Abstract
Background
Eosinophils in blood and sputum in chronic obstructive pulmonary disease (COPD) have been associated with more frequent exacerbations, lower lung function, and corticosteroid responsiveness. We hypothesized increased eosinophils are associated with a severe COPD phenotype, including exacerbation frequency, and tested whether blood eosinophils reliably predict sputum eosinophils.
Methods
Comprehensive baseline data on SPIROMICS subjects, recruited for a range of COPD severity for smokers with ≥20 pack year history, included demographics, questionnaires, clinical assessments, quantitative computed tomography (QCT), blood and induced sputum.
Findings
Significantly, stratification by mean sputum eosinophils ≥1·25% (N=827) was associated with reduced FEV1 % predicted (differences: 10% pre-bronchodilator, 4·7% post-bronchodilator), QCT density measures for emphysema and air trapping, and exacerbations treated with corticosteroids (p=0·002). In contrast, stratification by mean blood eosinophils ≥200/µL (N=2499) showed that FEV1 % predicted was significant between low and high blood subgroups, but less than observed between sputum subgroups (blood eosinophil group differences: 4·2% pre-bronchodilator, 2·7% post-bronchodilator), slightly increased airway wall thickness (0·02 mm, p=0·032), greater symptoms (p=0·037), and wheezing (p=0·018), but no evidence of association with COPD exacerbations or other indices of severity. Blood eosinophils showed weak although significant association with sputum eosinophils (ROC AUC=0·64, p<0·001), but with a high false discovery rate (72%). Elevated sputum eosinophils, with or without blood eosinophils, were associated with lower lung function. Elevated blood eosinophils only in combination with elevated sputum eosinophils were associated with COPD exacerbations.
Interpretation
Stratification of SPIROMICS subjects by blood eosinophils alone showed minimal clinical differences and no association with exacerbations, whereas stratification by sputum eosinophils was associated with larger phenotypic differences and COPD exacerbations. Importantly, increased blood eosinophils did not reliably predict airway eosinophils in induced sputum.
Keywords: COPD severity, airway eosinophilia, emphysema, hyperinflation, air-trapping
INTRODUCTION
Airways inflammation in chronic obstructive pulmonary disease (COPD) is thought to be characterized by increased neutrophils,1 macrophages,2 proteases, IL-6, IL-8, and Th1 cytokines3 while airways inflammation in asthma is traditionally characterized by increased eosinophils, and Th2 cytokines.4 However, reports challenge these presumptive differences between asthma and COPD. The ECLIPSE study reported that in COPD, sputum neutrophils are weakly associated with lung function and health status, and not associated with exacerbations, emphysema or systemic inflammation.1 ECLIPSE also reported a mean 1·3% sputum eosinophil level in 359 subjects with COPD,1 but did not observe blood eosinophil associations with radiologic measure of emphysema or with COPD exacerbations and hospitalizations. ECLIPSE reported ≥2% (150/µL) blood eosinophils associated with evidence of higher FEV1, lower St. Georges Respiratory Questionnaire (SGRQ) and modified Medical Research Council scores.5 Other COPD studies have reported increased eosinophils associated with exacerbations and greater hyperinflation on QCT,6,7 suggesting Th2 inflammation may contribute to disease progression. Moreover, increased epithelial Th2 signature gene expression has been associated in two COPD cohorts with more severe airflow obstruction.8 Eosinophils may represent a potential biomarker in COPD since eosinophilia is related to corticosteroid responsiveness.1,9–11 In a phase II clinical trial, anti-IL-5 receptor therapy reduced the COPD exacerbation rate in a subgroup of patients with elevated blood and sputum eosinophilia.12
Determination of disease severity in COPD is complex and involves more than lung function assessments; additional clinical characteristics have been incorporated in successive revisions of the GOLD severity stages.13 Current classification includes lung function, symptom scores and exacerbation frequency. Thus, severity of COPD is dependent on multiple characteristics; eosinophilic inflammation may contribute.
Reports suggest blood eosinophil counts may represent a useful surrogate measure of airway eosinophils in COPD,11,14 although blood eosinophils appear to correlate poorly with sputum eosinophils in asthma,15,16 and do not distinguish between asthma-dominant, COPD-dominant or asthma/COPD overlap populations.17 However, larger studies of comprehensively phenotyped COPD patients often lack robust sputum eosinophil data.18–21 Thus it is uncertain whether peripheral eosinophils accurately predict airway eosinophils.
We investigated the hypotheses that blood and sputum eosinophils in subjects with a history of tobacco use were associated with a more severe COPD phenotype identified by diminished lung function, QCT measurements of emphysema or air-trapping, clinical COPD characteristics, and exacerbations. We also investigated relationships of blood and sputum eosinophils to determine whether blood eosinophils reliably predicted sputum eosinophils. These hypotheses were evaluated in the comprehensively characterized SPIROMICS cohort.22 A portion of these studies were presented as an abstract at the 2016 American Thoracic Society meeting.23
METHODS
Subjects
Subjects with current or former history of tobacco use (≥20 pack-year), recruited to include specific groups of smokers with preserved lung function (31%), GOLD stages 1 and 2 (41%), and GOLD stages 3 and 4 (21%) and a control group of nonsmokers, age 40–80 (N=2737), were enrolled in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) at six clinical sites and additional subsites.22 COPD was defined in long-term smokers as a post-bronchodilator FEV1/FVC ratio <0·7. Subjects underwent extensive baseline phenotypic characterization including lung function assessment pre- and post-bronchodilator with albuterol and ipratropium, CT total lung capacity and residual volume (TLC and RV) using QCT indicators for emphysema (% voxels less than −950 Hounsfield Units [HU]) at TLC, and air-trapping (% voxels less than −856 HU) at RV, airway metrics (VIDA Diagnostics, Iowa)24 and parametric response mapping (PRM) for functional small airways disease (fSAD),25 collection of blood (for DNA, RNA, plasma, sera, IgE and complete blood cell counts [CBC]), urine, 6 minute walk distance, GOLD stage, BODE index, COPD Assessment Score (CAT), St. Georges Respiratory Questionnaire (SGRQ), and administered questionnaires for medical history, exacerbations (retrospective from past year), hospitalizations, respiratory exposures, and medications.22 In a subset (see online supplement Figure S1 detailing subset of SPIROMICS with induced sputum cell counts), induced sputum was performed. Subjects with a primary asthma diagnosis were excluded, but all subjects were asked if they had ever had a health care professional say that they had asthma (“prior asthma label”). In the N=2499 enrolled ever smokers, the mean ± standard deviation for blood eosinophil count was 200 ± 240/µL (median=190/µL; full range 0–8300/µL); a higher eosinophil cutpoint of 300/µL was also examined. See supplement for additional information on the selection of stratification cutpoints, reproducibility, and other details.
Sputum Induction and Processing
SPIROMICS subjects with post-bronchodilator FEV1 % predicted ≥35% were eligible for sputum induction with saline solutions, nebulized for three 7 min intervals each. Expectorated sputum samples were processed as described in detail in the supplement. Cytospin slides were read by the central reading center. Slides were available for 1001 subjects. Differential counts (500–600 total cells) which had ≤100 leukocytes total (N=179) or ≥80% squamous (N=11) were excluded as unacceptable. The mean sputum eosinophil % (± standard deviation) for the subgroup with acceptable sputum (N=827) was 1·25 ± 4·25% (median=0·3%; full range 0–75%); a higher cutpoint of 2% for sputum eosinophils was also examined. (See supplement on subject distribution with induced sputum.)
Statistical Analyses
Subjects were stratified by eosinophil mean blood counts or mean sputum %. Demographic and biomarker data are presented as means ± standard deviations, or medians (25%–75% interquartile range) for continuous variables, and as % positive for categorical variables. Measures not meeting Kolmogorov-Smirnov test for normal distribution, were transformed by log, or square root values. Continuous variables were tested by parametric or non-parametric tests (see supplement; SAS 9·2, or Sigmastat 12·5). Categorical variables were analyzed using Chi-square or Fisher Exact tests. Correlations were examined by Pearson Correlation test or linear regression. Receiver Operating Curve (ROC) analysis was performed for blood eosinophil prediction of sputum eosinophils. The False Discovery Rate (= False Positives / False Positives + True Positives) was examined.26 Classification tree analysis examining sputum and blood eosinophils to model exacerbations was performed using R part routines in R software package. Variables with a p value <0·05 were accepted as significant.27
RESULTS
Subject Demographics
Demographic characteristics of SPIROMICS subjects stratified by mean blood eosinophils (< or ≥200/µL) and by mean sputum eosinophils (< or ≥1·25%) are shown in Table 1. For those subjects with low compared to high blood eosinophils, age, gender, race, BMI, cigarette smoking pack-years, % current smoker, and % use of inhaled corticosteroids (ICS) statistically differed, although differences between the groups were small (<10%). The proportion of current smokers was less in the ≥200/µL eosinophil group, but the number of cigarettes smoked each day was the same. Medications (supplement Table S1) did not differ, except for ICS treatment, higher in the ≥200/µL eosinophil group (Table 1). Total serum IgE levels (range 5·5–1660) and sputum eosinophils % differed in blood eosinophil groups, but those with a “prior asthma label” or childhood asthma did not differ (Table 1). Median sputum eosinophil % in low and high blood eosinophil groups differed (p<0.001), but were lower than the overall sputum eosinophil mean of 1·25%.
Table 1.
Demographics for subjects stratified by mean blood or sputum eosinophils (Eos; cutoff 200/µL or 1·25%, respectively).
| Variable | Blood Eos<200/µL |
Blood Eos≥200/µL |
P Value* |
Sputum Eos<1.25% |
Sputum Eos≥1.25% |
P Value* |
|---|---|---|---|---|---|---|
| Number | 1262 | 1237 | 656 | 171 | ||
| Age (yr) | 65 (56 – 70) | 65 (59 – 71) | 0·001 | 65 (57–71) | 64 (57–71) | 0·87 |
| Male Sex, N (%) | 631 (50) | 730 (59) | <0·001 | 377 (57) | 99 (58) | 0·99 |
| RACE N (%) Cau/N (%) AA/N (%) other | 934 (74)/ 285 (23)/ 43 (3) | 1004 (81)/ 173 (14) / 60 (5) | <0·001 | 511 (78)/ 110 (17)/ 35 (5) | 132 (77)/ 29 (17)/ 10 (6) | 0·90 |
| BMI | 26·8 (24 – 31) | 28·2 (25 – 32) | <0·001 | 28·3 (25–32) | 28·1 (25–32) | 0·92 |
| Smoking (pack-years) | 41 (30 – 60) | 45 (34 – 60) | 0·008 | 43 (32–60) | 44 (33–60) | 0·70 |
| Cigarettes/day | 15 (9 – 20) | 15 (8 – 20) | 0·97 | 15 (10–20) | 15 (6–20) | 0·52 |
| Current Smoker, N (% positive) | 522 (42) | 451 (37) | 0·003 | 293 (45) | 70 (41) | 0·39 |
| ICS, N (% positive) | 404 (32) | 470 (38) | 0·002 | 169 (26) | 66 (39) | 0·002 |
| IgE (geometric mean) | 34 (14–93) | 49 (19–166) | <0·001 | 41 (16–104) | 56 (15–203) | 0·32 |
| Sputum Eos % | 0·23 (0·0–0·76) | 0·65 (0·12–2·42) | <0·001 | |||
| Blood Eos count/µL | 150 (100–200) | 230 (160–350) | <0·001 | |||
| “Prior asthma”, N (% positive) | 249 (20) | 255 (21) | 0·48 | 122 (19) | 48 (29) | 0·003 |
| Childhood asthma, N (% positive) | 97 (7·7) | 118 (9·5) | 0·10 | 52 (7·9) | 20 (11·7) | 0·07 |
Mann-Whitney rank sum test for continuous variables, results as median (25–75% interquartile range); Chi-square for categorical variables, results as N (% positive response).
In subjects stratified by mean sputum eosinophils at 1·25%, age, gender, race, BMI, smoking pack-years, cigarettes/day, and % current smokers did not differ (Table 1. Differential counts for leukocytes in sputum eosinophil groups are presented in supplement Table S2). Medication use in the sputum cohort did not differ, except increased use of ICS, and inhaled or nebulized bronchodilators in the ≥1·25% sputum eosinophils group (supplement Table S1). Higher sputum eosinophils, unlike higher blood eosinophils, did not have significantly increased IgE levels compared to low sputum eosinophil group. Nevertheless, IgE levels in low and high sputum eosinophil groups were similar to IgE levels in low and high blood eosinophil groups, respectively. In addition, the sputum eosinophil ≥1·25% group had elevated blood eosinophils (230/µL, p<0·001) and a greater proportion of subjects reporting a “prior asthma label” (p=0·003). Similar results for blood or sputum eosinophil stratification were obtained with higher cutpoints (≥300/mL blood eosinophils levels, or ≥2% sputum eosinophils, respectively; supplement Table S3).
Spirometry
Dividing by blood eosinophils, the ≥200/µL high eosinophil group had marginally lower values for pre-bronchodilator FEV1 % predicted (4·2%), and no difference post-bronchodilator (0·6%), compared to the <200/µL low eosinophil group (Table 2). The sputum eosinophils ≥1·25% group had greater differences between pre bronchodilator and post-bronchodilator FEV1 % predicted (10 and 5·7%, respectively), compared to <1.25% group. Due to safety exclusion of subjects with post-bronchodilator FEV1 % predicted <35% from sputum induction, fewer GOLD Stages 3 and 4 subjects were included in the sputum cohort. However, baseline and post-bronchodilator FEV1 % predicted showed larger differences between low and high sputum eosinophil groups than observed between low and high blood eosinophil groups. Reversibility of baseline FEV1 % predicted, was larger in the elevated sputum eosinophil group (p<0·001), but did not differ for the blood eosinophil groups. Similar observations were found between subgroups stratified by ≥300/µL blood and ≥2% sputum eosinophil cutpoints (supplement Table S4).
Table 2.
Lung function for subjects stratified by mean blood or sputum eosinophils (Eos; 200/µL cutoff or 1·25% cutoff, respectively).
| Variable | Blood Eos<200/µL |
Blood Eos≥200/µL |
P Value* |
Sputum Eos<1.25% |
Sputum Eos≥1.25% |
P Value* |
|---|---|---|---|---|---|---|
| Number | 1262 | 1237 | 656 | 171 | ||
| Pre-bronchodilator: | ||||||
| FEV1 (L) | 1.86 (1.22–2.54) | 1.81 (1.16–2.55) | 0.38 | 2.15 (1.57–2.77) | 1.83 (1.38–2.32) | <0.001 |
| FEV1 % predicted | 70·5 (46·6 – 88·2) | 66·3 (42 – 85·6) | 0·006 | 75·7 (59·3–90·2) | 65·7 (52–81·3) | <0·001 |
| FVC % predicted | 87.3 (73–99) | 85.5 (72–97) | <0·001 | 90.9 (79–100) | 87.1 (77–97) | 0·06 |
| FEV1/FVC | 0·64 (0·49 – 0·73) | 0·61 (0·47 – 0·72) | 0·016 | 0·66 (0·58 – 0·74) | 0·61 (0·52 – 0·69) | <0·001 |
| Post-bronchodilator: | ||||||
| FEV1 (L) | 2.05 (1.43–2.72) | 2.03 (1.39–2.75) | 0.62 | 2.34 (1.78–2.59) | 2.11 (1.69–2.59) | 0.003 |
| FEV1 % predicted | 75.9 (53–94) | 76.5 (53–92) | 0·85 | 82.9 (68–96) | 77.2 (63–88) | 0·001 |
| FVC % predicted | 92·9 (81– 104) | 90·5 (79 – 102) | 0·001 | 94·5 (85 – 105) | 94·2 (86 – 104) | 0·84 |
| FEV1/FVC | 0·66 (0·5 – 0·76) | 0·63 (0·49 – 0·74) | 0·004 | 0·68 (0·59 – 0·76) | 0·64 (0·55 – 0·72) | <0·001 |
| % FEV1 reversibility | 9·3 (4·2 – 17·7) | 9·8 (4·5 – 19) | 0·46 | 8 (3·7 – 15·4) | 11·6 (6 – 21·7) | <0·001 |
Mann-Whitney rank sum test for continuous variables, results as median (25–75% interquartile range).
Imaging
Indices of emphysema and air trapping at TLC and RV24, respectively, did not differ between blood eosinophil groups (Table 3). In contrast, significantly higher emphysema indices (% voxels <−950 HU) in left upper and lower lobes, and right upper lobe were observed in sputum eosinophil ≥1·25%. In addition, air trapping (% voxels <−856 HU) and functional small airways disease assessed by parametric response mapping (PRM fSAD,25) were higher in subjects with ≥1·25% sputum eosinophils.
Table 3.
Imaging parameters for subjects stratified by mean blood or sputum eosinophils (Eos; cutoff 200/µL, or cutoff 1·25%, respectively).
| Variable | Blood Eos<200/µL (N=1262) |
Blood Eos≥200/µL (N=1237) |
P value* |
Sputum Eos<1.25% (N=656) |
Sputum Eos≥1.25% (N=171) |
P value* |
|---|---|---|---|---|---|---|
| DENSITY MEASURES | ||||||
| TLC Left Upper Lobe %<−950 HU | 3·22 (1·11 –11·84) | 3·77 (1·20 –11·50) | 0·66 | 2·24 (0·89 – 5·74) | 2·88 (1·09 – 7·65) | 0·046 |
| TLC Right Upper Lobe %<−950 HU | 2·78 (0·72 –12·97) | 2·87 (0·73 –11·96) | 0 76 | 1·73 (0·6 – 5·58) | 2·43 (0·9 – 7·24) | 0·011 |
| TLC Left Lower Lobe %<–950 HU | 2·09 (0·81–7·09) | 2·42 (0·88–7·5) | 0·23 | 1·60 (0·72 – 3·78) | 1·98 (0·76 – 5·32) | 0·044 |
| RV BOTH Lungs %<−856 HU | 17·4 (6·71–39·38) | 18·91 (7·39–40·71) | 0·28 | 12·52 (5·34–25·27) | 17·20 (8·57–33·13) | 0·001 |
| PRM functional small airway disease | 14 (4–33) | 15 (4–34) | 0·21 | 9 (3–22) | 13 (6–26) | 0·011 |
| AIRWAY MEASURES | ||||||
| AVG WALL THICK apical right upper lobe | 1·26 (1·15–1·37) | 1·28 (1·16–1·39) | 0·032 | 1·28 (1·18–1·38) | 1·29 (1·19–1·42) | 0·08 |
| Taper Ratio - apical right upper lobe | 0·038 (−0·01–0·086) | 0·039 (−0·01–0·09) | 0·87 | 0·03 (−0·01–0·08) | 0·03 (−0·01–0·08) | 0·93 |
Mann-Whitney rank sum test for continuous variables, results as median (25–75% interquartile range).
There was a small, 0·02 mm increase in average airway wall thickness at RB1 (prespecified pathway in apical segment of right upper lobe) for elevated blood eosinophils, but not for elevated sputum eosinophils. Neither blood nor sputum stratification showed any difference in airway tapering (an index of bronchiectasis).
The higher ≥300/µL blood eosinophil cutpoint did not alter density measures for emphysema or air trapping, but reduced significance for RB1 airway wall thickness. The higher ≥2% sputum eosinophil cutpoint maintained significance for both emphysema and air trapping indices (supplement Table S5).
Clinical Characteristics
Among subjects with ≥1·25% sputum eosinophils, there were fewer GOLD Stage 0 and increased GOLD Stage 2 subjects compared to subjects with <1·25% sputum eosinophils (p=0·0006, Table 4). The 6 min walk distance, BODE Index and COPD Assessment Score did not differ for either blood or sputum eosinophil stratifications. The blood eosinophil ≥200/µL group showed significantly higher frequency of self-reported wheezing (Table 4). St. George Respiratory Questionnaire (SGRQ) symptom score was also higher in the ≥200/µL blood eosinophil subgroup; both SGRQ total and symptom scores were significantly higher in the ≥1·25% sputum eosinophils subgroup.
Table 4.
Clinical characteristics for subjects stratified by mean blood or sputum eosinophils (Eos; cutoff 200/µL or 1·25%, respectively).
| Variable | Blood Eos<200/µL (N=1262) |
Blood Eos≥200/µL (N=1237) |
P value* |
Sputum Eos<1.25% (N=656) |
Sputum Eos≥1.25% (N=171) |
P value* |
|---|---|---|---|---|---|---|
| GOLD Stage 0 N(%)/ 1 N (%)/ 2 N(%)/ 3 N(%)/ 4 N(%) | 505 (40)/ 150 (12)/ 323 (26)/ 190 (15)/ 79 (6) | 425 (34)/ 153 (12)/ 359 (29)/ 200 (16)/ 86 (7) | 0·10 | 295 (45)/ 106 (16)/ 200 (31)/ 47 (7)/ 0 (0) | 51 (30)/ 31 (18)/ 76 (44)/ 11 (6)/ 1 (1) | 0·001 |
| 6 Minute Walk Distance (m) | 418 (354 – 482) | 410 (341 – 471) | 0·12 | 426 (372 – 482) | 426 (363 – 478) | 0·40 |
| BODE Index | 1 (0 – 2) | 1 (0 – 2) | 0·29 | 0 (0 – 1) | 1 (0 – 1.5) | 0·09 |
| COPD Score (CAT) | 13 (7–20) | 13 (7–19.5) | 0·45 | 12 (7–19) | 13 (8–20) | 0·18 |
| SGRQ (Total) | 31·5 (14·9 – 48·2) | 31·2 (16·4 – 47·1) | 0·81 | 26·2 (14·0 – 43·6) | 31·8 (17·2 – 47·1) | 0·05 |
| SGRQ (Symptoms) | 45·3 (22·9 – 66·3) | 48·8 (27·0 – 66·3) | 0·037 | 45·2 (23·5 – 65·1) | 53·6 (34 – 70) | 0·004 |
| Symptoms: | ||||||
| Wheezing, N (% positive) | 741 (59) | 788 (64) | 0·018 | 389 (60) | 116 (68) | 0·07 |
Mann-Whitney rank sum test for continuous variables, results as median (25–75% interquartile range); Chi-square for categorical variables, results as N (% positive response).
The higher ≥300/µL blood eosinophils cutpoint showed a significant difference for GOLD Stages (supplement Table S6). The higher ≥2% sputum eosinophil cutpoint maintained significance for GOLD Stages, SGRQ total and symptoms scores, and became significant for BODE Index, SGRQ Impact and self-reported wheezing.
Exacerbations
Blood eosinophils ≥200/µL and sputum eosinophils ≥1·25% were tested for association with exacerbations (Table 5). Elevated blood eosinophils were not associated with any of the different categories of reported exacerbations. In contrast, elevated sputum eosinophils were associated with increased proportions of subjects with exacerbations requiring corticosteroids, exacerbations requiring treatment with any drug, and severe exacerbations requiring emergency department visit. The higher ≥300/µL blood eosinophil cutpoint did not show any association with exacerbations, but the higher ≥2% sputum eosinophil cutpoint demonstrated significance for all categories of reported COPD exacerbations (supplement Table S7). Tree classification of sputum and blood eosinophil association with exacerbations selected sputum eosinophils before blood eosinophils and showed similar cutpoints, sequentially < or ≥1·9% for sputum eosinophils and < or ≥ 176/µL for blood eosinophils, supporting the 2% and 200/µL cutpoints investigated for sputum and blood eosinophils in this study (supplement Figure S2). Sputum eosinophils >1·9% identified a subgroup of subjects with exacerbations (27 of 119 or 23%). In contrast, those with <1.9% sputum eosinophils showed a lower proportion of subjects with exacerbation (65 of 692 or 9%).
Table 5.
Comparison of exacerbations ≥1 (in the previous year) for subjects stratified by mean blood or sputum eosinophils (Eos; cutoff 200/µL or 1·25%, respectively). All values are percentage positive.
| Variable | BLOOD Eos<200 |
BLOOD Eos≥200 |
P value* |
SPUTUM Eos<1.25 % |
SPUTUM Eos≥1.25 % |
P value* |
|---|---|---|---|---|---|---|
| N | 1262 | 1237 | 656 | 171 | ||
| Definition of exacerbation: | ||||||
| Total, N (%) | 311 (25) | 309 (25) | 0·35 | 125 (19) | 44 (26) | 0·05 |
| Healthcare Utilization, N (%) | 294 (23) | 291 (24) | 0·36 | 125 (19) | 43 (25) | 0·07 |
| Antibiotic treatment, N (%) | 232 (18) | 240 (19) | 0·29 | 92 (14) | 34 (20) | 0·09 |
| Corticosteroid treatment, N %) | 199 (16) | 209 (17) | 0·27 | 66 (10) | 32 (19) | 0·002 |
| Any drug-treatment, N (%) | 265 (21) | 273 (22) | 0·29 | 105 (16) | 39 (23) | 0·033 |
| Severe, N (%) | 137 (11) | 162 (13) | 0·15 | 52 (8) | 22 (13) | 0·044 |
Chi-square, results as % positive response.
Blood Eosinophil Prediction of Sputum Eosinophils
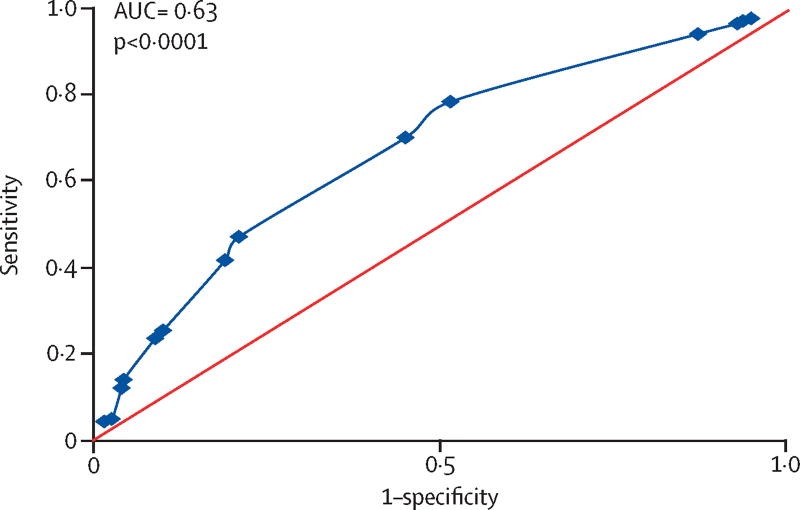
ROC analyses demonstrated a relatively weak, although significant relationship for blood eosinophils to predict sputum eosinophils ≥1·25% (Figure 1, AUC=0·63, p<0·0001); ROC to predict ≥2% sputum eosinophils was similar (supplement Figure S3). Highest sensitivity and specificity for predicting sputum eosinophils ≥1·25% and ≥2% were found at 150/µL and 250/µL blood eosinophils, respectively, with equivalent, significant AUCs observed at adjacent cutpoints (supplement Table S8). Nevertheless, both associations had very large false discovery rates; 72% for blood eosinophils ≥150/µL to predict sputum eosinophils ≥1·25% (false negative rate of 22%), and 74% for blood eosinophils ≥250/µL to predict sputum eosinophils ≥2% (false negative rate of 50%).
Figure 1.
ROC analysis for blood eosinophil prediction of sputum eosinophil. Blood eosinophils at cutpoints from 50/µL (highest sensitivity) to 500/µL (lowest sensitivity) were examined for correct prediction of sputum eosinophils < or ≥1·25%. Although significant (p<0·001), the area under the curve (AUC) was only 0·63, demonstrating a lack of strength for the prediction. Maximum sensitivity and specificity were observed at a blood eosinophil cutpoint of 150/µL.
Combined Blood and Sputum Eosinophil Phenotypes
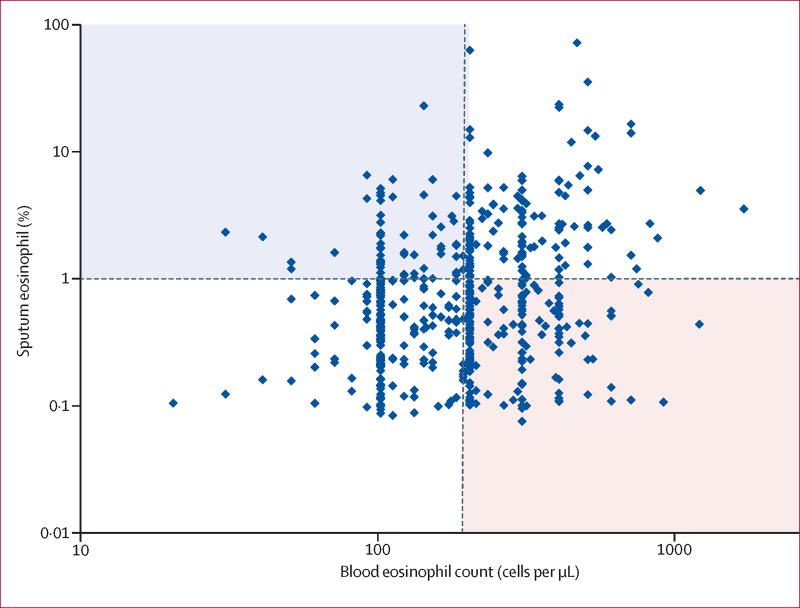
The correlation between sputum eosinophils and blood eosinophils was poor, but significant (Figure 2; correlation coefficient r=0·178, p<0·001). However, numerous subjects (42%) had discordant blood and sputum eosinophil levels; either high in blood or in sputum, but not in the other compartment. Lung function and reported exacerbations for subjects in the two discordant quadrants and two concordant quadrants were compared (Table 6). High sputum eosinophil groups, without or with high blood eosinophils, had the lowest lung function. Lung function for the high blood eosinophil group without high sputum eosinophils did not differ from the group with low eosinophils in both blood and sputum. However, high blood eosinophil groups only in combination with elevated sputum eosinophils had more COPD exacerbations; exacerbations treated with corticosteroids (p=0·006) or severe (p=0·013) were significant.
Figure 2.
Distribution of subject’s blood eosinophils with respect to sputum eosinophils. Although there is a significant association between blood and sputum eosinophils (EOS) (Pearson correlation coefficient r=0·178, p<0·001), use of the cutpoint ≥200/µL blood eosinophils (vertical red line) to predict sputum eosinophils ≥1·25% (horizontal red line) will mistakenly identify many subjects with lower sputum eosinophil% (lower right quadrant, pink shade) and miss many subjects with actual sputum eosinophils ≥1·25% (upper left quadrant, blue shade).
Table 6.
Spirometry and exacerbations for subjects in sputum (spt) versus blood (cbc) eosinophil (Eos) distribution stratified by quadrant (blood Eos cutpoint ≥200/µL; sputum Eos cutpoint ≥1·25%):
| Low spt Eos+ Low cbc Eos |
Low spt Eos+ High cbc Eos |
High spt Eos+ Low cbc Eos |
High spt Eos+ High cbc Eos |
P value* | |
|---|---|---|---|---|---|
| N | 355 | 290 | 50 | 116 | |
| Pre-bronchodilator: | |||||
| FEV1 % pred | 77 (62–91) | 74 (57–89) | 62 (51–81) | 66 (50–79) | <0·001 |
| FVC % pred | 93 (81–101) | 88 (77–100) | 89 (76–99) | 87 (76–96) | 0·033 |
| ratio FEV1/FVC | 0·67 (0·58–0·74) | 0·66‡ (0·57–0·73) | 0·61‡ (0·47–0·66) | 0·61 (0·52–0·69) | <0·001 |
| Post-bronchodilator: | |||||
| FEV1 % pred | 84 (70–97) | 81 (66–95) | 76 (63–87) | 77 (63–90) | 0·005 |
| FVC % pred | 96 (86–106) | 93 (84–104) | 98 (88–104) | 93 (85–104) | 0·24 |
| ratio FEV1/FVC | 0·69 (0·59–0·77) | 0·67‡ (0·59–0·76) | 0·63‡ (0·49–0·72) | 0·64 (0·58–0·70) | <0·001 |
| % reversibility | 7·8 (3·6–15·3) | 8·5‡ (4·0–16·6) | 14·5‡ (6·8–22·6) | 11·4 (5·8–20·5) | <0·001 |
| Definition of exacerbation: | |||||
| Total N (%) | 76 (21) | 47 (16) | 10 (20) | 32 (28) | 0·07 |
| Requiring HCU N (%) | 74 (21) | 45 (16) | 9 (18) | 31 (27) | 0·07 |
| Antibiotics treatment N (%) | 56 (16) | 36 (12) | 10 (20) | 23 (20) | 0·21 |
| Corticosteroid treatment N (%) | 37 (10) | 25 (9) | 6 (12) | 24 (21) | 0·006 |
| Any Drug treatment N (%) | 61 (17) | 38 (13) | 10 (20) | 27 (23) | 0·08 |
| Severe (ED or hospital) N (%) | 35 (10) | 15 (5) | 3 (6) | 17 (15) | 0·013 |
Kruskal-Wallis for continuous variables, results as median (interquartile range); Chi-square for exacerbation categories, results as N (% positive response).
Indicates High sputum Eos+ Low cbc Eos quadrant was significantly different from Low spt Eos+ High cbc Eos quadrant by Dunn’s method.
Additional Stratifications for Blood and Sputum Eosinophil Subgroups
Subjects were stratified by “prior asthma label” or ICS use and examined for interaction with high eosinophils (blood eosinophils ≥200/mL or sputum eosinophils ≥1·25%) on lung function and exacerbations. No interactions were significant (supplement Tables S9, and S10). Subjects who did not have acceptable sputum slides for various reasons (N=1498) were stratified by blood eosinophil counts to determine whether these subjects represented a phenotype with different characteristics (supplement Table S11). There was slightly higher proportion of subjects using ICS, lower lung function and increased proportions of GOLD Stage 3 and 4 subjects as would be expected in these groups which contained subjects with lower lung function and therefore ineligible for sputum induction, but otherwise resembled the larger cohort of smokers.
Another stratification examined whether blood eosinophil groups < or ≥200/µL showed differences when restricted to just those subjects in the sputum cohort (supplement Table S12). The sputum cohort divided into blood eosinophil subgroups had slightly greater proportion of current smokers, less ICS use and slightly better lung function, but did not show the same radiologic, clinical or exacerbation diffierences observed for sputum eosinophil stratification.
We examined whether associations with worse lung function and quality of life, and greater exacerbations, emphysema, and air trapping in the high sputum eosinophil group were due to elevated sputum neutrophils in addition to high sputum eosinophils. There was no difference in mean sputum neutrophil % between high and low sputum eosinophil groups (Table S2). Stratification of the sputum cohort into 4 groups based on < or ≥1·25% eosinophils + < or > 68% neutrophils (mean ± std deviation for sputum neutrophils: 68% ± 21%), confirmed differences across low and high sputum eosinophil groups but did not show significant post-hoc differences between the high eosinophil + high neutrophil and high eosinophil + low neutrophil subgroups (Supplement additional results, Table S13).
DISCUSSION
The results of this study from the SPIROMICS cohort, a smoking cohort that includes a spectrum of COPD severity defined by GOLD stages, confirms that elevated sputum eosinophils, but not blood eosinophils alone, identify a subset of COPD subjects with more severe airflow obstruction, worse quality of life, greater emphysema and air trapping, and exacerbations. Using sputum eosinophil stratification at either the mean, ≥1·25%, or ≥2%, we found significant associations with COPD exacerbations, including severe and those requiring corticosteroid therapy. In addition, significant associations were found for lower lung function, baseline and post-bronchodilation including increased bronchodilator reversibility; respiratory symptoms; emphysema and air trapping by QCT; and COPD severity by GOLD Stage. In contrast, blood eosinophils alone, at ≥200/µL, or the even higher cutpoint at ≥300/µL, showed no association with COPD exacerbations, and associations with other phenotypic markers were smaller or non-significant. Although there was an increase in SGRQ symptom scores for higher eosinophils in both blood and sputum compartments, and wheeze (found only for blood, possibly due to the larger N for that group) the differences between low and high eosinophil groups for these variables were greater in the sputum group. In addition, there was no difference in CAT scores for either blood or sputum, which tends to diminish the validity for this observation. Importantly, although the relationship between blood and sputum eosinophilia was statistically significant, blood eosinophils did not reliably predict sputum eosinophils, showing a 72–74% false-discovery rate and a 50% false negative rate for sputum eosinophils ≥2%. Lung function data stratified by high and low sputum and blood eosinophils showed no relationship with high blood eosinophils unless combined with high sputum eosinophils, while high sputum eosinophils even in the absence of blood eosinophils was associated with lower lung function. However, COPD subjects with both high sputum and blood eosinophils exhibited both decreased lung function and more frequent exacerbations. These findings among current and former smokers in a large multicenter cohort with a specified range of COPD severity have important implications for proposed use of blood eosinophils alone as a predictive biomarker to guide individualized COPD therapies.
Our results extend observations from previous studies in COPD cohorts, including ECLIPSE, which focused primarily on neutrophilic airways inflammation,1 and, though reporting eosinophil presence,5 did not address association of eosinophilia with indices of COPD severity.4,5 The importance of our findings and of Th2 inflammation in COPD are emphasized by the recent report of Th2 gene expression overlap in airway epithelial samples from asthma and COPD cohorts,8 and by shared clinical and biologic characteristics between asthma and COPD reported in several recent studies.5,7,17,28–29 However, differences are noted between SPIROMICS and other COPD cohorts. COPD gene enrolled a larger cohort (N=10,000) which was slightly older (minimum 45 yr) and had a lower smoking history (>10 pack year), but phenotyping with induced sputum was not performed.30 Sputum was also unavailable in Copenhagen General Population Study,20 WISDOM,21 INSPIRE, and TRISTAN.31 Although these studies report exacerbations correlating with blood eosinophils, it is important to note that the entry requirements included past history of COPD exacerbations which can impact the results since a past history of exacerbation is the most important factor predicting future exacerbations.18
In addition emphasis on persistent Th2 inflammation in COPD32, has focused on eosinophils as predictors of exacerbations. Bafadhel and colleagues reported a cluster analysis using blood and sputum biomarkers; peripheral blood eosinophils predicted sputum eosinophil-associated exacerbations of COPD.6 Sputum airway and peripheral blood eosinophils have been used to direct corticosteroid treatment and reduce occurrence of COPD exacerbations.9–11 The ECLIPSE study reported that 1483 subjects if stratified by blood eosinophils did not differ for COPD exacerbation rate in the previous year.5 We confirm that higher blood eosinophils are not associated with COPD exacerbations except combined with elevated sputum eosinophils or with other characteristics such as a previous history of exacerbation.18 However, in SPIROMICS higher sputum eosinophils alone are associated with exacerbations even in mild to moderate COPD.
Eosinophil levels have been suggested to indicate response to corticosteroids, anti-IL5, or anti-IL5 receptor therapy.9–12,33 In a retrospective analysis of two COPD exacerbation studies with long-acting beta-agonists and inhaled corticosteroids, Pascoe showed that subjects with higher blood eosinophils had greater reduction in COPD exacerbations.34 These observations suggest eosinophils may be important in development or potential biomarker of some COPD exacerbations. However, two factors may influence previous observations correlating elevated blood eosinophil groups with greater COPD exacerbations; selection criteria requiring recent exacerbation and perhaps lower lung function are both related to future COPD exacerbations and may be surrogate markers of increased sputum eosinophils. Elevated blood eosinophils, if also combined with elevated sputum eosinophils, associate with COPD exacerbations, as shown for the SPIROMICS cohort in this report. However, blood eosinophils alone were not associated with exacerbations, even when combined with “prior asthma label”. This latter observation contrasts with the association of blood eosinophils ≥275/µL with all cause mortality in 662 subjects, but that study found no change after exclusion of subjects with asthma.35 Blood eosinophils in our study were associated with COPD exacerbations only in the context of higher sputum eosinophils.
We also examined other characteristics in the blood and sputum eosinophil subgroups of SPIROMICS subjects that might suggest overlap with asthma: bronchodilator reversibility, IgE levels, and childhood asthma. Blood eosinophils at two different cutpoints (either ≥200 or ≥300/µL) did not have higher levels of acute bronchodilator reversibility, while elevated sputum eosinophils showed greater reversibility. IgE levels were significantly higher in the elevated blood eosinophil group, although not in sputum, but IgE levels in SPIROMICS blood and sputum eosinophil subgroups were well below median (91 IU/ml) and high IgE cutpoint (173 IU/ml) reported in a recent study of asthma-COPD overlap syndrome.36 Only ≥2% sputum eosinophils showed increased report of childhood asthma, which represent a small subgroup (12·5%).
There was greater use of prescribed inhaled corticosteroids in both high blood and sputum eosinophil groups. This was observed despite an expected reduction in eosinophils with corticosteroid therapy. Use of corticosteroids in the higher eosinophil groups potentially reflects individuals more likely to have had exacerbations, consistent with GOLD guideline recommendations for corticosteroids in COPD patients with frequent exacerbations.13 Limitations of this report include somewhat milder COPD in the group who were able to successfully perform induced sputum. For safety reasons, SPIROMICS subjects with post-bronchodilator FEV1 % predicted <35% did not have sputum induction, limiting the sputum subgroup to GOLD Stages 0–3. Although our cohorts (both for blood and sputum eosinophil analyses) included smoking subjects (>40 pack years) with preserved lung function, these subjects were included because they have been shown to have greater symptoms, exacerbations, activity limitations and radiologic evidence of airway disease.37 These findings are consistent with early COPD in this subgroup.37 Although SPIROMICS exacerbations data was retrospective, validity of retrospective data for future risk of COPD exacerbation has been shown in the ECLIPSE where self-reported exacerbation from the previous year had predicted exacerbations during the first year of follow up, more accurately than all other variables examined.18 An additional limitation, at least in clinical settings, is the difficulty in performing accurate sputum analysis. Even in the SPIROMICS network with centralized training for sputum induction and processing there were still reasons preventing sputum analysis on all eligible subjects as indicated in the supplemental methods. However analysis of those who did not have sputum analysis, stratified by blood eosinophils did not differ substantially from the larger cohort.
Of interest, longitudinal follow-up of the SPIROMICS cohort may be used to confirm the observations of Hospers and colleagues that peripheral eosinophils are associated with all cause mortality over a period of 30 years.35 Alternatively, the differences in decline of lung function associated with blood eosinophils < or ≥2% observed in the much smaller study over 9 years by Rogliani and colleagues should be examined in the larger SPIROMICS cohort longitudinally.38 In summary, using the larger and comprehensive phenotypic characterization of the SPIROMICS cohort, we show that stratification by elevated sputum eosinophil inflammation identified a subgroup with more severe COPD, having decreased lung function, greater emphysema and air trapping, and greater COPD exacerbations. Peripheral blood eosinophils identified a subgroup with decreased lung function without other indices of more severe COPD, specifically exacerbations unless examined on the background of elevated sputum eosinophils. Moreover, blood eosinophils did not accurately predict sputum eosinophils. These observations confirm the importance of assessing eosinophils in the airways. In the future, it will be important to follow these subjects with higher sputum eosinophilia longitudinally to determine whether long term effects on the progression of COPD.
Supplementary Material
Supplement Figure S1. Consort Diagram for subjects recruited and reason for removal from analysis. All normal, never smokers were removed. Although 90% of subjects (including normal never smokers) may have been eligible for sputum induction, there were several reasons that reduced the actual number of sputum slide samples available for analysis: no sputum produced upon completion of induction, no sputum processing form entered, removal of aliquots for mucus analysis and microbiome prior to processing leaving too little remaining sample for cytospin slide preparation, slides not sent to central slide reading center, and finally slide counts that were deemed unacceptable (leukocyte cell count <100 or too high, >80% squamous epithelial cells). There were 16 subjects with acceptable sputum differential count but without blood counts who were added to those with both acceptable sputum counts and blood counts (N=811).
Supplement Figure S2. Classification tree diagram for model of exacerbations by sputum and blood eosinophils. The Root has 811 subjects with 92 exacerbations in previous year. The first number in each node is the number of subjects without exacerbations; the second number is the number with exacerbations. The model first divides the subjects based on sputum eosinophils < and ≥1.9% and secondly divides the subjects by both sputum and blood eosinophils (< or >176/µL).
Supplement Figure S3. ROC analysis for blood Eos prediction of sputum Eos. Blood Eos at cutpoints from 50/µL (highest sensitivity) to 500/µL (lowest sensitivity) were examined for correct prediction of sputum Eos < or ≥2%. Although significant (p<0·001), the area under the curve (AUC) was only 0·64, demonstrating a lack of strength for the prediction. Maximum sensitivity and specificity were observed at a blood Eos cutpoint of 250/µL, but with very large false discovery rate (74%) and false negative rate (50%).
Research in Context.
Evidence before this study
A PubMed search for original research reports containing information on eosinophils, sputum, blood and COPD through May 2017 yielded 154 articles, of which 32 were reviews. Addition of either “severity” or “exacerbation” reduced publication numbers to 33 (7 reviews) or 35 (1 review), respectively. However, many of these reports have further limitations, either lacking sputum or blood eosinophil data for comparison, not specifically focused on severity of COPD including exacerbations, or containing small numbers of subjects (<100/group) which limit the power to make conclusions for broader COPD populations. Generally eosinophils in COPD have been linked to more frequent exacerbations and responsiveness to corticosteroid therapy, suggesting more severe disease. Often studies are primarily in populations who have met selection criteria for clinical trials that include the presence of COPD exacerbations. Thus, comparison of blood eosinophils and sputum eosinophils for association with a more severe COPD phenotype has not been well studied in a general smoking population with a broad range of COPD severity, nor has possible substitution of blood eosinophils as a biomarker for sputum eosinophils in COPD populations been carefully examined.
Added value of this study
This study demonstrates that in a large, comprehensively characterized smoking cohort with a broad range of COPD severity, elevated sputum eosinophils, but not blood eosinophils alone, had significant associations with multiple measures of COPD severity, including exacerbations, increased emphysema and air trapping, St. George Respiratory Questionnaire scores and GOLD spirometric stage. Blood eosinophils demonstrated weak association with sputum eosinophils and as a single biomarker had few significant associations with COPD severity and exacerbations. However, this study does demonstrate that elevated blood eosinophils in combination with elevated sputum eosinophils show associations with COPD exacerbations and severity.
Implications of all the available evidence
Increased sputum eosinophils from subjects with a broad range of COPD severity identify those more likely to have severe disease and exacerbations. Blood eosinophils as a single biomarker do not accurately predict sputum eosinophils, and do not show any association with disease severity or exacerbations unless observed in the background of increased sputum eosinophils. The findings from this study will be important in the design of therapeutic trials which target eosinophilic inflammation in COPD. This article has an online data supplement.
Acknowledgments
SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C HHSN268200900019C, HHSN268200900020C), which were supplemented by contributions made through the Foundation for the NIH from AstraZeneca; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc; Chiesi Farmaceutici SpA; Forest Research Institute, Inc; GSK; Grifols Therapeutics, Inc; Ikaria, Inc; Nycomed GmbH; Takeda Pharmaceutical Company; Novartis Pharmaceuticals Corporation; Regeneron Pharmaceuticals, Inc; Sanofi, and the COPD Foundation. Additional CT analyses were made possible by NIH/NHLBI HL122438. The authors thank the SPIROMICS participants and participating physicians, investigators and staff for making this research possible. More information about the study and how to access SPIROMICS data is at www.spiromics.org. We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Wayne H Anderson, PhD; Russell P Bowler, MD, PhD; Alejandro P Comellas, MD; Gerard J Criner, MD; Ronald G Crystal, MD; Mark T Dransfield, MD; Christine M Freeman, PhD; Robert J Kaner, MD; Jerry A Krishnan, MD, PhD; Stephen C Lazarus, MD; John D Newell Jr, MD; Elizabeth C Oelsner, MD, MPH; Stephen I. Rennard, MD; Donald P Tashkin, MD; Mary Beth Scholand, MD; J Michael Wells, MD; and Robert A Wise, MD.
CMD, ATH, DAM, report support from NHLBI and FNIH during conduct of study; RGB reports grants from NIH, grants from Foundation for the NIH and the COPD Foundation during the conduct of the study, grants from Alpha1 Foundation, and personal fees from UpToDate; CBC reports grants from Equinox Health Clubs, Amgen, and Spiration, and personal fees from Equinox Health Clubs, PulmonX, Boehringer Ingelheim, GlaxoSmithKline, SPIRATION and part-time work for scientific engagement for GlaxoSmithKline Global Respiratory Franchise outside of submitted work; DC reports support from NHLBI and the COPD Foundation during conduct of the study; EEC reports support from NHLBI, FNIH, Genentech and the COPD Foundation during conduct of study; SC reports grant from MedImmune, personal fees from AstraZeneca and nonfinancial support from Genentech outside the submitted work; JLC reports grants from NHLBI and NIAID, grants from Department of Veterans Affairs, grants from Foundation of NIH and COPD Foundation during the conduct of the study outside the submitted work; MKH reports support from NIH, FNIH and COPD Foundation, consulting fees from GSK, BI, Novartis, Astra Zeneca, and Sunovion, and royalties from UptoDate outside the submitted work; NNH reports grants from AstraZeneca, grants from NIH, and COPD Foundation, Boehringer Ingelheim, and GSK, outside the submitted work; EAH is a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software developed, in part, at the University of Iowa outside the submitted work; EK reports grants from the NIH and the Foundation for the NIH; non-financial support (inhalers for spirometry testing) from Boehringer Ingelheim and GlaxoSmithKline during the conduct of the study; grants from Boehringer Ingelheim, Novartis, Pearl/AstraZeneca and Sunovion/Sepracor outside the submitted work; FJM reports grants from NIH, personal fees from Forest, Janssens, GlaxoSmithKline, Nycomed/Takeda, Amgen, AstraZeneca, Boehringer Ingelheim, Ikaria/Bellerophon, Genentech, Novartix, Pearl, Pfizer, Roche, Sunovion, Theravance, Axon, CME Incite, California Society for Allergy and Immunology, Annenberg, Informa, Integritas, In Thought, Miller Medical, National Association for Continuing Education, Paradigm, Peer Voice, UpToDate, Haymarket Communications, Western Society of Allergy and Immunology, Unity Biotechnology, ConCert, Lucid, Methodist Hospital, Prime, WebMD outside the submitted work; VEO reports personal fees from CSL Behring outside the submitted work; RBIII reports support from NHLBI and the COPD Foundation, and a grant from Department of Veterans Affairs during conduct of study; SPP reports grants from NIH, NHLBI and COPD Foundation during the conduct of the study; PGW reports grants from Medimmune, personal fees from Genentech/Roche, Astra Zeneca, Novartis, Neostem, and Janssen outside the submitted work; In addition, Dr. Woodruff has a patent Asthma diagnostics pending.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors fulfill authorship criteria through contributions to concept and design of the study: ATH, FJM, JLC, CBC, CMD, NNH, RGB, EAH, REK, EK, WKO’N, SPP, PGW, MKH, ERB; recruitment of the cohort and acquisition of data: ATH, FJM, JLC, CMD, NNH, SC, NP, VEO, RGB, CBC, EEC, DC, EAH, REK, EK, RP, NEA, PGW, MKH, DAM, ERB; analyses and interpretation of the data: ATH, FJM, JLC, NNH, NP, XL RGB, EEC, CBC, DC, EAH, RP, PGW, MKH, DAM, ERB;, drafting and critical revision of the manuscript: ATH, FJM, JLC, CMD, NNH, SC, NP, XL, VEO, RGB, EEC, CBC, DC, EAH, REK, EK, WKO’N, RP, SPP, NEA, PGW, MKH, DAM, ERB;, approval of the final version to be published, and agreement to be accountable: ATH, FJM, JLC, CMD, NNH, SC, NP, XL, VEO, RGB, EEC, CBC, DC, EAH, REK, EK, WKO’N, RP, SPP, NEA, PGW, MKH, DAM, ERB, ensuring the accuracy and integrity of the work.
Declaration of interests
NEA, ERB, REK, WO’N NP and XL report nothing to disclose.
References
- 1.Singh D, Edwards L, Tal-Singer R, Rennard S. Sputum neutrophils as a biomarker in COPD: findings from the ECLIPSE study. Respir Res. 2010;11:77–89. doi: 10.1186/1465-9921-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiemstra PS. Altered macrophage function in chronic obstructive pulmonary disease. Annals ATS. 2013;10(Supplement):S180–185. doi: 10.1513/AnnalsATS.201305-123AW. [DOI] [PubMed] [Google Scholar]
- 3.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50s–59s. [PubMed] [Google Scholar]
- 4.Sethi S, Mahler DA, Marcus P, Owen CA, Yawn B, Rennard S. Inflammation in COPD: implications for management. Am J Med. 2012;125:1162–1170. doi: 10.1016/j.amjmed.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R on behalf of the ECLIPSE investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44:1697–1700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- 6.Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A, Lindblad K, Patel H, Rugman P, Dodson P, Jenkins M, Saunders M, Newbold P, Green RH, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, Brightling CE. Acute exacerbations of chronic obstructive pulmonary disease; identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 7.Hardin M, Silverman EK, Barr RG, Hansel NN, Schroeder JD, Make BJ, Crapo JD, Hersh CP for the COPDGene investigators. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christenson SA, Steiling K, van den Berge M, Hijazi K, Hiemstra PS, Postma DS, Lenburg ME, Spira A, Woodruff PG. Asthma-COPD Overlap: clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:758–766. doi: 10.1164/rccm.201408-1458OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brightling CE, Monteiro W, Ward R, Parker D, Morgan MDL, Wardlaw AJ, Pavord ID. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomized controlled trial. The Lancet. 2000;356:1480–1485. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 10.Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, Monteiro W, Berry M, Parker D, Wardlaw AJ, Pavord ID. Eosinophilic airway inflammation and exacerbations of COPD: a randomized controlled trial. Eur Respir J. 2007;29:906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 11.Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, Brightling CE. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease, a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brightling CE, Bleecker ER, Panettieri RA, Jr, Bafadhel M, She D, Ward CK, Xu X, Birrell C, van der Merwe R. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomized, double-blind, placebo-controlled phase 2a study. The Lancet/respiratory. 2014;2:891–901. doi: 10.1016/S2213-2600(14)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Global Strategy for the Diagnosis, Management, and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) Updated. 2016 Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html.
- 14.Negewo NA, McDonald VM, Baines KJ, Wark PAB, Simpson JL, Jones PW, Gibson PG. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1495–1504. doi: 10.2147/COPD.S100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, Peters SP, Meyers DA, Bleecker ER National Heart, Lung, and Blood Institute's Severe Asthma Research Program. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. 2013;132:72–80. doi: 10.1016/j.jaci.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee M, Nair P. Blood or sputum eosinophils to guide asthma therapy? The Lancet/Respiratory. 2015;3:824–825. doi: 10.1016/S2213-2600(15)00419-1. [DOI] [PubMed] [Google Scholar]
- 17.Ghebre MA, Bafadhel M, Desai D, Cohen SE, Newbold P, Rapley L, Woods J, Rugman P, Pavord ID, Newby C, Burton PR, May RD, Brightling CE. Biological clustering supports both “Dutch” and “British” hypotheses of asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2015;135:63–72. doi: 10.1016/j.jaci.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, MacNee W, Calverley P, Rennard S, Wouters EFM, Wedzicha JA. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui SH, Guasconi A, Vestbo J, Jones P, Agusti A, Paggiaro P, Wedzicha JA, Singh D. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:523–5. doi: 10.1164/rccm.201502-0235LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in COPD: the Copenhagen general population study. Am J Respir Crit Care Med. 2016;193:965–974. doi: 10.1164/rccm.201509-1869OC. [DOI] [PubMed] [Google Scholar]
- 21.Watz H, Tetzlaff K, Wouters EFM, Kirsten A, Magnussen H, Rodriguez-Roisin R, Vogelmeier C, Fabbri LM, Chanez P, Dahl R, Disse B, Finnigan H, Calverley PMA. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4:390–398. doi: 10.1016/S2213-2600(16)00100-4. [DOI] [PubMed] [Google Scholar]
- 22.Couper D, LaVange LM, Han ML, Barr RG, Bleecker ER, Hoffman EA, Kanner R, Kleerup E, Martinez FJ, Woodruff PG, Rennard S for the SPIROMICS Research Group. Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hastie AT, Alexis NE, Doerschuk C, Hansel NN, Christenson S, Putcha N, Ortega VE, Peters SP, Barr RG, Couper DJ, Hoffman EA, Kanner R, Kleerup E, Martinez FJ, Woodruff PG, Han MK, Meyers DA, Curtis JL, Bleecker ER. Blood Eosinophils Poorly Correlate with Sputum Eosinophils, and Have Few Associations with Spirometry, Clinical and Quantitated Computed Tomography Measures Compared to Sputum Eosinophils in the SPIROMICS Cohort [abstract] Am J Respir Crit Care Med. 2016:A6168. [Google Scholar]
- 24.Sieren JP, Newell JD, Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, Couper D, Goldin J, Guo J, Han MK, Hansel NN, Kanner RE, Kazerooni EA, Martinez FJ, Rennard S, Woodruff PG, Hoffman EA SPIROMICS Research Group. SPIROMICS Protocol for Multicenter Quantitative Computed Tomography to Phenotype the Lungs. Am J Respir Crit Care Med. 2016 Oct 1;194(7):794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boes JL, Hoff BA, Bule M, Johnson TD, Rehemtulla A, Chamberlain R, Hoffman EA, Kazerooni EA, Martinez FJ, Han MK, Ross BC, Galban CJ. Parametric response mapping monitors temporal changes on lung CT scans in the Subpopulations and Intermediate Outcomes Measures in COPD Study (SPIROMICS) Acad Radiol. 2015;22:186–194. doi: 10.1016/j.acra.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCSS 11 Statistical Software. Documentation. NCSS, LLC; Kaysville, Utah, USA: 2016. Chapter 546 ncss.com/software/ncss. [Google Scholar]
- 27.Ott RL, Longnecker MT. An introduction to statistical methods and data analysis. 7. BrooksCole; 2015. [Google Scholar]
- 28.Carolan BJ, Sutherland ER. Clinical phenotypes of chronic obstructive pulmonary disease and asthma: recent advances. J Allergy Clin Immunol. 2013;131:627–634. doi: 10.1016/j.jaci.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Postma DS, Rabe KF. The Asthma-COPD Overlap Syndrome. N Engl J Med. 2015;373:1241–9. doi: 10.1056/NEJMra1411863. [DOI] [PubMed] [Google Scholar]
- 30.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD for COPDGene Investigators. Genetic epidemiology of COPD (COPDGene) Study Design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavord ID, Lettis S, Locantore N, Pascoe S, Jones PW, Wedzicha JA, Barnes NC. Blood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPD. Thorax. 2015;71:118–125. doi: 10.1136/thoraxjnl-2015-207021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bafadhel M, Pavord ID, Russell REK COPD 2017. Eosinophils in COPD: just another biomarker? Lancet Respir Med. 2017 doi: 10.1016/S2213-2600(17)30217-5. Published Online June 7, 2017. [DOI] [PubMed] [Google Scholar]
- 33.Dasgupta A, Kuarsgaard M, Capaldi D, Radford K, Aleman F, Boylan C, Altman LC, Wight TN, Parraga G, O’Byrne PM, Nair P. A pilot randomized clinical trial of mepolizumab in COPD with eosinophilic bronchitis. Eur Respir J. 2017;49:1602486. doi: 10.1183/13993003.02486-2016. [DOI] [PubMed] [Google Scholar]
- 34.Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomized controlled trials. Lancet Respir Med. 2015;3:435–442. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 35.Hospers JJ, Schouten JP, Weiss ST, Postma DS, Rijcken G. Eosinophilia is associated with increased all-cause mortality after a follow-up of 30 years in a general population sample. Epidemiol. 2000;11:261–268. doi: 10.1097/00001648-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Tamada T, Sugiura H, Takahashi T, Matsunaga K, Kimura K, Katsumata U, Takekoshi D, Kikuchi T, Ohta K, Ichinose M. Biomarker-based detection of asthma-COPD overlap syndrome in COPD populations. Int J Chron Obstruct Pulmon Dis. 2015;10:2169–2176. doi: 10.2147/COPD.S88274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodruff PG, Barr RG, Bleecker ER, Christenson SA, Couper DJ, Curtis JL, Gouskova NA, Hansel NN, Hoffman EA, Kanner RE, Kleerup E, Lazarus SC, Martinez FJ, Paine R, III, Rennard S, Tashkin DP, Han MK for the SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–21. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogliani P, Puxeddu E, Ciaprini C, Ora J, Onorato A, Pezzuto G, Calzetta L, Cazzola M. The time course of pulmonary function tests in COPD patients with different levels of blood eosinophils. Bio Med Res Int. 2016;2016:4547953. doi: 10.1155/2016/4547953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure S1. Consort Diagram for subjects recruited and reason for removal from analysis. All normal, never smokers were removed. Although 90% of subjects (including normal never smokers) may have been eligible for sputum induction, there were several reasons that reduced the actual number of sputum slide samples available for analysis: no sputum produced upon completion of induction, no sputum processing form entered, removal of aliquots for mucus analysis and microbiome prior to processing leaving too little remaining sample for cytospin slide preparation, slides not sent to central slide reading center, and finally slide counts that were deemed unacceptable (leukocyte cell count <100 or too high, >80% squamous epithelial cells). There were 16 subjects with acceptable sputum differential count but without blood counts who were added to those with both acceptable sputum counts and blood counts (N=811).
Supplement Figure S2. Classification tree diagram for model of exacerbations by sputum and blood eosinophils. The Root has 811 subjects with 92 exacerbations in previous year. The first number in each node is the number of subjects without exacerbations; the second number is the number with exacerbations. The model first divides the subjects based on sputum eosinophils < and ≥1.9% and secondly divides the subjects by both sputum and blood eosinophils (< or >176/µL).
Supplement Figure S3. ROC analysis for blood Eos prediction of sputum Eos. Blood Eos at cutpoints from 50/µL (highest sensitivity) to 500/µL (lowest sensitivity) were examined for correct prediction of sputum Eos < or ≥2%. Although significant (p<0·001), the area under the curve (AUC) was only 0·64, demonstrating a lack of strength for the prediction. Maximum sensitivity and specificity were observed at a blood Eos cutpoint of 250/µL, but with very large false discovery rate (74%) and false negative rate (50%).