Summary
An integrated antibiotic allergy testing program resulted in increased prescribing of narrow-spectrum β-lactams and reduction in restricted antibiotics and inappropriate prescriptions. The program effectively and safely de-labeled patients, with >80% of antibiotic allergy labels removed following testing.
Keywords: antibiotic allergy, penicillin allergy, allergy testing, antimicrobial resistance
Abstract
Background
Despite the high prevalence of patient-reported antibiotic allergy (so-called antibiotic allergy labels [AALs]) and their impact on antibiotic prescribing, incorporation of antibiotic allergy testing (AAT) into antimicrobial stewardship (AMS) programs (AAT-AMS) is not widespread. We aimed to evaluate the impact of an AAT-AMS program on AAL prevalence, antibiotic usage, and appropriateness of prescribing.
Methods
AAT-AMS was implemented at two large Australian hospitals during a 14-month period beginning May 2015. Baseline demographics, AAL history, age-adjusted Charlson comorbidity index, infection history, and antibiotic usage for 12 months prior to testing (pre–AAT-AMS) and 3 months following testing (post–AAT-AMS) were recorded for each participant. Study outcomes included the proportion of patients who were “de-labeled” of their AAL, spectrum of antibiotic courses pre– and post–AAT-AMS, and antibiotic appropriateness (using standard definitions).
Results
From the 118 antibiotic allergy–tested patients, 226 AALs were reported (mean, 1.91/patient), with 53.6% involving 1 or more penicillin class drug. AAT-AMS allowed AAL de-labeling in 98 (83%) patients—56% (55/98) with all AALs removed. Post-AAT, prescribing of narrow-spectrum penicillins was more likely (adjusted odds ratio [aOR], 2.81, 95% confidence interval [CI], 1.45–5.42), as was narrow-spectrum β-lactams (aOR, 3.54; 95% CI, 1.98–6.33), and appropriate antibiotics (aOR, 12.27; 95% CI, 5.00–30.09); and less likely for restricted antibiotics (aOR, 0.16; 95% CI, .09–.29), after adjusting for indication, Charlson comorbidity index, and care setting.
Conclusions
An integrated AAT-AMS program was effective in both de-labeling of AALs and promotion of improved antibiotic usage and appropriateness, supporting the routine incorporation of AAT into AMS programs.
Over the past 2 decades, the prevalence of patient-reported antibiotic allergy (so-called antibiotic allergy labels [AALs]) in hospitalized patients has increased and is now estimated to be 18%–25% [1–3]. This type of overlabeling, which may persist over a lifetime, has been associated with poorer clinical outcomes and increased healthcare resource utilization [4–6]. Imprecision in antibiotic allergy labeling creates a recognized need to review antibiotic allergy labeling practices in hospitalized patients, leading to the emerging need for increased antibiotic allergy testing (AAT) [1, 7–9].
The association of AALs with restricted and inappropriate antibiotic usage [10–12] has provided an impetus to incorporate antibiotic allergy de-labeling into antimicrobial stewardship (AMS) [8]. Pilot studies of inpatient AAT [13, 14], protocolized provocation [15, 16], and targeted inpatient AMS programs [17–19] have proved successful, primarily targeting those with penicillin allergy. Earlier studies have demonstrated the high negative predictive value of skin prick testing/intradermal testing (SPT/IDT) when paired with an oral provocation for patients with immunoglobulin E (IgE)–mediated penicillin allergy [2, 14, 20]. However, the impact of coordinated multidisciplinary AAT programs on AMS has not been well studied [21].
We hypothesized that an AMS-led AAT program could effectively remove patient AALs and improve antibiotic utilization. To test this hypothesis, we performed a multicenter prospective cohort study, evaluating the effects of a multidisciplinary AAT-AMS program on (1) safe de-labeling of patient AALs, (2) narrow-spectrum and restricted antibiotic usage, and (3) antibiotic prescribing appropriateness.
METHODS
Setting and Participants
In May 2015, a standardized AAT program was simultaneously introduced at 2 tertiary referral centers in Melbourne, Australia. The Austin Hospital is a tertiary referral center encompassing spinal and transplantation services (liver, small bowel, renal, and stem cell transplant), and the Peter MacCallum Cancer Centre is a tertiary referral center for a range of malignant diseases (including stem cell transplant). Referrals to each program were also received from outside healthcare providers and community practices. An integrated AAT-AMS service was developed in collaboration with infectious diseases (ID), allergy/respiratory, and pharmacy departments. Established AMS programs were already operational at each site, comprising a dedicated AMS pharmacist and physician. During the study period, no new hospital-wide AMS interventions were introduced or changes made to antibiotic drug formularies.
At each center, referrals were generated from adverse drug reaction (ADR) committees and a range of clinical specialists (AMS clinicians, ID physicians, allergists, immunologists, and transplant physicians). Referred patients were assessed as having 1 of 3 primary AAL types: a non-immune-mediated antibiotic side effect (nausea, vomiting, diarrhea without allergic features) (AAL-1); an antibiotic allergy disproved by proven tolerance through the inadvertent/deliberate administration of the suspect antibiotic (AAL-2); and patients with a history consistent with immediate hypersensitivity (IgE), delayed hypersensitivity (T-cell), or unknown effect, who required formal AAT-AMS assessment (AAL-3). The current study was designed to assess the impact of an AAT-AMS program on AAL-3 patients, all of whom followed a uniform testing protocol (Supplementary Appendix 1).
Baseline demographics, age-adjusted Charlson comorbidity index [22], AAL history, and immunosuppression history were collected at first review. An immunocompromised host was defined as solid organ transplant recipient, hematological stem cell transplant recipient, asplenic patient, patient with autoimmune/connective tissue disorder, patient with cancer, or recipient of >10 mg prednisolone/day for >1 month.
Definitions
Allergy Types
AALs were defined as type A (non-immune-mediated, pharmacologically mediated reactions) ADRs or type B ADRs (immune-mediated reactions) or unknown, according to accepted criteria [23]. Type B reactions were further classified as per modified Gell and Coombs criteria [24]. All potentially implicated drugs were allocated a Naranjo score [25] and phenotype-specific scores, where appropriate (eg, algorithm of drug causality for epidermal necrolysis [ALDEN]; RegiSCAR drug reaction with eosinophilia and systemic symptoms [DRESS] [26, 27]).
Relabeling and De-labeling Definitions
A patient was considered to have a revised AAL (“relabeled”) if there was ≥1 change to the documented AAL. A patient was deemed to have been “de-labeled” if ≥1 AAL was removed.
Antibiotic Usage and Appropriateness Definitions
Antibiotic appropriateness was defined according to previously published guidelines; a score of 1 or 2 was considered to be “appropriate”, and 3 or 4 as “inappropriate” [10, 28]. Narrow-spectrum penicillin was defined as any of penicillin VK, penicillin G, flucloxacillin, dicloxacillin, or amoxicillin. Narrow-spectrum β-lactam included narrow-spectrum penicillins, in addition to cephalexin and cefazolin. In accordance with hospital policy at study centers, restricted antibiotics included carbapenems, fluoroquinolones, glycopeptides, lincosamides, lipopeptides, oxazolidinones, and third-/fourth-generation cephalosporins.
Preferred antibiotic treatment for an infection episode was defined as first-line therapy recommended by Australian national guidelines for antimicrobial prescribing [29]. Standardized criteria for defining infections were based upon National Healthcare Safety Network surveillance methods [30]. A patient was deemed to have avoided a penicillin if an alternative antibiotic was administered when a penicillin- containing regimen was the preferred treatment, irrespective of AAL (eg, use of cefepime for febrile neutropenia, in place of piperacillin-tazobactam).
Intervention: Antibiotic Allergy Testing Model
Following review by the ID/AMS team regarding allergy phenotype and antibiotic needs, AAT was performed by trained personnel (ID physicians and allergy nurses), co-located within existing on-site allergy services. Therapies with antihistaminic activity were avoided at least 4 days prior to testing. AAT protocols were based upon previously published pathways [20], with specific algorithms for immediate and delayed hypersensitivity (Supplementary Appendix 1). The validated Diater (DAP, Madrid, Spain) was used for the major (benzylpenicilloyl-poyl-l-lysine [PPL]) and minor determinant mixtures in patients with a β-lactam hypersensitivity [31]. SPT/IDT, patch testing (PT), and oral provocation were performed for both β-lactam and non–β-lactam AALs using testing concentrations consistent with existing recommendations [32]. Where a sterile intravenous preparation of the drug was available, delayed IDT was performed with or without PT. Following AAT, an observed single-dose oral antibiotic provocation was administered to patients with a history of immediate hypersensitivity (2-hour observation, doctor/nurse present), and a prolonged oral provocation (minimum 5 days) was employed in those with a nonsevere delayed or unknown hypersensitivity history (Supplementary Appendix 1).
Following AAT, patients and their healthcare providers were given written recommendations regarding the revised AALs and antibiotics that could be safely administered. Changes to AAL were forwarded to the referring hospital ADR committee and AMS pharmacist to facilitate updating of medical records. Patients and clinicians were requested to complete a common survey 3 months after AAT to assess postdischarge AALs, antibiotic usage, and antibiotic-associated ADRs.
Evaluation of AAT Program
Evaluable outcomes included (1) antibiotic de-labeling, (2) antibiotic usage, and (3) antibiotic appropriateness. Outpatient and inpatient antibiotic usage for infective episodes was assessed with respect to number of courses, spectrum, and appropriateness for the 12-month and 3-month period prior to AAT and for 3 months post-AAT for all AAL-3 patients. If the AAL history was <12 months prior to review, then antibiotic usage data was only collected from a time-point after the latest antibiotic ADR. Antibiotic courses (>1 dose of antibiotic) for 12 months and 3 months prior to testing were compared with those administered during 3-month post testing with regard to antibiotic usage and appropriateness.
Ethics Review
Multisite ethics approval was obtained via the Austin Health Human Research Ethics Committee (No. 15/AUSTIN/75).
Statistical Analysis
Categorical variables were summarized and compared between groups using the χ2 test or Fisher exact test. Continuous variables were compared using a Student t test or Wilcoxon signed-rank test. Mixed-effects linear regression models were used to quantify the association between AAT and each of the following characteristics of antimicrobial courses (binary outcomes): narrow-spectrum penicillin, narrow-spectrum β-lactam, restricted antibiotic, and appropriate prescription. As we sought to evaluate the impact of AAT on types of antimicrobials selected to treat infected patients rather than rate of antimicrobial use, the unit of analysis was the antimicrobial course. Study participants were included as random effects to account for nonindependence of sequential antimicrobial courses prescribed to the same patient. In addition to AAT, we selected the following covariates a priori for model inclusion: age-adjusted Charlson comorbidity index, setting (hospital/community), and antibiotic indication. Coefficients were reported as odds ratios (ORs) with 95% confidence intervals (CIs). A P value of <.05 (2-tailed) was deemed statistically significant. Statistical analyses were performed using Stata software version 13.0 (StataCorp, College Station Texas) and R version 3.2.2 (R Foundation for Statistical Computing, Vienna) with the “lme4” package.
RESULTS
Baseline Characteristics
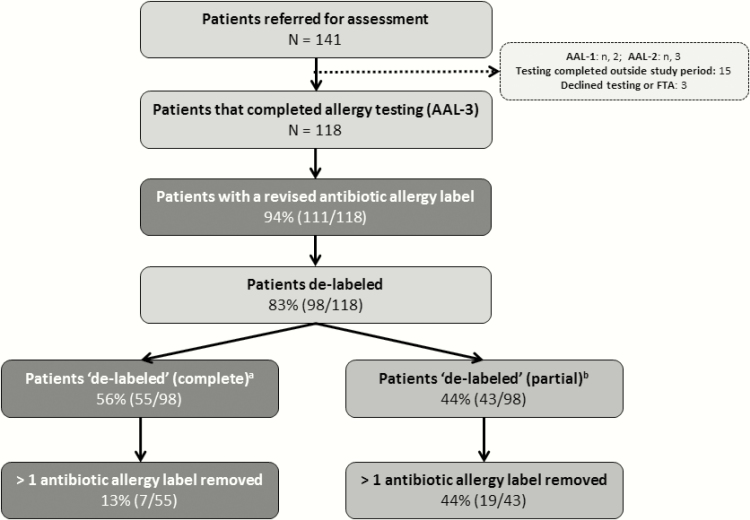
Overall, 141 patients were referred for assessment, 18 completed testing outside the study period or declined testing/failed to attend; 2 patients were identified to be AAL-1 only, 3 patients AAL-2, and 118 patients AAL-3 (Figure 1).
Figure 1.
The proportion of patients de-labeled via the antimicrobial stewardship–led antibiotic allergy testing program. aRemoval of all of the patient’s reported antibiotic allergy labels. bRemoval of ≥1 antibiotic allergy label. Abbreviations: AAL-1, primary antibiotic adverse drug reaction, the result of an non-immune-mediated antibiotic side effect (nausea, vomiting, diarrhea without allergic features); AAL-2, antibiotic adverse drug reaction that had since been disproven by the inadvertent/deliberate administration of the suspected antibiotic by a physician without any untoward consequences; AAL-3, patients with a likely antibiotic allergy that requires formal antibiotic allergy testing into antimicrobial stewardship assessment (ie, immediate hypersensitivity [immunoglobulin E], delayed hypersensitivity [T-cell], or unknown effect); FTA, failed to attend testing.
AAL-3 patients had the following characteristics: None had previously undertaken AAT, 61.9% were female, 48.3% were immunocompromised, and 43% had an AAL for >10 years’ duration (Table 1). In AAL-3 patients, an ADR resulted in hospitalization or occurred during a period of hospitalization in 47% (56/118), while 51% of patients (60/118) required specific therapy (eg, antihistamine, adrenaline, steroid) at time of ADR.
Table 1.
Characteristics of the 118 Antibiotic Allergy Label-3 (AAL-3) Patients
| Demographics | Total, No. (%) |
|---|---|
| Age, y, median (IQR) | 59 (47–70) |
| Sex, female | 73 (61.9) |
| Age-adjusted CCI, median (IQR) | 5 (4–7) |
| Race | |
| White | 111 (94.1) |
| Asian | 7 (5.9) |
| Immunocompromiseda | 57 (48.3) |
| Psychiatric historyb | 14 (11.8) |
| Infective syndromes (n = 224)c | |
| Gastrointestinal infection | 30 (13.4) |
| Bacteremia | 7 (3.1) |
| Central nervous system infection | 4 (1.8) |
| Febrile neutropenia | 17 (7.6) |
| Urinary system infection | 31 (13.8) |
| Otherd | 44 (19.6) |
| Lower/upper respiratory tract infection, including pneumonia | 48 (21.4) |
| Skin, soft tissue, bone, and joint | 43 (19.2) |
| Family history of antibiotic allergy | 24 (20.3) |
| Referrer (setting) | |
| Tertiary referral public hospital | 83 (70.3) |
| Nontertiary referral public hospital | 4 (3.4) |
| Primary care clinic/private rooms | 15 (12.7) |
| Private hospital | 16 (13.6) |
| Referrere | |
| Pharmacist | 12 (10.2) |
| Adverse drug reaction committee | 13 (11.0) |
| Hematologist/oncologist | 13 (11.0) |
| Transplant physician | 6 (5.1) |
| Infectious diseases physician/AMS | 35 (29.6) |
| Allergist/immunologist | 21 (17.8) |
| General practitioner | 14 (11.9) |
| Other | 8 (6.8) |
| No. of antibiotic allergy labels | 226 |
| Mean labels per patient | 1.91 |
| Range | 1–6 |
| Implicated antibiotics per label, median (IQR) | 1 (1–2) |
| ADR episodes for each allergy label, median (IQR) | 1 (1–1) |
| Time since last antibiotic ADR | |
| <1 mo | 2 (1.7) |
| 1–3 mo | 12 (10.2) |
| >3–12 mo | 27 (22.9) |
| >1–5 y | 11 (9.2) |
| >5–10 y | 4 (3.4) |
| >10 y | 51 (43.2) |
| Unknown | 11 (9.3) |
| Naranjo scoref, median (IQR) | 5 (5–5) |
| ALDEN scoreg (n = 8), median (IQR) | 3 (2.5–3) |
| RegiSCAR DRESS scoreh (n = 9), median (IQR) | 4 (2–5) |
Abbreviations: ALDEN, algorithm of drug causality for epidermal necrolysis; AMS, antimicrobial stewardship; CCI, Charlson comorbidity index; DRESS, drug reaction with eosinophilia and systemic symptoms; ADR, adverse drug reaction; IQR, interquartile range; RegiSCAR, registry of severe cutaneous adverse reactions.
aImmunocompromised: hematological malignancy, oncological malignancy, solid organ or stem cell transplant recipient, autoimmune disease, condition requiring >15 mg steroid daily for 1 month.
bPsychiatric history: history of depression, anxiety, or mood disorder.
cInfection history: infective episodes encountered pre– and post–antibiotic allergy testing.
dOther = prophylaxis (23), hepatitis (1), dental infection (1), fever unknown (2), pelvic inflammatory disease (1), endocarditis (4), epiglottitis (3), gynecological infection (3), syphilis (1).
eSome patients were referred by >1 source.
fNaranjo score: 0, doubtful; 1–4, possible; 5–8, probable; >9 definite [25].
gALDEN score: <0, very unlikely; 0–1, unlikely; 2–3, possible; 4–5, probable; ≥6 very probable [26].
hRegiSCAR DRESS score: <2, excluded; 2–3, possible; 4–5, probable; ≥6, definite [27].
Eighty-four percent (99/118) of AAL-3 patients had an infection episode in either the 12 months pre-AAT or 3 months post-AAT, totaling 224 infective episodes (Table 1). There was no statistical difference in the type of infection encountered for the 12 months pre- and 3 months post-AAT, apart from a higher proportion of febrile neutropenia in the pre-AAT period (11% [14/129] vs 41% [39/95], P = .04).
Antibiotic Allergy De-labeling
Pre-AAT AALs.
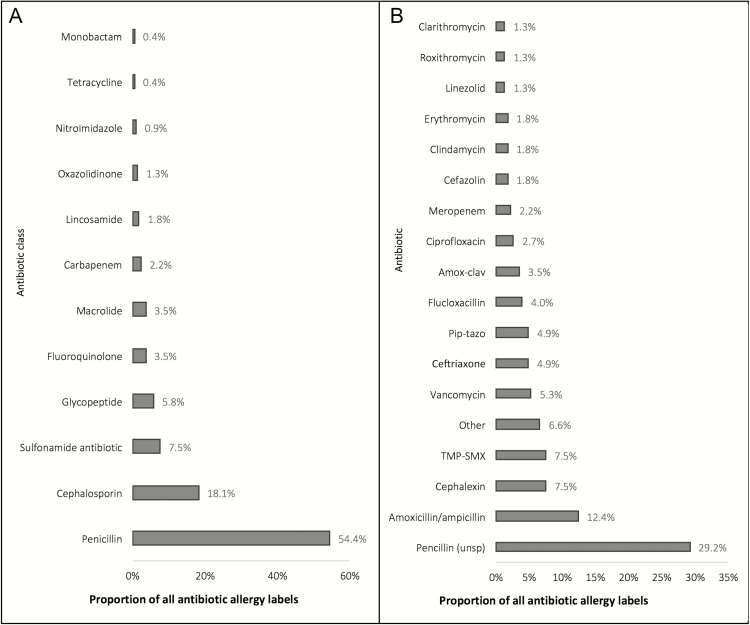
Two hundred twenty-six AALs were identified in the 118 AAL-3 patients, of whom 56% (66/118) harbored a penicillin (unspecified) AAL, 44% (52/118) a cephalosporin AAL, and 30% (35/118) aminopenicillin AAL (Figure 2). AAL phenotypes were as follows: 8% (18/226) type A, 75% (170/226) type B (30%, B1; 35% BIV-maculopapular exanthema; 9% BIV-severe cutaneous adverse drug reaction [SCAR]), and 17% (38/226) were unknown. A predominance of β-lactam AALs was noted for all phenotypes (type A, type B, and unknown reactions, P < .05). At a patient level, 44% (52/118) had ≥1 type BI ADR, 49% (58/118) ≥1 type BIV, and 27% (32/118) ≥1 unknown AAL.
Figure 2.
The antibiotic allergy labels (AALs) encountered in the AAL-3 antibiotic allergy testing cohort. A, AALs (n = 226) per associated antibiotic class. B, AALs (n = 226) per individual implicated antibiotic. “Penicillin” indicates any of penicillin V/G, amoxicillin, ampicillin, flucloxacillin, dicloxacillin, piperacillin-tazobactam, ticarcillin-clavulanate. “Other” indicates metronidazole (2), ceftazidime (2), cefepime (2), cefaclor (2), cefuroxime (1), cephalosporin (unspecified) (1), moxifloxacin (1), teicoplanin (1), aztreonam (1), doxycycline (1), norfloxacin (1). Abbreviations: Amox-clav, amoxicillin-clavulanate; Pip-tazo, piperacillin-tazobactam; TMP-SMX, trimethoprim-sulfamethoxazole; Unsp, unspecified.
AAT de-labeling.
Formal AAT of patients was as follows: 93% (110/118) had SPT and IDT, 87% (103/118) oral provocation after SPT/IDT, 5% (6/118) direct oral provocation, and 6% (7/118) PT. Patch testing was only performed on patients (n = 7) with a history of SCAR. No systemic adverse events following testing were observed.
Consequences of AAT are summarized in (Figure 1). Ninety-four percent (111/118) of patients had AALs revised and 54% (121/226) of all AALs were removed. Of the 6% of patients (7/118) with AALs unable to be revised, 4 of 7 of patients with an AAL phenotype consistent with SCAR were negative upon AAT, 2 of 7 had reported allergy reconfirmed with testing, and 1 of 7 refused an oral provocation. With regard to de-labeling, 83% (98/118) had 1 or more AALs removed, including 83% (55/66) of all patients with penicillin AALs. Of the 13 patients with a revised AAL history that could not be de-labeled, 77% (10/13) had a positive skin test (including to a different antibiotic) and 23% (3/13) a history of SCAR. Post-AAT there was a reduction in patients reporting a penicillin and/or aminopenicillin AAL (65% [77/118] vs 19% [22/118], P = .0001).
AAT-positive patients.
Within the AAT cohort, 27 patients had at least 1 positive test (SPT, IDT, PT, and/or oral provocation), equating to 48 positive tests. All those with a positive test tolerated an alternative antibiotic post-AAT (100% [27/27]) (Table 2). For 3 of the 4 oral provocation–positive patients, the ADR was a subjective report (itch) not requiring therapy; for the remaining patient, amoxicillin hypersensitivity (rash) was detected on day 2 of prolonged oral provocation. The aforementioned single positive delayed hypersensitivity to amoxicillin was the only positive 5-day prolonged provocation (2.85% [1/35]).
Table 2.
β-Lactam Tolerance in the 27 Test-Positive Patients
| Patients With a Positive Testa (n = 27 [48 Individual Positive Results]) |
Antibiotic Provocations Tolerated Post-AAT in Positive Patients | ||
|---|---|---|---|
| Any β-Lactam, No. (%) |
Penicillin or Aminopenicillin, No. (%) |
Cephalosporin, No. (%) |
|
| Penicillin G, PPL, and/or MDM only (n = 2)b | 2 (100) | 1 (50) | 1 (50) |
| Amoxicillin or ampicillin only (n = 8) | 7 (88) | 7 (88) | 5 (63) |
| Alternative penicillin only (n = 2)c | 2 (100) | 2 (100) | 2 (100) |
| Multiple penicillins (n = 8)d | 7 (88) | 1 (13) | 6 (75) |
| Penicillin and cephalosporin (n = 2)e | 1 (100) | 2 (100) | 0 (0) |
| Cephalosporin onlyf (n = 3) | 3 (100) | 3 (100) | 2 (67) |
| Other (n = 2)g | 2 (100) | 2 (100) | 2 (100) |
Abbreviations: AAT, antibiotic allergy testing; MDM, minor determinant mixture; PPL, benzylpenicilloyl poly-l-lysine.
aIncludes patients with a positive skin prick, intradermal test or oral provocation. Patients could have >1 positive test.
bPenicillin G 1 mg/mL, penicillin G 10 mg/mL, PPL, and/or MDM only.
cFlucloxacillin, piperacillin-tazobactam.
dMore than 1 positive to any penicillin, including PPL and/or MDM.
eIncludes a patient positive to cephalosporin and carbapenem.
fOne patient also was positive to clindamycin.
gClavulanic acid and ciprofloxacin.
Of AAL-3 patients with at least 1 positive test to an aminopenicillin (41% [11/27]), 82% (9/11) tolerated penicillin V and 55% (6/11) a first- or second-generation cephalosporin. There were 4 ADRs in the period 3 months post-AAT, including isolated exanthems (2, amoxicillin; 1, cephalexin) and subjective itch (amoxicillin, 1), none requiring therapy. Of these patients, 1 underwent a repeat prolonged challenge with the implicated drug (amoxicillin) without ADR, 1 self-challenged without prior AAT de-labeling (cephalexin), and the remaining 2 patients had not previously reported a hypersensitivity to the ADR-related antimicrobial (amoxicillin).
Impact of AAT on Antibiotic Usage
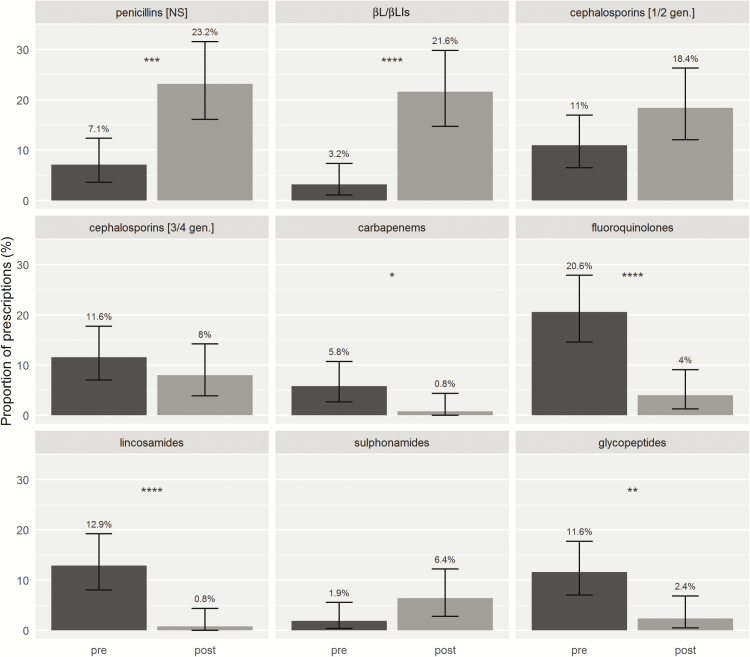
There was an increase in prescribing of guideline-preferred antibiotic therapies 3 months post-AAT compared with the 3 months pre-AAT (83% [104/125] vs 11.6% [18/155], P = .0001) and 12 months pre-AAT (83% [104/125] vs 18% [47/263], P = .0001). A significant reduction in glycopeptide, carbapenem, lincosamide, and fluoroquinolone antibiotic courses and increase in penicillin and β-lactam/β-lactamase inhibitor use was observed 3 months post-AAT compared with 3 months pre-AAT (P < .05) (Figure 3). A similar pattern was noted when antibiotic courses 12 months pre-AAT were compared with 3 months post-AAT with an additional increase in first-/second-generation cephalosporin use (P < .05) (Supplementary Figure 1). After adjusting for indication, setting, and Charlson comorbidity index, prescribers were more likely to select narrow-spectrum penicillins, narrow-spectrum β-lactams, and β-lactam/β-lactamase inhibitors post-AAT, and less likely to select restricted antibiotics (Table 3). Furthermore, when comparing the 3-months pre-AAT with 3 months post-AAT, there was a reduction in patients avoiding penicillin (89% [106/118] vs 9% [10/118], P = .0001), aminopenicillins (92% [109/118] vs 15% [18/118], P = .0001), and first-generation cephalosporins (61% [72/118] vs 14% [16/118], P = .0001) post-AAT.
Figure 3.
Antibiotics administered 3 months before and after antibiotic allergy testing, expressed as a proportion of total antibiotic courses in each period. Abbreviations: NS, narrow spectrum; βL/βLIs, β-lactam/β-lactamase inhibitor; gen., generation; pre, pre–antibiotic allergy testing (3 months prior); post, post–antibiotic allergy testing (3 months after). *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001.
Table 3.
Effect of Antibiotic Allergy Testing on Antibiotic Usage
| Antibiotic Classification | Period of Analysis | Crude ORa (95% CI) | Adjusted ORb (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Pre-AAT | Post-AAT | 3 mo Pre- vs 3 mo Post-AAT | 12 mo Pre- vs 3 mo Post-AAT | 3 mo Pre- vs 3 mo Post-AAT | 12 mo Pre- vs 3 mo Post-AAT | ||
| 12 mo, No. (%) (n = 263) | 3 mo, No. (%) (n = 155) | 3 mo, No. (%) (n = 125) | |||||
| NS penicillin | 27 (10.3) | 11 (7.1) | 29 (23.2) | 4.69 (2.02–10.92) | 3.34 (1.69–6.63) | 2.81 (1.45–5.42) | 4.07 (1.78–9.32) |
| NS β-lactam | 49 (18.6) | 25 (16.1) | 49 (39.2) | 4.67 (2.27–9.58) | 3.68 (2.05–6.60) | 3.54 (1.98–6.33) | 5.27 (5.22–5.31) |
| βL/βLI | 13 (4.9) | 5 (3.2) | 27 (21.6) | 11.17 (3.25–38.36) | 6.58 (2.83–15.26) | 9.25 (3.57–24.01) | 25.02 (4.71–132.87) |
| Restricted antibiotic | 150 (57) | 98 (63%) | 20 (16) | 0.11 (.06–.20) | 0.13 (.07–.24) | 0.16 (.09–.29) | 0.11 (.06–.22) |
Abbreviations: AAT, antibiotic allergy testing; βL/βLI, β-lactam/β-lactamase inhibitor; CI, confidence interval; NS, narrow-spectrum; OR, odds ratio.
aFrom univariable mixed-effects logistic regression models.
bFrom multivariable mixed-effects logistic regression models, controlled for age-adjusted Charlson comorbidity index, infective episode setting (inpatient/outpatient), and indication category.
Impact of AAT on Antibiotic Appropriateness
The proportion of antibiotic prescriptions that were appropriate was higher 3 months post-AAT than 3 months pre-AAT (95% [119/125] vs 62% [96/155]; OR, 13.25; 95% CI, 5.22–33.61). Analysis by multivariable logistic regression adjusting for indication, setting, and Charlson comorbidity index demonstrated appropriate prescribing to be significantly more likely in the post-AAT period (adjusted OR, 12.27; 95% CI, 5.00–30.09). Results from the 12 months pre- vs 3 months post-AAT analysis were similar (adjusted OR, 15.23; 95% CI, 5.65–41.10).
DISCUSSION
With increasing healthcare-associated infections due to antimicrobial-resistant organisms, attention has turned toward developing novel AMS programs. Recent Infectious Diseases Society of America guidelines acknowledge the utility of allergy assessment, but highlight this to be largely unstudied in AMS interventions [21]. We provide evidence that a multidisciplinary, real-world AAT program integrated into AMS enables safe and effective de-labeling. Importantly, this program increased narrow-spectrum β-lactam use and improved appropriateness of antibiotic prescribing.
We were able to not only completely remove 85% of penicillin allergy labels but also provide an alternative class-related antimicrobial in those with confirmed true allergy. Our de- labeling rate was consistent with previous reports suggesting that 80%–90% of penicillin AALs can be removed [20]. Importantly, even in those with a confirmed β-lactam allergy, alternative narrow-spectrum β-lactams could be safely employed post-AAT. Contrary to historically reported high rates of β-lactam ring cross-reactivity [33], current evidence suggests the true rate of penicillin and first- and third-generation cephalosporin cross-reactivity to be <5% and <1%–2%, respectively. For example, selective allergy to cefazolin is based on unique side chains (R groups) not shared by other cephalosporins [34]. Our study supports AAT in patients with confirmed β-lactam hypersensitivity, as a means of widening clinician antibiotic choice.
We demonstrated that AAT-AMS increased narrow- spectrum penicillin use, increased uptake of preferred therapies, and reduced restricted antibiotic use. The inability to employ preferred β-lactam therapies is not only associated with inferior outcomes (eg, use of vancomycin for treatment of methicillin-sensitive Staphylococcus aureus infections), but the utilization of agents associated with development of antibiotic resistance and Clostridium difficile infections (eg, cephalosporins). Desensitization to narrow-spectrum β-lactams is an effective temporary solution complicated by potential treatment delays and lack of de- or relabeling potential [35]. Our integrated AAT-AMS program was able to avert the issues of desensitization and effect antibiotic change and behavior modification on antibiotic prescribing. Published studies repeatedly demonstrate AALs to be associated with reduced β-lactam utilization and increased restricted prescriptions [5, 6, 10, 11, 36–38]. Our program was able to increase narrow-spectrum β-lactam uptake and reduce restricted antibiotic utilization. These findings are consistent with previous studies of education programs, pharmacist-led AMS/allergy rounds, inpatient SPT, and oral provocation/rechallenge, which have been shown to increase β-lactam uptake [14, 16, 19, 39, 40]. A better measure of the success of an AMS program may be examining antibiotic prescribing appropriateness [41, 42]. To our knowledge, this is the first study to demonstrate the impact of AAT-AMS on improving the appropriateness of antibiotic prescribing.
Our AAT-AMS service provided weekly clinical consultation and testing, underpinned by multidisciplinary collaboration, utilizing ID/AMS knowledge of current patient antibiotic needs. Although comparable coordinated programs may not always be feasible (eg, in smaller centers), the establishment of decision support and limited AAT is achievable [43], with recent examples of successful AAT programs led by ID fellows and pharmacists [44, 45]. Safe implementation of an AAT-AMS service requires significant and continued clinician education regarding antibiotic allergy. For instance, selective IgE-mediated reactions based on the R1 or R2 groups of penicillins and cephalosporins occur not uncommonly in contemporary practice. The use of major/minor determinants with or without benzylpenicillin for skin testing would miss selective aminopenicillin allergy, and there remains no reliable skin testing for aminocephalosporin anaphylaxis (oral drug formulations only are available). Given the lack of 100% negative predictive and/or absence of validated reagents for aminopenicillin or aminocephalosporin allergy, respectively, oral provocation after negative skin testing where the pretest proability of true IgE-mediated reaction is high should be performed with caution in settings experienced with prompt treatment of anaphylaxis.
The morbidity associated with not testing a patient with a self-reported antibiotic allergy has been previously highlighted [46]. Although we have not performed formal cost- effectiveness analyses, we believe that long-term benefits regarding antimicrobial costs, patient outcomes, and antimicrobial resistance outweigh the acquisition of costs of testing reagents [14, 47, 48].
Limitations to the current study include the fact that it was nonrandomized, with potential selection bias toward patients with a recently encountered infection. Testing was performed in the recovery phase post-ADR and reproducibility in critically or acutely unwell patients is therefore unknown. Although our study cohort comprised a significant number of immunocompromised patients, we encountered no adverse events or false-negatives on skin testing or oral provocation. A high proportion mounted delayed (T-cell regulated) skin responses, suggesting that findings are relevant to the broader group of immunocompetent and immunocompromised patients. Finally, assessment of antibiotic appropriateness was based on Australian national prescribing guidelines [31] and it is not known if equivalent outcomes would be identified using an alternative gold-standard comparator.
We demonstrate the success of a real-world integrated program to show significant improvement in antibiotic appropriateness in a patient cohort frequently in need of antibiotic therapy, of which a large portion were immunocompromised with serious ADR history. Key stakeholder engagement in a multidisciplinary program ensures maintenance of a quality service and clinician support. Future work must focus on improving AAL assessment at the onset, enhanced clinician and pharmacist education to sustain improvements in antibiotic utilization, and evaluation of the long-term impact and cost- benefit of AAT.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Supplementary Material
Notes
Acknowledgments. We thank the Pharmacy and Infectious Diseases Departments of Austin Health and Peter MacCallum Cancer Centre for supporting this program. We also thank the dedicated infectious diseases registrars, Victoria Hall, Rekha Pai Mangalore and Abby Douglas, who assisted with study recruitment.
Financial support. This work was supported by the Austin Medical Research Foundation. J. A. T. is supported by a National Health and Medical Research Council (NHMRC) postgraduate scholarship. E. J. P. is supported in part by the National Institutes of Health (NIH) (award numbers 1P50GM115305-01 and 1R01AI103348-01), the NIH-funded Tennessee Center for AIDS Research (P30 AI110527), 1R13 AR071267-01, NHMRC, ACH2, and the Angela Anderson Foundation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Trubiano J, Phillips E. Antimicrobial stewardship’s new weapon? A review of antibiotic allergy and pathways to “de-labeling”. Curr Opin Infect Dis 2013; 26:526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Macy E. Penicillin and beta-lactam allergy: epidemiology and diagnosis. Curr Allergy Asthma Rep 2014; 14:476. [DOI] [PubMed] [Google Scholar]
- 3. Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother 2008; 42:1017–25. [DOI] [PubMed] [Google Scholar]
- 4. Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy 2011; 31:742–7. [DOI] [PubMed] [Google Scholar]
- 5. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol 2014; 133:790–6. [DOI] [PubMed] [Google Scholar]
- 6. Trubiano JA, Leung VK, Chu MY, Worth LJ, Slavin MA, Thursky KA. The impact of antimicrobial allergy labels on antimicrobial usage in cancer patients. Antimicrob Resist Infect Control 2015; 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banks TA, Ressner RA, Gada SM. Antibiotic reclamation: penicillin allergy, antibiotic stewardship, and the allergist. Ann Allergy Asthma Immunol 2015; 115:451–2. [DOI] [PubMed] [Google Scholar]
- 8. Ressner RA, Gada SM, Banks TA. Antimicrobial stewardship and the allergist: reclaiming our antibiotic armamentarium. Clin Infect Dis 2016; 62:400–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Unger NR, Gauthier TP, Cheung LW. Penicillin skin testing: potential implications for antimicrobial stewardship. Pharmacotherapy 2013; 33:856–67. [DOI] [PubMed] [Google Scholar]
- 10. Trubiano JA, Chen C, Cheng AC, Grayson ML, Slavin MA, Thursky KA; National Antimicrobial Prescribing Survey (NAPS) Antimicrobial allergy “labels” drive inappropriate antimicrobial prescribing: lessons for stewardship. J Antimicrob Chemother 2016; 71:1715–22. [DOI] [PubMed] [Google Scholar]
- 11. Trubiano JA, Pai Mangalore R, Baey YW, et al. Old but not forgotten: antibiotic allergies in general medicine (the AGM Study). Med J Aust 2016; 204:273. [DOI] [PubMed] [Google Scholar]
- 12. Manzaneque A, López-Cabezas C, Mensa M, et al. Potentially inappropriate prescription in patients with a history of allergy to β-lactam antibiotics: a health care challenge. J Investig Allergol Clin Immunol 2016; 26:55–6. [PubMed] [Google Scholar]
- 13. Rimawi RH, Cook PP, Gooch M, et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med 2013; 8:341–5. [DOI] [PubMed] [Google Scholar]
- 14. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with β-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol 2016; 117:67–71. [DOI] [PubMed] [Google Scholar]
- 15. Blumenthal KG, Shenoy ES, Hurwitz S, Varughese CA, Hooper DC, Banerji A. Effect of a drug allergy educational program and antibiotic prescribing guideline on inpatient clinical providers’ antibiotic prescribing knowledge. J Allergy Clin Immunol Pract 2014; 2:407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blumenthal KG, Shenoy ES, Varughese CA, Hurwitz S, Hooper DC, Banerji A. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol 2015; 115:294–300 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Estep PM, Ferreira JA, Dupree LH, Aldridge PJ, Jankowski CA. Impact of an antimicrobial stewardship initiative to evaluate β-lactam allergy in patients ordered aztreonam. Am J Health Syst Pharm 2016; 73:S8–13. [DOI] [PubMed] [Google Scholar]
- 18. Staicu ML, Brundige ML, Ramsey A, et al. Implementation of a penicillin allergy screening tool to optimize aztreonam use. Am J Health Syst Pharm 2016; 73:298–306. [DOI] [PubMed] [Google Scholar]
- 19. Swearingen SM, White C, Weidert S, Hinds M, Narro JP, Guarascio AJ. A multidimensional antimicrobial stewardship intervention targeting aztreonam use in patients with a reported penicillin allergy. Int J Clin Pharm 2016; 38:213–7. [DOI] [PubMed] [Google Scholar]
- 20. Bourke J, Pavlos R, James I, Phillips E. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol 2015; 3:365–34 e1. [DOI] [PubMed] [Google Scholar]
- 21. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 23. Rawlins MD, van der Cammen TJ. Textbook of adverse drug reactions. Oxford: Oxford University Press, 1977. [Google Scholar]
- 24. Pichler WJ, ed. Drug hypersensitivity. Basel: Karger Publishers, 2007:168–89. [Google Scholar]
- 25. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30:239–45. [DOI] [PubMed] [Google Scholar]
- 26. Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther 2010; 88:60–8. [DOI] [PubMed] [Google Scholar]
- 27. Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. ; RegiSCAR Study Group Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol 2013; 169:1071–80. [DOI] [PubMed] [Google Scholar]
- 28. National Antimicrobial Prescribing Survey. Available at: https://naps.org.au/ Accessed 1 March 2015. [Google Scholar]
- 29. Antibiotic Expert Groups. Therapeutic guidelines: antibiotic. Version 15 Melbourne: Therapeutic Guidelines Limited, 2014. [Google Scholar]
- 30. National Healthcare Safety Network. CDC/NHSN surveillance definitions for specific types of infections. Available at: www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf Accessed 1 January 2016. [Google Scholar]
- 31. Fernández J, Torres MJ, Campos J, Arribas-Poves F, Blanca M; DAP-Diater Group Prospective, multicenter clinical trial to validate new products for skin tests in the diagnosis of allergy to penicillin. J Investig Allergol Clin Immunol 2013; 23:398–408. [PubMed] [Google Scholar]
- 32. Brockow K, Garvey LH, Aberer W, et al. ; ENDA/EAACI Drug Allergy Interest Group Skin test concentrations for systemically administered drugs—an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2013; 68:702–12. [DOI] [PubMed] [Google Scholar]
- 33. Trubiano JA, Worth LJ, Urbancic K, et al. ; Australasian Society for Infectious Diseases Clinical Research Network; Australasian Society of Clinical Immunology and Allergy Return to sender: the need to re-address patient antibiotic allergy labels in Australia and New Zealand. Intern Med J 2016; 46:1311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uyttebroek AP, Decuyper II, Bridts CH, et al. Cefazolin hypersensitivity: toward optimized diagnosis. J Allergy Clin Immunol Pract 2016; 4:1232–6. [DOI] [PubMed] [Google Scholar]
- 35. Legendre DP, Muzny CA, Marshall GD, Swiatlo E. Antibiotic hypersensitivity reactions and approaches to desensitization. Clin Infect Dis 2014; 58:1140–8. [DOI] [PubMed] [Google Scholar]
- 36. Trubiano JA, Cairns KA, Evans JA, et al. The prevalence and impact of antimicrobial allergies and adverse drug reactions at an Australian tertiary centre. BMC Infect Dis 2015; 15:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blumenthal KG, Shenoy ES, Huang M, et al. The impact of reporting a prior penicillin allergy on the treatment of methicillin-sensitive Staphylococcus aureus bacteremia. PLoS One 2016; 11:e0159406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. MacFadden DR, LaDelfa A, Leen J, et al. Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis 2016; 63:904–10. [DOI] [PubMed] [Google Scholar]
- 39. Park MA, McClimon BJ, Ferguson B, et al. Collaboration between allergists and pharmacists increases β-lactam antibiotic prescriptions in patients with a history of penicillin allergy. Int Arch Allergy Immunol 2011; 154:57–62. [DOI] [PubMed] [Google Scholar]
- 40. Vezir E, Dibek Misirlioglu E, Civelek E, et al. Direct oral provocation tests in non-immediate mild cutaneous reactions related to beta-lactam antibiotics. Pediatr Allergy Immunol 2016; 27:50–4. [DOI] [PubMed] [Google Scholar]
- 41. James R, Upjohn L, Cotta M, et al. Measuring antimicrobial prescribing quality in Australian hospitals: development and evaluation of a national antimicrobial prescribing survey tool. J Antimicrob Chemother 2015; 70:1912–8. [DOI] [PubMed] [Google Scholar]
- 42. Morris AM. Antimicrobial stewardship programs: appropriate measures and metrics to study their impact. Curr Treat Options Infect Dis 2014; 6:101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Narayan P, Jeffres M. Feasibility, benefits, and limitations of a penicillin allergy skin testing service [manuscript published online ahead of print 1 February 2017]. Ann Pharmacother 2017. doi:10.1177/1060028017690854. [DOI] [PubMed] [Google Scholar]
- 44. Chen JR, Tarver SA, Alvarez KS, Tran T, Khan DA. A proactive approach to penicillin allergy testing in hospitalized patients [manuscript published online ahead of print 22 November 2016]. J Allergy Clin Immunol 2016. doi:10.1016/j.jaip.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 45. Heil EL, Bork JT, Schmalzle SA, et al. Implementation of an infectious disease fellow-managed penicillin allergy skin testing service. Open Forum Infect Dis 2016; 3: ofw155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Macy E, Khan DA, Castells MC, Lang DM. Penicillin allergy testing, a key component of antibiotic stewardship. Clin Infect Dis 2017; 64(4):531–2. [DOI] [PubMed] [Google Scholar]
- 47. van Dijk SM, Gardarsdottir H, Wassenberg MW, Oosterheert JJ, de Groot MC, Rockmann H. The high impact of penicillin allergy registration in hospitalized patients. J Allergy Clin Immunol Pract 2016; 4:926–31. [DOI] [PubMed] [Google Scholar]
- 48. Blumenthal KG, Parker RA, Shenoy ES, Walensky RP. Improving clinical outcomes in patients with methicillin-sensitive Staphylococcus aureus bacteremia and reported penicillin allergy. Clin Infect Dis 2015; 61:741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.