Summary
Sepsis may be associated with long-term health consequences. In this analysis from the national REasons for Geographic and Racial Differences in Stroke cohort, we found that incident sepsis hospitalizations were associated with increased long-term risks of subsequent acute and fatal coronary heart disease events.
Keywords: sepsis, infections, heart disease, myocardial infarction, epidemiology
Abstract
Background
Sepsis is associated with long-term health consequences. We sought to determine the long-term risks of acute and fatal coronary heart disease (CHD) events after sepsis hospitalizations among community-dwelling adults.
Methods
We analyzed data from 30329 participants in the population-based REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Sepsis events included hospitalizations for a serious infection with ≥2 systemic inflammatory response syndrome criteria. Acute CHD events included myocardial infarctions (MIs; nonfatal and fatal) and acute CHD deaths. Fatal CHD included deaths ≤28 days of an acute MI and acute CHD deaths. We age- and time-matched each sepsis participant with 5 nonsepsis participants. We assessed the associations between sepsis hospitalizations and future acute and fatal CHD events using Cox regression, Gray’s model, and competing risks analysis, adjusting for comorbidities.
Results
The matched cohort contained 1070 sepsis and 5350 nonsepsis participants. Risk of acute CHD was higher for sepsis than nonsepsis controls after adjusting for sex, race, education, income, region, tobacco use, and select chronic medical conditions (0–1 year adjusted hazard ratio [HR], 4.38 [95% confidence interval (CI), 2.03–9.45]; 1–4 years, 1.78 [1.09–2.88]; and 4+ years, 1.18 [0.52–2.67]). Risk of fatal CHD was similarly higher for sepsis than nonsepsis individuals (0–1 year adjusted HR, 3.12 [95% CI, 1.35–7.23]; 1–4 years, 3.29 [1.89–5.74]; and 4+ years HR, 1.15 [0.34–3.94]).
Conclusions
The long-term risks of acute and fatal CHD are elevated after sepsis hospitalization. Management of acute CHD risk may be important for individuals surviving a sepsis event.
Sepsis is the clinical syndrome of infection complicated by systemic inflammation. Sepsis may lead to systemic vasodilation, organ injury, shock, and death. Sepsis is a major public health burden, resulting in more than 750000 hospitalizations and 200000 deaths annually in the United States [1]. Persons who survive sepsis hospitalization may experience poor quality of life and prolonged health sequelae such as cognitive dysfunction, chronic kidney disease, and increased long-term mortality [2–6].
Cardiovascular disease is a leading cause of morbidity and mortality [7]. Infections can raise the risk of cardiovascular events through a variety of mechanisms, including increased demand ischemia, depression of ventricular function, arrhythmias, endothelial dysfunction, procoagulant changes in the blood, inflammatory changes in atherosclerotic plaques, impaired cardiovascular autonomic response, and impaired renal function [8, 9]. The heightened systemic inflammation of infection may persist long after clinical resolution [10, 11]. While prior studies have linked select infection types with coronary heart disease (CHD) risk, there have been no studies to evaluate sepsis hospitalizations with long-term CHD risk in community-dwelling adults [12–16].
The REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort is one of the nation’s largest ongoing national longitudinal cohorts of community-dwelling adults. Our objective in this study was to identify the association between incident sepsis and subsequent risk of acute CHD events in the REGARDS cohort.
METHODS
Study Design
We performed a matched cohort study using participants from the REGARDS cohort. The University of Alabama at Birmingham Institutional Review Board approved the study.
Data Source
REGARDS is one of the largest ongoing national longitudinal cohorts, consisting of 30239 community-dwelling adults aged ≥45 years [17]. The REGARDS cohort was designed to evaluate the predictors of racial and geographic differences in stroke mortality. Among REGARDS participants, approximately 45% are male, 41% are black, and 69% are aged >60 years. REGARDS recruited participants between January 2003 and October 2007. At 6-month intervals, REGARDS contacted the participants by telephone to identify any hospitalizations of participants. Further details for the REGARDS study methods are described elsewhere [17]. While the goal of the REGARDS cohort was to examine and characterize stroke events, the population within REGARDS contained healthy adults at baseline. The REGARDS–Sepsis ancillary study used the infrastructure of the parent REGARDS study to independently identify sepsis hospitalizations [18].
Identification of Sepsis Cases
The primary exposure in this study was first hospitalization for sepsis. We included hospitalization events reported from 1 January 2003 through 31 December 2012. Using the taxonomy of Angus et al, we identified all hospitalizations (emergency department visits and/or hospital admission) attributed by participants to a serious infection [1]. We defined sepsis cases as hospital admission for a serious infection with the presence of at least 2 systemic inflammatory response syndrome (SIRS) criteria. The SIRS criteria included the following: heart rate >90 beats/minute; fever (temperature >38.3°C or <36.0°C); tachypnea (>20 breaths/min) or PCO2 < 32 mm Hg; and leukocytosis or leukopenia (white blood cells >12000 or <4000 cells/mm3 or >10% band forms) [19, 20]. Since the study focused on community-acquired (vs hospital-acquired) sepsis, we used vital signs and laboratory test results for the initial 28 hours of hospitalization. Two trained reviewers evaluated information from the corresponding medical record, confirming the presence of a serious infection based on diagnoses documented in the emergency department or admission physician record. Discordances were adjudicated among abstractors, with additional physician review as needed.
International consensus conferences (Sepsis-3) have proposed new definitions for sepsis [21]. Because of their common use in prior sepsis epidemiology studies, we used the SIRS-based sepsis definition in the primary analysis. However, in a sensitivity analysis, we repeated the analysis using the Sepsis-3 definition of sepsis as the presence of a serious infection plus a sequential organ failure assessment (SOFA) score ≥2.
Primary Outcome—Acute Coronary Heart Disease Events
The primary outcome was the occurrence of an acute CHD event [22]. Acute CHD included fatal and nonfatal myocardial infarctions (MI) and acute CHD death events. Expert teams reviewed medical records and identified and adjudicated CHD events using published guidelines [23, 24].
MI consisted of the presence of signs or symptoms suggestive of ischemia; a rising and/or falling pattern in cardiac troponin level or creatine phosphokinase-MB level over 6 or more hours, with a peak level greater than twice the upper limit of normal (diagnostic cardiac enzymes); and electrocardiogram changes consistent with ischemia or MI. Electrocardiogram evaluation followed the Minnesota code, classifying cases as evolving diagnostic, positive, nonspecific, or not consistent with ischemia [25]. We included only definite or probable MIs. Definite MIs included cases with diagnostic enzymes or electrocardiogram. Probable MIs included cases with elevated but not diagnostic (ie, equivocal) enzymes with a positive but not diagnostic electrocardiogram or, if enzymes were missing, a positive electrocardiogram in the presence of ischemic signs or symptoms. We classified MIs caused by an invasive procedure as procedure related. Elective and urgent coronary revascularization procedures were not included in the definition of MI.
For acute CHD deaths, adjudicators reviewed the medical history, hospital records, interviews with next of kin or proxies, and death certificate or national death index data to determine if CHD was a definite or probable cause of death. Cases were assigned to 2 adjudicators, and disagreements were adjudicated by committee. The test for agreement between adjudicators yielded a κ level >0.80 for the presence of definite or probable MI or definite or probable acute CHD death.
In a secondary analysis, we examined fatal CHD as the primary outcome, defined as acute CHD deaths or death within 28 days of an acute MI. To allow for at least 1 year of follow-up after the last adjudicated sepsis event, we included all acute CHD events during 1 January 2003 through 31 December 2013 (1 year beyond the last adjudicated sepsis event).
Prior studies examined the association between sepsis and subsequent cardiovascular disease using a composite outcome variable combining CHD and stroke events [12–16]. There are important clinical and pathophysiologic distinctions between stroke and heart disease. For example, stroke episodes may involve ischemic or hemorrhagic subtypes and may entail antecedent embolic events. Therefore, in this study we chose to focus on CHD.
Selection of Matched Cohort
We created a matched cohort that consisted of sepsis participants matched with individuals who had not experienced a sepsis event. Requirements for matching included that the participant must not have experienced an acute CHD prior to enrollment in REGARDS, the participant must not have experienced an acute CHD prior to the first sepsis event, and the participant must have complete sepsis and acute CHD event data during the follow-up period. For each sepsis participant, we calculated the time from REGARDS enrollment to first sepsis event. For each eligible sepsis participant, we used incidence density matching to select 5 nonsepsis controls, matching by age ±5 years. Potential controls were participants who were alive for at least the same presepsis time of the sepsis case but without a sepsis or acute CHD event. Cases and controls were selected with replacement; sepsis participants could also be selected as a matched control for another sepsis case during their presepsis period, and nonsepsis participants could serve as a matched control for more than 1 sepsis participant.
Participant Characteristics
Participant characteristics used in the analysis included sociodemographics, health behaviors, chronic medical conditions, and select biomarkers. Sociodemographics included age, race, sex, income, education, and geographic location. Health behaviors included tobacco and alcohol use. Chronic medical conditions included atrial fibrillation, chronic lung disease, coronary artery disease, deep vein thrombosis, diabetes, dyslipidemia, hypertension, MI, obesity, chronic kidney disease, peripheral artery disease, and stroke. Biomarkers in the analysis included cystatin-C, high-sensitivity C-reactive protein (CRP), serum creatinine (converted to estimated glomerular filtration rate [eGFR]), and urinary albumin-to-creatinine ratio (ACR). Medications included aspirin and statins. (Detailed participant characteristic definitions are provided in Supplementary Appendix 1.)
Statistical Analyses
We compared characteristics between matched sepsis and nonsepsis participants using conditional logistic regression. We fit Cox proportional hazards models to determine the risk of acute and fatal CHD events between matched sepsis and nonsepsis persons. We censored individuals at the time of their event, death, or end of follow-up (31 December 2012). Because we performed sampling with replacement, we incorporated matching stratum into all analytical models.
In developing risk adjustment strategies for the Cox models, we noted that the limited number of CHD and fatal CHD events was insufficient to allow adjustment for all potential sociodemographics, health behaviors, chronic medical conditions, and biomarkers. Therefore, we adjusted the multivariate models using a backwards variable selection process. First, we fit a model with all variables with univariate P ≤ .2. We then backwards eliminated variables with a multivariable P > .05. The resulting model included sequential adjustment for sex, race, education, income, and region; tobacco use; and atrial fibrillation, chronic lung disease, diabetes, hypertension, obesity, peripheral artery disease, stroke, chronic kidney disease, and abnormal ACR. To examine alternate risk adjustment strategies, we fit a Cox model adjusting for the REGARDS sepsis risk score only [26]. The REGARDS sepsis risk score predicts 10-year risk of sepsis based on patterns of sociodemographics, health behavior, chronic medical conditions, and biomarkers.
We fit a series of analytic models to test the robustness of the results. We fit a piecewise Cox model with 3 follow-up time segments (≤1 year, 1–4 years, ≥4 years). Because of the unclear proportionality of hazards at later follow-up time points, we fit a model using Gray’s piecewise constant time-varying coefficients model, which uses piecewise constant penalized splines to better estimate temporal exposure effects [27]. We used Fine and Gray’s model to examine all-cause mortality as a potential competing risk for acute CHD events [28]. Finally, following the Sepsis-3 consensus guidelines, we repeated the analysis defining sepsis as the presence of infection plus SOFA score ≥2. We used R, SAS version 9.4, and Stata version 13 for all analyses.
RESULTS
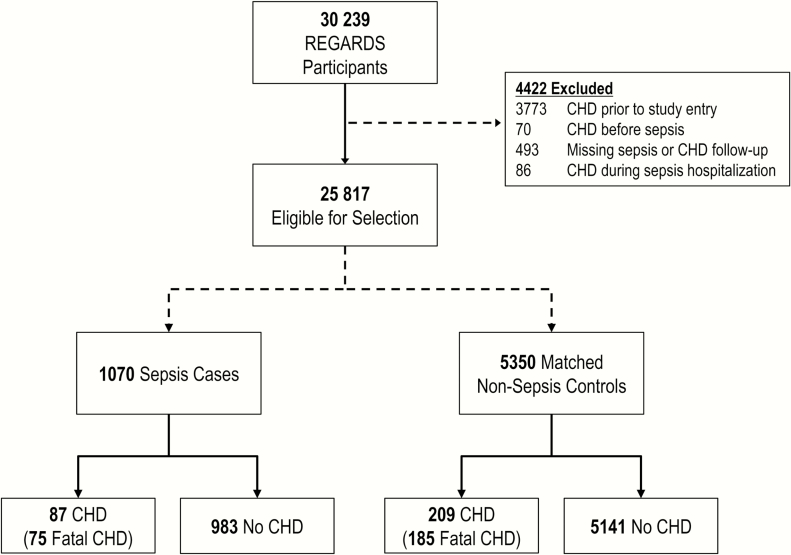
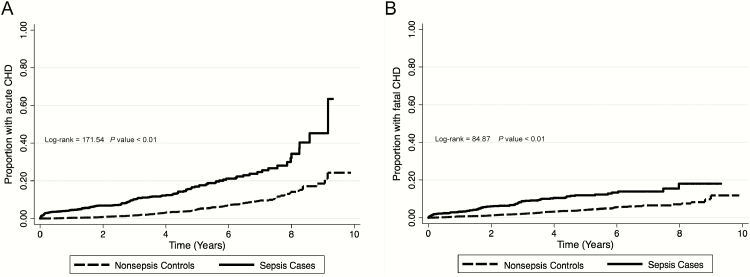
Among 30239 REGARDS participants, 1529 experienced a sepsis hospitalization. From 25817 eligible participants, we selected a matched cohort of 1070 sepsis cases and 5350 age-matched controls (Figure 1). Among these individuals, 143 developed sepsis after serving as a control. There were 87 CHD events (including 75 fatal events) among sepsis participants and 209 CHD events (including 185 fatal events) among nonsepsis controls. Incidence of acute and fatal CHD were 3.6 events per 100000 person-years (95% confidence interval [CI], 3.2–4.0) and 3.1 events per 100000 person-years (95% CI, 2.7–3.5), respectively (Figure 2). Median time to first sepsis event was 3.5 years (interquartile range [IQR], 1.6–5.4). After the first sepsis event, median times to acute and fatal CHD events were 2.6 years (IQR, 0.9–4.7) and 2.7 years (IQR, 1.0–4.8), respectively.
Figure 1.
Overview of study population. Abbreviations: CHD, coronary heart disease; REGARDS, REasons for Geographic And Racial Differences in Stroke.
Figure 2.
Kaplan-Meier failure curves depicting time to acute coronary heart disease (CHD) (A) and fatal CHD (B) events. Abbreviation: CHD, coronary heart disease.
The most common infection types among the sepsis cases were pneumonia (38.13%), urinary tract infections (16.82%), and abdominal infections (16.82%; Table 1). Sepsis cases were more likely to be male and white, exhibit lower education and income, reside in the Stroke Belt, and use tobacco (Table 2). Alcohol use was similar between sepsis cases and controls. Baseline medical conditions were more common in sepsis cases than controls. Sepsis cases had higher ACR and CRP levels, and lower eGFR than nonsepsis controls. Sepsis cases were more likely to be current aspirin users compared with nonsepsis controls.
Table 1.
Clinical Characteristics of 1070 Sepsis Case Hospitalizations
| Infection Type | N (%) |
|---|---|
| Infection type | |
| Pneumonia | 408 (38.13) |
| Urinary tract infections | 180 (16.82) |
| Abdominal | 180 (16.82) |
| Bronchitis | 104 (9.72) |
| Skin | 89 (8.32) |
| Sepsis | 56 (5.23) |
| Fever of unknown origin | 21 (1.96) |
| Catheter/Other | 32 (3.00) |
| Sequential organ failure assessment score | |
| 0 | 344 (32.15) |
| 1 | 269 (25.14) |
| ≥2 | 457 (42.71) |
| Intensive care unit admission | 84 (7.85) |
Table 2.
Characteristics of Sepsis Cases and Matched Nonsepsis Controls
| Characteristic |
Sepsis Cases
(N = 1070) |
Nonsepsis
Controls (N = 5350) |
P Value |
|---|---|---|---|
| N (%) | N (%) | ||
| Agea | 67.2 (9.2) | 67.0 (9.1) | — |
| Sex | |||
| Male | 495 (46.26) | 2038 (38.09) | <.01 |
| Female | 575 (53.74) | 3312 (61.91) | |
| Race | |||
| Black | 355 (33.18) | 2064 (38.58) | <.01 |
| White | 715 (66.82) | 3286 (61.42) | |
| Education | |||
| Less than high school | 150 (14.03) | 560 (10.47) | <.01 |
| High school graduate | 286 (26.75) | 1357 (25.36) | |
| Some college | 318 (29.75) | 1432 (26.77) | |
| College or higher | 315 (29.47) | 2001 (37.40) | |
| Income | |||
| ≤$20 000 | 251 (23.46) | 792 (14.80) | <.01 |
| $20 000–$34 000 | 294 (27.48) | 1295 (24.21) | |
| $35 000–$74 000 | 301 (28.13) | 1684 (31.48) | |
| ≥$75 000 | 116 (10.84) | 823 (15.38) | |
| Refuse | 108 (10.09) | 756 (14.13) | |
| Geographic region | |||
| Stroke Belt (non-coastal portions of North Carolina, South Carolina and Georgia; Tennessee, Mississippi, Alabama, Louisiana and Arkansas) | 415 (38.79) | 1713 (32.02) | <.01 |
| Stroke Buckle (coastal plains of North Carolina, South Carolina and Georgia) | 219 (20.47) | 1353 (25.29) | |
| Non-Stroke Belt (other states) | 436 (40.75) | 2284 (42.69) | |
| Tobacco use | |||
| Never | 410 (38.46) | 2643 (49.56) | <.01 |
| Past | 468 (43.90) | 2090 (39.19) | |
| Current | 188 (17.64) | 600 (11.25) | |
| Alcohol use | |||
| None | 686 (65.46) | 3336 (63.60) | .27 |
| Moderate (up to 1 drink per day for women or 2 drinks per day for men) | 320 (30.53) | 1725 (32.89) | |
| Heavy (>1 drink per day for women and >2 drinks per day for men) | 42 (4.01) | 184 (3.51) | |
| Baseline medical condition | |||
| Atrial fibrillation | 120 (11.42) | 417 (7.97) | <.01 |
| Chronic lung disease | 191 (17.85) | 468 (8.75) | <.01 |
| Coronary artery disease | 96 (9.28) | 349 (6.68) | <.01 |
| Deep vein thrombosis | 86 (8.12) | 269 (5.05) | <.01 |
| Diabetes | 299 (28.08) | 1108 (20.79) | <.01 |
| Dyslipidemia | 646 (62.84) | 2992 (57.57) | <.01 |
| Hypertension | 701 (65.82) | 3112 (58.26) | <.01 |
| Obesity | 649 (60.82) | 2754 (51.51) | <.01 |
| Peripheral artery disease | 38 (3.55) | 88 (1.64) | <.01 |
| Stroke | 93 (8.73) | 262 (4.91) | <.01 |
| Chronic kidney disease | 175 (16.36) | 601 (11.23) | <.01 |
| Biomarkersb | |||
| High-sensitivity C-reactive protein | 3.39 (1.54–6.90) | 1.98 (0.90–4.58) | <.01 |
| Albumin-to-creatinine ratio | 9.38 (5.17–23.29) | 7.30 (4.66–14.80) | <.01 |
| Estimated glomerular filtration rate | 83.12 (66.21–95.48) | 85.61 (72.81–96.34) | <.01 |
| Medications | |||
| Aspirin | 493 (46.07) | 2277 (42.58) | .03 |
| Statins | 349 (32.62) | 1652 (30.88) | .26 |
aMean (standard deviation).
bMedian (interquartile range).
The risk of acute CHD was higher for sepsis cases than nonsepsis controls after adjusting for age, sex, race, tobacco use, education, income, region, ACR, and baseline medical conditions. In piecewise models, this association persisted for 4 years after sepsis hospitalization (0–1 year adjusted hazard ratio [HR], 4.38 [95% CI, 2.03–9.45]; 1–4 years, 1.78 [1.09–2.88]; and 4+ years, 2.28 [0.52–2.67]; Table 3). The associations between sepsis and subsequent acute CHD persisted with adjustment by REGARDS sepsis risk score. The associations between sepsis and acute CHD risk persisted over 10 years of follow-up when the relationship was modeled using Gray’s model, showing a stronger association more proximal to the sepsis event (Figure 2, Supplementary Appendices 2 and 3). Sepsis remained associated with increased 4-year risk of acute CHD events when death as a competing risk for acute CHD was considered (0–1 year adjusted HR, 1.75 [95% CI, 1.35–2.26]; 1–4 years, 1.57 [1.25–1.98]; and 4+ years, 1.27 [0.69–2.32]; Supplementary Appendix 4).
Table 3.
Associations Between Sepsis Hospitalization and Risk of Subsequent Acute Coronary Heart Disease Events
| Sepsis Cases | Nonsepsis Controls | Risk of Acute CHD, Hazard Ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|---|
|
Acute CHD Events
/No. at Risk (%) |
Acute CHD Events
/No. at Risk (%) |
Crude | Model 1 | Model 2 | Model 3 | Model 4 | |
| Traditional Cox proportional hazards model | |||||||
| 87/1070 (8.13) | 209/5350 (3.91) | 2.85 (2.17–3.75) | 2.74 (2.07–3.64) | 2.64 (1.99–3.52) | 2.14 (1.53–3.01) | 2.08 (1.55–2.79) | |
| Piecewise Cox proportional hazards model | |||||||
| 0–1 year | 29/1070 (2.71) | 45/5350 (0.84) | 3.62 (2.24–5.86) | 3.67 (2.16–6.26) | 3.55 (2.07–6.11) | 4.38 (2.03–9.45) | 2.67 (1.58–4.49) |
| 1–4 years | 43/779 (5.52) | 90/4630 (1.94) | 3.22 (2.19–4.73) | 2.94 (1.97–4.39) | 2.86 (1.91–4.29) | 1.78 (1.09–2.88) | 2.05 (1.34–3.12) |
| 4+ years | 15/329 (4.56) | 74 / 2,210 (3.35) | 1.59 (0.86–2.94) | 1.59 (0.84–3.01) | 1.57 (0.82–3.02) | 1.18 (0.52–2.67) | 1.34 (0.70–2.56) |
Model 1: adjusted for sex, race, education, income, and region. Model 2: additional adjustment for tobacco use. Model 3: additional adjustment for atrial fibrillation, chronic lung disease, diabetes, hypertension, obesity, peripheral artery disease, stroke, chronic kidney disease, and albumin-to-creatinine ratio. Model 4: adjusted only for REasons for Geographic And Racial Differences in Stroke sepsis risk score.
Abbreviation: CHD, coronary heart disease.
The risk of fatal CHD was higher for sepsis cases than nonsepsis controls for 4 years after sepsis hospitalization (0–1 year adjusted HR, 3.12 [95% CI, 1.35–7.23]; 1–4 years, 3.29 [1.89–5.74]; and 4+ years, 1.15 [0.34–3.94]; Table 4). The association between sepsis and subsequent fatal CHD persisted with adjustment by REGARDS sepsis risk score. The associations between sepsis and fatal CHD persisted when the relationship was modeled using Gray’s model (Figure 2, Supplementary Appendices 2 and 3). Sepsis remained associated with increased 4-year risk of fatal CHD events when death as a competing risk was considered (0–1 year adjusted HR, 1.81 [95% CI: 1.34–2.43]; 1–4 years, 1.76 [1.28–2.42]; and 4+ years, 1.38 [0.64–2.99]; Supplementary Appendix 5).
Table 4.
Associations Between Sepsis Hospitalizations and Risk of Subsequent Fatal Coronary Heart Disease Events
| Sepsis Cases | Nonsepsis Controls | Risk of Fatal Coronary Heart Disease, Hazard Ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|---|
| Fatal CHD Events /No. at Risk (%) |
Fatal CHD Events
/No. at Risk (%) |
Crude | Model 1 | Model 2 | Model 3 | Model 4 | |
| Traditional Cox proportional hazards model | |||||||
| 75/1070 (7.01) | 185/5350 (3.46) | 2.63 (1.97–3.52) | 2.70 (1.99–3.67) | 2.60 (1.91–3.55) | 2.07 (1.42–3.03) | 2.05 (1.51–2.78) | |
| Piecewise Cox proportional hazards model | |||||||
| 0–1 year | 25/1070 (2.86) | 38/5350 (0.71) | 3.45 (2.07–5.77) | 4.20 (2.35–7.49) | 4.50 (2.49–8.14) | 3.12 (1.35–7.23) | 3.07 (1.81–5.20) |
| 1–4 years | 39/779 (5.01) | 87/4630 (1.88) | 3.56 (2.36–5.36) | 3.66 (2.35–5.69) | 3.54 (2.25–5.56) | 3.29 (1.89–5.74) | 2.36 (1.51–3.67) |
| 4+ years | 11/329 (3.34) | 60/2210 (2.71) | 1.11 (0.56–2.21) | 1.63 (0.74–3.60) | 1.39 (0.61–3.19) | 1.15 (0.34–3.94) | 0.92 (0.45–1.89) |
Fatal CHD included acute CHD deaths and death ≤28 days of an acute myocardial infarction. Model 1: adjusted for sex, race, education, income, and region. Model 2: additional adjustment for tobacco use. Model 3: additional adjustment for atrial fibrillation, chronic lung disease, diabetes, hypertension, obesity, peripheral artery disease, stroke, chronic kidney disease, and albumin-to-creatinine ratio. Model 4: adjusted only for REasons for Geographic And Racial Differences in Stroke sepsis risk score.
Abbreviation: CHD, coronary heart disease.
The risk of acute CHD was more pronounced when we modeled sepsis as the presence of infection plus SOFA score ≥2 (Supplementary Appendix 6).
DISCUSSION
In this study we found that sepsis hospitalizations were associated with increased risks of subsequent acute CHD events for at least 4 years. Furthermore, the risk of fatal CHD was also increased for at least 4 years after sepsis hospitalization. Our observations originate from REGARDS, one of the nation’s largest population-based cohorts of community-dwelling adults and were robust to a range of analytic approaches, including the use of Gray’s models and accounting for competing risk of death.
The connection between sepsis and increased acute CHD risk is biologically plausible. The pathophysiological mechanisms that underlie acute CHD include vascular and systemic inflammation, promotion of a prothrombotic state, vascular stress, alteration in vascular tone, altered hemodynamic homeostasis, and altered metabolic balance [8, 9]. These vascular pathologies are similarly prominent in sepsis [29, 30]. Survivors of sepsis are known to have health sequelae including persistent organ dysfunction, suggesting that these pathophysiological abnormalities may persist long after recovery from an acute sepsis episode [2–6]. Prior studies have linked biomarkers of inflammation and hemostasis with long-term mortality after pneumonia hospitalizations [10, 11].
Prior studies have linked specific infection types with subsequent risk of acute CHD [12–15]. In a case series study of the United Kingdom General Practice Research Database, Smeeth et al observed an increased risk of acute MI and stroke after acute respiratory infections [13]. Corrales-Medina et al used matched case cohorts from the Cardiovascular Health Study and the Atherosclerosis Risk in Communities study to identify increased long-term acute MI, stroke, or fatal coronary heart disease [12]. Using data from a single academic medical center, Jafarzadeh et al found that acute cardiovascular events (stroke, transient ischemic attack, and MI) were associated with prior episodes of bacteremia; however, the study relied on discharge diagnoses and included only individuals who had had at least 2 hospitalizations at the medical center [14]. Yende et al used Medicare claims data to assess 4179 intensive care unit survivors of severe sepsis, finding high 1-year rates of cardiovascular events compared with matched and unmatched hospitalized controls [16].
Our study has numerous strengths that add to these prior efforts. We used population-based data, which allowed us to compare CHD risk against all community-dwelling individuals, not just those admitted to the hospital. Rather than discharge diagnoses, we identified sepsis and acute CHD events through structured adjudicated review of hospital records. We were able to adjust for a range of participant characteristics, affirming that the increase in cardiovascular risk is independently associated with the sepsis event and not comorbid burden. We were able to identify sepsis and acute CHD events over a 10-year span. Our focus on sepsis illuminates the link of acute CHD risk with a range of infection types. Furthermore, we were able to differentiate fatal CHD events from nonfatal CHD events. The larger HRs observed with fatal CHD affirm that sepsis hospitalizations are likely linked with more deleterious future cardiovascular events.
Our findings highlight important unanswered questions regarding post-sepsis care. Current clinical and scientific initiatives focus on the course and care of acute sepsis [31]. Recent efforts highlight that sepsis survivors often have lingering health sequelae, such as chronic kidney disease and cognitive decline, and increased all-cause mortality. Our study highlights acute CHD as a potential target for mitigating the long-term effects of sepsis. For example, cornerstones of CHD prevention include hypertension and hyperlipidemia control; the use of antihypertensive and lipid-lowering medications may be considered for sepsis survivors [32]. Exercise and mobility are important for reducing cardiovascular risk; physical rehabilitation may potentially play an important part of sepsis recovery. Likewise, smoking cessation may potentially play a role. While proven effective in reducing cardiovascular disease risk, additional study must evaluate if these measures are effective, or feasible, in post-sepsis patients.
Our study did have limitations. As discussed previously, because of their differing pathophysiology compared with CHD, we did not include strokes in this analysis. We assumed that baseline participant characteristics did not vary over the 10-year follow-up period. We adjusted for a comprehensive set of confounders, but additional unidentified confounders may have influenced the analysis. We were able to obtain data on the presence or absence of chronic medical conditions, but not disease severity. REGARDS included only black and white participants. Because of our focus on community-acquired sepsis, we did not include sepsis that occurred during hospitalization. Also, because REGARDS is not a surveillance study, we may not have been able to detect all sepsis events. Because of the limited number of CHD events, we may have not been able to identify associations with longer-term risk of CHD after sepsis.
In the REGARDS cohort, incident sepsis hospitalizations were associated with increased risk of subsequent acute and fatal CHD. Acute CHD prevention may be an important consideration in post-sepsis care.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. H. E. W. and M. M. S. conceived the study, obtained funding, and oversaw data collection. J. X. M. and J. P. D. conducted the analysis. H. E. W. drafted the manuscript and all authors contributed to its critical review. H. E. W. assumes overall responsibility for the paper.
Acknowledgments. We thank the other investigators, staff, and participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org and http://www.regardssepsis.org.
Funding. This study was supported by the National Institute for Nursing Research (R01-NR012726) and the National Center for Research Resources (UL1-RR025777), as well as grants from the Center for Clinical and Translational Science and the Lister Hill Center for Health Policy of the University of Alabama at Birmingham. The parent REGARDS study was supported by a cooperative agreement from the National Institute of Neurological Disorders and Stroke and the National Institutes of Health (NIH), Department of Health and Human Services (U01-NS041588). J. P. D. received support from the Agency for Healthcare Research and Quality (AHRQ; T32-HS013852). J. X. M. received support from the National Cancer Institute (NCI R25-CA47888).
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Representatives of the funding agencies were involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data.
Potential conflicts of interest. M.M.S. reports the following potential conflicts of interest: Amgen, salary support to study patterns of statin use in Medicare and other large databases; diaDexus, salary support for a research grant on lipids and CHD outcomes; diaDexus, consulting to help with US Food and Drug Administration application; and NIH, AHRQ, salary support for research grants. E. B. L. receives research support from Amgen. All other authors: No reported conflicts. No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–10. [DOI] [PubMed] [Google Scholar]
- 2. Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 2012; 60:1070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med 2010; 38:1276–83. [DOI] [PubMed] [Google Scholar]
- 4. Wang HE, Szychowski JM, Griffin R, Safford MM, Shapiro NI, Howard G. Long-term mortality after community-acquired sepsis: a longitudinal population-based cohort study. BMJ Open 2014; 4:e004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304:1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta RL, Bouchard J, Soroko SB, et al. ; Program to Improve Care in Acute Renal Disease (PICARD) Study Group Sepsis as a cause and consequence of acute kidney injury: Program to Improve Care in Acute Renal Disease. Intensive Care Med 2011; 37:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129:399–410. [DOI] [PubMed] [Google Scholar]
- 8. Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet 2013; 381:496–505. [DOI] [PubMed] [Google Scholar]
- 9. Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis 2010; 10:83–92. [DOI] [PubMed] [Google Scholar]
- 10. Yende S, D’Angelo G, Kellum JA, et al. ; GenIMS Investigators Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med 2008; 177:1242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yende S, D’Angelo G, Mayr F, et al. ; GenIMS Investigators Elevated hemostasis markers after pneumonia increases one-year risk of all-cause and cardiovascular deaths. PLoS One 2011; 6:e22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015; 313:264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med 2004; 351:2611–8. [DOI] [PubMed] [Google Scholar]
- 14. Jafarzadeh SR, Thomas BS, Warren DK, Gill J, Fraser VJ. Longitudinal study of the effects of bacteremia and sepsis on 5-year risk of cardiovascular events. Clin Infect Dis 2016; 63:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ludwig A, Lucero-Obusan C, Schirmer P, Winston C, Holodniy M. Acute cardiac injury events ≤30 days after laboratory-confirmed influenza virus infection among U.S. veterans, 2010–2012. BMC Cardiovasc Disord 2015; 15:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yende S, Linde-Zwirble W, Mayr F, Weissfeld LA, Reis S, Angus DC. Risk of cardiovascular events in survivors of severe sepsis. Am J Respir Crit Care Med 2014; 189:1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005; 25:135–43. [DOI] [PubMed] [Google Scholar]
- 18. Moore JX, Donnelly JP, Griffin R, et al. Black-white racial disparities in sepsis: a prospective analysis of the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Crit Care 2015; 19:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bone RC. The sepsis syndrome. Definition and general approach to management. Clin Chest Med 1996; 17:175–81. [DOI] [PubMed] [Google Scholar]
- 20. Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 2014; 5:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Safford MM, Brown TM, Muntner PM, et al. ; REGARDS Investigators Association of race and sex with risk of incident acute coronary heart disease events. JAMA 2012; 308:1768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luepker RV, Apple FS, Christenson RH, et al. ; AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003; 108:2543–9. [DOI] [PubMed] [Google Scholar]
- 24. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Writing Group on Behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction Third universal definition of myocardial infarction. Glob Heart 2012; 7:275–95. [DOI] [PubMed] [Google Scholar]
- 25. Prineas RJ, Crow RS, Blackburn HW. The Minnesota code manual of electrocardiographic findings: standards and procedures for measurement and classification. 2nd ed London: Springer, 2010. [Google Scholar]
- 26. Wang HE, Donnelly JP, Griffin R, et al. Derivation of novel risk prediction scores for community-acquired sepsis and severe sepsis. Crit Care Med 2016; 44:1285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ren Y, Chang CC, Zenarosa GL, et al. Gray’s time-varying coefficients model for posttransplant survival of pediatric liver transplant recipients with a diagnosis of cancer. Comput Math Methods Med 2013; 2013:719389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fine JP, Gray RJ. . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 29. Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ 2016; 353:i1585. [DOI] [PubMed] [Google Scholar]
- 30. Miranda M, Balarini M, Caixeta D, Bouskela E. Microcirculatory dysfunction in sepsis: pathophysiology, clinical monitoring, and potential therapies. Am J Physiol Heart Circ Physiol 2016; 311:H24–35. [DOI] [PubMed] [Google Scholar]
- 31. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41: 580–637. [DOI] [PubMed] [Google Scholar]
- 32. Smith SC, Jr, Allen J, Blair SN, et al. ; AHA/ACC; National Heart, Lung, and Blood Institute AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006; 113:2363–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.