Abstract
Background
Previous studies have linked both extreme and sub-optimal air temperature to cardiopulmonary morbidity and mortality, especially in older individuals. However, the underlying mechanisms are yet to be determined.
Objectives
We hypothesized that short-term increases in air temperature may induce blood mitochondrial DNA (mtDNA) lesions in older individuals, which could contribute to temperature-related pathogenesis.
Methods
We repeatedly measured mtDNA lesions in blood samples from 654 participants in the Normative Aging Study from 1999 to 2013 (1142 observations) by quantitative long-amplicon polymerase chain reaction assay. Hourly temperature data were obtained from the Boston Logan Airport weather station (located approximately 12 km from the clinical site). We calculated 2-, 7-, and 14-day moving averages of 24-hour mean and 24-hour variability of temperature. We fit covariate-adjusted linear-mixed models accounting for repeated measures to evaluate the association between short-term increases in mean and variability of temperature with mtDNA lesions within each season.
Results
Interquartile increases in 7- and 14-day moving averages of 24-hour mean temperature in summer were associated with a 0.17 (95% CI: 0.07, 0.27; p = 0.0007) and 0.21 (95% CI: 0.10, 0.32; p = 0.0001) increase in the number of mtDNA lesions per 10 kb, respectively. Results were similar when we further adjusted for temperature variability. We also observed significant associations between increases in temperature variability and mtDNA lesions independent of mean air temperature. An interquartile range increase in the 7-day moving average of 24-hour standard deviation in summer was associated with a 0.19 (95% CI: 0.07, 0.31; p = 0.0023) increase in the number of mtDNA lesions per 10 kb.
Conclusions
Short-term exposure to higher mean air temperature was associated with increased mtDNA lesions in older adults, supporting the hypothesis that sub-optimal meteorological conditions may induce pathophysiological responses among susceptible populations.
1 Introduction
A growing body of evidence has shown that changes in air temperature are associated with increased cardiopulmonary morbidity and mortality (Braga et al., 2002; Koken et al., 2003; Schneider et al., 2008; Schwartz et al., 2004; Wolf et al., 2009), especially in older populations (Kim and Joh, 2006). However, the underlying mechanisms have not been clearly delineated. Previous epidemiological studies have linked changes in air temperature with abnormal cardiac output (Alperovitch et al., 2009; Barnett et al., 2007; Halonen et al., 2011; Ren et al., 2011), myocyte dysfunction (Wilker et al., 2012), increased blood pro-coagulatory and pro-inflammatory markers (Hampel et al., 2010; Keatinge et al., 1984; Schauble et al., 2012), and epigenetic modifications (Bind et al., 2014). Yet the role of mitochondrial-related oxidative stress—an emerging contributor to a range of cardiovascular disorders, including atherosclerosis, ischemic heart disease, and hypertension (Dhalla et al., 2000)—remains unknown.
Mitochondria, unlike other cellular organelles, contain their own circular double-stranded DNA (Mengel-From et al., 2014). Mitochondrial DNA (mtDNA) is a critical cellular target for oxidative stress mediated via reactive oxygen species (ROS) (Yakes and Van Houten, 1997; Zastawny et al., 1998). Indeed, mitochondrial genomes appear to be more vulnerable to oxidative attack than nuclear DNA due to a lack of protective barriers, such as histone proteins and chromatin organization, as well as relatively limited capability to repair mtDNA damage (Friedman and Nunnari, 2014; Mengel-From et al., 2014). Moreover, because 1%–2% of molecular oxygen consumed by aerobic respiration is released as ROS via the electron transport chain (ETC) reaction in mitochondria, disturbance of normal ETC activity may further exacerbate mitochondrial ROS formation (Demple and Harrison, 1994). As a result, damaged mitochondria may themselves become an important source of endogenous ROS generation and lead to further damage. Oxidative damage to mitochondria can induce mtDNA lesions, including single- and double-strand breaks, abasic sites, and base damage/modification (Yakes and Van Houten, 1997). Several lines of evidence indicate associations between mtDNA lesions and cardiovascular diseases (Corral-Debrinski et al., 1991; Corral-Debrinski et al., 1992; Lesnefsky et al., 2001), suggesting that mitochondrial genome damage, as reflected by increased mtDNA lesions, may represent a plausible biological mechanism for air temperature-related cardiovascular events.
In a repeated-measure study of older men in the greater Boston area, we investigated the association between short-term increases in 24-hour mean and variability of air temperature and blood mtDNA lesions. We hypothesized that older adults may be less adaptive to short-term increases in air temperature—especially at the extreme or sub-optimal ranges—which may lead to increased mtDNA lesions in blood.
2 Materials and methods
2.1 Study population
Our study population is derived from the Normative Aging Study, a prospective longitudinal cohort from the greater Boston area established in 1963 by the U.S. Veterans Administration. A detailed protocol has been published previously (Power et al., 2011). In brief, participants underwent clinical examinations every 3–5 years. At each visit, self-administered questionnaires were collected to provide information on socio-demographic characteristics, medical history, medication, and lifestyle. Blood samples were drawn after an overnight fast and smoking abstinence. A total of 654 participants had complete information on temperature measurements and blood mitochondrial markers for 1–4 visits during 1999–2013. Specifically, 173 participants came to one clinical visit, 387 participants came to two clinical visits, and 94 participants came to three or more clinical visits. This study was approved by the Institutional Review Boards of the participating institutions.
2.2 Ambient temperature measurements
We obtained hourly temperature and humidity data from the National Weather Service Station at Boston Logan airport, which is located approximately 12 km from the examination site. For each calendar day, we calculated 24-hour mean and 24-hour standard deviation (SD) of temperature. We then computed moving averages of the 24-hour mean and 24-hour SD of temperature (i.e., 2, 7, and 14 days prior to clinical visit) (Mehta et al., 2014).
As a secondary analysis, we estimated air temperature at each participant's residential address for each calendar day using a hybrid land-use regression and satellite-based model (Kloog et al., 2012). Specifically, we utilized Moderate Resolution Imaging Spectroradiometer (MODIS) satellite-derived land surface temperature measurements to predict daily air temperature at a 1 km-by-1 km spatial resolution. The model showed excellent prediction performance with cross validation of R2 = 0.94 and a root mean square-predicted error of 1.48. For additional details, refer to Kloog et al., 2014. We then computed moving averages of 24-hour mean temperatures for 2, 7, and 14 days before each clinical visit.
2.3 mtDNA lesion measurements
mtDNA lesions were measured as the number of lesions per 10 kb. A detailed protocol has been published previously (Furda et al., 2014). In brief, we adapted a quantitative long-amplicon polymerase chain reaction assay (QPCR) to amplify a long fragment (8.9 kb) and short fragment (222 bp) of the mitochondrial genome (Ponti et al., 1991). Because each mitochondrion contains multiple copies of circular DNA, the short fragment was amplified for normalization based on the premise that the short fragment would have no lesions due to its short length. Oxidative damage-associated mtDNA lesions, such as single- and double-stranded breaks, abasic sites, and base damage/modification, halt the progression of DNA polymerase, which in turn leads to decreased amplification of the desired sequence. Therefore, only those mtDNA fragments with no lesions would be amplified as a result. We measured the intensity of fluorescence using PicoGreen dsDNA (Thermo Fisher Scientific, Life Technologies, Carlsbad, California), and the amount of fluorescence released was inversely proportional to the number of mtDNA lesions. All samples were run in duplicate. The between-batch coefficient of variation for the current assay was 10%.
2.4 Statistical analysis
We evaluated the associations of short-term increases in mean and variability of temperature with number of mtDNA lesions per 10 kb in blood. Because previous studies often indicated J- or U-shaped relationships when modeling temperature with health-related outcomes (i.e., increased risk was observed at high or low temperature ranges) (Gasparrini et al., 2015; O'Neill and Ebi, 2009; Yang et al., 2016), we tested for effect modification by each season —spring, summer, fall, or winter. Within each seasonal category, we observed no deviations from linear dose–response using generalized additive mixed-effects models with penalized cubic splines. We therefore stratified our analysis by season and modeled mean and variability of air temperature as a linear term in the analysis.
We fitted linear mixed-effects models with subject-specific intercepts to account for repeated measurements of mtDNA lesions from the same individual and examined the association of increases in mean and variability of air temperature and mtDNA lesions. We modeled cumulative effects of air temperature as the 2-, 7- and 14-day moving average of 24-hour mean and 24-hour variability of temperature. We considered each moving average separately in the main analysis. Model estimates are expressed per interquartile range (IQR) increase in mean and variability of air temperature for specific moving averages.
In each model, we controlled for potential confounders and risk factors for mtDNA lesions: relative humidity (continuous); age (continuous); body mass index (BMI) [weight (kg) / height (m)2, continuous]; race (white or others); regular patterns of physical activity (< 12 h/week, ≥ 12 and < 30 h/week, or ≥ 30 h/week); smoking status (never, former, or current smoker); cumulative pack–years of smoking (continuous); alcohol consumption (two or more alcoholic drinks per day, less than two alcoholic drinks per day); education level (high school diploma or less, college degree, or graduate degree); platelet, lymphocyte and neutrophil counts in the blood; and batch effects.
The main regression model took the general form:
where i indexed participants, j indexed visit number, β0 was the intercept for population mean, and ui was the subject-specific random intercept. We assumed a linear effect of the moving average of mean temperature or temperature variability represented by βtemperature Temperatureij. Finally, β1X1ij − βpXpij corresponded to covariates we selected a priori. εij was the within-subject error term. We considered separate models for moving averages of mean temperature and temperature variability as well as a joint model that included both a moving average of mean and variability temperature. Model estimates are expressed per IQR increase in mean and variability of air temperature for specific moving averages.
2.5 Sensitivity analysis
Among the 654 participants included in our analysis, 27% of participants did not come for a subsequent clinical visit. We therefore accounted for potential selection bias due to follow-up loss using inverse probability of weighting (IPW). Specifically, we predicted the probability of coming to a subsequent clinical visit using covariates from the previous visit in logistic regression: age, BMI, regular patterns of physical activity, smoking status, pack–years smoked, forced expiratory volume in 1 s (FEV1) versus forced vital capacity (FVC) ratio, clinically diagnosed hypertension, clinically diagnosed diabetes, and medication (diuretics and beta blockers). In a subsequent analysis, we also restricted the analysis to participants' first visit to examine if differential loss to follow-up was an issue.
In the main analysis, we obtained temperature measurements from the stationary monitoring station, which was located approximately 12 km from the clinical site. This may have introduced potential exposure misclassifications, as two participants that came on the same day would be assigned to the same value. Therefore, for sensitivity analysis, we obtained temperature measurements from a spatial and temporal resolved model and examined the association between increases in mean air temperature from modeling data and mtDNA lesions.
We additionally adjusted for PM2.5 total mass concentration in the sensitivity analysis, as PM2.5 total mass concentration may potentially confound the association between air temperature and mtDNA lesions. Further, to understand the biological significance of the changes in the number of mtDNA lesions, we examined the spearman correlations between mtDNA lesions and systolic and diastolic blood pressure.
All analyses were conducted with SAS version 9.3 (SAS Institute Inc., Cary, NC) or R 3.0.1 (http://www.r-project.org/).
3 Results
3.1 Study population
Table 1 shows participant characteristics at each visit. Of the 654 participants who had blood mtDNA lesion measurements, 481 participants (74%) came to more than one clinical visit. Mean age at first visit was 73.1 ± 6.8 years, and mean BMI at first visit was 28.1 ± 4.0 kg/m2. Current smokers accounted for 4% of participants, and 66% were former smokers at the first visit. The study population was predominately white (97%). Mean mtDNA lesions was 1.9 ± 0.9 per 10 kb at baseline.
Table 1.
Personal characteristics of participants (N = 654).
| Variable | Visit 1 | Visit 2 | Visit 3 or more |
|---|---|---|---|
| (n = 654) | (n = 387) | (n = 94) | |
| Age (years), mean ± SD | 73.1 ± 6.8 | 76.1 ± 6.6 | 79.8 ± 6.2 |
| BMI (kg/m2), mean ± SD | 28.1 ± 4.0 | 27.9 ± 4.2 | 28.1 ± 4.2 |
| Weight (kg), mean ± SD | 85.0 ± 14.4 | 84.2 ± 14.9 | 83.3 ± 14.3 |
| Height (m), mean ± SD | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 |
| Smoking status, N (%) | |||
| Never | 196 (30%) | 121 (31%) | 31 (33%) |
| Former | 432 (66%) | 249 (65%) | 60 (65%) |
| Current | 26 (4%) | 14 (4%) | 2 (2%) |
| Smoking pack–years, mean ± SD | 19.9 ± 24.2 | 20.3 ± 24.7 | 21.0 ± 25.0 |
| Race, n (%) | |||
| White | 631 (97%) | 372 (97%) | 91 (97%) |
| Other | 17 (3%) | 11 (3%) | 3 (3%) |
| Physical exercise, n (%) | |||
| Low (12 h/week) | 412 (63%) | 247 (66%) | 66 (70%) |
| Medium (≥ 12 and < 30 h/week) | 145 (22%) | 82 (22%) | 13 (14%) |
| High (≥ 30 h/week) | 95 (15%) | 48 (12%) | 15 (16%) |
| Two or more alcoholic drinks per day, n (%) | 133 (20%) | 61 (16%) | 14 (15%) |
| Education, n (%) | |||
| < 12 years | 216 (33%) | 128 (33%) | 33 (35%) |
| 13–16 years | 306 (47%) | 180 (47%) | 49 (52%) |
| > 16 years | 132 (20%) | 79 (20%) | 12 (13%) |
| Season, n (%) | |||
| Spring (March–May) | 156 (24%) | 95 (25%) | 27 (29%) |
| Summer (June–August) | 187 (28%) | 104 (27%) | 26 (28%) |
| Fall (September–November) | 214 (33%) | 124 (32%) | 24 (25%) |
| Winter (December–February) | 97 (15%) | 63 (16%) | 17 (18%) |
| Lymphocyte count, mean ± SD (cells/cm3) | 25.9 ± 8.7 | 24.7 ± 7.8 | 23.6 ± 7.5 |
| Neutrophil count, mean ± SD (cells/cm3) | 61.7 ± 9.0 | 62.9 ± 8.5 | 64.1 ± 8.2 |
| Platelet count, mean ± SD (1000 cells/mm3) | 220.6 ± 55.7 | 220.8 ± 54.9 | 207.0 ± 57.5 |
| mtDNA lesionsa, mean ± SD | 1.9 ± 0.9 | 1.4 ± 0.9 | 1.8 ± 0.9 |
mtDNA lesions were measured per 10 kb.
3.2 Meteorological data
Table 2 describes summary statistics and Spearman correlations of meteorological variables. Twenty-four-hour mean air temperature was 13.0 °C ± 8.6 °C across the four seasons and was 9.8 °C ± 6.1 °C, 21.8 °C ± 4.5 °C, 13.5 °C ± 5.6 °C, and 1.2 °C ± 5.0 °C in spring, summer, fall, and winter, respectively. Minimum 24-hour mean temperature in the winter was − 13.9 °C, while maximum 24-hour mean temperature in the summer was 31.2 °C. We observed the highest 24-hour variability in air temperature in summer (median = 2.6 °C; interquartile range = 1.6 °C). Twenty-four-hour mean and 24-hour standard deviation of temperature showed moderate correlation in spring, summer, and fall (ρ = 0.36, 0.44, and 0.27, respectively).
Table 2.
Summary statistics and Spearman correlation of environmental variables.
| Summary statistics | Spearman correlation | |||
|---|---|---|---|---|
| Mean ± SD | Median (p25, p75) | 24-h TMEAN | 24-h TSD | |
| Overall (n = 1141) | ||||
| 24-h TMEAN (°C) | 13.0 ± 8.6 | 13.3 (6.7, 20.0) | 1.00 | 0.33** |
| 24-h TSD (°C) | 2.4 ± 1.2 | 2.2 (1.5, 3.1) | – | 1.00 |
| Spring (n = 281) | ||||
| 24-h TMEAN (°C) | 9.8 ± 6.1 | 10.2 (5.8, 13.1) | 1.00 | 0.36** |
| 24-h TSD (°C) | 2.4 ± 1.3 | 2.1 (1.5, 2.9) | – | 1.00 |
| Summer (n = 318) | ||||
| 24-h TMEAN (°C) | 21.8 ± 4.5 | 22.1 (19.0, 24.8) | 1.00 | 0.44** |
| 24-h TSD (°C) | 2.6 ± 1.2 | 2.6 (1.7, 3.3) | – | 1.00 |
| Fall (n = 365) | ||||
| 24-h TMEAN (°C) | 13.5 ± 5.6 | 13.7 (9.2, 17.7) | 1.00 | 0.27** |
| 24-h TSD (°C) | 2.3 ± 1.0 | 2.1 (1.4, 3.0) | – | 1.00 |
| Winter (n = 177) | ||||
| 24-h TMEAN (°C) | 1.2 ± 5.0 | 1.6 (−2.1, 4.0) | 1.00 | 0.02* |
| 24-h TSD (°C) | 2.2 ± 1.3 | 1.9 (1.4, 2.6) | – | 1.00 |
p < 0.05.
p < 0.01.
3.3 Air temperature and mtDNA lesions
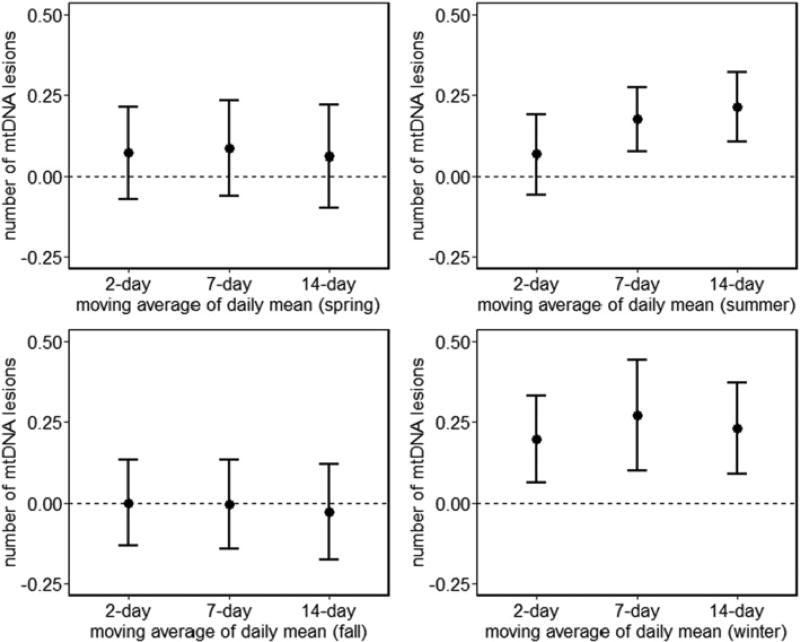
Short-term increases in mean air temperature were associated with increased mtDNA lesions in blood in summer. An IQR increase in 7-day (3.6 °C) and 14-day (3.8 °C) moving average of 24-hour mean air temperature was associated with a 0.17 (95% CI: 0.07, 0.27; p = 0.0007) and 0.21 (95% CI: 0.10, 0.32; p = 0.0001) increase in number of mtDNA lesions per 10 kb, respectively (Fig. 1). The estimates are slightly attenuated when we further adjusted for temperature variability of the corresponding moving average (Supplementary Fig. 1). In winter, we observed positive associations between increases in mean air temperature and mtDNA lesions both before (Fig. 1) and after adjusting for temperature variability (Supplementary Fig. 1).
Fig. 1.
Estimated change in mtDNA lesions per IQR increase of daily mean air temperature over 2-, 7-, or 14-day moving averages by season. Bars represent 95% CI. Results from linear mixed-effects regression models account for correlation across multiple visits and are adjusted for age, BMI, race, physical activity (low, < 12 h/week; medium, ≥ 12 and < 30 h/week; or high, ≥ 30 h/week), smoking status (current, former, or never), pack–years smoked, alcohol consumption, education level, platelet count, lymphocyte count, neutrophil count, and 24-h relative humidity.
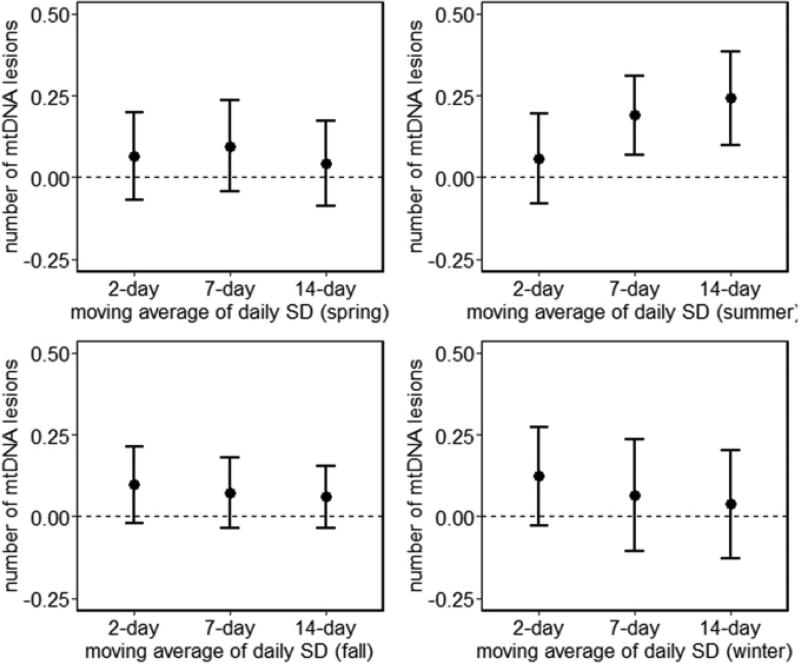
Short-term increases in temperature variability were associated with a higher number of mtDNA lesions in summer (Fig. 2). These associations were observed before and after adjustment for mean air temperature (Supplementary Fig. 2). For example, an IQR increase in 7-day (0.60 °C) and 14-day (0.58 °C) moving average of 24-hour temperature variability was associated with a 0.16 (95% CI: 0.04, 0.29; p = 0.0104) and 0.21 (95% CI: 0.06, 0.36; p = 0.0054) increase in number of mtDNA lesions per 10 kb, respectively.
Fig. 2.
Estimated change in mtDNA lesions per IQR increase of daily variability of air temperature over 2-, 7-, or 14-day moving averages by season. Bars represent 95% CI. Results from linear mixed-effects regression models account for correlation across multiple visits and are adjusted for age, BMI, race, physical activity (low, < 12 h/week; medium, ≥ 12 and < 30 h/week; or high, ≥ 30 h/week), smoking status (current, former, or never), pack–years smoked, alcohol consumption, education level, platelet count, lymphocyte count, neutrophil count, and 24-h relative humidity.
3.4 Sensitivity analysis
We used IPW to account for potential selection bias due to follow-up loss as a sensitivity analysis. Estimates were similar to those from the primary analysis (data not shown). We also observed similar results when we restricted the analysis to participants' first visit (Supplementary Table 3). In addition, we obtained temperature measurements from a spatial and temporal resolved model and compared results from modeling data to monitoring stations. Results were slightly attenuated when we obtained exposure measurements from the stationary monitoring station (Supplementary Fig. 3). For example, a 5 °C increase in 7-day moving average of 24-hour mean air temperature was associated with a 0.21 (95% CI: 0.07, 0.35) increase in number of mtDNA lesions per 10 kb in summer, based on stationary monitoring station data. A 5 °C increase in 7-day moving average of 24-hour mean air temperature was associated with a 0.31 (95% CI: 0.50, 0.57) increase in number of mtDNA lesions per 10 kb from the spatiotemporal modeling approach in summer. Adjusting for PM2.5 total mass concentration also did not seem to influence our results much (Supplementary Table 1). Further, we observed positively associations between mtDNA lesions with both systolic (ρ = 0.11) and diastolic (ρ = 0.18) blood pressure in the study population (Supplementary Table 2).
4 Discussion
In this analysis of the association between increases in air temperature and blood mtDNA lesions in a cohort of older individuals, we showed that a short-term increase in mean temperature in summer was associated with increased blood mitochondrial oxidative damage. Interestingly, we also observed positive associations between increases in mean air temperature and mtDNA lesions in winter. Results were similar when we further adjusted for within-day temperature variability. Moreover, we found significant associations between increased within-day temperature variability and mtDNA lesions after controlling for mean air temperature. To our knowledge, this is the first study describing increases in air temperature and mitochondrial genome damage.
The observed increase in mtDNA lesions in the summer makes sense biologically. In humans, core body temperature is usually tightly regulated and maintained within a narrow range (~ 37 °C). When external temperature increases, thermoreceptors under the skin sense such temperature changes and send information to the integrating center of the brain via the afferent neural pathway. The integrating center, located in the hypothalamus, senses temperature discrepancies between the external environment and core body temperature and sends signals via the efferent neural pathway to dissipate heat (Leon and Helwig, 2010).
There are both physiological and behavioral responses for heat dissipation. For example, increases in air temperature impose large demands on the heart and vasculature, resulting in blood shuttling away from core organs to the body's surface for heat dissipation (Leon and Helwig, 2010). Animal studies have shown that heat stress increases metabolic demand and reduces splanchnic blood flow, which in turn reduces visceral perfusion. The prolonged ischemic environment may result in generation of reactive oxygen and nitrogen species, which may lead to visceral and, in turn, systemic oxidative stress (Hall et al., 1999; Hall et al., 2001). Therefore, the observed increased mtDNA lesions in response to higher mean temperature during hot days may reflect systemic oxidative stress due to heat stress. Further, higher temperatures on hot days may increase basal metabolic rate, which, in turn, leads to increased mitochondrial activity. Because mitochondria are the primary site of endogenous ROS generation, increased mitochondrial activity on hot days may also contribute to mtDNA lesions.
Interestingly, we observed positive associations between increased mean air temperature and mtDNA lesions at low temperatures (~ − 10 °C–0 °C). Previous studies indicate J- or U-shaped relationships between changes in mean air temperature and mortality (Gasparrini et al., 2015). Epidemiological studies, however, report inconsistent associations between changes in mean air temperature and blood biomarkers. For instance, Hampel et al. (2010) and Schauble et al. (2012) report blood inflammatory and coagulation biomarkers increase with decreased air temperature in patients with cardiopulmonary and metabolic diseases. However, in individuals with stable heart failure, Wilker et al. (2012) show increased blood B-type natriuretic peptide and C-reactive protein levels with increased mean air temperature. Nevertheless, these studies focused on diseased populations, and individuals with chronic diseases, such as coronary heart disease (CHD) or diabetes, likely spend less time outdoors at extreme or sub-optimal temperatures, which may result in potential exposure misclassifications.
As part of our sensitivity analysis, excluding participants with chronic disease status (CHD and diabetes) resulted in slightly stronger associations between increases in mean air temperature and mtDNA lesions. For instance, an IQR increase in 7-day and 14-day moving average of 24-h mean temperature was associated with a 0.17 and 0.21 increase in mtDNA lesions, respectively. We observed positive correlations between mtDNA lesion (per 10 kb) and systolic and diastolic blood pressures (Supplementary Table 2). Since mitochondrial-related oxidative stress—as reflected by increased number of mtDNA lesions—potentially serve as a novel biomarker to reflect cardiovascular problems, the observed positive associations between mtDNA lesion and blood pressure make sense biologically. From the same cohort, Bind et al. found increased DNA methylation—which often silences gene expression—in several inflammatory and oxidative stress-related genes with decreased air temperature. Further, after environmental insults, DNA methylation in inflammatory genes changes rapidly to fine-tune inflammatory responses (Hou et al., 2014; Peng et al., 2016; Tarantini et al., 2009).
The observed positive associations between increases in mean air temperature and mtDNA lesions in cold weather may have resulted from decreased metabolic activity (as elderly people tend to stay indoor with little physical exercise), which in turn reduces mitochondrial activity. Mitochondria are the primary sites for cellular energy production as well as endogenous ROS generation (Murphy, 2009). Because ROS are the byproducts of ETC, where glucose is metabolized to cellular energy as adenosine triphosphate (ATP), a decrease in ROS generation due to reduced mitochondrial activity may be coupled with reduced ATP production. Hence, although we observed decreased blood mtDNA lesions with decreased mean air temperature, other cellular processes that are ATP-dependent—such as vascular control—may also be depressed (Crecelius et al., 2015), and impaired vascular control may be particularly problematic to elderly populations.
Alternatively, such positive associations between increased mean air temperature and mtDNA lesions in winter may be due to the “cold-weather paradox”: if outdoor temperatures get very cold, people (especially the elderly) tend to stay indoors and therefore have less exposure. With less cold winter temperatures, people tend to go outside more often and are thus more likely to be exposed to cold air.
Our study participants consist mainly of older adults (mean age = 73.1 years at first visit). In healthy young adults, increased temperatures may result in climate adaptation, which may involve enhanced cardiovascular performance, activation of the renin-angiotensin axis, increased capacity to secrete sweat, and expansion of plasma volume (Bouchama and Knochel, 2002; Leon and Helwig, 2010). However, older individuals are less adaptive to changes in air temperature due to impaired homeostatic control mechanisms, such as impaired baroreceptor reflex mechanism (Stauss et al., 1997), lower sweating rate (Inoue et al., 1999), and diminished renal sympathetic nerve activity (Kenney and Fels, 2002). As a result, increased air temperatures can disproportionally affect elderly individuals. Our study highlights the relevance of investigating temperature influences in such susceptible populations.
Our study has some limitations. In the primary analysis, we obtained air temperature data from a fixed monitoring station located approximately 12 km from the clinical site. This may introduce potential exposure misclassifications. However, in the sensitivity analysis, we estimated air temperature at each participant's residential address for each calendar day using a hybrid land-use regression and satellite-based model and examined the association between increases in mean air temperature and mtDNA lesions. Temperature estimates from our modeling approach introduced sufficient spatial and temporal variability and minimized exposure misclassifications, so we found similar results from stationary monitoring station estimates and modeling approaches. Further, although our exposure matrix used ambient air temperature, we have no indoor temperature measurements and no information about how much time each participant spent outdoors. Therefore, our study may suffer from potential exposure misclassifications, as elderly tend to stay indoors during hot and cold days. In addition, since our study is based in Boston, Massachusetts, the ambient air temperature range may not be generalizable to other climate zones.
However, our study also has a number of strengths. We applied a novel PCR technique to quantify mtDNA lesions, and our assay for analyzing mtDNA lesion yields high precision—the between-batch coefficient of variation was 10%. We used hourly temperature data from the National Weather Service Station at Boston Logan airport, which allowed us to examine short-term increases in both 24-hour mean and variability of air temperature with blood mtDNA lesions. Further, we accounted for potential selection bias due to follow-up loss using IPW. Point estimates were similar for models with or without IPW, indicating that little selection bias was introduced due to follow-up loss.
5 Conclusion
We observed increased mtDNA lesions in elderly adults exposed to short-term higher mean air temperatures, supporting the hypothesis that changes in meteorological conditions may induce molecular mitochondrial alterations in older individuals. The potential health consequences associated with increased mtDNA lesions must be further elucidated.
Supplementary Material
Highlights.
Short-term increases in mean ambient air temperature is associated with higher blood mitochondrial DNA (mtDNA) lesions in older individuals
Short-term increase in temperature variability is also associated with higher blood mitochondrial DNA (mtDNA) lesions independent of mean air temperature
Our study supports the hypothesis that sub-optimal meteorological conditions may induce pathophysiological responses among susceptible populations
Acknowledgments
The authors would like to thank all Normative Aging Study participants.
This work was supported by National Institutes of Health [R01ES021733, R01ES015172, R01ES025225, U34AG051918, P30ES009089, and T32ES007142]; U.S. Environmental Protection Agency [RD-83479801]; and the Department of Agriculture, Agricultural Research Service [contract 53-K06-510]. The VA Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center in Boston, Massachusetts. The views expressed in this paper are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs.
Abbreviations
- mtDNA
mitochondrial DNA
- ROS
reactive oxygen species
- ETC
electron transport chain
- QPRC
quantitative polymerase chain reaction
- IQR
interquartile range
- BMI
body mass index
- IPW
inverse probability of weighting
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- CHD
coronary heart disease
- ATP
adenosine triphosphate
Footnotes
Disclosures
None of the authors has any actual or potential competing financial interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.envint.2017.03.017.
References
- Alperovitch A, Lacombe JM, Hanon O, Dartigues JF, Ritchie K, Ducimetiere P, Tzourio C. Relationship between blood pressure and outdoor temperature in a large sample of elderly individuals: the Three-City study. Arch. Intern. Med. 2009;169(1):75–80. doi: 10.1001/archinternmed.2008.512. [DOI] [PubMed] [Google Scholar]
- Barnett AG, Sans S, Salomaa V, Kuulasmaa K, Dobson AJ, Project WM. The effect of temperature on systolic blood pressure. Blood Press. Monit. 2007;12(3):195–203. doi: 10.1097/MBP.0b013e3280b083f4. [DOI] [PubMed] [Google Scholar]
- Bind MA, Zanobetti A, Gasparrini A, Peters A, Coull B, Baccarelli A, Tarantini L, Koutrakis P, Vokonas P, Schwartz J. Effects of temperature and relative humidity on DNA methylation. Epidemiology. 2014;25(4):561–569. doi: 10.1097/EDE.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchama A, Knochel JP. Heat stroke. N. Engl. J. Med. 2002;346(25):1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- Braga AL, Zanobetti A, Schwartz J. The effect of weather on respiratory and cardiovascular deaths in 12 U.S. cities. Environ. Health Perspect. 2002;110(9):859–863. doi: 10.1289/ehp.02110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Debrinski M, Stepien G, Shoffner JM, Lott MT, Kanter K, Wallace DC. Hypoxemia is associated with mitochondrial DNA damage and gene induction. Implications for cardiac disease. JAMA. 1991;266(13):1812–1816. [PubMed] [Google Scholar]
- Corral-Debrinski M, Shoffner JM, Lott MT, Wallace DC. Association of mitochondrial DNA damage with aging and coronary atherosclerotic heart disease. Mutat. Res. 1992;275(3–6):169–180. doi: 10.1016/0921-8734(92)90021-g. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Dinenno FA. Intravascular ATP and the regulation of blood flow and oxygen delivery in humans. Exerc. Sport Sci. Rev. 2015;43(1):5–13. doi: 10.1249/JES.0000000000000031. [DOI] [PubMed] [Google Scholar]
- Demple B, Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu. Rev. Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000;18(6):655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505(7483):335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furda A, Santos JH, Meyer JN, Van Houten B. Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol. Biol. 2014;1105:419–437. doi: 10.1007/978-1-62703-739-6_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, Tobias A, Tong S, Rocklov J, Forsberg B, Leone M, De Sario M, Bell ML, Guo YL, Wu CF, Kan H, Yi SM, de Sousa Zanotti Stagliorio Coelho M, Saldiva PH, Honda Y, Kim H, Armstrong B. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet. 2015;386(9991):369–375. doi: 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DM, Baumgardner KR, Oberley TD, Gisolfi CV. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am. J. Phys. 1999;276(5 Pt 1):G1195–G1203. doi: 10.1152/ajpgi.1999.276.5.G1195. [DOI] [PubMed] [Google Scholar]
- Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 2001;280(2):H509–H521. doi: 10.1152/ajpheart.2001.280.2.H509. [DOI] [PubMed] [Google Scholar]
- Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Relationship between outdoor temperature and blood pressure. Occup. Environ. Med. 2011;68(4):296–301. doi: 10.1136/oem.2010.056507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel R, Breitner S, Ruckerl R, Frampton MW, Koenig W, Phipps RP, Wichmann HE, Peters A, Schneider A. Air temperature and inflammatory and coagulation responses in men with coronary or pulmonary disease during the winter season. Occup. Environ. Med. 2010;67(6):408–416. doi: 10.1136/oem.2009.048660. [DOI] [PubMed] [Google Scholar]
- Hou L, Zhang X, Zheng Y, Wang S, Dou C, Guo L, Byun HM, Motta V, McCracken J, Diaz A, Kang CM, Koutrakis P, Bertazzi PA, Li J, Schwartz J, Baccarelli AA. Altered methylation in tandem repeat element and elemental component levels in inhalable air particles. Environ. Mol. Mutagen. 2014;55(3):256–265. doi: 10.1002/em.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Shibasaki M, Ueda H, Ishizashi H. Mechanisms underlying the age-related decrement in the human sweating response. Eur. J. Appl. Physiol. Occup. Physiol. 1999;79(2):121–126. doi: 10.1007/s004210050485. [DOI] [PubMed] [Google Scholar]
- Keatinge WR, Coleshaw SR, Cotter F, Mattock M, Murphy M, Chelliah R. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. Br. Med. J. (Clin. Res. Ed) 1984;289(6456):1405–1408. doi: 10.1136/bmj.289.6456.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney MJ, Fels RJ. Sympathetic nerve regulation to heating is altered in senescent rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283(2):R513–R520. doi: 10.1152/ajpregu.00683.2001. [DOI] [PubMed] [Google Scholar]
- Kim Y, Joh S. A vulnerability study of the low-income elderly in the context of high temperature and mortality in Seoul. Korea, Sci. Total Environ. 2006;371(1–3):82–88. doi: 10.1016/j.scitotenv.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Kloog I, Chudnovsky A, Koutrakis P, Schwartz J. Temporal and spatial assessments of minimum air temperature using satellite surface temperature measurements in Massachusetts, USA. Sci. Total Environ. 2012;432:85–92. doi: 10.1016/j.scitotenv.2012.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Nordio F, Coull BA, Schwartz J. Predicting spatiotemporal mean air temperature using MODIS satellite surface temperature measurements across the Northeastern USA. Remote Sens. Environ. 2014;150:132–139. [Google Scholar]
- Koken PJ, Piver WT, Ye F, Elixhauser A, Olsen LM, Portier CJ. Temperature, air pollution, and hospitalization for cardiovascular diseases among elderly people in Denver. Environ. Health Perspect. 2003;111(10):1312–1317. doi: 10.1289/ehp.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J. Appl. Physiol. 1985;109(6):2010, 1980–1988. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia–reperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 2001;33(6):1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- Mehta AJ, Kloog I, Zanobetti A, Coull BA, Sparrow D, Vokonas P, Schwartz J. Associations between changes in city and address specific temperature and QT interval—the VA Normative Aging Study. PLoS One. 2014;9(9):e106258. doi: 10.1371/journal.pone.0106258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel-From J, Thinggaard M, Dalgard C, Kyvik KO, Christensen K, Christiansen L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum. Genet. 2014;133(9):1149–1159. doi: 10.1007/s00439-014-1458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MS, Ebi KL. Temperature extremes and health: impacts of climate variability and change in the United States. J. Occup. Environ. Med. 2009;51(1):13–25. doi: 10.1097/JOM.0b013e318173e122. [DOI] [PubMed] [Google Scholar]
- Peng C, Bind MC, Colicino E, Kloog I, Byun HM, Cantone L, Trevisi L, Zhong J, Brennan K, Dereix AE, Vokonas PS, Coull BA, Schwartz JD, Baccarelli AA. Particulate air pollution and fasting blood glucose in non-diabetic individuals: associations and epigenetic mediation in the Normative Aging Study, 2000–2011. Environ. Health Perspect. 2016;124(11):1715–1721. doi: 10.1289/EHP183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti M, Forrow SM, Souhami RL, D'Incalci M, Hartley JA. Measurement of the sequence specificity of covalent DNA modification by antineoplastic agents using Taq DNA polymerase. Nucleic Acids Res. 1991;19(11):2929–2933. doi: 10.1093/nar/19.11.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spiro A, 3rd, Schwartz J. Traffic-related air pollution and cognitive function in a cohort of older men. Environ. Health Perspect. 2011;119(5):682–687. doi: 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, O'Neill MS, Park SK, Sparrow D, Vokonas P, Schwartz J. Ambient temperature, air pollution, and heart rate variability in an aging population. Am. J. Epidemiol. 2011;173(9):1013–1021. doi: 10.1093/aje/kwq477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauble CL, Hampel R, Breitner S, Ruckerl R, Phipps R, Diaz-Sanchez D, Devlin RB, Carter JD, Soukup J, Silbajoris R, Dailey L, Koenig W, Cyrys J, Geruschkat U, Belcredi P, Kraus U, Peters A, Schneider AE. Short-term effects of air temperature on blood markers of coagulation and inflammation in potentially susceptible individuals. Occup. Environ. Med. 2012;69(9):670–678. doi: 10.1136/oemed-2011-100469. [DOI] [PubMed] [Google Scholar]
- Schneider A, Panagiotakos D, Picciotto S, Katsouyanni K, Lowel H, Jacquemin B, Lanki T, Stafoggia M, Bellander T, Koenig W, Peters A, Group AS. Air temperature and inflammatory responses in myocardial infarction survivors. Epidemiology. 2008;19(3):391–400. doi: 10.1097/EDE.0b013e31816a4325. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Samet JM, Patz JA. Hospital admissions for heart disease: the effects of temperature and humidity. Epidemiology. 2004;15(6):755–761. doi: 10.1097/01.ede.0000134875.15919.0f. [DOI] [PubMed] [Google Scholar]
- Stauss HM, Morgan DA, Anderson KE, Massett MP, Kregel KC. Modulation of baroreflex sensitivity and spectral power of blood pressure by heat stress and aging. Am. J. Phys. 1997;272(2 Pt 2):H776–H784. doi: 10.1152/ajpheart.1997.272.2.H776. [DOI] [PubMed] [Google Scholar]
- Tarantini L, Bonzini M, Apostoli P, Pegoraro V, Bollati V, Marinelli B, Cantone L, Rizzo G, Hou L, Schwartz J, Bertazzi PA, Baccarelli A. Effects of particulate matter on genomic DNA methylation content and iNOS promoter methylation. Environ. Health Perspect. 2009;117(2):217–222. doi: 10.1289/ehp.11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker EH, Yeh G, Wellenius GA, Davis RB, Phillips RS, Mittleman MA. Ambient temperature and biomarkers of heart failure: a repeated measures analysis. Environ. Health Perspect. 2012;120(8):1083–1087. doi: 10.1289/ehp.1104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Schneider A, Breitner S, von Klot S, Meisinger C, Cyrys J, Hymer H, Wichmann HE, Peters A Cooperative Health Research in the Region of Augsburg Study G. Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation. 2009;120(9):735–742. doi: 10.1161/CIRCULATIONAHA.108.815860. [DOI] [PubMed] [Google Scholar]
- Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 1997;94(2):514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yin P, Zhou M, Ou CQ, Li M, Li J, Liu X, Gao J, Liu Y, Qin R, Xu L, Huang C, Liu Q. The burden of stroke mortality attributable to cold and hot ambient temperatures: epidemiological evidence from China. Environ. Int. 2016;92–93:232–238. doi: 10.1016/j.envint.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Zastawny TH, Dabrowska M, Jaskolski T, Klimarczyk M, Kulinski L, Koszela A, Szczesniewicz M, Sliwinska M, Witkowski P, Olinski R. Comparison of oxidative base damage in mitochondrial and nuclear DNA. Free Radic. Biol. Med. 1998;24(5):722–725. doi: 10.1016/s0891-5849(97)00331-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.