Abstract
Actinic keratosis (AK) is a clinical condition characterized by keratinocytic dysplastic lesions of the epidermis, affecting individuals chronically exposed to sunlight. Topical therapies allow the treatment of a whole area of affected skin and currently include diclofenac sodium gel, 5-fluorouracil cream, 5-fluorouracil and acetylsalicylic acid solution, imiquimod cream, and ingenol mebutate gel. Due to the comparable efficacy of 3% diclofenac, ingenol mebutate, and 3.75% imiquimod in treating AK multiple lesions, a pharmacoeconomic evaluation of cost-effectiveness of the three treatments was needed. A cost-efficacy analysis comparing 3% diclofenac sodium with ingenol mebutate and 3.75% imiquimod was performed. In this analysis, efficacy data were combined with quality-of-life measurement derived from previous studies as well as the costs associated with the management of these lesions in Italy. Patients’ demographics and clinical characteristics were assumed to reflect those from the clinical studies considered.
Keywords: actinic keratosis, dermatology, diclofenac gel, pharmacoeconomy
Introduction
Actinic keratosis (AK) is a clinical condition characterized by keratinocytic dysplastic lesions of the epidermis, affecting individuals chronically exposed to sunlight, particularly with phototypes I–II according to the Fitzpatrick scale. Thanks to the many studies on AK’s natural history, it is evident that up to 10% of lesions can degenerate in invasive squamous cell carcinoma (SCC), with the risk increasing over time.1 Subclinical lesions of the photo-damaged area may degenerate as well in SCC according to the concept of “field cancerization.”2
AK is primarily treated to prevent progression to SCC, for cosmetic reasons, and to eliminate symptoms such as itching and pain. With an approach focused on the photo-damaged skin instead of single lesions, topical therapies have the advantage, over surgical or ablative therapies, of treating subclinical lesions as well, with recent evidence showing the latter also have the ability to degenerate into SCC.2
The mechanism of action of diclofenac sodium is complex, with evidence showing that arachidonic acid metabolites are involved in the response of keratinocytes to ultra violet (UV) exposure and skin irritation and that the overactivation of cyclooxygenase enzymes (especially COX-2) is carcinogenic.3 The action of diclofenac also appears to induce neoplastic cell apoptosis, downregulation of angiogenesis,4 and the activation of peroxisome proliferator-activated receptor (PPAR)-gamma receptors which reduce the proliferation of neoplastic cells.5
Ingenol mebutate, another common topical treatment for AK derived from the plant Euphorbia peplus, induces necrosis of tumoral cells through immune system activation with a dual mechanism of action: at first lesion necrosis mainly through a proinflammatory cytokines cascade and then by neutrophil activation and antibody production.6
Imiquimod, a synthetic compound member of the imidazoquinolone family of drugs, is an immune modulator, acting via its binding to Toll-like Receptor 7 present on dendritic cells, macrophages, and monocytes, with a subsequent mounting immune response and the induction of Fas receptor on tumor cells.7
Due to the comparable efficacy of 3% diclofenac, ingenol mebutate, and 3.75% imiquimod in treating AK multiple lesions, a pharmacoeconomic evaluation of cost-effectiveness of the three treatments was needed.
A cost-efficacy analysis comparing 3% diclofenac sodium with ingenol mebutate and 3.75% imiquimod was performed. In this analysis, efficacy data were combined with quality-of-life measurement derived from previous studies as well as the costs associated with the management of these lesions in Italy. Patients’ demographics and clinical characteristics were assumed to reflect those from the clinical studies considered.
Materials and methods
The following treatments have been considered in the analysis:
Diclofenac 3% gel in 2.5% hyaluronic acid (Solaraze®);
Ingenol mebutate gel 150 μg/g (Picato®);
Imiquimod 3.75% cream (Zyclara®).
A decisional tree model is presented based on the results of clinical studies of the three treatments and the costs in the Italian National health care service. The model takes into account a period of 1 year, evaluating treatment results including relapses, based on the availability of data in the published trials on topical treatments for AK.
The model uses the clinical results of these comparable treatments and the data obtained from the literature to estimate the quality-adjusted life years (QALY), expressing the results as incremental cost for QALY gained with 3% diclofenac, ingenol mebutate, and 3.75% imiquimod.
This analysis is carried out from the prospect of the Italian National Health Care Service (the prospect of the public payer), including only the health costs. For all the cited studies, patients provided written informed consent. This study was approved by our Institution Ethics Committee.
Parameters of the model
To identify the efficacy and recurrence rates of the three treatments, a review of the literature was carried out. Various clinical trials which describe the efficacy of diclofenac sodium,8–10 of ingenol mebutate,6 and of imiquimod11 were found.
Data of efficacy in terms of complete response and of recurrence for 3% diclofenac were taken from a phase-4, single-arm, multicenter, open-label study;8,9 data for ingenol mebutate were obtained from four multicenter, randomized, double-blind studies6 and data for imiquimod were obtained from one phase-III randomized, multicenter, placebo-controlled study11 and are shown in Table 1.
Table 1.
Percentages of complete cure, adverse effects during treatment, and recurrence rates for the three treatments.
| 3% Diclofenac | Ingenol mebutate | 3.75% Imiquimod | |
|---|---|---|---|
| Probability of adverse effects | 5% | 2.2% | 3.6% |
| Probability of a complete cure | 58% | 42.2% | 35.6% |
| Probability of recurrence at 12 months | 21% | 12.8% | 20.9% |
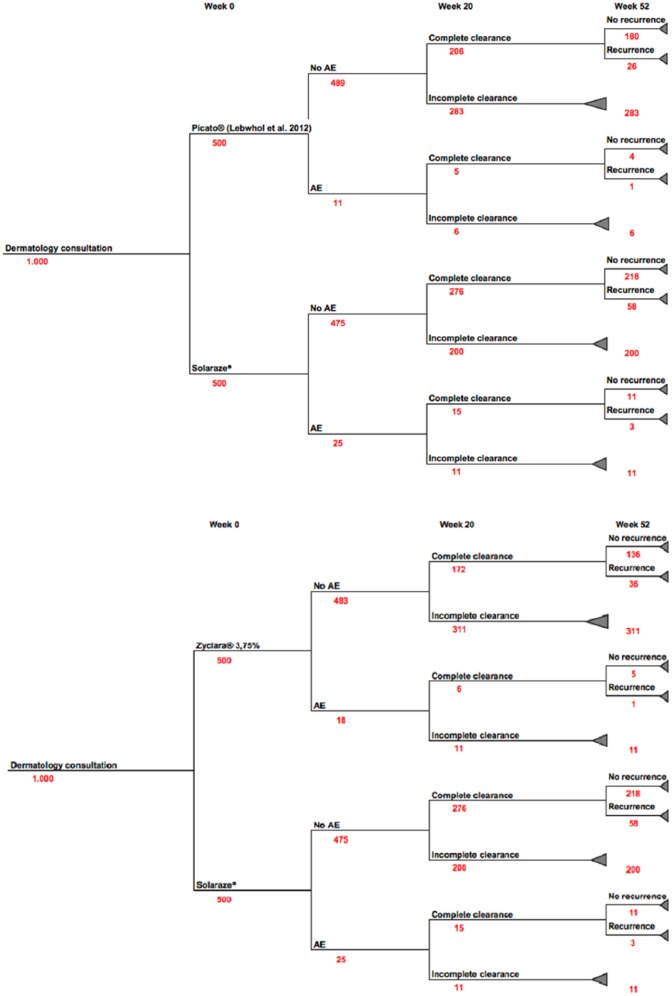
Figure 1 summarizes the complete response rate, the probability of adverse effects, and the probability of partial clearance of the lesions for the three treatments.
Figure 1.
Structure of the model, describing the main clinical results associated with the comparative medicinal products at 20 and 52 weeks.
In order to convert the complete cure rates and the incidence of adverse effects for each QALY, the following utility values obtained from a recently published cost-utility analysis were applied.12
Data on the costs
Costs included in the model for each therapeutic option include the drug treatment and the medical visits (dermatologist or general practitioner), necessary for its administration both in the initial management of lesions and in the consequent recurrences, assuming for both treatments one initial visit to a dermatologist and one follow-up visit.
Concerning the cost of a dermatological visit, the Health Ministry reference document “Nomenclatore tariffario dell’assistenza specialistica ambulatoriale,”13 published on the Ministry website, updated 8 April 2013 does not specifically mention the cost of a dermatologist consult; for this reason, a general visit cost of €20.66, the same price for other specialist visits, is reported. In the sensitivity analysis, the cost of a private dermatological visit of €70–85, as reported in the price list of Regione Veneto (the most updated available online), was considered. Though in the analysis, no cost is associated with the onset of adverse events for the baseline analysis, as it was considered that they do not involve the use of resources specific for the treatments, the sensitivity analysis includes the cost of a medical visit in case of adverse effects.
Sensitivity analysis
In order to evaluate the influence of the assumptions of the analysis and the variability of the data employed, various univariate sensitivity analyses were carried out to assess the influence on results of the relevant parameters employed. The probability of a complete response, the adverse effects rate, and the risk of recurrence were the variables considered. For most parameters, a ±50% variation was applied, which was considered to be significant and capable of reflecting large changes.
Results
The costs and health results of diclofenac sodium compared to ingenol mebutate and imiquimod in the treatment of AK were assessed for a period of 52 weeks. The number of patients for each treatment arm was set to n = 500. The model considered a comparison between 500 patients treated with diclofenac sodium, 500 patients with ingenol mebutate, and 500 patients with imiquimod, as shown in Figure 1. The results of the incremental cost-efficacy relationship in the base scenario are shown in Table 2.
Table 2.
Costs and QALY gained for treatment with 3% diclofenac, ingenol mebutate, and 3.75% imiquimod for the treatment arm (n = 500) and for the single patient.
| 3% Diclofenac | Ingenol mebutate | 3.75% Imiquimod | |
|---|---|---|---|
| Total cost of the treatment arm (n = 500) | €49.492 | €69.597 | €70.426 |
| QALY gained for the treatment arm (n = 500) | 478 | 470 | 467 |
| Cost per patient | €99.0 | €139.2 | €140.9 |
| QALY gained per patient | 0.956 | 0.939 | 0.934 |
QALY: quality-adjusted life years.
In case of recurrence, the cost of a new visit to a dermatologist and the cost of the second cycle of treatment were added to the total cost. During the 52 weeks of treatment, patients in the diclofenac sodium study arm reached a total cost of €49.492, including the cost of treatment and the direct cost of a first consultation with a dermatologist. For patients who received ingenol mebutate, the total cost was €69.597. For patients in the 3.75% imiquimod arm, the total cost was €70.426. Treatment cost for each patient was €99.0 for 3% diclofenac, €139.2 for ingenol mebutate, and €140.9 for 3.75% imiquimod.
Patients in the 3% diclofenac treatment arm had a total gain of 478 QALY, those in the ingenol mebutate arm had a gain of 470 QALY, and the 3.75% imiquimod arm had a gain of 467 QALY. When these results are calculated as QALY gained per patient, it translates into 0.956 QALY per patient treated with diclofenac sodium, 0.939 for subjects treated with ingenol mebutate, and 0.934 for subjects treated with imiquimod.
These small differences of QALY per patient can be considered to be clinically non-significant. Considering the results of QALY as equivalent, we could interpret the analysis as a cost minimization analysis.
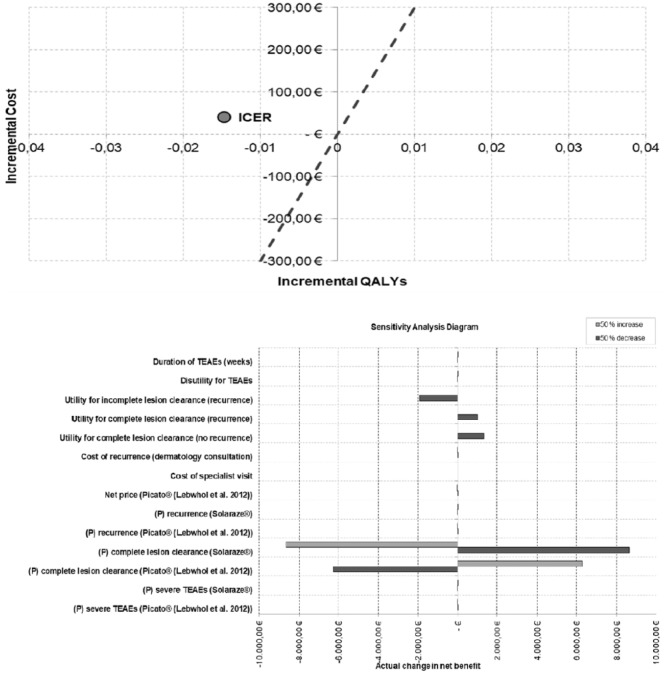
This translates into a higher cost per patient of €40.2 for ingenol mebutate and of €41.9 for imiquimod, in order to obtain a benefit equivalent to that achieved with diclofenac sodium. This is shown in the cost-efficacy plan of Figure 2.
Figure 2.
Cost-efficacy plan and bar diagram.
To consider for the uncertainty in different scenarios of the entry parameters of an economic model, a sensitivity analysis is useful to evaluate the impact of the variations in the baseline parameters on the results.
For this model, a univariate sensitivity analysis was performed by modifying the parameters listed in Table 3. All the results of the sensitivity analyses are shown with the profit net result.
Table 3.
Parameters changed in the sensitivity analysis and changes on net cash benefit.
| Base case (net
benefit) |
−€482 |
Min |
Max |
||||
|---|---|---|---|---|---|---|---|
| Parameter | Base value | Value (–50%) | Net benefit | Actual change | Value (+50%) | Net benefit | Actual change |
| Probabilities | |||||||
| (P) severe TEAEs (Picato® (Lebwohl et al.6)) | 0.022 | –50% | –€480 | €2 | +50% | –€485 | –€2 |
| (P) severe TEAEs (Solaraze®) | 0.050 | –50% | –€487 | –€5 | +50% | –€478 | €5 |
| (P) complete lesion clearance (Picato® (Lebwohl et al.6)) | 0.422 | –50% | –€6.771 | –€6.288 | +50% | €5.806 | €6.288 |
| (P) complete lesion clearance (Solaraze®) | 0.580 | –50% | €8.161 | €8.643 | +50% | –€9.126 | –€8.643 |
| (P) recurrence (Picato® (Lebwohl et al.6)) | 0.128 | –50% | –€477 | €6 | +50% | –€488 | –€6 |
| (P) recurrence (Solaraze®) | 0.210 | –50% | –€494 | –€12 | +50% | –€471 | €12 |
| Costs | |||||||
| Net price (Picato® (Lebwohl et al.6)) | €65 | –50% | –€450 | €32 | +50% | –€515 | –€32 |
| Cost of specialist visit | €21 | 15.00 | –€482 | –€ | 85.00 | –€482 | –€ |
| Cost of recurrence (dermatology consultation) | €21 | 15.00 | –€482 | €1 | 85.00 | –€488 | –€6 |
| Utilities | |||||||
| Utility for complete lesion clearance (no recurrence) | 0.997 | –50% | €866 | €1.349 | N/A | N/A | N/A |
| Utility for complete lesion clearance (recurrence) | 0.993 | –50% | €527 | €1.010 | N/A | N/A | N/A |
| Utility for incomplete lesion clearance (recurrence) | 0.901 | –50% | –€2.416 | –€1.934 | N/A | N/A | N/A |
| Disutility for TEAEs | 0.085 | –50% | –€485 | –€3 | +50% | –€480 | €3 |
| Duration of TEAEs (weeks) | 4 | –50% | –€485 | –€3 | +50% | –€480 | €3 |
| Base case (net
benefit) |
–€680 |
Min |
Max |
||||
| Parameter | Base value | Value (–50%) | Net benefit | Actual change | Value (+50%) | Net benefit | Actual change |
| Probabilities | |||||||
| (P) severe TEAEs (Zyclara®, 3.75%) | 0.035 | –50% | –€677 | €3 | +50% | –€683 | –€3 |
| (P) severe TEAEs (Solaraze®) | 0.050 | –50% | –€685 | –€5 | +50% | –€675 | €5 |
| (P) complete lesion clearance (Zyclara®, 3.75%) | 0.356 | –50% | –€5.982 | –€5.302 | +50% | €4.622 | €5.302 |
| (P) complete lesion clearance (Solaraze®) | 0.580 | –50% | €7.963 | €8.643 | +50% | –€9.323 | –€8.643 |
| (P) recurrence (Zyclara®, 3.75%) | 0.210 | –50% | –€672 | €8 | +50% | –€688 | –€8 |
| (P) recurrence (Solaraze®) | 0.210 | –50% | –€692 | –€12 | +50% | –€668 | €12 |
| Costs | |||||||
| Net price (Zyclara®, 3.75%) | €61 | –50% | –€649 | €31 | +50% | –€711 | –€31 |
| Cost of specialist visit | €21 | 15.00 | –€680 | –€0 | 85.00 | –€680 | –€0 |
| Cost of recurrence (dermatology consultation) | €21 | 15.00 | –€679 | €1 | 85.00 | –€691 | –€11 |
| Utilities | |||||||
| Utility for complete lesion clearance (no recurrence) | 0.997 | –50% | €1.965 | €2.645 | N/A | N/A | N/A |
| Utility for complete lesion clearance (recurrence) | 0.993 | –50% | €21 | €701 | N/A | N/A | N/A |
| Utility for incomplete lesion clearance (recurrence) | 0.901 | –50% | –€3.422 | –€2.742 | N/A | N/A | N/A |
| Disutility for TEAEs | 0.085 | –50% | –€682 | –€1 | +50% | –€679 | €1 |
| Duration of TEAEs (weeks) | 4 | –50% | –€682 | –€1 | +50% | –€679 | €1 |
TEAEs: treatment-emergent adverse events.
A positive value shows that the health benefit overcomes the additional costs, whereas a negative value implies that the costs are higher than the health benefit. To show a sensitivity example about the key parameters of the economic model, the bar diagram (Figure 2) evidences the variation of the net cash benefit at the variation of the tested parameters (mostly at ±25%). The probability of a total clearance of the lesion for diclofenac sodium, ingenol mebutate, and imiquimod as well as the profit changes appeared to be less influencing factors.
Discussion
As treatment possibilities for AK are expanding in recent years, the choice of a treatment regimen must take into account patient preference, with respect to the therapy schedule, tolerance of side effects, and the costs of treatment. Other critical aspects are the features of AK lesions such as distribution, number, and thickness, as well as the patient’s past history of treatment and recurrences.
Various clinical trials have shown the efficacy and safety of 3% diclofenac in both immunocompetent and immunosuppressed patients,8,10 ingenol mebutate,6 and of 3.75% imiquimod.11
One important factor to consider is the duration of treatment, with a short course of therapy that may reduce the burden of treatment and increase patients’ adherence. Ingenol mebutate in this regard is the most convenient treatment, allowing 2–3 days application.6
Our pharmacoeconomic model demonstrated that in the Italian health care service, with comparable efficacy in terms of complete remission and QALY gained, diclofenac sodium is as effective and less expensive than either ingenol mebutate or imiquimod.12
Data of efficacy, at the base of this analysis, were taken from multicenter phase-III clinical trials, felt by the authors to approximate the actual real practice effectiveness of the three treatment arms. The study has a few limitations, the most important of which concerns the quality of the data entered into the model. The efficacy parameters, for example, are based on studies with a limited time frame and may therefore be inadequate for modeling treatment of a disease for a longer period.
A further limitation could be consistency in duration of reported adherence with 3% diclofenac sodium, ingenol mebutate, and 3.75% imiquimod. Indeed, a decrease in patient adherence has been reported with any topical therapy over time.14
Inclusion of further factors relating to decreased adherence would have penalized all formulations, with consequent underestimation of the adherence effect from a pharmacoeconomic standpoint, and reduced clarity concerning the potential role of the treatments in improving patient compliance with therapy in clinical practice. Poor adherence inevitably leads to a poor treatment outcome. Prospective studies are needed to demonstrate the impact of interventions designed to enhance adherence and the effect of improved adherence on treatment outcomes.15
The inferior cost associated with diclofenac treatment should be an important consideration to take into account, considering the mildness of adverse effects for many topical treatments, the relapsing nature of AK and the long life expectancy of many AK patients.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Werner RN, Stockfleth E, Connolly SM, et al. (2015) Evidence- and consensus-based (S3) guidelines for the treatment of actinic keratosis: International League of Dermatological Societies in cooperation with the European Dermatology Forum: Short version. Journal of the European Academy of Dermatology and Venereology 29(11): 2069–2079. [DOI] [PubMed] [Google Scholar]
- 2. Stockfleth E, Ortonne JP, Alomar A. (2011) Actinic keratosis and field cancerisation. European Journal of Dermatology 21(Suppl. 1): 3–12.21628133 [Google Scholar]
- 3. Buckman SY, Gresham A, Hale P, et al. (1998) COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis 19: 723–729. [DOI] [PubMed] [Google Scholar]
- 4. Fecker LF, Stockfleth E, Braun FK, et al. (2010) Enhanced death ligand-induced apoptosis in cutaneous SCC cells by treatment with diclofenac/hyaluronic acid correlates with downregulation of c-FLIP. Journal of Investigative Dermatology 130(8): 2098–2109. [DOI] [PubMed] [Google Scholar]
- 5. Stockfleth E, Ferrandiz C, Grob JJ, et al. (2008) Development of a treatment algorithm for actinic keratoses: A European consensus. European Journal of Dermatology 18(6): 651–659. [DOI] [PubMed] [Google Scholar]
- 6. Lebwohl M, Swanson N, Anderson LL, et al. (2012) Ingenol mebutate gel for actinic keratosis. New England Journal of Medicine 366(11): 1010–1019. [DOI] [PubMed] [Google Scholar]
- 7. Navi D, Huntley A. (2004) Imiquimod 5 percent cream and the treatment of cutaneous malignancy. Dermatology Online Journal 10(1): 4. [PubMed] [Google Scholar]
- 8. Nelson C, Rigel D, Smith S, et al. (2004) Phase IV, open-label assessment of the treatment of actinic keratosis with 3.0% diclofenac sodium topical gel (solaraze). Journal of Drugs in Dermatology 3(4): 401–407. [PubMed] [Google Scholar]
- 9. Nelson C, Rigel D. (2009) Long-term follow up of diclofenac sodium 3% in 2.5% hyaluronic acid gel for actinic keratosis: One-year evaluation. Journal of Clinical and Aesthetic Dermatology 2(7): 20–25. [PMC free article] [PubMed] [Google Scholar]
- 10. Ulrich C, Johannsen A, Röwert-Huber J, et al. (2010) Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. European Journal of Dermatology 20(4): 482–488. [DOI] [PubMed] [Google Scholar]
- 11. Swanson N, Smith CC, Kaur M, et al. (2014) Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: Two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. Journal of Drugs in Dermatology 13(2): 166–169. [PubMed] [Google Scholar]
- 12. Wilson EC. (2010) Cost effectiveness of imiquimod 5% cream compared with methyl aminolevulinate-based photodynamic therapy in the treatment of non-hyperkeratotic, non-hypertrophic actinic (solar) keratoses: A decision tree model; PharmacoEconomics 28(11): 1055–1064. [DOI] [PubMed] [Google Scholar]
- 13. Nomenclatore tariffario dell’assistenza specialistica ambulatoriale, 8 April 2013. Available at: http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=1767&area=programmazioneSanitariaLea&menu=lea
- 14. Storm A, Benfeldt E, Andersen SE, et al. (2008) A prospective study of patient adherence to topical treatments: 95% of patients underdose. Journal of the American Academy of Dermatology 59: 975–980. [DOI] [PubMed] [Google Scholar]
- 15. Colombo GL, Di Matteo S, Bruno G, et al. (2012) Calcipotriol and betamethasone dipropionate in the treatment of mild-to-moderate psoriasis: A cost-effectiveness analysis of the ointment versus gel formulation. ClinicEconomics and Outcomes Research 4: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]