Abstract
IMPORTANCE
Cerebrovascular disease (CVD) and Alzheimer disease are significant causes of cognitive impairment in the elderly. However, few studies have evaluated the relationship between CVD and β-amyloid burden in living humans or their synergistic effects on cognition. Thus, there is a need for better understanding of mild cognitive impairment (MCI) before clinical deterioration begins.
OBJECTIVE
To determine the synergistic effects of β-amyloid burden and CVD on cognition in patients with subcortical vascular MCI (svMCI).
DESIGN, SETTING, AND PARTICIPANTS
A cross-sectional study was conducted using a hospital-based sample at a tertiary referral center. We prospectively recruited 95 patients with svMCI; 67 of these individuals participated in the study. Forty-five patients with amnestic MCI (aMCI) were group matched with those with svMCI by the Clinical Dementia Rating Scale Sum of Boxes.
MAIN OUTCOMES AND MEASURES
We measured β-amyloid burden using positron emission tomography with carbon 11–labeled Pittsburgh Compound B (PiB). Cerebrovascular disease was quantified as white matter hyperintensity volume detected by magnetic resonance imaging fluid-attenuated inversion recovery. Detailed neuropsychological tests were performed to determine the level of patients’ cognitive impairment.
RESULTS
On evaluation, 22 of the svMCI group (33%) and 28 of the aMCI group (62%) were found to be PiB positive. The mean PiB retention ratio was lower in patients with svMCI than in those with aMCI. In svMCI, the PiB retention ratio was associated with cognitive impairments in multiple domains, including language, visuospatial, memory, and frontal executive functions, but was associated only with memory dysfunction in aMCI. A significant interaction between PiB retention ratio and white matter hyperintensity volume was found to affect visuospatial function in patients with svMCI.
CONCLUSIONS AND RELEVANCE
Most patients with svMCI do not exhibit substantial amyloid burden, and CVD does not increase β-amyloid burden as measured by amyloid imaging. However, in patients with svMCI, amyloid burden and white matter hyperintensity act synergistically to impair visuospatial function. Therefore, our findings highlight the need for accurate biomarkers, including neuroimaging tools, for early diagnosis and the need to relate these biomarkers to cognitive measurements for effective use in the clinical setting.
Alzheimer disease (AD) and subcortical vascular dementia are considered to be the most common types of dementia. Most studies on mild cognitive impairment (MCI) have focused on the amnestic MCI (aMCI) form, which is a prodromal stage of AD. In contrast, few studies have involved patients in the prodromal stages of subcortical vascular dementia.1–3 Results from prior studies4,5 from our group suggest that the prodromal stage of subcortical vascular dementia, referred to as subcortical vascular MCI (svMCI), is distinctive from aMCI in terms of neuropsychological and neurobehavioral findings as well as the topography of glucose metabolism.
Evidence suggests that vascular risk factors or atherosclerosis and AD dementia have a strong association,6–8 suggesting that ischemia might be related to AD β-amyloid (Aβ) burden. Therefore, it is also possible that patients with svMCI harbor more Aβ burden than do those with aMCI. However, an alternative hypothesis is that cerebrovascular disease (CVD) itself might cause cognitive impairment, suggesting that patients with svMCI could have less Aβ than patients with aMCI without significant CVD. As a result of developments in molecular imaging, premortem detection of Aβ (a pathologic hallmark of AD) is now possible through positron emission tomography (PET) imaging using carbon 11–labeled Pittsburgh Compound B ([11C]PiB).9 The frequency of PiB positivity (PiB+) is reported10–15 to be approximately 90% in patients with AD and 52% to 75% in those with aMCI. Although 2 studies16,17 have evaluated the relationship between CVD (including white matter hyperintensity [WMH] or lacunes) and brain Aβ burden in individuals with normal or mildly impaired cognition, to our knowledge, an investigation of brain Aβ burden using PET amyloid imaging in patients with svMCI has not been conducted.
Most aMCI research10,13 has demonstrated that Aβ burden is selectively associated with memory dysfunction. Cerebrovascular disease is associated with impairment of executive function.1,2,16,18 Both brain Aβ and CVD affect cognition, and animal studies19,20 suggest that there might be interactive effects of ischemia and Aβ on cognition. Therefore, it is possible that in patients with CVD progressing to svMCI, Aβ burden and CVD could synergistically affect cognition. Alternatively, Aβ burden in svMCI might not affect cognitive impairment; previous studies16 have shown that Aβ burden was not associated with cognitive impairment.
In the present study, we investigated patients with svMCI who underwent PiB PET imaging and structural magnetic resonance imaging (MRI) for markers of CVD, quantified as WMH. The goals were to (1) determine the extent of PiB retention and frequency of PiB+ in patients with svMCI and (2) evaluate the relationship between Aβ burden, CVD (measured as WMH), and cognition in patients with svMCI.
Methods
Participants
We prospectively recruited 95 patients with svMCI, with diagnosis and follow-up conducted at Samsung Medical Center from October 7, 2009, to May 11, 2011. Patients with svMCI were evaluated using the criteria of Petersen et al,21 with the following modifications that have been previously described4 in detail: (1) a subjective report of cognitive difficulty by the patient or caregiver; (2) normal activities of daily living (ADL), with the score determined clinically and by the Seoul-Instrumental Activities of Daily Living scale22; (3) an objective cognitive decline below the 16th percentile on the Seoul Neuropsychological Screening Battery23; (4) no dementia; (5) a subcortical vascular feature defined as a focal neurologic symptom or sign including corticobulbar signs, pyramidal signs, or parkinsonism24; and (6) significant ischemia shown on MRI. Significant ischemia was defined as WMH on fluid-attenuated inversion recovery (FLAIR) images that satisfied the following criteria: (1) WMH of 10 mm or more in the periventricular white matter (caps or rim) and (2) WMH of 25 mm or more (maximum diameter) in the deep white matter, consistent with an extensive white matter lesion or diffusely confluent lesion. The MRI findings of patients with svMCI are shown in Author Figure 1 (http://lrc.skkumed.ac.kr/dataroom/AuthorContents.pdf).
A total of 45 patients with aMCI who were matched to svMCI patients with the Clinical Dementia Rating Scale Sum of Boxes were recruited at Samsung Medical Center during the same period. They met the Petersen et al21 clinical criteria for MCI with the following modifications: (1) subjective memory problems reported by the patient or caregiver, (2) normal general cognitive function above the 16th percentile on the Mini-Mental State Examination,25 (3) normal ADL as judged by an interview with a clinician and the standardized ADL scale,22 (4) objective memory decline below the 16th percentile determined with neuropsychological tests, and (5) no dementia. In addition, we determined that the patients had mild or no WMH on MRI (periventricular WMH <10 mm and deep WMH <10 mm in maximum diameter). Demographics and clinical characteristics of the patients are listed in the Supplement (eTable 1).
Patients were evaluated by clinical interview and neurologic and neuropsychological examinations as previously described.26 Brain MRI confirmed the absence of structural lesions, including territorial cerebral infarction.
Among the 95 patients with svMCI, 28 individuals declined participation in the study, with a final cohort of 67 patients. Characteristics of included vs excluded patients are described in the Supplement (eTable 1).
We also recruited 75 participants with normal cognition and no history of neurologic or psychiatric illnesses, as well as with normal neurologic examination results. They were required to undergo the same neuropsychological testing and MRI scanning as the svMCI group.
After a complete description of the study, written informed consent was obtained from each patient. The participants did not receive compensation. The institutional review board of the Samsung Medical Center approved the study protocol.
Neuropsychological Tests
All patients underwent neuropsychological testing using the Seoul Neuropsychological Screening Battery.23 Quantitatively scorable tests, including digit span (forward and backward), the Boston Naming Test (BNT),27 the Rey-Osterrieth Complex Figure Test (RCFT),28 the Seoul Verbal Learning Test (SVLT),27 a phonemic and semantic Controlled Oral Word Association Test,29 and a StroopTest (color reading),30 were used in the analysis.
[11C]PiB PET Imaging
All patients with MCI completed the [11C]PiB PET scan at Samsung Medical Center or Asan Medical Center and underwent PET scanning with identical settings (Discovery STe PET/CT scanner; GE Healthcare).18 Detailed methods are described in the Supplement (eMethods 1). Data processing was performed using Statistical Parametric Mapping, version 5 (SPM5) under MATLAB, version 6.5 (MathWorks, http://www.mathworks.com/products/matlab/). To measure PiB retention, we used the cerebral-cortical region to cerebellum uptake ratio. The cerebellum was used as a reference region because it did not show group differences. Regional cerebral-cortical uptake ratios were calculated by dividing each cortical volume of interest uptake ratio by mean uptake of cerebellar cortex (cerebellum crus 1 and crus 2). Global PiB retention ratios were calculated from the volume-weighted average uptake ratio of bilateral 28 cerebral cortical volumes of interest from bilateral frontal, temporal, parietal, and occipital lobes using the Annotated Anatomical Labeling atlas.31 Patients were considered PiB+ if their global PiB retention ratio was more than 2 SDs (PiB retention ratio >1.5) from the mean of the healthy controls.18 We also defined PiB retention ratio as a continuous variable.
MRI Acquisition
The transverse relaxation time (T2), longitudinal relaxation time (T1), FLAIR, and T2*-weighted gradient–recalled echo MRIs were acquired from all participants at Samsung Medical Center using the same 3.0-T MRI scanner (Achieva 3.0T; Philips). Detailed MRI factors are described in the Supplement (eMethods 2).
Measurement of Regional WMH Volume
We quantified WMH volumes (in milliliters) on FLAIR images using an automated method as previously described.32 Detailed WMH measurement methods are described in the Supplement (eMethods 3).
Assessment of Lacunes and Microbleeds on MRI
Lacunes were defined as lesions (≥3 mm and ≤15 mm in diameter) with low signal on T1-weighted images, high signal on T2-weighted images, and a perilesional halo on 80 axial sections of FLAIR images. Microbleeds were defined as 10 mm or less in diameter, using criteria proposed by Greenberg et al,33 on 20 axial sections of time constant for T2*-weighted gradient–recalled echo sequence MRIs. Detailed measurement methods for lacunes and microbleeds are described in the Supplement (eMethods 4). Two experienced neurologists (J.H.P. and an independent practitioner) who were blinded to other patient data reviewed the number and location of the lacunes and microbleeds. The κ value for the agreement between the 2 neurologists was 0.78 for lacunes and 0.92 for microbleeds, and consensus was reached in all cases of discrepancy.
Median time intervals from PiB PET to neuropsychological tests and from MRI to PiB PET were not significantly different between the groups. Although differences in time from neuropsychological tests to MRI occurred, their median differences were negligible (Supplement [eMethods 5]).
Statistical Analysis
Descriptive statistics were determined, and the χ2 test and unpaired, 2-tailed t test were conducted for comparisons between groups. Neuropsychological test results were compared between groups with adjustment for age and years of education using an analysis of covariance test. Because of the skew distribution, WMH volume, PiB retention ratios, and results of each neuropsychological test were log transformed before analysis. We added the value 1 to all the neuropsychological test results to enable us to log transform the data. Absence vs presence of lacunes or microbleeds was also used as a categorical variable instead of the number of lacunes or microbleeds. To examine the relationship between PiB and cognition, we performed multiple linear regression analyses using dependent variables for neuropsychological test scores (log transformed). The forward stepwise approach was used to select all possible predictors to explain cognitive deficits. The selection criteria involved entering variables below P < .05 and exit variables greater than P > .10. Considered as important clinical predictors, the total PiB retention ratio (log transformed) and WMH (log transformed) were entered into the final model to prevent them from being missed. Other possible predictors included were age; sex; years of education; vascular risk factors, including history of hypertension, diabetes mellitus, dyslipidemia, cardiac disease, and stroke; apolipoprotein E4 genotype; and presence of lacunes and microbleeds. To evaluate interactions between WMH volume and PiB retention ratio, interaction terms (log WMH × log PiB retention ratio) were added in the aforementioned multiple regression model (enter method) and other covariates listed above were also corrected (stepwise method). The false discovery rate (FDR) was used to correct for multiple testing. We defined statistical significance as an FDR-corrected value of P < .05. Statistical analyses were conducted using SAS, version 9.1 (SAS Institute Inc).
To compare the distribution of PiB retention between PiB+ svMCI and PiB+ aMCI patients (Figure 1), an analysis of covariance on a voxel-by-voxel basis after controlling for age was performed using SPM5 and MATLAB 6.5 for Windows. AnSPM5 regression analysis was performed without global normalization, since the [11C]PiB PET images had already been normalized to cerebellar region of interest PiB binding. The detailed methods of analysis are described in the Supplement (eMethods 6). We defined statistical significance as an FDR-corrected value of P < .05 at a cluster extent threshold of 150 voxels.
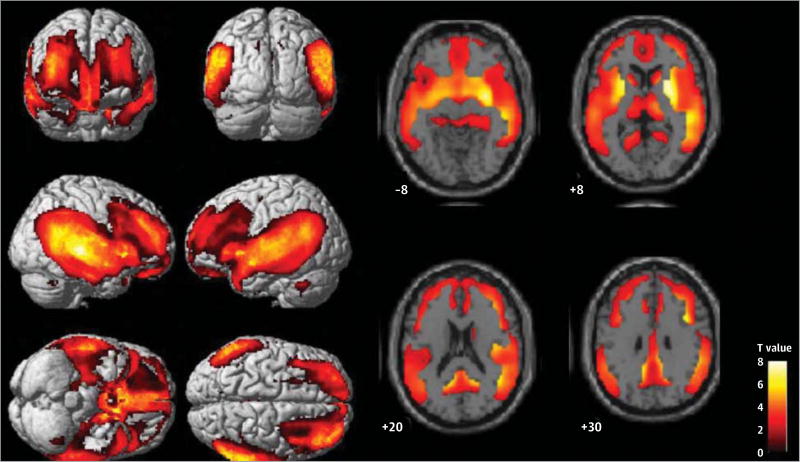
Figure 1. Statistical Parametric Mapping Analysis of Pittsburgh Compound B (PiB) Retention in the PiB-positive (PiB+) Amnestic Mild Cognitive Impairment (aMCI) and PiB+ Subcortical Vascular Mild Cognitive Impairment (svMCI) Groups.
The PiB+ aMCI patients exhibited greater PiB retention in the lateral temporal, posterior cingulate, and medial temporal and frontal cortices than did the PiB+ svMCI patients. The color bar intensity represents the value of the T statistic. The numbers represent the stereotactic z coordinate corresponding to each axial section. Statistical significance was set as a false discovery rate–corrected P < .05 at a cluster extent threshold of 150 voxels.
Results
Comparisons of PiB Retention and Neuropsychological Results
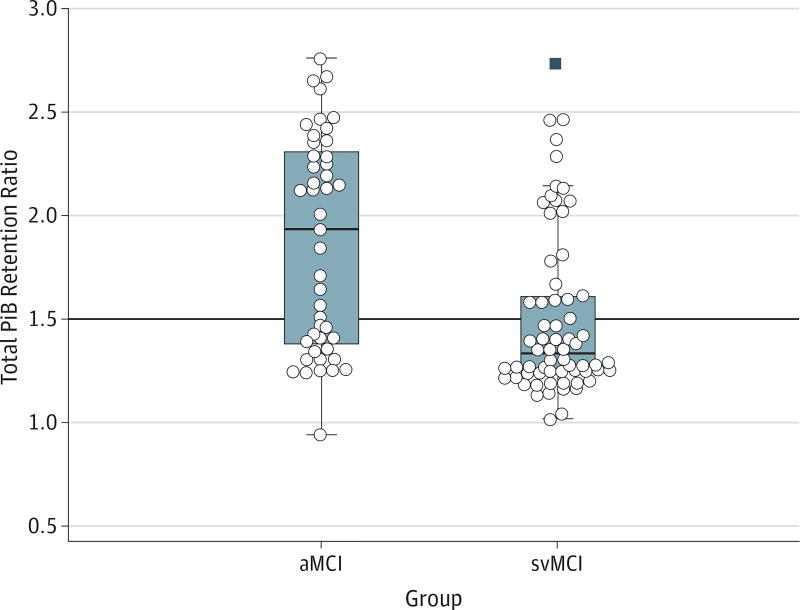
The mean (SD) PiB retention ratio was greater in aMCI patients than in svMCI patients (1.87[0.51] vs 1.50 [0.40]; P < .001). Twenty-eight of 45 aMCI patients (62%) were PiB+, and 22 of 67 svMCI patients (33%) were PiB+ (P = .002) (Figure 2 and Table 1). Compared with PiB+ svMCI patients, PiB+ aMCI patients had higher PiB retention in global and lobar PiB retention ratios except in the parietal region (global PiB, 2.20 [0.33] vs 1.98 [0.35]; P = .02; frontal PiB, 2.19 [0.34] vs 1.98 [0.38]; P = .048; temporal PiB, 2.18 [0.34] vs 1.93 [0.33]; P = .02; parietal PiB, 2.11 [0.40] vs 1.90 [0.38]; P = .07).
Figure 2. Pittsburgh Compound B (PiB) Retention Ratio in the Amnestic Mild Cognitive Impairment (aMCI) and Subcortical Vascular Mild Cognitive Impairment (svMCI) Groups.
The bold horizontal line indicates the cutoff value. PiB retention ratios were compared between aMCI and svMCI by applying analysis of covariance adjusted for age. Significant differences were noted between aMCI and svMCI patients (mean [SD], 1.87 [0.51] vs 1.50 [0.40], P < .001). Circles indicate patients; bars within the boxes, median values; boxes, interquartile range; bars outside the boxes, extreme values; and black square, outlier.
Table 1.
Characteristics of aMCI Patients, svMCI Patients, and Individuals With NC
| Characteristic | aMCI | svMCI |
P Valueb |
NC (n = 75) |
P Valuec |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Total (n = 45) |
PiB− (n = 17) |
PiB+ (n = 28) |
P Valuea |
Total (n = 67) |
PiB− (n = 45) |
PiB+ (n = 22) |
P Valuea |
||||
| Age, mean (SD), y | 70.0 (8.0) | 70.6 (7.8) | 69.7 (8.3) | .70 | 73.7 (6.7) | 72.1 (6.6) | 76.9 (5.9) | .006 | .01 | 63.6 (8.2) | <.001 |
|
| |||||||||||
| Female sex, No. (%) | 20 (44) | 9 (53) | 11 (39) | .37 | 41 (61) | 29 (64) | 12 (54) | .44 | .08 | 57 (76) | .17 |
|
| |||||||||||
| Educational level, mean (SD), y | 12.5 (4.6) | 11.4 (5.9) | 13.1 (3.5) | .29 | 9.4 (5.4) | 9.0 (5.2) | 10.2 (5.8) | .38 | .001 | 12.0 (4.8) | .002 |
|
| |||||||||||
| Vascular risk factors, No. (%) | |||||||||||
|
| |||||||||||
| Hypertension | 17 (38) | 10 (59) | 7 (25) | .02 | 50 (75) | 38 (84) | 12 (54) | .008 | <.001 | 14 (19) | <.001 |
|
| |||||||||||
| Diabetes mellitus | 5 (11) | 4 (24) | 1 (4) | .06 | 17 (25) | 12 (27) | 5 (23) | .73 | .06 | 31 (41) | .11 |
|
| |||||||||||
| Hyperlipidemia | 10 (22) | 3 (18) | 7 (25) | .72 | 20 (30) | 14 (31) | 6 (27) | .75 | .37 | 18 (24) | .40 |
|
| |||||||||||
| Cardiac disease | 7 (16) | 3 (18) | 4 (14) | >.99 | 17 (25) | 12 (27) | 5 (23) | .73 | .21 | 14 (19) | .30 |
|
| |||||||||||
| Stroke | 2 (4) | 2 (12) | 0 | .14 | 13 (19) | 9 (20) | 4 (18) | >.99 | .02 | 3 (4) | .009 |
|
| |||||||||||
| APOE genotyping, No. (%)d | |||||||||||
|
| |||||||||||
| APOE4 allele | 17 (44) | 0 | 17 (71) | <.001 | 15 (22) | 5 (11) | 10 (46) | .004 | .02 | NA | |
|
| |||||||||||
| APOE2 allele | 3 (8) | 3 (20) | 0 | .05 | 10 (15) | 6 (13) | 4 (18) | .72 | .27 | NA | |
|
| |||||||||||
| PiB retention ratio, mean (SD) | |||||||||||
|
| |||||||||||
| Total | 1.87 (0.51) | 1.31 (0.12) | 2.20 (0.33) | 1.50 (0.40) | 1.27 (0.10) | 1.98 (0.35) | <.001 | ||||
|
| |||||||||||
| Frontal | 1.84 (0.52) | 1.27 (0.12) | 2.19 (0.34) | 1.48 (0.42) | 1.23 (0.10) | 1.98 (0.38) | <.001 | ||||
|
| |||||||||||
| Parietal | 1.78 (0.53) | 1.24 (0.17) | 2.11 (0.40) | 1.40 (0.42) | 1.16 (0.12) | 1.90 (0.38) | <.001 | ||||
|
| |||||||||||
| Temporal | 1.86 (0.50) | 1.33 (0.14) | 2.18 (0.34) | 1.49 (0.38) | 1.27 (0.12) | 1.93 (0.33) | <.001 | ||||
|
| |||||||||||
| MRI markers of ischemia, mean (SD) | |||||||||||
|
| |||||||||||
| Total WMH, mL | 3.1 (3.2) | 3.5 (3.1) | 2.9 (3.2) | .65 | 34.9 (17.8) | 33.8 (17.6) | 36.8 (18.4) | .44 | <.001 | 1.3 (1.6) | <.001 |
|
| |||||||||||
| Frontal | 20.0 (9.4) | 19.7 (9.8) | 20.6 (8.9) | .74 | |||||||
|
| |||||||||||
| Parietal | 8.9 (5.7) | 8.7 (5.5) | 9.3 (6.1) | .68 | |||||||
|
| |||||||||||
| Temporal | 3.5 (1.9) | 3.3 (1.8) | 3.8 (2.1) | .30 | |||||||
|
| |||||||||||
| Total supratentorial lacunes, No. | 0.5 (2.2) | 1.3 (3.4) | 0.1 (0.4) | .07 | 7.2 (8.1) | 8.0 (8.2) | 5.5 (7.8) | .40 | <.001 | 0.5 (1.1) | <.001 |
|
| |||||||||||
| Frontal | 6.0 (6.6) | 7.0 (7.3) | 4.1 (4.4) | .05 | |||||||
|
| |||||||||||
| Parietal | 1.0 (3.3) | 0.9 (2.1) | 1.4 (4.9) | .58 | |||||||
|
| |||||||||||
| Temporal | 0.2 (0.9) | 0.3 (1.1) | 0 | .10 | |||||||
|
| |||||||||||
| Total supratentorial MBs, No. | 0.8 (3.5) | 1.2 (4.6) | 0.6 (2.7) | .66 | 5.3 (11.2) | 4.8 (9.5) | 6.1 (14) | .44 | .01 | 0.7 (1.1) | .003 |
|
| |||||||||||
| Frontal | 2.7 (5.6) | 3.3 (6.8) | 2.4 (5.1) | .58 | |||||||
|
| |||||||||||
| Parietal | 0.5 (1.7) | 0.3 (0.7) | 1.0 (2.8) | .29 | |||||||
|
| |||||||||||
| Temporal | 1.2 (3.1) | 1.0 (2.1) | 1.7 (4.9) | .50 | |||||||
Abbreviations: aMCI, amnestic mild cognitive impairment; APOE, apolipoprotein E; MBs, microbleeds; MRI, magnetic resonance imaging; NA, not applicable; NC, normal cognition; NCF, normal cognitive function; PiB, Pittsburgh Compound B; svMCI, subcortical vascular mild cognitive impairment; WMH, white matter hyperintensity; +, positive; −, negative.
Comparison of PiB+ vs PiB− participants.
Comparison of aMCI vs svMCI participants.
Comparison of NCF vs PiB− svMCI participants.
Six patients with aMCI (4 PiB+ and 2 PiB−) refused the APOE4 genotype testing.
Comparisons of neuropsychological results between the svMCI group and the aMCI or normal cognition groups are described in Table 2. The PiB+ svMCI patients showed lower performances in BNT, RCFT delayed recall, and Stroop color reading tests compared with PiB− svMCI patients (Table 2). Relative to individuals with normal cognition, PiB− svMCI patients had lower performances in all cognitive domains, including the digit span (backward); BNT; RCFT copy; SVLT immediate recall, delayed recall, and recognition; RCFT immediate and delayed recall; Controlled Oral Word Association Test; and Stroop color reading tests (Table 2).
Table 2.
Neuropsychological Testsa
| Mean (SD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| aMCI | svMCI | ||||||||||
|
|
|
||||||||||
| Test (Possible Range of Score) |
Total (n = 45) |
PiB− (n = 17) |
PiB+ (n = 28) |
P Valueb |
Total (n = 67) |
PiB− (n = 45) |
PiB+ (n = 22) |
P Valueb |
P Valuec |
NC (n = 75) |
P Valued |
| Digit span | |||||||||||
|
| |||||||||||
| Forward (0–9) | 5.8 (1.4) | 5.5 (1.4) | 6.0 (1.5) | .71 | 5.2 (1.3) | 5.1 (1.3) | 5.5 (1.2) | .16 | .83 | 6.4 (1.4) | .07 |
|
| |||||||||||
| Backward (0–8) | 4.0 (1.4) | 3.4 (1.1) | 4.4 (1.5) | .08 | 3.2 (1.1) | 3.2 (1.1) | 3.3 (1.2) | .55 | .28 | 4.4 (1.4) | .04 |
|
| |||||||||||
| BNT (0–60) | 41.4 (9.6) | 38.2 (9.1) | 43.4 (9.5) | .19 | 39.1 (10.4) | 41.2 (9.2) | 34.9 (11.7) | .006 | .49 | 50.5 (5.7) | <.001 |
|
| |||||||||||
| RCFT (0–36) | 30.9 (4.5) | 31.4 (3.8) | 30.6 (5.6) | .30 | 27.5 (7.6) | 28.2 (6.9) | 26.1 (8.8) | .22 | .34 | 33.5 (2.1) | <.001 |
|
| |||||||||||
| SVLT | |||||||||||
|
| |||||||||||
| Immediate recall (0–36) | 15.0 (4.2) | 15.4 (4.0) | 14.8 (4.3) | .37 | 16.5 (5.4) | 16.9 (5.4) | 15.7 (5.3) | .91 | .09 | 22.5 (4.4) | <.001 |
|
| |||||||||||
| Delayed recall (0–12) | 2.4 (2.4) | 3.5 (2.0) | 1.7 (2.3) | .01 | 4.3 (2.9) | 4.8 (2.8) | 3.2 (2.9) | .25 | .001 | 7.6 (2.0) | <.001 |
|
| |||||||||||
| Recognition (0–24) | 18.5 (2.9) | 19.9 (2.2) | 17.7 (3.0) | .007 | 19.3 (2.9) | 19.9 (2.1) | 18.1 (3.8) | .11 | .43 | 21.6 (1.7) | .001 |
|
| |||||||||||
| RCFT | |||||||||||
|
| |||||||||||
| Immediate recall (0–36) | 8.4 (5.7) | 10.5 (4.5) | 7.2 (6.0) | .06 | 10.6 (6.2) | 11.4 (6.0) | 8.8 (6.5) | .06 | .03 | 18.4 (5.0) | <.001 |
|
| |||||||||||
| Delayed recall (0–36) | 7.0 (5.4) | 9.1 (4.2) | 5.8 (5.8) | .049 | 10.2 (5.8) | 11.4 (5.3) | 7.6 (6.0) | .008 | <.001 | 17.7 (4.8) | <.001 |
|
| |||||||||||
| Recognition (0–24) | 18.0 (2.1) | 19.3 (2.0) | 17.3 (1.9) | .001 | 19.2 (2.2) | 19.6 (1.9) | 18.4 (2.4) | .05 | .001 | 20.2 (1.5) | .45 |
|
| |||||||||||
| COWAT | |||||||||||
|
| |||||||||||
| Animal | 13.4 (4.3) | 11.9 (3.4) | 14.4 (4.6) | .09 | 11.7 (3.6) | 12.2 (3.5) | 10.6 (3.8) | .10 | .17 | 17.3 (4.9) | <.001 |
|
| |||||||||||
| Supermarket | 13.2 (5.4) | 12.8 (4.2) | 13.5 (6.0) | .83 | 13.1 (4.9) | 13.3 (5.2) | 12.5 (4.2) | .42 | .39 | 18.6 (5.6) | <.001 |
|
| |||||||||||
| Phonemic | 23.6 (11.6) | 19.6 (7.5) | 26.0 (13.1) | .15 | 16.4 (8.9) | 15.9 (9.1) | 17.5 (8.5) | .63 | .07 | 28.7 (11.6) | <.001 |
|
| |||||||||||
| Stroop test: color (0–112) | 67.7 (24.3) | 63.3 (21.9) | 70.4 (25.6) | .64 | 59.4 (26.5) | 64.3 (24.7) | 49.5 (27.8) | .048 | .85 | 93.5 (18.3) | <.001 |
|
| |||||||||||
| MMSE (0–30) | 25.5 (4.6) | 26.9 (2.0) | 24.6 (5.6) | .07 | 26.2 (3.0) | 26.6 (2.4) | 25.4 (3.9) | .17 | .04 | 28.8 (1.5) | <.001 |
|
| |||||||||||
| CDR-SOB (0–30) | 1.7 (1.1) | 1.4 (0.9) | 1.9 (1.1) | .14 | 1.4 (1.1) | 1.3 (1.0) | 1.5 (1.1) | .58 | .09 | 0.5 (0.4) | <.001 |
Abbreviations: aMCI, amnestic mild cognitive impairment; BNT, Boston Naming Test; CDR-SOB, Clinical Dementia Rating Scale Sum of Boxes; COWAT, Controlled Oral Word Association Test; MMSE, Mini-Mental State Examination; NC, normal cognition; PiB, Pittsburgh Compound B; RCFT, Rey-Osterrieth Complex Figure Test; SVLT, Seoul Verbal Learning Test; svMCI, subcortical vascular mild cognitive impairment; +, positive; −, negative.
P values were adjusted for age and years of education.
Comparison of PiB+ vs PiB− participants.
Comparison of aMCI vs svMCI participants.
Comparison of NC vs PiB− svMCI participants.
There was no positive correlation between regional PiB retention ratios and regional WMH volumes, lacunes, or micro-bleeds in any region (Supplement [eTable 2A–D]). Rather, a negative correlation was found between the number of lacunes and PiB retention ratios (Supplement [eTable 2A and B]).
Voxelwise Relationship
The SPM analysis revealed that, when compared with patients with PiB+ svMCI, patients with PiB+ aMCI showed greater levels of PiB retention in the medial and lateral frontal and temporal, posterior cingulate, precuneus, and inferior parietal regions, as well as in the basal ganglia (Figure 1 and Author Table 1). There were no regions where patients with PiB+ svMCI had more PiB retention than did those with PiB+ aMCI. The topography of PiB retention in svMCI patients was similar to that seen in aMCI patients (Author Figure 2).
Correlation Between PiB Retention Ratio and Neuropsychological Results
In aMCI patients, the log total PiB retention ratio was associated with lower performances in verbal memory (log SVLT delayed recall) and visual memory (log RCFT recognition) (Table 3). There was no significant negative relationship with nonmemory cognitive domains.
Table 3.
Multiple Linear Regression Analysis of PiB Retention Ratios and Neuropsychological Assessments in Patients With aMCI and svMCI
| Dependent Variable: Neuropsychological Assessments (Possible Range of Score) |
Independent Variable: Total PiB Retention Ratio (Log Transformed) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| aMCI | svMCI | |||||
|
|
|
|||||
| β (SE) |
P Value (FDR-Corrected)a |
Covariates Included |
β (SE) |
P Value (FDR-Corrected)a |
Covariates Included |
|
| Attention | ||||||
|
| ||||||
| Digit span | ||||||
|
| ||||||
| Forward (0–9) | −0.01 (0.06) | .94 (.99) | WMH, educational level | 0.09 (0.07) | .20 (.29) | WMH, educational level |
|
| ||||||
| Backward (0–8) | 0.35 (0.11) | .002 (.01) | WMH, educational level, APOE4 | 0.07 (0.12) | .58 (.63) | WMH, educational level |
|
| ||||||
| Language and related disorders | ||||||
|
| ||||||
| BNT (0–60) | 0.06 (0.10) | .53 (.74) | WMH | −0.35 (0.09) | <.001 (.002) | WMH, educational level, sex, MB |
|
| ||||||
| Visuospatial function | ||||||
|
| ||||||
| RCFT (0–36) | −0.07 (0.10) | .49 (.74) | WMH | −0.34 (0.14) | .01 (.04) | WMH, educational level |
|
| ||||||
| Memory | ||||||
|
| ||||||
| SVLT | ||||||
|
| ||||||
| Immediate recall (0–36) | −0.12 (0.09) | .19 (.38) | WMH, sex, educational level | −0.10 (0.15) | .50 (.58) | WMH, hypertension |
|
| ||||||
| Delayed recall (0–12) | −0.77 (0.25) | .004 (.02) | WMH, sex, hyperlipidemia | −0.88 (0.24) | .001 (.005) | WMH, MB |
|
| ||||||
| Recognition (0–24) | −0.13 (0.06) | .03 (.10) | WMH, sex | −0.12 (0.06) | .05 (.09) | WMH, age |
|
| ||||||
| RCFT | ||||||
|
| ||||||
| Immediate recall (0–36) | −0.52 (0.27) | .06 (.15) | WMH, hyperlipidemia | −0.54 (0.25) | .04 (.07) | WMH, sex, diabetes mellitus |
|
| ||||||
| Delayed recall (0–36) | −0.67 (0.33) | .05 (.14) | WMH | −0.70 (0.26) | .008 (.03) | WMH, sex |
|
| ||||||
| Recognition (0–24) | −0.15 (0.04) | .001 (.01) | WMH | −0.11 (0.04) | .01 (.04) | WMH |
|
| ||||||
| Frontal/executive function | ||||||
|
| ||||||
| COWAT | ||||||
|
| ||||||
| Animal | 0.04 (0.09) | .71 (.90) | WMH | −0.17 (0.11) | .15 (.24) | WMH |
|
| ||||||
| Supermarket | −0.16 (0.14) | .27 (.47) | WMH, age, lacune | 0.10 (0.14) | .47 (.58) | WMH, cardiac disease |
|
| ||||||
| Phonemic | 0.05 (0.18) | .78 (.91) | WMH | 0.07 (0.29) | .81 (.81) | WMH, educational level, lacune |
|
| ||||||
| Stroop test: color (0–112) | <0.001 (0.10) | >.99 (>.99) | WMH, sex, educational level, stroke | −1.02 (0.27) | <.001 (.002) | WMH, educational level |
Abbreviations: aMCI, amnestic mild cognitive impairment; APOE4, apolipoprotein ε4 allele; BNT, Boston Naming Test; COWAT, Controlled Oral Word Association Test; FDR, false discovery rate; MB, microbleed; PiB, Pittsburgh Compound B; RCFT, Rey-Osterrieth Complex Figure Test; SVLT, Seoul Verbal Learning Test; svMCI, subcortical vascular mild cognitive impairment; WMH, white matter hyperintensity.
P values were adjusted for age, sex, educational level, vascular risk factors (including history of hypertension, diabetes mellitus, dyslipidemia, cardiac disease, and stroke), APOE4 genotype, WMH volume (log transformed), the presence of supratentorial lacune, and the presence of supratentorial MB. Log-transformed WMH volumes and log-transformed PiB retention ratios were entered into the regression model, and other covariates were included using a stepwise method.
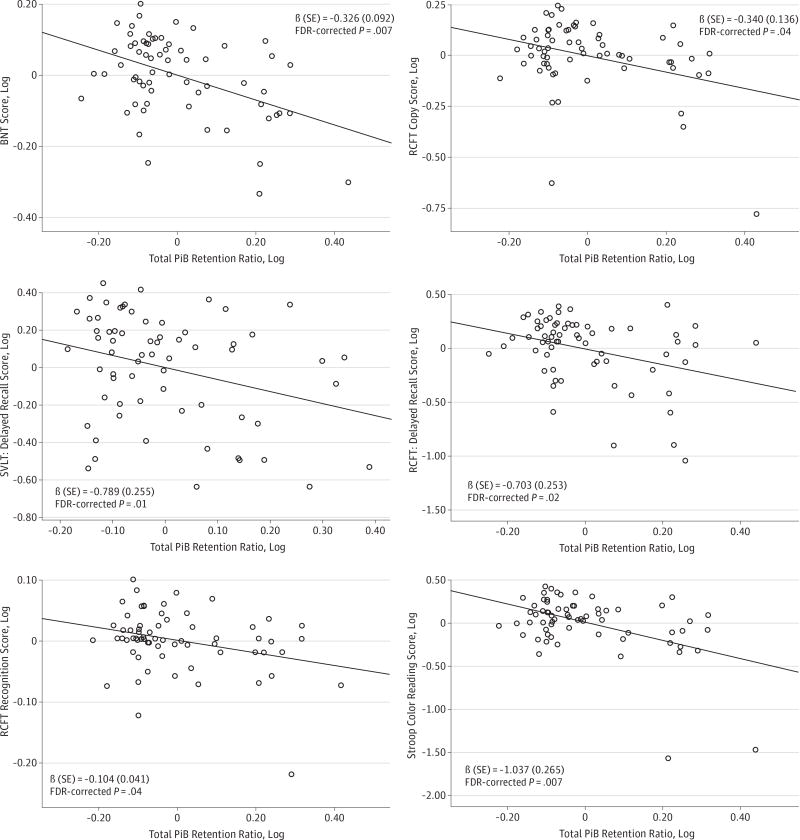
In contrast, svMCI patients showed a negative association between log PiB retention ratio and cognition in language (log BNT), visuospatial (log RCFT copy), and frontal executive (log Stroop color test) functions as well as in memory (log SVLT delayed recall, log RCFT delayed memory, and log RCFT recognition) domains (Table 3 and Figure 3).
Figure 3. Partial Regression Plots of Total Pittsburgh Compound B (PiB) Retention Ratios and Neuropsychological Results in Subcortical Vascular Mild Cognitive Impairment (svMCI) Patients.
Significant relationships were shown in language, visuospatial, verbal and visual memory, and frontal executive functions in svMCI patients. The y-axis values are log scores. FDR indicates false discovery rate; BNT, Boston Naming Test; RCFT, Rey-Osterrieth Complex Figure Test; and SVLT, Seoul Verbal Learning Test.
In patients with svMCI, each PiB retention ratio in the frontal, temporal, and parietal regions was separately associated with broad aspects of cognition (ie, language, visuospatial, memory, and frontal dysfunction). However, WMH volume, particularly in the frontal regions, was associated with visuospatial dysfunction; temporal and parietal WMH did not show any association with cognitive impairment (Author Table 2).
Interaction Between WMH and PiB Retention Ratio
When interaction analysis using cognitive domains showed a significant association with PiB retention ratios, interactive effects between PiB retention ratio and WMH volume on the RCFT copy test were detected (β = −0.89, P = .006 for interaction after FDR correction). Other cognitive domains did not demonstrate significant interaction, including BNT, SVLT delayed recall, RCFT delayed recall, RCFT recognition, and Stroop color reading (Author Table 3).
When we analyzed the correlations between regional PiB retention ratio and neuropsychological results, interactive effects between frontal PiB retention ratio and frontal WMH were detected (β = −1.72, P = .04 for interaction after FDR correction). However, domains in other regions did not demonstrate significant interactions (Author Table 3).
Discussion
There were 4 major findings of our study. First, svMCI patients showed significantly less Aβ burden compared with aMCI patients. The evidence for this was the lower frequency of PiB+ as well as lower PiB retention ratios in the svMCI patients. Second, the Aβ burden in svMCI patients was independently and significantly associated with cognitive impairment in multiple domains, including language, visuospatial, memory, and frontal executive functions. Third, in contrast to svMCI, Aβ burden in patients with aMCI was associated only with memory dysfunction. Fourth, there was an interaction between WMH (presumed to reflect small-vessel ischemia) and Aβ burden measured by PiB PET on visuospatial dysfunction in patients with svMCI, with no interactive effects of Aβ burden and CVD in language, memory, and frontal executive dysfunction. Taken together, our findings suggest that most patients with svMCI do not have coexistent AD Aβ burden and that CVD and AD synergistically impair visuospatial function in patients with svMCI.
Brain Aβ Burden
Our first major finding was that svMCI patients had less Aβ burden than aMCI patients. The frequency (62%) of PiB+ in aMCI patients was similar to data from previous studies10–14 showing that 52% to 75% of aMCI patients were PiB+. In contrast, the frequency (33%) of PiB+ in svMCI patients was comparable to that in previous reports11,13,15 of PiB+ in cognitively normal individuals, ranging from 22% to 30%. These results were also consistent with previous reports18 from our group regarding the proportion (31%) of PiB+ in patients with subcortical vascular dementia. Considering our other finding of PiB+ aMCI patients having greater PiB retention ratios than PiB+ svMCI patients (Figure 1), our results suggest that CVD is not associated with an increased frequency of Aβ positivity or with the quantity of Aβ in individual participants. Our suggestion may be supported by our other findings that CVD burden (WMH volume or the number of lacunes and microbleeds) was not positively correlated with Aβ burden (PiB retention ratio) (Supplement [eTable 2]). Our findings are consistent with previous PiB PET studies16,17 showing no direct correlation between CVD and Aβ burden in individuals with normal or mildly impaired cognition. Furthermore, a recent study34 has shown that people without severe CVD had no correlation between WMH and cerebrospinal fluid Aβ, either cross-sectionally or longitudinally. When considered together with previous results, our present findings could be explained by the index event bias.35 That is, because both CVD and Aβ burden cause cognitive impairment, patients with svMCI plus severe CVD have less Aβ burden than do aMCI patients with mild CVD.
Pattern of Brain Aβ Deposition
In this study, compared with PiB+ aMCI patients, those with PiB+ svMCI showed less PiB retention in the frontal, temporal, and parietal regions. β-Amyloid burden in these areas, according to pathologic studies,36 increases as AD progresses, although amyloid PET studies37,38 have shown inconsistent results. Therefore, the Aβ burden of svMCI in our results may reflect the similar levels of Aβ burden seen in preclinical AD (ie, the prodromal stage of aMCI). However, the topography of PiB retention in svMCI patients seems different from that in patients with cerebral amyloid angiopathy (Author Figure 2). Patients with cerebral amyloid angiopathy have been reported39 to have more PiB retention in occipital regions than those with AD. Furthermore, a direct comparison between patients with PiB+ svMCI and those with PiB+ aMCI showed no regions where patients with PiB+ svMCI had greater PiB retention.
Relationship of Aβ Burden to Cognition
We found differences in the effects of Aβ burden on cognition between svMCI patients and aMCI patients. That is, Aβ burden in aMCI was selectively related to memory function, a finding generally consistent with previous studies.10–14,40 In contrast, the Aβ burden in svMCI was associated with cognitive impairment in multiple domains, including language, visuospatial, frontal executive, and memory function. We also found that, compared with individuals with normal cognition, patients with PiB− svMCI had cognitive impairment in multiple domains, including language, visuospatial, memory, and frontal functions, although WMH was associated only with visuospatial dysfunction (Table 1). Therefore, these results suggest that the cognitive domain associated with CVD may overlap with domains related to Aβ burden.
The second major finding was that there was a positive interaction between CVD (measured as WMH) and Aβ burden on visuospatial function, with no interactive effects of Aβ burden and CVD in language, memory, and frontal executive dysfunction. In other words, the combined effects of WMH and Aβ burden on visuospatial function were greater than the sum of the 2 individual effects. These results are consistent with those of previous epidemiologic studies6–8 showing that patients with vascular risk factors or atherosclerosis had a greater extent of AD dementia. Our finding is also consistent with preclinical studies19,20 showing direct interactive effects of ischemia and Aβ on cognition. However, our detection of no interaction in other cognitive domains suggested that Aβ burden and CVD additively affect language, memory, and frontal dysfunction. Recent PiB PET studies16,17,41,42 revealed that the effects of CVD and PiB retention do not interact with regard to cognition. However, those studies did not evaluate the synergistic effects of Aβ burden and CVD on visuospatial function.
There are several possible interpretations regarding the synergistic interaction of WMH and Aβ burden, particularly those in the frontal region, on visuospatial function, especially the visual constructional function, which is associated with frontal dysfunction.43 First, WMH might accelerate an increase in neurofibrillary tangles that develop after the formation of the amyloid plaques. It has been shown44 that hypoxia promotes phosphorylation of tau through activation of mitogen-activated protein kinase. Alternatively, WMH and Aβ burden could be synergistically affecting common pathways, such as neuroinflammation, micro-structural changes, or cortical atrophy. Finally, it is possible that CVD, by interruption of critical brain networks, substantially reduces cognitive reserve. In this case, the additional brain insult associated with AD Aβ accumulation might be associated with a greater effect on cognition than would occur in the presence of nonreduced cognitive reserve alone.
The strengths of our study are its prospective design and the standardized PiB PET imaging and MRI protocols. However, we acknowledge some limitations. First, because we did not perform postmortem studies, we could not measure different abnormalities, including other AD (soluble amyloid and neurofibrillary tangles), CVD (microinfarct), or possible combined degenerative dementia (dementia with Lewy bodies or frontotemporal dementia), which are also associated with cognitive impairment. Second, although no patients met the clinical criteria for cerebral amyloid angiopathy,45 we were not able to exclude the possibility of inclusion of such patients, because 5 participants with svMCI exhibited strictly lobar microbleeds and 3 of these were PiB+ (Author Table 4). Third, the patients all had cognitive impairment and severe CVD, which may limit the generalizability of the results to other populations. Fourth, we did not include lacunes or microbleeds in the criteria for svMCI. However, a previous study46 has shown that our visual assessment of WMH used in the criteria for svMCI reflects the grade of ischemia, such as for the presence of vascular risk factors, and the extent of CVD, including lacunes and microbleeds. Fifth, we had no cognitively normal control group with which to directly compare the frequency of PiB+ svMCI. Sixth, PiB PET was performed 60 minutes after injection of the tracer, which could lower the signal to noise ratio, hampering the ability for reliable voxel-by-voxel analysis using SPM5. However, to overcome low signal to noise ratio, we used a higher injection dose (561 MBq) than that in a previous PiB PET study (370 MBq)47 and conducted imaging for 30 minutes. Seventh, because of the cross-sectional design of our study, we can suggest only the possibility of an interactive relationship for Aβ burden and subcortical CVD on cognition. Further follow-up investigations with repeated-measure data are necessary to reveal possible longitudinal relationships among the variables. Finally, we cannot exclude some selection bias. However, we consecutively recruited patients with svMCI, and PIB testing was not used in the classification of the patients as aMCI or svMCI. Therefore, we believe that the diagnostic selection process was unlikely to be affected by selection bias.
Conclusions
Patients with svMCI showed less Aβ burden than did those with aMCI. However, in svMCI patients, Aβ burden and WMH act synergistically or additively to impair cognition. Therefore, our findings highlight the need for accurate biomarkers, including neuroimaging tools, for early diagnosis and the need to relate these biomarkers to cognitive measurements for effective use in the clinical setting.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grant NRF-2013R1A1A2065365 from the Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education; by grants HI10C2020 and HIC120713 from the Korean Healthcare Technology Research and Development Project Ministry for Health and Welfare Affairs; by Ministry of Education, Science, and Technology program grant MEST 2011-0028333 from the Korea Science and Engineering Foundation National Research Laboratories; by grants CRL-108011 and CRS110-14-1 from Samsung Medical Center; and by grant 2013K000338 from the Converging Research Center Program through the Ministry of Science, Information, Communication, Technology, and Future Planning, Korea.
Footnotes
Author Contributions: Drs M. J. Lee and Seo had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: M. J. Lee, Seo, Na, J.-H. Lee.
Acquisition of data: Na, G. H. Kim, C. H. Kim, Noh, Cho, H. J. Kim, Yoon, Ye, J. S. Kim, S. T. Kim.
Analysis and interpretation of data: M. J. Lee, C. Kim, Park, G. H. Kim, Chin, Jeon, J.-M. Lee, Choe, K.-H. Lee, J. S. Kim, J.-H. Lee, Ewers, Werring, Weiner.
Drafting of the manuscript: M. J. Lee, Seo, Na, Park, G. H. Kim, C. H. Kim, Noh, Cho, H. J. Kim, Yoon, Ye, Chin, Jeon, J.-M. Lee, K.-H. Lee, J. S. Kim, J.-H. Lee.
Critical revision of the manuscript for important intellectual content: Na, C. Kim, J. S. Kim, S. T. Kim, J.-H. Lee, Ewers, Werring, Weiner.
Statistical analysis: M. J. Lee, C. Kim, J.-H. Lee.
Obtained funding: Na.
Administrative, technical, or material support: Seo, G. H. Kim, J. S. Kim, J.-H. Lee.
Study supervision: Seo, C. Kim, J. S. Kim, J.-H. Lee, Ewers.
Conflict of Interest Disclosures: None reported.
References
- 1.Frisoni GB, Galluzzi S, Bresciani L, Zanetti O, Geroldi C. Mild cognitive impairment with subcortical vascular features: clinical characteristics and outcome. J Neurol. 2002;249(10):1423–1432. doi: 10.1007/s00415-002-0861-7. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2(2):89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 3.Wentzel C, Rockwood K, MacKnight C, et al. Progression of impairment in patients with vascular cognitive impairment without dementia. Neurology. 2001;57(4):714–716. doi: 10.1212/wnl.57.4.714. [DOI] [PubMed] [Google Scholar]
- 4.Seo SW, Cho SS, Park A, Chin J, Na DL. Subcortical vascular versus amnestic mild cognitive impairment: comparison of cerebral glucose metabolism. J Neuroimaging. 2009;19(3):213–219. doi: 10.1111/j.1552-6569.2008.00292.x. [DOI] [PubMed] [Google Scholar]
- 5.Seo SW, Ahn J, Yoon U, et al. Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J Neuroimaging. 2010;20(1):37–45. doi: 10.1111/j.1552-6569.2008.00293.x. [DOI] [PubMed] [Google Scholar]
- 6.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349(9046):151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 7.Roher AE, Tyas SL, Maarouf CL, et al. Intracranial atherosclerosis as a contributing factor to Alzheimer’s disease dementia. Alzheimers Dement. 2011;7(4):436–444. doi: 10.1016/j.jalz.2010.08.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah NS, Vidal JS, Masaki K, et al. Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension. 2012;59(4):780–786. doi: 10.1161/HYPERTENSIONAHA.111.178962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 10.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29(10):1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131(pt 3):665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemppainen NM, Aalto S, Wilson IA, et al. PET amyloid ligand 11C PIB uptake is increased in mild cognitive impairment. Neurology. 2007;68(19):1603–1606. doi: 10.1212/01.wnl.0000260969.94695.56. [DOI] [PubMed] [Google Scholar]
- 13.Pike KE, Savage G, Villemagne VL, et al. β-Amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(pt 11):2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 14.Wolk DA, Price JC, Saxton JA, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65(5):557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe CC, Ng S, Ackermann U, et al. Imaging β-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 16.Marchant NL, Reed BR, DeCarli CS, et al. Cerebrovascular disease, β-amyloid, and cognition in aging. Neurobiol Aging. 2012;33(5):e25–e36. doi: 10.1016/j.neurobiolaging.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchant NL, Reed BR, Sanossian N, et al. The aging brain and cognition: contribution of vascular injury and Aβ to mild cognitive dysfunction. JAMA Neurol. 2013;70(4):488–495. doi: 10.1001/2013.jamaneurol.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Kim SH, Kim GH, et al. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology. 2011;77(1):18–25. doi: 10.1212/WNL.0b013e318221acee. [DOI] [PubMed] [Google Scholar]
- 19.Choi BR, Lee SR, Han JS, et al. Synergistic memory impairment through the interaction of chronic cerebral hypoperfusion and amyloid toxicity in a rat model. Stroke. 2011;42(9):2595–2604. doi: 10.1161/STROKEAHA.111.620179. [DOI] [PubMed] [Google Scholar]
- 20.Lee JS, Im DS, An YS, Hong JM, Gwag BJ, Joo IS. Chronic cerebral hypoperfusion in a mouse model of Alzheimer’s disease: an additional contributing factor of cognitive impairment. Neurosci Lett. 2011;489(2):84–88. doi: 10.1016/j.neulet.2010.11.071. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [Google Scholar]
- 22.Ku HM, Kim JH, Kwon EJ, et al. A study on the reliability and validity of Seoul-Instrumental Activities of Daily Living (S-IADL) J Korean Neuropsychiatr Assoc. 2004;43:189–199. [Google Scholar]
- 23.Ahn HJ, Chin J, Park A, et al. Seoul Neuropsychological Screening Battery–dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010;25(7):1071–1076. doi: 10.3346/jkms.2010.25.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SH, Seo SW, Go SM, et al. Pyramidal and extrapyramidal scale (PEPS): a new scale for the assessment of motor impairment in vascular cognitive impairment associated with small vessel disease. Clin Neurol Neurosurg. 2011;113(3):181–187. doi: 10.1016/j.clineuro.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients [Korean] J Korean Neurol Assoc. 1997;15(2):300–308. [Google Scholar]
- 26.Seo SW, Im K, Lee JM, et al. Cortical thickness in single- versus multiple-domain amnestic mild cognitive impairment. Neuroimage. 2007;36(2):289–297. doi: 10.1016/j.neuroimage.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 27.Kim H, Na DL. Normative data on the Korean version of the Boston Naming Test. J Clin Exp Neuropsychol. 1999;21(1):127–133. doi: 10.1076/jcen.21.1.127.942. [DOI] [PubMed] [Google Scholar]
- 28.Kang Y, Na DL. Seoul Neuropsychological Screening Battery (SNSB) Incheon, Korea: Human Brain Research & Consulting Co; 2012. [Google Scholar]
- 29.Kang Y, Chin J, Na DL, Lee J, Park J. A normative study of the Korean version of Controlled Oral Word Association Test (COWAT) in the elderly. Korean J Clin Psychol. 2000;19:385–392. [Google Scholar]
- 30.Lee J, Kang Y, Na DL. Efficiencies of Stroop interference indexes in healthy older adults and dementia patients. Korean J Clin Psychol. 2000;19:807–818. [Google Scholar]
- 31.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 32.Jeon S, Yoon U, Park J-S, et al. Fully automated pipeline for quantification and localization of white matter hyperintensity in brain magnetic resonance image. Int J Imaging Syst Technol. 2011;21:193–200. [Google Scholar]
- 33.Greenberg SM, Vernooij MW, Cordonnier C, et al. Microbleed Study Group Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo RY, Jagust WJ, Alzheimer’s Disease Neuroimaging Initiative Vascular burden and Alzheimer disease pathologic progression. Neurology. 2012;79(13):1349–1355. doi: 10.1212/WNL.0b013e31826c1b9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahabreh IJ, Kent DM. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA. 2011;305(8):822–823. doi: 10.1001/jama.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 37.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Alzheimer’s Disease Neuroimaging Initiative Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132(pt 5):1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engler H, Forsberg A, Almkvist O, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129(pt 11):2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 39.Dierksen GA, Skehan ME, Khan MA, et al. Spatial relation between microbleeds and amyloid deposits in amyloid angiopathy. Ann Neurol. 2010;68(4):545–548. doi: 10.1002/ana.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mormino EC, Kluth JT, Madison CM, et al. Alzheimer’s Disease Neuroimaging Initiative Episodic memory loss is related to hippocampal-mediated β-amyloid deposition in elderly subjects. Brain. 2009;132(pt 5):1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedden T, Mormino EC, Amariglio RE, et al. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci. 2012;32(46):16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Provenzano FA, Muraskin J, Tosto G, et al. White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013;70(4):455–461. doi: 10.1001/jamaneurol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freeman RQ, Giovannetti T, Lamar M, et al. Visuoconstructional problems in dementia: contribution of executive systems functions. Neuropsychology. 2000;14(3):415–426. doi: 10.1037//0894-4105.14.3.415. [DOI] [PubMed] [Google Scholar]
- 44.Fang H, Zhang LF, Meng FT, Du X, Zhou JN. Acute hypoxia promotes the phosphorylation of tau via ERK pathway. Neurosci Lett. 2010;474(3):173–177. doi: 10.1016/j.neulet.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 45.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56(4):537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 46.Noh Y, Lee Y, Seo SW, et al. A new classification system for ischemia using a combination of deep and periventricular white matter hyperintensities [published online July 13, 2013] J Stroke Cerebrovasc Dis. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Mathis CA, Kuller LH, Klunk WE, et al. In vivo assessment of amyloid-β deposition in nondemented very elderly subjects. Ann Neurol. 2012;73(6):751–761. doi: 10.1002/ana.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.