Summary
Fifteen virologically suppressed, HIV-1–infected patients received 4 weeks of Toll-like receptor 9 agonist treatment. During treatment, we observed elevated antiviral cytokine levels, upregulation of interferon-stimulated genes, activation of innate immune cells, and plasma HIV RNA blips indicating enhanced HIV-1 transcription.
Keywords: latent HIV-1 infection, latency reversal, TLR9 agonist, immune therapeutic treatment, NK cell activation.
Abstract
Background.
Treatment with latency reversing agents (LRAs) enhances human immunodeficiency virus type 1 (HIV-1) transcription in vivo but leads to only modest reductions in the size of the reservoir, possibly due to insufficient immune-mediated elimination of infected cells. We hypothesized that a single drug molecule—a novel Toll-like receptor 9 (TLR9) agonist, MGN1703—could function as an enhancer of innate immunity and an LRA in vivo.
Methods.
We conducted a single-arm, open-label study in which 15 virologically suppressed HIV-1–infected individuals on antiretroviral therapy received 60 mg MGN1703 subcutaneously twice weekly for 4 weeks. We characterized plasmacytoid dendritic cell, natural killer (NK), and T-cell activation using flow cytometry on baseline and after 4 weeks of treatment. HIV-1 transcription was quantified by measuring plasma HIV-1 RNA during MGN1703 administration.
Results.
In accordance with the cell type–specific expression of TLR9, MGN1703 treatment led to pronounced activation of plasmacytoid dendritic cells and substantial increases in plasma interferon-α2 levels (P < .0001). Consistently, transcription of interferon-stimulated genes (eg, OAS1, ISG15, Mx1; each P < .0001) were upregulated in CD4+ T cells as demonstrated by RNA sequencing. Further, proportions of activated cytotoxic NK cells and CD8+ T cells increased significantly during MGN1703 dosing, suggesting an enhancement of cellular immune responses. In 6 of 15 participants, plasma HIV-1 RNA increased from <20 copies/mL to >1500 copies/mL (range, 21–1571 copies/mL) during treatment.
Conclusions.
TLR9 agonist treatment in HIV infection has a dual potential by increasing HIV-1 transcription and enhancing cytotoxic NK cell activation, both of which are key outcomes in HIV-1 eradication therapy.
Clinical Trials Registration.
Antiretroviral therapy (ART) effectively suppresses human immunodeficiency virus type 1 (HIV-1) [1]. However, replication-competent proviruses persist in long-lived resting memory CD4+ T cells [2–5] and neither ART nor the immune system is capable of eliminating this latent viral reservoir [6]. Proof-of-concept trials testing the “shock and kill” approach [7] to eradicate the reservoir have demonstrated that treatment with latency reversing agents (LRAs) can enhance transcription of HIV-1 RNA from latently infected cells in vivo [8–12]. Yet clinical trials have found no, or only modest, reductions in the size of the HIV-1 reservoir, possibly due to insufficient immune-mediated killing of infected cells [13]. In vivo and ex vivo studies demonstrate that priming of specific effector cells, such as cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, can enhance their capacity to recognize and kill infected cells [13–17]. This indicates that therapeutic interventions boosting NK and CTL activity could lead to elimination of cells expressing HIV-1 antigens. MGN1703 (lefitolimod), a novel Toll-like receptor 9 (TLR9) agonist, belongs to a class of drugs referred to as immune surveillance reactivators and is currently in phase 3 testing for treatment of metastatic colorectal cancer [18]. Preclinical testing and data from cancer trials demonstrates that MGN1703 activates immune functions through TLR9 stimulation. MGN1703-induced TLR9 signaling leads to increased secretion of a range of cytokines (eg, interferon [IFN] α) and activation of plasmacytoid dendritic cells (pDCs) and B cells [18]. Furthermore, MGN1703 increases activation and cytotoxicity of NK cells in vitro [19]. We recently demonstrated ex vivo that MGN1703 stimulation of peripheral blood mononuclear cells from HIV-1–infected donors enhanced the cellular immune response and increased HIV-1 transcription in CD4+ T cells [20]. Based on our ex vivo findings, we hypothesized that adjunctive MGN1703 treatment in individuals with HIV infection would (1) augment NK and CD8+ T-cell activation and (2) induce plasma viremia. To test our hypotheses, we enrolled 15 virologically suppressed HIV-1–infected individuals on ART to receive MGN1703 for 4 consecutive weeks.
MATERIALS AND METHODS
Study Design
This trial was an investigator-initiated, single-arm, open-label phase 1b/2a trial conducted from April to December 2015. We enrolled adults from the HIV outpatient clinics of Aarhus University Hospital and Hvidovre Hospital, Denmark (Figure 1). Inclusion criteria were CD4+ T-cell count >350 cells/μL, plasma HIV-1 RNA < 50 copies/mL, and on ART >12 months. Exclusion criteria were hepatitis B or C coinfection, fever of unknown origin, antibiotic treatment or significant acute medical illness 2 weeks prior to inclusion, autoimmune disease, and pregnancy (complete list of inclusion/exclusion criteria is available at ClinicalTrials.gov, identifier NCT02443935). Prior to enrollment, subjects underwent a general physical examination, electrocardiogram recording, and safety laboratory tests.
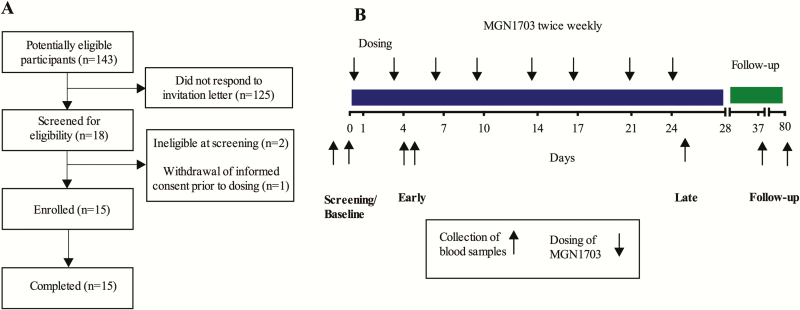
Figure 1.
Recruitment of participants and outline of study design. A, Flow diagram depicting the recruiting of human immunodeficiency virus–infected participants for MGN1703 treatment. B, Outline of study design. Participants received a total of 8 doses of MGN1703 during 4 weeks (down arrows). Blood samples (up arrows) for plasma cytokines and flow cytometry were drawn at the screening visit, baseline, during the first week (day 4 or 5, “early”) and last (day 25, “late”) week of MGN1703 treatment and at follow-up (day 37).
The study was approved by the National Health Ethics Committee, Denmark (case number 1-10-72-10-15), the Danish Medicines Agency (case number 2015014125), and the Danish Data Protection Agency. The trial was monitored in accordance with the principles for good clinical practice. Each patient provided written informed consent prior to any study procedures.
Procedures
As 120 mg MGN1703 per week is considered safe in cancer patients and healthy controls [18, 21, 22], study participants received 4 weeks of 60 mg (concentration 15 mg/mL) of MGN1703 (MOLOGEN AG, Berlin, Germany), administered subcutaneously by the study investigator as two 2-mL bilateral injections twice weekly. ART was maintained during the entire study period. The study comprised 15 visits and the total duration was 141 days for each participant. Blood samples for safety and laboratory analyses were collected 2–5 times per week during dosing, always prior to MGN1703 administration if scheduled. Participants returned for postdosing visits 14 days and 6 weeks after completion of MGN1703 treatment. Safety was assessed at each visit. Laboratory abnormalities were registered as adverse events (AEs) when they were associated with clinical symptoms or objective signs, with the exception of neutropenia. AEs were graded in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, and the investigators assessed causality to the study drug. Nonstandard laboratory analyses are described in the Supplementary Materials.
Biostatistical Analyses
As NK cell activation was the primary outcome, the study was powered to detect change in CD69+ expression on NK cells from baseline. Given a standard deviation (SD) of 0.1, enrolling 11 participants would give a power of 90% to detect ≥50% change at a 5% significance level. We enrolled 15 study subjects to accommodate for dropouts. Statistical analyses and graphs were done using GraphPad Prism 6.0 software. We used 2-tailed Wilcoxon signed-rank test to analyze changes from baseline and Spearman rank test for correlations.
RESULTS
Participants
Fifteen HIV-1–infected individuals (13 male, 2 female) with a median age of 52 years were enrolled in this study (Figure 1). Median duration of ART was 8.6 years (interquartile range [IQR], 5.3–14 years) and median time with HIV-1 RNA <50 copies/mL was 3.3 years (IQR, 1.6–8.4 years). Median baseline CD4+ T-cell count was 583 cells/μL (Table 1). All participants completed MGN1703 treatment and both follow-up visits.
Table 1.
Participant Baseline Characteristics (N = 15)
| Characteristic | No. (%) or Median (IQR) |
|---|---|
| Sex, No. (%) | |
| Female | 2 (13) |
| Male | 13 (87) |
| Ethnic origin, No. (%) | |
| White | 13 (87) |
| African Danish | 2 (13) |
| Age, y, median (IQR) | 52 (46–55) |
| Years since HIV-1 diagnosis, median (IQR) | 11 (5.3–15.58) |
| Years from HIV diagnosis to ART initiation, median (IQR) | 0.25 (0–1.5) |
| ART regimen, No. (%) | |
| PI-based | 7 (47) |
| NNRTI-based | 6 (40) |
| INI-based | 2 (13) |
| Years on ART, median (IQR) | 8.6 (5.25–14.08) |
| Years with HIV RNA <50 copies/mL, median, (IQR)a,b | 3.25 (1.6–8.3) |
| Nadir CD4+ T-cell count, cells/µL, median (IQR) | 212 (29–400) |
| Baseline CD4+ T-cell count, cells/µL, median (IQR) | 583 (470–890) |
| Pre ART viral load, log10 copies/mL, median (IQR) | 4.92 (2.77–5.47) |
| Baseline level of total HIV-1 DNA, copies/million CD4+ T cells, median (IQR) | 400.51 (181–1010) |
| Baseline level of CA-US HIV-1 RNA, copies/million CD4+ T cells, median (IQR) | 24.6 (5.9–39.49) |
Abbreviations: ART, antiretroviral therapy; CA-US, cell-associated unspliced; HIV, human immunodeficiency virus; INI, integrase inhibitor; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
aConsecutive years of documented viral load (plasma HIV-1 RNA) suppression <50 copies/mL. Viral load was analyzed using the same assay as for regular visits in the outpatient clinic.
bTwo participants had 1 blip and 1 participant had 2 blips since full suppression by ART.
MGN1703 Was Safe and Well-Tolerated
A total of 81 AEs were registered (Table 2), of which 57 were considered related to MGN1703. The most frequent drug- related AE was injection site reaction (23 reported events, primarily transient erythema). All drug-related AEs were grade 1, with the exception of 4 cases of neutropenia, of which 3 were grade 2 and 1 grade 3. Neutropenia can be observed within 24 to 72 hours after administration of a TLR9 agonist as a known class-effect of CpG-oligodeoxynucleotide analogues [22]. The neutropenia resolved spontaneously within few days (Supplementary Figure 1) and was likely indicative of a redistribution of leukocytes due to homing of the cells to tissues rather than a bone marrow cytotoxic effect. No AEs required additional follow-up beyond end of study as all AEs resolved spontaneously.
Table 2.
Adverse Events and Their Severity During MGN1703 Treatment (N = 15)
| Type of Adverse Event | Grade 1 | Grade 2 | Grade 3 | Any Grade | No. of Patients |
|---|---|---|---|---|---|
| Related to MGN1703 | |||||
| Fatigue | 7 | 7 | 5 | ||
| Fever | 1 | 1 | 1 | ||
| Malaise | 4 | 4 | 4 | ||
| Headache | 3 | 3 | 3 | ||
| Injection site reaction | 23 | 23 | 9 | ||
| Neutropeniaa | 7 | 3 | 1 | 11 | 10 |
| Localized lymphadenopathy (<0.5 x 0.5 cm) | 1 | 1 | 1 | ||
| Taste alteration | 1 | 1 | 1 | ||
| Dry mouth | 1 | 1 | 1 | ||
| Nausea | 1 | 1 | 1 | ||
| Diarrhea | 3 | 3 | 3 | ||
| Muscle pain | 1 | 1 | 1 | ||
| Not related to MGN1703 | |||||
| Gingival bleeding | 1 | 1 | 1 | ||
| Abdominal pain | 7 | 7 | 6 | ||
| Genital herpes infection (recurrence)b | 1 | 1 | 1 | ||
| Headache | 3 | 3 | 3 | ||
| Fatigue | 3 | 3 | 3 | ||
| Upper extremity pain | 2 | 2 | 2 | ||
| Dizziness | 1 | 1 | 1 | ||
| Oral blisters | 1 | 1 | 1 | ||
| Tooth infection | 1 | 1 | 1 | ||
| Insomnia | 1 | 1 | 1 | ||
| Reduced hair growth | 1 | 1 | 1 | ||
| Weight loss | 1 | 1 | 1 | ||
| Muscle pain | 1 | 1 | 1 | ||
aNeutropenia: grade 1, <lower limit of normal–1.5 × 109/L; grade 2, 1.5–1.0 × 109/L; grade 3, 1.0–0.5 × 109/L.
bDiscrete symptoms began before the first dose. This was a recurrence of a latent genital herpes infection that the participant reported to be frequently experienced. It was therefore considered not related to the study drug.
Early Plasmacytoid Dendritic Cell Activation
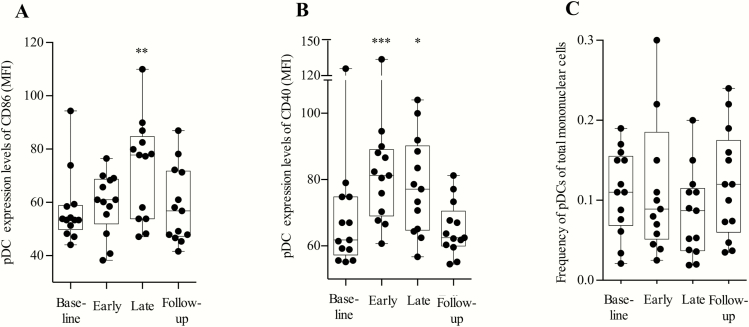
The MGN1703 molecule activates TLR9 in the endosomal compartment of pDCs. To confirm the direct effects of MGN1703 on pDCs, we performed flow cytometry analyses (Figure 2). As anticipated, expression of pDC costimulatory activation markers CD86 (P = .002) and CD40 (P = .0005) (Figure 2A–C) increased during MGN1703 treatment (gating strategy depicted in Supplementary Figure 2). After MGN1703 discontinuation, CD86 and CD40 expression returned to baseline levels, indicative of a reversible effect of TLR9 stimulation. In conclusion, MGN1703 effectively activates pDCs in HIV-infected individuals.
Figure 2.
MGN1703 increased expression of CD86 and CD40 on plasmacytoid dendritic cells. Flow cytometry analyses of fresh peripheral blood mononuclear cells were performed at the indicated time-points: baseline, day 0; early, day 5 (48 hours after second dose); late, day 24 (24 hours after eighth/last dose); and follow-up, 14 days after the last dose (n = 15). A, Plasmacytoid dendritic cell (pDC) expression levels of the marker CD86 (median fluorescence intensity [MFI]). B, pDC expression levels of the marker CD40 (MFI). C, Proportions of pDCs of total mononuclear cells. Statistical comparisons were 2-tailed, Wilcoxon signed-rank test as paired analysis against baseline. *P < .05; **P < .01; ***P < .001.
Potent Endogenous Interferon-α2 Response
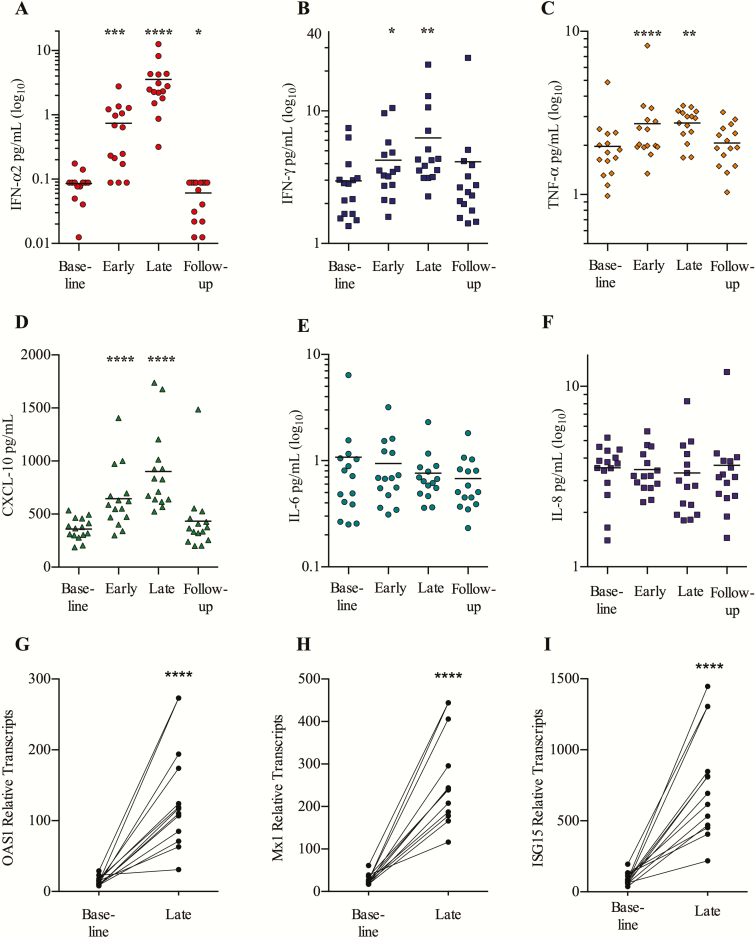
We observed potent induction of plasma IFN-α2 from baseline to the last week of treatment (4-fold median increase [IQR, 2.1- to 14.1-fold]; P = .0001) (Figure 3A). The levels of plasma INF-γ and tumor necrosis factor-α also increased (median, 1.8-fold, P = .003 and 1.5-fold, P < .0001, respectively) (Figure 3B and 3C). Accordingly, we observed elevated plasma CXCL10 (IFN-γ–induced protein 10 [IP-10]) (P < .0001; Figure 3D), which is a chemokine produced in response to type I and II interferon [23]. Levels of the proinflammatory cytokines interleukin 6 (IL-6) and interleukin 8 (IL-8) did not change (Figure 3E and 3F), which is in line with the observed low frequency of reactogenic AEs.
Figure 3.
Potent endogenous interferon (IFN)-α2 response and transcription of IFN-stimulated genes. Levels of the plasma cytokines IFN-α2 (A), IFN-γ (B), tumor necrosis factor (TNF)-α (C), IFN-γ–induced protein 10 (CXCL-10) (D), interleukin (IL) 6 (E), and IL-8 (F) were run in duplicates, using Meso Scale Multiplex assay. Indicated time-points were as follows: baseline, day 0; early, day 4 (24 hours after second dose); late, day 24 (24 hours after eighth/last dose) and follow-up, 14 days after the last dose (n = 15). Intracellular transcription of interferon-stimulated genes in CD4+ T cells was analyzed on baseline (day 0) and late (day 24) (24 hours after eighth/last dose). OAS1 (G), Mx1 (H), and ISG15 (I) were significantly increased following Toll-like receptor 9 stimulation (n = 13). Statistical comparisons were 2-tailed, Wilcoxon signed-rank test as paired analysis against baseline. *P < .05; **P < .01; ***P < .001.
To assess the downstream effect of the TLR9-induced type I interferons, we did RNA sequencing on purified blood CD4+ T cells and observed markedly increased transcription of numerous interferon-stimulated genes (ISGs) including OAS1 (P = 7.43 × 10–7, false discovery rate [FDR] = 0.00016), Mx1 (P = 2.54 × 10–8, FDR = 1.7 × 10–5), and ISG15 (P = 6.1 × 10–7, FDR = 0.00016) (Figure 3G–I and Supplementary Figure 3). In conclusion, MGN1703 treatment induced potent interferon responses, confirmed by enhanced transcription of ISGs, but did not cause unspecific secretion of potentially detrimental proinflammatory cytokines.
Enhanced Activation of Cytotoxic CD56dimCD16+ NK cells
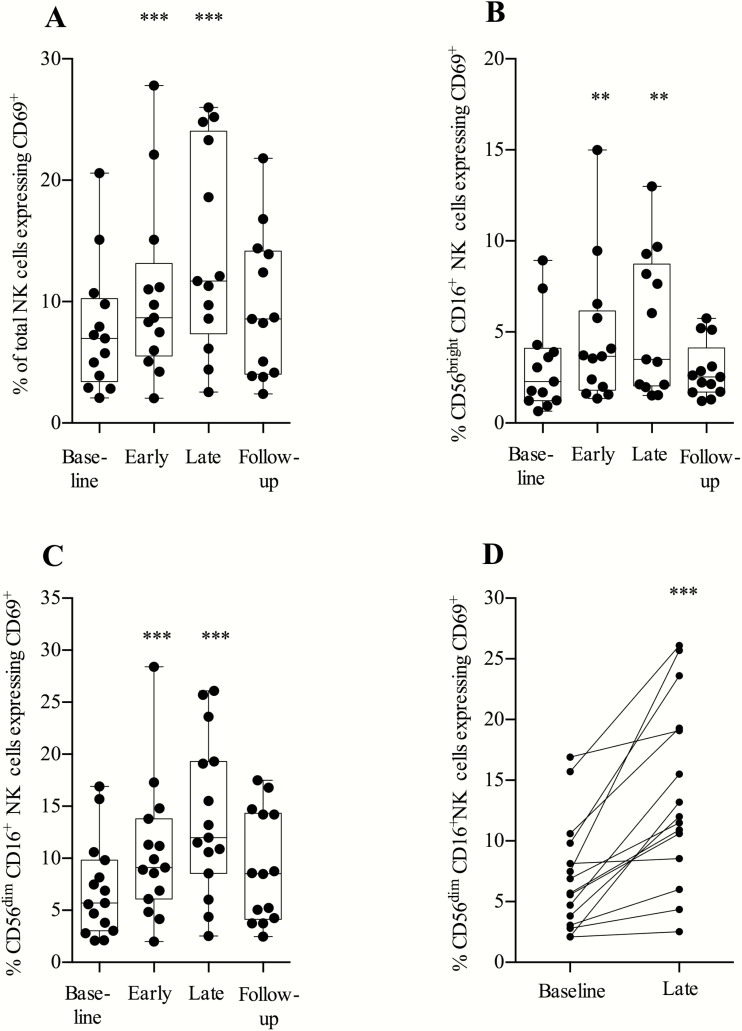
Proportions of total NK cells and cytokine-secreting CD56brightCD16+ NK cells expressing the activation marker CD69+ increased significantly during the treatment period (Figure 4A and 4B). Proportions of cytotoxic (CD56dimCD16+) NK cells [24] expressing the activation marker CD69 doubled during MGN1703 treatment (P < .0001; Figure 4C and 4D; gating strategy shown in Supplementary Figure 4).
Figure 4.
Toll-like receptor 9 agonist treatment significantly increased expression of CD69 on CD56dimCD16+ natural killer (NK) cells. Flow cytometry analyses of fresh peripheral blood mononuclear cells were performed at the indicated time-points: baseline, day 0; early, day 5 (48 hours after second dose); late, day 24 (24 hours after eighth/last dose); and follow-up, day 37 (14 days after the last dose). NK cells can be subdivided into populations of CD56brightCD16+ NK cells and CD56dimCD16+ NK cells. Cytotoxic (CD56dimCD16+) NK cells are known to have the broadest killing potential and higher expression of perforin of these defined NK cell populations. A, Expression of CD69 on the total NK cell population. B, Expression of CD69 on CD56brightCD16+ NK cells. C, CD69 expression on CD56dimCD16+ NK cells. D, Data as depicted in C, paired for each individual with baseline against last week of treatment (24 hours after last dose) (n = 15). Statistical comparisons were 2-tailed, Wilcoxon signed-rank test as paired analysis against baseline. *P < .05; **P < .01; ***P < .001.
We observed a reduction in the percentage of cytotoxic NK cells expressing the inhibitory receptor NKG2A (P = .0005) (Supplementary Figure 5). The proportion of NK cells of total lymphocytes remained stable throughout the treatment period. We conclude that MGN1703 increased the activation of cytotoxic NK cells and downregulated their expression of the inhibitory receptor NKG2A.
MGN1703 Treatment Causes CD4+ and CD8+ T-Memory Cell Activation
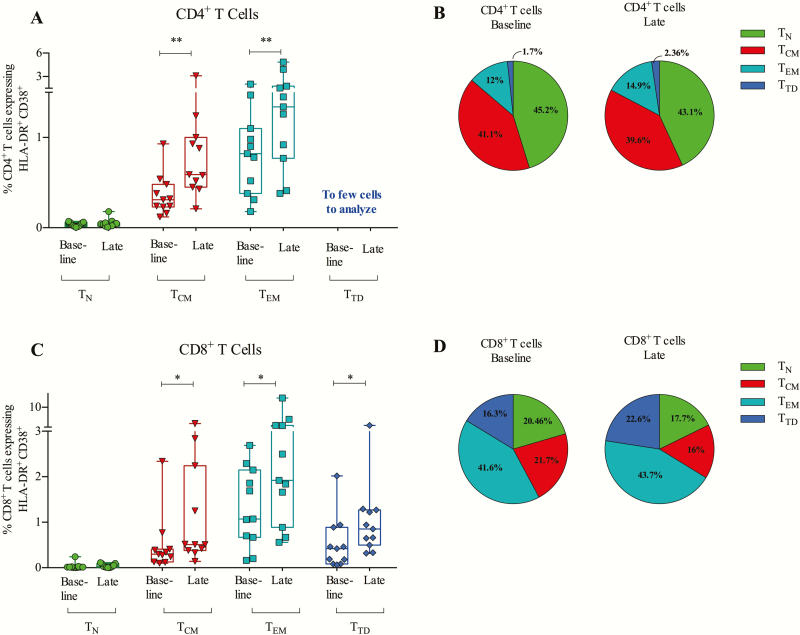
Because TLR9-activated pDCs can stimulate both CD4+ and CD8+ T cells, we measured coexpression of activation markers CD38 and HLA-DR on CD4+ and CD8+ T-cell subsets (Figure 5; gating strategy shown in Supplementary Figure 6). During treatment, CD4+ T cells upregulated coexpression of HLA-DR and CD38 on the central memory (TCM) and effector memory (TEM) populations (Figure 5A and 5B). For CD8+ T cells, we observed upregulation of HLA-DR and CD38 coexpression across all the memory subsets: TEM (P = .032), TCM (P = .032), and terminally differentiated TTD (P = .042; Figure 5C). The mean proportion of CD8+ TTD of the total CD8+ T cell pool increased significantly from 16.3% to 22.6% (P = .007) while TCM decreased from 21.7% to 16.0% (P = .001; Figure 5D). In conclusion, MGN1703 treatment activated CD4+ and CD8+ T-memory subsets and increased proportions of CD8+ TTD cells.
Figure 5.
MGN1703 treatment activates memory subsets of CD4+ and CD8+ T cells. Flow cytometry analyses of fresh peripheral blood mononuclear cells were performed at the indicated time-points: baseline, day 0; late, day 24 (24 hours after eighth/last dose). A, HLA-DR+ and CD38+ coexpression analyses on CD4+ T-cell subsets: naive (TN), central memory (TCM), effector memory (TEM), and terminal differentiated (TTD). B, Proportions of the 4 CD4+ T-cell subsets of total CD4+ T cells. C, HLA-DR+ and CD38+ coexpression analyses on CD8+ T-cell subsets: TN, TCM, TEM, TTD. D, Proportions of the 4 CD8+ T-cell subsets of total CD8+ T cells (n = 11). Statistical comparisons were 2-tailed, Wilcoxon signed-rank test as paired analysis against baseline. *P < .05; **P < .01; ***P < .001.
MGN1703 Treatment Induces Detectable Plasma Viremia
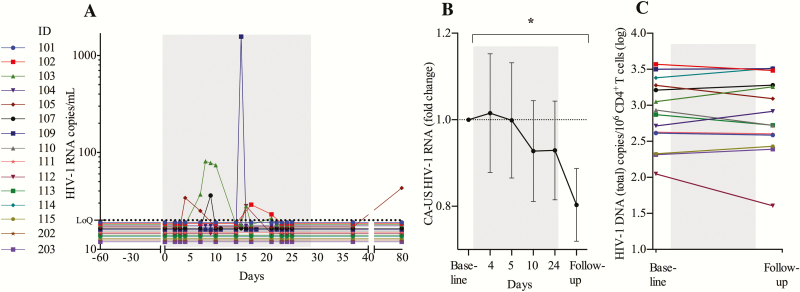
We quantified plasma viral load (pVL) using a standard clinical assay (Roche COBAS TaqMan HIV-1 Test, version 2.0), with a lower limit of quantification of 20 HIV-1 RNA copies/mL. During the 4 weeks of MGN1703 treatment, 6 of 15 participants had quantifiable pVL (range, 21–1571 copies/mL) (Figure 6A). One participant (ID 103) had quantifiable pVL at 6 separate time points during MGN1703 dosing. In conclusion, MGN1703 induced plasma HIV-1 RNA in a subset of participants, indicating that the drug may have LRA properties in vivo.
Figure 6.
MGN1703 treatment induce detectable plasma viremia. A, Analyses of plasma human immunodeficiency virus type 1 (HIV-1) RNA revealed that 6 of 15 participants had quantifiable plasma HIV-1 RNA (range, 21–1571 copies/mL) during MGN1703 treatment. Gray area represents the 4-week dosing period. B, Cell-associated unspliced (CA-US) HIV-1 RNA from 1 × 106 CD4+ T cells. Time-points depicted in the graph are as follows: “baseline”: average of day –30 and day 0 data (prior to dosing); day 4 (24 hours after second dose); day 5 (48 hours after second dose); day 10 (48 hours after third dose); day 24 (24 hours after eighth/last dose); follow-up: average of follow-up visit data on day 37 and 79 (14 and 42 days after last dose). No measurable induction of HIV-1 transcription was observed in peripheral blood during MGN1703 treatment; however, a significant reduction was observed at follow-up (n = 13). C, Total HIV-1 DNA (n = 14) was analyzed with digital droplet polymerase chain reaction from 5 × 106 CD4+ T cells on baseline and follow-up (14 days after last dose). Statistical comparisons were 2-tailed, Wilcoxon signed-rank test as paired analysis against baseline. *P < .05.
Cell-Associated Unspliced HIV-1 RNA Decreased After End of MGN1703 Treatment
Next, we determined cell-associated unspliced (CA-US) HIV-1 RNA in blood CD4+ T cells, using digital droplet polymerase chain reaction (ddPCR) at baseline, week 1, week 2, week 4, and at follow-up (Figure 6B). Whereas no change in CA-US HIV-1 RNA in peripheral CD4+ T cells was observed during MGN1703 administration, we found significant reduction in this measure at the post-MGN1703 follow-up time point relative to baseline (P = .041). We interpret this as a reduction in cells harboring the actively transcribed proviruses.
No Detectable Change in the Size of the Latent HIV-1 Reservoir
For measuring the replication-competent reservoir, we performed the quantitative viral outgrowth assay (qVOA) [9]. For total HIV-1 DNA, we used the ddPCR assay [25] and for integrated HIV-1 DNA, we performed an Alu-PCR analysis [26]. All assays were performed on total CD4+ T cells. Despite interindividual differences, at the cohort level there was no change in total HIV-1 DNA (Figure 6C), integrated HIV-1 DNA, or qVOA (Supplementary Figure 7A and 7B and Supplementary Figure 8) from baseline to follow-up.
DISCUSSION
To date, all clinical HIV cure interventions in the context of shock and kill have focused on the ability of a single intervention to either reverse latency or enhance cell-mediated immunity [9–14]. However, in the HIV eradication context, a single agent that is capable of affecting both outcomes has great potential benefits. Here we report the results of the first clinical trial using a single drug, a TLR9 agonist, as adjunctive immune therapeutic treatment in HIV-1–infected individuals on ART with the aim of both enhancing innate immunity and activating the HIV-1 reservoir. Our results demonstrate that MGN1703 treatment increased the activation of pDCs, upregulated levels of cytokines, and enhanced activation of cytotoxic NK cells and effector CD8+ T cells. Importantly, MGN1703 treatment induced plasma HIV-1 RNA blips up to >1500 copies/mL in a subset of participants. Thus, we report the first in vivo evidence that a single drug may both enhance immunity and increase HIV-1 transcription.
IFN-α is essential for immunological control of HIV infection (eg, by induction of antiretroviral restriction factors) [27, 28], and studies have demonstrated that long-term treatment with exogenous IFN-α can lead to viral suppression and a decline in integrated HIV-1 DNA [29], total HIV-1 DNA [30], and CA-US HIV-1 RNA [31]. We observed increased levels of IFN-α2 and a significant upregulation of numerous ISGs. Interestingly, levels of CA-US HIV-1 RNA declined at follow-up. Therefore, we conclude that the repetitive TLR9 stimulation induced an advantageous IFN-α secretion, without causing the severe side effects commonly observed in exogenous IFN-α treatment. However, the biological effects of IFN-α on HIV infection are very complex. Recent data from humanized mice indicate a potential beneficial effect of blocking the IFN-α receptor as mediator of enhanced viral reservoir control [32, 33], whereas studies in nonhuman primates suggest that short courses of INF-2α therapy enhance antiviral gene expression and prevent systemic infection [34]. This strategy needs to be further explored in HIV-infected patients to fully evaluate this modality.
During the acute phase of HIV-1 infection, the NK cell population expands rapidly, especially the cytotoxic subsets [16], and NK cells are possibly responsible for the initial containment of HIV-1 before potent CD8+ T-cell responses develop [35]. In an ex vivo study from our group, NK cells pretreated with MGN1703 demonstrated an HIV-1 specific response, evidenced by inhibition of virus production from autologous HIV-1–infected CD4+ T cells in a p24 viral inhibition assay [20]. In this present clinical study, TLR9 treatment activated cytotoxic CD56dimCD16+ NK cells [24], and downregulated the inhibitory receptor NKG2A, suggesting that MGN1703 might improve immunological control of HIV-1 infection in vivo.
We have previously demonstrated that MGN1703 increases HIV-1 transcription ex vivo [20]. In the present study, we found that 6 of 15 participants had increased plasma HIV-1 RNA during the MGN1703 treatment period. Contrary to findings from trials aiming to reverse HIV-1 latency by histone deacetylase (HDAC) inhibitor administration [10–14], we did not observe increases in CA-US HIV-1 RNA in MGN1703-treated individuals. However, this was not surprising given that HDAC inhibitors interrupt epigenetic gene regulation in all cells (including HIV-1–infected CD4+ T cells) while MGN1703 only directly activates TLR9-expressing cells (eg pDCs). Because peripheral blood does not have the same well-defined architecture as lymphoid tissues, the cell-to-cell interaction between TLR9-activated pDCs and latently infected CD4+ memory T cells is limited. MGN1703 treatment induced activation of all memory CD4+ and CD8+ T-cell subsets in peripheral blood, including both CD4+ TEM and TCM cells. These cells frequently harbor latent HIV-1 [4] and are homing to immunological tissues [36]. Thus, we speculate that the TLR9-mediated activation of latently infected CD4+ T cells residing in lymphoid tissues resulted in the production of HIV virions observed in plasma.
While participants were closely monitored and multiple virological and immunological parameters were investigated, a few limitations of this study should be mentioned. The lack of a control group reduced our ability to demonstrate that the observed plasma viremia was not caused by natural variation. However, a previous study from our group revealed that levels of plasma HIV-1 RNA is generally very low in ART-treated individuals, as only 2 of 26 individuals had blips of quantifiable viremia over a 6-month period during which a total of 225 plasma HIV-1 RNA quantifications were performed (Ole Søgaard, written personal communication, January 2017). Both cohorts are similar with respect to time on ART and level of viral suppression prior to enrollment. Thus, the virological signal detected in this current MGN1703-treated cohort is beyond that expected by natural variation. The discrepancy between the observed increases in pVL and the stability in CA-US HIV-1 RNA from peripheral blood CD4+ T cells during MGN1703 highlights a second limitation of our study: the general reliance upon peripheral blood-based measures of intervention outcomes, as lymphoid tissues represent key sites for harboring replication-competent and infectious HIV-1 in patients on ART [37–40]. Last, we only treated the participants for a 4-week period and protective CD4+ T-cell, CTL, and B-cell responses take weeks to fully develop. Thus, the limited duration of the TLR9 treatment might offer an explanation to the unaffected reservoir measurements.
In addition to TLR9 agonists, TLR7 agonists are also being investigated in HIV cure-related research [41]. The TLR7 agonist GS-9620 induce a pDC-dependent anti-HIV activity in vitro but also leads to an increase in the proinflammatory cytokines such as IL-6 [42]. Interestingly, a recent nonhuman primate study suggested that the TLR7 agonist GS-986 in combination with Ad26/MVA vectored therapeutic simian immunodeficiency virus vaccination improved immunologic control and delayed viral rebound compared to placebo-treated animals whereas animals receiving GS-986 alone appeared to have no improvement in virological control or change in the size of the viral reservoir. Collectively, these findings suggest that TLR agonists might have several possible roles to play in HIV cure-related research [43].
Whether immunity against HIV-1 infection can be augmented by therapeutic strategies is a focal point in current HIV research. We found that MGN1703 induced a balanced, targeted reactivation of cells and secretion of cytokines that are known to be critical for controlling HIV-1 infection [44] without objective clinical signs of general inflammation (eg, fever). Four weeks of MGN1703 treatment was considered safe and well tolerated in HIV-infected individuals, as only mild AEs occurred and all study subjects completed the treatment. In conclusion, this is the first study to report that a single-drug intervention increases HIV-1 transcription and enhances innate immunity altogether. To further enhance the anti–HIV-1 effect of MGN1703, a combination of immune therapeutic strategies with more potent LRAs and/or additional immune-based interventions could be a path for future HIV eradication studies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Steffen Leth for assisting in design of the ddPCR analyses and Lene Svinth Jøhnke for her laboratory technical assistance.
Disclaimer. The funders had no role in the study design, data analyses, data interpretation, or writing of the article.
Financial support. This work was supported by the American Foundation for AIDS Research (grant number 109118); Aarhus University, Denmark and the Research Foundation–Flanders (grant numbers 12G9716N to W. D. S. and 1802014N to L. V.). Mologen AG provided the study drug. Standards for quantitative PCR methods for tissue-integrated HIV-1 DNA were available with help from the University of California, San Francisco–Gladstone Institute of Virology and Immunology Center for AIDS Research, a National Institutes of Health–funded program (award number P30 AI027763).
Potential conflicts of interest. B. W. has intellectual property on MGN1703, receives funding from Mologen AG, and holds shares. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Palella JF, Delayney K, Anne M, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 2. Bruner KM, Hosmane NN, Siliciano RF. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol 2015; 23:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278:1295–300. [DOI] [PubMed] [Google Scholar]
- 4. Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Archin NM, Sung MJ, Garrido C, Soriano-Sarabia N, Margolis DM. Eradicating HIV-1 infection: seeking to clear a persistent pathogen. Nat Rev Microbiol 2015; 12:750–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003; 9:727–8. [DOI] [PubMed] [Google Scholar]
- 7. Deeks SG. HIV: shock and kill. Nature 2012; 487:439–40. [DOI] [PubMed] [Google Scholar]
- 8. Archin NM, Liberty AL, Kashuba AD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012; 487:482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rasmussen TA, Tolstrup M, Brinkmann CR, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 2014; 1:e13–21. [DOI] [PubMed] [Google Scholar]
- 10. Søgaard OS, Graversen ME, Leth S, et al. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog 2015; 11:e1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elliott JH, McMahon JH, Chang CC, et al. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV 2015; 2:e520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leth S, Schleimann MH, Nissen SK, et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV 2016; 3:e463–72. [DOI] [PubMed] [Google Scholar]
- 13. Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012; 36:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng K, Pertea M, Rongvaux A, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 2015; 517:381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vieillard V, Fausther-Bovendo H, Samri A, Debré P; French Asymptomatiques à Long Terme (ALT) ANRS-CO15 Study Group Specific phenotypic and functional features of natural killer cells from HIV-infected long-term nonprogressors and HIV controllers. J Acquir Immune Defic Syndr 2010; 53:564–73. [DOI] [PubMed] [Google Scholar]
- 16. Carrington M, Alter G. Innate immune control of HIV. Cold Spring Harb Perspect Med 2012; 2:a007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buzon MJ, Yang Y, Ouyang Z, et al. Susceptibility to CD8 T-cell-mediated killing influences the reservoir of latently HIV-1-infected CD4 T cells. J Acquir Immune Defic Syndr 2014; 65:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wittig B, Schmidt M, Scheithauer W, Schmoll HJ. MGN1703, an immunomodulator and Toll-like receptor 9 (TLR-9) agonist: from bench to bedside. Crit Rev Oncol Hematol 2015; 94:31–44. [DOI] [PubMed] [Google Scholar]
- 19. Kapp K, Kleuss C, Schroff M, Wittig B. Genuine immunomodulation with dSLIM. Mol Ther Nucleic Acids 2014; 3:e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Offersen R, Nissen SK, Rasmussen TA, et al. A novel Toll-like receptor 9 agonist, MGN1703, enhances HIV-1 transcription and NK cell-mediated inhibition of HIV-1-infected autologous CD4+ T cells. J Virol 2016; 90:4441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmoll HJ, Wittig B, Arnold D, et al. Maintenance treatment with the immunomodulator MGN1703, a Toll-like receptor 9 (TLR9) agonist, in patients with metastatic colorectal carcinoma and disease control after chemotherapy: a randomised, double-blind, placebo-controlled trial. J Cancer Res Clin Oncol 2014; 140:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qin M, Li Y, Yang X, Wu H. Safety of Toll-like receptor 9 agonists: a systematic review and meta-analysis. Immunopharmacol Immunotoxicol 2014; 36:251–60. [DOI] [PubMed] [Google Scholar]
- 23. Neville LF, Mathiak G, Bagasra O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev 1997; 8:207–19. [DOI] [PubMed] [Google Scholar]
- 24. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008; 9:503–10. [DOI] [PubMed] [Google Scholar]
- 25. Leth S, Nymann R, Jørgensen S, et al. HIV-1 transcriptional activity during frequent longitudinal sampling in aviremic patients on ART. AIDS 2015; 30:713–21. [DOI] [PubMed] [Google Scholar]
- 26. De Spiegelaere W, Malatinkova E, Lynch L, et al. Quantification of integrated HIV DNA by repetitive-sampling Alu-HIV PCR on the basis of Poisson statistics. Clin Chem 2014; 60:886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malim MH, Bieniasz PD. HIV restriction factors and mechanisms of evasion. Cold Spring Harb Perspect Med 2012; 2:a006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abdel-Mohsen M, Deng X, Liegler T, et al. Effects of alpha interferon treatment on intrinsic anti-HIV-1 immunity in vivo. J Virol 2014; 88:763–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Azzoni L, Foulkes AS, Papasavvas E, et al. Pegylated interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis 2013; 207:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun H, Buzon MJ, Shaw A, et al. Hepatitis C therapy with interferon-α and ribavirin reduces CD4 T-cell-associated HIV-1 DNA in HIV-1/hepatitis C virus-coinfected patients. J Infect Dis 2014; 209:1315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morón-López S, Gómez-Mora E, Salgado M, et al. Short-term treatment with interferon alfa diminishes expression of HIV-1 and reduces CD4+ T-cell activation in patients coinfected with HIV and hepatitis C virus and receiving antiretroviral therapy. J Infect Dis 2016; 213:1008–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deeks SG, Odorizzi PM, Sekaly RP. The interferon paradox: can inhibiting an antiviral mechanism advance an HIV cure? J Clin Invest 2017; 127:103–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng L, Ma J, Li J, et al. Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. J Clin Invest. 2017; 127:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sandler NG, Bosinger SE, Estes JD, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 2014; 511:601–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alter G, Teigen N, Ahern R, et al. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J Infect Dis 2007; 195:1452–60. [DOI] [PubMed] [Google Scholar]
- 36. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708–12. [DOI] [PubMed] [Google Scholar]
- 37. Banga R, Procopio FA, Noto A, et al. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med 2016; 22:754–61. [DOI] [PubMed] [Google Scholar]
- 38. Perreau M, Savoye AL, De Crignis E, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med 2013; 210:143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lorenzo-Redondo R, Fryer HR, Bedford T, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016; 530:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gosselin A, Wiche Salinas TR, Planas D, et al. HIV persists in CCR6+CD4+ T cells from colon and blood during antiretroviral therapy. AIDS 2017; 31:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buitendijk M, Eszterhas SK, Howell AL. Toll-like receptor agonists are potent inhibitors of human immunodeficiency virus-type 1 replication in peripheral blood mononuclear cells. AIDS Res Hum Retroviruses 2014; 30:457–67. [DOI] [PubMed] [Google Scholar]
- 42. Bam RA, Hansen D, Irrinki A, et al. TLR7 agonist GS-9620 is a potent inhibitor of acute HIV-1 infection in human peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2016. doi:10.1128/AAC.01369-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Borducchi EN, Cabral C, Stephenson KE, et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 2016;540:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walker BD, Yu XG. Unravelling the mechanisms of durable control of HIV-1. Nat Rev Immunol 2013; 13:487–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.