Abstract
There is still no “gold standard” for the diagnosis and prognosis of post-operative periprosthetic joint infection (PJI). Among serum biomarkers, an emerging molecule is presepsin, the soluble fraction of CD14, recently described in other settings as a powerful diagnostic tool to detect sepsis at different degrees of severity.
The aim of this study was to investigate the diagnostic and prognostic value of presepsin in PJI. A total of 30 patients with PJI and 30 patients without PJI were enrolled. Presepsin, C-reactive protein (CRP), serum interleukin (IL)-6, triggering receptor expressed on myeloid cells 1 (TREM-1), CCL2, matrix metalloproteinase 9 (MMP-9), CD163, osteopontin (OPN), and toll-like receptor 2 (TLR2) were measured at different times after surgery. Receiver operating characteristic (ROC) curves and area under the curve (AUC) were analyzed for each biomarker.
Presepsin showed greater diagnostic value than CRP and IL-6; CD163, TREM-1, and MMP-9 had very low diagnostic potential. Presepsin, OPN, CCL2, suPAR, and TLR2 all decreased significantly with increasing time of recovery after surgery in PJI patients. Presepsin can be considered a useful tool for the diagnosis and clinical monitoring of PJI and can be backed by a panel of new inflammatory markers involved in monocyte-/macrophage-mediated inflammatory responses, such as OPN, CCL2, TLR2, and suPAR.
Keywords: diagnosis, post-operative periprosthetic joint infection, presepsin, prognosis, serum biomarker
Introduction
Post-operative periprosthetic joint infection (PJI) is one of the most challenging complications with joint arthroplasty. Appropriate diagnosis and management are essential to prevent excess morbidity and restore adequate function. The diagnosis of PJI is currently based on several criteria provided by the Musculoskeletal Infection Society (MSIS) in 2011,1,2 but there is still no “gold standard” for its diagnosis and prognosis.3 There is therefore a pressing need for improved diagnostic tools. Molecular biomarkers may hold out promise in the management of PJI.
Among blood biomarkers, an emerging molecule is presepsin, recently described as a powerful diagnostic tool, not only to detect sepsis but also to distinguish different degrees of severity.4–8 Presepsin, also known as sCD14-ST, is a subtype of the soluble form of CD14, which is shredded from the monocyte surface during the inflammatory response and released into the blood. Therefore, presepsin can serve as a circulating marker of infection.9
Still little is known about the full mechanism of sCD14-ST shedding.10 Better understanding of presepsin’s action in the inflammatory response and its correlation with other inflammatory mediators could improve its diagnostic potential and clinical application.
In a recently published study,11 we reported the specific involvement in PJI of the soluble form of the toll-like receptor 2 (TLR2), in the recognition and response to Gram-positive bacteria. There is a close interaction between the soluble form of CD14 and TLR2,12–14 suggesting a possible mechanism of action of presepsin in response to Gram-positive bacterial infection.
A connection between TLR, monocytes, inflammatory cytokines such as interleukin (IL)-1 and IL-6 and the chemokine CCL2 in the mechanism of action of presepsin could be provided by the new infection marker triggering receptor expressed on myeloid cells 1 (TREM-1).15 TREM-1 is related to different aspects of the inflammatory response: it is involved in TLR signaling,16,17 acting as an “amplifier of inflammatory response”; it is over-expressed on monocytes in parallel with CD14 in septic conditions, then it is shredded from the monocytes by the action of the matrix metalloproteinase 9 (MMP-9) and induces the inflammatory cytokines IL-1, IL-6, tumor necrosis factor α (TNF-α) and CCL2.15,18
The over-expression of CD14 and the shedding of a soluble form during the inflammatory response are also correlated with another marker of infection, the soluble urokinase plasminogen activating receptor (suPAR).19–22 Our group recently reported that suPAR has good diagnostic value in the detection of PJI and that it correlates with the same inflammatory cytokines involved in the sCD14-mediated response.23 This suggests that suPAR and presepsin might act together in the inflammatory host response to infection.
Osteopontin (OPN) is a multifunctional glycoprotein with pro-inflammatory properties and is correlated with suPAR during the inflammatory response.24 In severe sepsis, levels of plasma OPN are significantly higher in non-survivors than survivors. OPN appears to be associated with a greater inflammatory response and increased mortality.24,25
Another emerging serum marker of infection is the hemoglobin (Hb) scavenger receptor, CD163.26,27 This is a macrophage-specific protein, and its upregulated expression is one of the main changes in the macrophage switch to alternative activated phenotypes in inflammation. Thus, high CD163 expression in macrophages is a characteristic of tissues responding to inflammation. CD163 is also a potential inflammation biomarker and a therapeutic target, particularly the soluble plasma CD163 that arises from the increased shedding of CD163 mediated by the TNF-α cleaving enzyme.10,27
The aim of this study was to investigate the diagnostic and prognostic value of presepsin in PJI, correlating presepsin levels with other emerging markers of infection such as TREM-1, TLR2, OPN, MMP-9, CD163, and suPAR, as well as the inflammatory mediators IL-6 and CCL2 involved in the progression of PJI.
Methods
Study design, enrollment of patients
A total of 100 patients undergoing prosthesis revision were enrolled during 1 year starting from September 2015, from the IRCCS Istituto Ortopedico Galeazzi (Milan), Istituto Ortopedico G. Pini (Milan), and IRCCS Policlinico San Donato (Milan). They were divided into two groups according to the cause of prosthesis failure: 48 patients had PJI confirmed by positive culture test, with isolation of the causal agent in the infectious focus, at preoperative joint aspiration, and 52 had aseptic loosening of the implant. The Muscle Skeletal Infection Society (MSIS) criteria were used to diagnose PJI.28 Patients were matched for age and sex. Inclusion criteria were as follows: patients of either sex; age >18 years; surgical indication for one-/two-stage prosthesis review. Exclusion criteria were inflammatory diseases, autoimmune diseases, and other types of ongoing infection.
Ethical approval
The study related to human use complied with all the relevant national regulations, institutional policies, and the Declaration of Helsinki. Details that might disclose the identity of the subjects under study were omitted. Written informed consent was obtained from all participants. The study was approved by the local ethics committee (CE of IRCCS San Raffaele Hospital, Milan, approval number CE/99/int/2015). Blood samples were taken from all patients at T0 (before surgery), T1 (1 week after surgery), T2 (1 month after surgery), and T3 (3 months after surgery). Plasma + ethylenediaminetetraacetic acid (EDTA) and serum samples were stored at −20°C.
Biomarker assays
CRP was measured by immunoturbidimetry on an automated biochemical analyzer (Olympus CRP-Latex assay, Central Valley, PA, CA, USA). Serum IL-6, TREM-1, CCL2, MMP-9, CD163, and OPN were measured with an ELISA sandwich Quantikine Assay according to the manufacturer’s protocol (R&D System, Minneapolis, MN, USA); TLR2 was measured with an ELISA Duo Set Assay, according to the manufacturer’s protocol (R&D System, Minneapolis, MN, USA). For IL-6, the sensitivity of the test was 0.7 pg/mL, and intra- and inter-assay coefficients of variation (CV) were 1.7% and 2.0%. For CCL2, the sensitivity was 10 pg/mL, and intra- and inter-assay CV were 4.2% and 4.5%. For TREM-1, the sensitivity was 7.69 pg/mL, and intra- and inter-assay CV were 3.5% and 4.9%. For MMP-9 the sensitivity was 0.156 pg/mL, and intra- and inter-assay CV were 2.9% and 6.9%. For CD163, the sensitivity was 0.177 pg/mL, and intra- and inter-assay CV were 3.4% and 4.1%. For OPN, the sensitivity was 0.024 pg/mL, and intra- and inter-assay CV were 2.9% and 5.4%.
Human suPAR serum concentration was determined using a commercial double monoclonal antibody sandwich immunoassay, according to the manufacturer’s protocol (suPARNOSTIC Standard Kit, ViroGates, Birkerød, Denmark). The sensitivity of the test was 0.1 ng/mL, and intra- and inter-assay CV were 3.5% and 5.1%. Plasma presepsin levels were measured with PATHFAST Presepsin according to the manufacturers’ protocol (Mitsubishi Chemical, Tokyo, Japan). This is a chemiluminescent enzyme immune assay (CLEIA) for the quantitative measurement of presepsin concentrations in whole blood or plasma. The assay uses monoclonal and polyclonal antibodies. All the necessary components are packed in one reagent cartridge. The principle of PATHFAST Presepsin is based on non-competitive CLEIA combined with B/F separation with magnetic particles washed in the pipette tip (a registered trademark of Precision System Science, Matsudo, Japan). During incubation of the sample with alkaline phosphatase–labeled anti-presepsin polyclonal antibody and anti-presepsin monoclonal antibody-coated magnetic particles, the presepsin molecules in the sample bind to the anti-presepsin antibodies forming an immunocomplex with the enzyme-labeled antibody and antibody-coated magnetic particles. After removing the unbound molecules by MAGITRON® technology, a chemiluminescent substrate is added. After short incubation, the intensity of the luminescence generated by the enzyme reaction is measured. The intensity is related to the presepsin concentration in the sample, which is calculated using a standard curve.
The sensitivity of the test was 20 pg/mL, intra- and inter-assay CV were, respectively, 3.9% and 5.0%, and the assay range was 20–20,000 pg/mL. The PATHFAST protocol indicates that the reference range of presepsin is independent of age and sex. In apparently healthy individuals, presepsin ranges from 60 to 365 pg/mL (mean 160 pg/mL, 95% confidence interval (CI): 148–171 pg/mL). The 5th percentile was 92 pg/mL (95% CI: 79–100 pg/mL) and the 95th percentile 320 pg/mL (95% CI: 233–363 pg/mL).
Statistical analysis
For all parameters, normality of distribution of the groups was verified by Kolmogorov–Smirnov tests. Statistical analysis was done using one-way analysis of variance (ANOVA); P < 0.05 was considered significant and P < 0.005 highly significant. Data are expressed as mean ± standard deviation (SD).
Correlation analysis was done using Prism 3.0 software, by linear regression analysis between the different groups of data and calculating the 95% CI of the regression line. The Pearson correlation coefficient (r) was calculated to establish the correlation between parameters measured by the different assays.
Receiver operating characteristic (ROC) curves and area under the curve (AUC) were analyzed with MedCalc 13.2.2 Software (Ostend, Belgium).
Results
Diagnostic values of presepsin and inflammatory markers in PJI
Presepsin was significantly higher in PJI patients than controls. In PJI, it reached 583.7 ± 250.64 pg/mL, compared to 196.54 ± 141.68 pg/mL in non-infected patients, the difference being very highly significant (P < 0.001, Figure 1(a)).
Figure 1.
Presepsin plasma levels and comparison with IL-6 and C-reactive protein: (a–c) presepsin plasma level, C-reactive protein (CRP), and serum IL-6 are shown. White bars: non-infected patients, dark gray bars: prosthetic joint infection (PJI). (d and e) Correlation analysis between presepsin and CRP (r2 = 0.862) or IL-6 (r2 = 0.887). (f–h) ROC curve and AUC for CRP, IL-6, and presepsin are shown.
To compare the diagnostic value of presepsin with inflammatory markers currently used for the clinical detection of PJI, we measured C-reactive protein (CRP) and IL-6. CRP was significantly higher in PJI patients (2.63 ± 1.79 mg/dL) than controls (0.5 ± 0.2 mg/dL) (P < 0.001, Figure 1(b)). IL-6 was also significantly higher in PJI patients (11.12 ± 2.36 pg/mL) than non-infected patients (1.81 ± 0.85 pg/mL) (P < 0.001, Figure 1(c)), confirming the ongoing inflammatory response in PJI patients.
Presepsin showed a very good positive linear correlation with both the inflammatory markers analyzed (r = 0.862 with CRP and r = 0.887 with IL-6), confirming its value as a marker of the inflammatory process in response to the infection (Figure 1(d) and (e)).
The AUC were, respectively, 0.750, 0.821, and 0.926 for CRP, IL-6, and presepsin (Figure 1(f)–(h)).
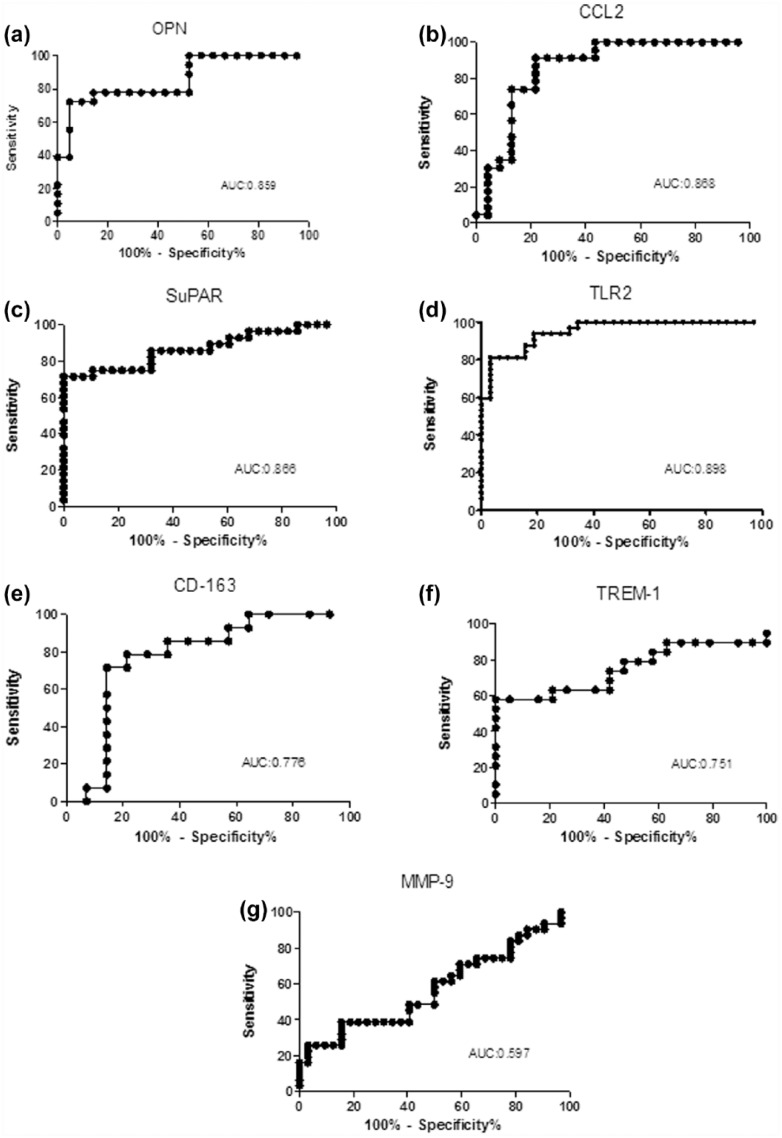
The diagnostic value of the other inflammatory markers was assessed on the basis of the ROC. AUC analysis is shown in Figure 2 (0.859, 0.868, 0.866, 0.898, 0.776, 0.751, 0.597 for OPN (Figure 2(a)), CCL2 (Figure 2(b)), suPAR (Figure 2(c)), TLR2 (Figure 2(d)), CD163 (Figure 2(e)), TREM-1 (Figure 2(f)), and MMP-9 (Figure 2(g))).
Figure 2.
Diagnostic value of infection markers in PJI—the diagnostic value of infection markers in PJI was assessed from the ROC curve and area under the curve (AUC) for: (a) OPN, (b) CCL2, (c) suPAR, (d) soluble TLR2, (e) CD163, (f) TREM-1, and (g) MMP-9.
Prognostic value of presepsin and inflammatory markers in PJI
Presepsin dropped significantly (P < 0.0001 between T0 and T1, P < 0.01 between T1 and T2), and from T0 to the time of recovery after surgery in PJI patients (Figure 3(a)), reaching values comparable to control at T2 and T3; it remained unaltered and significantly lower (Figure 3(b)) in the patients undergoing revision surgery for aseptic loosening of the implant.
Figure 3.
Prognostic value of presepsin and other infection markers in PJI.
(a) Presepsin plasma levels and serum levels of a panel of infection/inflammatory markers, (b) suPAR, (c) CCL2, (d) OPN, (e) CD163, (f) TREM-1, (g) MMP-9, (h) IL-6, and (i) soluble TLR2 were tested at time points T0 (before surgery), T1 (48 h after surgery), T2 (1 month after surgery), and T3 (3 months after surgery). White bars: non-infected patients; dark gray bars: PJI patients.
In the longitudinal study, in order to follow the resolution of the inflammatory process, we analyzed the primary inflammatory cytokine, IL-6, and all the inflammatory markers examined in the diagnostic phase from the preoperative condition (T0) to T1, T2, and T3 after revision surgery. Serum IL-6 (Figure 3(h)) was higher in PJI patients (16.58 ± 4.37 pg/mL) and gradually decreased during recovery after surgery, particularly between T0 and T1 (P < 0.01) In non-infected patients, Il-6 was undetectable at all the time points.
As expected, the serum level of CCL2 (Figure 3(c)) was higher in PJI patients (318.58 ± 86.23 pg/mL) than in the control group with aseptic loosening of the implants (162.48 ± 48.21 pg/mL). At later times, the level of the chemokine dropped rapidly. Similarly to presepsin, there was a significant decrease (P < 0.001) in serum CCL2 from T0 to T1, and by T2, it reached levels comparable to non-infected patients. OPN (Figure 3(d)) was significantly higher in infected patients (179.71 ± 56.11 pg/mL) than non-infected ones (46.18 ± 18.02 pg/mL). It then showed the same gradual decrease as presepsin. Even though there was a substantial SD at T0 and T1, the decrease from T0 to T1 was in fact significant (P < 0.01).
CD163 (Figure 3(e)) gradually decreased at the various time points, but the difference between infected (626.28 ± 18.02 pg/mL) and non-infected patients (426.8 ± 16.23 pg/mL) was not significant. This indicates that CD163 cannot be considered a good marker for PJI infection. TREM-1 showed a significant decrease at T1 and subsequent time points, reaching levels comparable to non-infected patients. However, the absolute level of serum TREM-1, even at its highest at T0 (17.07 ± 5.01 pg/mL), was far below the diagnostic cut-off of 30 pg/mL reported in the literature. In contrast, the inflammatory marker suPAR (Figure 3(b)), described as having a potential diagnostic role in PJI, showed the same gradual decrease as presepsin. At T0, it was significantly higher in PJI patients: 9.27 ± 2.78 pg/mL compared to 3.12 ± 1.00 pg/mL in non-infected patients—a highly significant difference (P < 0.001). Similar to presepsin, suPAR dropped steeply and significantly (P < 0.01) from T0 to T1, reaching values close to those of non-infected patients at T2 and T3.
CRP (Figure 3(l)) rose in control patients from T0 to T1, almost comparably to PJI patients, though the difference was not significant, but returned to levels comparable to T0 by T2 and T3. In PJI patients, the changes in CRP never reached statistical significance at the different follow-up times because of the wide SD.
Discussion
The diagnostic tools for early detection of PJI still need to be improved. New emerging biomarkers have been identified as inflammatory molecules involved in the host response to bacteria. Presepsin, the soluble fraction of CD14, has been recently described as a powerful diagnostic tool to detect sepsis and distinguish its severity.8,29,30
To study whether presepsin could serve as a valid biomarker in PJI, we compared it with biomarkers currently used in clinical practice (CRP and IL-6) and with a panel of new generation markers of infection.
As expected, in the PJI group, CRP was higher than the cut-off of 1 mg/dL for periprosthetic infection;31 IL-6 showed a significative increase, confirming the ongoing inflammatory response in PJI patients. Presepsin was significantly higher in PJI patients than non-infected controls, so it may well prove to be a good diagnostic marker for PJI.
To compare the clinical diagnostic value of presepsin with other inflammatory markers of infection, we calculated the ROC curve. These indicate the efficacy of a diagnostic test, by measuring the AUC. In clinical practice, a diagnostic test is considered acceptable if its AUC is ⩾0.8.32 Presepsin had greater diagnostic value—as indicated by the ROC AUC—than CRP and IL-6 in the diagnosis of PJI. Therefore, it can be considered a better diagnostic marker and could be usefully introduced in clinical settings for the detection of PJI.
ROC analysis indicated that OPN, CCL2, TLR2, and suPAR gave good diagnostic values in PJI, while CD163, TREM-1, and MMP-9 had very low diagnostic potential. These results indicate that, among the inflammatory markers analyzed, the most important ones for PJI diagnosis are related to the monocyte-/macrophage-mediated inflammatory response, such as the monocyte-specific chemokine CCL2, suPAR, TLR2, and OPN.
Then, to assess the prognostic value of presepsin, we ran a longitudinal study analyzing presepsin levels at different times after the surgery, in PJI and non-infected patients. The timing of the longitudinal studies was related to the clinical follow-up of the patients undergoing arthroplasty. Presepsin dropped significantly with longer recovery time after surgery in PJI patients, while it remained unaltered and significantly lower (P < 0.0001) in non-infected patients. These results clearly indicate that presepsin offers good prognostic value, decreasing gradually as the inflammatory process in response to the prosthetic infection is resolved.
At T1, 1 week after surgery, presepsin was almost half the T0 figure, but it did not reach the same level as in non-infected patients; this probably reflects the clearance time of the molecule from the circulation in the early stages after surgery. At subsequent times after surgery, which eradicates the infection, presepsin in PJI patients was comparable to the non-infected patients. These results are important in the follow-up of revision surgery because a persistent infection calls for immediate treatment.
CRP, as a general marker of inflammation, can remain high for roughly 30–60 days in the immediate post-operative period.33 As expected, CRP was high from T0 (pre-surgery) to T1 (1 week later), more evident in control patients, though the difference was not statistically meaningful, reaching levels comparable to PJI patients at T1. At later times, however, as post-surgical recovery proceeded, CRP returned to a low level in both PJI and non-infected patients.
IL-6 decreased gradually after surgery in PJI patients and was undetectable at all times in non-infected patients.
Since presepsin, the soluble form of CD14 ligand, originates from mononuclear cells, we examined, in parallel the main monocyte attractant chemokine, CCL2. As expected, the serum level of CCL2 was higher in PJI patients than in the control group, while at later times it fell rapidly, similarly to presepsin. As CCL2 is the main chemokine regulating monocyte and macrophage activity, this result agrees with recent evidence that presepsin is released upon macrophage phagocytosis.
suPAR, previously described as having diagnostic potential in PJI,23 decreased gradually like presepsin, confirming the potential role of presepsin in the prognostic assessment of PJI. Similarly, OPN showed the same gradual decrease as presepsin, though the SD was significant at T0 and T1.
A strong correlation has been described between CD14 and TLR, particularly TLR2 and TLR4.12,13 TLR2 decreased slightly at the first time point, 48 h, while at later times, it reached levels comparable to non-infected patients. This implies that it cannot be considered an early marker of eradication of infection, but at the later time points, it followed the same pattern of decrease as presepsin.
CD163 gradually decreased with time, but with no significant difference in infected patients and controls. Therefore, CD163 cannot be considered a good marker for PJI infection. This result reflects the specificity of PJI infection, where not all the usual infection markers, such as the canonical CRP, adequately detect the infection.34,35
CD163 is mainly reported to be a good marker for viral infections and chronic inflammation.36 Therefore, the slight elevation at T0 can only be due to the inflammatory response in PJI patients.
In PJI patients, TREM-1 was below the diagnostic cut-off, so it cannot be considered a useful tool for the diagnosis and prognosis of PJI. Since the soluble form of TREM-1 is released through the action of MMP-9,16 we assayed MMP-9 at the same time points as TREM-1. We found no significant differences between PJI patients and controls at any times, confirming that the MMP-9/TREM-1 axis is not involved in PJI.
The main limitation of our study is the small number of infected patients, but this reflects the low frequency of PJI.37 To overcome this limitation, we enrolled PJI patients from several different orthopedic institutes.
In conclusion, presepsin can be considered a useful tool for the diagnosis and clinical monitoring of PJI and could also be backed by a panel of new inflammatory markers involved in the monocyte-/macrophage-mediated inflammatory response such as OPN, CCL2, and suPAR. These markers, together with presepsin, could be introduced as a comprehensive panel after first-line investigations, for example, with CRP and IL-6, when there is a suspicion of PJI, in order to assess the need for second-line examinations, such as joint aspiration.
Acknowledgments
The authors thank Mitsubishi Chemical and the Italian Mitsubishi Chemical distributor GEPA for providing PATHFAST and kits for presepsin immunoassay. They also thank Dr Judith Baggott for English language revision.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Filippo Randelli  http://orcid.org/0000-0002-7643-0512
http://orcid.org/0000-0002-7643-0512
References
- 1. Bauer TW, Parvizi J, Kobayashi N, et al. (2006) Diagnosis of periprosthetic infection. Journal of Bone and Joint Surgery 88(4): 869–882. [DOI] [PubMed] [Google Scholar]
- 2. Parvizi J, Ghanem E, Menashe S, et al. (2006) Periprosthetic infection: What are the diagnostic challenges? Journal of Bone and Joint Surgery 88 (Suppl. 4): 138–147. [DOI] [PubMed] [Google Scholar]
- 3. Parvizi J, Ghanem E, Azzam K, et al. (2008) Periprosthetic infection: Are current treatment strategies adequate? Acta Orthopaedia Belgica 74(6): 793–800. [PubMed] [Google Scholar]
- 4. Sargentini V, Ceccarelli G, D’Alessandro M, et al. (2015) Presepsin as a potential marker for bacterial infection relapse in critical care patients. A preliminary study. Clinical Chemistry and Laboratory Medicine 53(4): 567–573. [DOI] [PubMed] [Google Scholar]
- 5. Pizzolato E, Ulla M, Galluzzo C, et al. (2014) Role of presepsin for the evaluation of sepsis in the emergency department. Clinical Chemistry and Laboratory Medicine 52(10): 1395–1400. [DOI] [PubMed] [Google Scholar]
- 6. Enguix-Armada A, Escobar-Conesa R, Garcia-De La, Torre A, et al. (2016) Usefulness of several biomarkers in the management of septic patients: C-reactive protein, procalcitonin, presepsin and mid-regional pro-adrenomedullin. Clinical Chemistry and Laboratory Medicine 54(1): 163–168. [DOI] [PubMed] [Google Scholar]
- 7. Endo S, Suzuki Y, Takahashi G, et al. (2012) Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. Journal of Infection and Chemotherapy 18(6): 891–897. [DOI] [PubMed] [Google Scholar]
- 8. Masson S, Caironi P, Fanizza C, et al. (2015) Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: Data from the multicenter, randomized ALBIOS trial. Intensive Care Medicine 41(1): 12–20. [DOI] [PubMed] [Google Scholar]
- 9. Vodnik T, Kaljevic G, Tadic T, et al. (2013) Presepsin (sCD14-ST) in preoperative diagnosis of abdominal sepsis. Clinical Chemistry and Laboratory Medicine 51(10): 2053–2062. [DOI] [PubMed] [Google Scholar]
- 10. Etzerodt A, Rasmussen MR, Svendsen P, et al. (2014) Structural basis for inflammation-driven shedding of CD163 ectodomain and tumor necrosis factor-alpha in macrophages. Journal of Biological Chemistry 289(2): 778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galliera E, Drago L, Vassena C, et al. (2014) Toll-like receptor 2 in serum: A potential diagnostic marker of prosthetic joint infection? Journal of Clinical Microbiology 52(2): 620–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bsibsi M, Bajramovic JJ, Van Duijvenvoorden E, et al. (2007) Identification of soluble CD14 as an endogenous agonist for Toll-like receptor 2 on human astrocytes by genome-scale functional screening of glial cell derived proteins. Glia 55(5): 473–482. [DOI] [PubMed] [Google Scholar]
- 13. Iwaki D, Nishitani C, Mitsuzawa H, et al. (2005) The CD14 region spanning amino acids 57–64 is critical for interaction with the extracellular Toll-like receptor 2 domain. Biochemical and Biophysical Research Communications 328(1): 173–176. [DOI] [PubMed] [Google Scholar]
- 14. Raby AC, Holst B, Le Bouder E, et al. (2013) Targeting the TLR co-receptor CD14 with TLR2-derived peptides modulates immune responses to pathogens. Science Translational Medicine 5(185): 185ra64. [DOI] [PubMed] [Google Scholar]
- 15. Ford JW, McVicar DW. (2009) TREM and TREM-like receptors in inflammation and disease. Current Opinion in Immunology 21(1): 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arts RJ, Joosten LA, van der Meer JW, et al. (2013) TREM-1: Intracellular signaling pathways and interaction with pattern recognition receptors. Journal of Leukocyte Biology 93(2): 209–215. [DOI] [PubMed] [Google Scholar]
- 17. Campanholle G, Mittelsteadt K, Nakagawa S, et al. (2013) TLR-2/TLR-4 TREM-1 signaling pathway is dispensable in inflammatory myeloid cells during sterile kidney injury. PLoS ONE 8(7): e68640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pelham CJ, Agrawal DK. (2014) Emerging roles for triggering receptor expressed on myeloid cells receptor family signaling in inflammatory diseases. Expert Review of Clinical Immunology 10(2): 243–256. [DOI] [PubMed] [Google Scholar]
- 19. Giamarellos-Bourboulis EJ, Georgitsi M. (2015) Host response biomarker in sepsis: suPAR detection. Methods in Molecular Biology 1237: 241–246. [DOI] [PubMed] [Google Scholar]
- 20. Hoenigl M, Raggam RB, Wagner J, et al. (2013) Diagnostic accuracy of soluble urokinase plasminogen activator receptor (suPAR) for prediction of bacteremia in patients with systemic inflammatory response syndrome. Clinical Biochemistry 46(3): 225–229. [DOI] [PubMed] [Google Scholar]
- 21. Wittenhagen P, Kronborg G, Weis N, et al. (2004) The plasma level of soluble urokinase receptor is elevated in patients with Streptococcus pneumoniae bacteraemia and predicts mortality. Clinical Microbiology and Infection 10(5): 409–415. [DOI] [PubMed] [Google Scholar]
- 22. Yilmaz G, Koksal I, Karahan SC, et al. (2011) The diagnostic and prognostic significance of soluble urokinase plasminogen activator receptor in systemic inflammatory response syndrome. Clinical Biochemistry 44(14–15): 1227–1230. [DOI] [PubMed] [Google Scholar]
- 23. Galliera E, Drago L, Marazzi MG, et al. (2015) Soluble urokinase-type plasminogen activator receptor (suPAR) as new biomarker of the prosthetic joint infection: Correlation with inflammatory cytokines. Clinica Chimica Acta 441: 23–28. [DOI] [PubMed] [Google Scholar]
- 24. Vaschetto R, Nicola S, Olivieri C, et al. (2008) Serum levels of osteopontin are increased in SIRS and sepsis. Intensive Care Medicine 34(12): 2176–2184. [DOI] [PubMed] [Google Scholar]
- 25. Matsue Y, Tsutsumi M, Hayashi N, et al. (2015) Serum osteopontin predicts degree of hepatic fibrosis and serves as a biomarker in patients with hepatitis C virus infection. PLoS ONE 10(3): e0118744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dultz G, Gerber L, Farnik H, et al. (2015) Soluble CD163 is an indicator of liver inflammation and fibrosis in patients chronically infected with the hepatitis B virus. Journal of Viral Hepatitis 22(4): 427–432. [DOI] [PubMed] [Google Scholar]
- 27. Etzerodt A, Moestrup SK. (2013) CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxidants & Redox Signaling 18(17): 2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parvizi J, Zmistowski B, Berbari EF, et al. (2011) New definition for periprosthetic joint infection: From the Workgroup of the Musculoskeletal Infection Society. Clinical Orthopaedics and Related Research 469(11): 2992–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Masson S, Caironi P, Spanuth E, et al. (2014) Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: Data from the albumin Italian outcome sepsis trial. Critical Care 18(1): R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shozushima T, Takahashi G, Matsumoto N, et al. (2011) Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. Journal of Infection and Chemotherapy 17(6): 764–769. [DOI] [PubMed] [Google Scholar]
- 31. Parvizi J, Walinchus L, Adeli B. (2011) Molecular diagnostics in periprosthetic joint infection. International Journal of Artificial Organs 34(9): 847–855. [DOI] [PubMed] [Google Scholar]
- 32. Carter JV, Pan J, Rai SN, et al. (2016) ROC-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surgery 159(6): 1638–1645. [DOI] [PubMed] [Google Scholar]
- 33. Bilgen O, Atici T, Durak K, et al. (2001) C-reactive protein values and erythrocyte sedimentation rates after total hip and total knee arthroplasty. Journal of International Medical Research 29(1): 7–12. [DOI] [PubMed] [Google Scholar]
- 34. Benoist JF, Mimoz O, Assicot M, et al. (1998) Serum procalcitonin, but not C-reactive protein, identifies sepsis in trauma patients. Clinical Chemistry 44(8 Pt. 1): 1778–1779. [PubMed] [Google Scholar]
- 35. Drago L, Vassena C, Dozio E, et al. (2011) Procalcitonin, C-reactive protein, interleukin-6, and soluble intercellular adhesion molecule-1 as markers of postoperative orthopaedic joint prosthesis infections. International Journal of Immunopathology and Pharmacology 24(2): 433–440. [DOI] [PubMed] [Google Scholar]
- 36. Tornai T, Vitalis Z, Sipeki N, et al. (2016) Macrophage activation marker, soluble CD163 is an independent predictor of short-term mortality in patients with cirrhosis and bacterial infection. Liver International 36(11): 1628–1638. [DOI] [PubMed] [Google Scholar]
- 37. Tande AJ, Patel R. (2014) Prosthetic joint infection. Clinical Microbiology Reviews 27(2): 302–345. [DOI] [PMC free article] [PubMed] [Google Scholar]