Summary
The development of nontoxic broad-spectrum antimicrobial drugs in the mid-20th century led to a culture of empiricism, underdevelopment of diagnostics, neglect of immunotherapy, and selection for drug resistance, which together created the conditions that underlie the current crisis.
In 1996 an article in this journal predicted a crisis in infectious diseases, which subsequently unfolded with a paucity of new drugs, increases in antimicrobial resistance, and underdevelopment in both immunotherapy and diagnostics. This article argues that the root cause of the current-day problems lies in the enormous success of the mid-20th century antibiotic revolution. The availability of low-toxicity broad-spectrum drugs fostered a culture of empiricism and widespread use, which in turn stunted the development of both the specialty and rapid diagnostics while promoting increases in resistance. Two decades later, there are promising signs that widespread recognition of these problems is leading to fundamental changes in the approach to the therapy of infectious diseases. Ultimately, a concerted effort to simultaneously develop pathogen-specific drugs, immunotherapy, and improved diagnostics could provide a qualitatively new platform for confronting the challenge of infectious diseases, which now also includes host dysbiosis.
Keywords: Immunotherapy, microbial resistance, antimicrobial therapy, immunosuppressed hosts.
In the mid-1990s I noted that my ability to treat certain infectious diseases had eroded from a decade earlier, when I was in training. Infectious diseases was the only subspecialty where treatment options could disappear as drugs lost efficacy due to microbial resistance. Whereas cimetidine, propranolol, and countless other drugs were as effective then, and now, as on the day they were introduced, penicillin was less valuable in 1996 than in 1986, when it was still effective for the treatment of pneumococcal pneumonia. Infectious diseases had already acquired the distinction of routinely dealing with new diseases, such as Lyme disease and AIDS. Furthermore, old diseases reemerged, often with drug-resistant microbes. The specialty also had to deal with changing hosts. By the late 20th century, the combination of the human immunodeficiency virus (HIV) epidemic and medical progress had produced an epidemic of immunocompromised hosts, susceptible to so-called opportunistic diseases, in whom antimicrobial therapy was often less effective. The combination of reduced drug efficacy due to resistance, emergence of new diseases and reemergence of old diseases, and the proliferation of immunosuppressed hosts heralded a crisis.
With a sense of angst I wrote “Crisis in Infectious Disease,” which was published in this journal in 1996 [1]. A central argument in that essay was that many of the problems facing the field were an unforeseen consequence of the antibiotic miracle, which had produced effective, low-toxicity broad-spectrum antimicrobial therapy [1]. This in turn had created a culture of empiricism that hobbled the development of diagnostics and fostered resistance through effects on bystander organisms. That article recounted how antimicrobial therapy had evolved from high specificity prior to 1940 when both serum therapy and the few drugs available were pathogen-specific to a situation epitomized by the introduction of imipenem, which was active against almost all pathogenic bacteria when introduced in 1985. “Crisis” also argued for the need to reintroduce immunotherapies in response to the problems of increasing drug resistance and as a means to restore immunity in immunocompromised individuals [1].
The reaction to the article was tepid. Colleagues wrote that there was no crisis, and many expected a new wave of antimicrobial drugs to rescue the situation. The mid-1990s were heady days for the field, which just had witnessed the introduction of effective antiretroviral therapy, a development that transformed the prognosis of patients with AIDS. Furthermore, in the area of antifungal therapy, the 1990s saw the development of new azoles, echinocandins, and lipid formulations of amphotericin B, which greatly improved the therapeutic options for mycotic diseases. At the time there was the feeling that if modern medicine could take on the complexity of a retroviral disease such as AIDS, and transform its prognosis from certain death to a chronic disease, then it could certainly address antimicrobial therapy, an old problem that had been solved decades earlier.
THE AGES OF ANTIMICROBIAL THERAPY
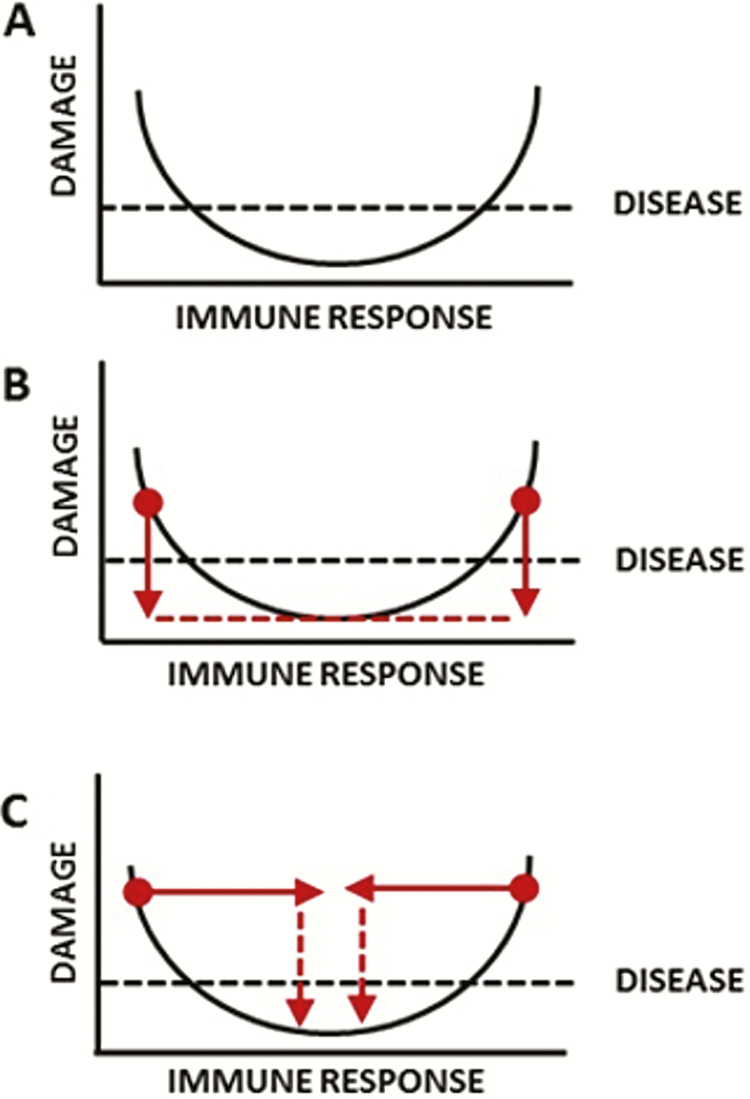
The first age of antimicrobial therapy begins in the 1890s with serum therapy, which involved administration of immune serum to treat infectious diseases [2, 3]. With the development of sulfonamides and β-lactams, the use of serum therapy came to an end because it could not compete with antimicrobial drugs in regard to cost and ease of use. Effective use of pathogen-specific sera required making a microbial diagnosis prior to use, whereas antimicrobial drugs could be used without a specific diagnosis. This greatly simplified therapy but, as described below, it also fostered a culture of empiricism that was to have tremendous consequences for the development and economics of future antimicrobial therapy. This second age is still with us as small-molecule antimicrobial therapeutics remain first-line therapies for most microbial diseases, albeit with reduced overall efficacy due to the emergence of resistance. In the late 20th century, advancements in immunology such as the development of monoclonal antibodies (mAbs) promised a third age defined by the introduction of immunomodulatory drugs into clinical practice [4], but this age was never fully realized. Microbial diseases occur when the host–microbe interaction results in sufficient host damage to impair homeostasis and result in symptoms (Figure 1) [5]. Host damage can come from the microbe, the host, or both. There are 2 major therapeutic approaches to microbial diseases: kill the microbe directly, as is done by antimicrobial drugs, or affect immunity to clear the infection and reduce damage. Currently the field is largely focused on killing the microbe, which reduces damage by reducing microbial burden (y-axis) and largely ignores the therapeutic opportunities available in modifying immunity (x-axis). Viewed through the prism of the “damage-response framework” of microbial pathogenesis [5], the shift from serum to antimicrobial drugs effectively meant that the infectious disease field shifted its focus from the x- to the y-axis.
Figure 1.
Therapy against infectious diseases as viewed through the prism of the “damage response framework” of microbial pathogenesis [5]. A, The basic relationship between a host and a pathogenic microbe is posited to be a parabolic function where damage is a function of the host response. Disease occurs when sufficient damage is accrued from a host–microbe interaction to affect homeostasis, which manifests as clinical symptoms. Disease can occur in settings of both inappropriately weak and exuberant immune responses. B, The administration of antimicrobial drugs meditates a therapeutic effect primarily by reducing microbial burden, which in turn reduces damage, and cure results when the damage is reduced beyond that which affects homeostasis. C, The administration of immunotherapy mediates a therapeutic effect by shifting the position of the immune response to a point where it can reduce damage through reducing the inoculum and/or reducing tissue-damaging inflammatory response. Serum therapy contained antibodies that served both functions through their effects on complement activation, phagocytosis, and regulation of the inflammatory response. Hence, from the viewpoint of the damage-response framework, the shift from serum therapy to antimicrobial drugs was a shift from x- to the y-axis as the mode to control infectious diseases. Today the field of infectious diseases is focused primarily on the y-axis and there are very few immunotherapies available for microbial diseases.
THE DEVELOPMENT OF A CULTURE OF EMPIRICISM
The fact that broad-spectrum antimicrobial drugs could be used effectively without having to make a specific diagnosis prior to therapy was advantageous to individual patients who benefited from earlier and effective therapies. However, this also fostered a neglect on new diagnostics. For example, by the early 1930s a diagnosis of pneumococcal pneumonia and isolate typing could be made in <6 hours by inoculating sputum into the peritoneum of mice and analyzing the exudate [5]. However, by the end of the 20th century a definitive diagnosis of pneumococcal pneumonia required 2 days and was made only if the isolate was recovered in blood cultures. Lack of rapid diagnostics in turn hindered any attempt to develop pathogen-specific therapy, such as antibody-based therapies, once mAb technology became available in the mid-1970s (see below). The widespread use of nonspecific therapy selected for resistance in nontargeted microbes and put pressure for the development of ever-broader classes of drugs. By the time I trained in the 1980s, the approach to the patient with a suspected infectious disease required a series of probability calculations, each with a significant error. For example, in assessing a febrile hospitalized patient, the physician would have to calculate the odds that the fever was originating from a microbial disease, the likely causative microorganism(s), and the antimicrobial susceptibility of likely culprits and then match those probabilities to the activity spectrum of available drugs leading to the selection of the narrowest possible regimen that covered all the bases. This Monte Carlo–like approach carried the inherent contradiction that the best empiric therapy would be that which would cover all likely pathogens using the narrowest regimen as possible. Thus emerged a culture of empiricism that persists to this day.
FIVE SIMULTANEOUS STORMS CREATE A CRISIS
The crisis in infectious diseases emerged from a confluence of 5 developments:
Widespread antimicrobial drug resistance: The pharmaceutical industry consistently developed new drugs from 1950 to 1985, thus maintaining a therapeutic edge despite increasing drug resistance. However, by the late 1980s, resistance was increasing much faster than new drugs were developed.
Increases in number of hosts with impaired immunity: Beginning in the 1950s, advances in medicine led to the development of corticosteroids and the first effective antineoplastic therapies, which came with the price of impaired immunity. Later, organ transplantation became routine and HIV infection became a worldwide epidemic, providing new types of impaired hosts. These individuals were susceptible to infectious diseases rarely seen in immunocompetent hosts and therapy was significantly less effective, which combined with a greater microbial burden, increasing the likelihood of emergence of resistance.
Emergence of new pathogenic microbes: The field of infectious diseases has confronted a steady parade of new pathogenic microbes [6], with at least 335 emergent infectious diseases documented between 1940 and 2006, of which most were zoonosis [7]. The emergence of each new infectious disease creates new challenges that must be confronted with research and clinical efforts, which in turn divert scarce resources from existing problems. For many of these emergent infectious diseases, there was no effective therapy, at least initially.
Reemergence of older pathogenic microbes: The field has also routinely confronted the return of old pathogenic microbes, which often reemerge accompanied by drug resistance. Recent years have seen reemergence of tuberculosis, syphilis, and gonorrhea, as well as occasional outbreaks of vaccine-preventable disease such as measles and pertussis, in individuals who eschew vaccination. The reemergence of old pathogens diverts scarce resources away from existing problems.
A drought on antimicrobial drug development: An influential study published in 2004 noted that only 6 of 506 drugs then in development were antimicrobial drugs [8]. Given the long lag times involved in drug discovery, it was clear by the early 2000s that medicine could not expect a new bounty of antimicrobial drugs to replace those being rendered less effective by increasing drug resistance.
IMMUNOTHERAPY: THE REVOLUTION THAT DID NOT HAPPEN
In recent decades, dozens of mAbs have been developed for treatment of cancer, inflammatory diseases, asthma, etc, and >30 are now approved [9]. However, only 3, palivizumab (respiratory syncytial virus prophylaxis), obiltoxaximab (inhalational anthrax therapy), and bezlotoxumab (prevention of recurrence of Clostridium difficile colitis) are approved for infectious diseases. Considering that antibody-based therapies have established efficacy against many infectious diseases and that there is great need for new therapeutic options, the paucity of mAbs for microbial diseases is perplexing. However, this scarcity is rooted in some of the fundamental problems that have plagued the field, which have created conditions that do not operate in oncology or rheumatology where mAb therapies have blossomed. Antibody-based therapeutics work best when administered early in infection and thus require the availability of early diagnostics, which are not available for many infectious diseases. In addition, the antigenic complexity of many microbes would require the creation of polyclonal reagents in the form of cocktails, which introduce significant expense and regulatory hurdles. Finally, there are already antimicrobial drugs available for most infectious diseases, which creates great challenges for the design of clinical trials, as such reagents much compete with established therapy, which is often much cheaper. In contrast, mAbs in non–infectious disease therapies are tested in an environment where available therapies are highly toxic and/or unsatisfactory and, by targeting host antigens, do not face the problems of antigenic diversity. Hence, the mAb revolution has largely bypassed the treatment of infectious diseases because efforts to develop antibody therapies have been hobbled by a lack of diagnostics, microbial antigenic complexity, higher costs, competition from existing drugs, and the difficulties involved in designing clinical trials that take into account these issues in combination.
THE DYSBIOTIC HOST AND ITS CONSEQUENCES
Antibiotic-associated colitis, thrush, and vaginal candidiasis are well-known complications of antimicrobial therapy. These complications have historically been considered manageable given the potential life-saving effects of nonspecific antimicrobial therapy. However, this benign view of microbiome disruption is being challenged by modern studies, which have raised the possibility that the widespread use of nonspecific antimicrobial therapy has caused dysbiosis in human populations, which is manifesting itself through an increased frequency of other diseases not usually attributed to microbes (Table 1). These associations must be viewed with the critical caveat that causation has yet to be established for any of these relationships. The problem of dysbiosis is potentially an existential challenge to the use of nonspecific antimicrobial therapy, for if a causal association is made between nonspecific antimicrobial therapy and a major chronic disease, it would have a major effect on how these drugs are used, as society is unlikely to tolerate such side effects, especially in children.
Table 1.
Associations Between Antimicrobial Drug Usage and Certain Noninfectious Diseases
| Conditiona | Referenceb |
|---|---|
| Asthma | [32, 33] |
| Atopy | [34] |
| Breast cancer | [35, 36] |
| Cancer (various) | [37] |
| Celiac disease | [38] |
| Colon cancer | [39] |
| Food allergy | [40] |
| Obesity | [41] |
aThis is not a complete list of conditions associated with antimicrobial drug–inferred dysbiosis.
bThese are representative references; for many of these conditions there is an extensive literature.
UNDERDEVELOPMENT OF INFECTIOUS DISEASES
The combination of effective and nontoxic broad-spectrum therapy meant that most physicians felt comfortable treating infectious diseases without consulting a specialist. In 1978, Petersdorf questioned the need for more specialists in the United States, stating, “Even with my great personal loyalty to infectious diseases, I cannot conceive a need for another 309 infectious disease experts unless they spend the time culturing each other” [10]. In 1980, Beeson analyzed the economics of subspecialty disciplines and noted the difficulties facing infectious diseases, stating that “the availability of antimicrobial therapy allows most doctors to treat most infections without asking for help” [11]. By the 1980s a sense of decline and unimportance was evident in articles with such titles as “The bell tolls for the infectious disease clinician” [12] and “Wither infectious diseases: memories, manpower and money” [13]. Although these analyses could not have anticipated the calamity of HIV or widespread antimicrobial resistance, they identified some structural problems in the field that persist today. Infectious disease specialists remain among the lowest-paid specialists and fellowship programs have struggled to fill their rosters with new trainees [14]. Unlike other specialties, no unique procedure was associated with the specialty, and in the United States it never controlled microbiology laboratories. Furthermore, it did not use its expertise to command an important position in the information and decision flows in medicine. Hence, the magisterial domain of infectious disease specialists became largely limited to providing intellectual input in the form of consultations, while other specialties developed expanded by developing procedures and controlling the generation and flow of information in their domains. Despite these headwinds, the discipline has continued to attract highly dedicated physicians who have made tremendous progress against HIV and hepatitis C infections, eradicated several diseases, and routinely confront new infectious diseases, antimicrobial resistance, and the challenges posed by immunocompromised hosts.
ONCOLOGY PROVIDES A CONTROL
Contrast the situation in infectious diseases with oncology, which is the other medical specialty that routinely uses antibiotics such as adriamycin in therapy. Antibiotics with antitumor activity are notoriously toxic and their use became highly specialized, such that nonspecialists would always defer to specialists and thus the specialty of oncology maintained its prominence in cancer therapeutics. The low therapeutic index of antineoplastic antibiotics created the need for precise diagnosis in oncology prior to therapy, and oncology avoided the culture of empiricism that took hold in infectious diseases. The difficulties in treating cancer fostered a continuous commitment to basic and clinical research that today is paying off with numerous antineoplastic agents including immunotherapies, which is in sharp contrast to the dearth of new antimicrobial drugs. Although biological differences in infectious and neoplastic diseases undoubtedly contributed to how the infectious disease and oncology specialties differ, it is intriguing to consider how their development would have differed if antimicrobial and antineoplastic antibiotics had been more and less toxic, respectively.
THE RECOGNITION OF CRISIS
By the turn of the 21st century, the word “crisis” began to be commonly used in publications relating to the field of infectious diseases [15, 16, 17]. The Infectious Diseases Society of America (IDSA) and international societies mobilized to bring attention to the emerging antibiotic problem, which in the United States included attempts to promote legislative solutions to improve antibiotic use and development [15, 16, 17]. Significant progress was made in the development of several drugs for gram-positive pathogenic bacteria. Despite some successes, the paucity of new antimicrobial drugs is embedded into a larger problem of inefficient drug discovery, whereas fewer drugs are being brought to market despite great expenditures in research and development [18-20]. By 2016 the United Nations had taken notice and convened a meeting on the topic of antimicrobial resistance [21], only the fourth time a health-related topic had been a specific focus of a resolution.
A WAY FORWARD
We cannot expect a quick resolution to the crisis that would bring us back to the 1950s, when Jawetz stated that “the position of antimicrobial agents in medical therapy is highly satisfactory” and added that the “majority of bacterial infections can be cured simply, effectively, and cheaply” [22]. Despite efforts to develop new antimicrobial agents, these are unlikely to address the problem of resistance, as such types of drugs are neither in development nor anticipated [23, 24].
For the near horizon, the field must contend with a meager pipeline of new antimicrobial drugs, which means redoubling our efforts to preserve the drugs we have by optimizing therapies, learning how to best use old drugs, enhance antibiotic stewardship, and identify the best combinations that reduce emergence of resistance and shortening therapeutic courses. These efforts must be coupled with outreach to educate the public, engagement of the political establishment to develop better policies for antibiotic use, and identify new business models that incentivize new antimicrobial drug development by the pharmaceutical industry [23]. Initiatives such as “Antibiotic Action” [25] involving alliances between international societies interested in infectious diseases and nonprofit organizations, as well as targeted education efforts [26], could be particularly important for informing the public and creating momentum to find solutions. Given the problems of resistance and the specter of chronic diseases associated with dysbiosis, antimicrobial drug use is likely to encounter increasing restrictions in the future and may become the exclusive purview of the infectious disease specialist, a development that will dramatically increase the importance of the specialty.
For the far horizon, the field needs a strategic plan centered on a triad that involves development of pathogen-specific drugs, improved diagnostics, and immunotherapy. Although nonspecific therapy will always have a niche for the treatment of mixed infections, pathogen-specific drugs are likely to be sufficient for the majority of cases and these would not affect nontargeted microbes, such as the indigenous microbiota. Pathogen-specific drugs would still be vulnerable to the emergence of resistance, but at least they should not select for resistance in bystander microbial populations. Although there have been few true pathogen-specific drugs, the efficacy of some such as isoniazid means that these are potentially feasible. The use of pathogen-specific drugs would require development in diagnostics to provide rapid information, which would also improve medical care. It is noteworthy that tremendous advances are already being made in diagnostic science, which improve current antimicrobial use and create the necessary conditions for development of pathogen-specific therapies. Furthermore, new economic models will have to be developed as the market of an antimicrobial drug is proportional to its spectrum and these drugs are not as attractive to industry [27]. In this regard, highly innovative solutions to the unfavorable economics of antimicrobial drug development have been proposed [28], which could also promote the development of pathogen-specific drugs. Microbial virulence remains an attractive target for drug discovery as it is less likely to select for resistance [29, 30], but this approach is usually pathogen-specific and faces the unfavorable economics of narrow specificity and requires rapid diagnostics. Finally, the field must embrace the x-axis and learn how to effect beneficial therapeutic outcomes by enhancing or diminishing immune responses (Figure 1).
The specialty of infectious diseases will always retain a critical importance in modern medicine given that its success is essential for the success of other specialties, such as surgery. The specialty is likely to thrive in the 21st century, for the storms that led to the current crisis also create the opportunities to develop new and more effective approaches to the treatment of infectious diseases. Far and near horizon developments will lead to a rapid rise in the importance of the infectious disease consultant, since their integration would require expertise not available to the nonspecialist, which would usher in a renaissance in the field. In this regard, the complexity in caring for HIV-infected individuals provides another control where the complexity of dozens of antiretroviral drugs has led to a vibrant subspecialty within the infectious disease specialty. Complexity demands expertise, which nourishes the specialty.
The conditions that have led to the crisis are so intertwined that that these cannot be solved piecemeal. Furthermore, the problems are also international as pathogenic microbes do not respect national borders. What is needed is a supranational entity with expertise in science, medicine politics, economics, industry, and sociology and sufficient staying power to chart a long-distance course. Precedents for successful international collaboration exist as evident in the successful campaigns to eradicate smallpox, limit fluorocarbon emissions that damaged the ozone layer, and save threatened species. In this regard, the recognition of the antimicrobial resistance problem in 2016 by the United Nations is potentially a landmark event, for it heralds international awareness of the problem [21]. Irrespective of future advances, the human struggle against infectious disease is likely to be eternal, for as long as there are human hosts and microbes, some of their interactions will result in disease [31]. Hence, humanity must create structures that are vigilant, resilient, and versatile that can adapt and respond to an ever-changing landscape in human–microbial interactions.
Notes
Financial support. The author is supported by the National Institutes of Health (grant numbers R37 AI033142, AI052733, and HL059842 17).
Potential conflicts of interest. Author certifies no potential conflicts of interest. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Casadevall A. Crisis in infectious diseases: time for a new paradigm? Clin Infect Dis 1996; 23:790–4. [DOI] [PubMed] [Google Scholar]
- 2. Casadevall A, Scharff MD. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother 1994; 38:1695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casadevall A, Scharff MD. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis 1995; 21:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casadevall A. The third age of antimicrobial therapy. Clin Infect Dis 2006; 42:1414–6. [DOI] [PubMed] [Google Scholar]
- 5. Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol 2003; 1:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morens DM, Folkers GK, Fauci AS. Emerging infections: a perpetual challenge. Lancet Infect Dis 2008; 8:710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature 2008; 451:990–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spellberg B, Powers JH, Brass EP, Miller LG, Edwards JE., Jr Trends in antimicrobial drug development: implications for the future. Clin Infect Dis 2004; 38:1279–86. [DOI] [PubMed] [Google Scholar]
- 9. Reichert JM. Marketed therapeutic antibodies compendium. MAbs 2012; 4:413–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petersdorf RG. The doctors’ dilemma. N Engl J Med 1978; 299:628–34. [DOI] [PubMed] [Google Scholar]
- 11. Beeson PB. The natural history of medical subspecialties. Ann Intern Med 1980; 93:624–6. [DOI] [PubMed] [Google Scholar]
- 12. Ervin FR. The bell tolls for the infectious diseases clinician. J Infect Dis 1986; 153:183–8. [DOI] [PubMed] [Google Scholar]
- 13. Petersdorf RG. Whither infectious diseases? Memories, manpower, and money. J Infect Dis 1986; 153:189–95. [DOI] [PubMed] [Google Scholar]
- 14. Chandrasekar P, Havlichek D, Johnson LB. Infectious diseases subspecialty: declining demand challenges and opportunities. Clin Infect Dis 2014; 59:1593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spellberg B, Guidos R, Gilbert D, et al. ; Infectious Diseases Society of America The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 2008; 46:155–64. [DOI] [PubMed] [Google Scholar]
- 16. Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1–12. [DOI] [PubMed] [Google Scholar]
- 17. Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG. Antimicrobial Availability Task Force of the Infectious Diseases Society of America Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 2006; 42:657–68. [DOI] [PubMed] [Google Scholar]
- 18. Bowen A, Casadevall A. Increasing disparities between resource inputs and outcome, as measured by certain health deliverables, in biomedical research. Proc Natl Acad Sci U S A 2015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scannell JW, Bosley J. When quality beats quantity: decision theory, drug discovery, and the reproducibility crisis. PLoS One 2016; 11:e0147215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 2012; 11:191–200. [DOI] [PubMed] [Google Scholar]
- 21. United Nations meeting on antimicrobial resistance. Bull World Health Organ; 2016; 94:638–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jawetz E. Antimicrobial chemotherapy. Annu Rev Microbiol 1956; 10:85–114. [DOI] [PubMed] [Google Scholar]
- 23. Theuretzbacher U. Resistance drives antibacterial drug development. Curr Opin Pharmacol 2011; 11:433–8. [DOI] [PubMed] [Google Scholar]
- 24. Högberg LD, Heddini A, Cars O. The global need for effective antibiotics: challenges and recent advances. Trends Pharmacol Sci 2010; 31:509–15. [DOI] [PubMed] [Google Scholar]
- 25. Piddock LJ. The crisis of no new antibiotics—what is the way forward? Lancet Infect Dis 2012; 12:249–53. [DOI] [PubMed] [Google Scholar]
- 26. Hoskisson PA, Aldridge P, Bowater L. Inspiring STEM undergraduates to tackle the AMR crisis. FEMS Microbiol Lett 2015; 362:fnv138. [DOI] [PubMed] [Google Scholar]
- 27. Casadevall A. The case for pathogen-specific therapy. Expert Opin Pharmacother 2009; 10:1699–703. [DOI] [PubMed] [Google Scholar]
- 28. Rex JH, Outterson K. Antibiotic reimbursement in a model delinked from sales: a benchmark-based worldwide approach. Lancet Infect Dis 2016; 16:500–5. [DOI] [PubMed] [Google Scholar]
- 29. Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol 2007; 3:541–8. [DOI] [PubMed] [Google Scholar]
- 30. Alksne LE, Projan SJ. Bacterial virulence as a target for antimicrobial chemotherapy. Curr Opin Biotechnol 2000; 11:625–36. [DOI] [PubMed] [Google Scholar]
- 31. Fauci AS, Morens DM. The perpetual challenge of infectious diseases. N Engl J Med 2012; 366:454–61. [DOI] [PubMed] [Google Scholar]
- 32. Pitter G, Ludvigsson JF, Romor P, et al. Antibiotic exposure in the first year of life and later treated asthma, a population based birth cohort study of 143,000 children. Eur J Epidemiol 2016; 31:85–94. [DOI] [PubMed] [Google Scholar]
- 33. Risnes KR, Belanger K, Murk W, Bracken MB. Antibiotic exposure by 6 months and asthma and allergy at 6 years: findings in a cohort of 1,401 US children. Am J Epidemiol 2011; 173:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kusel MM, de Klerk N, Holt PG, Sly PD. Antibiotic use in the first year of life and risk of atopic disease in early childhood. Clin Exp Allergy 2008; 38:1921–8. [DOI] [PubMed] [Google Scholar]
- 35. Sergentanis TN, Zagouri F, Zografos GC. Is antibiotic use a risk factor for breast cancer? A meta-analysis. Pharmacoepidemiol Drug Saf 2010; 19:1101–7. [DOI] [PubMed] [Google Scholar]
- 36. Velicer CM, Heckbert SR, Lampe JW, Potter JD, Robertson CA, Taplin SH. Antibiotic use in relation to the risk of breast cancer. JAMA 2004; 291:827–35. [DOI] [PubMed] [Google Scholar]
- 37. Boursi B, Mamtani R, Haynes K, Yang YX. Recurrent antibiotic exposure may promote cancer formation—another step in understanding the role of the human microbiota? Eur J Cancer 2015; 51:2655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mårild K, Ye W, Lebwohl B, et al. Antibiotic exposure and the development of coeliac disease: a nationwide case-control study. BMC Gastroenterol 2013; 13:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boursi B, Haynes K, Mamtani R, Yang YX. Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiol Drug Saf 2015; 24:534–42. [DOI] [PubMed] [Google Scholar]
- 40. Rachid R, Chatila TA. The role of the gut microbiota in food allergy. Curr Opin Pediatr 2016; 28:748–53. [DOI] [PubMed] [Google Scholar]
- 41. Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nat Rev Endocrinol 2015; 11:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]