Summary
We present long-term follow-up from a randomized trial of switching clinically stable HIV-infected children to efavirenz-based therapy, once 3 years of age or older, after initially suppressing on ritonavir-boosted lopinavir. All children had exposure to nevirapine for prevention of mother-to-child transmission.
Keywords: HIV infections, antiretroviral therapy, efavirenz, lopinavir-ritonavir, pediatrics.
Abstract
Background.
We previously demonstrated the noninferiority of switching to efavirenz (EFV) versus remaining on ritonavir-boosted lopinavir (LPV/r) for virologic control in children infected with human immunodeficiency virus (HIV) and exposed to nevirapine (NVP) for prevention of mother-to-child transmission. Here we assess outcomes up to 4 years post-randomization.
Methods.
From 2010–2013, 298 NVP-exposed HIV-infected children ≥3 years of age were randomized to switch to EFV or remain on LPV/r in Johannesburg, South Africa (Clinicaltrials.gov NCT01146873). After trial completion, participants were invited to enroll into observational follow-up. We compared HIV RNA levels, CD4 counts and percentages, lipids, and growth across groups through four years post-randomization.
Results.
HIV RNA levels 51–1000 copies/mL were less frequently observed in the EFV group than the LPV/r group (odds ratio [OR] 0.67, 95% confidence interval [CI]: 0.51–0.88, P = .004), as was HIV RNA >1000 copies/mL (OR 0.52 95% CI: 0.28–0.98, P = .04). The probability of confirmed HIV RNA >1000 copies/mL by 48 months was 0.07 and 0.12 in the EFV and LPV/r groups, respectively (P = .21). Children randomized to EFV had a reduced risk of elevated total cholesterol (OR 0.45 95% CI: 0.27–0.75, P = .002) and a reduced risk of abnormal triglycerides (OR 0.42, 95% CI 0.29–0.62, P < .001).
Conclusions.
Our results indicate that the benefits of switching virologically suppressed NVP-exposed HIV-infected children ≥3 years of age from LPV/r to EFV are sustained long-term. This approach has several advantages, including improved palatability, reduced metabolic toxicity, simplified cotreatment for tuberculosis, and preservation of second line options.
Clinical Trials Registration.
The World Health Organization (WHO) now recommends antiretroviral treatment (ART) for all children and adults infected with human immunodeficiency virus (HIV), regardless of clinical disease stage [1]. Pediatric ART access has expanded considerably in recent years, though only half of 1.8 million HIV-infected children worldwide were on treatment in 2015 [2]. Enhanced efforts towards earlier diagnosis may improve pediatric ART uptake [3], increasing the number of young children initiating lifelong ART. Treatment options that address the spectrum of pediatric health and development are needed.
WHO recommends a ritonavir-boosted lopinavir (LPV/r)-based regimen for treatment initiation <3 years of age, and an efavirenz (EFV)-based regimen for initiation at ≥3 years [1]. LPV/r was initially recommended for young children exposed to nevirapine (NVP) for prevention of mother-to-child transmission (PMTCT) [4] due to a high probability of NVP resistance [5]. Now evidence of superior virologic control with LPV/r in children with and without NVP exposure supports this strategy regardless of PMTCT [6]. However, long-term LPV/r use in children raises several concerns, including poor palatability, interactions with tuberculosis treatment, and potential metabolic complications. For young children initiated on LPV/r, WHO now recommends consideration of switching to EFV at 3 years of age when viral load monitoring is available [1].
Prior to this recommendation, we conducted a noninferiority randomized trial to evaluate this strategy. Perinatally HIV-infected children ≥3 years exposed to NVP for PMTCT and suppressed on LPV/r were randomized to remain on LPV/r or switch to EFV. At 48 weeks of follow-up, we found no evidence of compromised virologic control in children who switched to EFV [7]. Additionally, we observed improved lipid profiles and higher CD4 percentages in children who switched. Evidence of long-term safety and efficacy of this strategy is needed to alleviate concerns around switching clinically stable children to a non-nucleoside reverse transcriptase inhibitor (NNRTI) after prior exposure to NVP. Here we present outcomes through 4 years post-randomization in this trial.
METHODS
Study Population
Between June 2010 and December 2013, 300 perinatally HIV-infected children participated in a randomized, open-label noninferiority trial of switching to EFV at Rahima Moosa Mother and Child Hospital in Johannesburg, South Africa (Clinicaltrials.gov NCT01146873) [7]. Eligibility requirements included: age ≥3 years, exposure to NVP for PMTCT, initiation of LPV/r-based therapy <3 years of age continued for ≥1 year, and plasma HIV RNA <50 copies/mL. Children were randomized to switch to EFV or remain on LPV/r and followed for 48 weeks.
Following trial completion, participants were invited into a prospective observational study at the same site. The enrollment period was February 2013 to May 2014 and follow-up is ongoing. Mothers or legal guardians provided written informed consent separately for the clinical trial and the observational study. Written assent was obtained in the observational study from children ≥7 years of age capable of understanding the assent form. Both studies were approved by Institutional Review Boards at Columbia University and the University of Witwatersrand. This analysis includes follow-up through December 2015.
Study Procedures
Clinical trial procedures have been described [7]. Briefly, children attended visits at 4, 8, 16, 24, 32, 40, and 48 weeks following randomization. Weight and height were measured, a detailed clinical exam was done, and antiretroviral medications were prescribed and dispensed with dose adjustments according to growth. EFV was prescribed once-daily in capsules. LPV/r was prescribed twice-daily in syrup or tablets for children able to swallow tablets. Unused medication was returned at each visit for adherence reconciliation. Plasma HIV RNA levels (AmpliPrep/COBAS® TaqMan® HIV-1 Test, v2.0, Roche) were measured at baseline, 4, 8, 16, 24, and 48 weeks, and CD4 counts and percentages were measured at baseline, 24 and 48 weeks. A fasting lipid panel was done at baseline and 40 weeks.
Following the clinical trial period, observational study visits were every 6 months and included a clinical exam and weight and height measurements. The study site did not serve as the child’s HIV treatment clinic, and antiretroviral medications were prescribed elsewhere. Data on regimens and switches were collected by caregiver report, review of patient records, and communication with the treatment clinic. At annual visits, plasma HIV RNA levels (ABBOTT RealTime), CD4 counts and percentages, and lipid panels were conducted by BARC South Africa, an independent clinical trials laboratory (part of a global network of laboratories) contracted for this study. These annual tests were scheduled to ensure HIV RNA and CD4 measurements were done at least every 6 months inclusive of those done in routine care. Routine HIV RNA (AmpliPrep/COBAS® TaqMan® HIV-1 Test v2.0 or Cobas 6800/8800, Roche) and CD4 measurements were conducted by National Health Laboratory Service (NHLS); results were obtained from records.
Routine care visits were generally scheduled every 3 months. For children receiving care at Rahima Moosa, study visits were scheduled on the same day as routine visits when feasible.
Statistical Analysis
As the noninferiority trial is complete and reported, for this analysis incorporating the subsequent observational study period we followed conventional superiority analyses for ease of interpretation. Primary analyses were intention-to-treat, that is, comparing children as randomized in the original trial. Per-protocol analyses, censoring all visits after children switched treatment regimen away from their randomized assignment, were also done. We assessed factors associated with nonenrollment in the observational study using multivariable logistic regression. The cumulative probability of switching treatment regimens from the randomized assignment was estimated with the Kaplan-Meier method.
The primary outcome was elevated plasma HIV RNA, which we evaluated with two approaches. First, the frequency of HIV RNA 51–1000 copies/mL (viral rebound) and >1000 copies/mL over time in each group was compared using generalized estimating equations (GEE). Second, time to virologic failure (HIV RNA >1000 copies/mL confirmed) was compared with Kaplan-Meier and the log-rank test. All HIV RNA tests regardless of the laboratory where they were run were included in the analyses. During the clinical trial, any HIV RNA >1000 copies/mL prompted recall of the child for repeat testing. During the observational study, the schedule of repeat testing was at the discretion of the treating physician. We retained the definition of virologic failure as two HIV RNA tests >1000 copies/mL regardless of the time between samples and did not require consecutive results. This maintained consistency with the definition during the clinical trial and minimized potential misclassification when defining failure on the basis of an isolated measurement. Plasma samples from children with virologic failure were tested for drug resistance at the National Institute for Communicable Diseases [8]. HIV RNA database entries “below detectable limits” were assumed to be <50 copies/mL. Among these, ~10% of records were reviewed and of these >90% indicated the lower limit was <20 or <40 copies/mL.
CD4 counts and percentages, abnormal and elevated lipids [9], and growth parameters were compared over time with GEE. Growth parameters included WHO weight-for-age, height-for-age, and body mass index (weight [kg]/height [meters] squared)-for-age Z-scores [10]. Assessments of lipids and growth were limited to those measured at study visits. Analyses were conducted in SAS 9.4 (SAS Institute, Cary NC).
RESULTS
Study Population and Follow-up
Of 300 children enrolled in the clinical trial, 148 were randomized to continue LPV/r, 150 were randomized to switch to EFV, and 2 discontinued prior to randomization. As previously reported, baseline sociodemographic and clinical characteristics were comparable across groups [7]. Median age at randomization was 4.1 years (interquartile range [IQR] 3.5–4.8), and 47% were male. Children initiated ART at a median age of 6.8 months (IQR 4.0–13.2) and 7% had a CD4 percentage <25.
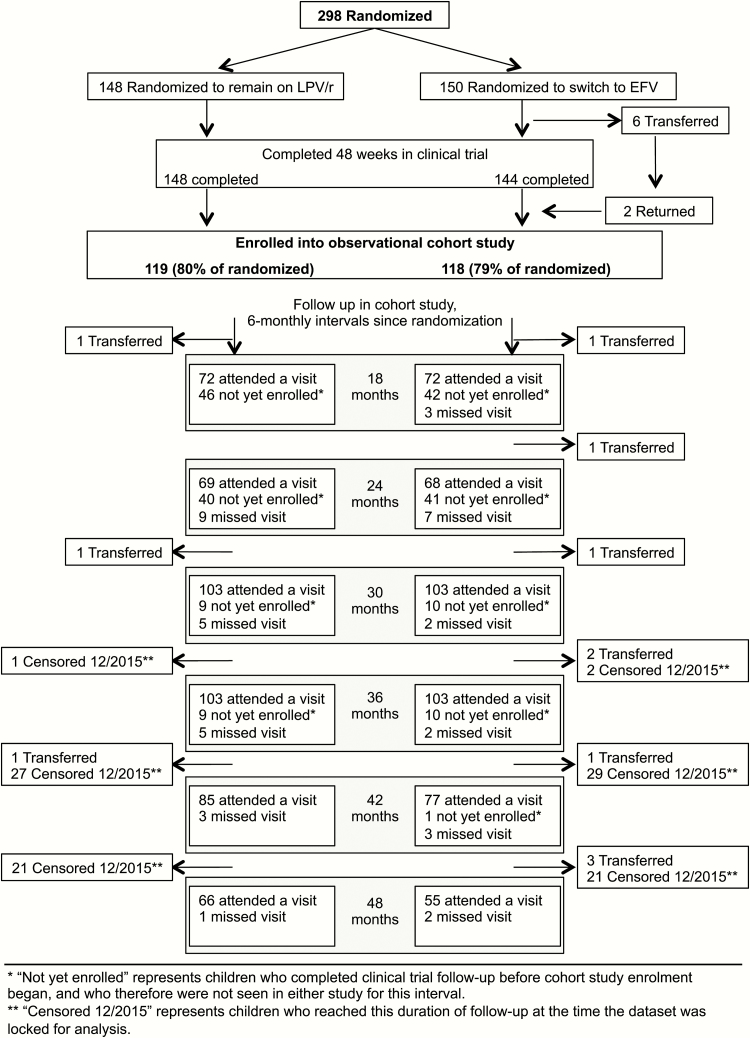
Among children randomized to remain on LPV/r, 119 (80%) enrolled into long-term follow-up, and 118 (78%) in the EFV group enrolled. In multivariable analysis, the primary factor associated with nonenrollment was a longer time between the last trial visit and observational study start (odds ratio [OR] 0.90, 95% confidence interval [CI] 0.86–0.95, per month, P < .001). Additionally, children with a baseline CD4 percentage <25 in the clinical trial were less likely to enroll (OR 0.16, 95% CI 0.05–0.49, P = .001) (Supplementary Table 1). Among children enrolled into long-term follow-up, characteristics at the time of randomization remained comparable across groups (Table 1). The median time between the last clinical trial visit and enrollment into the observational study was 7 months, and the median follow-up time from randomization through December 2015 was 46 months. By the end of 2015, among the 298 children randomized in the clinical trial, 116 (78%) in the LPV/r group and 109 (73%) in the EFV group were retained. Follow-up detail is shown in Figure 1.
Table 1.
Characteristics of Clinical Trial Participants Who Subsequently Enrolled Into Long-term Follow-up, by Randomization Group
| LPV/r, N = 119 | EFV, N = 118 | |
|---|---|---|
| n ( %) or Median (IQR) | ||
| Demographic characteristics at the time of randomization | ||
| Child’s sex | ||
| Male | 56 (47.1) | 61 (51.7) |
| Female | 63 (52.9) | 57 (48.3) |
| Child’s age at randomization, years | 3.9 (3.4, 4.7) | 4.0 (3.5, 4.6) |
| Caregiver education, highest grade | ||
| Any primary (1–7) | 8 (6.7) | 11 (9.5) |
| Any high school (8–11) | 63 (52.9) | 48 (41.4) |
| Completed high school | 48 (40.3) | 57 (49.1) |
| Water tap in home | ||
| Yes | 59 (49.6) | 65 (55.6) |
| No | 60 (50.4) | 52 (44.4) |
| Child has gone hungry | ||
| Yes | 14 (11.8) | 20 (17.1) |
| No | 105 (88.2) | 97 (82.9) |
| Clinical characteristics at the time of randomization | ||
| Age at ART initiation, months | 7.0 (3.7, 14.2) | 5.7 (3.7, 11.7) |
| CD4 percent | ||
| <25 | 5 (4.5) | 5 (4.6) |
| ≥25 | 105 (95.5) | 103 (95.4) |
| Weight for age Z-score | −0.8 (−1.6, −0.4) | −0.9 (−1.4, −0.2) |
| Height for age Z-score | −1.5 (−2.0, −0.5) | −1.4 (−2.0, −0.9) |
| BMI for age Z-score | −0.1 (−0.6, 0.6) | 0.0 (−0.7, 1.0) |
| Clinical characteristics at final (week 48) clinical trial visit | ||
| CD4 percent | ||
| <25 | 8 (7.0) | 3 (2.8) |
| ≥25 | 106 (93.0) | 106 (97.2) |
| Plasma HIV RNA, copies/mL | ||
| ≤50 | 104 (88.1) | 110 (95.7) |
| >50 | 14 (11.9) | 5 (4.3) |
| Weight for age Z-score | −0.8 (−1.6, −0.3) | −0.8 (−1.4, −0.2) |
| Height for age Z-score | −1.3 (−1.9, −0.5) | −1.4 (−2.0, −0.8) |
| BMI for age Z-score | −0.0 (−0.8, 0.6) | 0.1 (−0.6, 1.0) |
| Follow-up time | ||
| Months between last clinical trial visit and observational study enrollment visit | 7.1 (2.1, 17.8) | 7.4 (1.9, 18.7) |
| Months from randomization to last follow-up visit by 31 December 2015 | 47.0 (38.2, 48.0) | 44.5 (36.9, 48.0) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; EFV, efavirenz; HIV, human immunodeficiency virus; IQR, interquartile range; LPV/r, ritonavir boosted lopinavir.
Figure 1.
Flow of follow-up from randomization in clinical trial through observational study visits. Abbreviations: EFV, efavirenz; LPV/r, ritonavir-boosted lopinavir.
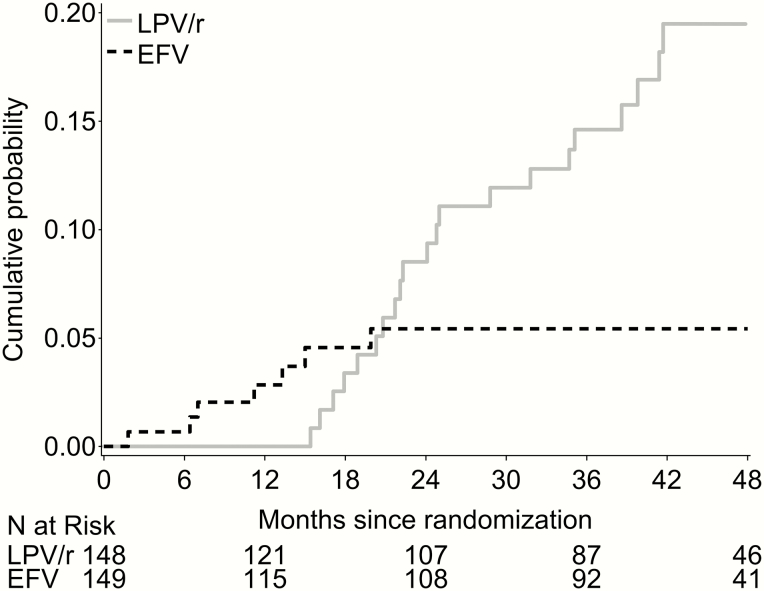
In the EFV group, 4 children switched back to LPV/r by 1 year (probability 0.03, 95% CI 0.01–0.07) and 3 switched back during the second year (probability 0.05, 95% 0.03–0.11); no further switching was observed. Of these 7, 2 experienced seizures, 2 had virologic failure, and the reason for switching was unknown for 3 (Figure 2).
Figure 2.
Cumulative probability of switching treatment regimen away from randomized assignment through 48 months. The solid gray line shows the probability of switching to EFV among children randomized to LPV/r; the dashed black line shows the probability of switching to LPV/r among children randomized to EFV. Abbreviations: EFV, efavirenz; LPV/r, ritonavir-boosted lopinavir.
In the LPV/r group, none switched to EFV during the trial. Thereafter, the cumulative number (and probability [95% CI]) who switched by the end of year 2, 3, and 4 was 10 (0.09 [0.05–0.15]), 17 (0.15 [0.09–0.22]), and 23 (0.22 [0.15–0.32]), respectively (Figure 2). Reasons for switching were lipid toxicity (n = 7), regimen simplification (n = 6), intolerable taste (n = 3), supply issues (n = 2), and 5 were unknown.
Virologic Outcomes
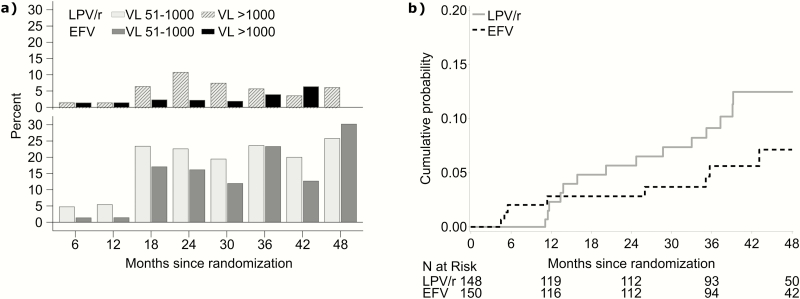
The odds of viral rebound (51–1000 copies/mL) through 48 months were lower in children randomized to EFV than in children randomized to remain on LPV/r (OR 0.67, 95% CI 0.51–0.88, P = .004) as were the odds of any HIV RNA >1000 copies/mL (OR 0.52, 95% CI 0.28–0.98, P = .04) (Figure 3a). By 48 months, virologic failure (>1000 copies/mL confirmed) was observed in 14 children randomized to LPV/r (probability 0.12, 95% CI 0.08–0.20) and 8 randomized to EFV (probability 0.07, 95% CI 0.04–0.14, P = .21) (Figure 3b). Per-protocol results were comparable.
Figure 3.
Elevated plasma HIV RNA by randomized assignment over time, represented by A, the frequency of HIV RNA 51–1000 and >1000 copies/mL at 6-monthly intervals, and B, the cumulative probability of virologic failure (HIV RNA >1000 copies/mL confirmed) up to 48 months. Abbreviation: EFV, efavirenz; LPV/r, ritonavir-boosted lopinavir; VL, viral load.
Resistance test results for children with virologic failure are displayed in Table 2. No children had mutations conferring resistance to protease inhibitors. Four who failed in the EFV group had detectable nucleoside reverse transcriptase inhibitor (NRTI) resistance (primarily M184V/I) and NNRTI resistance (primarily K103N): 2 switched back to LPV/r and resuppressed, 1 switched to lamivudine monotherapy and transferred out, and 1 failed to resuppress in follow-up. Two children randomized to EFV who failed and did not have resistance testing resuppressed on EFV.
Table 2.
Summary of Children With Virologic Failures by Group, Ordered by Time From Randomization to First Measured Plasma HIV RNA >1000 copies/mL
| Sex | First VL >1000 Copies/mL | Second VL >1000 Copies/mL | Months Since Randomization at First VL >1000 Copies/mL | Months Since Randomization at Second VL >1000 Copies/mL | Regimen Switches | Suppressed Between First and Second VL >1000 Copies/mL | Suppressed After 2nd VL>1000 Copies/mL | Resistance Testing Concurrent With First or Second VL >1000 Copies/mL | NRTI Mutationsa | NNRTI Mutationsa |
|---|---|---|---|---|---|---|---|---|---|---|
| Ritonavir-boosted lopinavir group | ||||||||||
| M | 72000 | 58996 | 1.2 | 20.2 | … | Yes | No | Second (first did not amplify) | Wild-type | Wild-type |
| M | 5770 | 2220 | 1.8 | 15.9 | … | Yes | Yes | First | M184V | Wild-type |
| M | 4070 | 7974 | 3.6 | 13.8 | … | Yes | No | First | Wild-type | Wild-type |
| Fb | 1925 | 77181 | 5.5 | 11.1 | … | Yes | Yes | First | M184V | Wild-type |
| M | 10015 | 5580 | 11.2 | 11.5 | Switched to EFV, 25 mo (reason unknown, post re-suppression on LPV/r) | No | Yes | First | Wild-type | Wild-type |
| Mb | 1370 | 2710 | 11.5 | 13.4 | … | No | Yes | First | M184V | Wild-type |
| Mb | 744682 | 24749 | 11.5 | 11.7 | … | No | Yes | First | Wild-type | E138Ac |
| F | 6634 | 3650 | 14.4 | 33 | … | No | Yes | Second, did not amplify (no storage first) | … | … |
| M | 154353 | 8805 | 18.9 | 24.7 | Switched to EFV, 18.9 mo (due to supply, prior to receiving first VL>1000 results); switched back to LPV/r at 35 mo. | No | No | Second (no storage first) | M184V | K103N |
| M | 3309 | 2055 | 23.2 | 28.7 | … | No | Yes | First | M41L, M184V | Wild-type |
| F | 7091 | 1035 | 25.6 | 39.1 | … | Yes | Yes | No sample available | … | … |
| F | 13255 | 15058 | 26.4 | 39.2 | … | No | No | No sample available | … | … |
| F | 4724 | 2329 | 32 | 35.2 | … | No | Yes | Second, did not amplify (first from records) | … | … |
| M | 1685 | 56211 | 32.3 | 37.3 | Switched to EFV, 22 mo (reason unknown, prior to first VL >1000) | No | No | First | M184V | K103N |
| Efavirenz group | ||||||||||
| F | 8606 | 4824 | 2.3 | 26 | … | Yes | Yes | First | Wild-type | Wild-type |
| Mb | 1025 | 1330 | 3.4 | 5 | Switched to LPV/r, 7 mo | No | Yes | First | M184V | K103N |
| Mb | 12785 | 10100 | 3.5 | 4.5 | Switched to LPV/r, 6 mo | No | Yes | First | M184I, N348I | K103N, E138Ac, N348I |
| Fb | 16120 | 4155 | 3.8 | 5.5 | Switched to 3TC alone, 8 mo, transferred out, 10 mo | No | No | First | M184V | K103N |
| Fb | 88390 | 225430 | 11.3 | 11.4 | Interruption, 11 mo, restarted EFV | No | Yes | First | Wild-type | Wild-type |
| F | 5463 | 33985 | 18.8 | 43.1 | … | Yes | Yes | No sample available | … | … |
| F | 37443 | 18152 | 27.9 | 35.7 | … | Yes | Yes | Second, did not amplify (first from records) | … | … |
| F | 30001 | 92858 | 28.3 | 35.1 | … | No | No | Second (first from records) | L74V, M184V | K103N |
Abbreviations: 3TC, Lamivudine; EFV, efavirenz; LPV/r, ritonavir-boosted lopinavir; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; VL, viral load.
aNo protease inhibitor mutations were found.
bPreviously reported failure during clinical trial.
cE138A is a polymorphic mutation associated with resistance to second generation NNRTIs.
Among children randomized to remain on LPV/r with virologic failure, 3 had NRTI resistance (M184V, M41L) and resuppressed. Two had switched to EFV prior to failure, both had detectable NRTI (M184V) and NNRTI (K103N) resistance: 1 switched due to supply issues and viral loads remained high over 1 year, then switched back to LPV/r at the last observed visit; the other switched to EFV for unknown reasons and viral loads remained between 162 and 742 copies/mL. Among the 4 without resistance testing, three resuppressed and subsequent viral loads for 1 are unknown.
Secondary Outcomes
Across all visits, CD4 percentages were 2.1 points higher on average in the EFV group compared to the LPV/r group (95% CI 0.7–3.5, P = .003) (Supplementary Figure 1). CD4 counts did not differ by group, nor did the proportion with any immunosuppression (CD4 percentage <25 if <5 years of age or CD4 count <500 if ≥5 years [11]).
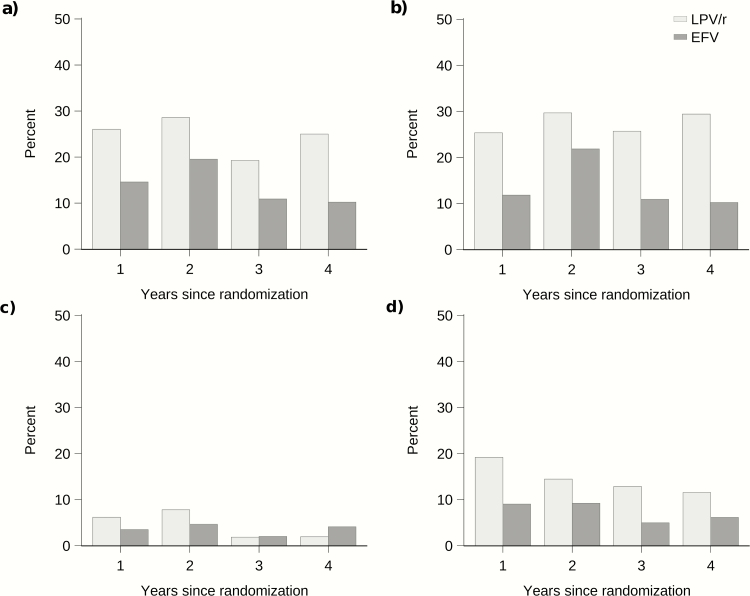
We observed a reduced risk of elevated total cholesterol in the EFV group compared to the LPV/r group (OR 0.45, 95% CI: 0.27–0.75, P = .002), as well as a reduced risk of abnormal triglycerides (OR 0.42, 95% CI 0.29–0.62, P < .001) and a reduced risk of elevated low-density lipoprotein (OR 0.45, 95% CI 0.24–0.84, P = .01) (Figure 4). Abnormal high-density lipoprotein was less frequently observed in the EFV group, though not statistically significant (OR 0.55, 95% CI 0.25–1.20, P = .13). Weight-for-age, height-for-age, and body mass index-for-age Z-scores did not differ across groups. Per-protocol results of secondary outcomes were comparable.
Figure 4.
Percent of children with abnormal or elevated lipids by group at annual intervals. A, Elevated total cholesterol (>=5.2 mmol/L), B, abnormal triglycerides (>1.69 mmol/L), C, abnormal high-density lipoprotein (<0.9 mmol/L), and D, elevated low-density lipoprotein (>=3.4 mmol/L). Abbreviations: EFV, efavirenz; LPV/r, ritonavir-boosted lopinavir.
Adherence based on medication reconciliation did not differ by group during the clinical trial though medicine containers were left at home more frequently in the LPV/r group [7]. During the observational study, caregivers reported that the child “ever” missed a dose since the last visit at 8% of visits in the EFV group and at 13% of visits in the LPV/r group (OR 0.63, 95% CI 0.40–0.97, P = .04).
DISCUSSION
Among HIV-infected children exposed to NVP for PMTCT and virally suppressed on LPV/r, we observed comparable virologic control (<1000 copies/mL) in children randomized to switch to EFV versus those randomized to remain on LPV/r through 4 years of follow-up. Importantly, children who switched to EFV experienced viral rebound (51–1000 copies/mL) less frequently than children who remained on LPV/r and had improved lipid profiles and somewhat higher CD4 percentages. Our results demonstrate that the benefits of switching to EFV reported through 48 weeks in the clinical trial [7] were sustained through 4 years. We observed clear benefits for reductions in lipid abnormalities. In this same study population, we have also reported improved bone mineral content in children who switched to EFV compared to those who remained on LPV/r [12]. Some trials have observed lower weight in children initiating LPV/r compared to NNRTI-based regimens [6, 13–15]. We did not observe this difference, similar to a trial from Uganda [16]. Notably, differences observed have been small [14] and may diminish over time [15].
In long-term follow-up, we found superior caretaker-reported adherence in children switched to EFV. In the clinical trial, medicine reconciliation, a more robust measure of adherence, did not differ by group, though containers were not returned more frequently in the LPV/r group [7]. Despite limitations of self-reported adherence, these observations are consistent with the poor taste of LPV/r and more frequent viremia seen in the LPV/r group. Sustained adherence in young children is a formidable challenge and even small improvements gained with a regimen change could lead to better outcomes over the long term. Notably, in both groups, few children experienced virologic failure. This cohort of young children remained highly adherent after transitioning from close clinical trial monitoring to standard clinical care, indicating that families can maintain the long-term commitment to supporting adherence in children. Simplified regimens can further support adherence [17, 18]; we hypothesize that once-daily EFV provided an adherence advantage over twice-daily LPV/r in our study.
We were surprised by the relatively low uptake of the switch strategy in the cohort after the trial ended. WHO guidelines currently recommend consideration of switching to EFV at age ≥3 years in clinically stable children [1], however South African guidelines do not yet recommend switching [19]. The limited uptake may reflect the relative recency of the publication of our trial results or concerns about the long-term viral efficacy of the strategy. These results through 4 years of follow-up, together with increasingly available viral load monitoring, may offer reassurance to clinicians considering a regimen switch.
In terms of limitations of EFV, 2 children developed seizures in our study, underscoring the importance of close clinical monitoring. Both had high plasma EFV concentrations, and polymorphisms in cytochrome P450 (CYP) 2B6 associated with reduced metabolism of EFV [20]. EFV dosing was consistent with South African guidelines [21]. Another potential concern with EFV is the lower genetic barrier to resistance compared to LPV/r [22]. We are reassured that virologic failures were uncommon and comparable across the groups 4 years after randomization. Also, we found that children with adequate adherence who restarted on LPV/r promptly after failure on EFV were not compromised by their experience with EFV. Further, in the context of routine viral load monitoring, we did not observe any developed resistance that would compromise newer NNRTI drugs [23].
An important limitation of our study is that we do not have data on children with extended regimens of NVP. Nevertheless, we believe that the biological plausibility of longer regimens substantially attenuating our results is low. Almost all infants exposed to single dose [24, 25] or extended [26] NVP have measurable levels of NNRTI resistance when measured close to the exposure with suitable assays. However, these mutations decline over time, albeit more slowly in infants with extended NVP exposure [26, 27]. Interestingly, the most common specific mutation selected post-PMTCT is Y181C [24], which confers about 2-fold reduced susceptibility to EFV compared to >40-fold reduced susceptibility to NVP [23, 28].
A similar trial in western Africa provides some evidence that the benefits of switching to EFV extend to the current PMTCT era. In this trial, 106 HIV-infected children suppressed on LPV/r were randomized to switch to EFV or remain on LPV/r at a median age of 27 months [29]. A substantial proportion received extended NVP during breastfeeding. Overall suppression (HIV RNA <500 copies/mL) 12 months post-randomization was similar in the LPV/r (85.2%) and EFV (82.7%) groups. Inference is limited by the small sample size.
A strength of our analysis is preservation of the randomized design while assessing the durability of effects when children were referred out into routine care. Our results provide reassurance about the generalizability of benefits of the EFV switch strategy to a real-world setting. Unfortunately, only 80% of the original trial participants enrolled in the observational study largely due to the gap between trial completion and observational study start. Nevertheless, once enrolled, retention has been excellent with 95% still in follow-up. Among those retained, 95% had the opportunity be followed up for 3 years by the end of 2015, and 54% were followed up to 4 years. Results were consistent at 3 and 4 years. We intend to continue to follow up the cohort for longer term outcomes.
New regimens are on the horizon in Sub-Saharan Africa. Integrase inhibitors, including dolutegravir, have few side effects, a higher barrier to resistance, and excellent virologic control in adults; data in children are promising [30]. We are hopeful that dolutegravir will be available in pediatric formulations in resource-limited settings soon. Although awaiting regimens in the pipeline, consideration of switching to EFV at 3 years of age remains an important strategy to minimize the risk of metabolic complications and maximize bone health.
In conclusion, our results support the strategy of switching clinically stable HIV-infected children 3 years of age and older from LPV/r to EFV. In 4 years of follow-up, we observed less frequent viral rebound, improved lipid profiles, and higher CD4 percentages in children who switched to EFV compared to those who remained on LPV/r.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Funding. This work was supported by grants HD061255, HD073977, and HD073952 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development with additional support from the National Institute of Mental Health under grant T32 MH19105-28.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Consolidated guideline on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed Geneva, Switzerland: World Health Organization, 2016. [PubMed] [Google Scholar]
- 2. UNAIDS. Children and HIV Fact sheet. Vol 2016 Geneva, Switzerland: UNAIDS, 2016. [Google Scholar]
- 3. Goga AE, Singh Y, Singh M et al. Enhancing HIV treatment access and outcomes amongst HIV infected children and adolescents in resource limited settings. Matern Child Health J 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palumbo P, Lindsey JC, Hughes MD et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med 2010; 363:1510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hunt GM, Coovadia A, Abrams EJ et al. HIV-1 drug resistance at antiretroviral treatment initiation in children previously exposed to single-dose nevirapine. AIDS 2011; 25:1461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Violari A, Lindsey JC, Hughes MD et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med 2012; 366:2380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coovadia A, Abrams EJ, Strehlau R et al. Efavirenz-based antiretroviral therapy among nevirapine-exposed HIV-infected children in South Africa: a randomized clinical trial. JAMA 2015; 314:1808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pillay V, Ledwaba J, Hunt G et al. Antiretroviral drug resistance surveillance among drug-naive HIV-1-infected individuals in Gauteng Province, South Africa in 2002 and 2004. Antivir Ther 2008; 13Suppl 2:101–7. [PubMed] [Google Scholar]
- 9. Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K; American Heart Association American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation 2003; 107:1562–6. [DOI] [PubMed] [Google Scholar]
- 10; World Health Organization. WHO Anthro. 3.2.2 ed, 2011. [Google Scholar]
- 11. World Health Organization. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance. African Region. Geneva, Switzerland: World Health Organization, 2005. [Google Scholar]
- 12. Arpadi SM, Shiau S, Strehlau R et al. Efavirenz is associated with higher bone mass in South African children with HIV. AIDS 2016; 30:2459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barlow-Mosha L, Angelidou K, Lindsey J et al. Nevirapine- versus lopinavir/ritonavir-based antiretroviral therapy in HIV-infected infants and young children: long-term follow-up of the IMPAACT P1060 randomized trial. Clin Infect Dis 2016; 63:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Babiker A, Castro nee Green H, Compagnucci A et al. ; Penpact Study Team First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis 2011; 11:273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuhn L, Coovadia A, Strehlau R et al. Switching children previously exposed to nevirapine to nevirapine-based treatment after initial suppression with a protease-inhibitor-based regimen: long-term follow-up of a randomised, open-label trial. Lancet Infect Dis 2012; 12:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Achan J, Kakuru A, Ikilezi G et al. Growth recovery among HIV-infected children randomized to lopinavir/ritonavir or NNRTI-based antiretroviral therapy. Pediatr Infect Dis J 2016; 35:1329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramjan R, Calmy A, Vitoria M et al. Systematic review and meta-analysis: Patient and programme impact of fixed-dose combination antiretroviral therapy. Trop Med Int Health 2014; 19:501–13. [DOI] [PubMed] [Google Scholar]
- 18. Nachega JB, Parienti JJ, Uthman OA et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis 2014; 58:1297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Department of Health South Africa. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria, South Africa: National Department of Health, 2015. [Google Scholar]
- 20. Pinillos F, Dandara C, Swart M et al. Case report: severe central nervous system manifestations associated with aberrant efavirenz metabolism in children: the role of CYP2B6 genetic variation. BMC Infect Dis 2016; 16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Department of Health South Africa. Guidelines for the management of HIV in children. 2nd ed Pretoria, South Africa: National Department of Health, 2010. [Google Scholar]
- 22. Deeks SG. International perspectives on antiretroviral resistance. Nonnucleoside reverse transcriptase inhibitor resistance. J Acquir Immune Defic Syndr 2001; 26 Suppl 1:S25–33. [DOI] [PubMed] [Google Scholar]
- 23. Basson AE, Rhee SY, Parry CM et al. Impact of drug resistance-associated amino acid changes in HIV-1 subtype C on susceptibility to newer nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother 2015; 59:960–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paredes R, Marconi VC, Lockman S, Abrams EJ, Kuhn L. Impact of antiretroviral drugs in pregnant women and their children in Africa: HIV resistance and treatment outcomes. J Infect Dis 2013; 207(suppl. 2:S93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ton Q, Frenkel L. HIV drug resistance in mothers and infants following use of antiretrovirals to prevent mother-to-child transmission. Curr HIV Res 2013; 11:126–36. [DOI] [PubMed] [Google Scholar]
- 26. Persaud D, Bedri A, Ziemniak C et al. ; Ethiopian Swen Study Team. Slower clearance of nevirapine resistant virus in infants failing extended nevirapine prophylaxis for prevention of mother-to-child HIV transmission. AIDS Res Hum Retroviruses 2011; 27:823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eshleman SH, Mracna M, Guay LA et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012). AIDS 2001; 15:1951–7. [DOI] [PubMed] [Google Scholar]
- 28. Rhee SY, Liu T, Ravela J, Gonzales MJ, Shafer RW. Distribution of human immunodeficiency virus type 1 protease and reverse transcriptase mutation patterns in 4,183 persons undergoing genotypic resistance testing. Antimicrob Agents Chemother 2004; 48:3122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dahourou DL, Amorissani-Folquet M, Malateste K et al. Efavirenz-based simplification after successful early lopinavir-boosted-ritonavir-based therapy in HIV-infected children in Burkina Faso and Côte d’Ivoire: the MONOD ANRS 12206 non-inferiority randomised trial. BMC Med 2017; 15(85). doi:10.1186/s12916-017-0842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dehority W, Abadi J, Wiznia A, Viani RM. Use of integrase inhibitors in HIV-infected children and adolescents. Drugs 2015; 75:1483–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.