Summary
Between 1996 and 2015, prevalence of transmitted drug resistance (TDR) increased significantly among HIV-infected people who use drugs in Vancouver, mainly driven by increases in nonnucleoside reverse transcriptase inhibitor–related TDR, strongly supporting baseline resistance testing to guide antiretroviral selection.
Keywords: transmitted drug resistance, people who use drugs, HIV, North America, antiretroviral treatment
Abstract
Background
Transmitted drug resistance (TDR) may compromise response to antiretroviral therapy (ART). However, there are limited data on TDR patterns and impacts among people who use illicit drugs (PWUD).
Methods
Data were drawn from 2 prospective cohorts of PWUD in Vancouver, Canada. We characterized patterns of TDR among human immunodeficiency virus (HIV)–infected PWUD, and assessed its impacts on first-line ART virological outcomes.
Results
Between 1996 and 2015, among 573 ART-naive PWUD (18% with recent HIV infection), the overall TDR prevalence was 9.8% (95% confidence interval [CI], 7.3%–12.2%), with an increasing trend over time, from 8.5% in 1996–1999 to 21.1% in 2010–2015 (P = .003), mainly driven by resistance to nonnucleoside reverse transcriptase inhibitors (NNRTIs). TDR-associated mutations were more common for NNRTIs (5.4%), followed by nucleoside reverse transcriptase inhibitors (3.0%) and protease inhibitors (1.9%). TDR prevalence was lower among recently infected PWUD (adjusted odds ratio, 0.39 [95% CI, .15–.87]). Participants with TDR had higher risk of virological failure than those without TDR (log-rank P = .037) in the first year of ART.
Conclusions
Between 1996 and 2015, TDR prevalence increased significantly among PWUD in Vancouver. Higher risk of virological failure among PWUD with TDR may be explained by some inappropriate ART prescribing, as well as undetected minority resistant variants in participants with chronic HIV infection. Our findings support baseline resistance testing early in the course of HIV infection to guide ART selection among PWUD in our setting.
Global scale-up of antiretroviral therapy (ART) coverage has led to remarkable declines in human immunodeficiency virus (HIV)–related morbidity and mortality, as well as reductions in new infections [1]. In addition, increasing availability of more potent, safer, and fixed-dosed ART regimens has resulted in improved tolerability and adherence, and consequently reduced the risk of virologic failure among people living with HIV (PLHIV) [2]. Thus, in recent years, rates of acquired drug resistance have declined sharply in most developed countries [3–5]. On the contrary, a similar declining trend has not been observed for transmitted drug resistance (TDR) [3, 6, 7].
Prevalence of TDR varies widely across geographic settings, populations, and calendar time [6]. For example, whereas studies in Europe have documented stable trends in TDR at around 8% for the period 2008–2010 [7, 8], studies conducted in the United States have shown higher and increasing rates of TDR of approximately 17% for the same period [9]. Surveillance data from Canada indicates an overall prevalence of TDR of around 10% for the period 1999–2008 (with higher rates in more recent years) [10].
TDR has important clinical and public health implications. At the individual level, the presence of TDR may limit first-line ART options and, if undetected, increase the risk of virologic failure, which in turn may compromise both the individual- and population-level effectiveness of standardized first-line ART regimens [11]. Thus, surveillance of TDR is critical to inform HIV-related policies and clinical guidelines, particularly with respect to recommended first-line ART regimens and the need of baseline genotypic resistance testing.
Although people who use illicit drugs (PWUD) represent a key population within the HIV pandemic, there is relatively less information on TDR patterns and impacts among this group compared to other key populations (eg, men who have sex with men [MSM]) [7]. While existing estimates indicate an overall TDR prevalence between 8% and 10% among people who inject drugs in North America [7, 12, 13], more updated estimates (ie, after 2010) are currently lacking. This represents a critical knowledge gap, particularly in the context of recent outbreaks of HIV infection driven by opioid injection in many North American settings [14]. Therefore, the objective of this study was to evaluate the prevalence, correlates, and trends of TDR, and secondarily to assess its impacts on virological outcomes of first-line ART among HIV-infected PWUD in Vancouver, Canada, from 1996 to 2015.
METHODS
Study Design and Population
Data for this study were drawn from 2 open prospective cohorts of PWUD with harmonized study procedures in Vancouver, Canada: the AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS) and the Vancouver Injection Drug Users Study (VIDUS) studies. Eligibility and study procedures have been described in detail previously [15, 16]. In brief, individuals are recruited through extensive street outreach and snowball sampling in the greater Vancouver region with a focus on the Downtown Eastside neighborhood, an area with an open drug market and high levels of illicit drug use, poverty, and HIV infection. VIDUS consists of HIV-negative adults (≥18 years) who injected drugs in the month prior to enrollment, and ACCESS of HIV-infected adults who used illicit drugs (other than cannabis) in the month prior to enrollment. VIDUS participants were considered for inclusion in the present analysis if they seroconverted to HIV during follow-up.
After providing written informed consent, at baseline and semiannually thereafter, participants complete an interviewer-administered questionnaire that collects information on sociodemographic characteristics, drug use patterns, and healthcare access and utilization, including HIV and addiction care as well as other relevant exposures. At each of these visits, participants provide blood samples for hepatitis C virus (HCV) and HIV serological testing and HIV disease monitoring as appropriate, and are examined by a study nurse, who provides basic medical care and referrals to additional health services when needed. As has been described elsewhere [15], information gathered at each visit is augmented by confidential data linkages with the British Columbia Centre for Excellence in HIV/AIDS Drug Treatment Program, which provides HIV care, including free ART and HIV clinical monitoring to all PLHIV in the province of British Columbia. Through these linkages, we are able to build a complete retrospective longitudinal HIV clinical and laboratory profile for each participant, including data on all CD4 counts, HIV viral load (VL), and genotypic tests conducted either through the aegis of the study or in the course of regular clinical care, as well as data on all ART dispensations (eg, dates, regimen, quantities). Of relevance to the present analysis, baseline genotypic testing was increasingly requested as part of regular clinical care in British Columbia after the year 2000, formally becoming standard of care in 2005. In addition, for participants enrolled before these years, genotypic testing was done retrospectively using archived samples when available; however, these retrospective test results were not available to clinicians to inform selection of first-line ART. Participants receive a stipend of 30 Canadian dollars at each study visit. Both cohort studies have received ethical approval by the University of British Columbia/Providence Health Care Research Ethics Board.
For the present analysis, we included HIV-infected participants who were recruited between 1 May 1996 and 31 May 2015 who had 1 or more genotypic resistance tests while ART naive. For participants with >1 test while ART naive, only the earliest available test was considered.
Transmitted Drug Resistance
Population-based nucleotide sequencing of the HIV reverse transcriptase and protease genes were performed at the British Columbia Centre for Excellence in HIV/AIDS following previously described laboratory and analytic protocols [13, 17]. The World Health Organization surveillance drug resistance mutation (SDRM) list was used for identification of TDR [18]. The overall prevalence of TDR was estimated as the percentage of participants with ≥1 SDRM. We also calculated the prevalence of TDR for each specific class of antiretroviral drugs: nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs).
Other Measures
We considered various factors that we hypothesized might be associated with TDR among PWUD, including sociodemographic characteristics (age, sex, self-reported ethnicity), HIV-related variables (recent HIV infection among VIDUS seroconverters [ie, <12 months between the last documented negative and the first documented positive HIV test], CD4 count and HIV plasma RNA VL at the time of the genotypic test), HCV coinfection, and history of injection drug use, sex work, and incarceration.
Statistical Analysis
As a first step, we examined baseline characteristics of the sample, stratified by presence of TDR. We compared categorical variables using the χ2 test or the Fisher exact test, as appropriate; continuous variable were compared using the Mann-Whitney test. Next, we used bivariable and multivariable logistic regression to identify the independent correlates of TDR. Starting with a full multivariable model containing all variables associated with the outcome at P < .10 in bivariable analysis, we used an a priori–defined backward stepwise procedure to select the final model with the best fit (ie, model with the lowest Akaike information criterion value).
Overall TDR prevalence values and 95% confidence intervals (CIs) were calculated based on the normal approximation method with continuity correction factor. Trends of prevalence of TDR over time were analyzed using the Cochran-Armitage test and χ2 test for trend. For this analysis, observations were grouped into periods based on ART availability in British Columbia: 1996–1999 (introduction of combination ART); 2000–2005 (steady state of ART use); 2006–2009 (second expansion of ART distribution); and 2010–2015 (aggressive scale-up of ART among key populations as part of treatment-as-prevention efforts), using data presented previously [19]. A similar analysis was conducted for each specific class of antiretroviral.
As a subanalysis, we assessed first-line ART regimens among the study sample and the impact of TDR on virological response to first-line ART. The latter was restricted to participants who had at least 1 VL test after 180 days of ART initiation. First, using the Stanford HIVdb algorithm version 8.3 [20], participants were classified as (1) no TDR; (2) TDR with fully active first-line ART (no resistance mutation affecting the prescribed ART); or (3) TDR with non–fully active ART (≥1 resistance mutation associated with reduced susceptibility to at least 1 of the drugs of their prescribed ART) [11]. Then, using Kaplan-Meier curves, we evaluated time to virological failure, defined as 2 consecutive VLs >50 copies/mL, after 180 days of ART initiation, considering the date of the first VL >50 copies/mL as failure date. Participants were censored if they died, stopped ART, or were lost to follow-up, or at their last VL test date in the 9- to 15-month window period after ART initiation. All analyses were conducted using R studio software (version 0.99.892) [21], and all P values are 2-sided.
RESULTS
Of 1125 participants recruited into ACCESS and completing ≥1 study interview between May 1996 and May 2015, 573 (50.9%) HIV-infected PWUD had at least 1 genotypic test while ART naive and were included in the present study. In comparison to the 552 participants who were excluded owing to lack of genotypic testing while ART-naive, participants included in these analyses were younger (median age, 37 vs 39 years, P = .028) and less likely to be coinfected with HCV (85% vs 89%; P = .003), with no other significant differences. Baseline characteristics of included participants, stratified by presence of TDR, are presented in Table 1. The median age was 37 years (interquartile range [IQR], 31–44 years), 370 (64.6%) were male, the majority (545 [95.1%]) had a history of injection drug use, and 101 (17.6%) had documented recent HIV infection. Median CD4 count and VL at the time of the genotypic test were 380 cells/μL (IQR, 230–530 cells/μL) and 4.6 log10 copies/mL (IQR, 4.0–5.0 log10 copies/mL), respectively.
Table 1.
Baseline Characteristics of 573 Human Immunodeficiency Virus–Infected People Who Use Illicit Drugs, Stratified by Presence of Transmitted Drug Resistance, Vancouver, Canada, 1996–2015
| Characteristic | Total, No. (%) (N = 573) | Transmitted Drug Resistance, No. (%) | P Value | |
|---|---|---|---|---|
| Yes (n = 56) | No (n = 517) | |||
| Individual-level factors | ||||
| Age, y, median (IQR)a | 37 (31–44) | 41 (34–47) | 37 (30–43) | .003b |
| Male sex | 370 (64.6) | 42 (75.0) | 328 (63.4) | .086 |
| White race | 329 (57.4) | 31 (55.4) | 298 (57.6) | .743 |
| Injection drug usec | 545 (95.1) | 55 (98.2) | 490 (94.8) | .508d |
| HCV seropositivec | 485 (84.6) | 45 (80.4) | 440 (85.1) | .331 |
| HIV-related factors | ||||
| Recent HIV infection | 101 (17.6) | 4 (7.1) | 97 (18.8) | .027d |
| CD4 count, cells/μLa | ||||
| Median (IQR) | 380 (230–530) | 430 (290–560) | 380 (230–530) | .088b |
| Categories | ||||
| <200 | 114 (19.9) | 7 (12.5) | 107 (20.7) | .303 |
| 200–349 | 132 (23.0) | 12 (21.4) | 120 (23.2) | |
| 350–499 | 154 (26.9) | 15 (26.8) | 139 (26.9) | |
| ≥500 | 162 (28.3) | 21 (37.5) | 141 (27.3) | |
| Viral load, log10 copies/mLa | ||||
| Median (IQR) | 4.6 (4.0–5.0) | 4.2 (3.8–4.7) | 4.6 (4.0–5.0) | .012b |
| >5 | 168 (29.3) | 10 (17.9) | 158 (30.6) | .047 |
| Year of resistance test | ||||
| 1996–1999 | 128 (22.3) | 11 (19.6) | 117 (22.6) | .004 |
| 2000–2005 | 167 (29.1) | 8 (14.3) | 159 (30.8) | |
| 2006–2009 | 207(36.1) | 22 (39.3) | 185 (36.8) | |
| 2010–2015 | 71 (12.4) | 15 (26.8) | 56 (10.9) | |
| Structural-level factors | ||||
| Sex workc | 241 (42.1) | 19 (33.9) | 222 (42.9) | .194 |
| Incarcerationc | 480 (83.8) | 48 (85.7) | 432 (83.6) | .678 |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range.
aAt the time of the genotypic resistance test.
bWilcoxon rank-sum test.
cRefers to lifetime behavior or exposure.
dFisher exact test.
The overall prevalence of TDR in the study sample was 9.8% (95% CI, 7.3%–12.2%), with only 3 (0.5% [95% CI, .0–1.2%]) participants harboring dual-class TDR. SDRMs were more common for NNRTIs (5.4% [95% CI, 3.5%–7.3%]), followed by NRTIs (3.0% [95% CI, 1.5%–4.4%]) and PIs (1.9% [95% CI, .7%–3.1%]). The most prevalent SDRM was the K103N (NNRTI-associated mutation) found in 3.7% of the participants (37.5% of those with TDR), followed by the M46I/L (PI-associated mutation) present in 1.2%. The most frequent NRTI-SDRMs were the thymidine analogue mutations D67N, K219Q, and T215 revertants T215S/C/E, each found in 1.0% of individuals.
The final multivariable model of correlates of TDR is presented in Table 2. The prevalence of TDR was significantly lower among PWUD with recent HIV infection (adjusted odds ratio [aOR], 0.39 [95% CI, .15–.87]) and those with high VL (>5 log10 copies/mL; aOR, 0.47 [95% CI, .25–.83]).
Table 2.
Unadjusted and Adjusted Logistic Regression Analyses of Factors Associated With Transmitted Drug Resistance Among Human Immunodeficiency Virus–Infected People Who Use Illicit Drugs, Vancouver, Canada, 1996–2015
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Age (per 10 years older)a | 1.34 (1.06–1.70)b | 1.25 (.98–1.61) |
| Male sex (yes vs no) | 1.73 (1.04–3.00)b | 1.61 (.94–2.84) |
| White race (yes vs no) | 0.91 (.57–1.46) | |
| Injection drug use (yes vs no)c | 3.03 (.77–29.42) | |
| HCV-seropositive (yes vs no)c | 0.71 (.40–1.31) | |
| Recent HIV infection (yes vs no)c | 0.33 (.12–.73)b | 0.39 (.15–.87) |
| CD4 count (ref: <200 cells/μL)a | ||
| 200–349 | 1.53 (.69–3.57) | |
| 350–499 | 1.65 (.77–3.76) | |
| ≥500 | 2.28 (1.11–5.04)b | |
| HIV VL (>5 log10 vs ≤5 log10 copies/ mL)a | 0.49 (.26–.87)b | 0.47 (.25–.83) |
| Sex work (yes vs no)c | 0.68 (.41–1.10) | |
| Incarceration (yes vs no)c | 1.18 (.63–2.39) | |
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; OR, odds ratio; VL, viral load.
aAt the time of the genotypic resistance test.
b P < .10 and considered in the multivariable model selection process.
cRefers to lifetime behavior or exposure.
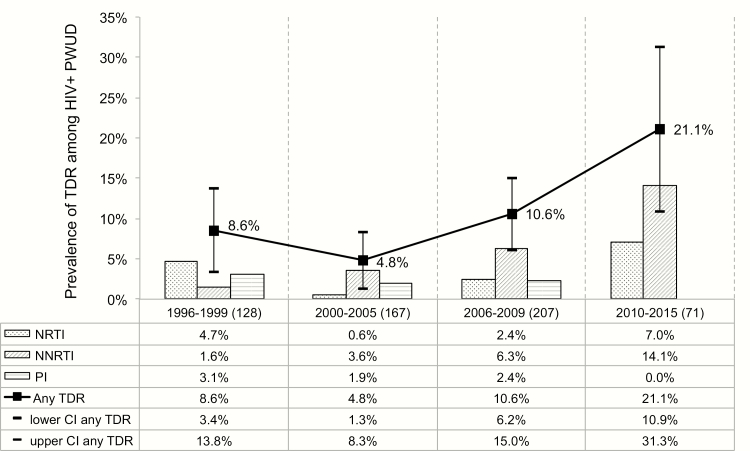
Figure 1 depicts the prevalence of TDR in the 4 time periods. As shown, after an initial decline in TDR, there was a significant increase in TDR prevalence over time, from 8.5% (95% CI, 3.4%–13.8%) in 1996–1999 to 21.1% (95% CI, 10.9%–31.3%) in 2010–2015 (P = .003), which remained even after adjusting for factors independently associated with TDR (ie, chronic HIV infection and low VL; P = .031). The increase in TDR prevalence over time was driven largely by an increase in NNRTI TDR from 1.6% (95% CI, .0–4.2%) in 1996–1999 to 14.1% (95% CI, 5.3%–22.9%) in 2010–2015 (P < .001). Prevalence of PI and NRTI TDR showed no significant changes over time (P = .270 and P = .410, respectively).
Figure 1.
Trends in transmitted drug resistance among human immunodeficiency virus–infected people who use illicit drugs, Vancouver, Canada, 1996–2015. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PWUD, people who use drugs; TDR, transmitted drug resistance.
Of the 573 included participants, 496 (86.6%) initiated ART, with no significant differences between those with or without TDR (83.9% vs 86.8%; P = .688). The most common first-line ART regimens in both groups were PI-based regimens. However, PWUD with TDR were more likely to be prescribed PI-based regimens (72.3% vs 51.2%; P = .006) and integrase strand transfer inhibitor (INSTI)–based regimens (8.5% vs 1.1%; P = .006), and less likely to receive NNRTI-based regimens (17.0% vs 41.2%; P = .001). Of the 47 participants with TDR, 35 (74.5%) were prescribed a fully active ART regimen, and 12 (25.5%) non–fully active regimens, half of whom had PI-associated TDR.
Of the 496 participants who initiated ART during the study period, 454 (91.5%) met the inclusion criteria for the analysis of virological outcomes: 409 (90.1%) with no TDR, 33 (7.3%) with TDR and fully active regimen, and 12 (2.6%) with TDR and non–fully active regimen. As indicated in Figure 2A, cumulative incidence of virological failure at 12 months of ART initiation was significantly higher among participants with TDR compared to those with no TDR (51.6% [95% CI, 34.0%–64.6%] and 36.1% [95% CI, 31.2%–40.6%], respectively; log-rank P = .037). However, when the TDR group was stratified according to predicted susceptibility of first-line ART, this association was no longer significant, likely due to the small number of cases in each of the subgroups (Figure 2B; log-rank P = .657).
Figure 2.
Cumulative incidence of virologic failure among human immunodeficiency virus–infected people who use illicit drugs initiating antiretroviral therapy (ART) in Vancouver, Canada, 1996–2015. A, Risk of virological failure according to presence or not of transmitted drug resistance (TDR). B, Risk of virological failure in participants with TDR by predicted susceptibility to first-line ART.
DISCUSSION
To our knowledge, the present study is among the largest studies assessing trends of TDR over time in a community-recruited cohort of ART-naive, HIV-infected PWUD. Over a 20-year period, spanning from 1996 (coinciding with the introduction of combination ART) to 2015 (during a community-wide treatment as prevention–based ART scale-up initiative), we observed moderate prevalence rates (9.8% overall) of TDR among PWUD in Vancouver [22]. However, given that most of our study participants had HIV infection of unknown duration, this figure may be an underestimation of real TDR prevalence in our setting. In addition—and of concern—prevalence of TDR increased substantially over time, reaching values of 21.1% for the period 2010–2015, and largely driven by increases in NNRTI-associated TDR.
The overall TDR prevalence found in this study is in line with findings reported in a recent systematic review that found pooled TDR estimates for PWID in North America from 1996 to 2011 ranging between 8.0% and 10.2% [7]. However, our results are in contrast with the findings that TDR prevalence rates have stabilized among PWID in high-income settings [7]. This disagreement may be explained by the fact that the aforementioned review included aggregated TDR prevalence estimates from both North America and Western Europe, potentially obscuring regional differences. Indeed, previous research has documented lower and fairly stable TDR prevalence trends among PWID in Europe in recent years [6–8]. Alternatively, it might reflect differences in the sampling frames, as the largest rise in TDR prevalence in our study was observed in the period 2010–2015, a time period not covered by the systematic review [7].
Consistent with most studies conducted in the combination ART era (ie, after 1996), SDRMs to NNRTIs were the most frequent SDRMs observed in our analysis, driving also the temporal increasing trend in global TDR [6, 7, 23]. Specifically, more than one-third of participants in our study with TDR harbored the K103N mutation. Also similar to previous studies, TDR to PIs remained low throughout the study period [6, 7, 23]. The high prevalence of NNRTI-associated TDR may be explained by a lower genetic barrier and increasing use of efavirenz-based regimens as first-line ART in British Columbia, as well as the minimal fitness costs associated with the K103N mutation [24]. It is worth noting that research has demonstrated that ART-naive PLHIV (including those undiagnosed, out of care, and PLHIV on pre-ART care) are a significant source of TDR (especially of low-fitness-cost SDRMs) in many settings [3, 25, 26]. The observed TDR pattern (ie, high prevalence of K103N, and extremely low prevalence or absence of high-fitness-cost SDRMs, such as M184V or K65R) suggests similar TDR transmission dynamics among PWUD in Vancouver. Future phylogenetic studies may help to better characterize sources of TDR in our setting. Collectively, these findings highlight the importance of expanding and sustaining efforts for earlier HIV diagnosis and effective treatment to achieve durable viral suppression and, consequently, prevent onward transmission of drug-resistant viruses. Importantly, the risk of cross-transmission of HIV drug resistance through sexual contact to other subpopulations (eg, MSM, heterosexual men and women) should not be overlooked [27].
Unexpectedly and in contrast to prior literature [28], we found that TDR prevalence rates were lower among recently infected PWUD compared to those with chronic or unknown duration of HIV infection. A possible explanation may be that almost half of PWUD with documented recent HIV infection were enrolled before the year 2000, when limited numbers of PWUD accessed ART due to multiple barriers [13, 16], thus contributing to limited population-level ART exposure among this group.
Our subanalysis revealed that three-quarters of HIV-infected PWUD with TDR were prescribed fully active first-line ART, underscoring the important role of baseline genotypic testing to guide clinical decisions. Some of the balance of inappropriate ART prescribing may be further accounted by the fact that, for some participants, genotypic testing was done retrospectively. Finally, overall participants with TDR had higher risk of virological failure compared with participants with no TDR. Among participants with fully active ART, a possible explanation for this finding may relate to the presence of minority resistant variants not detected with standard genotypic resistance testing, particularly among chronically infected participants [11, 29, 30].
Results from this study should be interpreted in light of a number of limitations. First, given the absence of official registries of PWUD in Vancouver and hidden nature of this population, we employed a nonrandom sampling strategy to recruit participants into our cohorts. Thus, findings of this analysis may not necessarily be representative of the larger population of HIV-infected PWUD in Vancouver or other settings. Second, the lack of phylogenetic analysis precludes the possibility of reliably identifying the source of TDR. However, the TDR pattern observed in our study suggest that ART-naive PWUD may be a significant reservoir of drug-resistant HIV in Vancouver. Third, although at the time of the analysis, INSTI resistance data were not available and thus we were not able to assess its prevalence and potential impact on first-line ART, epidemiological studies suggest that the prevalence of INSTI TDR is still minimal [31]. Finally, as reflected by the wide CI, the relatively small sample size for the period 2010–2015 may affect the accuracy of the TDR prevalence estimate. That said, recent studies in North America have documented a similarly high prevalence of TDR [9, 32, 33].
In summary, this study found overall moderate levels of TDR among ART-naive PWUD in Vancouver, Canada, over a 20-year period, but with a significant temporal rise in TDR, mainly driven by NNRTI-related TDR, reaching high levels of TDR in more recent years. Additionally, participants with TDR had increased risk of virological failure in the first year of ART initiation. These results support current recommendations for resistance testing among newly diagnosed PLHIV to guide individual clinical management [34], as well as the need for ongoing monitoring of TDR among PWUD and other subpopulations of PLHIV to inform HIV treatment guidelines [22]. Moreover, our findings underscore the importance of universal HIV testing and rapid linkage to care and ART initiation among newly diagnosed HIV-infected PWUD to limit the spread of TDR.
Notes
Acknowledgments. The authors thank the study participants for their contributions to the research, as well as current and past researchers and staff. We specifically thank Evan Wood, Deborah Graham, Tricia Collingham, Jennifer Matthews, Steve Kain, Sarah Sheridan, and Paul Sereda for their research and administrative assistance.
Financial support. This work was supported by the US National Institute on Drug Abuse (NIDA) at the US National Institutes of Health (NIH) (grant numbers U01-DA038886 and R01-DA021525). M. E. S. is supported by a Michael Smith Foundation for Health Research (MSFHR) postdoctoral fellowship award and a Canada Addiction Medicine Research Fellowship from NIDA (R25-DA037756). M.-J. M. is supported in part by the NIH (grant number R01-DA021525), a Scholar Award from MSFHR, and a New Investigator award from the Canadian Institutes of Health Research (CIHR). P. R. H. is supported by a CIHR/GlaxoSmithKline Research Chair in clinical virology. J. M. is supported by the British Columbia Ministry of Health and through an Avant-Garde Award from NIDA at the NIH (1DP1DA026182). K. H. is supported by a CIHR New Investigator Award (MSH-141971).
Potential conflicts of interest. The University of British Columbia has received unstructured funding from NG Biomed, Ltd, an applicant to the Canadian federal government, for a license to produce medical cannabis, to support M.-J. M.’s research. J. M. has received limited unrestricted funding, paid to his institution, from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. P. R. H. has received grants from, served as an ad hoc advisor to, or spoke at various events sponsored by Pfizer, GlaxoSmithKline, Abbott, Merck, Virco, and Monogram. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Joint United Nations Programme on HIV/AIDS. 90-90-90. On the right track towards the global target. Geneva, Switzerland: UNAIDS, 2016. [Google Scholar]
- 2. Viswanathan S, Justice AC, Alexander GC et al. Adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2015; 69:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang WL, Kouyos R, Scherrer AU et al. Assessing the paradox between transmitted and acquired HIV type 1 drug resistance mutations in the Swiss HIV Cohort Study from 1998 to 2012. J Infect Dis 2015; 212:28–38. [DOI] [PubMed] [Google Scholar]
- 4. De Luca A, Dunn D, Zazzi M et al. ; SEHERE Collaboration in Chain Declining prevalence of HIV-1 drug resistance in antiretroviral treatment-exposed individuals in Western Europe. J Infect Dis 2013; 207:1216–20. [DOI] [PubMed] [Google Scholar]
- 5. Montaner JS, Lima VD, Harrigan PR et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the “HIV treatment as prevention” experience in a Canadian setting. PLoS One 2014; 9:e87872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhee SY, Blanco JL, Jordan MR et al. Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: an individual-patient- and sequence-level meta-analysis. PLoS Med 2015; 12:e1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pham QD, Wilson DP, Law MG, Kelleher AD, Zhang L. Global burden of transmitted HIV drug resistance and HIV-exposure categories: a systematic review and meta-analysis. AIDS 2014; 28:2751–62. [DOI] [PubMed] [Google Scholar]
- 8. Hofstra LM, Sauvageot N, Albert J et al. ; SPREAD Program Transmission of HIV drug resistance and the predicted effect on current first-line regimens in Europe. Clin Infect Dis 2016; 62:655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ocfemia MCB, Saduvala N, Oster AM et al. HIV-1 drug resistance among men who have sex with men, 11 US jurisdictions, 2008–2011 [abstract 579]. In: 21st Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA, 2014. [Google Scholar]
- 10. Public Health Agency of Canada. HIV-1 strain and transmitted drug resistance in Canada: surveillance report to December 31, 2008. Ottawa, Canada: Centre for Communicable Diseases and Infection Control, 2012. [Google Scholar]
- 11. Wittkop L, Günthard HF, de Wolf F et al. ; EuroCoord-CHAIN study group Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis 2011; 11:363–71. [DOI] [PubMed] [Google Scholar]
- 12. Tossonian HK, Raffa JD, Grebely J et al. Primary drug resistance in antiretroviral-naive injection drug users. Int J Infect Dis 2009; 13:577–83. [DOI] [PubMed] [Google Scholar]
- 13. Alexander CS, Dong W, Schechter MT et al. Prevalence of primary HIV drug resistance among seroconverters during an explosive outbreak of HIV infection among injecting drug users. AIDS 1999; 13:981–5. [DOI] [PubMed] [Google Scholar]
- 14. Strathdee SA, Beyrer C. Threading the needle—how to stop the HIV outbreak in rural Indiana. N Engl J Med 2015; 373:397–9. [DOI] [PubMed] [Google Scholar]
- 15. Wood E, Hogg RS, Lima VD et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA 2008; 300:550–4. [DOI] [PubMed] [Google Scholar]
- 16. Strathdee SA, Palepu A, Cornelisse PG et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA 1998; 280:547–9. [DOI] [PubMed] [Google Scholar]
- 17. Milloy MJ, Wood E, Kerr T et al. Increased prevalence of controlled viremia and decreased rates of HIV drug resistance among HIV-positive people who use illicit drugs during a community-wide treatment-as-prevention initiative. Clin Infect Dis 2016; 62:640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bennett DE, Camacho RJ, Otelea D et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 2009; 4:e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Montaner JS, Lima VD, Barrios R et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 2010; 376:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang MW, Liu TF, Shafer RW. The HIVdb system for HIV-1 genotypic resistance interpretation. Intervirology 2012; 55:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2016. [Google Scholar]
- 22. World Health Organization. Global strategy for the surveillance and monitoring of HIV drug resistance: an update. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 23. Frentz D, Boucher CA, van de Vijver DA. Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS Rev 2012; 14:17–27. [PubMed] [Google Scholar]
- 24. Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. HIV-1 drug resistance and resistance testing. Infect Genet Evol 2016; 46:292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mourad R, Chevennet F, Dunn DT et al. ; UK HIV Drug Resistance Database & the Collaborative HIV, Anti-HIV Drug Resistance Network A phylotype-based analysis highlights the role of drug-naive HIV-positive individuals in the transmission of antiretroviral resistance in the UK. AIDS 2015; 29:1917–25. [DOI] [PubMed] [Google Scholar]
- 26. Drescher SM, von Wyl V, Yang WL et al. ; Swiss HIV Cohort Study Treatment-naive individuals are the major source of transmitted HIV-1 drug resistance in men who have sex with men in the Swiss HIV Cohort Study. Clin Infect Dis 2014; 58:285–94. [DOI] [PubMed] [Google Scholar]
- 27. Oster AM, Wertheim JO, Hernandez AL, Ocfemia MC, Saduvala N, Hall HI. Using molecular HIV surveillance data to understand transmission between subpopulations in the United States. J Acquir Immune Defic Syndr 2015; 70:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yanik EL, Napravnik S, Hurt CB et al. Prevalence of transmitted antiretroviral drug resistance differs between acutely and chronically HIV-infected patients. J Acquir Immune Defic Syndr 2012; 61:258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taniguchi T, Nurutdinova D, Grubb JR et al. Transmitted drug-resistant HIV type 1 remains prevalent and impacts virologic outcomes despite genotype-guided antiretroviral therapy. AIDS Res Hum Retroviruses 2012; 28:259–64. [DOI] [PubMed] [Google Scholar]
- 30. Geretti AM, Paredes R, Kozal MJ. Transmission of HIV drug resistance: lessons from sensitive screening assays. Curr Opin Infect Dis 2015; 28:23–30. [DOI] [PubMed] [Google Scholar]
- 31. Stekler JD, McKernan J, Milne R et al. Lack of resistance to integrase inhibitors among antiretroviral-naive subjects with primary HIV-1 infection, 2007–2013. Antivir Ther 2015; 20:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kassaye SG, Grossman Z, Balamane M et al. Transmitted HIV drug resistance is high and longstanding in Metropolitan Washington, DC. Clin Infect Dis 2016; 63:836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panichsillapakit T, Smith DM, Wertheim JO, Richman DD, Little SJ, Mehta SR. Prevalence of transmitted HIV drug resistance among recently infected persons in San Diego, CA 1996–2013. J Acquir Immune Defic Syndr 2016; 71:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Günthard HF, Saag MS, Benson CA et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA panel. JAMA 2016; 316:191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]