Abstract
Young infants are generally more susceptible to viral infections and experience more severe disease than adults. CD8+ T cells are important for viral clearance and although often ineffective in neonates can be protective if adequately stimulated. Using a murine CB6F1/J hybrid model of respiratory syncytial virus (RSV) infection, we previously demonstrated that the CD8+ T cell immunodominance hierarchy to two RSV-derived epitopes, KdM282–90, and DbM187–195, was determined by the age at infection. To determine if age-dependent RSV specific CD8+ T cell responses could be modified through enhanced innate signaling, we used toll-like receptor (TLR) agonist 4 or 9 treatment at the time of infection, which remarkably changed the neonatal codominant response to an adult-like KdM282–90 CD8+ T cell immunodominant response. This shift was associated with an increase in the number of conventional dendritic cells (cDCs), CD11b+ and CD103+ DCs, in the lung-draining lymph node, and increased expression of the co-stimulatory molecule CD86. The magnitude of the KdM282–90 CD8+ T cell response in TLR-treated neonates could be blocked with antibodies against CD80 and CD86. These studies demonstrate the age-dependent function of cDCs, their role in determining immunodominance hierarchy, and epitope-specific CD8+ T cell requirements for co-stimulation that all influence the immune response magnitude. The unique impact of TLR agonists on neonatal T cell responses is important to consider for RSV vaccines designed for young infants.
Introduction
Globally, lower respiratory tract infections are the largest contributor to mortality in the first year of life (1). Viral infections cause 50% of this mortality, with RSV being the single most important viral pathogen followed by influenza (1). Immunity to viral infection requires clearance of infected cells by CD8+ cytotoxic T lymphocytes (CTL), and in young infants, the appearance of CD8+ T cells correlates with the time of recovery and convalescence (2, 3). The highly regulated immune environment of the neonate has been implicated in limiting robust CD8+ T cell responses, thus playing a role in susceptibility to viral infection (4–6). Neonatal humans and mice, however, have been found to mount more adult-like T cell responses in the setting of infections, such as human cytomegalovirus or Trypanozoma cruzi (7, 8), and after particular immunizations or stimuli (9–11). These studies implicate a role for innate signaling to override the limitations of pathogen-specific CD8+ T cell responses in young infants.
Following primary infection, CD8+ T cell activation occurs in the lymph nodes draining the site of infection upon encountering antigenic peptide presented in the context of a MHC class I (MHCI) molecule in conjunction with accessory signaling (12–14). Only a tiny fraction of all the potential viral epitopes are recognized by naïve epitope-specific CD8+ T cells and the magnitude of each epitope-specific response varies resulting in a numerical hierarchy with immunodominant epitopes provoking the largest CD8+ T cell responses. Intrinsic CD8+ T cell factors such as the number and phenotype of naïve pathogen-specific CD8+ T cells and the affinity of the T cell receptor (TCR) for the peptide-MHCI complex have been shown to predict the resulting immunodominance hierarchy (15–17). In addition, factors extrinsic to the T cell such as antigen availability and the affinity of peptide for the MHCI complex of APCs have been shown to influence T cell response magnitudes (18–20).
We have shown previously that adult CB6F1/J mice have an immunodominant response to an epitope in the M2 protein of RSV (KdM282–90, RSV transcription processivity factor, amino acid residues 82–90) and a subdominant response to an epitope in the M protein (DbM187–195, RSV matrix protein, amino acid residues 187–195) (21). Neonatal mice make a distinct response during RSV infection in which the KdM282–90 CD8+ T cell response is lower in magnitude, resulting in a codominant T cell response (22). The adult response hierarchy is preserved during congenic transfer of adult CD8+ T cells into neonatal mice experiencing RSV infection, suggesting that intrinsic factors determined the KdM282–90-immunodominance (22).
In addition, we have shown that lung conventional dendritic cell (cDC) responses are more mature upon RSV infection outside of the neonatal period and this coincides with the age-dependent transition from neonatal to adult CD8+ T cell response hierarchy (23). In mice, two cDC subsets, designated CD103+ DCs and CD11b+ DCs, take up antigen in the lung and migrate to the mediastinal lymph node that drains the lung (dLN) to present antigen to T cells (24, 25). CD103+ DCs have been shown to be more effective at cross-presenting antigen to CD8+ T cells (25). Recent studies, however, have suggested that both CD103+ DCs and CD11b+ DCs can contribute to the quantity and quality of the CD8+ T cell response (26, 27). Despite their essential role in engaging CD8+ T cells, it is not known how these cDC subsets influence immunodominance.
In this study, we show that augmenting toll-like receptor (TLR) 4 or 9 triggering during RSV infection can increase the representation of neonatal cDCs expressing CD86 in the dLN during T cell priming. This is associated with an increase in the KdM282–90-specific T cell response to RSV resulting in an adult-like immunodominance hierarchy. Additionally, we demonstrate that epitope-specific CD8+ T cells have individual requirements for co-stimulation that may be differentially provided by distinct cDC subsets. Together the data show that neonatal DCs exhibit limited markers of maturation in response to RSV and that this influences the immunodominance hierarchy. Enhancing functional responses of neonatal cDCs to RSV through TLR activation can meet intrinsic epitope-specific CD8+ T cell requirements for recruitment during infection providing insight into adjuvant design for neonatal vaccines.
Methods and Materials
Mice
Neonatal hybrid CB6F1/J mice (6–7 days of age) were the product of timed in-house breeding of BALB/c (female) mice and C57BL/6 (male) mice purchased from The Jackson Laboratory (Bar Harbor, ME). Adult hybrid CB6F1/J mice (8–12 weeks old) were purchased from The Jackson Laboratory or bred in-house. CB6F1/J mice express both H-2b and H-2d class I MHC proteins. C.129S- Batf3tm1Kmm/J (BALB/c Batf3−/−) mice and B6.129S(C)-Batf3tm1Kmm/J (B6 Batf3−/−) mice were purchased from The Jackson Laboratory. BALB/c Batf3−/− (female) mice and B6 Batf3−/− (male) mice were bred in-house to produce adult and neonatal hybrid Batf3−/− mice expressing both the H-2b and H-2d class I MHC proteins. All mice were bred and maintained under specific pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care-accredited animal facility at the Vaccine Research Center (VRC), NIAID or the Uniformed Services University of the Health Sciences (USUHS) and housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals, National Research Council, 8th Edition, 2011. All experiments were performed under an animal study proposal approved by either the VRC or USUHS Institutional Animal Care and Use Committee.
Virus infection
The A2 strain of RSV was propagated in HEp-2 cells (ATCC, Manassas, VA) as previously described (28). Mice were anesthetized with 3% isoflurane inhalation prior to intranasal inoculation with 2×106 PFU live RSV in a volume titrated to the lung volume by age, approximately 5–6µl per gram of body weight or 25–30µl for neonates and 100µl for adults. Neonates were infected at 6–7 days of age and adults were infected at 8–12 weeks of age.
TLR agonists and administration
TLR agonists were administered intranasally at the time of RSV infection. TLR agonists were reconstituted as recommended by the manufacturer and then added to the viral stock prior to infection. Lipopolysaccharide from E.coli 055:B5 ready-made solution 1mg/ml (Sigma-Aldrich Corp., St. Louis, MO) was administered as a TLR4 agonist. Neonatal mice received 5µg and adult mice received 10µg intranasally at the time of infection. Alternatively, TLR9 agonist CpG oligonucleotide [ODN 1826 5’-tcc atg acg ttc ctg acg tt-3’ (20 mer)] was used, and compared with control ODN 1826, 5’- tcc atg agc ttc ctg agc tt −3’ (20 mer) reconstituted as directed from InvivoGen (San Diego, CA), or PBS as indicated. Neonatal mice received 10µg and adult mice received 20µg intranasally at the time of infection.
RSV-specific CD8+ T cell tetramer assays
Mice were euthanized by intraperitoneal injection of pentobarbitol (250mg/g) prior to removal of lung tissues at the indicated times post-infection. Lung tissues were dissociated with a GentleMACS machine (Miltenyi Biotec, San Diego, CA) in RPMI-1640 medium with 10% fetal bovine serum (FBS) and passed through a 70-µm filter to obtain a single cell suspension. FBS lot was tested for RSV neutralizing antibodies prior to use. Leukocytes were isolated by centrifugation over Fico/Lite-LM density gradient (Atlanta Biologicals). For tetramer analysis, washed cells were initially treated with Fc Block (BD Biosciences, San Jose, CA) for 10 min at room temperature then surface stained with KdM282–90 and DbM187–195 tetramers (Beckman Coulter, San Diego, CA) together with fluorescently conjugated antibodies specific for CD3, CD4, and CD8 in PBS with 2% FBS for 20 min at 4°C. LIVE/DEAD Fixable Aqua Dead Cell stain kit from Invitrogen was used to exclude dead cells. Fluorescence was measured on an LSR-II flow cytometer (BD, San Jose, CA) and data were analyzed using FlowJo version 9.8.3 (Tree Star, San Carlos, CA). Analysis was performed on singlet living lymphocytes and then gated for tetramer positive cells out of CD3+CD8+cells. Data acquisition parameters were set using fluorescent beads (BD Biosciences). Tetramer-specific cells were enumerated by counting the total number of cells in single cell suspension on a Beckman Coulter Counter (Z1 Coulter Counter) then calculated based on the percentage of tetramer positive cells identified by flow cytometric analysis. For Batf3−/− experiments tetramer-specific cell enumeration was obtained by running sample to completion on the flow cytometer. Sample isolation through acquisition on flow cytometer was performed on a single day.
Dendritic cell assays
The lung draining mediastinal lymph nodes (dLN) were dissociated between the frosted ends of two glass slides at the indicated times post-infection. Single cell suspensions were then washed and resuspended in PBS with 2% FBS. Cells were surface stained with fluorescently conjugated antibodies specific for the following molecules: I-Ab (clone AF6–120.1) or I-A/E Cy55PerCP (M5/114.15.2), CD80 (16-10A1), CD11b (M1–70), CD70 (FR70), CD103 (2E7), CD3 Cy7APC, CD86 (GL-1), mPDCA-1 (JF05-1C2.4.1), CD8 (53–6.7), CD11c (N418) from Biolegend, eBioscience, or BD Biosciences after Fc Block as described above. Fluorescence was measured on an LSR-II flow cytometer and data analyzed using FlowJo version 9.8.3. Analysis was performed on singlet living cells, CD3+ cells were then excluded followed by selection of CD11c+ cells that were MHC class II high and then identified as CD103+ or CD11b+ cells (cDC subset gating strategy in Supplemental Figure 1). Data acquisition parameters were set using fluorescent beads and fluorescence-minus-one (FMO) controls. Cell counts were obtained by running each individual sample to completion and dividing cell count by number of pooled dLNs. Sample isolation through acquisition on flow cytometer was performed on a single day.
In vivo blockade of costimulatory molecules CD80 and CD86
Neonatal mice were infected and treated with a TLR agonist as described above. Anti-CD86 and anti-CD80 were then administered by intraperitoneal injection at indicated day post-RSV infection. Anti CD86 (clone GL-1) and anti CD80 (clone 16-10A1) antibodies were purchased from Bio X Cell (West Lebanon, NH) (product numbers BE0024 and BE0025, respectively). Isotype controls were administered two days post infection by intraperitoneal injection and also purchased from Bio X Cell (Rat IgG2a product number BE0089, Hampster IgG, product number BE0091). Partial blockade of CD80 and CD86 was achieved by in vivo titration to diminish but not eliminate the CD8+ T cell responses (data not shown). After LPS treatment, 10µg of individual antibodies were required to induce partial blockade. After CpG treatment 7.5µg of individual antibodies were required to induce partial blockade.
Statistical analysis
Statistical analyses between two groups were conducted using a two-tailed Student’s t-test. Comparisons between multiple groups were performed using a one-way or two-way ANOVA followed by post-tests for multiple comparisons between all groups as described in figure legends. These statistical analyses were performed using Prism software (GraphPad Software, Inc.). Results are expressed as means + SEM. Values of p <0.05 were considered statistically significant (*).
Results
TLR agonist treatment at the time of RSV infection establishes an immunodominant KdM282–90–specific T cell response in neonatal mice
We have previously demonstrated a role for CD8+ T cell intrinsic factors in determining the adult KdM282–90 immunodominance (22). In early life, however, cDC responses correlated with a codominant RSV-specific CD8+ T cell response (23). Based on studies showing that certain conditions of immune stimulation led to more robust CD8+ T cell responses in neonates (29), we hypothesized that controlled alteration of immune stimulation early in RSV infection in neonates may influence the RSV-specific CD8+ T cell response hierarchy. Therefore, we treated neonatal mice with pathogen-associated molecular patterns (PAMPs) that trigger pattern recognition receptors (PRRs) at the time of RSV infection. We then analyzed the RSV-specific CD8+ T cell responses in the lung 7 days post-infection (DPI) in comparison to adults.
First, we treated neonatal mice with LPS that signals via the PRR, TLR 4. TLR4 is a robust innate immune stimulator, also shown to be a host molecule linked to pathogenesis during RSV infection (30, 31). Interestingly, LPS treatment at the time of RSV infection significantly increased the percentage of KdM282–90-specific T cells in neonatal mice resulting in a KdM282–90 -dominant T cell response hierarchy similar to that seen in untreated adult mice (Fig. 1A). Correspondingly, LPS treatment decreased the percentage of DbM187–195 –specific T cells responding to infection in the lung (Fig. 1A). Analysis of the absolute number of RSV-specific CD8+ T cells per lung showed that KdM282–90 -specific T cells increased nearly 3-fold after LPS treatment of neonatal mice, while the number of DbM187–195 -specific T cells was relatively unchanged (Fig. 1B). Consistent with an increase in the percentage of KdM282–90 -specific T cells after LPS treatment of neonates, the response ratio of KdM282–90 to DbM187–195 -specific cells increased from a mean ratio of one to 8.9 approximating the mean ratio of 10 seen in adults (Fig. 1D). LPS treatment of adults did not change the immunodominance hierarchy and in contrast to neonates, the percentage and number of KdM282–90-specific CD8+ T cells did not increase, but rather, slightly decreased in the adult lung.
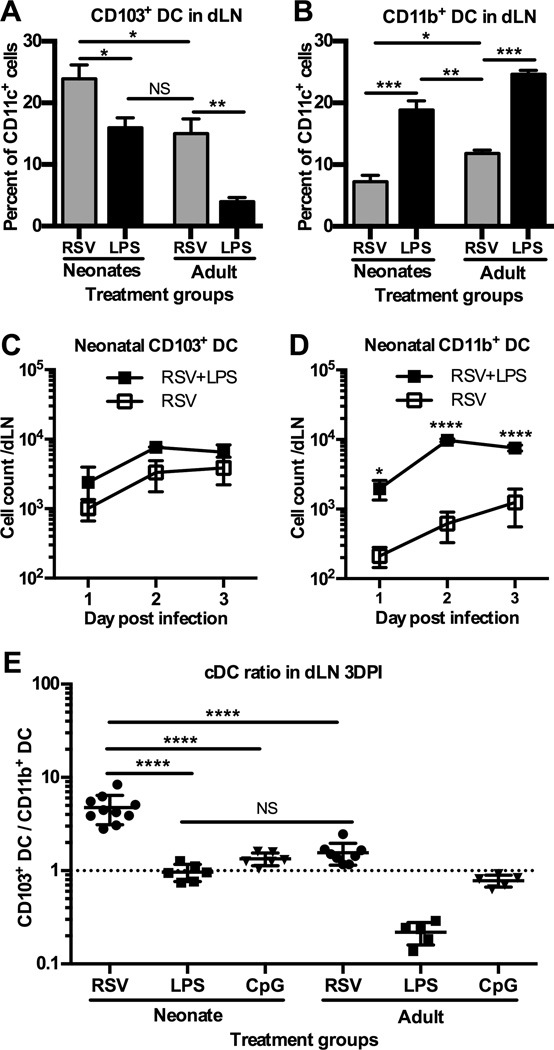
Figure 1.
Treatment with either LPS or CpG concurrent with RSV infection changes the RSV-specific immunodominance hierarchy in neonatal mice. (A) Percentage of tetramer-specific CD8+ T cells in the lung of neonatal or adult mice 7 days post-infection (DPI) with RSV alone or treated with LPS. (B) RSV-specific CD8+ T cell counts per lung 7DPI with or without LPS treatment. (C) Percentage of tetramer-specific CD8+ T cells in the lung 7DPI with RSV or RSV and CpG treatment. (D) Ratio of RSV-specific CD8+ T cells indicating the relative frequency of KdM282–90-specific T cells compared to DbM187–195-specific T cells from neonatal or adult mice with and without TLR agonist treatment. Differences between adult groups were not statistical significant. Each experiment had 4–5 mice per group and data are representative of 3 experiments. Response ratio represents data from 3 experiments. Statistical significance assessed by one-way ANOVA with Tukey’s multiple comparisons test with a single pooled variance. (*p≤0.05, **p≤0.01, **** p≤0.0001, NS indicates not significant). Error bars indicate SEM.
We next examined if CpG, a TLR9 agonist, shown to induce more robust CTL responses in neonates (11), also resulted in a KdM282–90–dominant response to RSV in neonates. CpG treatment of neonatal mice increased the percentage of KdM282–90-specific T cells in the lung at 7DPI from a mean percentage of 16% to 27% compared to a mean of 42% in the LPS-treated neonates (Fig. 1C). The percentage of DbM187–195 -specific CD8+ T cells in the neonatal lung was unchanged by CpG treatment (Fig. 1C). Analysis of the absolute number of KdM282–90 and DbM187–195 -specific T cells per lung after CpG treatment of neonates reflected an increase in the KdM282–90-specific response by nearly 3 fold and no change in the DbM187–195 –specific, similar to that seen after LPS treatment (Data not shown). The response ratio of KdM282–90-specific T cells to DbM187–195 -specific T cells reached a mean of 8.6, approximating the ratio seen in adults (Fig. 1D). Similar to LPS-treated adults, CpG treatment did not change the immunodominance hierarchy in adults and resulted in a slight decrease in the percentage of responding KdM282–90 -specific T cells. These data show that increasing TLR stimulation early in RSV infection alters the neonatal CD8+ T cell immunodominance hierarchy to more closely resemble an adult-like response to RSV. In addition, triggering of PRRs in adults during early RSV infection reduced the KdM282–90 -specific T cell response, suggesting that PRRs have distinct effects on the RSV-specific CD8+ T cell response in adults versus neonates.
TLR agonists increase the percentage of neonatal cDCs in the dLN towards adult-like levels
The quantity and quality of DCs found in tissue-draining lymph nodes determine the engagement and activation of T cells. In neonatal mice the representation of DC subsets in tissue-draining lymph nodes differs compared to adults (23, 32). Moreover, DC subset accumulation in the dLN during RSV infection is characterized by a predominance of CD103+ DCs prior to 10 days of age and the change in representation of cDCs in the dLN with age is associated with the transition towards KdM282–90 dominance (23).
To explore whether TLR agonist treatment alters the representation of cDC subsets in the dLN during RSV infection, we treated neonatal and adult mice intranasally with LPS at the time of RSV infection and then analyzed the cDC response in the dLN 3DPI by flow cytometry (Supplemental Fig. 1). Analysis of the cDC subsets showed that as a percent of CD11c+ cells, the neonatal CD103+ DCs decreased with LPS treatment, resulting in a similar percentage of CD103+ DCs to that of untreated adults (Fig. 2A). In contrast, neonatal CD11b+ DCs increased significantly in percentage and number (Fig. 2B and D) resulting in a slightly greater representation of CD11b+ DCs in the neonatal dLN compared to untreated RSV-infected adults. In adult mice treated with LPS, the percentage of CD103+ DCs also decreased while the CD11b+ DC subset increased (Fig. 2A and B).
Figure 2.
Treatment with either LPS or CpG concurrent with RSV infection increases the percentage and number of responding CD11b+ DCs in the dLN. (A–B) Neonatal and adult mice were infected with RSV and treated with LPS. cDCs were analyzed from the dLN 3DPI. CD103+ DCs (A) and CD11b+ DCs (B) as a percent of CD11c+ gated cells (Supplemental Fig. 1). Experiments were performed with 3–5 pooled dLN per sample and 2–3 samples per group and are representative of 3 experiments. (C–D) Comparison of the numbers of CD103+ DCs (C) or CD11b+ DCs (D) from the neonatal dLN with or without LPS treatment. cDC cell count was analyzed from the dLN 1, 2 and 3DPI. Three to 6 dLN were pooled per sample. (E) Relative frequency of CD103+ DCs to CD11b+ DCs demonstrated as a ratio of CD103+ DCs divided by CD11b+ DCs 3DPI. Differences between adult groups were not statistically significant. Each point represents a pool of 3–5 mice and is representative of 3 experiments. Statistical significance compared using one-way or two-way ANOVA and Tukey’s multiple comparisons test (*p≤0.05, **p≤0.01, ***p≤0.001, **** p<0.0001, NS indicates not significant). Error bars indicate SEM.
To determine if the kinetics of cDC arrival into the dLN was altered by LPS treatment, we quantified the number of cDCs responding 1–3DPI. We found that intranasal LPS treatment of neonates significantly increased the number of CD11b+ DCs in the dLN in the first 3DPI, while the increase in CD103+ DCs was not significant (Fig. 2C and D). The number of CD11b+ DCs was increased significantly in the dLN as early as 1DPI and was sustained through 3DPI resulting in an earlier accumulation in the dLN during T cell priming (Fig. 2D).
We examined if CpG also increased the representation of cDCs in the dLN during RSV infection. As a percentage of CD11c+ DCs, neonatal CD103+ DCs were relatively unchanged, while CD11b+ DCs significantly increased after CpG treatment, represented as the ratio of CD103+ DCs to CD11b+ DCs in Fig. 2E.The treatment of neonates with CpG resulted in a ratio of CD103+ DCs to CD11b+ DCs in the dLN similar to that of untreated adults (Fig. 2E). The numbers of neonatal CD103+ DCs and CD11b+ DCs accumulating in the dLN 3DPI and CpG treatment were similar to that seen after LPS treatment (data not shown). Together these data demonstrate that treatment of neonatal mice, with TLR4 or 9 agonists, results in a representation of cDCs in the dLN similar to that found in untreated adult mice during primary RSV infection.
Neonatal mice lacking CD103+ DCs mount a KdM282–90-dominant CD8+ T cell response to RSV infection
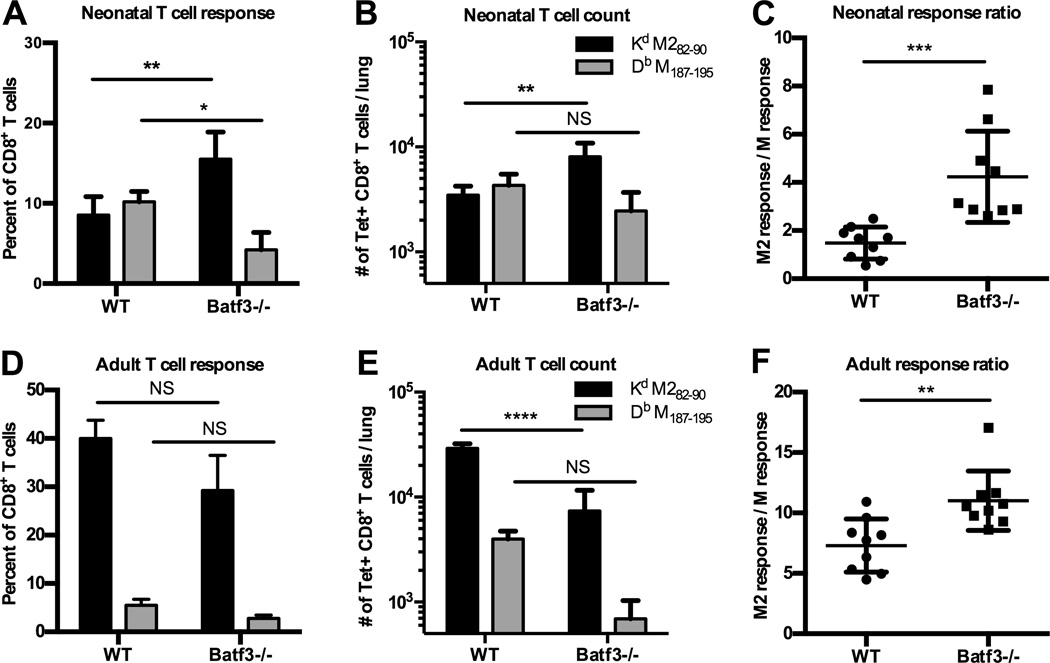
The data indicate that TLR agonists affect both cDC subsets, but more significantly increase the number of CD11b+ DCs in the neonatal dLN during early infection. The increased accumulation of CD11b+ DCs in the dLN at 10 days of age coincided with the age-dependent change in RSV-specific immunodominance (23). To further examine the role of these cDC subsets to influence the immunodominance hierarchy, we evaluated T cell responses in Batf3−/− mice, which lack CD103+ DCs (33). Hybrid CB6F1 Batf3−/− neonatal and adult mice were infected with RSV and RSV-specific CD8+ T cell responses were evaluated 7DPI in the lung. The lack of CD103+ DCs in hybrid Batf3−/− mice after RSV infection was confirmed by flow cytometric analysis of cDC subsets from the dLN of adults and neonates 3DPI (Supplemental Fig. 2). Infection of neonatal Batf3−/− mice resulted in an increased percentage and number of KdM282–90-specific T cells in the lung at 7DPI (Fig. 3A and B). The increase in KdM282–90-specific T cells resulted in a response ratio of approximately 4 and was statistically significant compared to the response ratio observed in wild type neonatal mice (Fig. 3C). RSV infection of adult hybrid Batf3−/− mice sharpened the immunodominance hierarchy (Fig. 3D and F); however, the total number of RSV-specific CD8+ T cells in the lungs of adults was dramatically reduced (Fig. 3E). Together our data indicate that the CD11b+ DC population preferentially supports KdM282–90-specific T cell dominance, although CD103+ DCs determine the magnitude of the total RSV-specific CD8+ T cell response, in both adults and neonates. Furthermore, these data suggest cooperative yet distinct roles for both cDC subsets to influence the immunodominance hierarchy.
Figure 3.
Lack of CD103+ DCs results in KdM282–90 -dominant T cell response in neonates and adults during RSV infection. (A–F) CD103+ DC-deficient CB6F1/J hybrid and wild type CB6F1/J hybrid mice were infected with RSV and epitope-specific T cell subsets were analyzed by tetramer staining from the lung at the peak of the CD8+ T cell response. (A and B) Adult and neonatal epitope-specific CD8+ T cell responses shown as a percentage of the total CD8+ T cell in the lung and (B and E) absolute number per lung. (C) Response ratio of the percentage of KdM282–90 to DbM187–195-specific CD8+ T cells. One-way or two-way ANOVA with Tukey’s multiple comparisons test was used to assess statistical significance (*p≤0.05, **p≤0.01, ***p≤0.001, **** p≤0.0001, NS indicates not significant). Error bars indicate SEM.
TLR agonists differentially increase the expression of the co-stimulatory molecule CD86 on neonatal CD103+ DCs and CD11b+ DCs
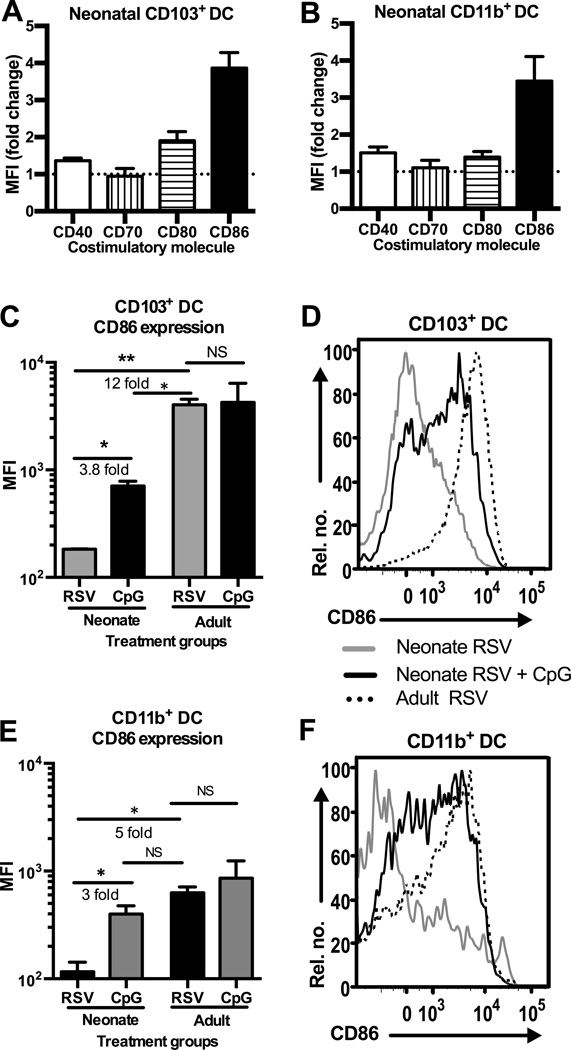
To determine if TLR signaling induced functional maturation in addition to increasing cDC numbers in the dLN, we next examined the expression of co-stimulatory molecules on the cDC subsets. Expression of key co-stimulatory molecules; CD40, CD70, CD80 and CD86 (34–36) on neonatal and adult cDCs, was analyzed by mean fluorescence intensity (MFI) as measured by flow cytometry. CD103+ DCs and CD11b+ DCs isolated from the dLN of neonatal mice at 3DPI showed non-significant differences in expression of CD40, CD70 or CD80 after LPS treatment (Fig. 4A and B). In contrast, the average expression of CD86 was increased 4–5 fold on neonatal CD11b+ DCs after LPS treatment in conjunction with RSV infection compared with RSV infection alone (Fig. 4B, E, and F). The increase in CD86 expression on neonatal CD11b+ DCs after LPS treatment resulted in levels of CD86 expression comparable to those exhibited by adult CD11b+ DCs in the absence of TLR agonist treatment (Figure 4E and F). CD103+ DCs demonstrated an increase in CD86 expression 3DPI that did not reach the expression levels seen on adult CD103+ DCs (Fig. 4C and D).
Figure 4.
CD86 expression is increased to adult-like levels on CD11b+ DCs after LPS treatment concurrent with RSV infection. (A–B) Neonatal cDC subsets were identified in the dLN 3DPI with or without LPS treatment and co-stimulatory molecule expression was measured as mean fluorescence intensity (MFI) by flow cytometry. Data represent fold-change increase of co-stimulatory molecule expression on cDCs from LPS treated mice over untreated mice. (C–D) MFI of CD86 expression on neonatal and adult CD103+ DCs with or without LPS treatment 3DPI represented as (C) geometric mean and (D) histogram. (E–F) MFI of CD86 expression on neonatal and adult CD11b+ DCs represented by (E) geometric mean and (F) histogram. Statistical significance determined by two-tailed unpaired Student’s t test (*p≤0.05, **p≤0.01, NS indicates not significant).
Therefore we next examined the co-stimulatory molecule expression on neonatal and adult cDCs after CpG treatment at the time of RSV infection. Similar to LPS treatment, CpG did not significantly alter the expression of CD40, CD70 or CD80 on neonatal cDCs in the dLN 3DPI (Fig. 5A and B), but did significantly increase the expression of CD86 on CD11b+ DCs (Fig. 5B, E, and F) and CD103+ DCs (Fig. 5B, and C). Treatment with CpG resulted in levels of CD86 expression on neonatal CD11b+ DCs nearly equivalent to that of adults after RSV infection alone, whereas neonatal CD103+ DC expression of CD86 did not reach adult levels. Flow cytometric analysis of MFI of the fluorochrome conjugated to the anti-CD86 antibody on non-cDC cells found in either the CD11c- or CD11c+ gates, did not identify alternate cell populations significantly expressing CD86 in the dLN (data not shown).
Figure 5.
CD86 expression is increased on CD103+ DCs and CD11b+ DCs after CpG treatment concurrent with RSV infection. (A–B) Neonatal cDC subsets were identified in the dLN 3DPI with or without CpG treatment and co-stimulatory molecule expression was measured as MFI by flow cytometry. Data represent fold change in costimulatory molecule expression on TLR agonist treated cDC subsets above untreated cDC subsets. (C–D) CD86 expression on neonatal and adult CD103+ DCs with or without CpG treatment 3DPI represented as (C) geometric mean and (D) histogram. (E–F) MFI of CD86 expression on neonatal and adult CD11b+ DCs with or without CpG treatment represented by (E) geometric mean and (F) histograms. Statistical significance determined by two-tailed unpaired Student’s t test (*p≤0.05, **p≤0.001, NS indicates not significant).
Co-stimulatory molecule expression on adult cDC subsets differed compared to neonates after TLR agonist treatment. CD80 was upregulated on both CD103+ DCs and CD11b+ DCs in the dLN 3DPI after CpG and LPS treatment (Supplemental Fig. 3A–D). In contrast to neonates, the expression of CD86 was primarily increased on adult CD11b+ DCs after LPS treatment but not CpG (Supplemental Fig. 3B and D). CD40 and CD70 expression were relatively unchanged on adult cDC subsets.
In addition to co-stimulation, antigen presentation is required to engage naïve T cells. Viral load and antigen abundance have been shown by others to influence the magnitude of CD8+ T cell responses suggesting a role in determining immunodominance hierarchy (16, 37). However, we have previously demonstrated that intranasal infection of neonates and adults with a replication-defective adenovirus vector expressing the KdM282–90 and DbM187–195 epitopes does not alter either immunodominance hierarchy (22). These data suggest that alterations in viral load and the abundance of available antigen do not change the RSV-specific CD8+ T cell response hierarchy. TLR agonist treatment has the potential to affect both viral load and antigen presentation. Therefore to further address whether the load of replicating virus and possible alterations in antigen availability influence immunodominance during RSV infection, we infected neonatal mice with 2×104, 2×105, 2×106 or 1×107 PFU of RSV and found no difference in the RSV-specific CD8+ T cell response hierarchy in neonates over this 1000 fold viral dose titration (Supplemental Fig. 4). These data suggest that RSV-specific immunodominance in neonates is more sensitive to co-stimulatory requirements than the abundance of antigen.
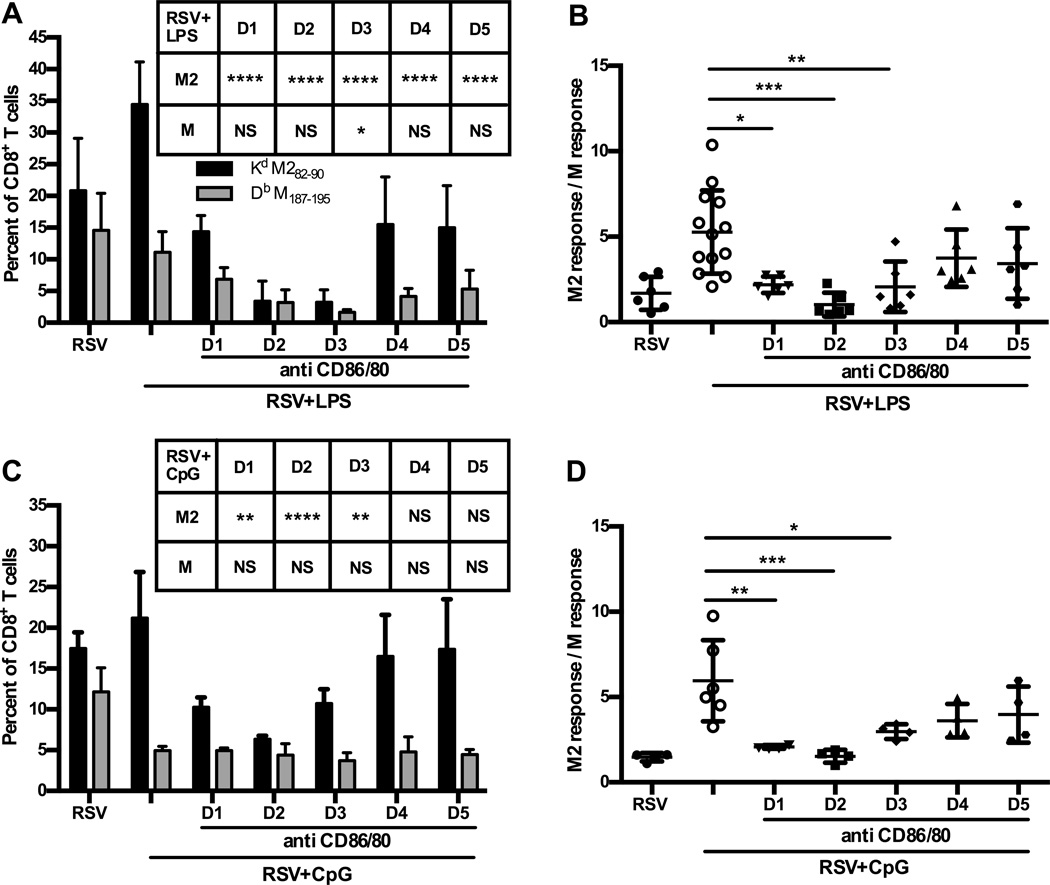
Partial blockade of CD80/86 in TLR agonist treated neonates during T cell priming restores codominant RSV-specific CD8+ T cell responses
Co-stimulatory molecules contribute to optimal T cell expansion (38, 39) and given that expression of CD86 was significantly increased after TLR stimulation, particularly on CD11b+ DCs, we hypothesized that CD86 co-stimulation may be key to KdM282–90 dominance. To examine the role of co-stimulation in determining KdM282–90-specific T cell immunodominance, partial blockade of CD86, and its redundant CD80, was achieved using receptor-specific antibodies. Blocking antibodies for CD86/80 molecules were administered in doses titrated to diminish but not eliminate their availability after TLR agonist treatment of neonates. Blocking antibodies were administered on a single day during each of the first 5 days post-TLR agonist treatment and RSV infection to determine when in the course of infection expression of CD86/80 was most important in influencing KdM282–90-dominance. RSV-specific CD8+ T cell responses were then assessed at 7DPI. Blocking antibodies administered during the first 3 days post-TLR agonist treatment and infection effectively reversed the KdM282–90-specific T cell response and reestablished the codominance of the KdM282–90 and DbM187–195-specific T cell responses characteristic of untreated neonates (Fig. 6A–D). This blockade of CD86/80 significantly diminished the percentage of responding KdM282–90-specific CD8+ T cells after LPS or CpG treatment of neonatal mice while the percentage of DbM187–195–specific T cells was not significantly affected. This striking reversal to the neonatal phenotype highlights the importance of co-stimulation provided by CD86/80 in the optimal T cell recruitment of KdM282–90-epitope specific CD8+ T cells in contrast to DbM187–195–specific T cells.
Figure 6.
Blockade of CD86 expression reverses the effect of LPS and CpG on immunodominance hierarchy in neonates during early T cell priming. (A–B) Neonatal mice were infected and treated with LPS then administered CD80/86 blocking antibodies on a single day during the first 5DPI followed by CD8 T cell analysis from the lung 7DPI. (A) RSV-specific CD8+ T cells were identified by tetramer staining and measured as a percentage of the total CD8+ T cell response in the lung. Experiments had 4–5 animals per group and are representative of 2 experiments. (C–D) Neonatal mice were infected and treated with CpG then administered CD80/CD86 blocking antibodies on a single day during the first 5DPI again T cell analysis was performed 7DPI. One-way or two-way ANOVA with Tukey’s multiple comparisons test was used to assess statistical significance (*p≤0.05, **p≤0.01, ***p≤0.001, **** p≤0.0001, NS indicates not significant). Error bars indicate SEM.
Discussion
Immunodominance hierarchies are generally reproducible amongst individuals with shared MHC alleles, however, we have previously demonstrated in a murine model that despite common MHC alleles immunodominance differs depending upon the age at RSV infection (22). Here, we show that TLR agonist treatment modifies diminished neonatal cDC responses to RSV and alters the immunodominance hierarchy by increasing the KdM282–90-specific CD8+ T cell responses. The data suggest co-stimulatory molecule expression on neonatal cDCs is limited compared to adult cDCs during RSV infection, and that epitope-specific CD8+ T cells have different requirements for co-stimulation. Increasing the number of neonatal CD86-expressing cDCs, particularly CD11b+ DCs, during RSV infection with TLR agonists was associated with an increase in KdM282–90-specific CD8+ T cells. The increase in number of KdM282–90-specific CD8+ T cells in neonates after TLR agonist treatment approximated that seen in untreated adult mice and recapitulated the adult immunodominance hierarchy.
Triggering PRRs has been shown to influence DC maturation, including antigen capture, processing and presentation, migration, and co-stimulatory molecule expression (40–43). Neonatal DCs from mice and humans, however, demonstrate limitations in markers of maturation such as cytokine production and co-stimulatory molecule expression upon TLR stimulation compared with adult DCs (44–47). Several PRRs including TLR2, TLR3, TLR4, TLR7, NOD2, RIG-I and MDA5 have been implicated in sensing RSV yet none conclusively (48–52). Less protective responses have been associated with genetic polymorphisms of TLR4 in some infant populations suggesting a role for sufficient TLR4 signaling in pathogenesis of disease (30). Augmenting TLR stimulation with either LPS or CpG at the time of RSV infection increased the recruitment of cDCs into the dLN of neonates resulting in a similar representation of neonatal cDCs in the dLN compared to adults infected with RSV alone. We have previously published that the numbers of CD11b+ DCs in the dLN 3 days post RSV infection are 10 fold greater in adults then neonates whereas the difference is only 1–2 fold greater in the adult lung suggesting that adult CD11b+ DCs are more affectively recruited to the dLN. TLR agonist treatment, however, mitigates this disparity by inducing 10 fold or greater rise in the accumulation of neonatal CD11b+ DC in the dLN. In addition to deficient recruitment of cDCs to the dLN, neonatal cDCs also exhibited lower expression of co-stimulatory molecule CD86 in response to RSV that could be increased with TLR agonist administration. Our data show that providing additional TLR engagement, with a robust TLR4 or a TLR9 agonist, in neonatal mice during RSV infection increases the accumulation of neonatal cDCs in the dLN and induces greater co-stimulatory molecule expression, similar to that seen in adults. This is consistent with published data evaluating DCs and other APC in cord blood and from other neonatal murine models suggesting that more robust responses can predominate if distinct or multiple TLR agonists are engaged (53, 54). Further research is necessary to understand the checkpoints that must be met through TLR activation to both recruit cDCs into peripheral tissues and further induce their expression of co-stimulatory molecules as our data and that of others suggests that these signaling pathways are not comparably activated between adults and neonates (55).
DCs have been shown to influence immunodominance hierarchies induced in other infection models (56, 57). These studies, however, have not evaluated the role of distinct DC subsets, either characterizing DCs only by CD11c+ expression or using bone marrow derived DCs. Furthermore individual DC subsets have been found to play distinct roles in mediating communication that activates naïve CD8+ T cells within the dLN (58). Functional roles of DC subsets have been attributed to lineage and genetic requirements (59). Context of immune stimulation has also been shown to determine function in lung cDCs (26, 27). Neonatal CD11b+ DCs were poorly recruited to the lung and dLN during RSV infection, which was in contrast to adult CD11b+ DCs that were recruited in greater number and percentage. Infection of mice lacking CD103+ DCs was used to evaluate the influence of these DC subsets on immunodominance hierarchy. Infection of both adult and neonatal CD103+ DC-deficient mice resulted in an increased percentage of responding KdM282–90-CD8+ T cells, resulting in a sharpening of the KdM282–90-dominance in adults and a more adult-like immunodominance hierarchy in neonates. The total number of RSV-specific CD8+ T cells was, however, altered in CD103+ DC- deficient mice. The data indicate that CD11b+ DCs were more apt to support a KdM282–90-dominant response, but participation of CD103+ DCs was necessary to achieve maximal CD8+ T cell responses. Current analysis of either CD103+ DC or CD11b+DC subsets most likely represent populations of cells that vary in maturation and function. Further development of phenotypic markers to better define antigen-presentation and function of CD103+DCs and particularly CD11b+DCs by our lab and others will improve the understanding of this complex interplay of DC subsets to elicit neonatal CD8+ T cell responses during RSV infection. Together these data suggest that cDC subsets may differentially provide requirements for epitope-specific CD8+ T cell recruitment thereby determining immunodominance and the magnitude of the pathogen-specific CD8+ T cell response.
Co-stimulatory molecule expression by APCs is required to elicit activated T cells (60, 61), however, the role of co-stimulation to influence immunodominance hierarchy has varied depending on the experimental infection model (62, 63). Of the co-stimulatory molecules evaluated in this report, the B7 family member, CD86, exhibited the greatest disparity in expression between adults and neonates during RSV infection. Neonatal cDCs in the dLN expressed lower levels of CD86 in comparison to adults, but were induced to express adult-like levels on CD11b+ DCs after TLR agonist treatment. Flow cytometric evaluation of additional cell populations in the dLN failed to identify alternate populations of cells that might express CD86 providing co-stimulation to T cells. The significant increase in CD86 expression on neonatal cDCs was associated with a dominant KdM282–90-specific T cell response. The TLR induced changes in co-stimulation provided by cDCs had limited effect on the DbM187–195-specific T cell response suggesting that intrinsic requirements for co-stimulation may differ between epitope-specific T cells and could explain differing reports on the role of co-stimulation to influence immunodominance. These data show that co-stimulation through the B7 family influences the magnitude of the RSV-specific CD8+ T cell response in an epitope-dependent manner to determine immunodominance.
Interestingly, upregulation of CD86 in TLR agonist-treated adult mice was not accompanied by an increase in KdM282–90 specific CD8+ T cell response thereby suggesting that a threshold for co-stimulation had been met. The titration of CD86/80 blockade to limit but not eliminate co-stimulatory molecule interaction in neonatal mice treated with TLR agonists also suggests that intrinsic epitope-specific thresholds for co-stimulation may drive KdM282–90 dominance. Thresholds of antigen-presentation have been shown to influence the magnitude of epitope-specific responses in another model and beyond those thresholds no additional increase in T cell number is seen (18). Similarly these data suggest that a threshold of CD86 co-stimulation may be required to elicit the dominance of the KdM282–90 specific T cell response.
Overall, our data demonstrate that neonatal immunodominance hierarchy can be regulated during RSV infection. Using TLR agonist treatment we altered, not only the accumulation of neonatal cDCs in the dLN, but also the quality of their ability to engage naïve CD8+ T cells. Furthermore, CD103+ DCs and CD11b+ DCs play distinct roles to influence the overall magnitude of the response and the immunodominant-epitope, respectively. By upregulated CD86 expression on neonatal cDCs, TLR agonist treatment enhanced co-stimulation required for KdM282–90 -specific CD8+ T cell dominance. In contrast, TLR-induced stimulation did not affect the magnitude of DbM187–195 specific T cell response demonstrating an epitope-dependent impact of TLR treatment. The neonatal cDC and T cell responses could be manipulated to a greater extent than adult responses suggesting that there is potential to selectively influence neonatal T cell responses to optimize protective immunity against viral infections.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Programs of the Vaccine Research Center, National Institutes of Health (NIH) and by the Uniformed Services University of the Health Sciences (USUHS) Department of Pediatrics Grant QP86GI-S24.
Abbreviations used in this article
- Batf3
Basic Leucine Zipper Transcription Factor,. ATF-Like 3
- cDC
conventional dendritic cell
- CpG
CpG ODN cytosine triphosphate deoxynucleotide linked by phosphodiester to a guanine triphosphate deoxynucleotide
- CB6F1/J hybrid
mouse strain that is the first generation of a BALB/c crossed with a C57BL/6
- DC
dendritic cell
- FMO
fluorescence-minus-one
- dLN
mediastinal lung-draining lymph node
- MFI
mean fluorescence intensity
- PAMPs
pathogen-associated molecular patterns
- PRRs
pattern recognition receptors
- RSV
respiratory syncytial virus
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The views presented in this manuscript are those of the authors; no endorsement by the Uniformed Services University of the Health Sciences or the Department of Defense has been given or should be inferred.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidema J, Lukens MV, van Maren WW, van Dijk ME, Otten HG, van Vught AJ, van der Werff DB, van Gestel SJ, Semple MG, Smyth RL, Kimpen JL, van Bleek GM. CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J Immunol. 2007;179:8410–8417. doi: 10.4049/jimmunol.179.12.8410. [DOI] [PubMed] [Google Scholar]
- 3.Lukens MV, van de Pol AC, Coenjaerts FE, Jansen NJ, Kamp VM, Kimpen JL, Rossen JW, Ulfman LH, Tacke CE, Viveen MC, Koenderman L, Wolfs TF, van Bleek GM. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J Virol. 2010;84:2374–2383. doi: 10.1128/JVI.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris DT, Schumacher MJ, Locascio J, Besencon FJ, Olson GB, DeLuca D, Shenker L, Bard J, Boyse EA. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci U S A. 1992;89:10006–10010. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 6.Prendergast AJ, Klenerman P, Goulder PJ. The impact of differential antiviral immunity in children and adults. Nat Rev Immunol. 2012;12:636–648. doi: 10.1038/nri3277. [DOI] [PubMed] [Google Scholar]
- 7.Marchant A, Appay V, Van Der Sande M, Dulphy N, Liesnard C, Kidd M, Kaye S, Ojuola O, Gillespie GM, Vargas Cuero AL, Cerundolo V, Callan M, McAdam KP, Rowland-Jones SL, Donner C, McMichael AJ, Whittle H. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest. 2003;111:1747–1755. doi: 10.1172/JCI17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermann E, Truyens C, Alonso-Vega C, Even J, Rodriguez P, Berthe A, Gonzalez-Merino E, Torrico F, Carlier Y. Human fetuses are able to mount an adultlike CD8 T-cell response. Blood. 2002;100:2153–2158. [PubMed] [Google Scholar]
- 9.Franchini M, Abril C, Schwerdel C, Ruedl C, Ackermann M, Suter M. Protective T-cell-based immunity induced in neonatal mice by a single replicative cycle of herpes simplex virus. J Virol. 2001;75:83–89. doi: 10.1128/JVI.75.1.83-89.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez X, Brandt C, Saddallah F, Tougne C, Barrios C, Wild F, Dougan G, Lambert PH, Siegrist CA. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc Natl Acad Sci U S A. 1997;94:8726–8731. doi: 10.1073/pnas.94.16.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci U S A. 1998;95:15553–15558. doi: 10.1073/pnas.95.26.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 13.Sigal LJ, Rock KL. Bone marrow-derived antigen-presenting cells are required for the generation of cytotoxic T lymphocyte responses to viruses and use transporter associated with antigen presentation (TAP)-dependent and -independent pathways of antigen presentation. J Exp Med. 2000;192:1143–1150. doi: 10.1084/jem.192.8.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenz LL, Butz EA, Bevan MJ. Requirements for bone marrow-derived antigen-presenting cells in priming cytotoxic T cell responses to intracellular pathogens. J Exp Med. 2000;192:1135–1142. doi: 10.1084/jem.192.8.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilchuk P, Spencer CT, Conant SB, Hill T, Gray JJ, Niu X, Zheng M, Erickson JJ, Boyd KL, McAfee KJ, Oseroff C, Hadrup SR, Bennink JR, Hildebrand W, Edwards KM, Crowe JE, Jr, Williams JV, Buus S, Sette A, Schumacher TN, Link AJ, Joyce S. Discovering naturally processed antigenic determinants that confer protective T cell immunity. J Clin Invest. 2013;123:1976–1987. doi: 10.1172/JCI67388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotturi MF, Scott I, Wolfe T, Peters B, Sidney J, Cheroutre H, von Herrath MG, Buchmeier MJ, Grey H, Sette A. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol. 2008;181:2124–2133. doi: 10.4049/jimmunol.181.3.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cukalac T, Chadderton J, Zeng W, Cullen JG, Kan WT, Doherty PC, Jackson DC, Turner SJ, La Gruta NL. The influenza virus-specific CTL immunodominance hierarchy in mice is determined by the relative frequency of high-avidity T cells. J Immunol. 2014;192:4061–4068. doi: 10.4049/jimmunol.1301403. [DOI] [PubMed] [Google Scholar]
- 18.Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J Immunol. 1999;163:3735–3745. [PubMed] [Google Scholar]
- 19.Probst HC, Tschannen K, Gallimore A, Martinic M, Basler M, Dumrese T, Jones E, van den Broek MF. Immunodominance of an antiviral cytotoxic T cell response is shaped by the kinetics of viral protein expression. J Immunol. 2003;171:5415–5422. doi: 10.4049/jimmunol.171.10.5415. [DOI] [PubMed] [Google Scholar]
- 20.Moutaftsi M, Peters B, Pasquetto V, Tscharke DC, Sidney J, Bui HH, Grey H, Sette A. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol. 2006;24:817–819. doi: 10.1038/nbt1215. [DOI] [PubMed] [Google Scholar]
- 21.Rutigliano JA, Ruckwardt TJ, Martin JE, Graham BS. Relative dominance of epitope-specific CD8+ T cell responses in an F1 hybrid mouse model of respiratory syncytial virus infection. Virology. 2007;362:314–319. doi: 10.1016/j.virol.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruckwardt TJ, Malloy AM, Gostick E, Price DA, Dash P, McClaren JL, Thomas PG, Graham BS. Neonatal CD8 T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog. 2011;7:e1002377. doi: 10.1371/journal.ppat.1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruckwardt TJ, Malloy AM, Morabito KM, Graham BS. Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response. PLoS Pathog. 2014;10:e1003934. doi: 10.1371/journal.ppat.1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Condon TV, Sawyer RT, Fenton MJ, Riches DW. Lung dendritic cells at the innate-adaptive immune interface. J Leukoc Biol. 2011;90:883–895. doi: 10.1189/jlb.0311134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desch AN, Randolph GJ, Murphy K, Gautier EL, Kedl RM, Lahoud MH, Caminschi I, Shortman K, Henson PM, Jakubzick CV. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med. 2011;208:1789–1797. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim TS, Gorski SA, Hahn S, Murphy KM, Braciale TJ. Distinct dendritic cell subsets dictate the fate decision between effector and memory CD8(+) T cell differentiation by a CD24-dependent mechanism. Immunity. 2014;40:400–413. doi: 10.1016/j.immuni.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desch AN, Gibbings SL, Clambey ET, Janssen WJ, Slansky JE, Kedl RM, Henson PM, Jakubzick C. Dendritic cell subsets require cis-activation for cytotoxic CD8 T-cell induction. Nat Commun. 2014;5:4674. doi: 10.1038/ncomms5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 29.Sarzotti M, Robbins DS, Hoffman PM. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science. 1996;271:1726–1728. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- 30.Caballero MT, Serra ME, Acosta PL, Marzec J, Gibbons L, Salim M, Rodriguez A, Reynaldi A, Garcia A, Bado D, Buchholz UJ, Hijano DR, Coviello S, Newcomb D, Bellabarba M, Ferolla FM, Libster R, Berenstein A, Siniawaski S, Blumetti V, Echavarria M, Pinto L, Lawrence A, Ossorio MF, Grosman A, Mateu CG, Bayle C, Dericco A, Pellegrini M, Igarza I, Repetto HA, Grimaldi LA, Gudapati P, Polack NR, Althabe F, Shi M, Ferrero F, Bergel E, Stein RT, Peebles RS, Boothby M, Kleeberger SR, Polack FP. TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization. J Clin Invest. 2015;125:571–582. doi: 10.1172/JCI75183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 32.Lantier L, Lacroix-Lamande S, Potiron L, Metton C, Drouet F, Guesdon W, Gnahoui-David A, Le Vern Y, Deriaud E, Fenis A, Rabot S, Descamps A, Werts C, Laurent F. Intestinal CD103+ dendritic cells are key players in the innate immune control of Cryptosporidium parvum infection in neonatal mice. PLoS Pathog. 2013;9:e1003801. doi: 10.1371/journal.ppat.1003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meunier S, Rapetti L, Beziaud L, Pontoux C, Legrand A, Tanchot C. Synergistic CD40 signaling on APCs and CD8 T cells drives efficient CD8 response and memory differentiation. J Leukoc Biol. 2012;91:859–869. doi: 10.1189/jlb.0611292. [DOI] [PubMed] [Google Scholar]
- 35.Ballesteros-Tato A, Leon B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat Immunol. 2010;11:216–224. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harding FA, Allison JP. CD28-B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help. J Exp Med. 1993;177:1791–1796. doi: 10.1084/jem.177.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flesch IE, Hollett NA, Wong YC, Quinan BR, Howard D, da Fonseca FG, Tscharke DC. Extent of Systemic Spread Determines CD8+ T Cell Immunodominance for Laboratory Strains, Smallpox Vaccines, and Zoonotic Isolates of Vaccinia Virus. J Immunol. 2015;195:2263–2272. doi: 10.4049/jimmunol.1402508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welten SP, Redeker A, Franken KL, Oduro JD, Ossendorp F, Cicin-Sain L, Melief CJ, Aichele P, Arens R. The viral context instructs the redundancy of costimulatory pathways in driving CD8(+) T cell expansion. Elife. 2015:4. doi: 10.7554/eLife.07486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003;14:265–273. doi: 10.1016/s1359-6101(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 40.West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 41.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 42.Sato A, Iwasaki A. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc Natl Acad Sci U S A. 2004;101:16274–16279. doi: 10.1073/pnas.0406268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jakubzick C, Helft J, Kaplan TJ, Randolph GJ. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J Immunol Methods. 2008;337:121–131. doi: 10.1016/j.jim.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dakic A, Shao QX, D’Amico A, O’Keeffe M, Chen WF, Shortman K, Wu L. Development of the dendritic cell system during mouse ontogeny. J Immunol. 2004;172:1018–1027. doi: 10.4049/jimmunol.172.2.1018. [DOI] [PubMed] [Google Scholar]
- 45.Upham JW, Rate A, Rowe J, Kusel M, Sly PD, Holt PG. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun. 2006;74:1106–1112. doi: 10.1128/IAI.74.2.1106-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, Goldman M, Willems F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–281. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demoor T, Petersen BC, Morris S, Mukherjee S, Ptaschinski C, De Almeida Nagata DE, Kawai T, Ito T, Akira S, Kunkel SL, Schaller MA, Lukacs NW. IPS-1 signaling has a nonredundant role in mediating antiviral responses and the clearance of respiratory syncytial virus. J Immunol. 2012;189:5942–5953. doi: 10.4049/jimmunol.1201763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lukacs NW, Smit JJ, Mukherjee S, Morris SB, Nunez G, Lindell DM. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J Immunol. 2010;185:2231–2239. doi: 10.4049/jimmunol.1000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vissers M, Remijn T, Oosting M, de Jong DJ, Diavatopoulos DA, Hermans PW, Ferwerda G. Respiratory syncytial virus infection augments NOD2 signaling in an IFN-beta-dependent manner in human primary cells. Eur J Immunol. 2012;42:2727–2735. doi: 10.1002/eji.201242396. [DOI] [PubMed] [Google Scholar]
- 52.Marr N, Turvey SE, Grandvaux N. Pathogen recognition receptor crosstalk in respiratory syncytial virus sensing: a host and cell type perspective. Trends Microbiol. 2013;21:568–574. doi: 10.1016/j.tim.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 54.Sun CM, Deriaud E, Leclerc C, Lo-Man R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity. 2005;22:467–477. doi: 10.1016/j.immuni.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 55.van Haren SD, Ganapathi L, Bergelson I, Dowling DJ, Banks M, Samuels RC, Reed SG, Marshall JD, Levy O. In vitro cytokine induction by TLR-activating vaccine adjuvants in human blood varies by age and adjuvant. Cytokine. 2016;83:99–109. doi: 10.1016/j.cyto.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmidt J, Iversen AK, Tenzer S, Gostick E, Price DA, Lohmann V, Distler U, Bowness P, Schild H, Blum HE, Klenerman P, Neumann-Haefelin C, Thimme R. Rapid antigen processing and presentation of a protective and immunodominant HLA-B*27-restricted hepatitis C virus-specific CD8+ T-cell epitope. PLoS Pathog. 2012;8:e1003042. doi: 10.1371/journal.ppat.1003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dinter J, Duong E, Lai NY, Berberich MJ, Kourjian G, Bracho-Sanchez E, Chu D, Su H, Zhang SC, Le Gall S. Variable processing and cross-presentation of HIV by dendritic cells and macrophages shapes CTL immunodominance and immune escape. PLoS Pathog. 2015;11:e1004725. doi: 10.1371/journal.ppat.1004725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H, Garbi N, Kaisho T, Germain RN, Kastenmuller W. Robust Anti-viral Immunity Requires Multiple Distinct T Cell-Dendritic Cell Interactions. Cell. 2015;162:1322–1337. doi: 10.1016/j.cell.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy KM. Transcriptional control of dendritic cell development. Adv Immunol. 2013;120:239–267. doi: 10.1016/B978-0-12-417028-5.00009-0. [DOI] [PubMed] [Google Scholar]
- 60.Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16–22. [PubMed] [Google Scholar]
- 61.Suzuki I, Fink PJ. Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J Exp Med. 1998;187:123–128. doi: 10.1084/jem.187.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen W, Bennink JR, Morton PA, Yewdell JW. Mice deficient in perforin, CD4+ T cells, or CD28-mediated signaling maintain the typical immunodominance hierarchies of CD8+ T-cell responses to influenza virus. J Virol. 2002;76:10332–10337. doi: 10.1128/JVI.76.20.10332-10337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin LC, Flesch IE, Tscharke DC. Immunodomination during peripheral vaccinia virus infection. PLoS Pathog. 2013;9:e1003329. doi: 10.1371/journal.ppat.1003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.