Abstract
Purpose
Late-life depression (LLD) even in subsyndromal stages revealed strong associations with Alzheimer’s disease (AD). Furthermore brain amyloidosis depicts an early biomarker in subjects subsequently suffering from AD and is sensitively detectable by amyloid-PET. Therefore we aimed to compare amyloid-load and glucose metabolism in subsyndromally depressed mild cognitive impaired (MCI) subjects.
Methods
371 MCI subjects from ADNI underwent [18F]-AV45-, [18F]-FDG-PET, and MRI. Subjects were judged β-amyloid positive (Aß(+); N=206) or negative (Aß(−); N=165) according to [18F]-AV45-PET. Depressive symptoms were assessed by the Neuropsychiatric Inventory Questionnaire (NPI-Q) depression-item. 65 Aß(+) and 41 Aß(−) subjects with depressive symptoms (DEP) were contrasted against their non-depressed (NON-DEP) counterparts. Conversion rates to AD were analysed (mean follow-up time: 21.5±9.1 months) with regard to coexisting depressive symptoms and brain amyloid-load.
Results
Aß(+) DEP subjects showed large clusters with higher amyloid-load in frontotemporal and insular cortices (p<0.001) and coincident hypermetabolism (p<0.001) in frontal cortices when contrasted against NON-DEP. Faster progression to AD was observed in subjects with either depressive symptoms (p<0.005) or Aß(+) status (p<0.001). Coincident depressive symptoms additionally shortened the conversion time in all Aß(+) subjects (p<0.005) and to a greater extent in a subgroup with high amyloid-load (p<0.001).
Conclusions
Our results clearly indicate that Aß(+) MCI subjects with depressive symptoms suffer from elevated amyloid-load combined with relative hypermetabolism of connected brain areas when contrasted against cognitively-matched non-depressed individuals. MCI subjects with high amyloid-load and coexistent depressive symptoms represent a high-risk group for faster conversion to AD.
Keywords: ß-amyloid, [18F]-AV45-PET, [18F]-FDG-PET, depressive symptoms, mild cognitive impairment
1. Introduction
Alzheimer’s disease (AD) is imposing an onerous burden on health care systems in societies with aging populations [1]. Neurofibrillary tangles and amyloid plaques together comprise the hallmark neuropathology of AD [2]. Elevated brain amyloid burden is now clearly associated with cognitive decline in the healthy elderly (CN) [3] and in cases of mild cognitive impairment (MCI) [4]. Recent studies have identified associations of late-life depression (LLD) and MCI [5] as well as AD [6]. A higher relative risk of conversion from CN to MCI and to a lesser degree from MCI to AD [7] was demonstrated for subsyndromally depressed elderly patients (rated by the Neuropsychiatric Inventory Questionnaire (NPI-Q)). However, the literature remains inconclusive whether LLD is a risk factor for emergence of AD, or if late-life depression, as an early symptom of AD, is implicated in the pathophysiology of Alzheimer’s type dementia. Prior investigations regarding the link between depression and brain amyloidosis have mainly focused on subjects with remitted earlier depressive episodes [8-10], mostly finding elevated β-amyloid levels. Despite these roughly consistent amyloid PET findings, there is less concordance in [18F]-fluorodeoxyglucose (FDG) PET studies in depressed subjects, which have shown regions of hypermetabolism [11-13] or hypometabolism [14-17].
Given this background, we aimed in the present study to investigate brain amyloidosis, in synopsis with studies of brain glucose metabolism at presence or absence of depressive symptoms (defined by NPI-Q) in a large cohort of MCI subjects from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Furthermore we investigated the impact of depressive symptoms at baseline on the progression of dementia in clinical follow-up data.
2. Methods
2.1 Alzheimer’s Disease Neuroimaging Initiative
Data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). The ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies and non-profit organizations, as a 5-year public-private partnership with a USD 60 million budget. The primary goal of ADNI has been to identify an optimal combination of serial magnetic resonance imaging (MRI), PET, and other biological markers, in conjunction with clinical and neuropsychological assessments to predict and measure the progression of MCI and early AD. The objective of determining sensitive and specific markers of very early AD progression will aid researchers and clinicians in developing new treatments and monitoring their effectiveness, while lessening the expense and duration of clinical trials.
The Principal Investigator of this initiative is Michael W. Weiner, MD, VA Medical Center and University of California – San Francisco, but ADNI is the fruit of efforts of many co-investigators from diverse academic institutions and private corporations; subjects have been recruited from over 50 sites across the U.S.A and Canada. Alzheimer’s Disease Neuroimaging Initiative studies are conducted in accordance with the Good Clinical Practice guidelines, the Declaration of Helsinki, and U.S. 21 CFR Part 50 (Protection of Human Subjects), and Part 56 (Institutional Review Boards). This study was approved by the Institutional Review Boards of all of the participating institutions. Informed written consent was obtained from all participants at each site. The initial goal of ADNI was to recruit 800 subjects, but with the project extensions ADNI-GO and ADNI-2 have recruited over 1500 adults, aged 55 to 90 years. The research population consists of either cognitively-normal older individuals, people with early or late MCI, or early AD patients. The follow-up duration of each group is specified in the protocols for ADNI-1, ADNI-2 and ADNI-GO. Subjects originally recruited for ADNI-1 and ADNI-GO had the option to be followed in ADNI-2. For up-to-date information, see www.adni-info.org.
Data from ADNI-GO/ADNI2 were included in the present work. Pre-processed brain PET recordings images and corresponding T1-weighted MPRAGE MR images (T1w-MRI) were downloaded from the ADNI database as defined on August 30th, 2013.
2.2 Patient selection and study design
371 clinically rated MCI subjects had received [18F]-AV45-PET, FDG-PET and T1w-MRI at baseline within ADNI-GO/ADNI2 at the database cutoff date. In addition, an assessment of apolipoprotein-ε (APO-ε) status, NPI-Q, mini mental state examination (MMSE) and education level were recorded at the time of the PET scans.
All subjects were categorized according to their depressive symptoms and brain ß-amyloid (Aß) status: present subsyndromal depression was defined by the NPI-Q [18], when item #4 (depressive symptoms) was either negative (NON-DEP) or positive (DEP) at the time (± 2 months) of PET scanning. Aß positive (Aß(+)) and Aß negative (Aß(−)) [18F]-AV45-PET status was defined according to the threshold of ≥ 1.10, a criterion deriving from the ADNI database for the composite-VOI-SUVR furnishing the highest diagnostic discrimination between CN and AD [19]. As proportional distributions of Aß(+) subjects were unequal between NON-DEP (53%; 141/265) and DEP (61%; 65/106) groups (p=0.16), analyses were performed separately in Aß(−) (N=165) and Aß(+) (N=206) subjects.
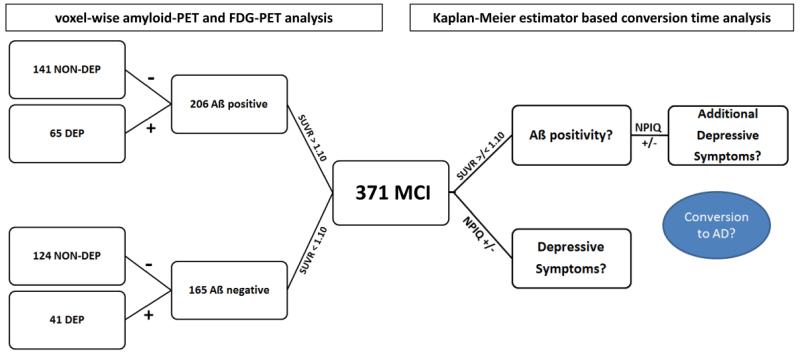
A mean 21.5 ± 9.1 month follow-up with regard to conversion to dementia was available for 366 subjects (database: June 2nd, 2014). Each of these subjects was identified as a non-converter (CONV(−)) when MCI diagnosis was stable over the whole observation time, or as a converter (CONV(+)), when MCI had progressed to AD. A detailed overview of all study groups including demographics is provided in Figure 1 and Table 1.
Figure 1.
Subject stratification among 371 MCI subjects with contemporaneous [18F]-AV45-PET, FDG-PET and T1w MRI at ADNI-GO/2 baseline assessment. All subjects were first categorized as positive or negative according to their amyloid-PET status [19] for the voxel-wise analysis (left branch). Subsequently, the Neuropsychiatric Inventory Questionnaire (NPI-Q; depression item #4) was used to identify subclinically depressed and non-depressed study-groups. The 366 subjects who received clinical follow-up were used for the conversion analysis (right branch) with respect to Aß and depression status.
Table1.
Demographics and covariates of the contrasted study groups for Aß(+) and Aß(−) subjects. Study groups consisted of subsyndromally depressed (DEP) and non-depressed (NON-DEP) subjects.
| Study Groups | N | Age (y±SD) |
Gender (%-m/%-f) |
Education (y±SD) |
MMSE (0-30±SD) |
APO-ε (N:E4(%)) |
|---|---|---|---|---|---|---|
| Aß positive (Aß+) | ||||||
| NON-DEP | 141 | 73.4±6.8 | 55%(m)/45%(f) | 16.1±2.8 | 27.8±1.9 | 0:N=55(39%) / 1:N=59(42%) / 2:N=27(19%) |
| DEP | 65 | 73.7±7.3 | 54%(m)/46%(f) | 16.2±2.6 | 27.6±1.7 | 0:N=23(35%) / 1:N=37(57%) / 2:N=5(8%) |
| Aß negative (Aß−) | ||||||
| NON-DEP | 124 | 70.3±7.7 | 56%(m)/44%(f) | 16.5±2.5 | 28.5±1.4 | 0:N=96(77%) / 1:N=25(20%) / 2:N=3(3%) |
| DEP | 41 | 70.1±9.4 | 54%(m)/46%(f) | 16.0±2.6 | 28.3±1.8 | 0:N=33(81%) / 1:N=7(17%) / 2:N=1(2%) |
2.3 Image data
2.3.1 ADNI [18F]-AV45 and FDG-PET acquisition and pre-processing
[18F]-AV45 and FDG-PET images had been acquired using Siemens, GE and Philips PET scanners (http://adni.loni.usc.edu/wp-content/uploads/2010/05/ADNI2_PET_Tech_Manual_0142011.pdf) and were pre-processed as described in: http://adni.loni.usc.edu/methods/pet-analysis/pre-processing/.
2.3.2 ADNI MRI acquisition and pre-processing
T1w-MRI scans had been acquired using Siemens, GE or Philips MRI scanners followed by MRI preprocessing according to a standard protocol [20].
2.3.3 Image processing
2.3.3.1 MRI co-registration and segmentation
All co-registration procedures were performed using the PMOD FUSION tool (V. 3.407 PMOD-Technologies). First, T1w-MRIs were rigidly co-registered to the corresponding PET images to gain linear MRI-to-PET and inverted PET-to-MRI transformations, which were saved in MATLAB format. Next, T1w-MRIs were non-linearly co-registered to the standard Montreal Neurological Institute (MNI)-spaceT1w-template, and the calculated transformation was also saved in MATLAB format (MRI-2-MNI). T1w-MRIs were segmented into gray matter (GM), white matter (WM) and cerebro-spinal fluid (CSF) within native MRI-space using the PMOD PNEURO tool [21]. All segmentations were visually checked for correctness and extracerebral artifacts. When artifacts were present, masking through the individual’s whole brain FDG-PET image binarisation (in T1w-MRI-space) was applied to the segmentation.
2.3.3.2 Partial volume effect correction of [18F]-AV45-PET and FDG-PET
T1w-MRI-segmentations were co-registered to the corresponding PET images by MRI-to-PET transformation in order to perform partial volume effect correction (PVEC) in non-interpolated PET space. Voxel-wise PVEC (MGM) [22] was executed in PET space by PMOD with a GM threshold of 0.3, WM regression of 0.95 and isotropic full-width-at-half-maximum (FWHM) of 8 mm. PET-to-MRI and MRI-to-MNI transformations were finally combined and applied to the atrophy-corrected PET images to achieve spatially normalized PET images with a minimum of interpolation and a maximum of accuracy.
2.3.4 Image analysis
2.3.4.1 Voxel-wise [18F]-AV45 and FDG-PET analyses and statistics
Group comparisons were performed voxel-wise using two-sample t-tests in SPM8 (Wellcome Department of Cognitive Neurology) implemented in MATLAB (R 2011a; MathWorks Inc.). APO-ε allelic status, age, gender, education and MMSE scores were entered as covariates. For SPM analysis, all images were Gaussian-filtered with 8mm FWHM to minimize inter-image variability. Intensity normalization was subsequently performed with scaling of [18F]-AV45 and FDG activities to the cerebellum defined by the Hammers Atlas [23]. Implicit masking was used to compare only those voxels with valid values in all subjects after PVEC.
DEP were contrasted against NON-DEP using a significance threshold of p < 0.005, uncorrected for multiple comparisons and a cluster size of >100 voxels, while β-amyloid and FDG differences in parametric images were analyzed separately with two-tailed tests.
2.3.4.2 VOI-based [18F]-AV45-PET analyses
Whole brain composite volume-of-interest (VOI) values were assessed using the Hammers atlas and PVE-corrected standard-uptake-value-ratios using whole cerebellum (SUVRCBL) as a reference region. SUVRCBL values were compared between CONV(−) and CONV(+) subjects and between NON-DEP and DEP. APO-ε alleles, age, gender, MMSE and education were used as covariates (SPSS, version 21.0; IBM, Chicago, IL). P-values < 0.05 were deemed to be significant after Bonferroni correction.
2.3.4.3 Conversion analyses
Kaplan-Meier plots were used to compare conversion rates between both NON-DEP and DEP, as well as Aß(+) and Aß(−) subjects. Additionally conversion rates between NON-DEP and DEP were separately compared in Aß(+) and a subgroup of subjects comprising a high amyloid load (SUVRCBL > 1.7). The multivariate Cox proportional hazard model was used to obtain hazard ratio estimates and 95% confidence intervals for Aß(+), DEP, and presence of established risk factors (APO-ε alleles, age, gender, MMSE and education).
3. Results
Frequence of DEP was 32% in Aß(+) and 25% in Aß(−) subjects. There were no significant differences in MMSE score, gender, age or education within the Aß(+) and Aß(−) groups. APO-ε status significantly differed between Aß(+) and Aß(−) subjects (Table 1).
3.1 Voxel-wise [18F]-AV45 and FDG-PET analyses
3.1.1 in Aß(+) subjects
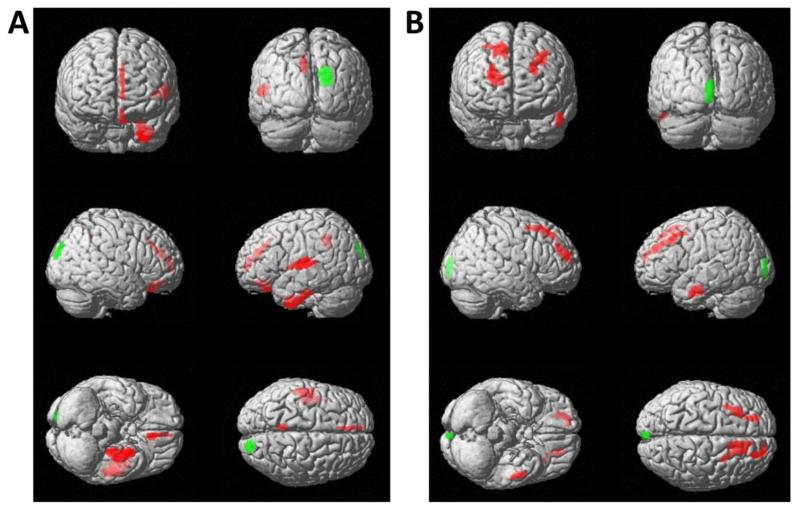
Within all Aß(+) subjects, the DEP subgroup revealed higher amyloid deposition in the left superior temporal gyrus, left uncus and gyrus parahippocampalis, left insula and the left cingulate gyrus (p < 0.001) as well as in the left medial frontal and rectal gyrus (p < 0.005) when compared to NON-DEP. Significantly lower levels of amyloid were found in a small cluster of the right cuneal cortex (p < 0.001) (Figure 2A).
Figure 2.
Results of statistical parametric mapping (SPM) for [18F]-AV45-PET (A) and FDG-PET (B) in Aß(+) subjects, corrected for MMSE scores, age, gender, APO-ε allelic status and years of education. Subsyndromally depressed subjects (DEP; N=65) were contrasted against NON-DEP (N=141) subjects. Voxels exceeding a significance threshold of p < 0.005, (unc.; k > 100 voxel) for increased amyloid levels or FDG hypermetabolism in depressed subjects are indicated in red, while voxels of decreased amyloid levels or FDG hypometabolism in subjects with depressive symptoms are indicated in green. Both contrasts are rendered on the surface of the standard SPM8 template.
Corresponding FDG data showed relative hypermetabolism in the bilateral frontal lobes as well as in the left fusiform gyrus (p < 0.001), when contrasting DEP versus NON-DEP subjects. Hypometabolism was found in a small left cuneal cluster (p < 0.001) (Figure 2B). All cluster sizes, localizations and T-scores are presented in Supplement Table 1.
3.1.2 in Aß(−) subjects
Within Aß(−) subjects, the [18F]-AV45-PET in the DEP group revealed small clusters with lower amyloid deposition in bilateral temporal, left precentral and right inferior frontal gyri (p < 0.001) when compared to NON-DEP, whereas no increases were found (Supplement Figure S1-A).
Corresponding FDG data did not show any metabolic differences in the whole DEP group (Supplement Figure S1-B). All cluster sizes, localizations and T-scores are presented in Supplement Table 2.
3.2 VOI based [18F]-AV45-PET analyses
Significant differences in PVE-corrected whole brain SUVRCBL were found between DEP CONV(+) subjects, who had the highest amyloid load (SUVRCBL 2.09 ± 0.48) and DEP CONV(−) subjects (SUVRCBL 1.77 ± 0.34; p < 0.001) as well as NON-DEP CONV(−) subjects (SUVRCBL 1.82 ± 0.30; p < 0.01). NON-DEP CONV(+) subjects also revealed significantly higher amyloid load (SUVRCBL 2.05 ± 0.42) in contrast to DEP CONV(−) subjects (SUVRCBL 1.77 ± 0.34; p < 0.01). No significant differences were found between subgroups of Aß(−) subjects (SUVRCBL range 1.15-1.23).
3.3 Conversion analyses
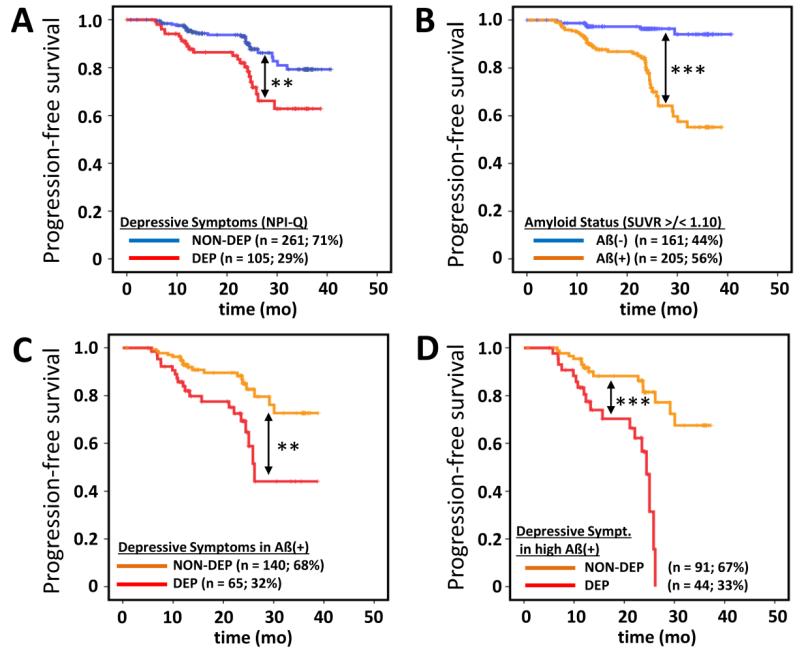
Out of 366 MCI subjects with a mean clinical follow-up of 21.9 ± 9.1 months, 50 (14%) progressed to AD. Considered separately, 24/105 (23%) DEP subjects and 26/261 (10%) NON-DEP subjects revealed a conversion during this time. 44/205 (21%) Aß(+) subjects and 6/161 (4%) Aß(−) subjects converted to AD. 20/44 (45%) subjects within Aß(+) converters revealed additional depressive symptoms.
Both the subsyndromally depressed (log-rank, p < 0.005; Figure 3A) and Aß(+) (log-rank, p < 0.001; Figure 3B) subjects showed significantly faster progression to AD when contrasted against their respective counterparts. Further categorization into DEP and NON-DEP groups within Aß(+) subjects revealed a significantly faster progression in DEP subjects (log-rank, p < 0.005) (Figure 3C). In subjects with a high amyloid load (SUVRCBL > 1.7) the effect of coexistent depressive symptoms was even more pronounced (log-rank, p < 0.001), revealing 100% converters to AD during follow-up, compared to 45% converters among the NON-DEP subjects (Figure 3D).
Figure 3.
Kaplan-Meier analyses of time to progression to dementia. Conversion to AD at a consecutive ADNI follow-up visit was used as the specific endpoint. DEP and NON-DEP subjects (A) as well as Aß positive (Aß+) and Aß negative (Aß−) subjects were contrasted using the log rank test. Additionally conversion rates between NON-DEP and DEP were separately compared for Aß(+) (C) and those subjects comprising a high amyloid load (SUVRCBL > 1.7) (D) in the brain. ** = p < 0.005; *** = p < 0.001
Hazard ratios for conversion to AD were 4.5 (95%-CI: 1.7–11.4; p < 0.005) for Aß(+), 2.9 (95%-CI: 1.6–5.3; p < 0.001) for DEP, and 0.8 (95%-CI: 0.7–0.9; p < 0.005) for NON-DEP. Considering Aß(+) subjects separately, those with additional depressive symptoms revealed a hazard ratio of 8.1 (95%-CI: 3.1–21.6; p < 0.001) in contrast to 3.0 (95%-CI: 1.1–8.2; p < 0.05) for Aß(+) subjects without depressive symptoms.
4. Discussion
We present results of the hitherto largest analysis of combined amyloid- and FDG-PET assessment in MCI subjects with respect to coexisting depressive symptoms. In addition, we provide the first longitudinal evaluation of progression to dementia in sub-syndromal but depressed subjects in synopsis with amyloid-PET as an integral biomarker. Our results clearly indicate that amyloid-positive MCI subjects with depressive symptoms suffer from elevated amyloidosis when contrasted against non-depressed individuals, with adjustment for various factors influencing cognition. The pronounced fronto-temporal amyloid deposition in these patients occurred in association with relative hypermetabolism in FDG-PET of connected brain areas. This finding may be related to an active inflammation, or represent a form of metabolic compensation in MCI subjects. The combination of elevated amyloid load and coexisting depressive symptoms proved to constitute a group with high-risk for faster progression to AD.
4.1 Amyloid-positive subjects
[18F]-AV45-PET revealed elevated amyloidosis in DEP Aß(+) subjects in left superior temporal, parahippocampal, insular and medial frontal gyri, brain regions comprising or connected with the mood disorder related medial prefrontal network [24]. The link between depression and dementia in Aß(+) subjects therefore might be based on the region-specific deposition of amyloid, when mood-related neurocircuits happen to be particularly affected by the amyloid pathology. Our findings are in line with previously reported associations of region-specific lateral and medial temporalamyloid/neurofibrillary tangle deposition with depression and anxiety in MCI subjects using [18F]-FDDNP-PET [25]. Earlier [11C]-PiB-PET findings with small sample size also provided evidence that amyloid load is elevated in late-life depressed MCI subjects [8]. CSF levels of Aß42 were concordantly reduced in MCI suffering from late life depression and correlated with cognitive status [26]. Postmortem analyses have indicated a pronounced deposition of amyloid plaques and tangles in the hippocampus, in brain of AD patients with history of major depression, while subjects with concurrent depressive symptoms at diagnosis of AD exhibited even higher neuropathological changes [27, 28]. Present elevated fronto-temporal levels of amyloid in DEP subjects are in line with these earlier histological findings. Another study revealed AD pathology to be prominent in the majority of cases with dementia and coexistent depression [29]. This is also in line with our data comprising a higher proportion of Aß(+) within the MCI DEP subjects (61%) in contrast to the MCI NON-DEP subjects (53%).
The question still remains if late-life depression constitutes part of the dementia prodrome and/or represents an individual risk factor for AD. From our data we can conclude that depressive symptoms in Aß(+) MCI patients were clearly associated with higher fronto-temporal amyloid levels. Recently published prospective results in the Australian Imaging, Biomarkers, and Lifestyle (AIBL) collective indicated notably slow increase of brain amyloidosis with age, taking extrapolated 12 years from absence to [11C]-PiB positivity and another 19 years from [11C]-PiB positivity to AD-like [11C]-PiB levels [30]. Therefore, we can speculate that a late-onset depression triggered by significant amyloid deposition may be part of the dementia prodrome, presenting as much as two decades before manifestation of AD. Indeed Wu and colleagues found increased [18F]-AV45 uptake in association with a lifetime occurrence of major depression (and present elevation of HAM-D score) in their contrast against depression-free MCI subjects [10] in similar brain regions as in the present investigation. However, an earlier [11C]-PiB study of similar design did not support this association [9]. This may reflect their investigation of significantly younger CN (mean age: 61y), with onset of depression six or more years previously, and with complete remission from depression at the time of PET scanning. Overall, findings are consistent with a model in which present depressive episodes in older subjects could constitute a prodrome and risk factor for AD development.
Our longitudinal follow-up data identified subsyndromally depressed MCI subjects (mean age: 72.1y) with a high amyloid load to be at high-risk for rapid conversion to AD. Recent findings in the AIBL population indicated that cognitively intact subjects with abnormal amyloid burden assessed by [11C]-PiB-PET showed a greater decline in episodic and verbal memory to three-year follow-up especially when comorbid for anxiety [31]. Hence, mood-related neuropsychiatric symptoms have an association with more aggressive AD pathology in Aß(+) subjects. Although the pathophysiological mechanism underlying this association cannot be resolved by this or other studies, present data confirms a link between rapid cognitive decline, amyloid burden and depressive symptoms, which may have important implications for new treatment targets [32].
4.2. Amyloid-negative subjects
Following the conjecture of higher amyloid deposition in subsyndromally depressed subjects, one might expect that amyloidosis in Aß(−) MCI subjects with depressive symptoms should all be close to the range or threshold for subjects with Aß(+) status. Surprisingly, we found significantly lower levels of amyloid in fronto-temporal areas of subsyndromally depressed Aß(−) subjects. These preliminary findings suggest that two different entities of “dementia” pathways can occur in late-life depressed subjects: 1) depression as a prodrome in the “normal” amyloid pathway and 2) depression without amyloid accumulation, in which subjective memory complaints may play an important role [33].
4.3 Amyloid levels in synopsis with FDG metabolism
Our results indicate that relative hypermetabolism is present in subsyndromally depressed Aß(+) MCI subjects within a similar domain of frontal brain areas revealing elevated amyloidosis in contrast with NON-DEP subjects. Increased glucose metabolism may reveal an inflammatory process in brain [34], plausibly caused by the amyloid pathology, or alternately reflecting a metabolic compensation in still healthy neurons embedded in amyloid-affected networks [35]. The correlation between binding of ligands such as [11C]-PBR28 for the 18 kDa translocator protein and AD severity was recently documented, lending support to the inflammation hypothesis [36]. It is noteworthy that [11C]-PBR28 and [11C]-PiB binding also correlated with clinical scores after partial volume effect correction, indicating that they reveal pathology rather than simply atrophy. Aß(−) MCI subjects positive to depressive symptoms were mostly inconspicuous with regard to FDG metabolism. These results likely explain the inconsistent results in previous FDG-PET studies regarding late-life depression where Aß status was not hitherto considered: Either hypermetabolism [11-13, 24] or hypometabolism [14-17] have been reported in late-life depressed subjects both in cognitively preserved, and impaired and AD patients. Moreover, PVEC was not applied in these FDG-PET studies investigating this topic, such that atrophy, as may occur in depressed subjects [37, 38], may have resulted in spurious findings of hypometabolism.
4.4 Limitations
NPI-Q was used to diagnose late-life depression, while there was no clinical diagnosis of depression, and especially no gold standard structured clinical interview data available. Therefore low sensitivity of a single item has to be considered as a limitation. The geriatric depression scale (GDS) might be a reasonable alternative. However a GDS > 5 was defined as an exclusion criterion for ADNI enrollment. Therefore, we think that defining clinical groups by the GDS might engender more selection bias due to this exclusion criterion, and we consecutively focused on NPI-Q. Additionally late-life depression as a clinical syndrome may reflect heterogeneous subtypes, including common vascular depression [39], which are probably different from amyloid pathology.
Subjects with depressive symptoms often suffer from additional neuropsychiatric symptoms such as anxiety or apathy [40, 41] which may be independent factors contributing to amyloid or metabolism status. However, had we restricted our search to patients positive for depressive symptoms only, the group size would have been too low to allow statistical comparison. Therefore, we contrasted our DEP subjects consistently against non-depressed controls irrespective of presence of other NPI-Q categories to minimize this bias.
Finally antidepressant medication was only documented since the baseline PET irrespective of prior duration, where 62% of DEP and 5% of NON-DEP subjects were treated with mostly serotonin-reuptake-inhibitors (SSRI). Therefore no final conclusions can be drawn on the effect of preexisting SSRI medication on amyloid levels in our subjects. However according to a recent report in cognitively normal subjects [32], amyloid levels in SSRI treated subjects would be expected to be decreased. Therefore our findings were more likely attenuated by this circumstance than positively biased. Longitudinal amyloid PET imaging in a prospective study of SSRI treated and untreated late-life depressed subjects would be of great value.
Supplementary Material
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.;NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to Rev December 5, 2013 support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. The authors acknowledge Inglewood Biomedical Editing for professional editing of the manuscript.
Peter Bartenstein received research support from the Federal Ministry of Education and Science (BMBF).
Axel Rominger received research support from the Friedrich-Baur Foundation and SyNergy cluster.
Footnotes
Conflict of Interest:
Matthias Brendel reports no disclosures
Oliver Pogarell reports no disclosures
Guoming Xiong reports no disclosures
Andreas Delker reports no disclosures
References
- 1.Ziegler-Graham K, Brookmeyer R, Johnson E, Arrighi HM. Worldwide variation in the doubling time of Alzheimer’s disease incidence rates. Alzheimers Dement. 2008;4:316–23. doi: 10.1016/j.jalz.2008.05.2479. doi:S1552-5260(08)02638-1 [pii] 10.1016/j.jalz.2008.05.2479. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 3.Lim YY, Ellis KA, Pietrzak RH, Ames D, Darby D, Harrington K, et al. Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012;79:1645–52. doi: 10.1212/WNL.0b013e31826e9ae6. doi:10.1212/WNL.0b013e31826e9ae6. [DOI] [PubMed] [Google Scholar]
- 4.Lim YY, Maruff P, Pietrzak RH, Ellis KA, Darby D, Ames D, et al. Abeta and cognitive change: Examining the preclinical and prodromal stages of Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2014 doi: 10.1016/j.jalz.2013.11.005. doi:10.1016/j.jalz.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of general psychiatry. 2006;63:530–8. doi: 10.1001/archpsyc.63.5.530. doi:10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olin JT, Katz IR, Meyers BS, Schneider LS, Lebowitz BD. Provisional diagnostic criteria for depression of Alzheimer disease: rationale and background. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2002;10:129–41. [PubMed] [Google Scholar]
- 7.Steenland K, Karnes C, Seals R, Carnevale C, Hermida A, Levey A. Late-life depression as a risk factor for mild cognitive impairment or Alzheimer’s disease in 30 US Alzheimer’s disease centers. Journal of Alzheimer’s disease: JAD. 2012;31:265–75. doi: 10.3233/JAD-2012-111922. doi:10.3233/JAD-2012-111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butters MA, Klunk WE, Mathis CA, Price JC, Ziolko SK, Hoge JA, et al. Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh Compound-B. Alzheimer disease and associated disorders. 2008;22:261–8. doi: 10.1097/WAD.0b013e31816c92bf. doi:10.1097/WAD.0b013e31816c92bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madsen K, Hasselbalch BJ, Frederiksen KS, Haahr ME, Gade A, Law I, et al. Lack of association between prior depressive episodes and cerebral [11C]PiB binding. Neurobiology of aging. 2012;33:2334–42. doi: 10.1016/j.neurobiolaging.2011.11.021. doi:10.1016/j.neurobiolaging.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Wu KY, Hsiao IT, Chen CS, Chen CH, Hsieh CJ, Wai YY, et al. Increased brain amyloid deposition in patients with a lifetime history of major depression: evidenced on F-florbetapir (AV-45/Amyvid) positron emission tomography. European journal of nuclear medicine and molecular imaging. 2013 doi: 10.1007/s00259-013-2627-0. doi:10.1007/s00259-013-2627-0. [DOI] [PubMed] [Google Scholar]
- 11.Smith GS, Kramer E, Hermann C, Ma Y, Dhawan V, Chaly T, et al. Serotonin modulation of cerebral glucose metabolism in depressed older adults. Biological psychiatry. 2009;66:259–66. doi: 10.1016/j.biopsych.2009.02.012. doi:10.1016/j.biopsych.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith GS, Kramer E, Ma Y, Kingsley P, Dhawan V, Chaly T, et al. The functional neuroanatomy of geriatric depression. International journal of geriatric psychiatry. 2009;24:798–808. doi: 10.1002/gps.2185. doi:10.1002/gps.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marano CM, Workman CI, Kramer E, Hermann CR, Ma Y, Dhawan V, et al. Longitudinal studies of cerebral glucose metabolism in late-life depression and normal aging. International journal of geriatric psychiatry. 2013;28:417–23. doi: 10.1002/gps.3840. doi:10.1002/gps.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirono N, Mori E, Ishii K, Ikejiri Y, Imamura T, Shimomura T, et al. Frontal lobe hypometabolism and depression in Alzheimer’s disease. Neurology. 1998;50:380–3. doi: 10.1212/wnl.50.2.380. [DOI] [PubMed] [Google Scholar]
- 15.Holthoff VA, Beuthien-Baumann B, Kalbe E, Ludecke S, Lenz O, Zundorf G, et al. Regional cerebral metabolism in early Alzheimer’s disease with clinically significant apathy or depression. Biological psychiatry. 2005;57:412–21. doi: 10.1016/j.biopsych.2004.11.035. doi:10.1016/j.biopsych.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Lee DY, Choo IH, Jhoo JH, Kim KW, Youn JC, Lee DS, et al. Frontal dysfunction underlies depressive syndrome in Alzheimer disease: a FDG-PET study. Am J Geriatr Psychiatry. 2006;14:625–8. doi: 10.1097/01.JGP.0000214541.79965.2d. doi:14/7/625 [pii] 10.1097/01.JGP.0000214541.79965.2d. [DOI] [PubMed] [Google Scholar]
- 17.Lee HS, Choo IH, Lee DY, Kim JW, Seo EH, Kim SG, et al. Frontal Dysfunction Underlies Depression in Mild Cognitive Impairment: A FDG-PET Study. Psychiatry investigation. 2010;7:208–14. doi: 10.4306/pi.2010.7.3.208. doi:10.4306/pi.2010.7.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of neuropsychiatry and clinical neurosciences. 2000;12:233–9. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 19.Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2013;9:e-1–16. doi: 10.1016/j.jalz.2013.01.002. doi:10.1016/j.jalz.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jack CR, Jr., Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. Journal of magnetic resonance imaging: JMRI. 2008;27:685–91. doi: 10.1002/jmri.21049. doi:10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. doi:10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Muller-Gartner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–83. doi: 10.1038/jcbfm.1992.81. doi:10.1038/jcbfm.1992.81. [DOI] [PubMed] [Google Scholar]
- 23.Smith GS, Ma Y, Dhawan V, Chaly T, Eidelberg D. Selective serotonin reuptake inhibitor (SSRI) modulation of striatal dopamine measured with [11C]-raclopride and positron emission tomography. Synapse. 2009;63:1–6. doi: 10.1002/syn.20574. doi:10.1002/syn.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. doi:10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavretsky H, Siddarth P, Kepe V, Ercoli LM, Miller KJ, Burggren AC, et al. Depression and anxiety symptoms are associated with cerebral FDDNP-PET binding in middle-aged and older nondemented adults. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2009;17:493–502. doi: 10.1097/jgp.0b013e3181953b82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun X, Steffens DC, Au R, Folstein M, Summergrad P, Yee J, et al. Amyloid-associated depression: a prodromal depression of Alzheimer disease? Archives of general psychiatry. 2008;65:542–50. doi: 10.1001/archpsyc.65.5.542. doi:10.1001/archpsyc.65.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rapp MA, Schnaider-Beeri M, Grossman HT, Sano M, Perl DP, Purohit DP, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Archives of general psychiatry. 2006;63:161–7. doi: 10.1001/archpsyc.63.2.161. doi:10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 28.Rapp MA, Schnaider-Beeri M, Purohit DP, Perl DP, Haroutunian V, Sano M. Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2008;16:168–74. doi: 10.1097/JGP.0b013e31816029ec. doi:10.1097/JGP.0b013e31816029ec. [DOI] [PubMed] [Google Scholar]
- 29.Sweet RA, Hamilton RL, Butters MA, Mulsant BH, Pollock BG, Lewis DA, et al. Neuropathologic correlates of late-onset major depression. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2004;29:2242–50. doi: 10.1038/sj.npp.1300554. doi:10.1038/sj.npp.1300554. [DOI] [PubMed] [Google Scholar]
- 30.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet neurology. 2013;12:357–67. doi: 10.1016/S1474-4422(13)70044-9. doi:10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 31.Pietrzak RH, Scott JC, Neumeister A, Lim YY, Ames D, Ellis KA, et al. Anxiety symptoms, cerebral amyloid burden and memory decline in healthy older adults without dementia: 3-year prospective cohort study. The British journal of psychiatry: the journal of mental science. 2014 doi: 10.1192/bjp.bp.113.134239. doi:10.1192/bjp.bp.113.134239. [DOI] [PubMed] [Google Scholar]
- 32.Sheline YI, West T, Yarasheski K, Swarm R, Jasielec MS, Fisher JR, et al. An Antidepressant Decreases CSF Abeta Production in Healthy Individuals and in Transgenic AD Mice. Science translational medicine. 2014;6:236re4. doi: 10.1126/scitranslmed.3008169. doi:10.1126/scitranslmed.3008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckley RF, Saling MM, Irish M, Ames D, Rowe CC, Lautenschlager NT, et al. Personal Memory Function in Mild Cognitive Impairment and Subjective Memory Complaints: Results from the Australian Imaging, Biomarkers, and Lifestyle (AIBL) Study of Ageing. Journal of Alzheimer’s disease: JAD. 2014 doi: 10.3233/JAD-131820. doi:10.3233/JAD-131820. [DOI] [PubMed] [Google Scholar]
- 34.Hermida AP, McDonald WM, Steenland K, Levey A. The association between late-life depression, mild cognitive impairment and dementia: is inflammation the missing link? Expert review of neurotherapeutics. 2012;12:1339–50. doi: 10.1586/ern.12.127. doi:10.1586/ern.12.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caroli A, Lorenzi M, Geroldi C, Nobili F, Paghera B, Bonetti M, et al. Metabolic compensation and depression in Alzheimer’s disease. Dementia and geriatric cognitive disorders. 2010;29:37–45. doi: 10.1159/000257761. doi:10.1159/000257761. [DOI] [PubMed] [Google Scholar]
- 36.Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain. 2013;136:2228–38. doi: 10.1093/brain/awt145. doi:10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lebedeva A, Westman E, Lebedev AV, Li X, Winblad B, Simmons A, et al. Structural brain changes associated with depressive symptoms in the elderly with Alzheimer’s disease. Journal of neurology, neurosurgery, and psychiatry. 2014 doi: 10.1136/jnnp-2013-307110. doi:10.1136/jnnp-2013-307110. [DOI] [PubMed] [Google Scholar]
- 38.Lee GJ, Lu PH, Hua X, Lee S, Wu S, Nguyen K, et al. Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer’s disease-related regions. Biological psychiatry. 2012;71:814–21. doi: 10.1016/j.biopsych.2011.12.024. doi:10.1016/j.biopsych.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Archives of general psychiatry. 1997;54:915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 40.Marshall GA, Donovan NJ, Lorius N, Gidicsin CM, Maye J, Pepin LC, et al. Apathy is associated with increased amyloid burden in mild cognitive impairment. The Journal of neuropsychiatry and clinical neurosciences. 2013;25:302–7. doi: 10.1176/appi.neuropsych.12060156. doi:10.1176/appi.neuropsych.12060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori T, Shimada H, Shinotoh H, Hirano S, Eguchi Y, Yamada M, et al. Apathy correlates with prefrontal amyloid beta deposition in Alzheimer’s disease. Journal of neurology, neurosurgery, and psychiatry. 2014;85:449–55. doi: 10.1136/jnnp-2013-306110. doi:10.1136/jnnp-2013-306110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.