Abstract
Microencapsulation of mesenchymal stem cells (MSC) in alginate facilitates cell delivery, localization and survival, and modulates inflammation in vivo. However, we found that delivery of the widely used ~0.5 mm diameter encapsulated MSC (eMSC) by intrathecal injection into spinal cord injury (SCI) rats was highly variable. Injections of smaller (~0.2 mm) diameter eMSC into the lumbar spine were much more reproducible and they increased the anti-inflammatory macrophage response around the SCI site. We now report that injection of small eMSC >2 cm caudal from the rat SCI improved locomotion and myelin preservation 8 weeks after rat SCI versus control injections. Because preparation of sufficient quantities of small eMSC for larger studies was not feasible and injection of the large eMSC is problematic, we have developed a procedure to prepare medium-sized eMSC (~0.35 mm diameter) that can be delivered more reproducibly into the lumbar rat spine. The number of MSC incorporated/capsule in the medium sized capsules was ~5-fold greater than that in small capsules and the total yield of eMSC was ~20-fold higher than that for the small capsules. Assays with all three sizes of eMSC capsules showed that they inhibited TNF-α secretion from activated macrophages in co-cultures, suggesting no major difference in their anti-inflammatory activity in vitro. The in vivo activity of the medium-sized eMSC was tested after injecting them into the lumbar spine 1 day after SCI. Histological analyses 1 week later showed that eMSC reduced levels of activated macrophages measured by IB4 staining and increased white matter sparing in similar regions adjacent to the SCI site. The combined results indicate that ~0.35 mm diameter eMSC reduced macrophage inflammation in regions where white matter was preserved during critical early phases after SCI. These techniques enable preparation of eMSC in sufficient quantities to perform pre-clinical SCI studies with much larger numbers of subjects that will provide functional analyses of several critical parameters in rodent models for CNS inflammatory injury.
Keywords: Microcapsules, alginate, mesenchymal stem cells, intrathecal, inflammation
1. Introduction
Polymerized alginate is biocompatible and has been approved for clinical treatments.1,2 Alginate micro-spheres of various sizes have been prepared with encapsulated cells for several applications including to control cell differentiation within the capsules3 and as a source for secretion of desired factors.4–6 Encapsulation involves extrusion of a mixture of alginate with cells into microdroplets that become cross-linked into microspheres after they fall into a bath containing divalent cations. Capsule sizes typically range from as small as ~0.1 mm, containing only a few cells, up to ~0.5 mm to 1 mm, containing hundreds to thousands of cells, but perfusion of factors throughout larger capsules may be limited by diffusion.4 Encapsulation promotes cell survival and protects cells from immune rejection in vivo while allowing secreted factors to be released from the capsules. The capsules are useful for delivering factors locally when they can be delivered to specific sites including in the brain, spinal cord, eye, cochlea, bone, kidney capsule, pancreas and heart.4,5
A major use of cells encapsulated in microspheres is to release growth factors, cytokines and small proteins into the surrounding milieu. Genetically engineered cell lines over-expressing neuroprotective factors including ciliary neurotrophic factor (CNTF), glial cell line-derived neurotrophic factor (GDNF) and fibroblast growth factor (FGF) have been encapsulated in alginate and implanted into the central nervous system (CNS) to yield improvement in several models of neurodegenerative disorders.4,6,7 Different types of cells [(e.g., fibroblasts, Schwann cells and mesenchymal stem cells (MSC)] and cell lines [baby hamster kidney cells (BHK) and myoblasts] have been transfected to release various factors from capsules that are bio-active.5 For example, eMSC transfected to release GLP-1 were found to be neuroprotective but protection was also reported for the eMSC alone.8
While only a few studies have used eMSC without transfection of exogenous proteins, many studies have demonstrated that migratory MSC improve recovery in wide range of injuries and disorders both in animal models and, in a few cases, in the clinic.9–12 There is convincing evidence in vitro that MSC act to a great extent via their secreted anti-inflammatory and growth factors but other possible mechanisms of action cannot be ruled out when the cells are injected in vivo.9,11,13 Effects using encapsulated cells in vivo can be attributed to their secreted factors because the cells are segregated from the host by the capsule.5,8 Encapsulation of MSC in alginate has been found to upregulate the expression of many cytokines dramatically and many of the secreted factors are anti-inflammatory both in vitro and in vivo.14
Proinflammatory cytokines play a critical role in expanding damage after CNS trauma and treatment with MSC can control inflammation and improve survival of neural tissue and functional outcomes.15,16 Spinal cord contusion in the rat is a widely used model to study molecular processes of secondary damage over time caused by inflammatory and other factors, and mediated by inflammatory cells including macrophages.17 Beneficial effects of MSC in spinal cord injury (SCI) have resulted following various injection methods but the fate of the MSC is unclear making it difficult to study their mechanisms of action. Studies with eMSC are not complicated by direct interactions of the cells with the host and therefore reveal actions only of MSC secreted factors.
Functional improvement with encapsulated cells in the CNS has been observed when the capsules are injected into or adjacent to injury sites, which can accommodate large capsules but this requires invasive surgical procedures and exposes the capsules and cells to an inflammatory and cytotoxic environment.5,7,18 This may be avoided by placing the capsules at a location distant from the site of inflammation as long as the factors secreted by the cells gain access to the site of inflammation. We have injected small (~0.2 mm) eMSC intrathecally into the rat lumbar spine (>2 cm from SCI site), which had anti-inflammatory effects after SCI.14 A major limitation in the use of ~0.2 mm alginate capsules is the small number of live MSC that could be encapsulated. To overcome this limitation and thereby facilitate more extensive quantitative studies, we report here a method that yields much higher numbers of cells in intermediate-sized micro-spheres, which when injected intrathecally, reduced pro-inflammatory macrophages and preserved white matter tissue after SCI.
2. Materials and Methods
2.1. MSC culture
Human bone marrow-derived mesenchymal stem/stromal cells (MSC) were purchased from Texas A&M at passage 1 and cultured, as previously described,14 in minimum essential medium (MEM)-α (Gibco Life Technologies, USA) medium containing no deoxy- or ribonucleosides, and supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals, USA), 1 ng/mL basic fibroblast growth factor (bFGF), 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco). MSC were plated at 5000 cells/cm2 and allowed to proliferate to ~70% confluence. Only low passages (2–4) were used for experiments.
2.2. Alginate microencapsulation
Microencapsulation of MSC was performed as described.14,19 Briefly, MSC were detached using trypsin–EDTA (Gibco), washed and mixed with 2.2% (w/v) alginate (Sigma Aldrich, USA) solution to obtain an initial cell density of 4–6×106 cells/mL. An electrostatic bead generator was used to form microdroplets, which were subsequently cross-linked in a CaCl2 bath. Microcapsules were washed with phosphate buffered saline (PBS, Gibco) and briefly incubated with 0.05% (w/v) poly-L-Lysine (Sigma Aldrich, USA) in PBS. Encapsulated cells were re-suspended in cell culture medium and transferred to 25 cm2 tissue culture flasks. MSC viability in the capsules was assessed using a calcein and ethidium homodimer assay (Molecular Probes, USA).19 Capsules were imaged in an inverted fluorescent microscope (IX81, Olympus, Tokyo, Japan) and diameters were measured using SlideBook image analysis software version 5.0 (Intelligent Imaging Innovations, USA). Large- (n = 5) and medium- (n = 4) sized capsules were prepared using 0.17 mm inner bore extrusion needles, which yielded a total of ~106 live cells in a single preparation, and 0.12 mm inner bore needles were used for the small capsules (n = 6), which yielded ~50, 000 cells/preparation (Table 1). Encapsulations were performed within 1 h to maximize cell viability, which decreased at longer times of exposure to unpolymerized alginate, and therefore the low flow rate (0.5 mL/h) with the narrower 0.12 mm needles yielded much fewer eMSC than with the larger 0.17 mm needles, which had a much higher flow rate of 20 mL/h. The percent encapsulated/input is the ratio of the total number of cells encapsulated divided by the total cell input in the alginate mixture. To reduce variability among preparations of small eMSC, three preparations were pooled, the average numbers of live cells/capsule were measured, and aliquots from the pool were used for 2–3 replicates. Individual preparations of large and medium sized capsules yielded sufficient quantities for many replicates. Ejection efficiency of eMSC through 23 gauge thin walled needles (0.52 mm inner diameter, NIPRO) was calculated as the number of ejected capsules recovered divided by (the average number of capsules/mL) × (the volume ejected in mL).
Table 1.
Analysis of MSC encapsulation parameters.
| MSC capsules | Diameter (μm) +/− Std. | Viability (%) | Live cells/capsule | Total live cells encapsulated | Encapsulated/input (%) |
|---|---|---|---|---|---|
| Large | 450 ± 65 | 88 ± 7 | 124 ± 43 | ~106 | ~25 |
| Medium | 378 ± 29 | 83 ± 7 | 73 ± 15 | ~106 | ~25 |
| Small | 214 ± 27 | 83 ± 13 | 14 ± 5 | ~50,000 | ~1.25 |
2.3. Co-culture of macrophages with eMSC
Macrophages were isolated and co-culture assays were performed as described20 except with adaptations made for the use of capsules. Briefly, peripheral blood mononuclear cells were collected from blood of healthy donors (Blood Center of New Jersey) after centrifugation in Ficoll density gradients (GE Healthcare, USA). Monocytes were isolated by magnetic cell sorting using anti-CD14 coated beads (Miltenyi Biotec, USA), and CD14+ monocytes were cultured in 175 cm2 flasks (BD Biosciences, USA) at 107 cells/flask in Advanced RPMI 1640 medium (Gibco) supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA), 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mM L-glutamine (Gibco). Macrophages were incubated with 5 ng/mL GM-CSF (R&D Systems, USA) for 7 days and replated at 1×104 cells/mL or 5×104 cells/mL in 96 or 24-well plates (Corning, USA), respectively, and allowed to attach overnight. To activate the macrophages, the medium was replaced with medium containing 1 μg/mL lipopolysaccharide (LPS) (Sigma-Aldrich). Trans-well inserts containing small, medium or large eMSC in medium with 1 μg/mL LPS were then added to the wells in a 1:1 macrophage:MSC ratio. Wells containing only macrophages and medium with or without LPS were included as positive and negative controls, respectively. After 48 h, co-culture supernatants were collected, stored at 20°C and then analyzed for secreted tumor necrosis factor (TNF)-α by ELISA14 (Biolegend, USA).
2.4. SCI and transplantation
Adult female Sprague-Dawley rats (77 ± 2 days old, Taconic, USA) were used for all experiments with protocols approved by the Animal Care and Use Committee of Rutgers, The State University of New Jersey. For SCI surgery, rats were anesthetized with 2% isoflurane (IsoFlo; Abbott Laboratories, USA), the spinal cord was exposed by laminectomy at T9–T10, and then contused by dropping a 10-g rod on the exposed cord from a height of 12.5 mm using a MASCIS Impactor as described.14,21 Following contusion, the muscles and skin were closed separately. Cefazolin (25 mg/kg) was administered daily for 7 days by subcutaneous injection after SCI to all rats. Food and water were provided ad libitum. One day after SCI, saline, capsules without MSC, 5 × 104 free MSC or 3×104 eMSC were injected intrathecally into the lumbar spine (L4–L5) over a period of 30 s using a thin-walled needle (23G × 1 inch, inner diameter = 0.52 mm, outer diameter = 0.64 mm, NIPRO) attached to 250 μL Hamilton syringe. The needle was left in place for another 60 s to prevent leakage. After the injection, the muscles and skin were sutured separately. Locomotor recovery was assessed weekly using the 21-point Basso, Beattie and Bresnahan (BBB) scoring method22 by a team that was unaware of experimental treatments. Rats injected with 0.2 mm eMSC were allowed to survive for 8 weeks and those injected with 0.35 mm eMSC survived for 8 days (7 days after injection).
2.5. Magnetic resonance imaging of intrathecal transplanted capsules
Alginate capsules were prepared with 100 nm chitosan-coated magnetite nanoparticles (ChMNP, 6 mg/mL) and ~4 × 103 ChMNP-containing 0.2 mm diameter capsules were prepared without or with MSC, and implanted intrathecally into the rat cauda equina. MRI was performed on rats under isoflurane anesthesia using a T1-weighted fast spin echo sequence with the M2™ Compact High-Performance MRI at the Rutgers Molecular Imaging Center. Imaging was performed at indicated times after ChMNP capsule implantation to track the capsules in the spinal cord over time. Noncontused rats were transferred to the Rutgers Molecular Imaging Center one day after ChMNP capsules injection and MRI was performed first on day 2 and at the indicated times up to 42 days. Contused rats remained in the Nelson Biological Labs animal facility for ~2–3 weeks until they gained bladder control and no longer required special care.
2.6. Tissue processing for immunofluorescence and quantitation
Animals were sacrificed, perfused with 4% paraformaldehyde either 8 days or 8 weeks after SCI. Spinal cords were dissected and post-fixed with 4% paraformaldehyde at 4°C overnight. For cryosections, spinal cords were equilibrated in graded sucrose (10–30%) for 72 h at 4°C, embedded in optimal cutting temperature compound (OCT) compound (Fisher Scientific, USA) and cut into 20 μm cross sections. For immunofluorescence, sections were blocked with 10% normal goat serum at room temperature for 2 h and incubated with Isolectin IB4 Alexa-488 conjugate (1:100, Invitrogen). Image analysis was performed using Zeiss 510 confocal laser scanning microscope and analyzed using LSM software. IB4 stained tile scans encompassing the entire cross section were taken from the 8-day rats at ~2.2 mm distal to the SCI epicenter. For quantitation in the dorsal midline of the SCI site, a circle (1900 μm circumference) was drawn between and below the dorsal horns using Zeiss LSM Image Browser tool. A constant threshold was applied to obtain superthreshold pixels of IB4 staining within the fixed circular areas and averages of the resulting percentages were calculated.
2.7. White matter sparing
For analysis of spared white matter, cryosections from 8-week rats ranging from −3 mm (rostral) to +3 mm (caudal) to injury epicenter at 1 mm intervals were stained with Eriochrome Cyanine-R (ECR) as described.23 Sections were dehydrated at room temperature in a series of graded ethanol solutions for 5 min each and cleared in histoclear (National Diagnostics, USA) solution for 5 min. The sections were then rehydrated in a reverse-graded ethanol series. Sections were stained for 10 min with a solution of 0.16% ECR, 0.4% sulfuric acid, 0.4% iron chloride and 0.12% HCL. Sections were then rinsed with water for 10 min, mounted in Permount media (Fisher Scientific, USA) and scanned with Super Coolscan 8000 (Nikon, Japan). Images were analyzed using NIH ImageJ software to obtain super-threshold areas for each section using a constant threshold for all sections that were stained at the same time with ECR. Percent white matter is defined as the super-threshold area divided by the total area outlined in each section. Averages of spared white matter tissue were calculated. For analysis of spared white matter, rats were sacrificed after 8 days and sections at 2.2 mm caudal to the SCI epicenter were used.
2.8. Statistical analysis
BBB scoring and white matter sparing data were analyzed using repeated measure ANOVA followed by Tukey HSD test. Macrophage TNF-α secretion data were analyzed using ANOVA followed by Fisher’s least significant difference (LSD) test. White matter sparing and IB4 staining data were analyzed using Student’s t-test. Statistical significance was accepted for p values of <0.05 and the mean ± SD are indicated.
3. Results
3.1. Localization of MSC capsules by MRI in the spinal cord
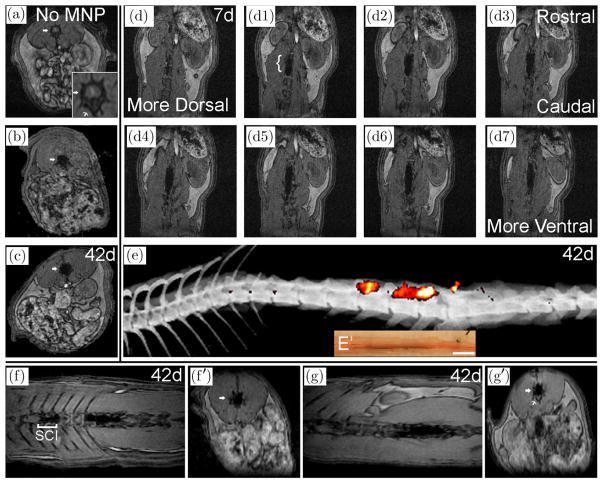
In a previous SCI study we showed one week after intrathecal injection that fluorescent capsules were detected in the lumbar spine.14 To determine their distribution over more extended times in the living rat, we injected capsules containing magnetic nanoparticles (ChMNP) intrathecally, and MRI localized them to the cauda equina for 6 weeks after injection (Fig. 1). Signals were detected at all time points, indicating that the capsules persisted for 6 weeks in vivo but no contrast enhancement was detected in an uninjected rat [Fig. 1(a)]. Cross-section images at day 2 and 42 indicated that the capsules were restricted to the spinal column [Figs. 1(b) and 1(c)] and horizontal imaging showed that they remained close to where they were injected in the cauda equina at 1–6 weeks after injection in a noncontused rat [Figs. 1(d) and 1(e)]. Optical sections through the spinal cord showed the distribution of ChMNP capsules at several levels from dorsal to ventral [Figs. 1(d)– 1(d7)]. The most intense mass of capsules was localized within ~1 cm along the rostro-caudal axis of the spinal cord within ~3 spinal segments after 1–6 weeks in the live rat [Fig. 1(e)]. Histological analysis after sacrifice confirmed that most capsules were found in this same region after the spinal cord was removed from the spinal column [Figs. 1(e) and 1(e′)].
Fig. 1.
MRI visualization of ChMNP capsules in rat spinal cords without and with contusion. Noncontused rats without (a) or with (b)–(e) intrathecal injection of ChMNP capsules were imaged at indicated times after injection. In the axial image, without ChMNP capsules note the gray region in panel A (arrow) corresponding to the spinal cord and the bony vertebral body, which is marked by a thin arrow (a) and at higher magnification in the insert. Signals (black) were detected with ChMNP capsules after 2 days (b) and they persisted for 42 days (c) in axial images. Horizontal images at 7 days (d1–d7) show localization of ChMNP capsules along the spinal axis (bracket in d1) localized in serial sections from dorsal to ventral encompassing ~ 2.2 mm. A 3D reconstruction of super-threshold MRI signals superimposed on an X-ray (e) shows a ventrolateral distribution, which is within the ventral distribution of ChMNP particles in brown (e′) in a bright field image of the same spinal cord after dissection from the spine. SCI Rats at 42 days after intrathecal injection of ChMNP capsules without (f, f′) or with (g, g′) eMSC were imaged (n = 2, 3, respectively). A bracket marks the SCI site at T9-10 in the horizontal image (f) with signals of ChMNP particles in dorsal regions of T13- L2; the signal extends more caudally to L3–4 (f′) but was primarily ventral (not shown). Horizontal image (g) with signals of ChMNP particles in eMSC in ventral regions of L3–L5; note the signal is in the spine above the vertebral body (g′).
To determine whether similar distributions of ChMNP capsules were obtained in SCI rats, we injected them intrathecally one day after contusion. Injections of ChMNP capsules without [Fig. 1(f)] and with MSC [Fig. 1(g)] in SCI rats were localized primarily in the lumbar spine after 42 days; the distributions in each rat were very similar to that observed ~3 weeks after injection (data not shown), suggesting no gross movement of the capsules. ChMNP capsules extended rostrally as far as L13 [Fig. 1(f)] and caudally to L5 [Fig. 1(g)] and L6 [Fig. 1(e)]. Distributions of ChMNP capsules without and with MSC were typically located more ventrally in the spine [Figs. 1(e) and 1(g)] and less frequently in dorsal regions [Fig. 1(f)]. The combined results indicate that the ChMNP alginate capsules without or with eMSC remained in and around the cauda equina in the lumbar spine for 6 weeks without any gross movement in both control and contused rats.
3.2. Injection of eMSC in SCI inhibits inflammation and promotes locomotor recovery
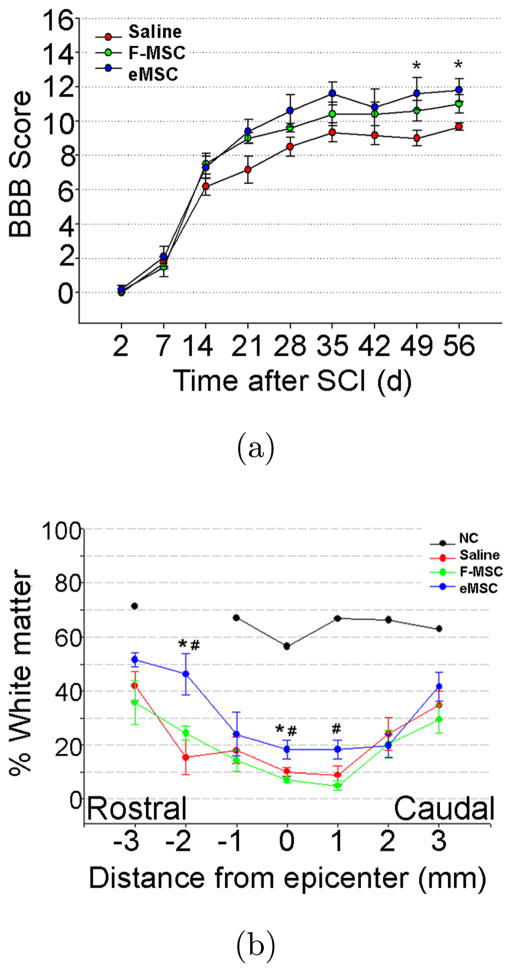
We observed previously that one week after intra-thecal injection of ~0.2 mm eMSC at a location >2 cm caudal from the SCI site at T9–10, expression in the injury site was increased for CD206, a marker for anti-inflammatory/reparative M2 macrophages.14 In a longer-term study with survival for 8 weeks following SCI and lumbar injection of small eMSC, we observed a temporal trend toward improved hindlimb locomotion that reached significance between eMSC and the saline control groups at 7 and 8 weeks [Fig. 2(a)]. The ~2 point differences observed in this region of the nonlinear BBB scale (i.e., between 9–10 and 11–12) represents a difference between weight support and walking.22 The BBB scores for a third group of SCI rats that were injected with free nonencapsulated MSC, fell in between the eMSC and saline controls but the differences were not statistically different by comparison to the other two groups [Fig. 2(a)]. This suggests that ~0.2 mm eMSC are more effective than free MSC in improving locomotion when injections were made 1 day after rat SCI. Histological analyses after sacrifice showed that levels of white matter were significantly higher in the eMSC group by comparison to the saline group but no significant differences were observed by comparison to the free MSC group [Fig. 2(b)]. The weaker improvements observed with the free MSC may be explained by their disappearance from the spinal cord within one week after injection while eMSC retrieved at the same time showed an average MSC survival of ~50% in the capsules by live/dead staining (data not shown).
Fig. 2.
Effects of small eMSC on recovery after SCI. (a) Post-injury locomotor function assessed using BBB scoring was done weekly for 8 weeks by trained observers in SCI rats injected with 5 × 104 free human MSC (F-MSC), small encapsulated MSC (3 × 104), or saline 1 day after SCI. Data is represented as mean ± SD. The eMSC (n = 5) showed consistently higher scores than F-MSC (n = 5) and saline group (n = 6) and attained significance at week 7 and 8 (*, p < 0.05 ANOVA repeated measures with Tukey HSD test). (b) Percent preserved white matter at 8 weeks after SCI was calculated by measuring ECR staining in cross sections from rats treated with saline (n = 4), F-MSC (n = 5) and eMSC (n = 3) at the indicated positions relative to the injury epicenter; (NC, normal control). Significant differences were detected in the percent of preserved white matter between eMSC versus saline groups (*, p < 0.05) or versus F-MSC (#, p < 0.05).
3.3. Optimization of MSC capsules for intrathecal injection in rats
3.3.1. Properties of small and large MSC capsules
A major limitation in these experiments using the small ~0.2 mm alginate capsules was the low number of MSC that could be encapsulated due to the slow flow rates through the narrow bore extrusion needles used to generate them. The average number of live eMSC per 1-h encapsulation for the small microspheres was ~50, 000, whereas the average for the large capsules with the larger inner diameter of 0.17 mm needle was ~106 (Table 1). Thus, we were able to generate on average >20 times more cells/encapsulation in large capsules than in the small capsules.
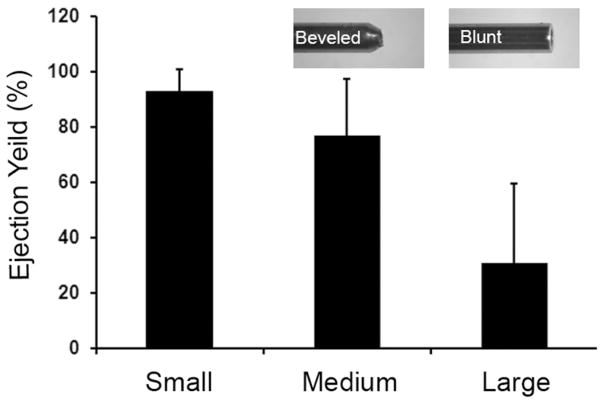
In control experiments, we analyzed the reproducibility of capsule ejection through 0.52 mm inner diameter thin-walled needles to model the intra-thecal delivery by measuring the numbers of capsules that were ejected into a tube in vitro. An average of >90% of the expected numbers of capsules were ejected with the small capsules but <30% were ejected with the large capsules and the yield was highly variable (Fig. 3). For these ejections that modeled the intrathecal rat injection procedure, it was necessary to concentrate the eMSC into a small volume (~0.15 mL), which often caused the large capsules to aggregate and become trapped in the hub of the injection needles. This trapping resulted in highly variable numbers of large capsules ejected in repeated samples of equal volume. Capsule trapping in the syringe hub was not a problem with small eMSC (Fig. 3) or with the large capsules prepared without cells (data not shown). The variable yields that occurred with large eMSC made them unsuitable for quantitative studies in vivo.
Fig. 3.
Efficiency of eMSC ejection through narrow bore needles. Graph shows the effect of capsule size on eMSC ejection yield (%, mean ± SD) in 2–3 experiments for three different eMSC sizes (small ~ 0.2 mm, medium ~ 0.35 mm and large ~ 0.5 mm). The ejection yield with larger capsules was much lower than the small and medium size capsules due to aggregation (see text). The inserts show tips of 0.17 inner diameter needles with blunt or beveled ends, which gave large- and medium-sized capsules, respectively.
Given the limited number of small capsules that could be prepared, we attempted to increase the number of cells/small capsule or to produce intermediate sized capsules. We varied multiple parameters using the small bore needle (inner diameter of 0.12 mm) for capsule generation including different flow rates and voltages, alginate densities and cell input (up to 8 million/preparation) but we could not get much higher yields and the properties of the capsules (i.e., shape and fragility) were often inferior (data not shown). We then varied these parameters using the larger needle with inner diameter of 0.17 mm in attempts to generate smaller capsules but we could not find conditions to produce eMSC that could pass reproducibly through the 0.52 mm injection needles (Fig. 3). However, we found that a needle with the same inner diameter of 0.17 mm with a beveled tip, instead of the blunt tip used previously (Fig. 3 inserts), yielded relatively uniform eMSC of ~0.35 mm, which contained five times as many cells as in the small eMSC (Table 1). The beveled needle tip has a narrower outer diameter at the ejection orifice than the blunt needle (Fig. 3 inserts), which resulted in the formation of smaller diameter micro-drops before they fell from the needle tip into the CaCl2 bath where they were cross-linked.
Then we analyzed the reproducibility of ejecting the ~0.35 mm eMSC through 0.52 mm inner diameter thin-walled needles and compared the results with those for the larger eMSC described above. An average of ~80% of the expected numbers of ~0.35 mm eMSC were ejected, which is much improved by comparison to the <30% ejected with the large capsules (Fig. 3). Unlike the larger ~0.5 mm eMSC which tended to aggregate and clog the thin-walled needles used for intrathecal injections, these problems were not observed with ~0.35 mm eMSC, which were collected with more reproducible yields than the larger ~0.5 mm eMSC (Fig. 3).
3.3.2. Properties of medium-sized ~0.35 mm MSC capsules
We characterized these medium-sized ~0.35 mm eMSC by comparison to the small and large eMSC. While the number of live cells/capsule was much higher in the medium and large than in small capsules, the percent of live MSC was comparable among the three different sized capsules (Table 1). Comparable yields of incorporated cells were obtained for the large and the medium sized capsules generated using the inner diameter needle of 0.17 mm. However, the smaller capsules generated using the inner diameter needle of 0.12 mm required much slower flow rates such that during a 1-h encapsulation only ~50, 000 cells were obtained in capsules (Table 1). Encapsulations were limited to 1 h to avoid lower yields of live cells that resulted when MSC were exposed to unpolymerized alginate for longer durations (J. Barminko, unpublished observations). Thus, large numbers of cells were obtained in medium-sized capsules (Table 1), which could be ejected through narrow bore needles used for intrathecal delivery in SCI experiments (Fig. 3).
3.4. MSC capsules inhibit secretion of TNF-α from activated macrophages in vitro
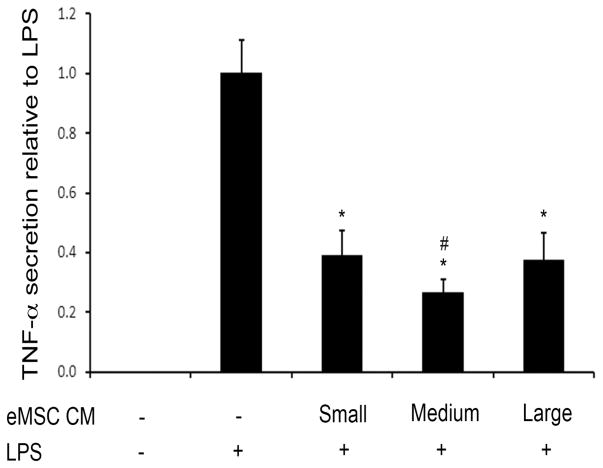
To compare the immunomodulatory properties of the different sized eMSC, we tested their effects in co-cultures with LPS-activated human macrophages.20 LPS induced secretion of TNF-α and these levels were decreased by co-culture with the different sized eMSC (Fig. 4). Decrease in TNF-α levels were obtained in all cases, with the medium eMSC showing the most robust affect (Fig. 4). Thus, the medium-sized eMSC were more effective than the large eMSC in reducing secretion of a key pro-inflammatory cytokine from activated human macrophages.14
Fig. 4.
Different sizes of eMSC reduce TNF-α secretion when cultured with activated macrophages. Culture supernatants collected from transwell co-cultures of LPS-activated macrophages with eMSC at 1:1 ratio were assayed for TNF-α by ELISA and normalized to levels in LPS-activated macrophages supernatants without added eMSC. Differences between the LPS only control and each of the three different sized eMSC were significant (*, p < 0.05) and the difference between the medium-sized capsules and the large and small capsules was also significant (#, p < 0.05).
3.5. MSC capsules reduce levels of activated macrophages in SCI
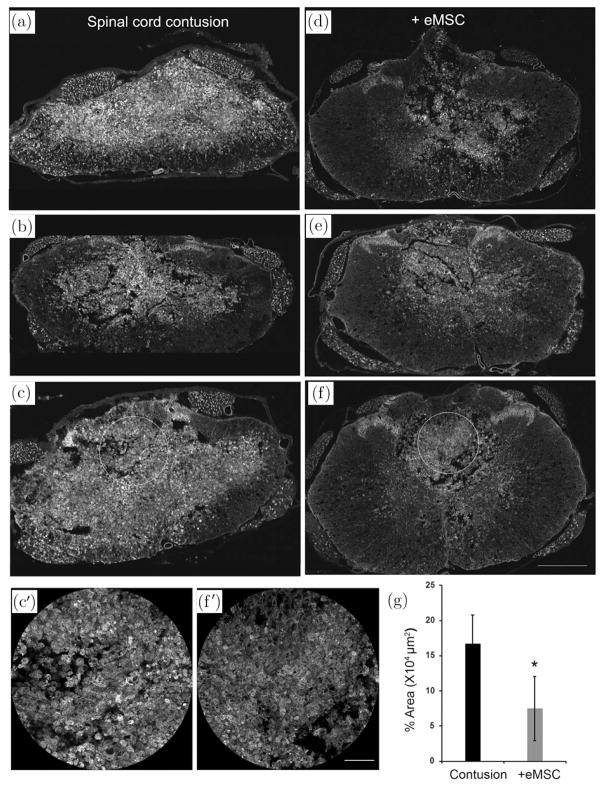
Given the immunomodulatory effects of the medium-sized eMSC in vitro, we injected them 1 day after SCI in rats to evaluate their effects on inflammation in vivo. To test the effects on pro-inflammatory microglia and macrophages we immunostained for the isolectin IB4 in sections from rat SCI one week after eMSC injection. IB4 binding is an early marker for activated macrophages24 and microglia.25 IB4+ staining in SCI cross sections indicated the presence of many robustly labeled pro-inflammatory macrophages one week after injection in SCI rats with control treatment [Figs. 5(a)–5(c)] but much less staining was observed after injection of eMSC [Figs. 5(d)–5(f)]. While the IB4 staining varied among different rats within each group, there appeared to be less staining in the eMSC group [Figs. 5(d)–5(f)] in white matter regions than in the controls [Figs. 5(a)–5(c)]. The IB4 staining was most intense in the dorsal central region of the SCI site, and quantitation in these regions (C′ versus F′) showed significantly lower average levels in the eMSC rats by comparison to controls [Fig. 5(g)].
Fig. 5.
Effect of medium-size eMSC transplantation on activated macrophages in SCI. Tissues from SCI rats one week after injection with ~ 0.35 mm eMSC (d)–(f) or saline as a control (a)–(c) were cryosectioned in cross sections and were incubated with Isolectin IB4 Alexa-488 conjugate. Confocal imaging showed more robust staining in control than eMSC treated sections in white matter and gray matter regions. Quantitation (g) of IB4 staining regions in equivalent dorsal midline circles drawn in spinal cord cross sections (for example, C′ and F′ are magnified images from C and F, respectively) were measured as super-threshold areas at ~2.2 mm caudal to the injury epicenter. IB4 levels decreased significantly (*, p < 0.05) in the eMSC group (n = 3) as compared to the control (n = 4) group. Scale bars are 500 μm in panels (a)–(f) and 100 μm in panels C′ and F′.
3.6. MSC capsules promote white matter sparing in SCI
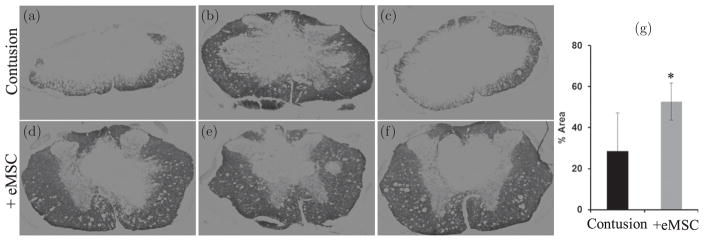
Pro-inflammatory macrophages and cytokines have been associated with CNS demyelination.26 We found that ECR staining for myelin showed more white matter sparing with eMSC [Figs. 6(d)–6(f)] by comparison to controls [Figs. 6(a)–6(c)]. Quantitation of ECR staining confirmed larger superthreshold areas of white matter with eMSC treatment compared to controls [Fig. 6(g)]. The combined results indicate that intrathecal injection of medium-sized eMSC reduced levels of pro-inflammatory macrophages and increased white matter sparing in the corresponding regions of the spinal cord [compare Figs. 5(a)–5(f) with 6(a)–6(f)] after a one-week treatment after SCI.
Fig. 6.
Medium-size eMSC increased white matter sparing. Spinal cord cross sections in regions adjacent to those analyzed in Fig. 5 from spinal cord contusion control (a)–(c) and ~0.35 mm eMSC (d)–(f) injected groups were stained for ECR. The percent of white matter (g) is significantly higher in eMSC group (n = 7) as compared to control group (n = 3) (*, p < 0.05).
4. Discussion
Transplantation of encapsulated cells has shown success in vivo4,27 but very few studies have demonstrated benefits in the CNS.14 In this and a previous study,14 we provide the first evidence that intrathecal eMSC injections are immunomodulatory and promote recovery after contusive SCI. We showed previously that small eMSC increased M2 anti-inflammatory macrophages 1 week after injection,14 and we report here that after 8 weeks they improved locomotor recovery and white matter sparing. Medium-sized eMSC decreased M1 pro-inflammatory macrophages and promoted white matter sparing one week after acute injection in SCI. These improvements in white matter sparing [Fig. 2(b)] and locomotion [Fig. 2(a)] are consistent with previous studies demonstrating a positive relationship between white matter sparing and locomotion after SCI of different severities.22 We also report methods for encapsulating ~20 times more human MSC in medium-sized capsules than was possible previously in the small capsules, which were used for minimally invasive intrathecal injection of eMSC into the rat spine after SCI. The results indicate that alginate capsules without or with MSC remain in the lumbar spine for at least 6 weeks and suggest that eMSC have immunomodulatory effects at a distance of >2 cm in more rostral thoracic SCI sites.
Relatively large (>0.5 mm diameter) capsules have been used for many applications where they can be delivered locally, although smaller capsules are preferred due to their excellent surface-volume ratio and oxygen permeability.4 However, few studies have compared the properties of different sized capsules in vivo28,29 and we are not aware of any comparison that has been reported for injection of different sized alginate capsules into the CNS. A comparison performed with various sizes of encapsulated islets concluded that capsules <0.35 mm were more biocompatible than larger capsules, which were more fibrotic.28 We have compared three different sizes of capsules (~0.2 mm, 0.35 mm and 0.5 mm) and found that they have relatively similar activities to modulate inflammation in vitro. However, their use differed substantially in vivo for minimally invasive intrathecal delivery, which required small volumes and narrow bore needles to avoid excessive damage and leakage of cerebrospinal fluid (CSF). We found that for quantitative studies injections of concentrated suspensions of large alginate capsules were problematic because of high variability in the number of capsules that could be injected. Similar problems have not been reported with injection of much larger volumes through wider bore needles.8,28 We have shown here and previously14 that MSC capsules ranging from 0.2–0.35 mm can be injected intrathecally through narrow-walled needles and that they had significant anti-inflammatory effects in SCI. The ~0.35 mm medium-sized capsules are advantageous because they allow encapsulation of many more cells than in the small capsules, thereby providing sufficient quantities for injection in large controlled physiological studies.
Encapsulation of MSC is also advantageous because the capsule protects cells from direct contact with the host while the MSC secrete immunomodulatory factors. MSC respond rapidly to pro-inflammatory cytokines by releasing anti-inflammatory cytokines and factors, making them particularly well suited to suppress inflammation.30 Encapsulated MSC were comparable to free MSC in vitro in decreasing macrophage secretion of TNF-α, a major pro-inflammatory cytokine,14 suggesting that prolonged survival of MSC in capsules may modulate inflammation in a host for longer times than free MSC, which disappear rapidly. Encapsulation of MSC also upregulates the expression of many cytokines for extended times even without activation, and eMSC are anti-inflammatory and neuroprotective both in vitro and in vivo as shown here and previously.8,14
A typical response to injury involves an early pro-inflammatory activation of M1 macrophages followed by a transition to a later phase with increased levels of M2 anti-inflammatory macrophages that promote resolution and repair, but in severe injuries the M1 phase persists and the M2 response is suppressed.31 Pro-inflammatory stimuli (e.g., LPS) activate macrophages into a pro-inflammatory M1 phenotype26 and this activation has been detected as an increase in the percent of IB4+ macrophages.24 IB4 binds robustly to both microglia indigenous to CNS tissue and macrophages that migrate into the CNS after injury.32 This suggests that the reduction in IB4+ staining in SCI with eMSC treatment represents a decrease in M1 macrophages that is mediated by factors secreted by the MSC. Human eMSC also increased M2 macrophages in rat SCI,14 which is consistent with increased levels of anti-inflammatory cytokines such as IL-10 in M1 activated macrophages in vitro14 and in rat SCI (unpublished observations).
Effects of MSC in a wide range of animal models have been attributed primarily to their anti-inflammatory properties mediated by secreted cytokines and growth factors but it has not been possible to evaluate direct interactions with host cells in vivo because MSC migrate widely, are difficult to locate and survive only transiently. Very small fractions of intravenously injected MSC migrate to sites of injury and disease while most get trapped in organs including the lungs and liver. Their transient survival makes it difficult to analyze their relative contributions in vivo via secretion versus cell–cell interactions.33 A comparison of different MSC delivery methods in the CNS indicated that intrathecal lumbar injection was superior to intraspinal or intravenous delivery.34 However, the transient fate of MSC in vivo may require very large or repeated cell doses to be maximally effective. Even when MSC have been localized with intrinsic markers in vivo, it is difficult to prove their mechanism of action.35 Given that encapsulation of cells excludes direct interactions with host cells, the effects of eMSC must be due to their secretion of relatively small proteins and factors that can diffuse into the host.30
Encapsulation is also advantageous because it allows MSC to survive longer and perhaps be effective at lower doses, to be localized in vivo, and to be retrieved from the host.8,36 In rat SCI for example, a comprehensive review of MSC transplantation indicated that improved locomotion was observed with intravenous doses of 106 MSC and intraspinal doses of at least 250,000 cells.16 We showed locomotor improvement with 30,000 MSC when they were encapsulated, suggesting that encapsulation may allow reduced MSC dosing for efficacy. Delayed injections of MSC indicated their benefit even four weeks after SCI,35 suggesting that multiple doses may be required for maximal activity but this may be avoided using the longer lasting eMSC. Because CSF circulates only within the CNS, delivery of eMSC may be particularly effective by injection into CSF in brain ventricles8 and the cauda equina.14 Imaging demonstrated that alginate capsules with or without MSC remain for as long as 6 weeks in the CNS near their injection sites in the cauda equina and they can be retrieved from the host for analysis8 (JHK, SK and MG unpublished observations).
MSC have only been approved for clinical therapy in graft versus host disease (GvHD),37 which probably differs from other applications because MSC normally reside in the bone marrow where they can survive for extended periods and integrate into that niche. Numerous studies indicate that MSC are safe but in many cases they have modest and variable immunomodulatory effects in the CNS for a variety of reasons including some suggested above but their mechanisms of action in vivo are not yet clear.15,16,38,39 Studies with eMSC have shown that protective effects of MSC can be enhanced by genetically overexpressing bioactive proteins, demonstrating the efficacy of proteins secreted from eMSC.8,36 However, the use of other cells as vehicles to deliver proteins may be problematic because they elicit much greater immune responses around capsules than with MSC, which are hypoimmunogenic.36
Safety for alginates has been demonstrated in the clinic for several applications40 and in a clinical trial (NCT01298830) using eMSC expressing GLP-1.18 These capsules were generated using a recently described proprietary method (USP 8790705) with concentric needles that yield high cell densities in an inner capsule core surrounded by a cell-free outer alginate shell.29 Capsules containing immortalized human MSC without or with transfection to express GLP-1 yielded improved outcome but their delivery adjacent to injury sites may have contributed to enlarge brain ventricles.8 It will be interesting to determine whether intrathecal delivery of similar capsules with large numbers of low passage human MSC at a distance from the target site avoids the deleterious consequences that may result when eMSC are delivered close to injury sites.
Alginate encapsulates MSC sufficiently well so that many of the human cells survive for at least 2 weeks18 to 2 months in a rat host while only a minute fraction of the human cells are detectable in the rat within 1 week without encapsulation (JHK, SK, MG, unpublished observations). Xenogenic transplantation of human cells in animals usually requires immunosuppression or the use of immuno-compromised animals, which may complicate interpretation of results in injuries and disease with major inflammatory components such as SCI. Alginate encapsulation provides protection in xeno-genic transplantation of human cells in animals and will be useful for preclinical testing of cells that secrete bioactive factors.
Besides these studies, little data are available on the use and optimization of encapsulated cells in the CNS. Our results underscore advantages of intra-thecal injections of medium-sized capsules for the widely used MASCIS model for rat SCI that may also be applicable to other CNS disorders by minimally invasive lumbar injection. Based on many reports of MSC efficacy,9,11,15,30,33,41 early intrathecal injections of eMSC may be effective in treating other acute CNS injuries including traumatic brain injury and stroke, and extended eMSC survival may be beneficial in chronic inflammatory disorders such as multiple sclerosis.10 Feasibility for these applications will require proof of concept with eMSC in preclinical models, which often involve delivery through small bore needles in rodent models as described here. While larger microcapsules are useful for various applications, the results described here highlight potential problems with injection of large eMSC and underscore advantages of delivering smaller capsules by minimally invasive procedures that use narrow bore needles.
Acknowledgments
These studies were supported by the New Jersey Commission for Spinal Cord Injury 10-2947-SCR-R-0 and the New Jersey Commission for Brain Injury Research CBIR12IRG019. JB and AG were supported in part by a DHHS-PHS-NIH Biotechnology Training Grant T32GM008339. We thank Derek Adler (Molecular Imaging Center), Dr. Noriko Kane-Goldsmith, Nabgha Farhat, Justyna Michalik and Sean O’Leary for technical assistance. Suneel Kumar and Joanne Babiarz contributed equally to this paper.
Contributor Information
Suneel Kumar, Department of Cell Biology & Neuroscience, Rutgers University, 604 Allison Rd., Piscataway, NJ 08854 USA.
Joanne Babiarz, Department of Cell Biology & Neuroscience, Rutgers University, 604 Allison Rd., Piscataway, NJ 08854 USA.
Sayantani Basak, Department of Cell Biology & Neuroscience, Rutgers University, 604 Allison Rd., Piscataway, NJ 08854 USA.
Jae Hwan Kim, Department of Cell Biology & Neuroscience, Rutgers University, 604 Allison Rd., Piscataway, NJ 08854 USA. Department of Anatomy, Yonsei University College of Medicine, Seoul 120-752, Republic of Korea.
Jeffrey Barminko, Department of Biomedical Engineering, Rutgers University, Piscataway, NJ 08854 USA. The Mount Sinai Hospital, One Gustave L. Levy Place New York, NY 10029.
Andrea Gray, Department of Biomedical Engineering, Rutgers University, Piscataway, NJ 08854 USA.
Parry Mendapara, Department of Cell Biology & Neuroscience, Rutgers University, 604 Allison Rd., Piscataway, NJ 08854 USA.
Rene Schloss, Department of Biomedical Engineering, Rutgers University, Piscataway, NJ 08854 USA.
Martin L. Yarmush, Department of Biomedical Engineering, Rutgers University, Piscataway, NJ 08854 USA.
Martin Grumet, W. M. Keck Center for Collaborative Neuroscience, Rutgers Stem Cell Research Center. Department of Cell Biology & Neuroscience, Rutgers University, Piscataway, NJ, 08854 USA.
References
- 1.Acarregui A, Murua A, Pedraz JL, Orive G, Hernandez RM. Biodrugs. 2012;26:283. doi: 10.1007/BF03261887. [DOI] [PubMed] [Google Scholar]
- 2.Visted T, Bjerkvig R, Enger PO. Neuro-Oncology. 2001;3:201. doi: 10.1093/neuonc/3.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maguire T, Novik E, Schloss R, Yarmush M. Biotechnol Bioeng. 2006;93:581. doi: 10.1002/bit.20748. [DOI] [PubMed] [Google Scholar]
- 4.Orive G, Santos E, Pedraz JL, Hernandez RM. Adv Drug Deliv Rev. 2014;67–68:3. doi: 10.1016/j.addr.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Zanin MP, Pettingill LN, Harvey AR, Emerich DF, Thanos CG, Shepherd RK. J Control Release. 2012;160:3. doi: 10.1016/j.jconrel.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Gilert A, Machluf M. J Angiogenesis Res. 2010;2:20. doi: 10.1186/2040-2384-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobias CA, et al. J Neurotrauma. 2005;22:138. doi: 10.1089/neu.2005.22.138. [DOI] [PubMed] [Google Scholar]
- 8.Heile AM, Wallrapp C, Klinge PM, Samii A, Kassem M, Silverberg G, Brinker T. Neurosci Lett. 2009;463:176. doi: 10.1016/j.neulet.2009.07.071. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI. J Pathol. 2009;217:318. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueroa FE, Carrion F, Villanueva S, Khoury M. Biol Res. 2012;45:269. doi: 10.4067/S0716-97602012000300008. [DOI] [PubMed] [Google Scholar]
- 11.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Cell Death Differ. 2014;21:216. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mundra V, Gerling IC, Mahato RI. Mol Pharm. 2013;10:77. doi: 10.1021/mp3005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prockop DJ. Stem Cells (Dayton, Ohio) 2013;31:2042. doi: 10.1002/stem.1400. [DOI] [PubMed] [Google Scholar]
- 14.Barminko J, Kim JH, Otsuka S, Gray A, Mendpara P, Schloss R, Grumet M, Yarmush ML. Biotechnol Bioeng. 2011;108:2747. doi: 10.1002/bit.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parr AM, Tator CH, Keating A. Bone Marrow Transplant. 2007;40:609. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 16.Tetzlaff W, et al. J Neurotrauma. 2011;28:1611. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander JK, Popovich PG. Prog Brain Res. 2009;175:125. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- 18.Heile A, Brinker T. Dialogues Clin Neurosci. 2011;13:279. doi: 10.31887/DCNS.2011.13.2/aheile. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maguire T, Davidovich AE, Wallenstein EJ, Novik E, Sharma N, Pedersen H, Androulakis IP, Schloss R, Yarmush M. Biotechnol Bioeng. 2007;98:631. doi: 10.1002/bit.21435. [DOI] [PubMed] [Google Scholar]
- 20.Barminko JA, Nativ NI, Schloss R, Yarmush ML. Biotechnol Bioeng. 2014;111:2239. doi: 10.1002/bit.25282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasegawa K, Chang Y-W, Li H, Berlin Y, Ikeda O, Kane-Goldsmith, Grumet M. Exp Neurol. 2005;193:394. doi: 10.1016/j.expneurol.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Basso DM, Beattie MS, Bresnahan JC. Exp Neurol. 1996;139:244. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 23.McEwen ML, Springer JE. J Histochem Cytochem. 2005;53:809. doi: 10.1369/jhc.4A6467.2005. [DOI] [PubMed] [Google Scholar]
- 24.Tabor DR, Larry CH, Jacobs RF. J Leukoc Biol. 1989;45:452. doi: 10.1002/jlb.45.5.452. [DOI] [PubMed] [Google Scholar]
- 25.Lee CH, et al. Brain Res. 2010;1342:138. doi: 10.1016/j.brainres.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 26.Gaudet AD, Popovich PG, Ramer MS. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan R, Alexander M, Robles L, Foster CE, 3rd, Lakey JR. J Soc Biomed Diabet Res. 2014;11:84. doi: 10.1900/RDS.2014.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robitaille R, Pariseau JF, Leblond FA, Lamoureux M, Lepage Y, Halle JP. J Biomed Mater Res. 1999;44:116. doi: 10.1002/(sici)1097-4636(199901)44:1<116::aid-jbm13>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Wallrapp C, Thoenes E, Thurmer F, Jork A, Kassem M, Geigle P. J Microencapsul. 2013;30:315. doi: 10.3109/02652048.2012.726281. [DOI] [PubMed] [Google Scholar]
- 30.Prockop DJ, Oh JY. Mol Ther. 2012;20:14. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novak ML, Koh TJ. J Leukoc Biol. 2013;93:875. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dehghani F, Conrad A, Kohl A, Korf HW, Hailer NP. Exp Neurol. 2004;189:241. doi: 10.1016/j.expneurol.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Cell Transplant. 2010;19:667. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B. Spine. 2009;34:328. doi: 10.1097/BRS.0b013e31819403ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osaka M, Honmou O, Murakami T, Nonaka T, Houkin K, Hamada H, Kocsis JD. Brain Res. 2010;1343:226. doi: 10.1016/j.brainres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Goren A, Dahan N, Goren E, Baruch L, Machluf M. FASEB J. 2010;24:22. doi: 10.1096/fj.09-131888. [DOI] [PubMed] [Google Scholar]
- 37.Kurtzberg J, et al. Biol Blood Marrow Transplant. 2014;20:229. doi: 10.1016/j.bbmt.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Drago D, Cossetti C, Iraci N, Gaude E, Musco G, Bachi A, Pluchino S. Biochimie. 2013;95:2271. doi: 10.1016/j.biochi.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoo J, Kim HS, Hwang DY. J Cell Bio-chem. 2013;114:743. doi: 10.1002/jcb.24427. [DOI] [PubMed] [Google Scholar]
- 40.Andersen T, Strand B, Formo K, Alsberg E, Christensen B. Carbohydr Chem. 2012;37:227. [Google Scholar]
- 41.Oliveri RS, Bello S, Biering-Sorensen F. Neurobiol Dis. 2014;62:338. doi: 10.1016/j.nbd.2013.10.014. [DOI] [PubMed] [Google Scholar]