Summary
We conducted the present trial to test whether early BCG-Denmark reduces mortality rate in low-weight (LW) neonates. We found that early administration of BCG-Denmark in LW infants is associated with major reductions in mortality rate.
Keywords: Bacille Calmette-Guérin, neonatal mortality, heterologous immunity, nonspecific effects
Abstract
Background
BCG vaccine may reduce overall mortality by increasing resistance to nontuberculosis infections. In 2 randomized trials in Guinea-Bissau of early BCG-Denmark (Statens Serum Institut) given to low-weight (LW) neonates (<2500 g at inclusion) to reduce infant mortality rates, we observed a very beneficial effect in the neonatal period. We therefore conducted the present trial to test whether early BCG-Denmark reduces neonatal mortality by 45%. We also conducted a meta-analysis of the 3 BCG-Denmark trials.
Methods
In 2008–2013, we randomized LW neonates to “early BCG-Denmark” (intervention group; n = 2083) or “control” (local policy for LW and no BCG-Denmark; n = 2089) at discharge from the maternity ward or at first contact with the health center. The infants were randomized (1:1) without blinding in blocks of 24. Data was analyzed in Cox hazards models providing mortality rate ratios (MRRs). We had prespecified an analysis censoring follow-up at oral poliovirus vaccine campaigns.
Results
Early administration of BCG-Denmark was associated with a nonsignificant reduction in neonatal mortality rate (MRR, 0.70; 95% confidence interval [CI], .47–1.04) and a 34% reduction (0.66; .44–1.00) when censoring for oral poliovirus vaccine campaigns. There was no reduction in mortality rate for noninfectious diseases, but a 43% reduction in infectious disease mortality rate (MRR, 0.57; 95% CI, .35–.93). A meta-analysis of 3 BCG trials showed that early BCG-Denmark reduced mortality by 38% (MRR, 0.62; 95% CI, .46–.83) within the neonatal period and 16% (0.84; .71–1.00) by age 12 months.
Conclusion
Early administration of BCG-Denmark in LW infants is associated with major reductions in mortality rate. It is important that all LW infants receive early BCG in areas with high neonatal mortality rates.
Clinical Trials Registration
BCG vaccine may not only prevent tuberculosis. Historical data from its introduction [1] and observational studies from low-income countries [2–6] suggest that BCG vaccine reduces mortality rates more than can be explained by prevention of tuberculosis. These nonspecific effects have recently been confirmed in randomized controlled trials (RCTs) [7, 8]. In 2014, the World Health Organization (WHO) conducted a review of potential nonspecific effects of BCG vaccine and concluded that it was associated with almost a halving of mortality rate, an effect not likely to be explained by prevention of tuberculosis [9].
WHO recommends BCG vaccine at birth to all infants born in tuberculosis-endemic countries [10]. In Guinea-Bissau, BCG vaccine is not administered at birth to low-birth-weight (<2500 g) neonates. Instead, it is given when the infants have gained weight, typically when they come for their first diphtheria-tetanus-pertussis, hepatitis B, and Haemophilus influenzae type b (pentavalent) vaccination, recommended at 6 weeks of age. This made it possible to compare mortality rates for “early BCG” versus “BCG later” schedules in RCTs among low-weight (LW) neonates (<2500 g at inclusion). We conducted a small RCT among infants receiving their first vaccination at the health centers from 2002 to 2004 (trial I) and a larger, mainly hospital-based, RCT from 2004 to 2008 (trial II) [7, 8]. The LW infants were randomized to receive early BCG (intervention group) or the usual delayed BCG vaccine (control group). The primary hypothesis was a 25% reduction in infant mortality. A combined analysis of the 2 trials showed a reduction in infant mortality rate of 21% (95% confidence interval, –2% to 39%) and a larger-than-expected reduction in neonatal mortality rate of 48% (18%–67%), before most control infants received BCG vaccine [8].
If BCG vaccine has major beneficial nonspecific effects, it could have a major impact on child survival in many low-income countries. The previous trials tested the effect on infant mortality rates. They have not led to more emphasis on BCG vaccination at birth or to a policy of giving BCG vaccine to all LW infants. We therefore conducted a new RCT (trial III) among LW infants to test the a priori hypothesis that early BCG reduces the neonatal mortality rate by 45%.
METHODS
Setting
The Bandim Health Project (BHP) maintains a Health and Demographic Surveillance System in 6 districts of Bissau, Guinea-Bissau. Since 2002, the BHP has maintained a cohort of LW infants from the capital and suburbs. In August 2008, the diphtheria-tetanus-pertussis vaccine given at 6, 10, and 14 weeks of age was replaced by the pentavalent vaccine, and yellow fever vaccine was added at 9 months.
Study Design
The present RCT was designed to test the effects of early BCG vaccination (intervention group) versus BCG vaccination later (control group) on neonatal mortality rates (within 28 days after birth) among LW infants. The trial was initiated on 19 February 2008, when trial II had ended (Supplementary Figure S1) and follow-up ended on 9 September 2014, when the last participant had the 12-month visit. Initially, we enrolled only LW girls, because growth data from trial II suggested that BCG vaccine was only beneficial to girls. LW boys were randomized to neonatal vitamin A supplementation (intervention group) versus oral poliovirus vaccine (OPV) at birth (control group) because previous trials had suggested that such supplementation might be beneficial for boys but not for girls [11, 12]. As reported elsewhere [13] the male trial was stopped in November 2008 owing to a cluster of deaths in the supplementation group. When the mortality analyses of trial II were completed, they showed major reductions in neonatal mortality rates in both sexes [7]. Hence, we decided to include both girls and boys, and ethical approval was obtained in May 2010 (Supplementary Figure S1).
Enrollment and Informed Consent
LW infants born at the national hospital and 3 private hospitals in Bissau were invited to participate. Infants born at the private hospitals whose mothers consented to participate were driven to the national hospital for enrollment. Infants born at home were included when they came for their first vaccination at one of the 3 health centers in the Health and Demographic Surveillance System catchment area or at one of the private hospitals. Infants weighing <2500 g at randomization were eligible to participate. Those who had major malformations, were overtly sick, or had received BCG vaccine before first contact were excluded.
Mothers or guardians were informed about the study in the local language, Creole, and received a written explanation in the official language, Portuguese. If they accepted participation, they were asked to sign the consent form using signature or fingerprint. The infant was weighed on an electronic scale (SECA; model 334). The infants enrolled at the national hospital were examined by a physician or a neonatal nurse, and Ballard score was used to assess gestational age [14].
Randomization Procedures
The infants were randomized (1:1) in blocks of 24 at enrollment. Girls and boys were randomized separately; same-sex twins were allocated to the same treatment to prevent potential confusion. Infants allocated to “early BCG” were vaccinated intradermally with 0.05 mL of BCG-Denmark vaccine (Statens Serum Institut). The infants allocated to “no BCG” were treated according to local practice; that is, mothers were encouraged to have the infants BCG vaccinated at the local health center when the infant had gained weight. All infants received OPV at birth. We did not give control infants a comparator vaccine, which might also have nonspecific effects [15]. Furthermore, if we had used placebo, the mothers in the control group might believe that their infant had already received BCG vaccine and not seek it later (Appendix 1).
Follow-up
All infants were visited 3 days after enrollment and at 2, 6, and 12 months of age. If an infant’s family moved within Bissau city, a neighbor was asked to guide the field assistant to the new house, ensuring that very few infants were lost to follow-up. Infants who were moved outside Bissau city were was considered lost to follow-up after migration.
Outcomes
The primary outcome was neonatal mortality (nonaccidental deaths) occurring within 28 days of life). For all registered deaths a WHO/INDEPTH verbal autopsy [16] was conducted 3 months after death by a specially trained field assistant. A local physician blinded to the trial allocation read the autopsy report and assigned a diagnosis. The death was classified according to the primary diagnosis unless the assigned cause was the underlying condition prematurity (n = 16), in which case the secondary diagnosis was used. In 4 cases, no secondary diagnosis was provided and the diagnosis from a second physician was used. The secondary outcome was infant mortality, that is, nonaccidental deaths occurring within the first 12 months of life.
Sample Size
The neonatal mortality rate was 4.20% in the control group in trial II [7]. To detect a 45% reduction in neonatal mortality rate for infants who received early BCG, we needed 2974 infants in the study. Considering the limited loss to follow-up within the first month of life, we aimed to include 3050 infants. During the trial, the neonatal mortality rate dropped to 3.15% in the control group. Therefore, the Data and Safety Monitoring Board recommended including another 1050 infants, and the final sample size was 4100 infants.
Statistical Analyses
Mortality rates were compared for infants randomized to early or late BCG in a Cox proportional hazard model, with age as the underlying time in the per-protocol analyses. We adjusted the standard errors for the dependence of twins by using robust standard errors. Because girls and boys were randomized separately, we allowed separate baseline hazards for the 2 sexes. Kaplan-Meier curves were drawn with time since randomization as the underlying time.
As prespecified in the study protocol, we examined whether the estimated effect of early BCG was influenced by health campaigns by censoring follow up on the first day of the campaign. Only OPV campaigns (8 campaigns during trial period) involved the neonatal period.
We conducted a meta-analysis of the 3 trials of early BCG to LW infants (trials I, II, and the present trial III; Supplementary Figure S1). We calculated meta-estimates for 3 days after enrollment, 28 days after birth and 12 months after birth. A fixed-effects model was used because the test for heterogeneity was not significant (3 days, P = .46; 28 days, P = .45; 12 months, P = .38).
We also conducted explorative analyses where we stratified by cause of death (infectious disease/noninfectious disease), sex, antibiotic use by mother, and trial period (girls only/all infants). Statistical analyses were performed using Stata12 software.
Ethics
The original protocol and the amendment were approved by the Guinean Ministry of Health’s Research Coordination Committee. Consultative approval was obtained from the Danish Central Ethical Committee. The BHP offered free healthcare consultations and essential drugs to all infants invited to participate in the study. The study was registered at clinicaltrials.gov (NCT00625482).
RESULTS
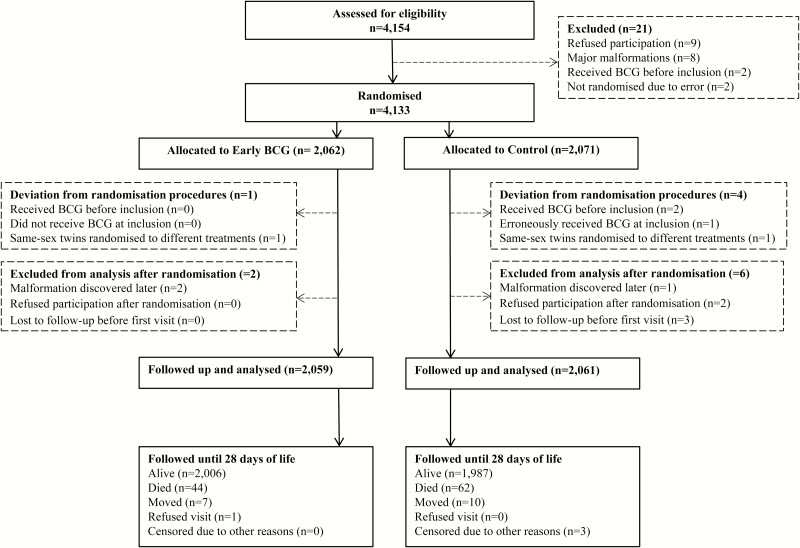
We enrolled 4172 infants (early BCG, 2083; controls, 2089) from 19 February 2008 to 7 September 2013. Twenty-one infants invited to participate in the trial did not meet the inclusion criteria. (Figure 1). Thirteen infants were included but afterward excluded owing to malformations (3 infants), receipt of BCG vaccine after allocation to the control group (1 infant), receipt of BCG vaccine before inclusion (2 infants), randomization of same-sex twins to different interventions (2 infants), refusing participation after randomization (2 infants), and leaving the hospital without notice (3 infants). Four infants were censored in the analyses (for more information, see Appendix 1) (Figure 1). A total of 39 infants were enrolled after 28 days and were not included in the analyses of neonatal mortality rates (Supplementary Figure S2).
Figure 1.
Flowchart for the neonatal period (first 28 days of life). The “loss to follow-up” category denotes infants who could not be followed up because their mothers left the hospital without the field assistant and their residence was unknown. The “censored for other reasons” category denotes infants who had been included twice and were excluded on the second inclusion.
The background factors were comparable between the randomization groups (Table 1). Furthermore, vaccination coverage during follow-up was the same in the 2 groups (Supplementary Table S1). Very few infants had enlarged lymph nodes at day 3 after inclusion (n = 0), at 2 months (n = 6), or at 6 months of age (n = 19); the proportions were the same in both groups (Supplementary Table S2). Moreover, health center referrals by the follow-up team were the same in the 2 randomization groups (P = .71; data not shown). During the neonatal period, 106 nonaccidental deaths occurred (Table 2). At 12 months, 322 nonaccidental deaths had occurred, the overall infant mortality rate being 8.5 per 100 person-years, and 9.1 per 100 person-years in the control group.
Table 1.
Background Factors According to Randomization Group
| Background Factor | Infants, % (No./Total)a | P Value | |
|---|---|---|---|
| Early BCG (n = 2059) | Control (n = 2061) | ||
| Male sex | 32.0 (658/2059) | 31.8 (655/2061) | .90 |
| Age at randomization, median (10th–90th percentile), d | 2 (1–10) | 2 (1–10) | .78 |
| Maturityb | |||
| Preterm | 23 (399/1737) | 22 (374/1740) | .31 |
| Term | 77 (1338/1737) | 78 (1366/1740) | |
| Cesarean delivery | 6 (119/2059) | 6 (117/2054) | .91 |
| Twins or triplets | 21 (425/2059) | 21 (423/2061) | .90 |
| Inclusion during rainy season | 47 (1083/2059) | 50 (1054/2061) | .35 |
| Mother dead at enrollment | 0.4 (9/2058) | 0.3 (7/2061) | .53 |
| Place of inclusion | |||
| Born and included at the national hospital | 70 (1444/2059) | 70 (1449/2061) | .83 |
| Born at private and included at the national hospital | 16 (321/2059) | 16 (330/2061) | |
| Included at health centers or on immunization daysc | 14 (294/2059) | 14 (282/2061) | |
| Weight at birthd | |||
| 2.00–2.49 kg | 78 (1361/1749) | 78 (1358/1751) | .68 |
| 1.50–1.99 kg | 18 (310/1749) | 17 (304/1751) | |
| <1.50 kg | 5 (78/1749) | 5 (89/1751) | |
| Weight at inclusion, median (10th–90th percentile), kg | 2.2 (1.6–2.4) | 2.2 (1.6–2.4) | .84 |
| Measurement, median (10th–90th percentile) | |||
| Length, cm | 46 (42–48) | 46 (42–48) | .36 |
| Head circumference, cm | 32 (29–33) | 32 (29–33) | .42 |
| Abdominal circumference, cm | 27 (24–30) | 27 (25–29) | .52 |
| MUAC, in mm | 82 (70–88) | 82 (70–88) | .85 |
| Maternal MUAC, mm | 244 (214–286) | 244 (214–286) | .46 |
| Maternal HIV-positive statuse | 5 (40/846) | 6 (48/811) | .32 |
Abbreviations: HIV, human immunodeficiency virus; MUAC, mid-upper-arm circumference.
aData represent the % (No./Total) of infants, unless otherwise specified.
bBallard score for assessment of maturity was available only for infants included at the national hospital (n = 3477).
cMost infants included at the health centers or on immunization days had been born at home.
dBirth weight was available only for infants born at the hospital (n = 3529).
eThe mother’s HIV status was registered only for infants born at the national hospital during periods when HIV tests were available in the country.
Table 2.
Mortality Rates and Mortality Rate Ratios in the Neonatal Period and at 12 Months by Randomization Status With Complete Follow-Up and Follow-Up Censored for National Oral Poliovirus Vaccine Campaignsa
| Follow-up | Mortality Rate per 100 Person-Years (No. of Deaths/Total Person-Years) | MRR for Early BCG vs Control (95% CI) | |
|---|---|---|---|
| Early BCG | Control | ||
| Complete follow-up | |||
| 28 d after birth | 32 (44/137) | 46 (62/135) | 0.70 (.47–1.04) |
| 12 mo after birth | 8 (152/1906) | 9 (170/1870) | 0.88 (.71–1.10) |
| Censoring follow-up on 1st day of national OPV campaigns | |||
| 28 d after birth | 31 (40/128) | 47 (59/126) | 0.66 (.44–1.00) |
| 12 mo after birth | 13 (108/939) | 15 (122/922) | 0.87 (.67–1.13) |
Abbreviations: CI, confidence interval; MRR, mortality rate ratio; OPV, oral poliovirus vaccine.
aAnalyses were performed using Cox regression.
Neonatal and Infant Mortality
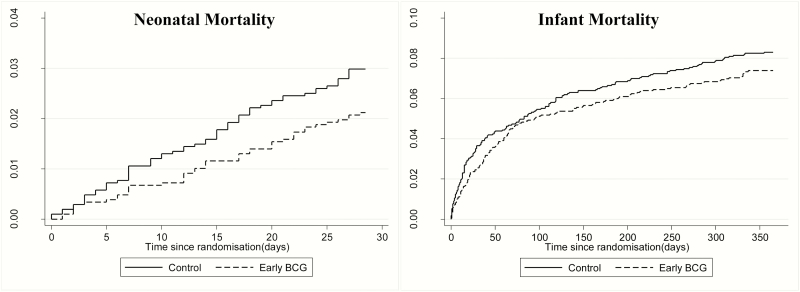
The mortality curves are shown in Figure 2. Early receipt of BCG vaccine was associated with a 30% reduction in neonatal mortality rate (mortality rate ratio [MRR], 0.70; 95% CI, .47–1.04) (Table 2). There was no significant difference between boys (MRR, 0.63; 95% CI, .35–1.14) and girls (0.76; .45–1.28; P for same effect = .65) (Supplementary Table S3). The effect of BCG vaccination on neonatal mortality rate may have been slightly better among infants weighing >1.5 kg and among mature infants (ie, born at term) (Supplementary Table S4).
Figure 2.
Cumulative mortality curves during the neonatal (28 days) and infant (365 days) periods according to randomization group. (Note differences in scale for the 2 curves.)
At both 6 and 12 months after birth, the reduction in mortality rate was 12%. There was no difference in the effect of early BCG between boys and girls. A sensitivity analysis to test effects of potential misclassifications of neonatal deaths showed that potential misclassification of the timing of the deaths did not affect the estimates (Appendix 1).
Oral Poliovirus Vaccine Campaigns
Eight OPV campaigns were conducted during the present trial. With censoring of follow-up on the first day of a campaign, the reduction in neonatal mortality rate was 34% (MRR, 0.66; 95% CI, .44–1.00) (Table 2).
Causes of Death in the Neonatal Period
Of the 106 neonatal deaths, we obtained information on the cause for 100 deaths (94%); 6 families had moved. Early receipt of BCG-Denmark vaccine was associated with a 43% reduction in infectious deaths (MRR, 0.57; 95% CI, .35–.93) (Table 3). Neonatal sepsis was the most common cause of neonatal deaths (54%) (early BCG, 21 deaths; control group, 33 deaths) (Table 4). There was no difference between randomization groups for noninfectious deaths.
Table 3.
Cause-Specific Mortality Rates and Mortality Rate Ratios in the Neonatal Perioda
| Cause of Deathb | Cause-Specific Mortality Rate per 100 Person-Years (No. of Deaths/Total Person-Years) | Cause-Specific MRR for Early BCG vs Control (95% CI) | |
|---|---|---|---|
| Early BCG | Control | ||
| Infection | 17 (25/137) | 31 (44/135) | 0.57 (.35–.93) |
| Noninfectious conditions | 14 (17/137) | 12 (14/135) | 1.20 (0.58–2.49) |
Abbreviations: CI, confidence interval; MRR, mortality rate ratio.
aAnalyses were performed using Cox regression.
bThe infection category included sepsis, respiratory infections, fever, malaria, omphalitis, and gastrointestinal infections. Noninfectious conditions included prematurity, sudden infant death syndrome, anemia, bleeding, congenital problems, kernicterus, hypoglycemia, hypothermia, and respiratory distress syndrome. The cause of death was not available for 6 deaths, 2 in the early BCG group and 4 in the control; verbal autopsies were not conducted for these deaths because the families moved before the verbal autopsy visit.
Table 4.
Specific Causes of Death in the Neonatal Period
| Specific Cause of Death | Deaths, No. | |
|---|---|---|
| Early BCG | Control | |
| Sepsis | 21 | 33 |
| Respiratory infections | 1 | 3 |
| Fever or malaria | 0 | 2 |
| Omphalitis | 0 | 5 |
| Gastrointestinal infections | 3 | 1 |
| Sudden infant death syndrome | 8 | 8 |
| Respiratory distress syndrome | 5 | 3 |
| Other noninfectious conditionsa | 4 | 3 |
| Not availableb | 2 | 4 |
| Total | 44 | 62 |
aOther noninfectious conditions included anemia, bleeding, congenital problems, kernicterus, hypoglycemia, and hypothermia.
bThe cause of death was not available for 6 deaths, 2 in the early BCG group and 4 in the control; verbal autopsies were not conducted for these deaths because the families moved before the verbal autopsy visit.
Meta-Analysis of 3 Trials of Early BCG Vaccination in Low Weight Infants
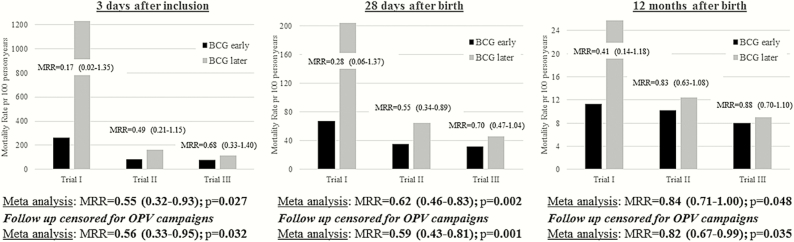
Figure 3 shows a meta-analysis of RCT I-III of early BCG in LW infants in Guinea-Bissau, conducted from 2002 to 2013. At 3 days after enrollment, BCG vaccine almost halved the mortality rate (MRR, 0.55; 95% CI, .32–.93). At 28 days of life, the reduction in mortality rate after BCG-Denmark was 38% (MRR, 0.62; 95% CI, .46–.83). At 12 months, the mortality was significantly reduced by 16% for the early BCG group (MRR, 0.84; 95% CI, .71–1.00). Censoring follow-up for OPV campaigns enhanced the effect on mortality rates slightly (Figure 3).
Figure 3.
Mortality rates and mortality rate ratios (MRRs) in the 3 trials of early BCG vaccination of low-weight infants in Guinea-Bissau, by trial and combined in a meta-analysis (fixed effect model) with and without censoring for participation in oral poliovirus vaccine (OPV) campaigns. MRRs are given with 95% CIs. Trial I is the small randomized controlled trial (RCT) conducted in Guinea-Bissau in 2002–2004 (n = 104; median age at randomization, 2 days for early BCG and 4 days for controls) [8]; trial II, the RCT conducted in Guinea-Bissau in 2004–2008 (n = 2320) [7]; and trial III, the RCT conducted in Guinea-Bissau in 2008–2013 and presented in the current article (n = 4120; median age at randomization, 2 days for both early BCG and controls). Note differences in scale for the three panels.
Explorative Analyses
Because the effect of BCG vaccine on neonatal mortality rates was smaller than hypothesized, we made explorative analyses of factors that could have affected the measured effect of BCG vaccination in the present and the previous trial [7] (Appendix 1).
DISCUSSION
In the present third trial of early BCG, BCG was associated with a 30% (-4% to 53%) reduction in all-cause neonatal mortality; there was a 43% reduction among infectious deaths but no effect among deaths with noninfectious causes. A meta-analysis of the 3 RCTs of early BCG supported marked reductions in mortality within 3 days after vaccination and at 28 days and 12 months of age.
Strengths and Weaknesses
The follow-up was based on home visits and loss to follow-up during the neonatal period was limited (0.5%) making it unlikely that deaths were underreported. Owing to declining mortality rate, we increased the sample size to achieve sufficient power
The trial was not blinded. However, this is unlikely to have affected the outcome. The infants were vaccinated only at discharge. The trial staff at the hospital was not involved in providing healthcare to the infants after randomization. The staff collecting follow-up data did not provide healthcare, apart from referring sick infants to the health centers. The proportions of infants referred to health center consultation were the same in the 2 groups. The physicians and nurses at the pediatric wards and the health centers were unaware of the study. There was no difference in healthcare-seeking behavior among infants who died in the neonatal period; according to the verbal autopsy, 13% of deaths in the early BCG group and 11% in the control group occurred at the hospital (P = .72). Preliminary data on hospitalization admissions show no difference between the 2 randomization groups (unpublished data).
The BCG vaccine exists in several strains [17], with differences in immunogenicity [18–21] and genetics [20, 22]. Infants in the intervention group received BCG-Denmark, whereas control infants were most likely to receive BCG-Russia, provided by the local vaccination program. It could have influenced the results that the 2 groups received different BCG strains.
Comparison With Previous Findings
WHO has recently reviewed the potential nonspecific effects of vaccines, including observational and randomized studies. It was concluded that BCG vaccine almost halved mortality rate and that the effect, if true, was unlikely to be explained by protection against tuberculosis [9]. Trials I and II were included in the current review. The present trial corroborates the results of the WHO review. A recent RCT of BCG vaccine in Denmark showed no effect on all-cause hospitalizations [23]. However, among BCG-vaccinated mothers, randomization to BCG vaccination of the newborn was associated with a 35% significant reduction in hospital admissions for infectious diseases, whereas there was no effect of BCG vaccine in infants of BCG-unvaccinated mothers (unpublished data).
Because most mothers in Guinea-Bissau are BCG vaccinated, but only 17% were vaccinated in Denmark, it could be speculated that differences in maternal BCG priming explain the difference in results. A recent study from Uganda found that children of mothers with a BCG vaccine scar had stronger nonspecific proinflammatory responses after BCG vaccination [24, 25]. Recent observational studies from Greenland and Finland, where most mothers were BCG vaccinated, found BCG to be associated with reductions in hospitalizations for infants <3 months of age, but there was no effect in older children who would have received other vaccines [26, 27].
We had hypothesized a 45% reduction in neonatal mortality rate. There are several possible reasons why we saw a less pronounced effect in the present trial than in previous trials. First, OPV has been found to reduce infant mortality rate (Andersen A, et al, unpublished data) [28]. The increased number of OPV campaigns (trial II had 2 campaigns, and trial III had 8) may have reduced the difference between the intervention and the control group; after censoring for OPV campaigns, the effect of BCG increased (Figure 3). Second, BCG reduced infectious causes of death but not noninfectious causes. With a declining proportion of deaths with infectious causes among neonatal deaths, the beneficial nonspecific effect of BCG vaccine would decrease. Third, more control infants received BCG vaccine during the neonatal period than in the previous trials, diluting any effect of the vaccine. Fourth, the effect of BCG vaccine was strongest for infants whose mothers had not taken prophylactic antibiotics after delivery, but antibiotic consumption has increased during the last decade in Bissau.
The observed effect of BCG vaccine may thus depend on several factors that were not under our control and may vary between settings. Nonetheless, the meta-analysis shows a 38% reduction in neonatal mortality rate, which should make early BCG vaccination an indispensable intervention. For comparison, an RCT of neonatal home visits (without vaccinations) found a reduction of 12% [29].
Potential Biological Mechanisms
BCG vaccine is a potent immune stimulant with adjuvantlike properties [30]. The rapidly occurring effects of BCG vaccine observed in the present and in previous trials indicate that innate immune mechanisms may be involved. Consistent with this, BCG vaccination of BCG-naive Dutch adults was found to increase innate immune responses to heterologous pathogens [31]. BCG induced epigenetic reprogramming of monocytes, a phenomenon termed “trained innate immunity” [32]. Furthermore, increased proinflammatory cytokine production by NK cells was observed after BCG vaccination [33]. We corroborated this in a subgroup of infants enrolled in the present trial. Four weeks after vaccination, BCG had induced a T-helper 1–polarized and proinflammatory immune response to innate stimulation [34]. Hence, beneficial effects of BCG vaccine in protecting against unrelated infections is biologically plausible and may be mediated by enhanced innate immune effector functions.
CONCLUSIONS AND IMPLICATIONS
BCG-Denmark already had major beneficial effects on mortality rate within 3 days after vaccination and throughout the neonatal period. Although WHO recommends BCG vaccination at birth in all infants, many low-income countries postpone giving BCG vaccine to premature and LW infants until they have gained weight. Furthermore, infants with normal birth weight often experience a delay in BCG vaccination as well. In Sub-Saharan Africa, <50% of infants receive BCG vaccine within the neonatal period [35]. Considering the large and rapid effects on neonatal mortality, BCG vaccine should be promoted not only as a tuberculosis vaccine but also as a vaccine against neonatal infections to assure that infants in areas with high infant mortality rates receive BCG vaccine at birth.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. P. A. and C. S. B. conceived and designed the BCG trial and are the guarantors of the study. S. B. S., N. L., I. M., K. J. J., H. B. E., F. S. B., A. S. P. J., A. B. F., and A. R. supervised the field data collection. S. B. S. analyzed the data. P. A. supervised the data analysis. S. B. S. wrote the first draft of the manuscript. All authors contributed to the final version of the manuscript. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments. We thank the mothers and infants who participated in the study. We also thank the Data Safety and Monitoring Board, consisting of Robin Bailey, the late Jørn Attermann, and Poul-Erik Kofoed, as well as Henrik Ravn and Aksel Jensen for help with supervising the statistical analyses.
Disclaimer. The funding agencies had no role in the study design, data collection, data analysis, data interpretation, or the writing of the manuscript.
Financial support. The study was supported by The European Research Council (starting grant ERC-2009-StG-243149, which also funded S. B. S., I. M., and C. S. B.); the Novo Nordisk Foundation (research professorship grant to P. A.); the Danish National Research Foundation (grant DNRF108 to the Research Center for Vitamins & Vaccines and to K. J. J. and F. S. B.); and DANIDA, European Union FP7, and OPTIMUNISE (grant Health-F3-2011–261375 to the Bandim Health Project).
Potential conflicts of interest. The salaries for all authors are administered, but not financed, by Statens Serum Institut, the producer of the BCG-Denmark vaccine used in the present study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Shann F. Nonspecific effects of vaccines and the reduction of mortality in children. Clin Ther 2013; 35:109–14. [DOI] [PubMed] [Google Scholar]
- 2. Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ 2000; 321:1435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garly ML, Martins CL, Balé C et al. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa: a non-specific beneficial effect of BCG? Vaccine 2003; 21:2782–90. [DOI] [PubMed] [Google Scholar]
- 4. Roth A, Gustafson P, Nhaga A et al. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int J Epidemiol 2005; 34: 540–7. [DOI] [PubMed] [Google Scholar]
- 5. Roth A, Sodemann M, Jensen H et al. Tuberculin reaction, BCG scar, and lower female mortality. Epidemiology 2006; 17: 562–8. [DOI] [PubMed] [Google Scholar]
- 6. Roth A, Jensen H, Garly ML et al. Low birth weight infants and Calmette-Guérin bacillus vaccination at birth: community study from Guinea-Bissau. Pediatr Infect Dis J 2004; 23:544–50. [DOI] [PubMed] [Google Scholar]
- 7. Aaby P, Roth A, Ravn H et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis 2011; 204:245–52. [DOI] [PubMed] [Google Scholar]
- 8. Biering-Sørensen S, Aaby P, Napirna BM et al. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guérin vaccination at first health center contact. Pediatr Infect Dis J 2012; 31:306–8. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Weiser KS, Reingold A.. Systematic review of the non specific effects of BCG, DTP and measles containing vaccines. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 10. World Health Organization. BCG vaccine: WHO position paper. Wkly Epidemiol Rec 2004; 4: 25–40. [PubMed] [Google Scholar]
- 11. Benn CS, Diness BR, Roth A et al. Effect of 50000 IU vitamin A given with BCG vaccine on mortality in infants in Guinea-Bissau: randomised placebo controlled trial. BMJ 2008; 336:1416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benn CS, Fisker AB, Napirna BM et al. Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: two by two factorial randomised controlled trial. BMJ 2010; 340:c1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lund N, Biering-Sørensen S, Andersen A et al. Neonatal vitamin A supplementation associated with a cluster of deaths and poor early growth in a randomised trial among low-birth-weight boys of vitamin A versus oral polio vaccine at birth. BMC Pediatr 2014; 14:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard score, expanded to include extremely premature infants. J Pediatr 1991; 119:417–23. [DOI] [PubMed] [Google Scholar]
- 15. Byberg S, Benn CB. Placebo use in vaccine trials: caution when using active vaccines as placebo. Vaccine 2017; 35:1211. [DOI] [PubMed] [Google Scholar]
- 16. INDEPTH network. INDEPTH standardized verbal autopsy questionnaire Available at: http://www.indepth-network.org/resources/indepth-standardized-verbal-autopsy-questionnaire. Accessed 7 July 2017.
- 17. Behr MA. BCG—different strains, different vaccines? Lancet Infect Dis 2002; 2:86–92. [DOI] [PubMed] [Google Scholar]
- 18. Anderson EJ, Webb EL, Mawa PA et al. The influence of BCG vaccine strain on mycobacteria-specific and non-specific immune responses in a prospective cohort of infants in Uganda. Vaccine 2012; 30:2083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ritz N, Dutta B, Donath S et al. The influence of bacille Calmette-Guerin vaccine strain on the immune response against tuberculosis: a randomized trial. Am J Respir Crit Care Med 2012; 185:213–22. [DOI] [PubMed] [Google Scholar]
- 20. Brosch R, Gordon SV, Garnier T et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci U S A 2007; 104: 5596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu J, Tran V, Leung AS, Alexander DC, Zhu B. BCG vaccines: their mechanisms of attenuation and impact on safety and protective efficacy. Hum Vaccin 2009; 5:70–8. [DOI] [PubMed] [Google Scholar]
- 22. Bedwell J, Kairo SK, Behr MA, Bygraves JA. Identification of substrains of BCG vaccine using multiplex PCR. Vaccine 2001; 19:2146–51. [DOI] [PubMed] [Google Scholar]
- 23. Stensballe LG, Sørup S, Aaby P et al. BCG vaccination at birth and early childhood hospitalisation: a randomised clinical multicentre trial. Arch Dis Child 2017; 102:224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mawa PA, Nkurunungi G, Egesa M et al. The impact of maternal infection with Mycobacterium tuberculosis on the infant response to bacille Calmette-Guerin immunization. Philos Trans R Soc Lond B Biol Sci 2015 June 19; 370(1671). doi:10.1098/rstb.2014.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mawa PA, Webb EL, Filali-Mouhim A et al. Maternal BCG scar is associated with increased infant proinflammatory immune responses. Vaccine 2017; 35:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haahr S, Michelsen SW, Andersson M et al. Non-specific effects of BCG vaccination on morbidity among children in Greenland: a population-based cohort study. Int J Epidemiol 2016: 1–9. [DOI] [PubMed] [Google Scholar]
- 27. Benn CS, Sørup S. Commentary: BCG has no beneficial non-specific effects on Greenland. An answer to the wrong question? Int J Epidemiol 2016; 45:2131–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lund N, Andersen A, Hansen AS et al. The effect of oral polio vaccine at birth on infant mortality: a randomized trial. Clin Infect Dis 2015; 61:1504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kirkwood BR, Manu A, ten Asbroek AH et al. Effect of the Newhints home-visits intervention on neonatal mortality rate and care practices in Ghana: a cluster randomised controlled trial. Lancet 2013; 381:2184–92. [DOI] [PubMed] [Google Scholar]
- 30. Ota MO, Vekemans J, Schlegel-Haueter SE et al. Influence of Mycobacterium bovis bacillus Calmette-Guérin on antibody and cytokine responses to human neonatal vaccination. J Immunol 2002; 168:919–25. [DOI] [PubMed] [Google Scholar]
- 31. Kleinnijenhuis J, Quintin J, Preijers F et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 2012; 109:17537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe 2011; 9:355–61. [DOI] [PubMed] [Google Scholar]
- 33. Kleinnijenhuis J, Quintin J, Preijers F et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun 2014; 6:152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jensen KJ, Larsen N, Biering-Sørensen S et al. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial. J Infect Dis 2015; 211:956–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet 2009; 373:1543–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.