Abstract
Pramlintide, an approved analog of amylin, is responsible for regulating the physiology of energy homeostasis. The goals of this study were to investigate the roles of pramlintide in the regulation of cell survival and matrix metabolism, and further explore their underlying mechanisms, in human nucleus pulposus (NP) cells.
NP cells were treated with different concentrations of pramlintide in normoxic or hypoxic conditions. Cell viability, LAC concentration, calcium concentration, mitochondrial membrane potential (ΔΨm), MMPs proteins, and apoptotic related proteins were detected.
The results indicate that pramlintide could improve NP cell proliferation, glycolytic activity, and the ECM synthesis under hypoxia, which is evident from the increased precipitation of proteoglycans; increased expression of AGG, Col2, and SOX9 proteins; and decreased expression of MMP3, MMP9, and MMP13 proteins, which are Ca2+-dependent enzymes. And, pramlintide could facilitate the survival of NP cells through mitochondrial-mediated, Bcl-2/caspase-3-dependent apoptosis. In addition, activation of AKT-AMPK/mTOR signaling pathway is also observed by the treatment.
These findings demonstrate that pramlintide may play a pivotal role in reversing intervertebral disk degeneration and may relieve the impairment of ECM metabolism and NP cells survival through mitochondrial-dependent apoptotic signaling pathway, thus offering a novel potential pharmacological treatment strategy.
Keywords: apoptosis, hypoxia, matrix metabolism, nucleus pulposus, pramlintide
Introduction
Intervertebral disk (IVD) degeneration is one of the most common causes for lower-back pain. The etiology of IVD degeneration is complicated and involves advanced age, injury, pathology, or more likely a combination of these factors. The pathophysiological process of the disease is mainly characterized by excessive destruction of the extracellular matrix (ECM). The IVDs consist of inner nucleus pulposus (NP) and outer annulus fibrosus (AF). NP, a gel-like substance, consists of proteoglycan, collagen, and NP cells. The NP cells play a pivotal role in IVD degeneration by regulating the anabolic and catabolic ECM activity.1 These cells reside in the inner AF without any blood supply and receive oxygen and nutrients from the endplates by diffusion. The degenerative changes of IVD cause a decrease in the nutrient supply at the disk periphery, significantly affecting the nutrient supply to the disk, which is further responsible for the decrease in the proteoglycan content.2 Although the etiology of IVD degeneration at molecular level is still unclear, it is well known that a decrease in the proteoglycan content and, therefore, water content in NP are critical for causing the disk degeneration.3 Therefore, biosynthesis of proteoglycan by the NP cells plays an important role during the process of disk degeneration.
There are several factors behind the onset of disk degeneration, including mechanical, nutritional, and genetic factors. Previous studies have reported a close association between diabetes and IVD degeneration. Patients with diabetes have a higher incidence of degenerative disk disease at a younger age than those without diabetes.4,5 In a prospective study of 200 patients, the incidence of lumbar disease was much higher in the diabetic patients than in patients with other diseases.4 Therefore, diabetes mellitus may be an essential risk factor for IVD degeneration. In addition, diabetes mellitus has been reported to influence the treatment outcomes for patients with spinal stenosis or degenerative spondylolisthesis, which may be a predisposing factor for the development of spinal disorders associated with back pain.6 Diabetes is responsible for causing a decrease in the number of viable cells, which may be the initial trigger for IVD degeneration. In addition, animal studies have shown that diabetes is strongly associated with disk degeneration, accompanied by the changes in proteoglycan biosynthesis and apoptosis in the IVDs of diabetic rats.7,8
Amylin acts as a metabolic mediator through nutrient-sensing mammalian target of rapamycin (mTOR) protein.9,10 Pramlintide, the first approved amylin analog drug, is widely used in the treatment of type 1 and type 2 diabetes and acts by regulating the energy metabolism.11 Thus, pramlintide might regulate the energy homeostasis through mTOR signaling. Activation of mTOR signaling regulates PI3K/AKT-dependent pathway and PI3K/ AKT-independent signals, such as Erk, mitogen-activated protein kinase (MAPK), and adenosine monophosphate (AMP)-activated protein kinase (AMPK). The AMPK is a serine/threonine protein kinase that serves as a cellular energy sensor under environmental and nutritional stress. Activated AMPK can regulate the intracellular energy homeostasis by decreasing energy consumption and increasing the energy production. Hypoxia and glucose deprivation lead to depleted adenosine triphosphate (ATP) and elevated AMP levels, resulting in an elevated AMP/ATP ratio, finally activating AMPK. A few studies have shown that AMPK activation is required for regulating apoptosis12 and hypoxia.13 In addition, pramlintide has been found to have anti-inflammatory and anti-oxidative effects on the central and peripheral nervous systems.14,15
Previous studies have indicated that pO2 is present in low levels in the disk, which enables the NP cells to survive in a hypoxic tissue niche through the activation of hypoxic signals.16–19 The major goal of our study was to evaluate the therapeutic potential of pramlintide by determining its effect on function and matrix metabolism of NP cells under hypoxic conditions. In addition, the study also explored the reactive oxygen species (ROS)-induced apoptotic and the AKT/AMPK-mTOR pathways.
Materials and methods
Isolation and treatment of human NP cells
The human NP cells were isolated from the idiopathic scoliosis NP specimens (n = 8) as described previously.20 All the experimental protocols were approved by the Clinical Research Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. After isolation, NP cells were plated and expanded for 3 weeks in Dulbecco’s modified Eagle’s medium (DMEM/F-12) containing 15% fetal bovine serum (FBS; Gibco, Waltham, MA, USA), and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in 5% CO2. The culture medium was changed every 4 days, providing enough time for the primary cells to adhere to the surface prior to the first change. Cells from the second passage were serum-starved for 12 h and then cultured with different concentrations of pramlintide (ranging from 0 to 500 nM with a difference of every 50 nM) in normoxic (21% oxygen) or hypoxic (1% oxygen) conditions generated by a hypoxic chamber (Invivo2 200, Ruskinn Technology Ltd, Pencoed, UK). After 72 h of exposure, the cells and supernatants were collected for the subsequent experiments.
Cell viability assay
Cell viability was determined by the Cell Counting Kit-8 (CCK-8) (C0037, Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. Briefly, human NP cells were seeded in 96-well plates (3 × 104/well) and incubated in DMEM at 37°C for 24 h. After that, the cells were exposure to different concentrations of pramlintide for another 72 h. Then, the cells were washed with phosphate-buffered saline (PBS) and then 190 µL of DMEM with 10 µL of CCK-8 solution was added to each well. The plates were further incubated at 37°C for 4 h. The absorbance of the wells was measured at 450 nm by a microplate reader.
Lactic acid assay
Cells from the second passage were seeded at the density of 105 cells/well in 24-well plate. After serum-starvation for 12 h, the cells were treatment with pramlintide. After incubation, the cultures were centrifuged at 1000g for 10 min in a Beckman GPR centrifuge, and the supernatant and the cells were collected separately. The lactic acid (LAC) concentration in the supernatant was measured using Lactate Assay kit (15-0908, Gcell, Beijing, China). Each assay contained 2 µL of supernatant and 200 µL of R1 reaction solution, containing 0.4 mmol/L 4-aminoantipyrine, 2.1 mmol/L TOOS, 10,000 U/L ascorbic acid oxidase, 1000 U/L peroxidase, and 600 U/L lactate oxidase in phosphate buffer. After incubating at 37°C for 5 min, the absorbance of the samples were detected at 540 nm (A2) and 700 nm (A1) by a microplate reader. The LAC concentration in the supernatant was calculated based on the following equation: LAC concentration = ΔΑ sample/ΔA calibrator × standard concentration, where ΔA = A-Sample − A-Blank.
Measurement of cellular ATP
After culturing for 48 h, the NP cells were collected, and cellular ATP levels were measured using the ATP assay kit (S0026, Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. In brief, 1 × 106 NP cells were homogenized with ATP assay buffer (50 µL of the reaction mix was added to 50 µL of the cell homogenate). The ATP production was measured at 562 nm by colorimetric assay.
Analysis of proteoglycan content
The NP cells were seeded in a six-well plate at the density of 5 × 105 cells/well and treated with different concentrations of pramlintide in normoxic or hypoxic conditions for 48 h. After incubation, the cells were fixed with 4% paraformaldehyde and were dehydrated using different concentrations of ethanol and xylol. Following dehydration, the cells were stained with 1% of Alcian blue solution for 30 min at 37°C, dehydrated, and observed under light microscopy.
Western blot analysis
The NP cells were cultured in six-well plates with the density of 5 × 105 cells/well. After intervention, the cells were washed twice with PBS and treated with 500 mL of RIPA Lysis Buffer (P0013B, Beyotime Biotechnology, Shanghai, China). Then, the samples were separated using 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) and transferred to Hybond enhanced chemiluminescence (ECL) membranes (Amersham, Arlington Heights, IL, USA). The membranes were first blocked with 6% non-fat milk dissolved in tris-buffered saline Tween-2 (TBST) buffer (10-mM tris Cl (pH 8.0), 150-mM NaCl, and 0.05% Tween-20) and then the blots were probed with primary antibodies specific against MMP3 (14351, CST, 1:1000), MMP9 (BS6893, Bioworld, 1:1000), MMP13 (ab39012, Abcam, 1:4000), aggrecan (AGG) (ab3778, Abcam, 1:100), collagen II (Col2) (sc-7764, Santa, 1:8000), SRY-related HMG box 9 (SOX9) (ab185966, Abcam, 1:3000), Bcl-2 (ab32124, Abcam, 1:1000), Bax (ab32503, Abcam, 1:1000), Caspase-3 (9664, Abcam, 1:200), AKT (10176-2-AP, ProteinTech Group, 1:1000), p-AKT (4060P, CST, 1:2000), AMPK (5832, CST, 1:1000), p-AMPK (2535,CST, 1:1000), mTOR (BS3611, Bioworld, 1:800), and p-mTOR (BS4706, Bioworld, 1:800), at 4°C for overnight. Then, the membranes were incubated with appropriate horseradish peroxidase–conjugated secondary antibodies (BA1054, Boster, 1:5000), at room temperature for 1 h. The blots were developed using the ECL method (NCI5079, Amersham, Arlington Heights, IL, USA), while the bands were quantified and analyzed using the Bio-Rad image software.
Calcium quantification assay
The logarithmic-phase NP cells used in the experiment, seeded in six-well plates with the density of 2 × 105 cells/well. The calcium concentrations in NP cells from each group were detected by calcium assay kit (40776ES50, YEASEN, China) according to the manufacturer’s instruction. After culturing for 48 h, Rhod-2 AM was added into the culture with the final concentration of 5 µM/L. Then, the cells were incubated in dark for 30 min at 37°C. After incubation, the cells were washed twice with Dulbecco’s phosphate-buffered saline (D-PBS) (without Ca2+ and Mg2+) by centrifugation. Finally, the cells were resuspended in D-PBS to the volume of 500 µL and analyzed by flow cytometry. Rhod-2 AM is cell-permeable version of Rhod-2. The concentration changes of Ca2+ in cells can be detected by fluorescence-label flow cytometry.
ROS detection by flow cytometry
The intracellular level of ROS was detected by 2′,7′-dichlorofluorescin diacetate (DCFH-DA), an ROS-specific fluorescent probe (S0033, Beyotime Biotechnology, Shanghai, China). The ROS oxidizes DCFH-DA to DCF, resulting in fluorescence, which is then detected by flow cytometry. The NP cells were resuspended in DCFH-DA at a density of 2 × 106 cells/mL and incubated in the dark at 37°C for 20 min with continuous mixing at every 5 min for efficient hybridization of the probes. After the incubation, the cells were washed three times with serum-free culture medium to remove the unhybridized probes. Then, flow cytometry was used to analyze the mean fluorescence intensity of DCF in different treatments.
Determination of mitochondrial membrane potential (ΔΨm)
The ΔΨm was determined by flow cytometry after staining the NP cells with Rhodamine 123 (Rh123) staining fluid (R0084, Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s instructions. Briefly, NP cells were cultured in six-well plates with the density of 2 × 105 cells/well. After intervention, the harvested NP cells were resuspended in 0.5 mL of the culture medium, and Rh123 staining fluid was added, and the final concentration was adjusted to 10 µg/mL. Then, the mixture was incubated in dark at 37°C for 30 min. Following the incubation, the cells were washed twice with PBS by centrifugation. Finally, the cells were resuspended in PBS and analyzed by flow cytometry.
Detection of apoptosis by TUNEL assay and flow cytometry analysis
TUNEL Assay (Terminal deoxynucleotidyl transferase dUTP nick end labeling) is a common method for detecting apoptotic signaling cascades by labeling DNA fragmentation. For TUNEL assay, NP cells were cultured in 12-well plates with the density of 105 cells/well. After treatment with pramlintide, the harvested NP cells were analyzed using in situ cell death detection kit (12156792910, Roche, Branford, CT, USA) after fixing the cells in 4% paraformaldehyde (pH 7.4). For cytometry analysis, the NP cells from each treatment group were labeled with Annexin V-APC/7-AAD double staining, according to the manufacturer’s instructions (KGA108, KeyGen Biotech, Nanjing, China). Each sample was applied to a FACSCalibur Flow Cytometer (BD Biosciences, USA) and fluorescence intensity of the apoptotic cells was detected.
Statistical analysis
All the experiments were carried out from three individual experiments on independent cell cultures, and the data are represented as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 5 software. One-way analysis of variance (ANOVA) and Student’s t-test were used for determining the significant differences among the groups. Relative influences of pramlintide on NP cells under normoxic versus hypoxic conditions were evaluated using a two-way ANOVA test. A P-value of less than 0.05 was considered as statistically significant.
Result
Cell functions regulated by pramlintide
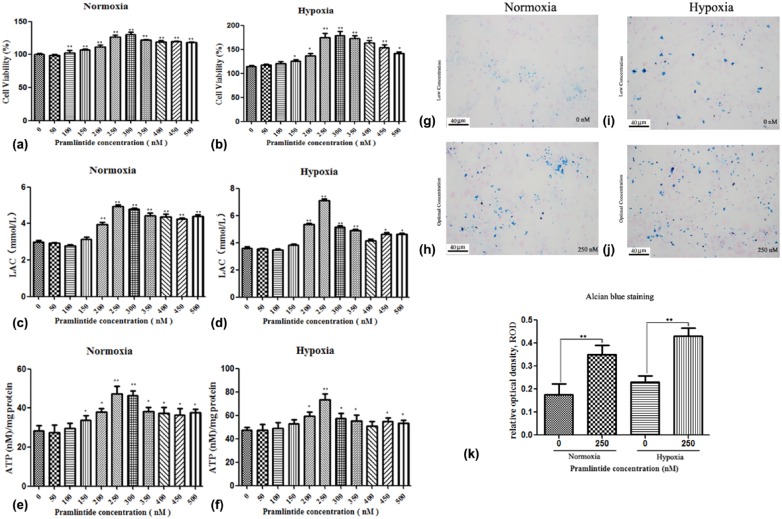
To investigate the effect of pramlintide on NP cell function, the cells were treated with varying concentrations of pramlintide. The proliferation of NP cells was analyzed by CCK-8. As shown in Figure 1(a) and (b), pramlintide increased the proliferation of NP cells in both normoxic and hypoxic conditions. The cell proliferation was found to be significantly increased in hypoxic condition (174.39%) compared to the normoxic condition (124.24%) at a drug concentration of 250 nM (P < 0.05). The NP cells generate energy mainly through glycolysis. The LAC level representing the glycolytic activity of the cells indicated that pramlintide promoted the energy metabolism of NP cells under both normoxic and hypoxic conditions (Figure 1(c) and (d)). Furthermore, the data indicated that ATP level was increased in NP cells by pramlintide treatment under hypoxia and normoxia at the concentration of 250 nM, with 73.36 ± 6.92 and 47.32 ± 5.37 nM/mg, respectively (Figure 1(e) and (f)). Although higher concentration of pramlintide facilitated the metabolism of NP cells, it was shown that a concentration of 250–300 nM was optimal under the experimental conditions.
Figure 1.
Determination of cell viability and energy metabolism in human nucleus pulposus (NP) cells treated with varying concentrations of pramlintide. Human NP cells were cultured with different concentrations of pramlintide (ranging from 0 to 500 nM with a difference of every 50 nM) in normoxic or hypoxic condition. (a) and (b) Cell viability of pramlintide treated NP cells, as measured by CCK-8. (c) and (d) Energy metabolism of NP cells treated with pramlintide, as measured by LAC levels using DCFH-DA. (e) and (f) ATP production in NP cells under normoxia or hypoxia, as determined using ATP assay kit. (g)–(j) Determination of AGG secretions in human NP cells treated with varying concentrations of pramlintide were detected by staining with 1% Alcian blue solution (Alcian blue staining 200×). (k) Quantification analysis of proteoglycan content. Data are represented as mean ± SD. *P < 0.05, **P < 0.01. Significance analysis was performed by comparing with normal control (NC).
The proteoglycan content was measured by Alcian blue staining of NP cells. The results showed an increased precipitation of proteoglycans in NP cells, in a dose-dependent manner. A significant increase in the proteoglycan precipitation was observed in the groups treated with concentrations of 250 and 300 nM of pramlintide compared to those treated with lower (0 and 50 nM) or higher concentrations (450 and 500 nM) of the drug under normoxic and hypoxic conditions. The optimal concentration of pramlintide considered was 250 nM. The precipitation of proteoglycans was significantly higher under hypoxia compared to normoxia (Figure 1(g)–(j)).
Effects of pramlintide on the expression of ECM anabolic proteins in NP cells
The expression of ECM anabolic proteins in NP cells was performed by western blotting. The expressions of AGG, Col2, and SOX9 were increased by pramlintide treatment under both normoxia and hypoxia (Figure 2). The drug concentration of 250–300 nM was further confirmed as optimum concentration for NP regeneration. Thus, pramlintide could promote ECM anabolism by increasing the expression of ECM anabolic proteins in NP cells.
Figure 2.
Anabolic phenotype with varying concentrations of pramlintide. Human NP cells were treated with different concentrations of pramlintide (ranging from 0 to 500 nM with a difference of every 50 nM) in normoxic or hypoxic condition. (a) and (b) Western blot analysis of AGG, Col2, and SOX9 proteins, in NP cells under normoxia and hypoxia. (c)–(h) Relative expression of AGG, Col2, and SOX9 proteins in normoxic and hypoxic conditions. Values are represented as mean ± SD. *P < 0.05, **P < 0.01. Significance analysis was performed by comparing with NC.
Effects of pramlintide on the expression of ECM catabolic proteins in NP cells
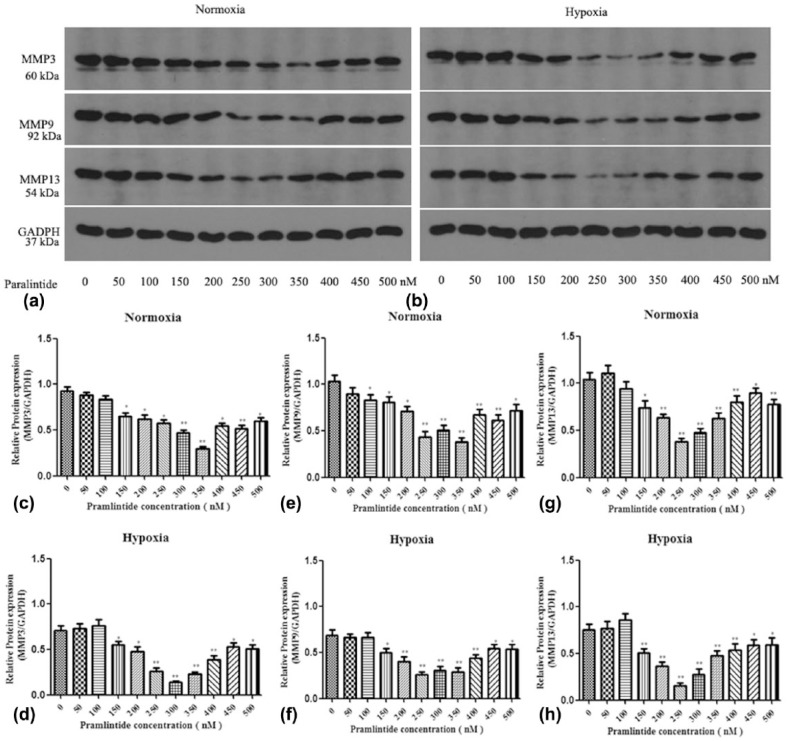
To further investigate the role of pramlintide on the ECM metabolism of NP cells, catabolic proteins in NP cells were detected by western blot analysis. The results indicated that pramlintide treatment decreased the expression of ECM catabolic proteins, including MMP3, MMP9, and MMP13 under both normoxia and hypoxia (Figure 3). Therefore, pramlintide was shown to promote ECM anabolism by inhibiting the expression of catabolic proteins in NP cells.
Figure 3.
Catabolic phenotype with varying concentrations of pramlintide treatment. Human NP cells were cultured with different concentrations of pramlintide (ranging from 0 to 500 nM with a difference of every 50 nM) in normoxic or hypoxic condition. (a) and (b) Western blot analysis of MMP3, MMP9, and MMP13 proteins in NP cells under normoxia and hypoxia. (c)–(h) Relative expression of MMP3, MMP9, and MMP13 proteins in normoxia and hypoxia. Values are represented as mean ± SD. *P < 0.05, **P < 0.01. Significance analysis was performed by comparing with NC.
Evaluation of ROS, ΔΨm, and Ca2+ concentration
The cellular oxidative stress was caused by ROS. As shown in Figure 4(a) and (b), flow cytometry results indicated that pramlintide countered the oxidative state in human NP cells under normoxia. The fluorescence intensity of ROS was significantly decreased in NP cells treated with 250 nM of pramlintide in hypoxic condition, compared to the control groups. Increased cellular Ca2+ concentration could damage mitochondrial function, which was measured by ΔΨm, an important parameter for determining the mitochondrial function. To observe the role of pramlintide on the mitochondrial membrane potential (ΔΨm), NP cells were treated with 250 nM of pramlintide under both normoxia and hypoxia. As shown by flow cytometry results, the fluorescence intensity of ΔΨm was significantly increased in NP cells treated with 250 nM of pramlintide in hypoxic condition compared to the control groups (Figure 4(c) and (d)). Quantitative measurement showed that ΔΨm was significantly improved in the cells treated with 250-nM pramlintide, which coincided with the changes of cellular Ca2+ concentration (Figure 4(e) and (f)). These results showed that pramlintide treatment on NP cells resulted in decreased release of Ca2+ from intracellular stores ([Ca2+]) and ameliorated loss of ΔΨm in NP cells under hypoxia.
Figure 4.
Determination of oxidative stress, mitochondrial membrane potential (MMP), and Ca2+ concentration in human nucleus pulposus (NP) cells treated with pramlintide. Human NP cells were cultured with 0 and 250 nM of pramlintide in normoxic or hypoxic conditions. (a) The cellular ROS was detected by measuring the fluorescence intensity using flow cytometry. (b) Quantitative analysis of ROS production in different groups. (c) The cellular MMP was detected by measuring the fluorescence intensity using flow cytometry. (d) Quantitative analysis of the changes in MMP in different groups. (e) The cellular Ca2+ concentration was detected by measuring the fluorescence intensity using flow cytometry. (f) Quantitative analysis of Ca2+ concentration in different groups. Data are represented as mean ± SD. *P < 0.05, **P < 0.01. Significance analysis was performed by comparing with NP cells treated with 250-nM pramlintide under normoxia.
Pramlintide facilitates survival of NP cells under hypoxia by inhibiting apoptosis
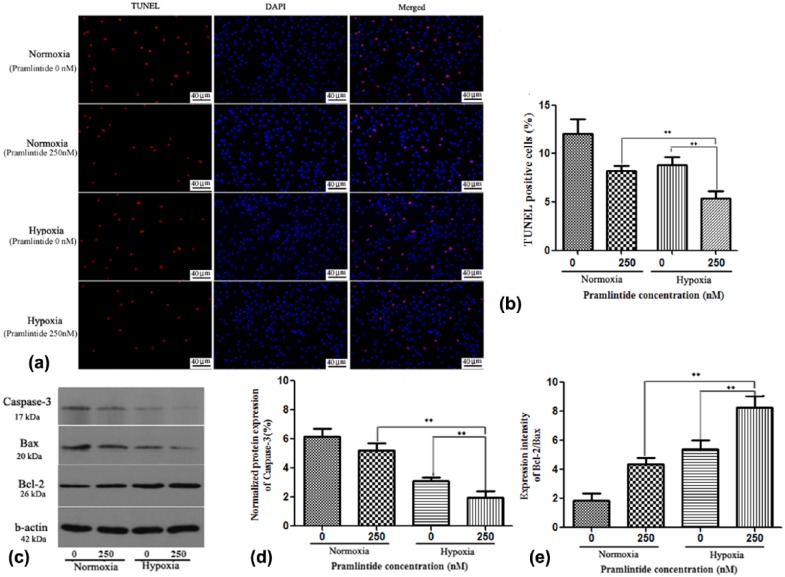
To investigate the effects of pramlintide on cell apoptosis, NP cells were treated with 250 nM of pramlintide under both normoxia and hypoxia. As shown in Figure 5(a) and (b), pramlintide treatment significantly decreased NP cell apoptosis compared to the untreated groups, with TUNEL positive rate being 12.02% and 8.19% under normoxia and 8.81% and 5.35% under hypoxia, in treated and untreated groups, respectively. The results indicated that apoptotic rate was decreased after pramlintide treatment under normoxia and hypoxia. Overall, the results indicated that pramlintide could facilitate the survival of NP cells under hypoxia.
Figure 5.
Apoptotic assays of human NP cells treated with pramlintide. Human NP cells were cultured with 0 and 250 nM of pramlintide in normoxic or hypoxic condition. (a) TUNEL assay to identify the apoptotic (red) NP cells upon treatment with pramlintide under normoxia and hypoxia (scale bar magnification: 200×). (b) Statistical analysis showing TUNEL positive rate. (c) Western blot analysis of cleaved Caspase-3, Bax, and Bcl-2 in different groups. (d) Statistical analysis showing the relative activity of cleaved Caspase-3. (e) Statistical analysis showing the ratio of Bcl-2/Bax. Data are represented as mean ± SD. *P < 0.05, **P < 0.01. Significance analysis was performed by comparing with NP cells treated with 250-nM pramlintide under normoxia.
Pramlintide decreases apoptosis in NP cells through the activation of Bcl-2 and inhibition of Bax and cleaved caspase-3 proteins
To investigate the effects of pramlintide on apoptotic pathway in NP cells, the expressions of apoptosis-related proteins were detected. As shown in Figure 5(c)–(e), pramlintide treatment reduced the expression of cleaved caspase-3 in NP cells under normoxia and hypoxia. In addition, the treatment increased the expression of Bcl-2 protein, which was accompanied by inhibition of Bax expression, as shown by the increased ratio of Bcl-2/Bax. These results indicated that pramlintide treatment inhibited the mitochondrial pathway of apoptosis in NP cells, especially in the hypoxic condition.
Pramlintide treatment activates the AKT-AMPK/mTOR signaling pathway in NP cells
The AKT and AMPK are the key upstream kinases involved in apoptosis regulation. To explore the effect of pramlintide on the regulation of AKT-AMPK signaling pathway, NP cells were cultured in the presence of 250-nM pramlintide with or without specific inhibitors of AKT and AMPK under normoxia and hypoxia. As shown in Figure 6, pramlintide treatment increased the level of p-AKT and decreased the level of p-AMPK, resulting in an increase in the phosphorylation of its substrate mTOR. To further explore the mechanism, AKT/mTOR and AMPK/mTOR were blocked with specific inhibitors (AMPK inhibitor: Compound C, Selleck, S7306; AKT inhibitor, GSK690693, Selleck, S1113, Houston, TX, USA). The results indicated that the phosphorylated levels of AKT (p-AKT) were partially activated and levels of p-AMPK were significantly reduced upon pramlintide treatment, resulting in the phosphorylation of mTOR and hence survival of NP cells.
Figure 6.
Effects of pramlintide on the AKT-AMPK-mTOR signaling. (a) Western blot analysis to measure the phosphorylated and unphosphorylated protein levels of AKT-AMPK-mTOR signaling pathway in human nucleus pulposus (NP) cells cultured with 0 and 250 nM of pramlintide in normoxic or hypoxic condition. (b) The NP cells were treated with pramlintide alone or in combination with AKT or AMPK inhibitor. The phosphorylated and unphosphorylated levels of protein were detected by western blot analysis. (c)–(h): Ratio of (c) and (f) p-AKT/AKT, (d) and (g) p-AMPK/AMPK, and (e) and (h) p-mTOR/mTOR in different treatment groups. Data are presented as mean ± SD. *P < 0.05, **P < 0.01. Significance analysis was performed by comparing with NP cells treated with 250-nM pramlintide under normoxia.
Discussion
The goal of this study was to investigate the effects of pramlintide on cell proliferation and matrix metabolism of human NP cells under hypoxic condition. To the best of our knowledge, this is the first study to investigate the effects of pramlintide on NP cells under normoxic/hypoxic conditions. Our results demonstrated that NP cells responded differently to normoxia and hypoxia. The pramlintide treatment showed significant increase in the cell proliferation and ECM synthesis in NP cells under hypoxia, which was further supported by the expression of ECM anabolic and catabolic genes, and proteoglycan retention in NP cells. In addition, our results clearly indicated that pramlintide treatment inhibited cell apoptosis via mitochondria-Ca2+-dependent apoptotic pathway, thereby decreasing the apoptosis via AKT/AMPK–mTOR signaling pathway.
Despite evidence that impaired nutrient supply is closely related to disk degeneration, not enough is known about the cellular consequences of nutrient failure. A large number of studies have shown that hypoxia affects cellular metabolism in the disk21,22 and low glucose is detrimental to the cell proliferation and survival.2 Hypoxic microenvironment plays a critical role in maintaining the IVD-associated physiological functions, including cellular metabolism and protein synthesis.20,23,24 In low-oxygen environments, ATP is generated mainly through glycolysis. Because of low oxygen, the NP cells depend on glycolysis rather than oxidative phosphorylation to generate their energy.25 Glycolysis may be an inefficient process, yielding only two ATP molecules of ATP/mole glucose compared to 30 ATP molecules of ATP/mole glucose by mitochondrial metabolism. However, the mitochondrial metabolism is very slow compared to the rate of glycolysis. Thus, the glycolytic pathway can provide sufficient energy to meet the needs of cell metabolism and protein synthesis.
Under normoxic condition, basal ATP levels in NP cells are between 20 and 25 nmol/L/mg protein. The NP cells cultured with 2-deoxyglucose, a potent inhibitor of glycolysis, resulted in 80% decrease in the ATP generation, which indicated that NP cells mostly rely on glycolysis to generate ATP.25 LAC production, a good indirect marker for glycolysis, has been shown to decrease with low concentration of substrate glucose. As shown by our results, pramlintide promoted energy metabolism in NP cells through glycolysis in normoxic and hypoxic conditions. The NP cells play an important role in the maintenance of ECM metabolism by regulating the expression of anabolic and catabolic genes.1 With respect to anabolism in response to hypoxia, collagen type II (Col2), proteoglycans, and SOX9 are involved. The proteoglycan AGG is responsible for maintaining the water content of the nucleus. Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes that degrade ECM components.26 In case of catabolism, it is known that the expression and activity of MMPs are increased in degenerative IVD, resulting in ECM degradation.27,28 Our results showed that pramlintide enhanced proteoglycan retention, increased the expression of ECM anabolic proteins, and decreased the expression of ECM catabolic proteins, resulting in ECM synthesis. Additionally, the current qualitative observation of an increase in the ECM might possible due to more NP cells. As we know, ECM components are MMP substrates. MMPs are Ca2+-dependent enzymes which can regulate ECM degradation under activation.29,30
In general, apoptosis occurs through the death receptor pathway (extrinsic) or the mitochondria dependent pathway (intrinsic). The IVD degeneration is thought to involve both the intrinsic and extrinsic apoptotic pathways. The mitochondrial pathway of apoptosis is mediated by a number of cellular signaling cascades, such as decrease in the ΔΨm and increase in the membrane permeability, resulting in the opening of the permeability transition pore and the release of Ca2+. Subsequently, the expression of pro-apoptotic proteins is increased and the apoptotic process is activated.31 Calcium is a ubiquitous second messenger, and Ca2+-dependent cellular events are necessary for cell survival. As reported, the increase in the cytosolic Ca2+ concentration could induce cell apoptosis.32–34 Therefore, mitochondrial-Ca2+is a key regulator of apoptosis in cells and activates mitochondrial apoptotic signaling pathway.35,36 Lots of literature reports the roles of mitochondria on NP cells. Compression-induced IVD degeneration is mediated by mitochondrial apoptotic pathway, via excessive generation of ROS and decreasedΔΨm.37 Pyrroloquinoline quinone (PQQ) could decrease H2O2-induced ROS formation and maintain ΔΨm in NP cells, resulting in reduced apoptosis.38 IL-1β can damage mitochondrial and decrease ΔΨm, eventually led to cell apoptosis in degenerative NP cells.39 In mitochondrial death pathway, the ratio of Bcl-2/Bax either leads to cell death or survival.40 The Bcl-2 family of proteins plays key roles in mitochondrial apoptotic pathway by preventing the efflux of cytochrome c and suppressing the release of other pro-apoptotic factors.40,41 In addition, Bcl-2 family-mediated caspase-3 activation has been confirmed as an essential factor for apoptosis.41 In this study, we have shown that anti-apoptotic protein Bcl-2 was increased during the treatment with pramlintide and further an increase in the ratio of Bcl-2/Bax was observed. In addition, the activation of cleaved caspase-3 was inhibited after pramlintide treatment. Overall, the results indicated that pramlintide treatment could suppress the mitochondrial-dependent apoptotic pathway in NP cells. Furthermore, the treatment improved the mitochondrial function by increasing ΔΨm and reducing ROS production.
The mTOR signaling is known to promote cell proliferation, differentiation, and survival. Constitutive activation of AKT, AMPK, and mTOR signaling regulates the cell energy balance. As reported in literature, pharmacological agents can lead to AMPK activation through mitochondrial stress in many cells.4 Suppression of AKT activity and activation of AMPK activity have been confirmed to contribute to the inhibition of mTOR signaling.42,43 Our study showed that pramlintide treatment could activate AKT/AMPK–mTOR signaling pathways by up-regulating AKT and down-regulating AMPK in NP cells. Based on the results, we deduced that pramlintide could promote NP cell proliferation, decrease cell apoptosis, and improve ECM metabolism through the activation of AKT-mTOR signaling pathway.
Without sufficient nutrient supply, NP cells fail to maintain the cellular activity or proliferation and synthesize the ECM, leading ultimately to disk degeneration. Hence, insufficient nutrient could be involved in the progression of disk degeneration, which may be the biological repair target for the impaired disk. This study demonstrates that pramlintide increases ECM metabolism and decreases apoptosis via mitochondria-Ca2+-dependent apoptotic pathway and the AMPK/mTOR signaling pathway in NP cells under hypoxia. Our results provide preliminary evidences supporting the possibility of using pramlintide as a novel pharmacological treatment strategy in IVD degeneration.
Acknowledgments
X.W., Y.S., and S.L. contributed equally to this work.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the National Science Foundation of China (NSFC; nos 81201393, 81541056, 2013YGYL015, and 2016-176).
References
- 1. Hu B, Shi C, Xu C, et al. (2016) Heme oxygenase-1 attenuates IL-1beta induced alteration of anabolic and catabolic activities in intervertebral disc degeneration. Scientific Reports 62: 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horner HA, Urban JP. (2001) 2001 Volvo award winner in basic science studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine 26(23): 2543–2549. [DOI] [PubMed] [Google Scholar]
- 3. Gruber HE, Hanley EN., Jr (1998) Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine 23(7): 751–757. [DOI] [PubMed] [Google Scholar]
- 4. Sakellaridis N. (2006) The influence of diabetes mellitus on lumbar intervertebral disk herniation. Surgical Neurology 66(2): 152–154. [DOI] [PubMed] [Google Scholar]
- 5. Mobbs RJ, Newcombe RL, Chandran KN. (2001) Lumbar discectomy and the diabetic patient: Incidence and outcome. Journal of Clinical Neuroscience 8(1): 10–13. [DOI] [PubMed] [Google Scholar]
- 6. Anekstein Y, Smorgick Y, Lotan R, et al. (2010) Diabetes mellitus as a risk factor for the development of lumbar spinal stenosis. The Israel Medical Association Journal 12(1): 16–20. [PubMed] [Google Scholar]
- 7. Robinson D, Mirovsky Y, Halperin N, et al. (1998) Changes in proteoglycans of intervertebral disc in diabetic patients. A possible cause of increased back pain. Spine 23(8): 849–855. [DOI] [PubMed] [Google Scholar]
- 8. Won HY, Park JB, Park EY, et al. (2009) Effect of hyperglycemia on apoptosis of notochordal cells and intervertebral disc degeneration in diabetic rats. Journal of Neurosurgery Spine 11(6): 741–748. [DOI] [PubMed] [Google Scholar]
- 9. Weyer C, Maggs DG, Young AA, et al. (2001) Amylin replacement with pramlintide as an adjunct to insulin therapy in type 1 and type 2 diabetes mellitus: A physiological approach toward improved metabolic control. Current Pharmaceutical Design 7(14): 1353–1373. [DOI] [PubMed] [Google Scholar]
- 10. Roth JD, Erickson MR, Chen S, et al. (2012) GLP-1R and amylin agonism in metabolic disease: Complementary mechanisms and future opportunities. British Journal of Pharmacology 166(1): 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Traina AN, Kane MP. (2011) Primer on pramlintide, an amylin analog. The Diabetes Educator 37(3): 426–431. [DOI] [PubMed] [Google Scholar]
- 12. Jeon SM, Chandel NS, Hay N. (2012) AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485: 661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu YL, DeLay M, Jahangiri A, et al. (2012) Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Research 72(7): 1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adler BL, Yarchoan M, Hwang HM, et al. (2014) Neuroprotective effects of the amylin analogue pramlintide on Alzheimer’s disease pathogenesis and cognition. Neurobiology of Aging 35(4): 793–801. [DOI] [PubMed] [Google Scholar]
- 15. Ceriello A, Piconi L, Quagliaro L, et al. (2005) Effects of pramlintide on postprandial glucose excursions and measures of oxidative stress in patients with type 1 diabetes. Diabetes Care 28(3): 632–637. [DOI] [PubMed] [Google Scholar]
- 16. Rajpurohit R, Risbud MV, Ducheyne P, et al. (2002) Phenotypic characteristics of the nucleus pulposus: Expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell and Tissue Research 308(3): 401–407. [DOI] [PubMed] [Google Scholar]
- 17. Bartels EM, Fairbank JC, Winlove CP, et al. (1998) Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine 23(1): 1–7; discussion 8. [DOI] [PubMed] [Google Scholar]
- 18. Lee DC, Adams CS, Albert TJ, et al. (2007) In situ oxygen utilization in the rat intervertebral disc. Journal of Anatomyt 210(3): 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hiyama A, Skubutyte R, Markova D, et al. (2011) Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: Implications in degenerative disc disease. Arthritis and Rheumatism 63(5): 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Risbud MV, Guttapalli A, Stokes DG, et al. (2006) Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: A metabolic adaptation to the intervertebral disc microenvironment. Journal of Cellular Biochemistry 98(1): 152–159. [DOI] [PubMed] [Google Scholar]
- 21. Holm S, Maroudas A, Urban JP, et al. (1981) Nutrition of the intervertebral disc: Solute transport and metabolism. Connective Tissue Research 8(2): 101–119. [DOI] [PubMed] [Google Scholar]
- 22. Ishihara H, Urban JP. (1999) Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. Journal of Orthopaedic Research 17(6): 829–835. [DOI] [PubMed] [Google Scholar]
- 23. Risbud MV, Schipani E, Shapiro IM. (2010) Hypoxic regulation of nucleus pulposus cell survival: From niche to notch. American Journal of Pathology 176(4): 1577–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grunhagen T, Shirazi-Adl A, Fairbank JC, et al. (2011) Intervertebral disk nutrition: A review of factors influencing concentrations of nutrients and metabolites. The Orthopedic Clinics of North America 42(4): 465–477. [DOI] [PubMed] [Google Scholar]
- 25. Agrawal A, Guttapalli A, Narayan S, et al. (2007) Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. American Journal of Physiology: Cell Physiology 293(2): C621–C631. [DOI] [PubMed] [Google Scholar]
- 26. Nagase H, Woessner JF., Jr (1999) Matrix metalloproteinases. Journal of Biological Chemistry 274(31): 21491–21494. [DOI] [PubMed] [Google Scholar]
- 27. Le Maitre CL, Pockert A, Buttle DJ, et al. (2007) Matrix synthesis and degradation in human intervertebral disc degeneration. Biochemical Society Transactions 35(Pt. 4): 652–655. [DOI] [PubMed] [Google Scholar]
- 28. Richardson SM, Doyle P, Minogue BM, et al. (2009) Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Research and Therapy 11(4): R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nagase H, Suzuki K, Cawston TE, et al. (1997) Involvement of a region near valine-69 of tissue inhibitor of metalloproteinases (TIMP)-1 in the interaction with matrix metalloproteinase 3 (stromelysin 1). Biochemical Journal 325(Pt. 1): 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Visse R, Nagase H. (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circulation Research 92(8): 827–839. [DOI] [PubMed] [Google Scholar]
- 31. Qiao Y, Xiang Q, Yuan L, et al. (2013) Herbacetin induces apoptosis in HepG2 cells: Involvements of ROS and PI3K/Akt pathway. Food Chemical Toxicology 51: 426–433. [DOI] [PubMed] [Google Scholar]
- 32. Wang H, Zhai N, Chen Y, et al. (2017) Cadmium induces Ca2+ mediated, calpain-1/caspase-3-dependent apoptosis in primary cultured rat proximal tubular cells. Journal of Inorganic Biochemistry 172: 16–22. [DOI] [PubMed] [Google Scholar]
- 33. Wei S, Wang Y, Chai Q, et al. (2014) Potential crosstalk of Ca2+-ROS-dependent mechanism involved in apoptosis of Kasumi-1 cells mediated by heme oxygenase-1 small interfering RNA. International Journal of Oncology 45(6): 2373–2384. [DOI] [PubMed] [Google Scholar]
- 34. Son YO, Lee JC, Hitron JA, et al. (2010) Cadmium induces intracellular Ca2+- and H2O2-dependent apoptosis through JNK- and p53-mediated pathways in skin epidermal cell line. Toxicological Science 113(1): 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kroemer G, Reed JC. (2000) Mitochondrial control of cell death. Nature Medicine 6(5): 513–519. [DOI] [PubMed] [Google Scholar]
- 36. Ma Q, Fang H, Shang W, et al. (2011) Superoxide flashes: Early mitochondrial signals for oxidative stress-induced apoptosis. Journal of Biological Chemistry 286(31): 27573–27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ding F, Shao ZW, Yang SH, et al. (2012) Role of mitochondrial pathway in compression-induced apoptosis of nucleus pulposus cells. Apoptosis 17(6): 579–590. [DOI] [PubMed] [Google Scholar]
- 38. Yang L, Rong Z, Zeng M, et al. (2015) Pyrroloquinoline quinone protects nucleus pulposus cells from hydrogen peroxide-induced apoptosis by inhibiting the mitochondria-mediated pathway. European Spine Journal 24(8) 1702–1710. [DOI] [PubMed] [Google Scholar]
- 39. Shen J, Xu S, Zhou H, et al. (2017) IL-1beta induces apoptosis and autophagy via mitochondria pathway in human degenerative nucleus pulposus cells. Scientific Reports 7: 41067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kluck RM, Bossy-Wetzel E, Green DR, et al. (1997) The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 275(5303): 1132–1136. [DOI] [PubMed] [Google Scholar]
- 41. Yang J, Liu X, Bhalla K, et al. (1997) Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 275(5303): 1129–1132. [DOI] [PubMed] [Google Scholar]
- 42. Boehlke C, Kotsis F, Patel V, et al. (2010) Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nature Cell Biology 12(11): 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumar D, Shankar S, Srivastava RK. (2013) Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: Molecular mechanisms. Molecular Cancer 12(1): 171. [DOI] [PMC free article] [PubMed] [Google Scholar]