Abstract
Objective
A third of Americans are obese and an increasing number opt for bariatric surgery. This study estimates the cost-effectiveness of common bariatric surgical procedures from a healthcare system perspective.
Methods
We evaluated the three most common bariatric surgical procedures in the US: laparoscopic gastric bypass (LRYGB), conventional (open) Roux en Y gastric bypass (ORYGB), and laparoscopic adjustable gastric banding (LAGB) compared to no surgery. The reference case was defined as a 53-year old female with BMI of 44 kg/m2. We developed a two-part model using a deterministic approach for the first 5-year period post-surgery and separate empirical forecasts for the natural history of body mass index (BMI), costs and outcomes in the remaining years. We used a combination of datasets including Medicare and MarketScanR together with estimates from the literature to populate the model.
Results
Bariatric surgery produced additional life expectancy (80 to 81 years) compared to no surgery (78 years). The incremental cost-effectiveness ratios (ICERs) of the surgical procedures were $6600 per QALY gained for LRYGB, $6200 for LAGB, and $17,300 for ORYGB (3% discount rate for cost and QALYs). ICERs varied according to choice of BMI forecasting method and clinically plausible variation in parameter estimates. In most scenarios, the ICER did not exceed a threshold of $50,000 per QALY gained.
Conclusion
Gastric bypass and banding procedures are likely to be cost-effective for obese patients compared to nonsurgical treatment at conventional cost-effectiveness thresholds.
Background
In the United States (U.S), over a third of the adult population has measurements of body mass index (BMI) equal or above 30 kg/m2, which defines them as obese [1,2]. The prevalence of obesity ranged from 4% to 28% among adult men and 6% to 37% among adult women across 28 countries in Europe. Obesity is associated with multiple chronic conditions including diabetes mellitus, coronary heart disease, sleep apnea, osteoarthritis, and other metabolic and cardiovascular risk factors, as well as specific types of cancers [3–7]. These conditions, associated with obesity, increase the risk of premature death and healthcare expenditures while reducing patient quality of life and productivity [3,4,7–10]. Pharmacological and non-pharmacological interventions have shown high attrition rates and limited efficacy and effectiveness in long-term weight loss [11,12]. Surgical interventions for obesity, on the other hand, offer the potential for significant and sustained weight loss, improving, or in some cases resolving associated conditions. These interventions have been shown to improve long-term survival [13,14], but at significant costs [10,15] that may limit access to treatment [16,17].
With the increasing availability and prevalence of bariatric procedures in the U.S., it is important for patients, payers and policymakers to understand the long-term cost-effectiveness of these approaches. Previous studies in the U.S. and other countries have found that bariatric procedures are generally cost-effective and potentially cost-saving [18–30]. However, many of these studies make the assumption that the benefits of bariatric surgery persist over time, particularly with regard to the persistence of weight loss. There are limited long-term data following subjects who have undergone bariatric surgery and even less for newer procedures, making it very difficult to assess with precision the long-term benefits and harms associated with these procedures. Another common methodological challenge is the selection of a control or reference group that would be comparable to the group of patients undergoing these procedures.
Our aim was to estimate the lifetime cost-effectiveness of the three most common bariatric procedures in the United States: open roux-in-y gastric bypass (ORYGB), laparoscopic roux-in-y gastric bypass (LRYGB), and laparoscopic adjustable gastric banding (LAGB), compared to non-surgical interventions for severe obesity. We used a non-surgical comparator group who meet current clinical indications for these procedures, including individuals with BMI 40 and above and for those with BMI 35 and above with specific comorbidities. Our approach modeled the lifetime cost-effectiveness in two parts. First, we modeled direct medical costs and outcomes in the first five years after a bariatric procedure using a deterministic approach. Second, because data pertaining to costs and outcomes associated with bariatric surgery beyond five years are limited, we developed a natural history model to project costs and outcomes for the remaining years.
Methods
We estimated the lifetime direct medical costs and outcomes associated with three bariatric procedures (ORYGB, LYRGB, LAGB) compared with non-surgical care using a simulation model for individuals eligible for bariatric surgery based on BMI. The non-surgical care group consisted of patients receiving usual medical care for obesity associated health conditions, such as hypertension, diabetes, and dyslipidemia. In most health plans or care systems in the U.S., usual care for severe obesity does not include any intensive weight loss treatment or pharmacotherapy, therefore any weight loss achieved by patients in the non-surgical care group reflected self-directed and self-financed weight loss treatment.
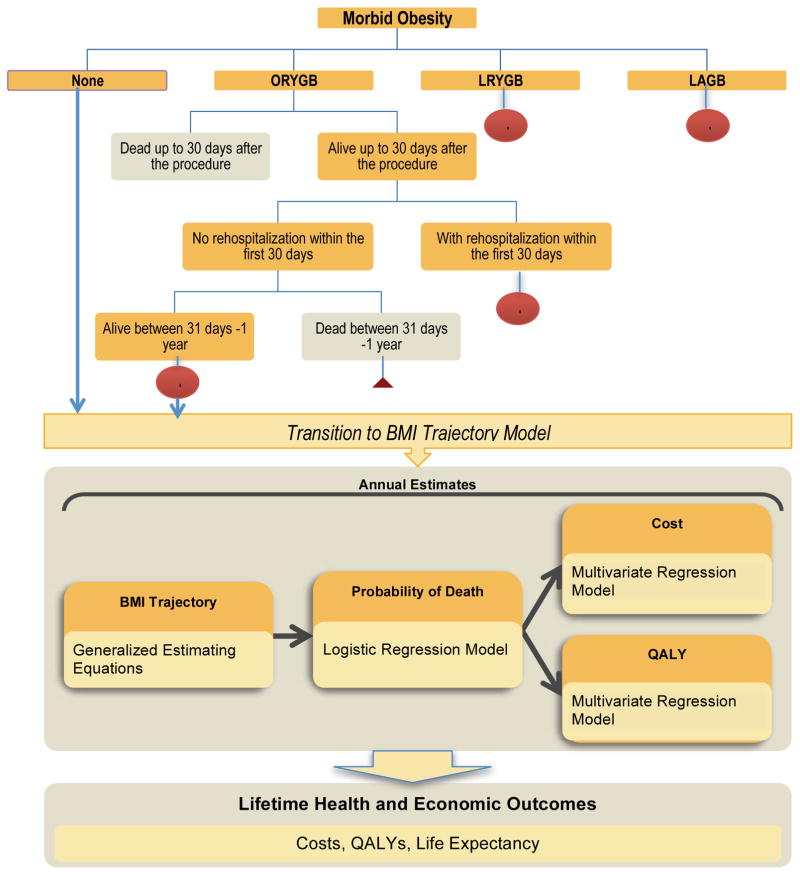
The simulation was based on a two-part, linked model (Figure 1): 1) a decision analytic model, which models the cost-effectiveness of the surgical procedures in the first five years post-surgery; and 2) an empirical “natural history” model that models long-term changes in BMI, costs, and quality of life for the non-surgical control population as well as the surgical population beyond the fifth year following surgery. This two-part model was comprised of a number of interconnected regression equations to forecast expected lifetime changes in BMI, life expectancy, costs, and patient quality of life and their response to various treatments. The rationale behind these two linked models was to maximize the use of clinically rich, individual-level data to model outcomes for the first 5 years after surgery, which allowed us to simulate more complex clinical scenarios, including early complications. The natural history model provided the flexibility to estimate long-term outcomes based on nationally representative cohorts of obese U.S. adults for year six post-surgery and beyond, where direct clinical data on bariatric surgical patients are limited. A more detailed description of the modeling equations, parameters, and estimation techniques is provided in the Technical Appendix. All analyses were performed from the payer perspective and assume a standard discount rate of 3% for costs and quality-adjusted life expectancy (QALY). We adjusted for inflation by converting all costs to 2010 dollars using the Consumer Price Index. All models were implemented in Microsoft Excel (Microsoft Corporation, Redmond, WA USA).
Figure 1.
Decision analytic model for the first five years after bariatric surgery and the empirical models for lifetime outcomes and costs.
+ represent branches collapsed for presentation purposes. For surgical procedures, subtrees are identical to ORYGB. In subtree of patients Alive between 30 days - 1 year, subsequent branches are divided on Dead or Alive yearly until end of year 5.
Decision Analytic Model
The decision analytic model assessed the impact surgical procedures compared to usual non-surgical care in the first five years. Each surgical procedure was simulated to estimate changes in BMI, costs, and quality-adjusted life expectancy from the time of procedure to five years post-surgery. All-cause mortality, complication rates in the first 30 days after each procedure and direct medical costs were estimated directly using the Medicare claims database (2004–2008). The presence of complications (defined as rehospitalization within 30 days of surgery or prolonged length of stay) during this period was used to identify subjects with increased health resource use over the first five years after each procedure. Because BMI data were not available in Medicare, annual changes in BMI associated with each procedure were drawn from a systematic review of literature examining the effectiveness of bariatric surgery [31].
All-cause mortality during the 0–30 and 31–365 day periods post-surgery was measured as unadjusted rates from Medicare data. Annual mortality in years two through five post-surgery were estimated using a regression model (see Natural History Model and Technical Appendix, Table 4). We used data from a prior systematic review of studies measuring the clinical effectiveness of bariatric surgery [31] to assess changes in utility for the first 30 days following each procedure. Utility changes from 31 days until year five post-surgery conditional on age, BMI and gender were also estimated using a regression model (see Natural History Model). Finally, we estimated annual direct medical costs in the first five years post-surgery using a generalized linear model (GLM) with a log link function and gamma distribution. Adjustment variables in the GLM cost model included procedure type (ORYGB, LRYGB, and LAGB), complications (death within 30 days, alive with complications, alive without complications), age and gender. Regression results for these GLM cost models are in Tables 5–10 of the Technical Appendix. Also, a summary of simulation parameter values for each procedure is presented in Tables 1–3 of the Technical Appendix.
Natural History Model
We assessed the direct medical costs and outcomes for non-surgical subjects and surgical patients beyond five years post-surgery using a lifetime trajectory model. For example, outcomes for a patient who underwent a bariatric procedure at age 40-years were predicted using the surgery-specific decision tree until age 45 and were predicted using the natural history trajectory model from age 46 until expected death. Cost and outcomes for non-surgical patients were derived from the natural history model in all years.
The natural history model was driven by our previously published annual estimates of BMI change conditional on survival [32]. Our primary model predicts BMI over time given starting age, baseline BMI, and gender using longitudinal data from patients with BMI >= 35 enrolled in Group Health [33] between 2005 and 2010.
Consistent with prior published methods [25], we used a logistic regression model to estimate mortality with 5-year probability of death as the primary outcome. Expected years of life based on BMI, age and gender were derived from static life tables constructed using predicted survival probabilities from the logistic model. Data used to estimate the survival model were from the National Health Interview Survey between 1997 and 2000 linked to the National Death Index with mortality follow-up through December 31, 2005. Survival model results are provided in Table 4 of the Technical Appendix. Given the predicted BMI and survival probability in each period, we estimated health utility and all-cause medical expenditure using data from the Medical Expenditure Panel Survey (MEPS) between 2000 and 2006. For utilities, we used the SF-12 physical and mental component summary scales to generate EQ-5D scores based on an existing algorithm [34]. We then estimated utilities using GLM with a log link and gamma distribution in order to address the skewness in the distribution of EQ-5D scores. Table 11 of the Technical Appendix presents health utility regression results. Annual medical costs were estimated using a two-part model [35,36] to address the high proportion of MEPS respondents with zero costs. The first part of the two-part model estimated the probability of non-zero costs using a probit model. We then estimated the second part of the two-part model using a GLM across the sample of respondents with non-zero costs, assuming a square root link and gamma distribution. Adjustment variables in the utility and cost models included BMI, age and gender. All GLM link functions were verified using the Box-Cox test and outcome distributions were verified using the Modified Park test [37]. Cost regression results are in Table 12 of the Technical Appendix.
We also computed the marginal effect of BMI and other adjustment variables on health utility and total costs. For models estimated using GLM, we first applied inverse link functions to linear predictors in order to compute marginal effects on expected outcomes. Because costs were estimated using a two-part model, marginal effects incorporate the impact of adjustment variables on the likelihood of non-zero costs and expected costs conditional on non-zero costs.
Validation, Sensitivity and Scenario Analysis
We undertook a technical validation by reproducing analytic models using SAS (SAS Institute Inc., Cary, NC), which produced identical results. We examined the robustness of the findings with multiple scenarios and sensitivity analyses. One-way sensitivity analyses were performed using age, gender, early mortality, early complication rates, baseline BMI, BMI loss after five years, and discount rates. We also examined three different scenarios for weight-loss maintenance after surgery using the BMI trajectory model described above. The scenarios examined were: 1) Primary Model: following the same trajectory of a non-surgical patient after reaching the BMI at year five post-surgery; 2) Weight Stable Model: maintaining the same weight achieved at year five post-surgery, or 3) Maximum Weight Regain Model: regaining 100% of weight lost in the first five years within 15 years of surgery. We relied primarily on Medicare data to inform our cost analyses in the decision tree, but additionally, we used Thomson Reuters MarketScan® data to test the sensitivity of our results.
Results
Decision Analytic Model
For the base case, we simulated lifetime costs and outcomes for the average individual undergoing bariatric surgery in the Medicare database. Specifically, results are presented for a 53 year-old female with a baseline BMI of 44 kg/m2. Table 1 presents cost and utility inputs to the decision analytic model. Costs in respective annual periods post procedure were generally largest for ORYGB followed by LRYGB and LAGB. Utility estimates were the same for ORYGB and LRYGB because the magnitude of weight loss for these procedures was assumed to be equal.
Table 1.
Clinical and cost inputs for the initial 5-year period post-surgery (53 year old female with a BMI of 44 kg/m2 and no post-surgical complications).
| LRYGB | ORYGB | LAGB | |
|---|---|---|---|
| Total Cost (<31 days) | $ 16,691 | $ 20,675 | $ 14,159 |
| Total Cost (31–365 days) | $ 9,494 | $ 12,849 | $ 7,835 |
| Total Cost (Year 2) | $ 9,478 | $ 12,302 | $ 8,551 |
| Total Cost (Year 3) | $ 8,507 | $ 11,237 | $ 7,917 |
| Total Cost (Year 4) | $ 5,622 | $ 9,887 | $ 6,291 |
| Total Cost (Year 5) | $ 1,221 | $ 5,764 | $ 2,685 |
| Cost of Death (All periods) | $38,049 | $ 38,049 | $ 38,049 |
| Utility (0–31 days) | 0.75 | 0.75 | 0.75 |
| Utility (31–365 days) | 0.78 | 0.78 | 0.75 |
| Utility (Year 2) | 0.74 | 0.74 | 0.73 |
| Utility (Year 3) | 0.73 | 0.73 | 0.70 |
| Utility (Year 4) | 0.70 | 0.70 | 0.67 |
| Utility (Year 5) | 0.67 | 0.67 | 0.65 |
Natural History Model
In our simulation, all-cause mortality rates were higher among men and were increasing with age and BMI, although the associated mortality risk that was attributable to obesity decreased as individuals increased in age (Technical Appendix, Table 4). Higher BMI was also associated with lower health utility (Technical Appendix, Table 11) and higher total costs (Technical Appendix, Table 12). For the base case, an increase in BMI by 10 units was associated with a 0.039 decrease in utility; an increase of 10 years in age was associated with a 0.030 decrease in utility; and being female was associated with a 0.031 decrease in utility. For annual direct medical cost, increasing BMI by one unit was associated with an additional $261; increasing age by one year was associated with an additional $171; and being female was associated with an additional $1573 in annual medical expenditures.
Simulation Results
Table 2 presents simulation results for the base case. There were higher lifetime direct medical costs for the surgical interventions compared to the non-surgical cohort. All surgical interventions were associated with longer life expectancy compared with the non-surgical cohort and yielded both greater life years and QALYs.
Table 2.
Results of the lifetime simulation for the base case (53 year old Female with BMI 44 kg/m2, using Medicare data)
| Lifetime Outcomes | ||||
|---|---|---|---|---|
| Intervention | Non-surgical | LRYGB | LAGB | ORYGB |
| Standard BMI Trajectory | ||||
| Direct Medical Costs | $150,934 | $169,074 | $164,313 | $194,858 |
| QALY | 10.6 | 13.4 | 12.8 | 13.2 |
| Life-years | 16.7 | 18.0 | 17.7 | 17.8 |
| Expected Age of Death | 78 | 81 | 80 | 80 |
| ICER | $6,600 | $6,200 | $17,300 | |
| Weight Stable Model | ||||
| Direct Medical Costs | $150,934 | $169,091 | $164,076 | $194,874 |
| QALY | 10.6 | 13.7 | 12.7 | 13.5 |
| Life-years | 16.7 | 18.2 | 17.7 | 18.0 |
| Expected Age of Death | 78 | 81 | 80 | 81 |
| ICER | $6,000 | $6,300 | $15,600 | |
| Maximum Weight Regain | ||||
| Direct Medical Costs | $150,934 | $175,815 | $171,001 | $201,493 |
| QALY | 10.6 | 11.7 | 11.4 | 11.5 |
| Life-years | 16.7 | 16.8 | 16.7 | 16.5 |
| Expected Age of Death | 78 | 78 | 78 | 77 |
| ICER | $24,100 | $26,700 | $59,500 | |
The incremental cost-effectiveness ratios (ICERs) for surgical procedures compared to non-surgical were $6600 per QALY gained for LRYGB, $6200 for LAGB, and $17,300 for ORYGB. The net present values of the total lifetime costs were higher for the surgical arms compared to no surgery. However, if we monetize the QALYs at a willingness-to-pay (WTP) threshold of $100,000/QALY, we show a large net economic benefit of each surgical procedure: $257,200 saved for LRYGB, $202,300 saved for LAGB, and $210,500 saved for ORYGB.
Results were sensitive to alternative weight change scenarios. The ICERs for surgical procedures compared to no surgery under the assumption of weight stability (Scenario 2) were $6000 per QALY gained for LRYGB, $6300 for LAGB and $15,600 for ORYGB. Under the assumption of full weight regain by year 15 post-procedure, ICERs relative to no surgery were $24,100 per QALY gained for LRYGB, $26,700 for LAGB and $59,500 for ORYGB.
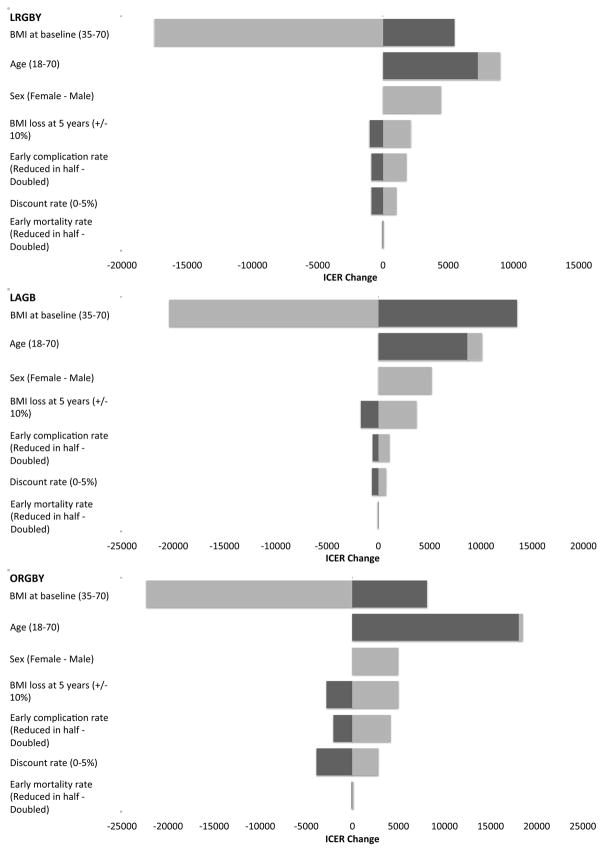
Figure 2 presents results from the one-way sensitivity analysis for key model parameters. Dark (light) bars indicate the change the ICER reflected by modifying the specified model parameter to the lower (upper) bound. A negative change reflects a lower ICER after modifying the model parameter. The one-way sensitivity analyses showed that the parameters with the largest impact were BMI at baseline, age at the time of the procedure and gender. ICERs were decreasing with baseline BMI and the magnitude of weight loss after 5 years, but were increasing with higher discount rate and higher rates of early complication. ICERs were also higher for men.
Figure 2.
Results from one-way sensitivity analyses
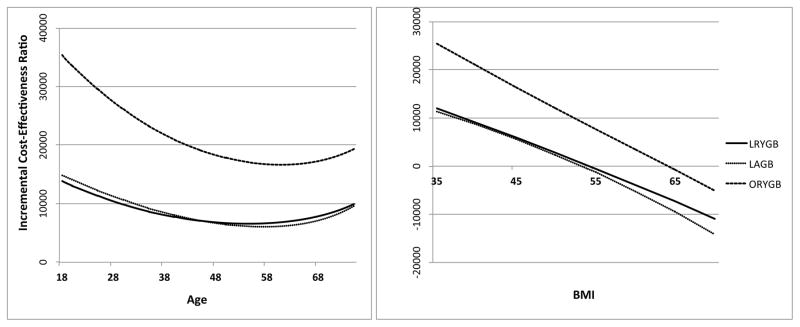
We further examined the sensitivity of ICER estimates to age and baseline BMI in Figure 3. ICER estimates for all procedures were a nonlinear function of age. Decreasing age to 18 and increasing age to 70 both resulted in higher ICER estimates relative to the base case. ICERs were minimized at age 54, 57 and 61 for LRYGB, LAGB and ORYGB, respectively. Relatively high ICER estimates for patients at the left tail of the age distribution reflect increases in BMI predicted by the natural history model after five years post-surgery. ICER estimates were monotonically decreasing with baseline BMI. LRYGB is cost saving at baseline BMI of 55 and above. LAGB is cost saving at baseline BMI of 54 and above.
Figure 3.
Incremental cost-effectiveness ratios as a function of baseline age and BMI.
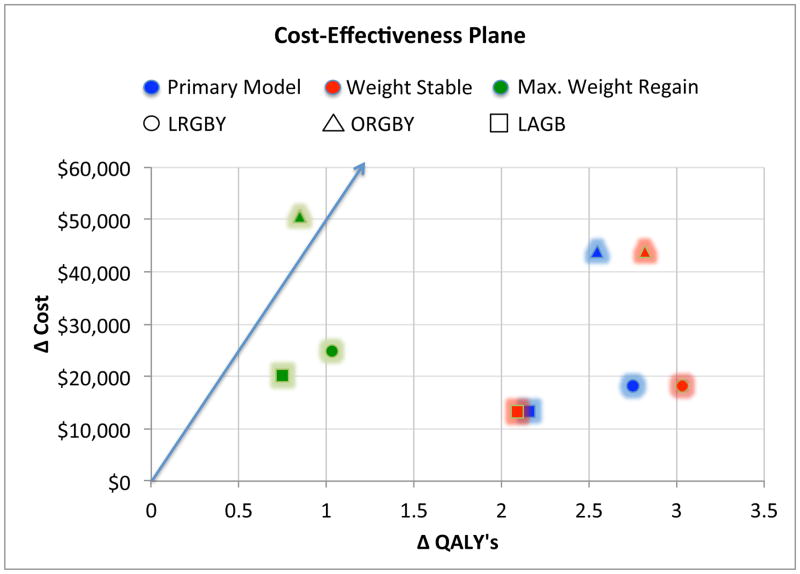
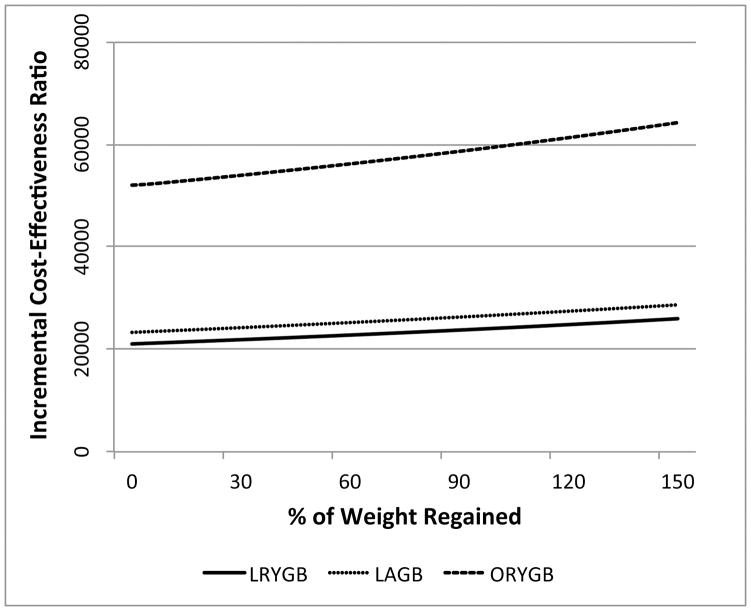
In Figure 4, we calculated costs per QALY for each procedure under the three weight change trajectory scenarios previously described. Points in Figure 4 vary by shape (procedure) and color (weight change scenario). Points below the blue line reflect combinations that were cost-effective given a willingness-to-pay (WTP) threshold of $50,000 per QALY gained. Maintaining base case assumptions, ICER point estimates were cost-effective in eight out of the nine simulated scenarios. The exception was ORYGB under the maximum weight regain scenario. We further examined the sensitivity of ICER estimates to long-term weight loss by varying the amount of weight regained in Scenario 3 from 0% to 150%. Figure 5 presents ICERs as a function of the percent of weight regained by year 15 post-surgery. For LRYGB and LAGB, ICERs ranged from $21,000 to $29,000 per QALY gained. For ORYGB, ICERs were above $50,000 per QALY gained for all levels of weight regain.
Figure 4.
Estimated cost-effectiveness for the surgical procedures under three different weight-loss maintenance scenarios.
**The red line represents the Threshold for $50,000 per QALY gained.
QALY = quality-adjusted life years gained compared to non-surgical cohort.
Figure 5.
Incremental cost-effectiveness ratios as a function of percentage weight regained by year 15.
To assess potential cost differences from using an alternative data source, we also used commercial claims data from MarketScan® (years 2002–2009), in which the average patient receiving bariatric surgery was a 43 year-old female with BMI 44 kg/m2. The average 5-year cost of a non-surgical patient was $55,700 compared with $45,900, $55,400, and $75,200 for LAGB, LRYGB, and ORYGB, respectively.
Discussion
A third of Americans are obese and an increasing number are opting for bariatric surgery [38]. Patients elect surgical intervention in part because they believe that bariatric surgery will likely reduce BMI and weight-associated clinical complications in the long-term. However, health care payers lack consistent reimbursement policies for bariatric surgery, in part because data on long-term costs, effectiveness, and safety are unavailable. We undertook a comprehensive, empirical assessment of the cost-effectiveness of the most common bariatric surgical approaches compared to a non-surgical cohort to inform payers who value comparative economic information for decisions, whether personal or for coverage policies. We set out to estimate the cost-effectiveness of these procedures using a mixed, empirical method that makes use of clinically rich data, administrative claims information and public data sources.
Our base case analysis showed that each of the bariatric procedures analyzed is cost-effective compared to no surgery for most patients eligible for bariatric surgery, assuming a willingness-to-pay (WTP) threshold of $50,000 per QALY gained. Each procedure was also generally cost-effective when compared to no surgery under alternative assumptions of weight change, including complete weight regain by 15 years post-procedure. In contrast to other studies [20,28], our results indicate that the initial cost of surgery is sufficiently high such that net cost-savings is not achieved over a lifetime horizon unless the economic impact of surgery on improved quality of life is taken into consideration.
Collectively, our findings demonstrating the cost-effectiveness of bariatric surgery compared to no surgical intervention are consistent with other prior studies [24,26,27,29,30]. Direct comparisons of cost-effectiveness are difficult because of differences in study populations; however, our base case ICER estimates were generally smaller than those reported in other studies. For example, a prior study used a simulated cohort representative of the population of newly diagnosed diabetes patients over BMI 30 in the National Health and Nutrition Examination Survey and estimated cost-effectiveness ratios of $7000/QALY and $11,000/QALY for gastric bypass and gastric banding, relative to no surgery [30]. One-way sensitivity analyses in our study showed ICERs were sensitive to model parameters, particularly BMI and age at baseline. Decreases in each of these parameters may yield ICER estimates closer to those reported in prior studies. Base case lifetime cost estimates in this study were also higher relative to cost estimates obtained in other studies that employ a lifetime analysis. There are at least two reasons for these differences. First, our study estimated all-cause medical expenditure associated with obesity, compared to other studies [27,29,30] that measured only costs associated with obesity related diseases. Second, our costs reflect 2010 dollars, thus differences in costs across studies may in part reflect inflation.
Our approach differed from prior studies in a number of ways. First, we estimated the direct costs of bariatric surgery using cost data from Medicare and commercial health insurance plans, as opposed to published estimates of costs per procedure. Second, we developed a simulation model to estimate lifetime costs and outcomes of patients undergoing bariatric surgery beyond five years post-intervention, which is in contrast to previous studies [24,26,27] that rely on cost estimates from literature. We used regression models to estimate lifetime costs and outcomes as a function of BMI, which an important indicator of the effectiveness of bariatric surgery [39]. Additionally, BMI has been shown to be an robust predictor of costs [8], mortality [40–42] and health utility [43,44]. Third, in contrast to other lifetime simulation models [29,30], our approach did not target a disease specific population and evaluated differences in all-cause medical expenditure between bariatric patients and non-surgical patients. These two prior studies simulated health utility among a cohort of type 2 diabetes patients and lifetime costs associated with the treatment and management of diabetes.
The ICER estimates for bariatric surgical procedures appear to be cost-effective under most modeled scenarios. Current trends toward better immediate post-surgical outcomes (i.e., lower mortality and fewer complications) and long-term management are likely to make surgical options even more cost-effective. For example, the growth of bariatric procedures in recent years may lead to lower prices due to economies of scale and price competition, as these procedures become subject to bundled payment or other managed pricing schemes. Moreover, in previous work, a trend towards better surgical outcomes following bariatric procedures with lower mortality rates and lower complication rates has been shown [45]. The combination of lower prices and better outcomes, other factors staying equal, would lower ICERs.
Despite the promising results of our study, suggesting that bariatric surgery is a cost-effective intervention to improve the health of the obese, there remain notable limitations. First, the cost-effectiveness of these procedures is highly dependent upon the clinical data for initial weight loss and the forecasted change in BMI over the simulation time period, which affects survival, cost and QALY estimates in the natural history model. We estimated BMI trajectories using a sample of severely obese patients, but were unable to estimate BMI change among patients undergoing bariatric surgery due to limited long-term BMI data. Given currently available data, there was no way of knowing whether the BMI trajectories of surgical patients behave similarly or differently from non-surgical patients in the long-term after the initial weight loss phase. We attempted to address this limitation by employing three alternative long-term BMI trajectory scenarios and found that our results were robust to different assumptions of weight change. Second, the lack of long-term follow-up data on costs and outcomes for bariatric patients (especially for LAGB) necessitated the development of a natural history trajectory model. Longer follow-up data from randomized control trials or rigorously conducted, controlled observational studies could yield more accurate projections. This is particularly relevant given emerging evidence of poor long-term outcomes for the LAGB procedure. For example, a previous study found that after 14 years, the reoperation rate was 30% and the incidence of band removal was 12% [46]. Depending on the trajectory of BMI following removal, the cost-effectiveness of LAGB could be worse, though there is little information to guide modeling of this scenario. Third, medical costs, complication rates and 1-year mortality in the decision analytic model were derived using Medicare data. While these data provide a national sample of patients undergoing bariatric surgery, subjects who are less than age 65 are only eligible for Medicare if they are disabled or have end-stage renal disease. As a result, the representative subject undergoing surgery in our simulation is likely different relative to the average subject outside of Medicare. Third, systematic differences between the non-surgical group and patients opting for bariatric surgery that are not controlled for in our model may, at least in part, impact ICER estimates. For example, if non-surgical patients have a greater number of comorbidities, then our models potentially understate the true ICER values. Lastly, this analysis aggregates outcomes of large cohorts together for a mean effect when for some procedures (e.g., LAGB) there appears to be a bimodal response effect [47]. Some patients respond with significant weight loss while others do not, however, there is no existing evidence available to predict which patients are more likely respond to LAGB by losing a significant amount of weight. Thus, our results should be interpreted accordingly.
Conclusion
Our results suggest surgical procedures to treat morbid obesity improve patient quality of life and their life expectancy by reducing BMI and other comorbidities, but are associated with higher lifetime direct medical costs. However, under most reasonable assumptions, bariatric surgery appears to be cost-effective compared to no surgery using a lifetime timeframe. Depending upon a specific willingness to fund QALY gains, bariatric surgery may be cost-effective compared to no surgery. The sustainability of the benefits from bariatric surgery, in terms of weight maintenance and comorbidity resolution, is essential to determine the value of these interventions. Additional data over a longer duration of follow-up measuring the effectiveness and safety of these procedures are needed to improve the precision of these estimations.
Supplementary Material
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ogden C, Carroll M. Prevalence of overweight, obesity, and extreme obesity among adults: United states, trends 1976 1980 through 2007 2008. National Center for Health Statistics, Centers for Disease Control and Prevention; Atlanta: 2010. [Google Scholar]
- 3.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117(13):1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 4.McQueen DA, Long MJ, Algotar AM, Schurman JR, 2nd, Bangalore VG. The effect of obesity on quality-of-life improvement after total knee arthroplasty. Am J Orthop (Belle Mead NJ) 2007;36(8):E117–120. E127. [PubMed] [Google Scholar]
- 5.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 7.Bessesen DH. Update on obesity. J Clin Endocrinol Metab. 2008;93(6):2027–2034. doi: 10.1210/jc.2008-0520. [DOI] [PubMed] [Google Scholar]
- 8.Arterburn DE, Maciejewski ML, Tsevat J. Impact of morbid obesity on medical expenditures in adults. Int J Obes (Lond) 2005;29(3):334–339. doi: 10.1038/sj.ijo.0802896. [DOI] [PubMed] [Google Scholar]
- 9.Fry J, Finley W. The prevalence and costs of obesity in the EU. Proc Nutr Soc. 2005;64(3):359–362. doi: 10.1079/pns2005443. [DOI] [PubMed] [Google Scholar]
- 10.Bachman KH. Obesity, weight management, and health care costs: a primer. Dis Manag. 2007;10(3):129–137. doi: 10.1089/dis.2007.103643. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, Hilton L, Suttorp M, Solomon V, Shekelle PG, Morton SC. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142(7):532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- 12.Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, Guven S, Spitz AF, Apovian CM, Livingston EH, Brolin R, Sarwer DB, Anderson WA, Dixon J. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical Guidelines for Clinical Practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Surg Obes Relat Dis. 2008;4(5 Suppl):S109–184. doi: 10.1016/j.soard.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 14.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lonroth H, Narbro K, Naslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 15.Powers KA, Rehrig ST, Jones DB. Financial impact of obesity and bariatric surgery. Med Clin North Am. 2007;91(3):321–338. ix. doi: 10.1016/j.mcna.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Frezza EE. Six steps to fast-track insurance approval for bariatric surgery. Obes Surg. 2006;16(5):659–663. doi: 10.1381/096089206776945129. [DOI] [PubMed] [Google Scholar]
- 17.Champion JK, Williams M. Economic impact of bariatrics on a general surgery practice. Obes Surg. 2006;16(2):113–118. doi: 10.1381/096089206775565186. [DOI] [PubMed] [Google Scholar]
- 18.Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, Smith WC, Jung RT, Campbell MK, Grant AM. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8(21):iii–iv. 1–182. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- 19.Paxton JH, Matthews JB. The cost effectiveness of laparoscopic versus open gastric bypass surgery. Obes Surg. 2005;15(1):24–34. doi: 10.1381/0960892052993477. [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein EA, Brown DS. A cost-benefit simulation model of coverage for bariatric surgery among full-time employees. Am J Manag Care. 2005;11(10):641–646. [PubMed] [Google Scholar]
- 21.van Mastrigt GA, van Dielen FM, Severens JL, Voss GB, Greve JW. One-year cost-effectiveness of surgical treatment of morbid obesity: vertical banded gastroplasty versus Lap-Band. Obes Surg. 2006;16(1):75–84. doi: 10.1381/096089206775222113. [DOI] [PubMed] [Google Scholar]
- 22.Ackroyd R, Mouiel J, Chevallier JM, Daoud F. Cost-effectiveness and budget impact of obesity surgery in patients with type-2 diabetes in three European countries. Obes Surg. 2006;16(11):1488–1503. doi: 10.1381/096089206778870067. [DOI] [PubMed] [Google Scholar]
- 23.Levy P, Fried M, Santini F, Finer N. The comparative effects of bariatric surgery on weight and type 2 diabetes. Obes Surg. 2007;17(9):1248–1256. doi: 10.1007/s11695-007-9214-z. [DOI] [PubMed] [Google Scholar]
- 24.Salem L, Devlin A, Sullivan SD, Flum DR. Cost-effectiveness analysis of laparoscopic gastric bypass, adjustable gastric banding, and nonoperative weight loss interventions. Surg Obes Relat Dis. 2008;4(1):26–32. doi: 10.1016/j.soard.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schauer DP, Arterburn DE, Livingston EH, Fischer D, Eckman MH. Decision modeling to estimate the impact of gastric bypass surgery on life expectancy for the treatment of morbid obesity. Arch Surg. 2010;145(1):57–62. doi: 10.1001/archsurg.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell J, McGarry LA, Shikora SA, Hale BC, Lee JT, Weinstein MC. Cost-effectiveness of laparoscopic gastric banding and bypass for morbid obesity. Am J Manag Care. 2010;16(7):e174–187. [PubMed] [Google Scholar]
- 27.Craig BM, Tseng DS. Cost-effectiveness of gastric bypass for severe obesity. Am J Med. 2002;113(6):491–498. doi: 10.1016/s0002-9343(02)01266-4. [DOI] [PubMed] [Google Scholar]
- 28.Cremieux PY, Buchwald H, Shikora SA, Ghosh A, Yang HE, Buessing M. A study on the economic impact of bariatric surgery. Am J Manag Care. 2008;14(9):589–596. [PubMed] [Google Scholar]
- 29.Ikramuddin S, Klingman D, Swan T, Minshall ME. Cost-effectiveness of Roux-en-Y gastric bypass in type 2 diabetes patients. Am J Manag Care. 2009;15(9):607–615. [PubMed] [Google Scholar]
- 30.Hoerger TJ, Zhang P, Segel JE, Kahn HS, Barker LE, Couper S. Cost-effectiveness of bariatric surgery for severely obese adults with diabetes. Diabetes Care. 2010;33(9):1933–1939. doi: 10.2337/dc10-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13(41):1–190. 215–357, iii–iv. doi: 10.3310/hta13410. [DOI] [PubMed] [Google Scholar]
- 32.Wong E, Wang B, Alfonso-Cristancho R, Flum D, Sullivan S, Garrison L, Arterburn D. BMI Trajectories Among the Severely Obese: Results From an Electronic Medical Record Population. Obesity. doi: 10.1038/oby.2012.29. (in press) [DOI] [PubMed] [Google Scholar]
- 33.Arterburn D, Ichikawa L, Ludman EJ, Operskalski B, Linde JA, Anderson E, Rohde P, Jeffery RW, Simon GE. Validity of Clinical Body Weight Measures as Substitutes for Missing Data in a Randomized Trial. Obes Res Clin Pract. 2008;2(4):277–281. doi: 10.1016/j.orcp.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franks P, Lubetkin EI, Gold MR, Tancredi DJ. Mapping the SF-12 to preference-based instruments: convergent validity in a low-income, minority population. Med Care. 2003;41(11):1277–1283. doi: 10.1097/01.MLR.0000093480.58308.D8. [DOI] [PubMed] [Google Scholar]
- 35.Mullahy J. Much ado about two: reconsidering retransformation and the two-part model in health econometrics. J Health Econ. 1998;17(3):247–281. doi: 10.1016/s0167-6296(98)00030-7. [DOI] [PubMed] [Google Scholar]
- 36.Duan N, Manning WG, Morris CN, Newhouse JP. A Comparison of Alternative Models for the Demand for Medical Care. J Bus Econ Stat. 1983;1(2):115–126. [Google Scholar]
- 37.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 38.Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009;19(12):1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 39.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 40.Wong ES, Wang BC, Garrison LP, Alfonso-Cristancho R, Flum DR, Arterburn DE, Sullivan SD. Examining the BMI-mortality relationship using fractional polynomials. BMC Med Res Methodol. 2011;11:175. doi: 10.1186/1471-2288-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durazo-Arvizu RA, McGee DL, Cooper RS, Liao Y, Luke A. Mortality and optimal body mass index in a sample of the US population. Am J Epidemiol. 1998;147(8):739–749. doi: 10.1093/oxfordjournals.aje.a009518. [DOI] [PubMed] [Google Scholar]
- 42.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 43.Lee AJ, Morgan CL, Morrissey M, Wittrup-Jensen KU, Kennedy-Martin T, Currie CJ. Evaluation of the association between the EQ-5D (health-related utility) and body mass index (obesity) in hospital-treated people with Type 1 diabetes, Type 2 diabetes and with no diagnosed diabetes. Diabet Med. 2005;22(11):1482–1486. doi: 10.1111/j.1464-5491.2005.01657.x. [DOI] [PubMed] [Google Scholar]
- 44.Sach TH, Barton GR, Doherty M, Muir KR, Jenkinson C, Avery AJ. The relationship between body mass index and health-related quality of life: comparing the EQ-5D, EuroQol VAS and SF-6D. Int J Obes (Lond) 2007;31(1):189–196. doi: 10.1038/sj.ijo.0803365. [DOI] [PubMed] [Google Scholar]
- 45.Flum DR, Kwon S, MacLeod K, Wang B, Alfonso-Cristancho R, Garrison LP, Sullivan SD. The use, safety and cost of bariatric surgery before and after Medicare's national coverage decision. Ann Surg. 2011;254(6):860–865. doi: 10.1097/SLA.0b013e31822f2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stroh C, Hohmann U, Schramm H, Meyer F, Manger T. Fourteen-year long-term results after gastric banding. J Obes. 2011;2011:128451. doi: 10.1155/2011/128451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bessler M, Daud A, DiGiorgi MF, Schrope BA, Inabnet WB, Davis DG. Frequency distribution of weight loss percentage after gastric bypass and adjustable gastric banding. Surg Obes Relat Dis. 2008;4(4):486–491. doi: 10.1016/j.soard.2008.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.