autoantibody and oral glucose tolerance test (OGTT) measurements on persons who are found to be antibody positive every 6–12 months thus providing an opportunity to assess antibody titers over time with respect to glucose tolerance and disease progression. Here we considered whether antibody titers in the TrialNet Pathway to Prevention study correlated with glucose tolerance and whether changes in an individual’s titers over time are predictive of disease progression. We further hypothesized that variations in autoantibody titers over time could be reflective of waxing and waning of disease activity.
Methods
Subjects, Autoantibodies and Metabolic Testing
Subject data collected in the TrialNet Pathway to Prevention study were analyzed. The TrialNet Pathway to Prevention Study has previously been referred to as the Natural History Study and has been well described (10–14). Briefly, subjects who are first-degree (up to age 45) or second degree (up to age 20) relatives of a proband with type 1 diabetes are eligible for annual screening with diabetes autoantibodies (GAD65, ICA512, and insulin (mIAA),). Any subject who is positive for one of these autoantibodies is also screened for the non-specific ICA and ZnT8. Starting in 2012, subjects positive for >= 2 autoantibodies are screened with an oral glucose tolerance test (OGTT) every 6 months. Prior to 2012, any subject with >=1 autoantibody was monitored every 6 months with an oral glucose tolerance test and serial autoantibody measurements. All antibodies were run in the central core lab and the Barbara Davis Center.
We analyzed subjects who were positive for at least 2 autoantibodies at some point during their follow up. This population was selected for analysis given the higher rate of progression to abnormal glucose tolerance tests in subjects with >1 autoantibody (15). Analysis of the predictive capacity of titers was limited to GAD65, ICA512, and insulin autoantibodies. ZnT8 and the so called harmonized GAD65 and ICA512 assays began to be collected (in addition to standard GAD65, ICA512, and insulin autoantibodies) several years into the Pathway to Prevention study, limiting the number of measurements per subject for these newer assays. Positivity to ZnT8 was used however to meet criteria for analysis (i.e. >= 2 autoantibodies).
The average titer score was calculated by averaging all of an individual’s titer scores (regardless of being designated positive or negative by the reference lab) during their follow up. Similarly cumulative standard deviations were calculated as the standard deviation of an individual’s titer scores during their follow up in the study. All subjects or their parent/guardianprovided informed consent prior to study enrollment. Children provided assent as required by the local institution.
Statistics
All statistical analysis was done in R. The multivariate proportional odds model utilized the R ordinal package developed by Christensen (16). The matched pairs data were analyzed using Wilcoxon rank tests (17).
Results
A total of 826 subjects with >= 2 autoantibodies were available for analysis of whom 57% were female. The mean age of entry and duration of follow up were 12 +/−14.1 years of age and 5.1+/−1.7 years respectively. The mean number of autoantibody measurements per subject with standard deviations for GAD65, ICA512, and mIAA were 6.3+/−3.6, 6.0+/−3.4, and 6.5+/−2.4 respectively. Of the 826 subjects, 46 subjects only had one observation with an oral glucose tolerance test and 2 subjects were missing glucose data from their OGTT tests. Therefore a total of 778 subjects were analyzed.
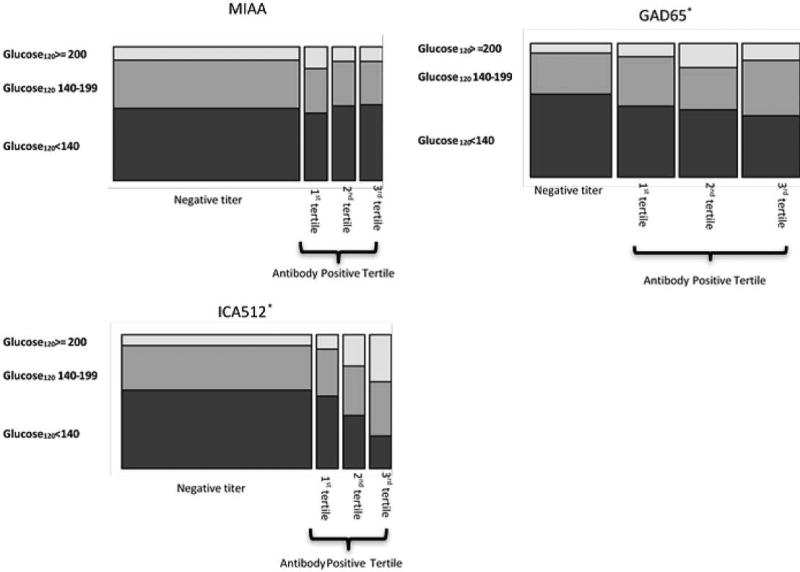
We first determined an individual’s mean titer level (referred to as the average titer score) for each autoantibody for the duration of the study by averaging their autoantibody titer results. All titer levels, regardless of “positive” or “negative” status, were included in the calculation. The average titer score for GAD65, ICA512, and mIAA were not normally distributed (Figure 1). Therefore, positive autoantibody titer values were divided into tertiles: bottom 33%, middle 33%, and upper 33%. Similarly, an individual’s highest 120 minute-glucose value during serial OGTTs for the duration of the study was placed into 3 categories: normal (glucose<140), impaired (glucose 140–199), and diabetes (>199). We then constructed a proportional odds ordinal regression model to study the relationship between cumulative autoantibody titer and peak 120 minute glucose tertiles. Average titer score tertiles for ICA512 (estimated increase in proportional odds = 1.61, 95% CI = 1.39–1.87, p < 1×10−9) and GAD65 (estimated increase in proportional odds = 1.17, 95% CI = 1.03–1.32, p = 0.016) correlated with greater likelihood of dysglycemia (Figure 2). Tertiles for mIAA did not correlate with OGTT results. This initial analysis demonstrated that higher cumulative titer levels of both ICA512 and GAD65 correlated with a greater dysglycemia (i.e. an impaired or diabetes range OGTT). Importantly, our model included tertiles for all three autoantibodies simultaneously to account for autoantibody number, suggesting that elevated titers, in addition to autoantibody number, are associated with abnormal glucose metabolism.
Figure 1.
Distribution of average titer scores in the study population. Average titer score refers to the average of a subject’s individual titer measurements (regardless of positive or negative status) for duration of the study. The Y axis represents number of subjects.
Figure 2.
Relationship between cumulative autoantibody titer means and peak 120 minute glucose value on oral glucose tolerance test during study follow up. On the x-axis are cumulative titer means classified as either negative (below core lab cutoff for titer positivity) or positive. Positive values are divided into tertiles. On the y-axis are peak glucose values tertiles at 120 minutes from all oral glucose tolerance tests. Boxes represent proportion of subjects in respective tertiles. *= p-value of relationship between titer tertiles and glucose120
We next performed a matched pairs analysis to compare individual changes in titers with OGTT results. We identified groups of patients that either progressed to have a diabetic range OGTT (cases) and those that did not (controls). The case group consisted of individuals with an initially normal OGTT who proceeded to have a 120 minute glucose value >199 on an OGTT at some point during their follow up. Controls had comparable duration of follow up but did not have abnormal OGTT results on follow up. Comparisons were made between autoantibody titers at the initial OGTT and the follow up OGTT. Of the 778 subjects with complete OGTT data, there were 90 cases and 688 controls (Table 1). When evaluating the difference between these two time points, we found that t ICA512 titer levels significantly increased in the case group over time compared to the control groups (Table 2). In contrast, titer levels for GAD65 and mIAA did not change significantly in either cases or controls between the initial value and the follow up OGTT.
Table 1.
Demographic and clinical data on cases (those with a diabetic range OGTT) and controls (those without a diabetic range OGTT) used in matched pairs analysis.
| Cases (n=90) | Controls (n=688) | |
|---|---|---|
| % Female | 52 | 57 |
| Median age, years (range) | 12 (1–45) | 13 (1–45) |
| Median number of OGTT results (range) | 8 (3–19) | 7 (2–18) |
Table 2.
Changes in titer level between initial OGTT and diabetic range OGTT in cases and controls.
| Mean difference between titers (SD) | p-value | |
|---|---|---|
| Cases | ||
| ICA512 | 0.185(0.443) | 0.009 |
| GAD65 | 0.04(0.406) | 0.305 |
| MIAA | 0.002(0.031) | 0.935 |
| Controls | ||
| ICA512 | 0.003(0.398) | 0.493 |
| GAD65 | −0.016(0. 149) | 0.149 |
| MIAA | 0.0003(0.079) | 0.471 |
Finally we examined the variation in antibody titers over time by evaluating the standard deviation of each individual’s cumulative antibody measurements. When comparing average titer scores levels with cumulative standard deviations, some subjects demonstrated elevated standard deviations even with similar average titer scores (Figure 3). To study the impact of standard deviation on development of abnormal OGTTs, we divided subjects with positive ICA512 titers into two groups: those in the upper 50th% of the range of standard deviations and those in the lower 50th% of the range of standard deviations. Subjects in the upper 50th percentile of the range of cumulative standard deviations demonstrated longitudinal fluctuations of autoantibody titers (Figure 3). Proportionally, there was no difference in diabetic range OGTT values between the two groups (p=1.00 CI 95th% −0.27–0.358).
Figure 3.
Relationship between cumulative titer mean and intra-subject titer standard deviation are shown for all 3 autoantibodies on the left with each circle representing an individual patient with their average titer score on the x-axis and their cumulative titer standard deviation on the y-axis. Representative longitudinal ICA512 titers from 10 random individuals with high standard deviations and 10 random individuals with low standard deviations are shown to illustrate titer fluctuations. Each individual is represented by a different color.
Discussion
Developing effective prevention strategies in type 1 diabetes require identifying individuals at high risk for developing disease. Increasingly, new models of risk prediction account for age, BMI, HbA1c and autoantibody titers and number (13, 14, 18). We observed that both cumulative mean titer levels of ICA512 and longitudinal increases in ICA512 titer levels correlate with abnormal OGTT results.
The correlation of ICA512 titers with abnormal OGTT results is important. It suggests cellular assays focused on key components of autoantibody formation may yield interesting insights into pathogenesis of type 1 diabetes. Specifically, the role of T follicular helper cells and their role in guiding B cell antibody formation would be particularly important to understand in light of this data (19). The lack of association of insulin autoantibody titer with abnormal glucose tolerance does not conflict with published data regarding the importance of insulin autoantibodies in development of type 1 diabetes (20). Our study looked at a different population namely those with multiple autoantibodies at some point during their follow up in the TrialNet Pathway to Prevention study. Unlike other studies, our cohort was not followed at birth so it is possible that the development of insulin autoantibodies in the very young might be especially important as has been published (20). Finally, given our observations of increased rates of abnormal oral glucose tolerance tests with increased ICA512 titers, it may be useful to utilize ICA512 titers in a post-hoc fashion to analyze outcomes in subjects from ongoing clinical trials that look to prevent development of dysglycemia or type 1 diabetes. The use of IA-2 tetramers may also be useful to better understand how changes in T-cells responses over time correlate with autoantibody responses (21).
In conclusion we demonstrate that autoantibody titers, particularly of ICA512, correlate with abnormal OGTT results in subjects at risk for disease. Future studies will investigate the utility of changes in autoantibody titers to predict outcomes in prevention studies of type 1 diabetes.
Supplementary Material
Acknowledgments
The sponsor of the trial was the Type 1 Diabetes TrialNet Study Group. Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085465, U01 DK085453, U01 DK085461, U01 DK085466, U01 DK085499, U01 DK085504, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK085476, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, UC4 DK106993, and the Juvenile Diabetes Research Foundation International (JDRF). The contents of this Article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF.
The authors thank Cliff Anderson-Bergman of the Gladstone Bioinformatics Core and Iryna Lobach of the UCSF CTSI for assistance with statistical analysis. The authors wish to acknowledge the participation of the subjects and families in the TrialNet Pathway to Prevention study. This study was funded by a JDRF grant: 2-SRA-2014-252-Q-R. This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR991872. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siljander HT, Simell S, Hekkala A, Lahde J, Simell T, Vahasalo P, et al. Predictive characteristics of diabetes-associated autoantibodies among children with HLA-conferred disease susceptibility in the general population. Diabetes. 2009;58(12):2835–42. doi: 10.2337/db08-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thivolet C, Nicolino M, Monbeig S, Estour B, Halimi S, Robert M, et al. Combination of autoantibody markers and risk for development of type 1 diabetes: results from a large french cohort of family members. Diabetes & metabolism. 2002;28(4 Pt 1):279–85. [PubMed] [Google Scholar]
- 4.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473–9. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonifacio E, Bingley PJ, Shattock M, Dean BM, Dunger D, Gale EA, et al. Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet. 1990;335(8682):147–9. doi: 10.1016/0140-6736(90)90013-u. PubMed PMID: 1967440. [DOI] [PubMed] [Google Scholar]
- 6.Sosenko JM, Skyler JS, Palmer JP, Krischer JP, Cuthbertson D, Yu L, et al. A longitudinal study of GAD65 and ICA512 autoantibodies during the progression to type 1 diabetes in Diabetes Prevention Trial-Type 1 (DPT-1) participants. Diabetes Care. 2011;34(11):2435–7. doi: 10.2337/dc11-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu P, Beam CA, Cuthbertson D, Sosenko JM, Skyler JS, Krischer JP, et al. Prognostic accuracy of immunologic and metabolic markers for type 1 diabetes in a high-risk population: receiver operating characteristic analysis. Diabetes Care. 2012;35(10):1975–80. doi: 10.2337/dc12-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achenbach P, Warncke K, Reiter J, Naserke HE, Williams AJ, Bingley PJ, et al. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes. 2004;53(2):384–92. doi: 10.2337/diabetes.53.2.384. [DOI] [PubMed] [Google Scholar]
- 9.Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, Norris JM, et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY) The Journal of clinical endocrinology and metabolism. 2004;89(8):3896–902. doi: 10.1210/jc.2003-031887. [DOI] [PubMed] [Google Scholar]
- 10.Mahon JL, Sosenko JM, Rafkin-Mervis L, Krause-Steinrauf H, Lachin JM, Thompson C, et al. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatric diabetes. 2009;10(2):97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 11.Sosenko JM, Mahon J, Rafkin L, Lachin JM, Krause-Steinrauf H, Krischer JP, et al. A comparison of the baseline metabolic profiles between Diabetes Prevention Trial-Type 1 and TrialNet Natural History Study participants. Pediatric diabetes. 2011;12(2):85–90. doi: 10.1111/j.1399-5448.2010.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vehik K, Beam CA, Mahon JL, Schatz DA, Haller MJ, Sosenko JM, et al. Development of autoantibodies in the TrialNet Natural History Study. Diabetes Care. 2011;34(9):1897–901. doi: 10.2337/dc11-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosenko JM, Skyler JS, DiMeglio LA, Beam CA, Krischer JP, Greenbaum CJ, et al. A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care. 2015;38(2):271–6. doi: 10.2337/dc14-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sosenko JM, Skyler JS, Beam CA, Boulware D, Mahon JL, Krischer JP, et al. The development and utility of a novel scale that quantifies the glycemic progression toward type 1 diabetes over 6 months. Diabetes Care. 2015;38(5):940–2. doi: 10.2337/dc14-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes care. 2015;38(10):1964–74. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agresti A. Categorical data analysis. 2. xv. New York: Wiley-Interscience; 2002. p. 710. [Google Scholar]
- 17.Hollander M, Wolfe DA. Nonparametric statistical methods. xviii. New York: Wiley; 1973. p. 503. [Google Scholar]
- 18.Sosenko JM, Skyler JS, Mahon J, Krischer JP, Beam CA, Boulware DC, et al. Validation of the Diabetes Prevention Trial-Type 1 Risk Score in the TrialNet Natural History Study. Diabetes Care. 2011;34(8):1785–7. doi: 10.2337/dc11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Ing S, Fraser A, Chen M, Khan O, Zakem J, et al. Follicular helper T cells: new insights into mechanisms of autoimmune diseases. Ochsner J. 2013;13(1):131–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Giannopoulou EZ, Winkler C, Chmiel R, Matzke C, Scholz M, Beyerlein A, et al. Islet autoantibody phenotypes and incidence in children at increased risk for type 1 diabetes. Diabetologia. 2015;58(10):2317–23. doi: 10.1007/s00125-015-3672-y. [DOI] [PubMed] [Google Scholar]
- 21.Acevedo-Calado M, James EA, Morran MP, Pietropaolo SL, Ouyang Q, Arribas-Layton D, et al. Identification of Unique Antigenic Determinants in the Amino Terminus of IA-2 (ICA512) in Childhood and Adult Autoimmune Diabetes: New Biomarker Development. Diabetes Care. 2017 doi: 10.2337/dc16-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.