Abstract
O2-evolving chlorite dismutases (Clds) efficiently convert chlorite (ClO2−) to O2 and Cl−. Dechloromonas aromatica Cld (DaCld) is a highly active chlorite-decomposing homopentameric enzyme, typical of Clds found in perchlorate and chlorate respiring bacteria. The Gram-negative, human pathogen Klebsiella pneumoniae contains a homodimeric Cld (KpCld) that also decomposes ClO2−, albeit with a 10-fold lower activity and a lower turnover number compared to DaCld. The interactions between the distal pocket and heme ligand of the DaCld and KpCld active sites have been probed via kinetic, thermodynamic and spectroscopic behaviors of their cyanide complexes for insight into active site characteristics that are deterministic for chlorite decomposition. At 4.7 × 10−9 M, the KD for KpCld–CN− is two orders of magnitude smaller than that of DaCld–CN− and indicates an affinity for CN− that is greater than that of most heme proteins. The difference in CN− affinity between Kp and DaClds is predominantly due to differences in koff. The kinetics of cyanide binding to DaCld, DaCld(R183Q) and KpCld between pH 4 and 8.5 corroborate the importance of distal Arg183 and a pKa ~ 7 in stabilizing complexes of anionic ligands, including substrate. The Fe–C stretching and FeCN bending modes of DaCld–CN− (νFe-C, 441 cm−1; δFeCN, 396 cm−1) and KpCld–CN− (νFe-C, 441 cm−1; δFeCN, 356 cm−1) reveal differences in their FeCN angle, which suggest different distal pocket interactions with their bound cyanide. Conformational differences in their catalytic sites are also reported by the single ferrous KpCld carbonyl complex, which is in contrast to the two conformers observed for DaCld–CO.
Keywords: heme, chlorite dismutase, cyanide, Dechloromonas, Klebsiella, resonance Raman
INTRODUCTION
O2-evolving chlorite dismutases (Clds) catalyze the rapid decomposition of a single chlorite ion (ClO2−) to O2 and Cl− with heme b as the sole cofactor required for this unique O–O bond-forming reaction.1, 2 Unlike the water-oxidation reaction catalyzed by the tetramanganese center in the oxygen-evolving complex of photosystem II,3 Cld catalyzes the reaction without being coupled to generation of a proton gradient. Instead, the Cld-catalyzed reaction serves to detoxify chlorite generated from respiratory reduction of perchlorate or chlorate.4
The Cld protein family consists of three major subfamilies identified in phylogentic studies.5–9 Although members of two of these subfamilies differ in subunit size and oligomeric state, they are referred to as functional Clds because they catalyze the decomposition of ClO2−. The first subfamily consists predominantly of the respiratory pentameric Clds found mainly in Proteobacteria.10 Dimeric Clds from non-perchlorate-respiring species constitute the second subfamily; their subunit size is significantly smaller than that of the respiratory Clds due to a truncated N-terminus.9, 11 The third Cld subfamily contains coproheme decarboxylases.12 While these enzymes share structural similarities with the first two subfamilies, they catalyze a completely different reaction, the oxidative decarboxylation of coproheme as the terminal step of heme biosynthesis in Gram-positive bacteria.12–16
In perchlorate-respiring bacteria like Dechloromonas aromatica, Cld’s function is the efficient detoxification of the ClO2− produced by metabolic reduction of ClO4−.4 Dechloromonas aromatica Cld (DaCld) is capable of turning over >20,000 equivalents of ClO2− per heme prior to irreversible inactivation (kcat/KM = 3.2(± 0.4)×107 M−1 s− at pH 5.2, 4 °C).17 Although members of the second subfamily are efficient catalysts for ClO2− decomposition (kcat/KM = 106 M−1 s−1, pH 5.0–6.0, 20–30 °C)5, 9, 11, their in-vivo function is not clear. Growth studies of a Δcld knockout strain of Klebsiella pneumoniae strain MGH 78578, a Gram-negative, non-perchlorate-respiring bacterium, suggest that its Cld (KpCld) may have a role in detoxification of ClO2− produced endogenously by nitrate reductases, acting upon ClO4− or ClO3−.9 It has been hypothesized that a general function of Clds from non-perchlorate-respiring species may be protection against the effects of environmental ClO4− or ClO3−.1, 18, 19
The heme active sites of representatives of the two ClO2−-decomposing Cld subfamilies are shown in Figure 1. In both representative enzymes the heme is bound to the protein through a highly conserved proximal histidine,20, 22 and the distal pocket contains a conserved arginine.23–25 The distal arginine side chain position is flexible resulting in the accessibility of two conformations: one in which its guanidinium group is oriented toward the heme and one where the guanidinium group is directed toward the main access channel into the heme pocket.5, 20, 23, 26 The catalytic importance of the distal Arg is illustrated by substantial loss of chlorite degrading activity upon its mutation to Ala or Gln.23–25 Recent evidence suggests that one role of the distal Arg is confining ClO− generated during ClO2− decomposition to the heme pocket.27 In DaCld the Arg is quite effective in this role as ClO− is not detected during catalytic turnover.17, 28 When ClO− escapes from the heme pocket, as observed for KpCld and the Cld from Candidatus Nitrospira defluvii (NdCld), a pentameric Cld, it is readily detected as HOCl.9, 27 Enzyme degradation by HOCl limits KpCld to a significantly lower number of turnovers (6700 ± 300 equivalents ClO2−/heme) relative to DaCld.9 Thus, although the general heme site characteristics of Clds are similar, subtle variations in their active site structures and/or dynamics appear to modulate their ClO2− -decomposing activity and turnover.
Figure 1. Active site structures of a pentameric and a dimeric Cld.

Heme environments for A) the ferric nitrite complex of the respiration-associated, pentameric DaCld (PBD ID 3Q08)20 and B) ferric aqua complex of NwCld (3QPI),5 which like KpCld is a dimeric enzyme, are shown. Heme carbons are cyan, protein carbons are gray; nitrogen and oxygen atoms are blue and red, respectively. The distal face contains conserved threonine, phenylalanine, leucine, and arginine which form a cage around the sixth coordination site of the heme. The two distal pockets differ in the orientation of the distal Arg side chain. In the DaCld structure (A), a MES buffer molecule (shown in black, yellow and red) is H-bonded to Arg183. A Gln, the H-bond partner to the distal Arg in NwCld (B), stabilizes the Arg in its open conformation. The structures are displayed using UCSF Chimera.21
The resting axial ligation states of five-coordinate high spin (5cHS) and 5cHS/six-coordinate high spin (6cHS) heme in ferric DaCld and KpCld, respectively, also point to differences in their active sites.9 Ligand binding to heme proteins is sensitive to various factors such as the identity of the heme proximal ligand, amino acid composition of the distal pocket and the ability of distal residues to stabilize exogenous heme ligands with H-bonding, electrostatic, or steric interactions. Numerous spectroscopic methods using nonphysiological heme ligands have been developed to probe the distal landscape of the heme pocket to ascertain its influence on enzymatic activity. The reaction of cyanide with ferric heme proteins is widely used to probe their active site structure.25, 29–35 Since it does not oxidize the heme iron center subsequent to coordination and its binding can readily be monitored spectrophotometrically to obtain kon, koff and equilibrium constants, cyanide can be used as an anionic reporter ligand. Additionally, subtle structural differences in heme–CN− complexes due to different distal heme environments are discernible via rR spectroscopy.36–43 Here we examine ferric Cld complexes with cyanide to probe the distal environments of Clds from the two functional subfamilies with the goal of identifying structural elements that differentially modulate their ClO2− -degrading activities.
EXPERIMENTAL METHODS
Growth, purification, and characterization of Da and KpCld enzymes
DNA containing the full-length coding region of chlorite dismutase from Klebsiella pneumoniae MGH 78578 (accession no. CP000650.1) was PCR-amplified and cloned into the pET-15b (Merck/Novagen) expression vector for production of protein with a N-terminal His-tag as previously described.9 The resulting vector was mutated to replace the thrombin protease site (CTG GTG CCG CGC GGC AGC) with a TEV protease site (GAA AAC CTG TAT TTT CAG GGC) for removal of the 6-His tag post purification. A Q5 Site-Directed Mutagenesis Kit was used with primers TEV_F (5′-T TTT CAG GGC CAT ATG AAT ACA CGA TTA TTT ACG TTC G-3′) and TEV_R (5′-TA CAG GTT TTC GCC GCT GCT GTG ATG ATG-3′). Mutated plasmid was transformed in Tuner DE3 E. coli cells and KpCld was expressed, isolated, and purified as previously described.9 The N-terminal His-tag of KpCld was removed by incubation with S219V TEV (at 1 mol TEV: 10 mol Cld) in 50 mM sodium phosphate pH 8.0, 2 mM DTT, and 0.5 mM EDTA overnight at 4 °C.44 The proteolysis mixture was passed over a 5 mL HisTrap column equilibrated in 20 mM imidazole, 100 mM sodium phosphate pH 7.4. A linear gradient (20–500 mM imidazole) was used to separate cleaved KpCld from tagged KpCld during elution. Imidazole was removed with a PD-10 desalting column and exchanged into 100 mM sodium phosphate pH 6.8. Purity and cleavage were verified by SDS-PAGE.
Plasmids for the over-expression of WT DaCld and DaCld(R183Q) were available from previous work.24, 28, 45 These DaClds were expressed, isolated, and purified using published protocols.17 All Cld concentrations are given as heme-bound monomer, where [Cld] was determined using reported extinction coefficients. The activity with chlorite was routinely monitored to confirm the competency of each enzyme preparation.9, 17 Initial rates of chlorite-decomposing activity by Clds were determined by monitoring O2 evolution with a luminescence-based probe under pseudo-first order conditions with 20 nM enzyme and [ClO2−] concentrations from 0.1 mM – 2.0 mM in 100 mM sodium phosphate pH 6.0 at 25 °C. Concentrations of freshly prepared stock chlorite solutions were determined via iodometric titration or spectrophotometrically by measuring absorbance at 260 nm using ε260 = 155 M−1cm−1.46
Equilibrium binding of cyanide to Cld
Potassium cyanide (KCN) stock solutions were prepared anaerobically under nitrogen in 0.2 M potassium phosphate pH 5 – 8 and 0.2 M glycine pH > 8. Stock KCN concentrations were determined analytically via titration against a known silver nitrate (AgNO3) solution in KOH with p-dimethylaminobenzylidene rhodanine as the indicator.47, 48 Titrations were done anaerobically to insure that the cyanide concentration did not change during the course of the experiment due to oxidation.49
For each spectrophotometric titration, a 4 – 8 μM Cld sample was prepared in a 1 mL volume of anaerobic buffer. Stock KCN solution was added in 1–2 μL aliquots. Spectra were repeatedly measured after each addition until the reaction mixtures had reached equilibrium. At the end of the titration, a cyanide stock of 10-fold higher concentration was added in 10 μL aliquots to insure a clear endpoint had been reached. Difference spectra were generated from spectra which had been corrected for sample dilution. The wavelength of maximum absorbance change was used to construct a plot of ΔA versus [L]T (total concentration of added ligand) and for tight binding ligands such as cyanide, where the binding of ligand to the enzyme (E) affects the overall concentration of ligand in solution, the data were fit to the quadratic form of the one-to-one titration equation:34, 50
| [1] |
Transient kinetic reactions with KCN
Rapid kinetic measurements were made using a Hi-Tech SF-61DX2 stopped-flow system. Spectra were measured from 320 – 700 nm with a diode array detector. Reactions were carried out under pseudo-first order conditions (≥ 10-fold ligand excess over heme) by rapidly mixing DaCld, KpCld, or DaCld(R183Q) (1–10 μM final concentrations) with varied final concentrations of potassium cyanide (10 μM –100 mM for WT and R183Q DaCld and 5–200 μM for KpCld). Both the enzyme and ligand were diluted into the same buffer prior to binding experiments.
Observed rate constants (kobs) were calculated by fitting ΔA time courses at either 420 nm (DaCld), or 405 and 419 nm (KpCld) to the single-exponential function:
| [2] |
Values for the second-order association rate constant (kon) and the first-order dissociation constant (koff) were determined from fitting plots of kobs versus [KCN] to the following:
| [3] |
Dissociation constants for the DaCld–CN− complexes were determined from the ratio of rate constants:
| [4] |
In the case of KpCld, values for koff extrapolated to negative values near the origin. In each case, koff is on the order of 10% or less of the corresponding value for kon. We interpret these results as indicative of a small koff across the pH range. Since the uncertainties in the intercepts for KpCld–CN− are larger than the koff itself, KDs were measured using spectrophotometric titrations and then used to calculate koff using equation 4.
The rate constants kon and koff were measured in citrate-phosphate buffers as a function of pH from 4 to 8.5, as described above. The log10 values of these rate constants were computed and plotted versus pH. The data were then fit to either a one (Equation 5) or two (Equation 6) Ka model, depending on whether single or dual inflection points were observed in the data. These fits in turn yielded values for acid dissociation constants:
| [5] |
| [6] |
All data were plotted using KaleidaGraph. Fits to equation 1 were made using the KinetAssyst software from Tgk Scientific. Fits to equations 2–5 were generated using the least squares fitting program in KaleidaGraph. All values for kobs were based on measurements made in triplicate or more. The reported uncertainties are based on the standard deviations of k, which were determined from these multiple runs.
Vibrational characterization of Cld cyanide and CO complexes
Resonance Raman (rR) spectra were recorded with 413.1-nm emission from a Kr+ laser, using the 135° backscattering geometry for collection of Raman scattered light. The spectrometer was calibrated against Raman frequencies of toluene, dimethylformamide, acetone, and methylene bromide. Spectra were recorded at ambient temperature from samples in spinning, 5-mm NMR tubes. Laser power at samples ranged from 2 mW for CO complexes to 5–12 mW for CN− complexes; no spectral artifacts due to photoinduced chemistry were observed with these irradiation powers. UV-visible spectra were recorded from the rR samples before and after spectral acquisition, and they confirmed that sample integrity had not been compromised by exposure to the laser beam.
The vibrational parameters for Cld cyanide complexes were examined at pH 5.8, 6.8, 8.8, and 10.0 using 13C and 15N isotopologs of cyanoferric Clds (40 μM enzyme and 25–50 mM cyanide). Isotopically labeled potassium cyanides K13CN (99 atom % 13C), KC15N (98 atom % 15N) and K13C15N (99 atom % 13C; 98 atom % 15N) were used. For the spectral range of 600–250 cm−1 no baseline corrections were applied. The original spectra were simulated using Origin® to calculate frequencies, intensities and widths of overlapping Gaussian bands. The number of bands and their widths were held constant whenever possible during peak fitting of the spectra for the four cyanide isotopolog complexes for a given Cld while the frequencies were allowed to vary. The simulated spectra were then subtracted from one another and the resulting simulated difference spectra were compared by superposition to the experimental difference spectra.
Enzyme samples in 2H2O were prepared from concentrated stock protein solutions. Samples were exchanged into 100 mM sodium phosphate buffer prepared in 2H2O (99.9% 2H) at the desired pH by a 25-fold dilution with the buffer followed by concentration of the sample back to its original volume. This procedure was performed three times to obtain a final solution enrichment of 99 % 2H2O.
The Fe–C and C–O stretching frequencies were assigned for ferrous KpCld–CO using the 13C isotopolog. Ferrous KpCld was generated anaerobically by reduction of ferric KpCld with an excess of buffered stock sodium dithionite solution. The corresponding CO complexes were prepared by flushing the ferrous proteins with natural abundance CO (12CO) or 13CO (99 atom % 13C). Protein concentration was 40 μM KpCld.
RESULTS
Comparison of kinetic parameters for ClO2− decomposition by Clds
Activities have been reported for a number of Clds.5, 9, 17, 23, 26, 51–53 Nevertheless, the kinetic parameters for the various Clds are difficult to compare because the pHs, buffers, ionic strengths, temperatures, and detection methods used in these assays differ among the literature reports. The DaCld and KpCld used here were assayed near the peaks of their pH activity profiles and under identical solution conditions to support comparisons of their kinetic parameters (Table S1). These data show that the enzyme efficiencies differ only by a factor of about four (kcat/KM: DaCld (4±1)×107 M−1·s−1; KpCld (9±1)×106 M−1·s−1), while their activities differ by an order of magnitude (kcat: DaCld (2.3±0.4)×104 s−1; KpCld (2.6±0.1)×103 s−1).
Low spin ferric Cld-CN as a probe of the catalytic site
Conversion of high spin ferric Da- and KpClds to six-coordinate, low spin (6cLS) heme complexes upon cyanide binding is readily monitored by the changes in their UV-vis spectra, as shown in Figure S1. Their cyanide complexes exhibit UV-vis spectra (B band at 418–420 nm, Q bands at 540 and 565 nm)24, 26 comparable to those reported for NdCld (420 nm, 540 nm)25 and the dimeric Cld from Cyanothece sp. PCC7425 (CCld) (421 nm, 538 nm).54 The UV-vis spectra of the three 6cLS Cld–CN complexes examined here do not change with pH.
Kinetics of Cyanide Association and Dissociation as a Function of pH
Changes in absorbance at 419 nm upon binding of cyanide to the ferric form of all three proteins at each pH and [CN−] examined were monophasic with time, yielding high quality fits to a single-term exponential decay function (Equation 1, Figure S2) having R2 values ≥ 0.98. The resulting values for kobs were plotted versus [KCN] and used to determine kon, koff, and KD (Equations 3 and 4). Above pH 8.5, the binding events became too fast to track with rapid-mixing techniques, reflected by linear changes in absorbance over time. The kon, koff, and KD values over the pH range of 4.0 to 8.5 are tabulated in Table S2.
Values of log kon measured for CN− and WT DaCld, DaCld(R183Q), and WT KpCld are plotted as a function of pH (Figure 2A). In every case, the log kon increased roughly linearly with pH, having slopes ≈ 1, consistent with affinity for CN− being modulated by a single deprotonation event. For DaCld at the highest pH values, the data plateau, suggesting a nearby pKa. Fitting the data to a single-pKa model (Equation 5) yielded 8.1 ± 0.2, which is an approximation since values for kon above pH 8.5 were too fast to be measured. The R183Q mutant showed a similar pH dependence for kon, albeit with values ~500-fold smaller than their WT counterparts. Fitting the data to Equation 5 reflects both the expected linearity of the data and the slope ≈ 1; however, the pKa was too high to be determined and is therefore designated as >8.5. Finally, the values and pH-dependent trends in log kon for KpCld were similar to those of WT DaCld. Given the inflection in its plot (Figure 2A), the data were fit to a two pKa model (Equation 6) which yielded pKa values of 7.7 ± 0.1 and 4.4 ± 0.2. The lower pKa corresponds to irreversible protein denaturation, which makes it an apparent pKa. A similar pH profile of kon for CN− binding to dimeric CCld has been recently reported.54
Figure 2. Kinetics of formation and dissociation for cyano-complex formation with ferric DaCld (closed circles, blue), DaCld(R183Q) (open circles, blue), and KpCld (closed circles, red).

Values for (A) logkon and (B) logkoff are plotted as a function of pH. Lines represent the fit of equations 5 and 6 to the data, as described in the text.
Values of log koff for DaCld, DaCld(R183Q), and KpCld cyanide complexes are plotted as a function of pH in Figure 2B. The koff for DaCld is approximately four orders of magnitude smaller than its kon at the lowest pH where rates were measured. The log koff remains unchanged until ~ pH 6.5, whereupon it begins to rise with a slope near 1. Fitting the curve to Equation 5 yields pKa = 6.7 ± 0.3. By contrast, koff for the R183Q variant appeared to be unaffected by pH, remaining small (<1 s−1) at all pH values measured. Finally, the koff values for KpCld are approximately two orders of magnitude smaller than those for DaCld. As a function of pH, they exhibit the similar behavior as those for DaCld, unchanging until ~ pH 7 followed by a linear rise; a fit of the log koff versus pH to equation 5 reveals a pKa = 7.6 ± 0.2.
Comparison of Cyanide Affinities
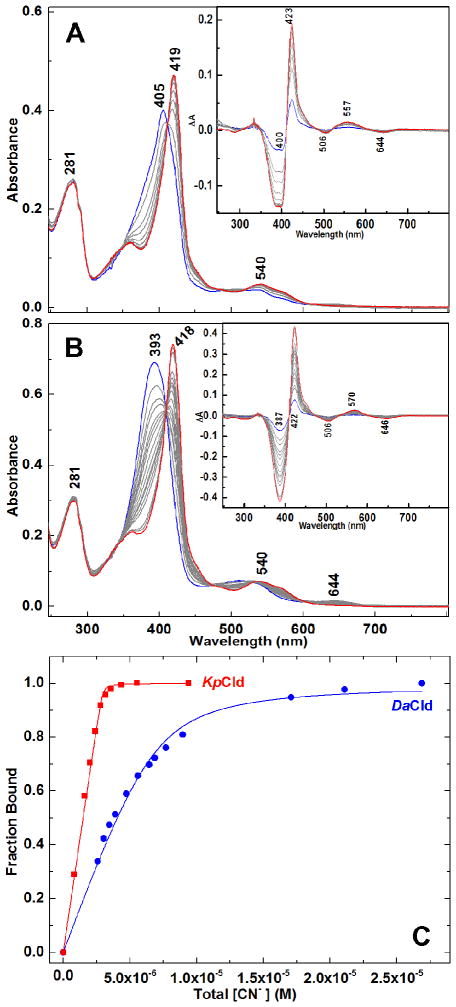
Under alkaline conditions (pH 8.5) DaCld and DaCld(R183Q) had similar binding affinities for cyanide (KD ≈ 3×10−6 M) while KpCld exhibited a much larger cyanide affinity (KD ≈ 1×10−8 M) (Table S2). This difference in cyanide affinity is clearly revealed by spectrophotometric titrations of KpCld and DaCld with cyanide (Figure 3).
Figure 3. Spectrophotometric determination of KD values for the reaction of Clds with cyanide.
A) UV-visible spectra of 2.97 μM KpCld in 0.2 M potassium phosphate titrated with KCN at pH 7.0. Starting spectrum, blue; final spectrum, red; spectra at intermediate cyanide concentrations, gray. [CN−] = 0, 1.59, 1.99, 2.39, 2.78, 3.17, 3.57, 4.35, 5.52, and 9.38 μM. Inset: ΔA spectra generated from spectra in A by subtraction of starting ferric KpCld spectrum from each spectrum for subsequent CN− addition. B) Spectrophotometric titration of 6.8 μM DaCld in 0.2 M potassium phosphate with KCN at pH 7.0. Color scheme is the same as in A. [CN−] = 0, 0.86, 1.73, 2.59, 3.02, 3.45, 3.88, 4.73, 5.58, 6.43, 6.85, 7.69, 8.95, 17.1, 21.1, and 26.9 μM. Inset: ΔA spectra generated from spectra in B by subtraction of starting ferric DaCld spectrum from each spectrum for subsequent CN− addition. C) Plots of fraction of protein bound with cyanide versus total [KCN] (M); KpCld, red squares; DaCld blue circles. Since cyanide binding to both enzymes is quite strong, the fraction bound (ΔAobs/ΔAmax) is a quadratic function of the total [KCN]. Nonlinear least squares regression was used to fit ΔA422 vs [CN−] to equation 1.
The difference in cyanide affinity is attributed to differences in the koff values, as kon for KpCld and DaCld were essentially the same across the pH range examined. Trends in KD with pH reflected the trends observed independently in kon and koff. Diminution followed by some degree of flattening in KD values was observed for KpCld, DaCld, and DaCld(R183Q) dominated by the much larger magnitude linear increases in log kon, relative to log koff, over much of the measurable range of pH. The KD data were not well modelled by either the single or double pKa functions of Equations 2 and 3, respectively.
The spectrophotometrically determined KD for DaCld–CN− of (6.0±0.5)×10−7 M at pH 7.0 (Figure 3) is in excellent agreement with KD calculated from kinetic constants using equation 4 (Table S2). The previously reported spectrophotometrically determined KD for DaCld(R183Q)–CN− of 8.4×10−5 M24 is close to the 6.0×10−5 M calculated here using kinetic constants (Table S2). Agreement between the kinetically and spectroscopically determined KD values was taken as an indication that use of our spectrophotometrically determined KD values for KpCld–CN− to calculate koff is reasonable.
Identification of the FeCN vibrational modes
The low frequency range of the Soret-excited rR spectra of KpCld–CN− and DaCld–CN− at pH 5.8 are shown in Figures 4A and 4C. The ferric Cld–CN− complexes were generated with cyanide isotopologs 12C14N−, 13C14N−, 12C15N− and 13C15N− to identify bands arising from the modes involving distortions of the FeIIICN fragment. As these frequencies and isotope shift patterns are responsive to properties of the distal heme pocket, they constitute a probe of its structural properties. Since several strong heme deformation modes are observed in the 270–500 cm−1 region, the difference spectra shown in Figures 4B and 4D were generated by digital subtraction of various pairs of Cld–CN− isotopolog spectra to facilitate identification of the isotope-sensitive modes. Peak-fitting analyses of the experimental spectra identified the bands responsible for features in the difference spectra; multiple isotope-sensitive bands were observed and their Raman shifts are listed in Table S3. The frequencies indicated at the minima and maxima of the difference spectra (Figures 4B and 4D) differ from the actual Raman shifts obtained from the fits of the experimental data (Figures 4A and 4C) because the isotope shifts are less than the widths of the 13CN−- and C15N−-sensitive bands. The FeCN angle in cyanide complexes of synthetic iron porphyrinates is typically near 180°. Distortions that alter the Fe–C–N angle can be imposed by electronic and/or steric factors in the heme pocket of proteins, lending both stretching (νFe–C) and bending (δFeCN) character to the 13CN−and C15N−-sensitive normal modes. The FeCN fragment exhibits a νFe–C band that shifts monotonically to lower frequency with increasing total mass of the cyanide ligand and a δFeCN band whose frequency shifts in a “zigzag” pattern upon isotopic substitution of the carbon (in order: 12C14N−, 13C14N−, 12C15N−, 13C15N−, the frequency decreases, increases, and then decreases). In heme protein cyanide complexes where there are small equilibrium distortions, the nearly linear FeCN fragments exhibit a νFe–C frequency that is greater than the δFeCN frequency (Table 1). For bent FeCN fragments (∠FeCN between ~160–130°), the relative frequencies of νFe–C and δFeCN are less clear, possibly because of the effect of lower force constants for the Fe–C stretching (fFe–C) and FeCN bending (fFeCN) internal coordinates relative to those for a linear FeCN fragment (vide infra).43
Figure 4. A) Low-frequency resonance Raman spectra of isotopically labeled cyanoferric KpCld pH 5.8.
The cyanoferric KpCld complexes were 20 μM KpCld and 25 mM cyanide in 100 mM sodium phosphate buffer. KpCld–CN− νFe–C and δFeCN frequencies are consistent with a bent FeCN unit. B) Isotope difference spectra for KpCld–CN−. Difference spectra generated by digital subtraction of the spectra shown in A. C) Low-frequency resonance Raman spectra of isotopically labeled cyanoferric DaCld pH 5.8. The cyanoferric DaCld complexes were 20 μM DaCld and 25 mM cyanide in 100 mM sodium phosphate buffer. DaCld–CN− νFe–C and δFeCN frequencies are consistent with a larger FeCN angle than that of KpCld–CN−. D) Isotope difference spectra for DaCld–CN−. Difference spectra generated by digital subtraction of the spectra shown in C. E) Low-frequency resonance Raman spectra of isotopically labeled cyanoferric DaCld(R183Q) pH 5.8. The cyanoferric DaCld(R183Q) complexes were 25 μM DaCld(R183Q) and 25 mM cyanide in 100 mM Tris/sulfate buffer. DaCld(R183Q)–CN− νFe–C and δFeCN are consistent with a nearly linear FeCN unit. F) Isotope difference spectra for DaCld(R183Q)–CN−. Difference spectra generated by digital subtraction of the spectra shown in E. The excitation wavelength was 413.1 nm; laser power at the sample was 12 mW. Spectra were acquired at 20 °C. Original spectral data are shown in black, bands used to fit the spectra are grey, and the calculated spectra are shown in red. Difference spectra generated from subtraction of spectral data are shown in black; the fit difference spectra are shown in red. Fits for the difference spectra were obtained by generating fits for the experimental spectra and then subtracting those best-fit spectra.
Table 1.
Stretching (ν) and bending (δ) frequencies (cm−1) for the FeCN fragment in the cyanide complex of various ferric heme proteins with proximal histidine ligation.
| proteina | “nearly linear” FeCN | “bent” Fe–CN | Potential distal H-bond donors to CN− | references | ||||
|---|---|---|---|---|---|---|---|---|
| νFe–C | δFeCN | Δ(ν – δ) | νFe–C | δFeCN | Δ(ν – δ) | |||
| Chlorite dismutases | ||||||||
| KpCld pH 5.8 | 439 | 356 | 83 | Arg/H2O | This work | |||
| KpCld pH 8.0 | 440 | 359 | 81 | Arg/H2O | This work | |||
| DaCld pH 5.8 | 441 | 396 | 45 | Arg | This work | |||
| DaCld pH 8.8 | 441 | 398 | 43 | Arg | This work | |||
| DaCld(R183Q) | 441 | 403 | 38 | Gln | This work | |||
| Nitrophorin | ||||||||
| Rhodnius prolixus Nitrophorin 1 | 454 | 397 | 57 | 443b | 357c | 86 | H2O | 39 |
| Globins | ||||||||
| TrHbC | 440d | nde | nde | Gln/Tyr/Gln | 42 | |||
| trHbC E7Gln→Gly | 452 | nde | nde | Tyr/Gln | 42 | |||
| Tf-trHb | 439 | 380 | 59 | Trp/Tyr | 55 | |||
| Horse Mb pH 8.0 | 452 | 441 | 11 | His | 41 | |||
| Human HbA, pH 8.0 | 452 | 434 | 18 | His | 41 | |||
| Chironomus Hb | 453 | 412 | 41 | His | 56 | |||
| C. jejuni single domain Hb | 440 | 403 | 37 | 353 | 417 | −64 | Gln/Tyr | 57 |
| Peroxidases | ||||||||
| HRP pH 11.6 | 444 | 405 | 35 | 355 | 420 | −65 | Arg | 38 |
| HRP pH 5.5 | 453 | 405 | 48 | 360 | 422 | −62 | His/Arg | 38, 58 |
| HPR(H42Q) pH 7.0 | 443 | nde | nde | Gln/Arg | 59 | |||
| LPO, pH 7.0 | 360 | 453 | −93 | His/Arg | 60 | |||
| MPO | 361 | 453 | −92 | His/Arg | 43 | |||
Hb, hemoglobin; Mb, myoglobin; Tf trHb, Thermobifida fusca truncated Hb II; trHbC, Chlamydomonas eugametos trHb-I; Chironomus Hb, Chironomus thummi thummi Hb; C. jejuni single domain Hb, Campylobacter jejuni single domain Hb; HRP, horseradish peroxidase; LPO, lactoperoxidase; MPO, myeloperoxidase.
Normal mode calculations indicate that band has contributions from modes ν(Fe–CN), δ(FeCN), and ν(Fe–His).
Normal mode calculations indicate that band has contributions from δ(FeCN), δ(His–Fe–C), and ν(Fe–His).
Assignment made based on comparison to ν(Fe–CN) frequencies for Mb and Hb. Isotopic shifts from 12C14N− to 13C15N− were comparable to those observed for KpCld-CN−.
not determined
FeCN geometry and vibrational signatures
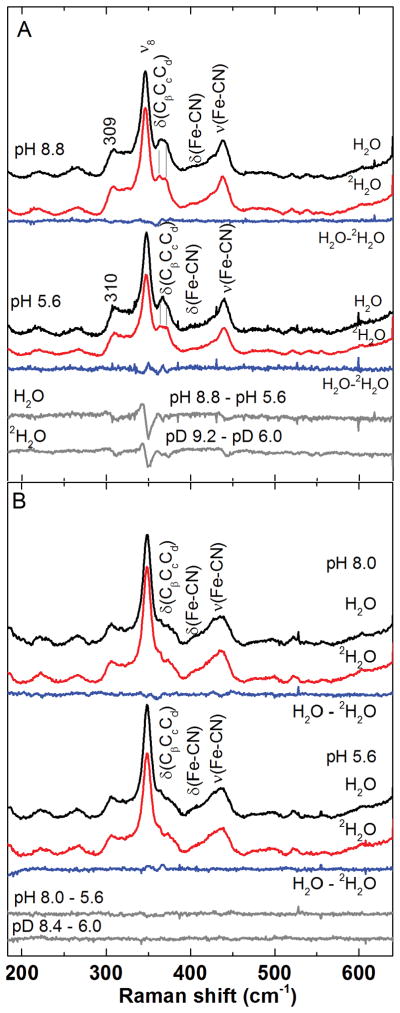
The νFe–C frequencies for KpCld–CN− and DaCld–CN− at pH 5.8 are observed at 439 and 441 cm−1, respectively. Both bands shift monotonically (Figures 4A and 4C) by 3 cm−1 (KpCld) and 5 cm−1 (DaCld) between 12C14N− and 13C15N− complexes. These assignments are further supported by the difference spectra of Cld–CN− isotopolog complexes which have differing total cyanide mass but carbon atoms of equal mass; in these difference spectra, the features arising from the stretching vibrations are predominant and, since the bending vibrations occur at the same frequencies, they are removed by the subtraction process. The difference spectra of [12C14N− – 12C15N−] and [13C14N− – 13C15N−] in Figure 4B and 4D exhibit a prominent feature corresponding to a single stretching mode in each Cld. The observed shifts are considerably smaller than the ~11 cm−1 calculated for a linear FeCN;42 the myoglobin cyanide complexes, which have “nearly linear” FeCN geometries (∠FeCN= 166° for sperm whale Mb–CN−; pdb code: 1EBC),61 exhibit isotope shifts of 8–10 cm−1.41 The smaller isotope sensitivity of KpCld–CN− relative to DaCld–CN− and cyanometMb is consistent with delocalized modes, attributable to the FeCN triad of KpCld-CN having the greater deviation from linearity.
In KpCld–CN− at pH 5.8, there are two possibilities for the δFeCN bending mode; bands at 356 and 372 cm−1 exhibit small, but clearly discernable, zigzag patterns in their cyanide isotopolog frequencies. The band at 356 cm−1 is tentatively assigned to the δFeCN mode because it is absent in the ferric KpCld spectrum whereas a 371 cm−1 band, assigned to a propionate bending mode, δ(CβCcCd), is present (Figure S3). The zigzag pattern due to the isotope sensitivity of the 356-cm−1 band yields a difference feature with a maximum at 361 cm−1 and a minimum at 353 cm−1 in [12C14N− – 13C14N−], [12C14N− – 13C15N−], and [12C15N− – 13C15N−] difference spectra (Figure 4B). A similar difference feature for the 372-cm−1 band (max. @ 377 cm−1, min. @ 368 cm−1) is tentatively attributed to kinematic or vibrational coupling of a FeCN coordinate, involving displacement of the iron along a vector having a nonzero projection on the mean porphyrin plane, and in-plane porphyrin coordinates.41 The 372 cm−1 difference feature is not observed in the DaCld–CN− spectra (Figure 4D), suggesting that the kinematic coupling is activated by a bent FeCN geometry in KpCld–CN−. One additional isotope-sensitive band at 306 cm−1, attributed to kinematic coupling of the FeCN and Fe–His distortions,42 is observed for KpCld–CN− at pH 5.8.
The δFeCN mode is assigned for DaCld–CN− pH 5.8 at 396 cm−1 based on its zigzag isotope shift pattern (Figure 4C). The difference feature due to this zigzag pattern has a maximum at 401 cm−1 and a minimum at ~390 cm−1 in the [12C14N− – 13C14N−], [12C14N− – 13C15N−], and [12C15N− – 13C15N−] difference spectra (Figure 4D). One additional band at 309 cm−1 is isotope-sensitive with shifts of ~2 cm−1 to lower frequency upon substitution of natural abundance CN− (hereinafter designated by 12C14N−) with 13C15N−. A similar isotope-sensitive mode has been reported for other ferric heme protein cyanides and, like the 306 cm−1 band in KpCld–CN−, is attributed to vibrational coupling between the local Fe–His stretching coordinate and the FeCN distortion coordinates.42, 43
Two major differences are observed when comparing vibrational data for cyanide complexes of DaCld(R183Q), shown in Figure 4E and 4F, and WT DaCld. First, although the νFe–C frequency at 441 cm−1 for the mutant is the same as that of the WT, the line width of the band increases by a factor of approximately 1.5 (from 12 to 17 cm−1); this is visualized by the greater difference between maxima/minima in the difference spectra; for example, in the [12C14N− – 13C15N−] spectrum the maximum and minimum for WT appear at 444 and 433 cm−1 while for the R183Q mutant they are observed at 448 and 429 cm−1. This band width increase is consistent with greater conformational inhomogeneity in the distal pocket of the mutant. It suggests that the Arg side chain constrains the FeCN fragment to a narrower distribution of geometries (FeCN angles) than the amide side chain of Gln. Secondly, the δFeCN frequency (403 cm−1) is 7 cm−1 higher than its WT counterpart. This is consistent with some contribution from a less strained FeCN unit. Several additional isotope sensitive bands are observed in the DaCld(R183Q)–CN− spectra. An isotope sensitive band at 305 cm−1 appears comparable to that observed in WT at 309 cm−1 due to coupling between the Fe–His stretching and FeCN distortions.42, 43 Isotope shifts of 1–2 cm−1 are observed for bands at 375 and 361 cm−1; sensitivity of these modes is similar to that reported for cyanide complexes of Mb and Hb and are likely due to kinematic coupling of in-plane coordinates having Fe–Npyrrole stretching character with the Fe–CN stretching coordinate.41 Although the shifts in these bands are small, the bands overlap with other bands, some of which are also isotope sensitive, and they appear in the difference spectra as features around 381 and 350 cm−1.
FeCN geometry and Δ(ν δ)
In contrast to bent FeCN of peroxidases, for which the δFeCN frequency is greater than the νFe–C frequency, the KpCld–CN− νFe–C frequency is higher than its δFeCN frequency (Table 1). These cyanide-sensitive normal vibrational modes comprise displacements along both the νFe–C and δFeCN coordinates, with their frequencies depending upon both the FeCN geometry and force constants of the Fe–C stretching (fFe–C) and FeCN bending (fFeCN) internal coordinates. Each of these frequencies has a potential energy distribution that includes contributions from internal Fe–C stretching, FeCN bending and Fe-Hisprox stretching coordinates. The relative contributions of displacements along these internal coordinates determine which of the normal modes comprises more stretch than bend or more bend than stretch as illustrated in Figure S5.43 As fFe–C increases, a point is reached where the Fe-Hisprox stretching contribution changes dramatically and the relative frequencies of the predominantly stretching and bending normal modes reverse. The value of fFe–C at which this reversal occurs depends on the equilibrium FeCN angle; for angles of 170° and 155°, reversal occurs near fFe–C = 1.80 mdyn/Å and 1.45 mdyn/Å, respectively.43 KpCld–CN− has observed frequencies and isotopic sensitivities comparable to the nonlinear form of NP1–CN−. In fitting the vibrational data of NP1–CN−, a fFe–C of 1.55 mdyn/Å and an ∠FeCN of 155.0° reproduced the observed isotope shift pattern (Table S4).39 Thus, the occurrence of the Fe–C stretch at a higher frequency than the FeCN bend in the spectra of KpCld–CN− and NP1–CN− (Table 1) indicates that fFe–C for these two cyanide complexes are greater than 1.45 mdyn/Å. By contrast, the bent cyanide complexes of other heme proteins (Table 1, Figure S5) exhibit lower values of fFe–C (< 1.45 mdyn/Å), thereby pushing their δFeCN modes to higher frequencies than their νFe–C modes.43
The frequency separations between modes of predominantly νFe–C and δFeCN character are 83, 45, and 38 cm−1 at pH 5.8 for KpCld–CN−, DaCld–CN− and DaCld(R183Q) CN−, respectively. Since the frequency difference between νFe–C and δFeCN modes is larger for bent FeCN geometries (65–106 cm−1) than for the “nearly linear” forms (11–58 cm−1) of cyanoheme proteins,37–39, 41 these frequency separations together with isotope sensitivities (vide supra) support a bent geometry for KpCld–CN− (~155°). Nearly linear geometries, wherein ∠FeCN lies between ~166° and 176°, are observed for DaCld–CN− and DaCld(R183Q)–CN−. The smaller Δ(ν–δ) for DaCld(R183Q)–CN− is consistent with the largest ∠FeCN of the three cyanide complexes probably due to the distal Gln exerting less off-axis force on the coordinated CN− than the native Arg side chain.
pH sensitivity of FeCN modes
As the pH of KpCld–CN− is increased from 5.8 to 8.0, the bands assigned to νFe–C and δFeCN modes both sharpen with line widths decreasing by ~2 cm−1, while their frequencies increase slightly from 439 cm−1 to 440 cm−1 and from 356 cm 1 to 359 cm 1, respectively (Figures 4A, 4B, and S4). Isotope shifts of 1–3 cm−1 were observed upon substitution of 12C14N− by 13C15N−. As already noted for the pH 5.8 case, these observed shifts are considerably smaller than the calculated 11 cm−1 for a linear FeCN.42 Under alkaline conditions, the isotope-sensitive νFe–C and δFeCN bands both exhibit a zigzag isotope shift pattern. Zigzag patterns for the band predominantly due to the stretch have on occasion been observed in proteins where the FeCN has a bent geometry and the νFe–C and δFeCN internal coordinates both contribute to the isotope-sensitivity of the frequencies. Thus the band assigned to the “stretching” mode has a significant contribution from the bending coordinate and vice versa.36
Comparison of the KpCld–CN− spectra at pH 5.8 and 8.0 suggests that increasing pH causes subtle changes in the heme environment which alter the contributions of the stretching and bending coordinates to the isotope-sensitive bands. The frequency separation between the predominantly νFe–C and δFeCN–dominated modes (ν–δ) for KpCld–CN− at pH 8.0 remains large at 82 cm−1. Large ν–δ, the small isotope shifts, and the zigzag shift pattern for the band at 440 cm−1 support the conclusion that the overall geometry of KpCld–CN− remains bent over the pH range examined in this study.
The FeCN modes of DaCld–CN− also exhibit very small shifts with increasing pH. The νFe–C frequency decreases from 442 to 441 cm−1 while that of δFeCN increases from 396 to 398 cm−1 going from pH 5.8 to 8.8 (Figures 4C and S6). Thus, no dramatic changes in FeCN geometry occur as the pH is raised. However, several low frequency porphyrin modes (309, 336, and 347 cm−1) exhibit band width increases of 2 to 4 cm−1 and 1–2 cm−1 shifts in frequency as the pH is increased. These changes are the source of the features observed in the [pH 8.8 – pH 5.6] rR difference spectra (Figure 5A). By analogy to previously assigned modes, these bands correspond to νFe–His, γ6, and ν8.42, 62 In contrast, neither the FeCN nor the out of plane porphyrin modes of DaCld(R183Q)–CN− are altered by increases in pH, consistent with the lack of features in the DaCld(R183Q)–CN− [pH 8.0 – pH 5.6] difference spectrum (Figure 5B). Since these pH-dependent shifts for WT are not observed for DaCld(R183Q)–CN−, they may be correlated with the kinetically observed pKa of 6.7 for DaCld–CN−.
Figure 5. Low-frequency resonance Raman spectra of A) cyanoferric DaCld and B) cyanoferric DaCld(R183Q) as a function of pH and.
2H2O.
The parent spectra at pH 8.8, 8.0, and 5.6 in H2O (black) and in 2H2O (red) were used to generate the [H2O – 2H2O] difference spectra (blue). Difference spectra highlighting changes that occur upon increased pH were obtained by subtraction of spectra of acidic enzymes from those under alkaline conditions (grey).
To probe for H-bonding interactions between bound cyanide and the distal pocket, rR spectra of the cyanide complexes of DaCld at pH 5.6 and 8.8 and DaCld(R183Q) at pH 5.6 and 8.0 were recorded in H2O and in 2H2O (Figure 5). These [H2O – 2H2O] difference spectra indicate that no significant perturbation occurs in the νFe–C and δFeCN frequencies of either enzyme upon isotope substitution, regardless of pH. A similar lack of H2O/2H2O sensitivity is observed for KpCld–CN− (data not shown). Although the lack of deuterium isotope shifts in νFe–C and δFeCN frequencies does not eliminate the possibility of hydrogen bonding between the bound cyanide and residues in the distal pocket,36, 42, 63 it is consistent with a bound cyanide that does not have strong hydrogen bonding along the Fe–C–N axis. This suggests that the distal Arg is not positioned for optimal hydrogen bonding to the cyanide nitrogen.
Low spin ferrous Cld–CO as a probe of the catalytic site
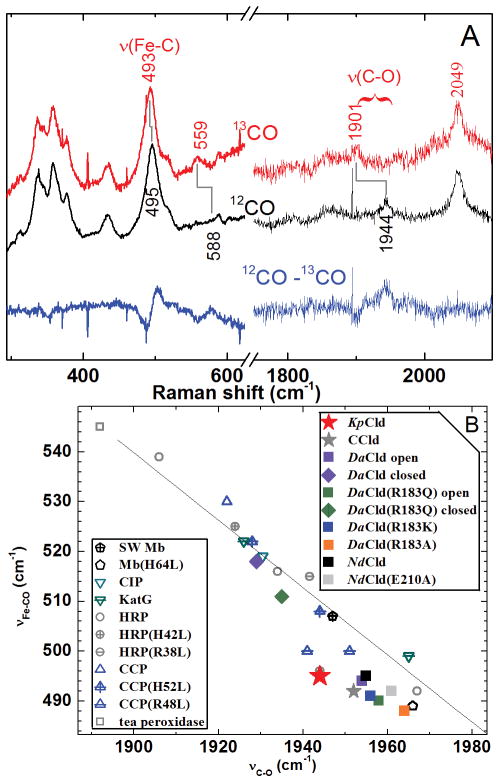
The Soret-excited rR spectra of KpCld–CO (Figure 6) reveal three isotope-sensitive bands at 495, 588, and 1944 cm−1. They are assigned to νFe–C, δFeCO, and νC–O modes, respectively. These data report a single form of KpCld–CO; its position on the νFe–C/νC–O correlation plot (Figure 6) is consistent with little or no non-bonded interaction between the distal Arg and the bound CO ligand. The CO complexes of WT DaCld and DaCld(R183Q) have open and closed conformers, likely corresponding to different orientations of the distal Arg side chain.24 In contrast, KpCld–CO exhibits a single form, having a Raman fingerprint comparable to the open form of WT DaCld–CO.
Figure 6. Resonance Raman characterization of ferrous CO complex of KpCld reports on its proximal ligands and distal environments.
A) Soret-excited rR spectra of the isotopomers of CO complexes of KpCld–CO at pH 8.0. Spectra were acquired with 413.1 nm excitation and 2 mW power at the sample. 12CO complex, black; 13CO complex, red; difference spectra 12CO–13CO, blue. B) Backbonding correlation plot of νFe-C versus νC-O for ferrous carbonyls of heme proteins shows the dependences of their positions on axial ligation and distal pocket properties. The black line correlates vFe–C with vC–O for six-coordinate FeCO adducts in which the sixth ligand is histidine (neutral imidazole). Solid symbols represent Clds: dimeric Clds, stars; pentameric Clds, squares and diamonds. Data for DaClds, NdClds and CCld are from references 24, 64 and 54, respectively. Raman shifts for other heme carbonyls are tabulated in Table S1 of reference 24 and their plot positions are shown as open symbols.
DISCUSSION
The O2-evolving Clds constitute an unusual example of rapid and efficient catalytic O–O bond-formation. Despite the similarities in protein folds and active sites in the catalytic domains of enzymes in both the homodimeric and homopentameric Cld subfamilies, there is variability in their catalytic activities, enzyme efficiencies, and turnover numbers. Cyanide complexes, which are sensitive probes of the heme environment, have been used here to evaluate the active sites of the KpCld dimer and DaCld pentamer as a means of gaining insight into the structural and electronic features responsible for their high-fidelity O2 generation.
Anion binding to Clds and catalytic efficiency
Cyanide is known to be a strong, competitive inhibitor of KpCld65 and Azospira oryzae strain GR-1 Cld.51 This is not unexpected for the strong field CN− ligand, which readily forms stable 6cLS complexes with the ferric Clds, thereby blocking access of the substrate to the exogenous (i.e. distal) ligand site of the catalytic hemin center. In the pH-dependent reactions between resting Clds and CN−, the CN− ligand can be considered in some ways a non-reactive surrogate for ClO2− (pKa = 1.96; HClO2 ⇌ H+ + ClO2−). The pH-dependent behavior of the chlorite reaction with DaCld was previously studied,26 and interconversions associated with two pKas were previously shown to modulate its activity and spectroscopic properties. The lowest pKa of 6.5 involves the conformation reorganization of distal Arg183,26 which is strictly conserved among all O2-evolving Clds.10, 23 Activity, spectroscopic, crystallographic, and molecular dynamics studies suggest that the Arg side chain can rotate from a catalytically optimal position in a closed conformer to a less active position in an open conformer.20, 23, 26, 66 Conversion of the most active 5cHS heme to a 6cLS heme hydroxide occurs with a pKa of 8.7.26 The analogous pKas for the distal heme pocket conformation and heme hydroxide formation in KpCld are 7.0 and 8.3.9 Based on the pH profiles of their chlorite decomposing activities, these Clds are most active in their acidic form, which has been hypothesized to have a heme-substrate complex wherein the side chain of the conserved distal Arg points inward over the heme plane (closed conformation).
In contrast to the chlorite reaction, the second-order rate constant for CN− binding, kon, increased with pH for both KpCld and DaCld, having values of 106 M−1·s−1 near the estimated pKas of 7.7 and ~8.1, respectively. These pKa values are consistent with kon being limited by the deprotonation of HCN to yield the anion (pKa = 9.2, 25 °C), exchange of the heme OH− ligand 9, 26 for CN−, or both. The similarity in magnitudes of kon and kcat/KM at their respective pH maxima, however, suggests that values of kon near 106 M−1·s−1 define the limiting rate for KpCld and DaCld complexation with anions. Moreover, based on the similarity among values of kon reported for CN− binding to other Clds,11, 23, 67, 54rapid anion binding appears to be characteristic of Clds (Table 2).
Table 2.
Kinetic and thermodynamic parameters for cyanide binding to selected heme proteins with varying distal pockets
| Protein | conditions | kon/M−1 s−1 | koff/s−1 | KD/M | reference |
|---|---|---|---|---|---|
| KpCld | 20 °C, pH 7.0 | (1.15±0.01)×106 | (5.8±3.2)×10−3a | (4.7±2.8)×10−9b | This work |
| DaCld | 20 °C, pH 7.0 | (1.30±0.02)×106 | (8±1)×10−1 | (6.1±0.4) ×10−7c | This work |
| CCld | 25 °C, pH 7.0 | 1.6×105 | 1.4 | 8.6×10−6 | 11 |
| NwCld | 25 °C, pH 7.0 | 1.0×106 | 2.4 | 2.4×10−6 | 67 |
| NdCld | 25 °C, pH 7.0 | 2.57×106 | 9.3 | 3.6×10−6 | 23 |
| CcP | 25 °C, pH 7.0 | 1.1×105 | 9.0×10−1 | 8×10−6 | 68 |
| CcP(H52L) | 25 °C, pH 7.0 | 7.0×103 | 1.5×10−1 | 6.3×10−6 | 34 |
| HRP | 25 °C, pH 7.05 | 9.8×104 | 2.8×10−1 | 2.9×10−6 | 35 |
| Cj trHbPd | 20 °C, pH 7.0 | ≥ 2×104 | ≥ 1×10−4 | 5.8×10−9 | 69, 70 |
| Mt trHbNd | 20 °C, pH 7.0 | 3.80×102 | 6.8×10−4 | 1.8×10−6 | 69, 70 |
| Mt trHbOd | 20 °C, pH 7.0 | 3.20×102 | 3.5×10−4 | 1.1×10−6 | 69, 70 |
| Human Mb | 20 °C, pH 7.0 | 2.30×102 | 3.8×10−4 | 1.67×10−6 | 71, 72 |
| SW Mb | 20 °C, pH 7.0 | 3.20×102 | 4.0×10−4 | 1.25×10−6 | 71, 72 |
| Human Mb(H64A) | 20 °C, pH 7.0 | 1.1×102 | 4.6×10−4 | 4.17×10−6 | 72 |
| Human Mb(H64Q) | 20 °C, pH 7.0 | 1.8×102 | 1.0×10−5 | 5.6×10−8 | 71, 72 |
| BjFixLHd | 25 °C, pH 7.0 | 1.8×101 | 1.2×10−4 | 5.2×10−6 | 73 |
| SmFixLHd | 25 °C, pH 7.0 | 1.4×101 | 1.7×10−4 | 1.04×10−5 | 73 |
| SmFixLNd | 22 °C, pH 7.0 | 3.2×101 | 14.7×10−4 | 1.48×10−5 | 49 |
| Microperoxidase-11 | 20 °C, pH 7.0 | 8.9×103 | 1.5×10−3 | 1.7×10−7 | 74 |
| DaCld(R183Q) | 20 °C, pH 7.0 | (3.0±0.02)×103 | (1.7±0.2)×10−1 | (6.0±0.6)×10−5 | This work |
| NdCld(R173Q) | 25 °C, pH 7.0 | 2.3×103 | 5×10−1 | 2.164×10−4 | 25 |
| NdCld(R173K) | 25 °C, pH 7.0 | 1.62×103 | 3.0×10−1 | 1.85×10−4 | 23 |
| NdCld(R173A) | 25 °C, pH 7.0 | 3.43×103 | 5.01×10−1 | 1.46×10−4 | 23 |
| NdCld(R173E) | 25 °C, pH 7.0 | 7.3×101 | 3.0×10−1 | 3.874×10−3 | 25 |
| CcP(H52Q) | 25 °C, pH 6.0 | 8.4×101 | 4.9×10−2 | 6.1×10−3 | 33 |
| Gd II Hb+d | 20 °C, pH 7.0 | 4.91 ×10−1 | 9.82 ×10−8 | 2 ×10−7 | 75 |
| Gd III Hb+d | 20 °C, pH 7.0 | 3.02 ×10−1 | 2.9 ×10−6 | 1×10−5 | 76 |
| Gd IV Hb+d | 20 °C, pH 7.0 | 1.82 | 2.2 ×10−5 | 1.2 ×10−5 | 76 |
Calculated from spectrophotometric KD and kinetically determined kon.
Measured via spectrophotometric titration.
Kinetic and spectrophotometric titration yield the same value for DaCld KD.
Cj, Campylobacter jejuni;Mt, Mycobacterium tuberculosis; Bj, Bradyrhizobium japonicum; Sm, Sinorhizobium meliloti; Gd, Glycera dibranchiate.
In DaCld(R183Q), where the side chain of the distal Gln bears no overall charge, kon diminishes by 2 to 3 orders of magnitude (Table S2). Similar decreases in kon relative to WT enzyme have been reported for R173Q, R173K, and R173A mutants (Nd distal Arg numbering) of NdCld (Table 2).23, 25 Notably, kon does not exhibit a pKa near 6.5, attributed to the impact of the open/closed transition on kcat/KM, for either ferric WT DaCld or its R183Q mutant.26 This suggests that closing of the Arg “gate” is not important for forming the Fe–CN bond in the Da enzymes but for a subsequent step in the catalytic cycle. Comparison of koff values at pH 7.0 for pentameric Cld–CN− s and their distal Arg mutants (Table 2) reveals that these mutations have a ~102 fold smaller effect on koff relative to their effect on kon.
The pH dependence of koff, in contrast, yields pKas for DaCld and KpCld, which are very close to those reported for these Clds and their chlorite decomposition reaction. Based on X-ray structures of pentameric Cld ligand complexes,20, 22, 53 a closed conformation of the charged distal Arg appears to be readily accessible in DaCld where its interaction with the bound CN− imparts maximum stability and activity under acidic conditions. Above the pKa koff increases; the coordinated CN− is more readily lost from DaCld suggesting that an open orientation is more accessible as conditions become more alkaline. In contrast, X-ray structures for dimeric Clds, NwCld and CCld, show the distal Arg held in an open conformation by H-bonding to a Gln residue (Figure 1).5, 54 In turn, the Arg is H-bonded to a water molecule that is within H-bonding distance of heme ligands such as hydroxide and fluoride.54 Examination of a KpCld homology model reveals a similar Gln residue poised to facilitate a comparable open conformer. In such an open conformer of KpCld–CN, the Fe-C bond would be exposed to considerably less influence of Coulombic or steric interaction with Arg. The distancing of the Arg charge from the Fe-C bond stabilizes the bond against dissociation; this is supported by the two orders of magnitude slower dissociation rate observed for KpCld relative to pentameric Clds. In addition stabilization of an open conformation via H-bonding between the distal Arg and a conserved Gln in dimeric Clds is an attractive explanation for the decreased activity and chlorite turnover number of KpCld relative to DaCld which lacks a H-bond partner in the comparable position.
FeCN vibrational distortions as indicators of FeCN geometry and distal pocket landscape
Open and closed conformers of the ferrous DaCld–CO complex, forming with Arg183 in its open and closed configuration, respectively, have been observed.24 Their positions on the π backbonding correlation plot are consistent with different distal H-bond donor strengths.24, 26 It was anticipated, that in DaCld–CN−, the open conformer would lack H-bonding between Arg183 and CN− while the closed conformer, with its inward-oriented guanidinium group, would interact more strongly with the coordinated CN− through H-bond donation. However, only one DaCld–CN− conformer was observed. Since the polar non-bonding interactions between distal H-bond donors and the anionic CN− ligand are expected to be stronger than their CO counterparts,36, 38 one explanation for the single DaCld–CN− conformer is that the stronger non-bonding interaction coupled with the ionic interaction between the anionic ligand and the cationic Arg result in a closed conformation.
The 2H2O insensitivity of the FeCN modes of the nearly linear DaCld–CN− leaves the question of H-bonding in the distal pocket unresolved.63, 77 Insensitivity of the νFe–C frequencies to 2H2O suggests that either there is no hydrogen bond or the hydrogen bond is so weak as to be undetectable by a shift of the νFe–C frequencies in 2H2O because its geometry is not optimal for polarizing electron density in the FeCN unit.36, 42, 63
The FeCN angle in heme protein cyanides is quite sensitive to distal steric interactions. In and out movement of the distal Arg in DaCld would certainly alter steric interactions between CN− and the distal heme pocket. Modulation of distal steric interactions with coordinated CN− could be directly associated with dynamics of the distal Arg183 side chain or indirectly, through its influence on the side chain conformations of other distal residues, including Phe200, Thr198 and Leu185. In either case, the ∠FeCN could be influenced by the position of the distal Arg. As judged by its νFe–C and δFeCN frequencies, the DaCld–CN− ∠FeCN is unaffected by pH; this also supports a single conformation for the cyanide complex. There is, however, a pH-dependent broadening of νFe–His and ν8 rR bands indicating some conformational inhomogeneity of the heme pocket. This decrease in homogeneity could correspond to the kinetic pKa estimated from koff. These data support the hypothesis that at low pH, where koff for DaCld–CN− is small, the active site is in a closed conformation while at alkaline pH open Arg conformational states become more accessible, lowering the barrier to CN− dissociation with a corresponding increase in koff values. However, it is likely that, even in the absence of distal H-bond interactions, the closed conformation dominates the rR spectrum of the ferric cyanide complex due to the ionic interaction between the cationic Arg side chain and the negatively charged cyanide ligand. Such a salt bridge-type interaction is not available to DaCld–CO with its neutral CO ligand, resulting in CO complexes mostly in the open Arg conformation.
Although the low pKa governing the Arg183 gating of the active site in ferric WT DaCld is not observed kinetically in the R183Q mutant, two forms of its heme carbonyl have been reported and interpreted as this mutant accessing two conformations analogous to those of the WT enzyme, one with the Gln183 side chain oriented towards the heme and a second in which it is oriented away.24 Although WT DaCld–CN− and DaCld(R183Q)–CN− exhibited similar νFe–C frequencies, the broadened νFe C band of the R183Q mutant reveals a decrease in structural homogeneity of the FeCN unit analogous to that seen for alkaline WT enzyme (vide infra). The smaller Δ(ν–δ) for DaCld(R183Q)–CN− relative to WT enzyme indicates that its FeCN geometry is less constrained. This suggests that lack of charge in the R183Q distal pocket or the difference in Gln side chain size and/or its hydrogen bond donor strength result in a weaker distal interaction with the bound cyanide in the mutant than in WT. Conformational inhomogeneity and the less constrained FeCN unit of DaCld(R183Q)–CN− indicate that the open conformer is accessible. However, based on the lack of pH sensitivity of the koff for DaCld(R183Q)–CN− and its similarity to the WT DaCld–CN− koff under acidic conditions, it is likely that an active-site conformer of DaCld(R183Q)–CN− analogous to the WT closed conformer is also accessible. Taken together, these lines of evidence suggest why DaCld(R183Q) retains a modicum of its chlorite-decomposing activity;24 while its closed conformation is responsible for the mutant’s residual activity, its diminished ability to bind and retain substrate in the open conformation significantly attenuates its activity.
In contrast to DaCld and its R183Q mutant, a single conformer is observed for KpCld–CO; its position on the νFe–C/νC–O correlation plot indicates that there is only weak interaction between heme–CO and distal pocket of KpCld (Figure 6). By analogy to its CO complex, KpCld–CN− would be expected to exist as a single conformer with a minimal H bonding environment around the CN−. The insensitivity of FeCN frequencies in KpCld–CN− to 2H2O substitution could be consistent with this; however, only very small isotope shifts are anticipated for H bonding to the cyanide making it difficult to detect. One FeCN geometry with very subtle frequency differences on either side of the pKa is observed for KpCld–CN−, suggesting that, like DaCld–CN−, its distal Arg is in a single conformation. Interestingly, NdCld–CO exhibits a single open conformation in which its distal Arg does not interact with the CO (Figure 6).64 Molecular dynamics simulations for ferrous NdCld predict that its distal Arg is in the open position 62% of the time, while in ferric NdCld–CN− the distal Arg is predominantly in the closed position (85% of the time). This suggests that, while the open conformation is observed for KpCld–CO, the form of KpCld–CN− observed spectroscopically is mostly likely closed due to nonbonding interactions comparable to those described above for DaCld.
Distal Arg versus Gln – effects of charge and steric bulk
The small differences between the FeCN frequencies of the DaCld and DaCld(R183Q) cyanides suggest that the structural features influencing the force constants and reduced masses of these modes are similar in the WT and mutant enzymes. Increased breadth of the mutant νFe–C band relative to that of WT (Figure 4) reflects greater structural flexibility (i.e. inhomogeneity) in the heme pocket structure. This points toward the Arg charge being important in controlling its side chain conformation.
Interestingly, several heme proteins having a distal Gln residue exhibit νFe–C frequencies comparable to those of the three Cld–CN− complexes reported here (Table 1). Increases in these νFe–C frequencies have been attributed to removal of constraints on the FeCN unit based on “strapped” heme cyanide model complexes with (CH2)n chains over the heme, where lengthening or eliminating the strap hindering the bound CN− caused the νFe–C to shift to higher frequencies (n=13 to 15, 445–447 cm−1; no strap, 451 cm−1).78 A similar trend is observed in heme protein cyanide complexes. For example, the crystal structure of Chlamydomonas Hb–CN− (pdb code:1DLY) revealed that its FeCN angle is 130° due to a H-bonding network involving a Tyr and two Gln in its distal pocket.79 Disruption of this H-bonding network resulted in a less hindered FeCN (i.e. more linear FeCN moiety), which was reported by the νFe–C of Chlamydomonas Hb–CN− shifting from 440 cm−1 to 454 cm−1 upon mutation of its distal Gln to Gly.42 Replacing the distal His in HRP–CN− with Gln elicits a downshift of its νFe–C frequency from 453 to 443 cm−1, consistent with steric reorganization of the distal heme pocket accompanying loss of the H-bond donation to the terminal N atom of the CN− ligand.59 A similar νFe–C frequency (444 cm−1)38 reported for HRP at pH 12.5 (c.f. pKa of ~14 for ImH of aqueous histidine) is probably due to loss of the H bond to the CN− ligand in addition to relaxing structural factors influencing the FeCN angle. Another possibility is that in the absence of H-bonding from the distal His, the distal Arg of HRP interacts with the coordinated CN− in a manner comparable to the distal Gln in the examples described here. This idea is supported by the fact that the WT Cld–CN− complexes with their distal Arg residues exhibit similar νFe–C frequencies (439–441 cm−1). Steric interactions dictated by Gln or Arg in these distal pockets appear to provide comparable nonbonding environments, which restrict the FeCN moiety, in their respective heme cyanides.
Relative insensitivities of νFe–C frequencies in the Cld–CN−s to Arg/Gln mutation and H2O/2H2O substitution suggest that neither the distal Arg nor distal Gln serve as significant H-bond donors to the terminal N atom of coordinated CN−. Interestingly, the distal Arg does donate two hydrogen bonds to coordinated F−.65 Therefore, the apparent lack of hydrogen bonding with coordinated CN− leads to the conclusion that heme pocket structure precludes it. In other words, the Cld heme pockets are organized to favor hydrogen bond donation to the coordinated atom of exogenous ligands.65 This is the first experimental evidence that supports a structurally based explanation for the poor peroxidase activity28 of the Clds. Specifically, their heme pocket structure disfavors peroxidase and catalase reactions by disfavoring the terminal hydrogen bond necessary to polarize the peroxo bond, which is responsible, in part, for its heterolytic cleavage in the conversion of compound 0 to compound I in heme peroxidases.
While DaClds and other heme proteins like peroxidases, Mb and Hb, have comparable affinities for cyanide (KD ~ 10−6 M), their kon and koff, values vary significantly (Table 2). The kon values for cyanide binding to Clds are 1 to 2 orders of magnitude greater than those for cytochrome c peroxidase (CcP) and HRP, which have distal pockets containing His and Arg, and 4 orders of magnitude greater than Mb and Hb with a His in their distal pockets. The distal Arg in Cld is partially responsible for these greater kon values, which drop from 106 to 103 M−1·s−1 upon its replacement with Gln. The series of NdCld Arg mutants and DaCld(R183Q) still have greater kon values than Mb or Hb indicating that factors intrinsic to Clds other than their distal Arg, such as the hydrophobicity of the distal pocket, contribute to their low kinetic barriers to cyanide binding.
The significantly higher affinity of KpCld for CN− relative to DaCld, driven by the much smaller koff value in the former, is surprising. An affinity for CN− of similar magnitude has been reported for the truncated Hb from Camplyobacter jejuni (Cj trHbP). However, its distal heme pocket is quite different than that of KpCld. In Cj trHbP, a Tyr and a Trp (pdb code: 2IG3) provide direct H bonds to stabilize bound CN−.80 How the pocket characteristics that promote the high CN− affinity of KpCld are relevant to its function is currently unclear.
Differing FeCN geometries in DaCld and KpCld
The stretching and bending frequencies reported here for DaCld and KpCld cyanides are compared to those of other cyanoferric heme proteins in Table 1. The frequencies for WT DaCld and its R183Q mutant are similar to other nearly linear (166° – 179°) cyanide complexes, especially those of HRP.38, 43 While the νFe–C and δFeCN frequencies of KpCld–CN− are close to those of bent NP1–CN−, the Raman shift patterns of their 13CN− and C15N− isotopologs reveal that their νFe–C and δFeCN frequencies are reversed from those for bent peroxidase, catalase, and P450 cyanides. The bent FeCN geometry of the KpCld–CN− indicates that there are interactions in the distal pocket that are strong enough to generate large off-axis distortions of the FeCN unit that are absent in DaCld. The presence of one or two water molecules in the KpCld active site, as is the case in dimeric ferric NwCld and CCld and pentameric NdCld–CN−,5, 23, 54 could provide the steric interactions and possibly H bonds responsible for the off-axis distortions in KpCld–CN− that contribute in slower koff relative to DaCld–CN−. This is also consistent with the “nearly linear” DaCld–CN− having an active site that is less accessible to water than the KpCld heme pocket.9, 26 Given that the chlorite decomposition activity of these enzymes differs by an order of magnitude and assuming that both enzymes react via similar intermediates, it appears that the factors responsible for the differences in FeCN geometry could play a significant role in their oxygen-evolving activity. The presence of water in the KpCld heme pocket would weaken the ion pair presumably formed between the distal Arg and ClO−, possibly explaining the decrease in activity. This may also be the reason that KpCld leaks ClO− more readily than DaCld, resulting in oxidative damage to the enzyme, lowering its turnover number.
While crystal structures of various Cld-ligand complexes have been reported,5, 20, 22, 23, 53, 54, 81 only one cyanide complex structure is available.23 In the NdCld–CN− crystal structure (3NN2), ∠FeCN ranges from 140.9 to 177.0° with an O atom of a glycerol molecule within H-bonding distance of the terminal cyanide nitrogen atom. The guanidinium group of the distal Arg is pointed away from the heme and lies beyond H-bonding distance from the cyanide ligand (Figure S7).23 It is unclear how the glycerol in the heme pocket of the NdCld–CN− structure influences the geometry of the FeCN unit or the orientation of the distal Arg. It does illustrate that a wide range of ∠FeCN can be accommodated. Since there is no glycerol present in the experiments described here and a bent FeCN unit is also observed for KpCld–CN−, it is possible that 1) its Arg is in a position analogous to the glycerol, pointed into the pocket toward the cyanide or 2) a water molecule can occupy the glycerol position and provide a bridge between the Arg and the cyanide.
CONCLUSIONS
KpCld has one of the highest affinities for CN− reported for heme proteins. Its particularly small KD of 4.7×10−9 M for CN− dissociation is attributable to slow dissociation kinetics. While the rates of CN− association with WT DaCld and KpCld are similar to one another, their kon values are at least an order of magnitude larger than those reported for peroxidases and four orders of magnitude greater than those of Mbs. The distal Arg is an important factor in the high CN− association rates, of both Cld subfamilies. Substitution of Arg with Gln reveals that the charge of the distal residue is important in controlling the homogeneity of the ligand-bound pentameric enzyme conformation and the pKa of ~7 is important in stabilizing the CN− complex. Differing FeCN geometries between DaCld (nearly linear) and KpCld (bent) reveal that their distal pocket environments, and possibly those between the subfamilies they represent, are distinct. Factors responsible for the bent FeCN geometry in KpCld, such as the possibility of water being positioned between the ligand and the distal Arg in the active site, could also affect the stability of the intermediates and/or transition states. Associated differences in kinetic barriers likely account for KpCld’s 10-fold lower oxygen-evolving activity relative to DaCld. That larger kinetic barrier is also likely the reason that escape of enzyme-inactivating HOCl from the catalytic heme pocket under turnover conditions competes with product formation, thereby limiting the turnover number. Whether these distal pocket characteristics are conserved within the Cld subfamilies remains to be determined.
Supplementary Material
Acknowledgments
Support is gratefully acknowledged from the National Institutes of Health Grants GM114787 (to G. S. L.-R.) and GM090260 (to J. L. D.).
ABBREVIATIONS
- Cld
chlorite dismutase
- KpCld
Klebsiella pneumoniae Cld
- DaCld
Dechloromonas aromatica Cld
- NwCld
Nitrobacter winogradskyi Cld
- NdCld
Candidatius Nitrospira defluvii Cld
- CCld
Cld from Cyanothece sp. PCC7425
- HRP
horseradish peroxidase
- Mb
myoglobin
- Hb
hemoglobin
- NP1
Rhodnius prolixux Nitrophorin 1
- rR
resonance Raman
- EPR
electron paramagnetic resonance
- WT
wild type
- HS
high spin
- LS
low spin
- 6cHS
six coordinate high spin
- 5cHS
five coordinate high spin
- 6cLS
six coordinate low spin
Footnotes
Notes
The authors declare no competing financial interests.
Supporting Information. Additional data referred to in the text: Figures S1–S7 and Tables S1–S4 include UV-vis spectra of Kp and DaCld–CN− (Fig S1), representative kinetic data for the formation of KpCld–CN− (Fig S2), comparison of low frequency window of rR of ferric and ferric KpCld–CN− (Fig S3), rate constants as a function of pH for the CN− reaction with KpCld, DaCld, DaCld(R183Q) (Table S1), rR of isotopically labeled alkaline Kp and DaCld–CN− (Fig S4 & S6), comparison of rR isotope-sensitive frequencies for cyanoferric Cld complexes as obtained by curve fitting analysis (Table S2) effect of increasing the Fe-C stretching force constant at an FeCN bond angle of 155°on the potential energy distribution and frequency of the stretching and bending normal modes (Fig S5), comparison of resonance Raman isotope-sensitive frequencies for cyanoferric Cld complexes as obtained by curve fitting analysis (Table S3), comparison of KpCld–CN− and NP1–CN− vibrational frequencies and isotope shifts (Table S4), and active site structure of NdCld-CN− complex (Fig S7). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.DuBois JL, Ojha S. Production of dioxygen in the dark: dismutases of oxyanions. Met Ions Life Sci. 2015;15:45–87. doi: 10.1007/978-3-319-12415-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaffner I, Hofbauer S, Krutzler M, Pirker KF, Furtmueller PG, Obinger C. Mechanism of chlorite degradation to chloride and dioxygen by the enzyme chlorite dismutase. Arch Biochem Biophys. 2015;574:18–26. doi: 10.1016/j.abb.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Tagore R, Crabtree RH, Brudvig GW. Oxygen Evolution Catalysis by a Dimanganese Complex and Its Relation to Photosynthetic Water Oxidation. Inorg Chem. 2008;47:1815–1823. doi: 10.1021/ic062218d. [DOI] [PubMed] [Google Scholar]
- 4.Coates JD, Achenbach LA. Microbial perchlorate reduction: rocket-fueled metabolism. Nat Rev Microbiol. 2004;2:569–580. doi: 10.1038/nrmicro926. [DOI] [PubMed] [Google Scholar]
- 5.Mlynek G, Sjoeblom B, Kostan J, Fuereder S, Maixner F, Gysel K, Furtmueller PG, Obinger C, Wagner M, Daims H, Djinovic-Carugo K. Unexpected diversity of chlorite dismutases: a catalytically efficient dimeric enzyme from Nitrobacter winogradskyi. J Bact. 2011;193:2408–2417. doi: 10.1128/JB.01262-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melnyk RA, Engelbrektson A, Clark IC, Carlson HK, Byrne-Bailey K, Coates JD. Identification of a perchlorate reduction genomic island with novel regulatory and metabolic genes. Appl Environ Microbiol. 2011;77:7401–7404. doi: 10.1128/AEM.05758-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark IC, Melnyk RA, Engelbrektson A, Coates JD. Structure and evolution of chlorate reduction composite transposons. mBio. 2013;4:e00379, 00312. doi: 10.1128/mBio.00379-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofbauer S, Schaffner I, Furtmueller PG, Obinger C. Chlorite dismutases - a heme enzyme family for use in bioremediation and generation of molecular oxygen. Biotechnol J. 2014;9:461–473. doi: 10.1002/biot.201300210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celis AI, Geeraerts Z, Ngmenterebo D, Machovina MM, Kurker RC, Rajakumar K, Ivancich A, Rodgers KR, Lukat-Rodgers GS, DuBois JL. A Dimeric Chlorite Dismutase Exhibits O2-Generating Activity and Acts as a Chlorite Antioxidant in Klebsiella pneumoniae MGH 78578. Biochemistry. 2015;54:434–446. doi: 10.1021/bi501184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goblirsch B, Kurker RC, Streit BR, Wilmot CM, DuBois JL. Chlorite dismutases, DyPs, and EfeB: 3 microbial heme enzyme families comprise the CDE structural superfamily. J Mol Biol. 2011;408:379–398. doi: 10.1016/j.jmb.2011.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaffner I, Hofbauer S, Krutzler M, Pirker KF, Bellei M, Stadlmayr G, Mlynek G, Djinovic-Carugo K, Battistuzzi G, Furtmueller PG, Daims H, Obinger C. Dimeric chlorite dismutase from the nitrogen-fixing cyanobacterium Cyanothece sp. PCC7425. Mol Microbiol. 2015;96:1053–1068. doi: 10.1111/mmi.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dailey TA, Boynton TO, Albetel AN, Gerdes S, Johnson MK, Dailey HA. Discovery and Characterization of HemQ: an essential heme biosynthetic pathway component. J Biol Chem. 2010;285:25978–25986. doi: 10.1074/jbc.M110.142604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dailey HA, Gerdes S, Dailey TA, Burch JS, Phillips JD. Noncanonical coproporphyrin-dependent bacterial heme biosynthesis pathway that does not use protoporphyrin. Proc Natl Acad Sci U S A. 2015;112:2210–2215. doi: 10.1073/pnas.1416285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dailey HA, Gerdes S. HemQ: An iron-coproporphyrin oxidative decarboxylase for protoheme synthesis in Firmicutes and Actinobacteria. Arch Biochem Biophys. 2015;574:27–35. doi: 10.1016/j.abb.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celis AI, Streit BR, Moraski GC, Kant R, Lash TD, Lukat-Rodgers GS, Rodgers KR, DuBois JL. Unusual Peroxide-Dependent, Heme-Transforming Reaction Catalyzed by HemQ. Biochemistry. 2015;54:4022–4032. doi: 10.1021/acs.biochem.5b00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobo SAL, Scott A, Videira MAM, Winpenny D, Gardner M, Palmer MJ, Schroeder S, Lawrence AD, Parkinson T, Warren MJ, Saraiva LM. Staphylococcus aureus haem biosynthesis: characterisation of the enzymes involved in final steps of the pathway. Mol Microbiol. 2015;97:472–487. doi: 10.1111/mmi.13041. [DOI] [PubMed] [Google Scholar]
- 17.Streit BR, DuBois JL. Chemical and Steady-State Kinetic Analyses of a Heterologously Expressed Heme Dependent Chlorite Dismutase. Biochemistry. 2008;47:5271–5280. doi: 10.1021/bi800163x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catling DC, Claire MW, Zahnle KJ, Quinn RC, Clark BC, Hecht MH, Kounaves S. J Geophys Res, [Planets] American Geophysical Union; 2010. Atmospheric origins of perchlorate on Mars and in the Atacama; pp. E00E11/01–E00E11/15. [Google Scholar]
- 19.Rao B, Hatzinger PB, Bohlke JK, Sturchio NC, Andraski BJ, Eckardt FD, Jackson WA. Natural Chlorate in the Environment: Application of a New IC-ESI/MS/MS Method with a Cl18O3- Internal Standard. Environ Sci Technol. 2010;44:8429–8434. doi: 10.1021/es1024228. [DOI] [PubMed] [Google Scholar]
- 20.Goblirsch BR, Streit BR, DuBois JL, Wilmot CM. Structural features promoting dioxygen production by Dechloromonas aromatica chlorite dismutase. J Biol Inorg Chem. 2010;15:879–888. doi: 10.1007/s00775-010-0651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 22.de Geus DC, Thomassen EAJ, Hagedoorn PL, Pannu NS, van Duijn E, Abrahams JP. Crystal Structure of Chlorite Dismutase, a Detoxifying Enzyme Producing Molecular Oxygen. J Mol Biol. 2009;387:192–206. doi: 10.1016/j.jmb.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Kostan J, Sjoeblom B, Maixner F, Mlynek G, Furtmueller PG, Obinger C, Wagner M, Daims H, Djinovic-Carugo K. Structural and functional characterisation of the chlorite dismutase from the nitrite-oxidizing bacterium “Candidatus Nitrospira defluvii”: Identification of a catalytically important amino acid residue. J Struct Biol. 2010;172:331–342. doi: 10.1016/j.jsb.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Blanc B, Mayfield JA, McDonald CA, Lukat-Rodgers GS, Rodgers KR, DuBois JL. Understanding How the Distal Environment Directs Reactivity in Chlorite Dismutase: Spectroscopy and Reactivity of Arg183 Mutants. Biochemistry. 2012;51:1895–1910. doi: 10.1021/bi2017377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofbauer S, Gysel K, Bellei M, Hagmueller A, Schaffner I, Mlynek G, Kostan J, Pirker KF, Daims H, Furtmueller PG, Battistuzzi G, Djinovic-Carugo K, Obinger C. Manipulating conserved heme cavity residues of chlorite dismutase: Effect on structure, redox chemistry, and reactivity. Biochemistry. 2014;53:77–89. doi: 10.1021/bi401042z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streit BR, Blanc B, Lukat-Rodgers GS, Rodgers KR, DuBois JL. How Active-Site Protonation State Influences the Reactivity and Ligation of the Heme in Chlorite Dismutase. J Am Chem Soc. 2010;132:5711–5724. doi: 10.1021/ja9082182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofbauer S, Gruber C, Pirker KF, Suendermann A, Schaffner I, Jakopitsch C, Oostenbrink C, Furtmueller PG, Obinger C. Transiently produced hypochlorite is responsible for the irreversible inhibition of chlorite dismutase. Biochemistry. 2014;53:3145–3157. doi: 10.1021/bi500401k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee AQ, Streit BR, Zdilla MJ, bu-Omar MM, DuBois JL. Mechanism of and exquisite selectivity for O-O bond formation by the heme-dependent chlorite dismutase. Proc Natl Acad Sci U S A. 2008;105:15654–15659. doi: 10.1073/pnas.0804279105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graves AB, Morse RP, Chao A, Iniguez A, Goulding CW, Liptak MD. Crystallographic and spectroscopic insights into heme degradation by Mycobacterium tuberculosis MhuD. Inorg Chem. 2014;53:5931–5940. doi: 10.1021/ic500033b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida T, Tsuge H, Konno H, Hisabori T, Sugano Y. The catalytic mechanism of dye-decolorizing peroxidase DyP may require the swinging movement of an aspartic acid residue. FEBS J. 2011;278:2387–2394. doi: 10.1111/j.1742-4658.2011.08161.x. [DOI] [PubMed] [Google Scholar]
- 31.Rwere F, Mak PJ, Kincaid JR. Resonance Raman Interrogation of the Consequences of Heme Rotational Disorder in Myoglobin and Its Ligated Derivatives. Biochemistry. 2008;47:12869–12877. doi: 10.1021/bi801779d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.La Mar GN, Chen Z, Vyas K, McPherson AD. An Interpretive Basis of the Hyperfine Shifts in Cyanide-Inhibited Horseradish Peroxidase Based on the Magnetic Axes and Ligand Tilt. Influence of Substrate Binding and Extensions to Other Peroxidases. J Am Chem Soc. 1995;117:411–419. [Google Scholar]
- 33.Foshay MC, Vitello LB, Erman JE. Effect of alternative distal residues on the reactivity of cytochrome c peroxidase: Properties of CcP mutants H52D, H52E, H52N, and H52Q. Biochim Biophys Acta, Proteins Proteomics. 2011;1814:525–535. doi: 10.1016/j.bbapap.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bidwai A, Witt M, Foshay M, Vitello LB, Satterlee JD, Erman JE. Cyanide Binding to Cytochrome c Peroxidase (H52L) Biochemistry. 2003;42:10764–10771. doi: 10.1021/bi034632k. [DOI] [PubMed] [Google Scholar]
- 35.Ellis WD, Dunford HB. The kinetics of cyanide and fluoride binding by ferric horseradish peroxidase. Biochemistry. 1968;7:2054–2062. doi: 10.1021/bi00846a006. [DOI] [PubMed] [Google Scholar]
- 36.Simianu MC, Kincaid JR. Resonance Raman Spectroscopic Detection of Both Linear and Bent Fe-CN Fragments for the Cyanide Adducts of Cytochrome P-450 Camphor and Its Substrate-Bound Forms. Relevance to the “Charge Relay” Mechanism. J Am Chem Soc. 1995;117:4628–4636. [Google Scholar]
- 37.Al-Mustafa J, Sykora M, Kincaid JR. Resonance Raman investigation of cyanide ligated beef liver and Aspergillus niger catalases. J Biol Chem. 1995;270:10449–10460. doi: 10.1074/jbc.270.18.10449. [DOI] [PubMed] [Google Scholar]
- 38.Al-Mustafa J, Kincaid JR. Resonance Raman Study of Cyanide-Ligated Horseradish Peroxidase. Detection of Two Binding Geometries and Direct Evidence for the “Push-Pull” Effect. Biochemistry. 1994;33:2191–2197. doi: 10.1021/bi00174a028. [DOI] [PubMed] [Google Scholar]
- 39.Maes EM, Walker FA, Montfort WR, Czernuszewicz RS. Resonance Raman Spectroscopic Study of Nitrophorin 1, a Nitric Oxide-Binding Heme Protein from Rhodnius prolixus, and Its Nitrosyl and Cyano Adducts. J Am Chem Soc. 2001;123:11664–11672. doi: 10.1021/ja0031927. [DOI] [PubMed] [Google Scholar]
- 40.Boffi A, Chiancone E, Takahashi S, Rousseau DL. Stereochemistry of the Fe(II)- and Fe(III)-cyanide complexes of the homodimeric Scapharca inaequivalvis hemoglobin. A resonance Raman and FTIR study. Biochemistry. 1997;36:4505–4509. doi: 10.1021/bi9618880. [DOI] [PubMed] [Google Scholar]
- 41.Hirota S, Ogura T, Shinzawa-Itoh K, Yoshikawa S, Kitagawa T. Observation of Multiple CN-Isotope-Sensitive Raman Bands for CN Adducts of Hemoglobin, Myoglobin, and Cytochrome c Oxidase: Evidence for Vibrational Coupling between the Fe-C-N Bending and Porphyrin In-Plane Modes. J Phys Chem. 1996;100:15274–15279. [Google Scholar]
- 42.Das TK, Couture M, Guertin M, Rousseau DL. Distal Interactions in the Cyanide Complex of Ferric Chlamydomonas Hemoglobin. J Phys Chem B. 2000;104:10750–10756. [Google Scholar]
- 43.Lopez-Garriga JJ, Oertling WA, Kean RT, Hoogland H, Wever R, Babcock GT. Metal-ligand vibrations of cyanoferric myeloperoxidase and cyanoferric horseradish peroxidase: evidence for a constrained heme pocket in myeloperoxidase. Biochemistry. 1990;29:9387–9395. doi: 10.1021/bi00492a012. [DOI] [PubMed] [Google Scholar]
- 44.Tropea JEC, Scott, Waugh David S. Expression and purification of soluble His6-tagged TEV protease. In: Doyle SA, editor. Methods in Molecular Biology: High Throughput Protein Expression and Purification. Humana Press; Totowa, NJ: 2009. pp. 297–306. [DOI] [PubMed] [Google Scholar]
- 45.Blanc B, Rodgers KR, Lukat-Rodgers GS, DuBois JL. Understanding the roles of strictly conserved tryptophan residues in O2 producing chlorite dismutases. Dalton Trans. 2013;42:3156–3169. doi: 10.1039/c2dt32312e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Philippi M, dos Santos HS, Martins AO, Azevedo CM, Pires M. Alternative spectrophotometric method for standardization of chlorite aqueous solutions. Anal Chim Acta. 2007;585:361–365. doi: 10.1016/j.aca.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 47.Ryan JA, Culshaw GW. The use of p-dimethylaminobenzylidene rhodanine as an indicator for the volumetric determination of cyanides. Analyst. 1944;69:370–371. [Google Scholar]
- 48.Breuer PL, Sutcliffe CA, Meakin RL. Cyanide measurement by silver nitrate titration: Comparison of rhodanine and potentiometric end-points. Hydrometallurgy. 2011;106:135–140. [Google Scholar]
- 49.Rodgers KR, Lukat-Rodgers GS, Barron JA. Structural basis for ligand discrimination and response initiation in the heme-based oxygen sensor FixL. Biochemistry. 1996;35:9539–9548. doi: 10.1021/bi9530853. [DOI] [PubMed] [Google Scholar]
- 50.Bidwai AK, Ok EY, Erman JE. pH Dependence of Cyanide Binding to the Ferric Heme Domain of the Direct Oxygen Sensor from Escherichia coli and the Effect of Alkaline Denaturation. Biochemistry. 2008;47:10458–10470. doi: 10.1021/bi800872d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Ginkel CG, Rikken GB, Kroon AGM, Kengen SWM. Purification and characterization of chlorite dismutase. A novel oxygen-generating enzyme. Arch Microbiol. 1996;166:321–326. doi: 10.1007/s002030050390. [DOI] [PubMed] [Google Scholar]
- 52.Stenklo K, Danielsson Thorell H, Bergius H, Aasa R, Nilsson T. Chlorite dismutase from Ideonella dechloratans. JBIC Journal of Biological Inorganic Chemistry. 2001;6:601–607. doi: 10.1007/s007750100237. [DOI] [PubMed] [Google Scholar]
- 53.Freire DM, Rivas MG, Dias AM, Lopes AT, Costa C, Santos-Silva T, Van Doorslaer S, Gonzalez PJ. The homopentameric chlorite dismutase from Magnetospirillum sp. J Inorg Biochem. 2015;151:1–9. doi: 10.1016/j.jinorgbio.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Schaffner I, Mlynek G, Flego N, Puehringer D, Libiseller-Egger J, Coates L, Hofbauer S, Bellei M, Furtmueller PG, Battistuzzi G, Smulevich G, Djinovic-Carugo K, Obinger C. Molecular Mechanism of Enzymatic Chlorite Detoxification: Insights from Structural and Kinetic Studies. ACS Catal. 2017;7:7962–7976. doi: 10.1021/acscatal.7b01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicoletti FP, Droghetti E, Howes BD, Bustamante JP, Bonamore A, Sciamanna N, Estrin DA, Feis A, Boffi A, Smulevich G. H-bonding networks of the distal residues and water molecules in the active site of Thermobifida fusca hemoglobin. Biochim Biophys Acta, Proteins Proteomics. 2013;1834:1901–1909. doi: 10.1016/j.bbapap.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 56.Yu NT, Benko B, Kerr EA, Gersonde K. Iron-carbon bond lengths in carbonmonoxy and cyanomet complexes of the monomeric hemoglobin III from Chironomus thummi thummi: a critical comparison between resonance Raman and x-ray diffraction studies. Proc Natl Acad Sci U S A. 1984;81:5106–5110. doi: 10.1073/pnas.81.16.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu C, Mukai M, Lin Y, Wu G, Poole RK, Yeh SR. Structural and Functional Properties of a Single Domain Hemoglobin from the Food-borne Pathogen Campylobactor jejuni. J Biol Chem. 2007;282:25917–25928. doi: 10.1074/jbc.M704415200. [DOI] [PubMed] [Google Scholar]
- 58.Al-Mustafa JI, Alshbool T. Fourier Transform Infrared Spectroscopic Investigation of the Binary and Ternary Cyanide Adducts of the Oxidized Horseradish Peroxidase: Identification of a Second Stretching Mode for the Carbon-Nitrogen Bond of the Bound Cyanide Ion. Spectrosc Lett. 2014;47:281–291. [Google Scholar]
- 59.Tanaka M, Ishimori K, Mukai M, Kitagawa T, Morishima I. Catalytic activities and structural properties of horseradish peroxidase distal His42 -> Glu or Gln mutant. Biochemistry. 1997;36:9889–9898. doi: 10.1021/bi970906q. [DOI] [PubMed] [Google Scholar]
- 60.Hu S, Treat RW, Kincaid JR. Distinct heme active-site structure in lactoperoxidase revealed by resonance Raman spectroscopy. Biochemistry. 1993;32:10125–10130. doi: 10.1021/bi00089a031. [DOI] [PubMed] [Google Scholar]
- 61.Bolognesi M, Rosano C, Losso R, Borassi A, Rizzi M, Wittenberg JB, Boffi A, Ascenzi P. Cyanide binding to Lucina pectinata hemoglobin I and to sperm whale myoglobin: an x-ray crystallographic study. Biophys J. 1999;77:1093–1099. doi: 10.1016/S0006-3495(99)76959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu S, Smith KM, Spiro TG. Assignment of protoheme resonance Raman spectrum by heme labeling in myoglobin. J Am Chem Soc. 1996;118:12638–12646. [Google Scholar]
- 63.Deng T-j, Macdonald IDG, Simianu MC, Sykora M, Kincaid JR, Sligar SG. Hydrogen-Bonding Interactions in the Active Sites of Cytochrome P450cam and Its Site-Directed Mutants. J Am Chem Soc. 2001;123:269–278. doi: 10.1021/ja001517d. [DOI] [PubMed] [Google Scholar]
- 64.Hofbauer S, Howes BD, Flego N, Pirker KF, Schaffner I, Mlynek G, Djinovic-Carugo K, Furtmueller PG, Smulevich G, Obinger C. From chlorite dismutase towards HemQ-the role of the proximal H-bonding network in haeme binding. Biosci Rep. 2016;36:e00312. doi: 10.1042/BSR20150330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geeraerts Z, Rodgers KR, DuBois J, Lukat-Rodgers GS. Active sites of O2-evolving chlorite dismutases probed by halides, hydroxides and new iron-ligand vibrational correlations. Biochemistry. 2017;56:4509–4524. doi: 10.1021/acs.biochem.7b00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suendermann A, Reif MM, Hofbauer S, Obinger C, Oostenbrink C. Investigation of Ion Binding in Chlorite Dismutases by Means of Molecular Dynamics Simulations. Biochemistry. 2014;53:4869–4879. doi: 10.1021/bi500467h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hofbauer S, Bellei M, Suendermann A, Pirker KF, Hagmueller A, Mlynek G, Kostan J, Daims H, Furtmueller PG, Djinovic-Carugo K, Oostenbrink C, Battistuzzi G, Obinger C. Redox Thermodynamics of High-Spin and Low-Spin Forms of Chlorite Dismutases with Diverse Subunit and Oligomeric Structures. Biochemistry. 2012;51:9501–9512. doi: 10.1021/bi3013033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erman JE. Kinetic and equilibrium studies of cyanide binding by cytochrome c peroxidase. Biochemistry. 1974;13:39–44. doi: 10.1021/bi00698a007. [DOI] [PubMed] [Google Scholar]
- 69.Milani M, Ouellet Y, Ouellet H, Guertin M, Boffi A, Antonini G, Bocedi A, Mattu M, Bolognesi M, Ascenzi P. Cyanide Binding to Truncated Hemoglobins: A Crystallographic and Kinetic Study. Biochemistry. 2004;43:5213–5221. doi: 10.1021/bi049870+. [DOI] [PubMed] [Google Scholar]
- 70.Bolli A, Ciaccio C, Coletta M, Nardini M, Bolognesi M, Pesce A, Guertin M, Visca P, Ascenzi P. Ferrous Campylobacter jejuni truncated hemoglobin P displays an extremely high reactivity for cyanide - a comparative study. FEBS J. 2008;275:633–645. doi: 10.1111/j.1742-4658.2007.06223.x. [DOI] [PubMed] [Google Scholar]
- 71.Brancaccio A, Cutruzzola F, Allocatelli CT, Brunori M, Smerdon SJ, Wilkinson AJ, Dou Y, Keenan D, Ikeda-Saito M, Brantley REJ, Olson JS. Structural factors governing azide and cyanide binding to mammalian metmyoglobins. J Biol Chem. 1994;269:13843–13853. [PubMed] [Google Scholar]
- 72.Dou Y, Olson JS, Wilkinson AJ, Ikeda-Saito M. Mechanism of Hydrogen Cyanide Binding to Myoglobin. Biochemistry. 1996;35:7107–7113. doi: 10.1021/bi9600299. [DOI] [PubMed] [Google Scholar]
- 73.Bidwai AK, Ahrendt AJ, Sullivan JS, Vitello LB, Erman JE. pH dependence of cyanide and imidazole binding to the heme domains of Sinorhizobium meliloti and Bradyrhizobium japonicum FixL. J Inorg Biochem. 2015;153:88–102. doi: 10.1016/j.jinorgbio.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ascenzi P, Sbardella D, Santucci R, Coletta M. Cyanide binding to ferrous and ferric microperoxidase-11. J Biol Inorg Chem. 2016;21:511–522. doi: 10.1007/s00775-016-1361-z. [DOI] [PubMed] [Google Scholar]
- 75.Mintorovitch J, Satterlee JD. Anomalously slow cyanide binding to Glycera dibranchiata monomer methemoglobin component II: implication for the equilibrium constant. Biochemistry. 1988;27:8045–8050. doi: 10.1021/bi00421a011. [DOI] [PubMed] [Google Scholar]
- 76.Mintorovitch J, Van Pelt D, Satterlee JD. Kinetic study of the slow cyanide binding to Glycera dibranchiata monomer hemoglobin components III and IV. Biochemistry. 1989;28:6099–6104. doi: 10.1021/bi00440a055. [DOI] [PubMed] [Google Scholar]
- 77.Unno M, Christian JF, Olson JS, Sage JT, Champion PM. Evidence for Hydrogen Bonding Effects in the Iron Ligand Vibrations of Carbonmonoxy Myoglobin. J Am Chem Soc. 1998;120:2670–2671. [Google Scholar]
- 78.Tanaka T, Yu NT, Chang CK. Resonance Raman studies of sterically hindered cyanomet “strapped” hemes. Effects of ligand distortion and base tension on iron-carbon bond. Biophysical Journal. 1987;52:801–805. doi: 10.1016/S0006-3495(87)83274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Egawa T, Yeh SR. Structural and functional properties of hemoglobins from unicellular organisms as revealed by resonance Raman spectroscopy. J Inorg Biochem. 2005;99:72–96. doi: 10.1016/j.jinorgbio.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 80.Nardini M, Pesce A, Labarre M, Richard C, Bolli A, Ascenzi P, Guertin M, Bolognesi M. Structural Determinants in the Group III Truncated Hemoglobin from Campylobacter jejuni. J Biol Chem. 2006;281:37803–37812. doi: 10.1074/jbc.M607254200. [DOI] [PubMed] [Google Scholar]
- 81.De Schutter A, Correia HD, Freire DM, Rivas MG, Rizzi A, Santos-Silva T, Gonzalez PJ, Van Doorslaer S. Ligand binding to chlorite dismutase from Magnetospirillum sp. J Phys Chem B. 2015;119:13859–13869. doi: 10.1021/acs.jpcb.5b04141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.