Abstract
In this population-based study in the contemporary era in the United States, the proportion of human immunodeficiency virus (HIV)–negative patients with cryptococcosis approaches that in HIV-infected patients. Cryptococcosis is associated with higher mortality rates in HIV-negative patients (including organ transplant recipients).
Keywords: Comparative epidemiology, outcomes, crypto- coccosis
Cryptococcosis is an important opportunistic fungal infection that causes significant mortality and morbidity in immunocompromised hosts. Nationally representative data show a decline in cryptococcal infections in developed nations with the advent of highly active antiretroviral therapy [1, 2]. Human immunodeficiency virus (HIV) negative patients with cryptococcosis experience delayed diagnosis and have higher mortality rates than HIV-infected patients [3–5]. A few university hospitals in the United States have reported that cryptococcosis in non-HIV immunocompromised hosts outnumber HIV-associated cases, possibly indicating a shift in epidemiology [3, 5–7]. However, these studies are limited by their single-center nature, shorter follow-up, and higher proportion of organ transplant recipients. The current study was done to generate more generalizable epidemiologic information regarding cryptococcosis in the United States, using a large and diverse cohort of patients admitted with cryptococcosis included the Healthcare Cost and Utilization Project State Inpatient Databases (SIDs).
METHODS
Study Design and Patient Population
We performed a retrospective cohort study in adults aged ≥18 years with newly coded cryptococcosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis code 117.5) and cryptococcal meningitis (ICD-9-CM 321.0) from 2004 to 2010 in California (n = 2556) and from 2006 to 2012 in Florida (n = 1172). The California and Florida SIDs were chosen for their population diversity and the ability to ascertain HIV status. These states also provide a consistent unique data element that permits linkage across hospitalizations. The SIDs contain longitudinal demographic and billing data that capture inpatient diagnoses and procedures through ICD-9-CM coding across hospitals within a state.
Demographic Data, Comorbid Conditions, and Follow-up
Demographic characteristics of the study population were determined during the admission for incident cryptococcosis. To compare the incidence of cryptococcosis over time, we used state-specific intercensal estimates of the resident population provided by the US Census Bureau [8]. Conditions listed in the Elixhauser Comorbidity Index, HIV infection, and organ transplantation were identified using ICD-9-CM diagnosis codes within 1 year before and during the hospitalization for incident cryptococcosis [9]. Inpatient readmissions were identified using the encrypted patient-level identifier. ICD-9-CM diagnosis codes for pneumonia and skin and soft tissue infection assigned during the admission coded for cryptococcosis were used to characterize potential sites of cryptococcal infection (Supplementary Table S1).
Statistical Analysis
Descriptive statistics were used to define the demographic and clinical characteristics of the study population. The Cochran-Armitage trend test was used to check whether incident trends varied over time. Inpatient mortality rates and clinical presentations at admission were compared among HIV-infected, non–HIV non-transplant (NHNT), and solid organ transplant (SOT) groups. Potential risk factors for inpatient death were analyzed separately for the 3 groups of patients, using univariable and multivariable Cox proportional hazards models. All analyses were performed using SAS Enterprise Guide 5 software.
RESULTS
Incidence of Cryptococcosis
A total of 3728 patients with cryptococcosis were identified across 276 hospitals in California and 175 hospitals in Florida; 56% (n = 2091) were HIV infected, 39.4 % (n = 1470) were in the NHNT group, and 4.5% (n = 167) were SOT recipients (Table 1). Incidence rates of cryptococcosis declined over the years in both states (both P < .001; Cochran-Armitage trend test), driven largely by the decline in HIV-infected patients with cryptococcosis, from 6.8 to 3.9 hospitalizations per million population in California and from 7.1 to 3.3 per million in Florida (both P < .001). The incidences of NHNT- and SOT-associated cryptococcosis hospitalizations per million also declined, albeit at a slower rate, from 4.6 to 3.5 in California (P = .04) and from 3.7 to 3.5 in Florida (P = .07) for NHNT, and from 0.64 to 0.34 in California (P = .01) and from 0.55 to 0.34 in Florida (P = .39) for SOT (Supplementary Figure S1).
Table 1.
Demographics, Clinical Characteristics, and Outcomes in Patients With Cryptococcal Disease
| Variable | Patients, %a | P Value | |||
|---|---|---|---|---|---|
| HIV Infected (n = 2091) | NHNT (n = 1470) | SOT (n = 167) |
All Groups | NHNT vs SOTb | |
| Age, mean (range), y | 42.8 (18–83) | 58.0 (18–98) | 58.0 (20–84) | <.001 | .28 |
| Race | NA | NA | NA | <.001 | .10 |
| White | 28.7 | 50.5 | 46.0 | NA | NA |
| Black | 37.8 | 13.2 | 9.3 | NA | NA |
| Hispanic | 30.2 | 27.1 | 29.2 | NA | NA |
| Primary payer | NA | NA | NA | <.001 | <.001 |
| Medicare | 18.4 | 42.5 | 61.1 | NA | NA |
| Medicaid | 43.4 | 18.3 | 14.4 | NA | NA |
| Private insurance | 22.1 | 29.7 | 23.4 | NA | NA |
| Self-pay | 9 | 4.0 | …c | NA | NA |
| Lowest income quartile or missing | 50.6 | 29.8 | 31.2 | <.001 | .78 |
| SOT | …c | NA | 100 | NA | NA |
| Kidney | …c | NA | 60.1 | NA | NA |
| Liver | NA | NA | 17.8 | NA | NA |
| Lung | NA | NA | 8.3 | NA | NA |
| Heart | NA | NA | 13.0 | NA | NA |
| Other organ or multiorgan | NA | NA | …c | NA | NA |
| Cancer | |||||
| Lymphoma/leukemia | 2.8 | 8.2 | …c | <.001 | .004 |
| Solid tumors | 1.2 | 6.2 | 8.4 | <.001 | .22 |
| Metastatic cancer | 0.9 | 5.1 | …c | <.001 | .001 |
| Other comorbid conditions | |||||
| Renal failure | 7.4 | 22.5 | 18.5 | <.001 | .25 |
| Congestive heart failure | 3.6 | 19.8 | 13.8 | <.001 | .06 |
| Chronic lung disease | 12.6 | 24.6 | 14.4 | <.001 | .003 |
| Diabetes | 9.1 | 31.9 | 61.1 | <.001 | .02 |
| Liver disease | 11.2 | 14.7 | 7.8 | .001 | .02 |
| Connective tissue disease | …c | 9.2 | …c | <.001 | .01 |
| Clinical syndromes identified | |||||
| Meningitis | 69.1 | 34.4 | 41.9 | <.001 | .06 |
| Pneumonia | 16.1 | 33.0 | 40.7 | <.001 | .04 |
| Skin/soft-tissue infection | 5.1 | 7.4 | 8.1 | .009 | .63 |
| Mortality rate | |||||
| 90-d | 14.6 | 20.7 | 13.7 | <.001 | .03 |
| 1-y | 19.6 | 27.8 | 24.6 | <.001 | .41 |
| Overall inpatient | 25 | 33.2 | 37.1 | <.001 | .34 |
| Time to death, median (range), d | 43.5 (0–2416) | 49 (0–2421) | 155 (4–2808) | <.001 | .83 |
Abbreviations: HIV, human immunodeficiency virus; NA, not available; NHNT, non–HIV-infected nontransplant; SOT, solid organ transplant.
aData represent No. (%) of patients except where otherwise specified.
b P values in this column represent comparison between NNHT and SOT groups, except for time to death (in days), where the comparison is between HIV-infected and NHNT groups.
cThe Agency for Healthcare Research and Quality confidentiality statute prohibits disclosure of information where the number of observations is <11.
Demographic and Clinical Characteristics
The HIV-infected group was significantly younger and more often male (84.6%) and nonwhite (71.3%) than the NHNT and SOT groups. More than half (50.6%) of HIV-infected patients had their estimated median household income in the poorest quartile. Greater proportions of NHNT and SOT patients with cryptococcosis had diabetes, congestive heart failure, liver disease, chronic lung disease, and renal failure (Table 1).
Among the HIV-infected group, 69.1% were coded with cryptococcal meningitis, in contrast to 34.4% in the NHNT group and 41.9% among SOT recipients (P < .001). Coding for lumbar puncture also differed among groups, with 71.0%, 40.6%, and 55.1% of HIV-infected, NHNT, and SOT groups, respectively, undergoing the procedure (P < .001). During the index hospitalization for cryptococcosis, greater proportions of patients in the HIV-negative (NHNT and SOT) groups were identified with pneumonia (33.5% and 40.7% vs 16.1% for HIV-infected patients) or skin and soft-tissue infections (7.4 % and 8.4% vs 5.1%).
Inpatient Mortality Rates in Patients With Cryptococcosis
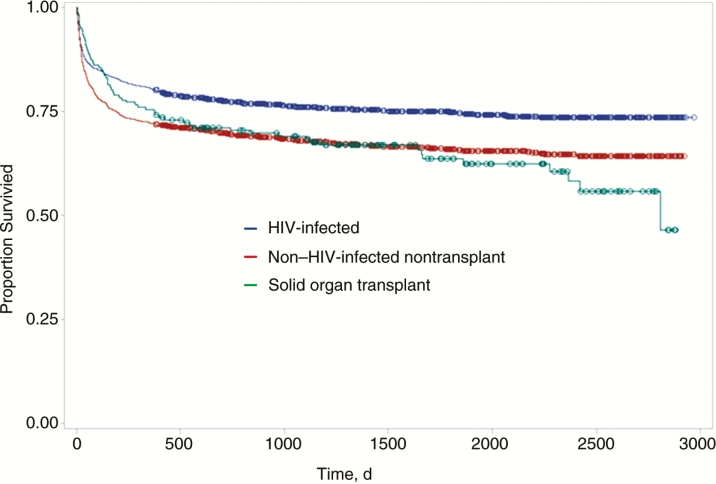
The overall mortality rate was 28.8 % (1073 of 3728) during the study period. Of 1637 patients, 489 (33.2%) died in the NHNT group with cryptococcosis (hazard ratio, 1.42; 95% confidence interval, 1.25–1.61; P < .001), 62 of 167 (37.1%) died among SOT recipients (1.49; 1.15–1.95; P = .002), and 522 of 2091 (25.0%) died among HIV-infected patients (Table 1 and Figure 1). The 90-day inpatient mortality rate was higher for the NHNT group (20.7%) than for the HIV-infected (14.6%) and SOT (13.7%) groups (P < .001).
Figure 1.
Kaplan-Meier survival curves for 3728 patients with cryptococcal disease stratified based on the presence of human immunodeficiency virus (HIV) infection, non–HIV-infected nontransplant status, and solid organ transplant.
The median duration of follow-up was 1294 days (range, 0–2971 days). Liver disease, congestive heart failure, and lymphoma or leukemia were risk factors for death in both HIV-infected and NHNT patients with cryptococcosis. Liver disease and having received a lung transplant were risk factors for death in the SOT group after adjustment for age and other comorbid conditions (Supplementary Table S1).
DISCUSSION
This study demonstrates that the overall incidence of cryptococcosis is declining over a 7-year period in 2 large and diverse states. The decline is driven by a strong and persistent decrease in the incidence of cryptococcosis among HIV-infected patients more than by its gradual decline in HIV-negative patients.
We observed important demographic differences in patients with cryptococcosis among the 3 groups. HIV-infected patients with cryptococcosis were more frequently male and African American, on average 15 years younger, and poorer than HIV-negative patients with cryptococcosis. Unsurprisingly, comorbid conditions, such as cancer, renal failure, congestive heart failure, chronic lung disease, diabetes, and liver disease, were more often identified in the NHNT and SOT groups, given their older age and multiple underlying illnesses. Of note, the subgroup of SOT recipients was much smaller (4.5%) in this study compared with reported proportions from academic medical centers (20%–28%) [3, 7].
The overall mortality rate in patients with cryptococcosis continues to be high (28.8%). The 90-day mortality rate (13.7%–20.7%) in the 3 groups is similar to rates in recent cohorts and probably represents real-world practices, because effective treatment of cryptococcosis continues to be an unmet need [7, 10, 11]. The NHNT group had significantly higher 90-day, 1-year, and overall mortality rates than the HIV-infected group, consistent with prior reports [1, 3, 5, 7]. Possible reasons for this include advanced age, severe underlying comorbid conditions, and atypical presentations of cryptococcosis, leading to delays in diagnosis and treatment. Less typical presentations of cryptococcosis, such as pneumonia in the absence of meningitis, or skin and soft-tissue infections, were identified more often in NHNT and SOT groups than in HIV-infected patients, similar to prior reports [3, 7, 10].
Clinical inexperience with unusual presentations of cryptococcosis may have led to delayed diagnosis, suboptimal management, and poorer outcomes. Irreversibility of immunocompromise may also account for worse outcomes in the NHNT group. In contrast, HIV-infected patients can receive effective antiretroviral therapy and gain reversal in immune dysfunction [12, 13]. The SOT recipients seemed to be an intermediate-risk group with lower 90-day mortality rates, similar to the HIV-infected group, but with 1-year and overall mortality rates similar to those in the NHNT group (Table 1 and Figure 1). The lower mortality rate earlier on might reflect the very close posttransplant follow-up these patients receive, and the higher mortality rate with further follow-up might reflect allograft failures and continued immunocompromise.
In multivariable analyses, patients with liver disease had a higher risk of death in all 3 groups. Patients with end-stage liver disease are a subgroup with a high risk for cryptococcosis and subsequent death (mortality rate, 57%–80%) [5, 14]. This calls for increased vigilance in patients with liver disease and cirrhosis.
The main strengths of this study are its large cohort size and long duration of follow-up. It has limitations, however. With 2 specific ICD-9-CM codes for cryptococcal disease and meningitis, we believe that errors from misclassification are less likely. A validation study of ICD-9-CM codes for serious infections found a positive predictive value of 100% for cryptococcosis [15]. In another validation study, 107 of 122 cases of cryptococcosis identified by ICD-9-CM coding were confirmed with a positive culture or positive results of serology or histopathology (positive predictive value, 87%; John W. Baddley, personal communication, 2017). Finally, the data source does not contain microbiology or laboratory test results, information regarding antiretroviral or antifungal medications, or direct causes of death.
In conclusion, our findings suggest that the overall incidence of cryptococcosis in the United States is declining but is associated with higher mortality rates in NHNT and SOT groups. There is a need to better sensitize clinicians to the atypical presentations and significantly increased mortality rate associated with cryptococcosis in HIV-negative patients. Future research should be directed toward better screening and treatment strategies for these groups.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We gratefully acknowledge Satish Munigala, MD, for the statistical help and John W. Baddley, MD, MSPH, for providing the unpublished data on the validity of ICD-9-CM codes for cryptococcosis.
Financial support. This study used the services of the Center for Administrative Data Research, supported in part by the Washington University Institute of Clinical and Translational Sciences (grant UL1 TR000448 from the National Center for Advancing Translational Sciences, National Institutes of Health (NIH); grant R24 HS19455 through the Agency for Healthcare Research and Quality; and grant KM1CA156708 through the National Cancer Institute, NIH).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PloS One 2013; 8:e56269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Elden LJ, Walenkamp AM, Lipovsky MM et al. Declining number of patients with cryptococcosis in the Netherlands in the era of highly active antiretroviral therapy. AIDS 2000; 14:2787–8. [DOI] [PubMed] [Google Scholar]
- 3. Bratton EW, El Husseini N, Chastain CA et al. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLoS One 2012; 7:e43582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liao CH, Chi CY, Wang YJ et al. Different presentations and outcomes between HIV-infected and HIV-uninfected patients with cryptococcal meningitis. J Microbiol Immunol Infect 2012; 45:296–304. [DOI] [PubMed] [Google Scholar]
- 5. Spec A, Raval K, Powderly WG. End-stage liver disease is a strong predictor of early mortality in cryptococcosis. Open Forum Infect Dis 2016; 3:ofv197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc 2013; 124:61–79. [PMC free article] [PubMed] [Google Scholar]
- 7. Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with cryptococcosis according to immune status. PLoS One 2013; 8:e60431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Census. Population and housing unit estimates Available at: https://www.census.gov/programs-surveys/popest.html.Accessed 2 February 2017.
- 9. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 10. Jongwutiwes U, Sungkanuparph S, Kiertiburanakul S. Comparison of clinical features and survival between cryptococcosis in human immunodeficiency virus (HIV)-positive and HIV-negative patients. Jpn J Infect Dis 2008; 61:111–5. [PubMed] [Google Scholar]
- 11. Spec A, Olsen MA, Raval K, Powderly WG. Impact of infectious diseases consultation on mortality of cryptococcal infection in patients without HIV. Clin Infect Dis 2017; 64:558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haddad NE, Powderly WG. The changing face of mycoses in patients with HIV/AIDS. AIDS Read 2001; 11:365–8, 375–8. [PubMed] [Google Scholar]
- 13. Murphy EL, Collier AC, Kalish LA et al. ; Viral Activation Transfusion Study Investigators Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med 2001; 135:17–26. [DOI] [PubMed] [Google Scholar]
- 14. Singh N, Sifri CD, Silveira FP et al. Cryptococcosis in patients with cirrhosis of the liver and posttransplant outcomes. Transplantation 2015; 99:2132–41. [DOI] [PubMed] [Google Scholar]
- 15. Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran’s affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol 2007; 60:397–409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.