Abstract
Background
Previous studies have attributed high maternal weight gain during pregnancy and pre-pregnancy obesity to a higher risk for autism spectrum disorders (ASD). Maternal underweight was not previously explored with respect to ASD risk.
Methods
We evaluated the association between maternal pre-pregnancy body mass index (BMI) and ASD occurrence among singletons born into the General Practice Research Database from 1993 through 2008. Case subjects were children with a diagnosis of ASD from birth through 2010. Up to four control subjects were matched to each case subject on birth year, sex, and general practice. Restricted cubic splines were used to assess the non-linearity of the association between maternal BMI and ASD. All study subjects were classified as underweight, normal weight, overweight or obese based on maternal pre-pregnancy BMI using the WHO Classification Standard. Conditional logistic regression was used to calculate crude and multivariable adjusted odds ratios for the association between categorical BMI (reference=normal weight) and the occurrence of ASD
Results
The association between maternal BMI and ASD occurrence was non-linear and J-shaped (p=0.003). The adjusted ORs for maternal underweight and obesity were 1.43 [95% CI: 1.01, 2.04] and 1.54 [95% CI: 1.26, 1.89], respectively.
Conclusions
Results suggest that extremes in maternal BMI may be associated with modest increases in the risk for ASD among offspring.
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by core deficits in social interaction, communication, and repetitive behavior.1 The prevalence of ASD has increased dramatically over the past few decades, rising from less than 20 per 10,000 persons in the 1980's to recent estimates as high as 1 per 68 persons, and has consistently been found to be approximately 4 times higher in males than females.2,3
The prevalence of obesity has exhibited a temporal increase that parallels that of ASD. In the UK, nearly 25% of the population is obese and about 16% of women are obese at the start of pregnancy, up from 9% in 1990.4 There are diverse theories supporting how maternal factors, including prepregnancy underweight and obesity, might alter the fetal environment to influence autism risk. Maternal undernutrition may elicit a physiological stress response leading to neuronal damage via excess release of proinflammatory factors.5 Additionally, maternal nutritional status and diet are important as the fetus is reliant on the mother for micronutrients and growth factors required for normal neurodevelopment. Insufficiency or overabundance, depending on the specific nutrient, can directly result in altered myelination, hippocampal and cerebellar development and synapse formation.6 Lastly, obesity may result in activation of the maternal immune system and a chronic inflammatory environment potentiating abnormal neuronal growth and differentiation in the fetus.7-10
Previous studies attributed high maternal weight gain during pregnancy and pre-pregnancy obesity to higher risk for ASD.11-13 However, some studies relied to some degree on retrospective self-report of maternal height and weight information. To our knowledge, ASD risk has not been explored relative to underweight.
The current study evaluated the relationship between maternal pre-pregnancy BMI measurements recorded prospectively within the UK-based General Practice Research Database and ASD occurrence among singletons born from 1993 through 2008.
METHODS
Data Source
This study utilized data from the version of the General Practice Research Database (GPRD) maintained by the Boston Collaborative Drug Surveillance Program (BCDSP). The GPRD (now called the Clinical Practice Research Datalink) is a primary care database containing anonymized records for nearly 4 million active patients (about 6% of the population of England, Northern Ireland, Scotland and Wales) from over 450 general practices (GP).14 GPRD data are recorded by trained GP using standardized software including the Read diagnosis coding system and multilex drug codes.15 Participating GP not only record each episode of illness and medical diagnoses, but also clinical contacts, examination findings, therapeutic and administrative procedures, test results, drug prescriptions, and referrals to specialists and outpatient clinics with the associated diagnoses. In the UK, GP assume the role of ‘gate-keeper’ to medical care. The typical trajectory to ASD diagnoses is that the GP refers a patient to a specialist who performs relevant evaluations and determines the diagnosis. The specialist is then required to provide a letter to the GP documenting the diagnosis and corresponding care plan. Therefore, GPRD should have near complete capture of ASD diagnoses.
GPRD members have both a unique individual identification code and a family identification code. A mother's records can be matched to offspring records based on family identification number, GP practice code, infant's birth year and mother's pregnancy outcome date.16, 17 Additionally, the start of the first trimester of pregnancy can be reliably identified in the majority of mother-offspring matched pregnancies, thus permitting the study of associations between maternal pre-pregnancy exposures and the development of offspring.17 Validation studies have verified most diagnoses coded in the GPRD electronic record, including ASD, to be highly accurate (i.e., a median of 90% of cases confirmed) when compared against GP questionnaire responses, medical records held at GP practices, or hospital letters, with diagnoses of chronic illness being somewhat more accurate than those of acute illness.14,18-20
Study Design and Population
All ASD cases and non-ASD controls were identified from a source population consisting of all singleton live births in the GPRD from 1993 through 2008 whose records were successfully matched to a mother with one or more years of recorded history in the database and at least one height and weight measurement recorded in the two years before the start of the pregnancy. Cases were identified as those with a diagnosis of ASD from birth through 2010 (Read Codes: E140.00 Infantile autism, E140000 Active infantile autism, E140.12 Autism, E140.13 Childhood autism, E140z00 Infantile autism NOS, Eu84000 Childhood autism, Eu84011 Autistic disorder, Eu84012 Infantile autism, Eu84z11 Autistic spectrum disorder NOS, Eu84500 Asperger syndrome, Eu84100 Atypical autism, Eu84y00 Other pervasive developmental disorder, and Eu84z00 Pervasive developmental disorder unspecified). Up to four random control subjects without an ASD diagnosis were selected from the source population and matched to each case on birth year, sex, and GP.
Determination of Pregnancy Start Date
We determined the first day of the last menstrual period (LMP) before the study pregnancy and used that as the pregnancy start date. For approximately half of the study population (54% of cases and 52% of controls), LMP was recorded in the maternal GPRD record or could be calculated directly from an expected date of delivery recorded in the medical record. Otherwise, LMP was calculated as the actual delivery date minus 280 days. If there was a code for preterm associated with the study pregnancy or the calculated LMP suggested the pregnancy duration was less than 37 weeks, then mother and child profiles were reviewed manually to verify the calculated LMP.
Body Mass Index
Pre-pregnancy height and weight measurements recorded within the two years before and closest to the LMP were used as the measure of maternal pre-pregnancy BMI, calculated as weight in kilograms divided by the square of height in meters (kg/m2). Intra-individual variability in BMI over time before the study pregnancy was evaluated to confirm that the selected BMI record was not an outlier value, for example a BMI measured very shortly after a previous pregnancy. Subjects were classified as underweight (BMI<18.5), normal weight (BMI 18.5–24.9), overweight (BMI 25–29.9) or obese (BMI ≥30) using the WHO Classification Standard. In a sub-analysis, adolescent mothers were re-classified to BMI categories based on an age-specific reference.21
Covariates
Potential confounders were identified a priori based on literature review. In addition to matched factors (child sex, birth year and GP), the following variables were abstracted from GPRD records of all case and control mothers and explored as potential confounders: maternal age at LMP, pre-pregnancy smoking status recorded nearest to the LMP (coded as current, past, never) and pre-pregnancy histories (yes, no) of alcoholism, drug abuse, diabetes and depression before the LMP. For each case and control, we also identified whether there were siblings with an ASD diagnosis (yes, no), as a crude proxy for genetic susceptibility.
Statistical Analyses
Descriptive analyses were performed to determine the covariate distributions for cases and controls, and by BMI category among controls. Restricted cubic spline regression was used to assess nonlinearity of the relationship between maternal BMI and ASD.22 Conditional logistic regression was used to calculate odds ratios (OR) controlling for matched variables only and multivariable adjusted ORs for the association between maternal categorical BMI (reference=normal weight) and the occurrence of ASD. The initial multivariable model included all potential confounders. Each covariate was independently removed from the full multivariable model and the variable with the smallest change in OR across BMI levels among those exhibiting a change of <10% was removed. A similar process was employed with successive models until a final model was achieved in which all retained variables would result in >10% change in OR for at least one level of BMI if removed. Since none of the covariates were retained in the final model using these criteria, results from both unadjusted models (cOR) and fully adjusted models (aOR) are presented. Subjects with missing covariate information were excluded from multivariable regression models. Effect modification by maternal age (≤30 years; >30 years) was evaluated on both multiplicative (incorporation of an interaction term in the statistical model) and additive scales (computation of the relative excess risk due to interaction, RERI). Initially ASD subtypes were examined separately with no differences identified; however, these analyses were not reported due to DSM-5 changes.
Sensitivity Analyses
We implemented a series of sensitivity analyses to address concerns about potential misclassification. Nondifferential misclassification of the timing of pre-pregnancy BMI could occur since the LMP for many mothers was estimated; therefore, we completed analyses restricted to mothers with a GP-recorded LMP. There was inter-individual variability in the proximity of the most recent GP-recorded BMI information to the LMP, so we completed separate analyses for individuals with BMI measurements within 12 months before the pregnancy start date and for those with measurements more than 12 months before the pregnancy start date.
RESULTS
During the study period 1993-2008, approximately 84% of children with a diagnosis of ASD who were registered with the GPRD at birth were able to be matched to their mother in the GPRD. Among the source population, we identified a total of 889 individuals with a diagnosis of ASD, of which 69.2% were diagnosed with autism, 27.4% were diagnosed with Asperger disorder, and 3.4% were diagnosed with PDD-NOS or more generally as having autism spectrum disorder. On average, cases were first diagnosed by 6.2 years of age (standard deviation ±3.2 years) with autism generally diagnosed at earlier ages than Asperger's disorder (5.4 ±2.9 years and 8.2 ±3.0 years respectively). ASD cases were predominantly male (n=724, 81.4%) independent of subtype. There were 3530 control subjects identified from the same source population as the ASD case subjects. All but 16 cases (98.1%) were matched to four controls.
Distributions of selected child and maternal characteristics among ASD case and non-ASD control subjects are presented in Table 1. Compared to control mothers, case mothers tended to be slightly older and somewhat more likely to be ever smokers (40.2% versus 37.5%) and to have a history of diabetes (1.1% versus 0.6%) or depression (17.8% versus 13.9%). Maternal histories for drug and alcohol abuse did not differ for cases and controls. A higher proportion of cases (8.4%) than controls (1.3%) had a sibling with an ASD diagnosis.
Table 1.
Distributions of selected child and maternal characteristics among ASD case and non-ASD control subjects, GPRD singleton live births 1993-2008
| Any ASD (N=889) | Controls (N=3530) | |
|---|---|---|
| % | % | |
| Child Sex | ||
| Male | 81.4 | 81.3 |
| Female | 18.6 | 18.7 |
| Child Year of Birth | ||
| 1993-1996 | 26.2 | 26.5 |
| 1997-2000 | 34.9 | 34.4 |
| 2001-2004 | 29.2 | 29.0 |
| 2005-2008 | 9.8 | 10.0 |
| Child Year of Diagnosisa | ||
| 1996-1998 | 3.0 | |
| 1999-2001 | 12.0 | |
| 2002-2004 | 20.7 | |
| 2005-2007 | 27.8 | |
| 2008-2010 | 36.5 | |
| Maternal Age | ||
| <20 years | 3.8 | 4.4 |
| 20-24 years | 16.6 | 16.5 |
| 25-29 years | 28.9 | 31.8 |
| 30-34 years | 32.5 | 31.6 |
| 35+ | 18.1 | 15.7 |
| Maternal Smoking | ||
| Current | 11.2 | 10.6 |
| Former | 28.9 | 26.9 |
| Never | 56.5 | 59.0 |
| Missing | 3.4 | 3.5 |
| Maternal Diabetes | ||
| Yes | 1.1 | 0.6 |
| No | 98.9 | 99.4 |
| Maternal Depression | ||
| Yes | 17.8 | 13.9 |
| No | 82.2 | 86.1 |
| Maternal Drug Abuse | ||
| Yes | 0.6 | 0.5 |
| No | 99.4 | 99.5 |
| Maternal Alcoholism | ||
| Yes | 2.0 | 2.1 |
| No | 98.0 | 97.9 |
| Sibling with ASD Diagnosis | ||
| Yes | 8.4 | 1.3 |
| No | 91.5 | 98.7 |
Year of diagnosis only relevant to children with autism spectrum disorder
Distributions of selected child and maternal characteristics by BMI category among controls are presented in Table 2. The majority of control mothers (57.8%) were normal weight, but 4% were underweight, 23.7% were overweight and 14.6% were obese. The maternal age distribution differed by BMI category. Specifically, compared to normal weight mothers, underweight mothers were younger (18.6% versus 4.8% younger than 20 years) whereas obese were older (17.3% over 35 years versus 14.6%). The extreme maternal BMI categories, underweight and obese, had higher proportions of ever smokers (43.6% and 41.6% versus 35.9%), pre-pregnancy depression (22.9% and 20.0% versus 11.1%) and alcoholism (2.1% and 3.5% versus 1.8%) versus normal weight. Pre-pregnancy diabetes was more prevalent with higher BMI and drug abuse was more common with lower BMI.
Table 2.
Distributions of selected child and maternal characteristics by BMI catergory among control subjects, GPRD singleton live births 1993-2008
| Underweight (n=140) | Normal Weight (n=2040) | Overweight (n=836) | Obese (n=514) | |
|---|---|---|---|---|
| % | % | % | % | |
| Maternal Age | ||||
| <20 years | 18.6 | 4.8 | 2.6 | 1.9 |
| 20-24 years | 20.7 | 17.1 | 15.9 | 14.2 |
| 25-29 years | 31.4 | 31.4 | 29.7 | 36.8 |
| 30-34 years | 19.3 | 32.2 | 33.4 | 29.8 |
| 35+ | 10.0 | 14.6 | 18.4 | 17.3 |
| Maternal Smoking | ||||
| Current | 6.4 | 9.7 | 12.1 | 12.6 |
| Former | 37.1 | 26.2 | 25.6 | 29.0 |
| None | 50.7 | 60.6 | 59.3 | 54.1 |
| Missing | 5.7 | 3.4 | 3.0 | 4.3 |
| Maternal Diabetes | ||||
| Yes | 0.0 | 0.2 | 1.1 | 1.4 |
| No | 100.0 | 99.8 | 98.9 | 98.6 |
| Maternal Depression | ||||
| Yes | 22.9 | 11.1 | 15.3 | 20.0 |
| No | 77.1 | 88.9 | 84.7 | 80.0 |
| Maternal Drug Abuse | ||||
| Yes | 1.4 | 0.5 | 0.5 | 0.2 |
| No | 98.6 | 99.5 | 99.5 | 99.8 |
| Maternal Alcoholism | ||||
| Yes | 2.1 | 1.8 | 1.9 | 3.5 |
| No | 97.9 | 98.2 | 98.1 | 96.5 |
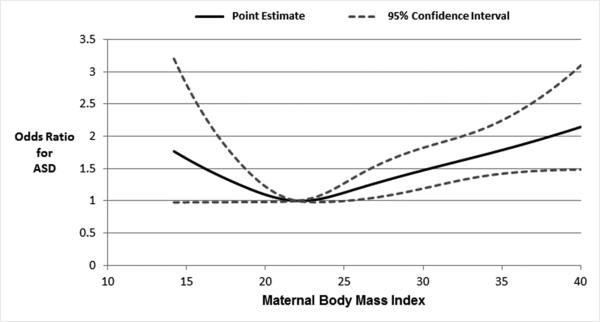
Conditional restricted cubic spline regression revealed that the association between maternal BMI and ASD occurrence was nonlinear and J-shaped (p-value for test for curvature=0.013; overall significance of curve=0.003), with extremes in maternal BMI being associated with the largest increases in ASD risk (Figure 1). The ORs conditional on matching factors for the associations of maternal underweight, overweight and obesity with ASD were 1.43 [95% CI 1.01, 2.04], 1.21 [95% CI 1.01, 1.45] and 1.54 [95% CI 1.26, 1.89], compared to normal weight mothers (Table 3). None of the variables assessed were meaningful confounders of the observed associations as evidenced by associations that were essentially unchanged following full multivariable adjustment for maternal age, genetic susceptibility, pre-pregnancy depression, diabetes, smoking status, drug abuse, and alcoholism (Table 3).
Figure 1.
Restricted cubic spline regression analysis assessing nonlinearity of the association between maternal BMI and occurrence of autism spectrum disorders among offspring, GPRD singleton live births 1993-2008
Test for curvature: p=0.018; Test for overall significance of the curve: p<0.0001
Table 3.
Conditional unadjusted and multivariable adjusted odds ratios for the association between maternal BMI and ASD among offspring, GPRD singleton live birth 1993-2008
| Cases (n)a | Controls (n) | ORadjusteda [95% CI] | ORunadjustedb [95% CI] | |
|---|---|---|---|---|
| Underweight, BMI<18.5 | 44 | 140 | 1.42 [0.99, 2.03] | 1.43 [1.01, 2.04] |
| Normal weight, BMI 18.5-24.9 | 450 | 2040 | reference | reference |
| Overweight, BMI 25-29.9 | 222 | 836 | 1.20 [1.00, 1.44] | 1.21 [1.01, 1.45] |
| Obese, BMI ≥30 | 173 | 514 | 1.52 [1.23, 1.86] | 1.54 [1.26, 1.89] |
adjusted model odds ratios adjusted for maternal age (continuous), maternal pre-pregnancy depression, diabetes, smoking status, drug abuse, alcoholism, in addition to matching factors (birth year, sex, and general practice)
unadjusted model odds ratios adjusted for matching factors (birth year, sex, and general practice) only
Unadjusted and multivariable adjusted results from conditional regression analyses stratified by maternal age are presented in Table 4. There was a qualitative difference in the ORs for the associations of maternal underweight, overweight and obesity with ASD occurrence by maternal age which approached statistical significance (p=0.0845). Underweight was associated with elevated ASD risk among mothers 30 years of age or older (cOR: 2.13 [95% CI 1.03, 4.43]), but not among mothers younger than 30 years (cOR for maternal age 25-29 years: 1.09, [95% CI 0.42, 2.85]), cOR for maternal age <25 years: 1.16 [95% CI 0.49, 2.77]. Maternal overweight was associated with an increase in ASD risk in offspring only for mothers 25-29 years of age (cOR: 1.89, [95% CI 1.19, 2.98]). Stratum-specific ORs for the association of maternal obesity suggested increases in ASD risk ranging from 1.32-2.20 dependent upon age, where obesity was associated with more pronounced increases among younger mothers. There was no evidence of additive interaction (RERI=0.094, 95% CI:-0.636, 0.449).
Table 4.
Conditional unadjusted and multivariable adjusted odds ratios for the association between maternal BMI and ASD among offspring stratified by maternal age at the start of the study pregnancy, GPRD singleton live birth 1993-2008
| Cases (n) | Controls (n) | ORadjusteda [95% CI] | ORunadjustedb [95% CI] | |
|---|---|---|---|---|
| Maternal age <25 years | ||||
| Underweight, BMI<18.5 | 15 | 55 | 1.05 [0.43, 2.58] | 1.16 [0.49, 2.77] |
| Normal weight, BMI 18.5-24.9 | 90 | 446 | reference | reference |
| Overweight, BMI 25-29.9 | 41 | 155 | 1.07 [0.60, 1.92] | 1.11 [0.63, 1.95] |
| Obese, BMI ≥30 | 36 | 83 | 2.16 [1.09, 4.30] | 2.20 [1.12, 4.31] |
| Maternal age 25-29 years | ||||
| Underweight, BMI<18.5 | 12 | 44 | 0.93 [0.34, 2.51] | 1.09 [0.42, 2.85] |
| Normal weight, BMI 18.5-24.9 | 117 | 640 | reference | reference |
| Overweight, BMI 25-29.9 | 73 | 248 | 1.89 [1.19, 2.98] | 1.82 [1.17, 2.84] |
| Obese, BMI ≥30 | 55 | 189 | 1.82 [1.08, 3.09] | 1.95 [1.18, 3.23] |
| Maternal age ≥30 years | ||||
| Underweight, BMI<18.5 | 17 | 41 | 1.94 [0.97, 3.89] | 2.13 [1.03, 4.43] |
| Normal weight, BMI 18.5-24.9 | 243 | 954 | reference | reference |
| Overweight, BMI 25-29.9 | 108 | 433 | 1.00 [0.75, 1.33] | 0.95 [0.71, 1.28] |
| Obese, BMI ≥30 | 82 | 242 | 1.29 [0.92, 1.81] | 1.32 [0.96, 1.86] |
adjusted model odds ratios adjusted for maternal age (continuous), maternal pre-pregnancy depression, diabetes, smoking status, drug abuse, alcoholism, in addition to matching factors (birth year, sex, and general practice)
unadjusted model odds ratios adjusted for matching factors (birth year, sex, and general practice)
Findings from analyses restricted to pregnancies with a GP-recorded LMP did not differ from those observed for the entire study population (Supporting Information, Table S1). Analyses stratified by timing of BMI measurement relative to the start of the pregnancy showed that associations were generally stronger for BMI measurements obtained closer to the start of the pregnancy (Supporting Information, Table S2).
COMMENT
In this study, we observed a modest non-linear association between maternal pre-pregnancy body mass index and ASD among offspring, with extremes being associated with higher risk compared to normal weight for the overall study population. The OR for maternal underweight and obesity were 1.4 [95% CI: 1.0, 2.1] and 1.5 [95% CI: 1.2, 1.9], respectively. The relationship between maternal BMI and ASD risk differed by maternal age. Maternal underweight was significantly associated with an increase in ASD only among mothers over 30 years of age. Maternal obesity was associated with increased risk for ASD irrespective of age, but the relative association was most prominent among mothers under 30 years of age.
To our knowledge, we are the first to identify an elevated risk associated with maternal underweight. A low maternal pre-pregnancy BMI may be a marker for undernutrition and limited availability of micronutrients, including prenatal deficiencies of folic acid, iodine, and iron, which have been implicated in neurologic deficits and developmental disorders.6 Why underweight would only be associated with an elevated risk for ASD among offspring of older mothers is unclear. One possibility is that our chosen BMI categorization method preferentially misclassified young normal weight women as underweight (i.e., younger than 20 years) thus producing a downward bias of the resulting OR. The BMI cut-points we utilized are widely accepted as standard and are based on relationships with morbidity and mortality in adults, but they may not be appropriate for adolescents for whom age-specific cut-points have been proposed due to their continued growth and development.21, 23 However, the results of our sub-analysis in which adolescent mothers were re-classified to BMI categories based on an age-specific reference were not materially different. Another possibility is that underweight in older women maybe more likely to represent underlying illness than underweight in younger women.
Our findings relating maternal pre-pregnancy obesity to ASD support those of others. Dodds et al. reported that maternal pre-pregnancy weight ≥90 kg was associated with a 58% higher risk for ASD compared to maternal weight <90 kg among a cohort of children born from 1990 to 2002 in Nova Scotia.12 More recently, the risk of neurodevelopmental disorders in relation to maternal metabolic conditions was investigated in the CHARGE study, a population-based case-control study in California, and maternal obesity (BMI >30 kg/m3) was found to be associated with a 67% higher risk for ASD.13 The observed age-related differences in the association between maternal obesity and ASD occurrence may be due in part to differences in baseline risk in the two subpopulations. Even among normal weight women, advanced age is associated with higher rates of antepartum complications, preterm delivery, and cesarean delivery each of which has been linked to increased risk for ASD among offspring.24, 25 Therefore, the relative association of obesity on ASD occurrence among offspring may be diluted for older women.
An association between maternal obesity and ASD is plausible based on evidence from animal experiments and human studies that suggest activation of the maternal immune system may be a risk factor for autism.7 Although maternal circulating cytokine levels vary over the course of pregnancy, concentrations are dependent upon BMI and age.8 Pro-inflammatory cytokine levels (i.e., IL-6) are higher in obese women compared to those of normal BMI.9,10 Therefore, pregnancies among overweight or obese women may be exposed to higher levels of inflammatory cytokines which pass to the fetal circulation and cross the fetal blood-brain barrier leading to abnormal neuronal growth and differentiation within the fetal brain.7
Our study has several strengths. Our study consisted of a large population-based sample in which all information was recorded prospectively by trained medical personnel thereby eliminating the possibility of bias due to differential parental recall. Nesting our study within an established UK primary care database also minimized the potential for self-selection bias that would be a greater concern in studies which rely on information from mothers who volunteered to participate. Whereas previous studies focused only on maternal obesity, we utilized spline regression to assess nonlinearity of the relationship across the complete range of maternal BMI.
Despite the strengths of the study, our results should be considered in light of some potential limitations. We relied exclusively on Read codes for identification of cases, thus they were not validated by clinical assessment which may have led to some misclassification and an attenuation of observed associations. However, a recent review of GPRD computerized records of ASD against all available clinical reports verified over 90% of diagnoses, suggesting over-ascertainment was low.20
Exposure misclassification is also a possibility. We used height and weight information recorded closest to the start of the pregnancy and there was some variation in this proximity across subjects. Analyses stratified by timing of BMI measurements revealed stronger associations for BMI measured closer to the actual start of pregnancy. Thus, suggesting that the observed associations may be real because measurements made closer to the start of the study pregnancy are the more valid measure of pre-pregnancy BMI status. We estimated the pregnancy start date for over half of the study population so for some of those mothers we may have utilized measurements that were not reflective of their pre-pregnancy BMI status. However, results of analyses restricted to mothers with a GP-recorded LMP did not differ from the full analysis suggesting that any misclassification of BMI due to our method of estimating the pregnancy start date was negligible.
The source population for our study was limited to those children who could be matched to a mother in the GPRD which could introduce selection bias if a successful match was related to maternal BMI and differed for cases and controls. However, the proportion of ASD cases in the GPRD that could be successfully matched to their mother was high and did not differ from the match rate for all births into the GPRD over the study period. While eligible controls were required to have no evidence of an ASD diagnosis, it is possible, albeit rare, that some of the matched controls may have a diagnosis for another developmental disability. The observed association with obesity may be an underestimate if control patients have developmental disabilities that are also associated with maternal obesity. Our study was also restricted to ASD cases and matched controls whose mothers had a BMI recorded within 2 years before the start of the study pregnancy. It is possible that the availability of such a BMI is related to actual BMI differentially for cases and controls. We evaluated the distribution of BMI measurements among ASD cases and controls whose mothers had measurements within 10 years before the study pregnancy. By relaxing the definition of the pre-pregnancy window, the proportions of maternal normal weight, overweight, and obesity changed similarly for both cases and controls compared to the distributions observed in the current study. However, the proportion of underweight mothers increased about 80% for controls and only 30% for cases. This trend suggests that the observed association between maternal underweight and ASD in offspring may be partially explained by differential missingness (selection bias). It should be noted that there were relatively small numbers of ASD cases within the category of maternal underweight. As such, associations were somewhat imprecise especially at the lowest BMI measures.
There is the potential for confounding by unmeasured factors such as parity which is not specifically coded in the GPRD. Additionally, unlike mothers, fathers are generally not reliably matched to offspring in the GPRD because their medical records do not contain diagnostic codes related to pregnancy and delivery to match to birth codes in the child record. Therefore, an assessment of potential confounding by paternal BMI could not be evaluated in this analysis. Our findings of an increased risk for ASD in relation to maternal obesity are comparable in magnitude to those report by Suren et al. for autistic disorder and Asperger disorder which were attenuated following accounting for paternal BMI.26
In conclusion, detailed medical information in a population-based data resource with the ability to link maternal records with those of offspring enabled us to evaluate the occurrence of ASD in relation to maternal BMI. Our results suggest that extremes in maternal BMI may be associated with modest increases in ASD among offspring.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Kristen Lyall and Susan E. Manning for their analytic review and feedback at early stages of the development of this manuscript. At the time of design and analysis, Kelly D. Getz was a pre-doctoral Boston University Reproductive, Perinatal and Pediatric Epidemiology trainee supported in part by the National Institutes of Health (Grant T32 HD052458), she completed the study in partial fulfillment of the requirements of the degree of Doctor of Philosophy.
REFERENCES
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edition, text revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 2.Gillberg C, Steffenburg S, Schaumann H. Is autism more common now than ten years ago? British Journal of Psychiatry. 1991;158:403–409. doi: 10.1192/bjp.158.3.403. [DOI] [PubMed] [Google Scholar]
- 3.Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report Surveillance Summaries. 2014;63(SS02):1–21. [PubMed] [Google Scholar]
- 4.Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. British Journal of Obstetrics & Gynaecology. 2007;114(2):187–194. doi: 10.1111/j.1471-0528.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- 5.Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? Journal of Child Psychology and Psychiatry. 2007;48(3-4):245–61. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. American Journal of Clinical Nutrition. 2007;85(2):614S–20S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 7.Buehler MR. A proposed mechanism for autism: an aberrant neuroimmune response manifested as a psychiatric disorder. Medical Hypotheses. 2011;76(6):863–870. doi: 10.1016/j.mehy.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 8.Curry AE, Vogel I, Skogstrand K, Drews C, Schendel DE, Flanders WD, et al. Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. Journal of Reproductive Immunology. 2008;77(2):152–160. doi: 10.1016/j.jri.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 9.Bugatto F, Fernandez-Deudero A, Bailen A, Fernandez-Macias R, Hervias-Vivancos B, Batha JL. Second-trimester amniotic fluid proinflammatory cytokine levels in normal and overweight women. Obstetrics & Gynecology. 2010;115(1):127–133. doi: 10.1097/AOG.0b013e3181c5367f. [DOI] [PubMed] [Google Scholar]
- 10.Ryan AS, Nicklas BJ. Reductions in plasma cytokine levels with weight loss improve insulin sensitivity in overweight and obese postmenopausal women. Diabetes Care. 2004;27(7):1699–1705. doi: 10.2337/diacare.27.7.1699. [DOI] [PubMed] [Google Scholar]
- 11.Stein D, Weizman A, Ring A, Barak Y. Obstetric complications in individuals diagnosed with autism and in healthy controls. Comprehensive Psychiatry. 2006;47(1):69–75. doi: 10.1016/j.comppsych.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. Journal of Autism and Developmental Disorders. 2011;41(7):891–902. doi: 10.1007/s10803-010-1114-8. [DOI] [PubMed] [Google Scholar]
- 13.Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129(5):e1121–e1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. British Journal of Clinical Pharmacology. 2010;69(1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barron SL. The Read clinical classification. British Journal of Obstetrics & Gynaecology. 1993;100(9):800. doi: 10.1111/j.1471-0528.1993.tb14300.x. [DOI] [PubMed] [Google Scholar]
- 16.Devine S, West S, Andrews E, Tennis P, Hammad TA, Eaton S, Thorp J, Olshan A. The identification of pregnancies within the general practice research database. Pharmacoepidemiology and Drug Safety. 2010;19(1):45–50. doi: 10.1002/pds.1862. [DOI] [PubMed] [Google Scholar]
- 17.Hardy JR, Holford TR, Hall GC, Bracken MB. Strategies for identifying pregnancies in the automated medical records of the General Practice Research Database. Pharmacoepidemiology and Drug Safety. 2004;13(11):749–759. doi: 10.1002/pds.935. [DOI] [PubMed] [Google Scholar]
- 18.Jick SS, Kaye JA, Vasilakis-Scaramozza C, Garcia Rodriguez A, Ruigomez A, Meier, et al. Validity of the general practice research database. Pharmacotherapy. 2003;23:686–689. doi: 10.1592/phco.23.5.686.32205. [DOI] [PubMed] [Google Scholar]
- 19.Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. British Journal of General Practice. 2010;60:e128–e136. doi: 10.3399/bjgp10X483562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fombonne E, Heavey L, Smeeth L, Rodriguez LC, Cook C, Smith PG, et al. Validation of the diagnosis of autism in general practitioner records. BMC Public Health. 2004;4:5. doi: 10.1186/1471-2458-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body mass index cut offs to define thinness in children and adolescents: international survey. The British Medical Journal. 2007;335(7612):194. doi: 10.1136/bmj.39238.399444.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in Medicine. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 23.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Advance Data From Vital and Health Statistics. 2000;(314):1–27. [PubMed] [Google Scholar]
- 24.Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse pregnancy outcome. Obstetrics & Gynecology. 2004;104(4):727–733. doi: 10.1097/01.AOG.0000140682.63746.be. [DOI] [PubMed] [Google Scholar]
- 25.Seoud MAF, Nassar AH, Usta IM, Melhem Z, Khalil AM. Impact of advanced maternal age on pregnancy outcome. American Journal of Perinatology. 2002;19(1):001–008. doi: 10.1055/s-2002-20175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.