Abstract
Knowledge of the link between a vector population’s pathogen-transmission potential and its biotic environment can generate more realistic forecasts of disease risk due to environmental change. It also can promote more effective vector control by both conventional and novel means.
This study assessed the effect of particular plant species assemblages differing in nectar production on components of the vectorial capacity of the mosquito Anopheles gambiae s.s., an important vector of African malaria.
We followed cohorts of mosquitoes for three weeks in greenhouse mesocosms holding nectar-poor and nectar-rich plant species by tracking daily mortalities and estimating daily biting rates and fecundities. At death, a mosquito’s insemination status and wing length were determined. These life history traits allowed incorporation of larval dynamics into a vectorial capacity estimate. This new study provided both novel assemblages of putative host plants and a human blood host within a nocturnal period of maximum biting.
Survivorship was significantly greater in nectar-rich environments than nectar-poor ones, resulting in greater total fecundity. Daily biting rate and fecundity per female between treatments was not detected. These results translated to greater estimated vectorial capacities in the nectar-rich environment in all four replicates of the experiment (means: 1,089.5 ± 125.2 vs. 518.3 ± 60.6). When mosquito density was made a function of survival and fecundity, rather than held constant, the difference between plant treatments was more pronounced, but so was the variance, so differences were not statistically significant. In the nectar-poor environment, females’ survival suffered severely when a blood host was not provided. A sugar-accessibility experiment confirmed that Parthenium hysterophorus is a nectar-poor plant for these mosquitoes.
Synthesis and applications. This study, assessing the effect of particular plant species assemblages on the vectorial capacity of malaria mosquitoes, highlights the likelihood that changes in plant communities (e.g. due to introduction of exotic or nectar-rich species) can increase malaria transmission and that a reduction of favourable nectar sources can reduce it. Also, plant communities’ data can be used to identify potential high risk areas. Further studies are warranted to explore how and when management of plant species assemblages should be considered as an option in an integrated vector management strategy.
Keywords: mosquito, fecundity, survival, nectar, plant species assemblages, vectorial capacity, integrated vector management, malaria, disease, pathogen transmission
Introduction
Understanding variation in population sizes between different environments is one of the main occupations of ecologists (Case 2000). Such an understanding is particularly important for populations of insect vectors of pathogens, as population density and the demographic parameters that influence it are major determinants of pathogen transmission potential (Godfray 2013). The focus of this study is on the mosquito Anopheles gambiae s.s. Giles that, despite tremendous recent progress in malaria control, continues to place a considerable health burden on sub-Saharan Africa, due to Plasmodium falciparum Welch infection (Bhatt et al. 2015). In addition to environmental factors such as temperature and rainfall (Mordecai et al., 2013; Parham & Michael, 2009), vegetation type and abundance have been considered to be prime predictors of mosquito abundance (Reisen 2010). In part, this may be related to the fact that nectariferous plants can provide both shelter (a hospitable diurnal microclimate) and sugar meals for most haematophagous flies, including malaria mosquitoes. Despite growing recognition that plant traits can mediate transmission of a wide range of pathogens in general, little is known about how plant abundance and species composition influence the transmission of mosquito-borne pathogens between vertebrates (McArt, Koch, Irwin, & Adler, 2014).
Understanding the manner in which the environment affects the vector’s transmission potential is best achieved by examining the vectorial capacity of populations in different environments. Vectorial capacity (C), as originally conceived for human malaria, is a function that describes a particular population of mosquitoes in terms of the number of new human infections, per day, derived directly from one original infection via mosquito transmission. It is a distillation of entomological components from the basic reproduction number of infected humans, R0 (Dye, 1992; Garrett-Jones, 1964). It is determined by the females’ density relative to humans, the interval between bites (i.e., biting rate) on humans, the females’ mortality rate, and the duration of the pathogen’s extrinsic cycle within her. It is not intended as an accurate assessment of the actual potential of a natural vector population, but rather a simple heuristic device for understanding the relative importance of its components and a tool for comparing hypothetical or real populations when inaccessible components are held constant. For example, vector competence, the likelihood that the insect can ingest, host, and subsequently inject the proliferated pathogen, is often considered an additional parameter of vectorial capacity (Smith & McKenzie 2004) but not easy to ascertain.
Mosquitoes ingest sugar, in the form of floral or extra-floral nectar, honeydew, or rotting fruit, as a source of immediately available energy to sustain flight, as well as a means of rapidly accumulating an energy reserve. It is the only source of food of adult males, while females of most species additionally rely on vertebrate blood to provide the protein required for egg production. The blood meal also contributes to the maternal energy reserve (Briegel 2003). Plant sugar has been shown, experimentally, to affect most components of vectorial capacity. It increases the longevity of females (Gary & Foster 2001, 2004; Impoinvil et al. 2004; Stone, Jackson & Foster 2012), decreases their biting rates (Gary & Foster, 2001, 2004; Straif & Beier, 1996), and increases the survival and mating capability of males (Gary, Cannon, & Foster, 2009; Stone, Taylor, Roitberg, & Foster, 2009). Owing to its detrimental effect on males, severe sugar deprivation can cause a proportion of females to remain uninseminated and therefore reduce the egg output of a population. A shortage of sugar also may affect the probability of a mosquito becoming infected with Plasmodium (Okech et al. 2004) and may lead to a greater pathogen-induced mortality in the vector (Ferguson & Read, 2002; Lalubin, Delédevant, Glaizot, & Christe, 2014; Lambrechts, Chavatte, Snounou, & Koella, 2006). Major gaps remain, however, in our understanding of the impact of plant sugar on population density, of the environment’s mosquito carrying capacity, and of the impact on vectorial capacity when feeding on various plant species, rather than on laboratory-supplied sugar solutions. These gaps must be filled, not only to understand the role of nectar sources on pathogen transmission, but also to determine whether selective removal of the best host plants would be enough to significantly lower the mosquito population’s vectorial capacity. Plants differ in their nectars’ sugars, secondary metabolites, and amino acid compositions, in their volumes and accessibilities, and in their blends of volatile organic chemicals that facilitate location of the plant. It is not surprising, therefore, that survivorship of mosquitoes depends on the species of plant available to them (Gary & Foster, 2004; Impoinvil et al., 2004; Manda, Gouagna, Foster, et al., 2007; Nikbakhtzadeh, Terbot, & Foster, 2016; Nyasembe et al., 2015). In a mesocosm, Stone et al. (2012) found that providing A. gambiae s.s. with access to an assemblage of nectar-rich plants likewise led to a higher survivorship, but to a lower biting rate. On average, vectorial capacity was higher in the sugar-restricted settings, but the outcome varied among replicates. Interpretation of those results was hampered by relatively low biting frequencies, possibly due to the presence of a blood host only at dawn and to the assumption of a constant mosquito density. In the present study, we investigated this issue again, under more natural conditions. In particular, the human blood host was made available during a nocturnal time-frame when most biting occurs in the field in this species, and we provided more plants, to reduce the impact of individual variation in their developmental and physiological states. Fecundity and insemination rates were used to expand on the traditional vectorial capacity formula to include density-dependent population dynamics.
Additionally, we investigated a possible source of variation in the role of the noxious invasive weed Parthenium hysterophorus L. (Asteraceae). This plant has been described as being sugar-poor yet attractive to A. gambiae, females of which readily settle on it (Manda, Gouagna, Nyandat, et al., 2007), but it sustains life only for short periods (Manda, Gouagna, Foster, et al., 2007; Nikbakhtzadeh et al., 2016; Stone et al., 2012). Other accounts suggest that P. hysterophorus is an important sugar source for A. gambiae, lengthening its longevity and thus its vectorial capacity, and thereby promoting malaria prevalence (Nyasembe et al. 2015). Here we test whether this discrepancy may be due to its flowering status.
Materials and Methods
Mbita strain of A. gambiae s.s. (S form) mosquitoes were reared following the methods of Stone, Taylor & Foster (2009). Four cohorts were followed over four separate 21-day periods in mesocosms that contained assemblages of either nectar-rich or nectar-poor plants occurring in the Mbita Point area of Kenya. The experiments were conducted in two mesocosms, each 82.69 m3, in the Biological Sciences Greenhouse at The Ohio State University (see Jackson, Stone, Ebrahimi, Briët, & Foster, 2015 for a complete description). Besides potted plants, the mesocosms contained four resting sites (large ceramic pots) and two shallow oviposition pans. Temperature, humidity and light conditions were maintained as described previously (Jackson et al., 2015; Stone et al., 2012). The average temperature and RH in the mesocosms, about 1 m above the floor, was 25.2 °C and 71%, respectively. Inside the resting sites, the average temperature and RH was 22.7 °C and 86%, respectively.
The plant composition varied among the four replicates in both treatments (Table 1). This variation was imposed by our intention to maintain roughly similar plant cover between replicates, rather than similar numbers of potted plants, because the size of the plants available for use varied. Additionally, the composition of the nectar-poor plants was changed between the first two and second two replicates, to see whether the flowering status and abundance of nectar-poor plants affected the performance of female mosquitoes. Flowering P. hysterophorus were in replicates one and two and P. hysterophorus lacking flowers were in replicates three and four; the latter two replicates also had fewer of those plants. To maintain approximately similar surface areas of foliage among all replicates in the nectar-poor environment, two varieties (Amate and Amate Soleil) of non-flowering Schefflera actinophylla (Endl.) Harms were introduced in the third and fourth replicates. These two varieties were chosen because they are not reported to produce extra-floral nectar, and no extra-floral nectaries were detected. The plants were watered daily, and every third day the sugar-poor plants were checked for the presence of plant-feeding insects (e.g., scale insects and aphids) or honeydew on the leaves. These pests were removed, and the leaves were cleaned with a damp paper towel. In the nectar-rich mesocosm, plant pests were removed at 3-day intervals, also, but honeydew was not. These interventions maximized differences in sugar production from the plants themselves. Plant infestations tend to be unrealistically heavy in greenhouses, and our current impression in the field in western Kenya is that honeydew is quickly removed by ants and wasps and that extra-floral nectar is the primary source of sugar for nocturnal mosquitoes from both nectar-poor and nectar-rich plants.
Table 1.
Plant compositions, with (+) and without (−) flowers, in the nectar-rich or nectar-poor mesocosms. Number of plants present in each mesocosm is indicated to the left of each species name
| Replicate | Nectar-rich | Nectar-poor |
|---|---|---|
| 1 | 4 Senna didymobotrya (−) | |
| 4 Ricinus communis (−) | 17 Parthenium hysterophorus (+) | |
| 2 Senna occidentalis (+) | 4 Lantana camara (−) | |
| 2 Tecoma stans (+) | ||
|
| ||
| 2 | 3 Senna didymobotrya (−) | |
| 3 Ricinus communis (−) | 18 Parthenium hysterophorus (+) | |
| 1 Senna occidentalis (+) | 4 Lantana camara (−) | |
| 1 Tecoma stans (+) | ||
|
| ||
| 3 | 3 Senna didymobotrya (−) | 3 Parthenium hysterophorus (−) |
| 3 Ricinus communis (−) | 3 Lantana camara (−) | |
| 1 Senna occidentalis (+) | 2 Schefflera actinophylla var. Amate (−) | |
| 1 Tecoma stans (+) | 4 S. actinophylla var. Amate Soleil (−) | |
|
| ||
| 4 | 4 Senna didymobotrya (−) | 3 Parthenium hysterophorus (−) |
| 3 Ricinus communis (−) | 2 Lantana camara (−) | |
| 1 Senna occidentalis (+) | 2 Schefflera actinophylla var. Amate (−) | |
| 1 Tecoma stans (+) | 3 S. actinophylla var. Amate Soleil (−) | |
After providing the mesocosms with plants, resting pots, and oviposition pans, equal numbers of pupae of a mixture of the sexes (totaling 1100–1300, depending on replicate) were placed in each of the two mesocosms in the afternoon. The following day (designated d 0), all remaining pupae were removed from the mesocosms and counted and subtracted from the pupal totals, to determine the number of adults emerging into a mesocosm overnight. They were as follows, according to replicate number, in nectar-rich and nectar-poor mesocosms, respectively: 1, 833 and 850; 2, 887 and 1,027; 3, 719 and 707; 4, 623 and 610. The proportions that were female, based on totals recovered both alive and dead at the end of 21 days, were as follows: 1, 48.1% and 50.0%; 2, 53.0% and 47.0%; 3, 48.0% and 45.5%; 4, 45.0% and 44.0%. To provide females with an opportunity to blood feed, a human host (B.E.) was available for 1 hour per mesocosm between the hours of 23:00 and 01:00 every night (Biomedical IRB protocol No. 200440193, Institutional Biosafety Protocol No. 2005R0020). The host wore a Tyvek ® coverall suit (TY122S, DuPont) with an 8- by 30-cm opening at the front part of each leg, so that mosquitoes would have access to blood only from the shins of the host’s legs. A video camera (Camcorder HDR-SR11, Sony) with a night vision feature and built-in infrared lamp was fixed on a tripod, about 1 m away from the host’s leg, and recorded the exposed part of the host’s legs during blood-feeding. Additional infrared lights (15-IL05, Cop Security, California, USA) were used to enhance the quality of recorded videos. The wavelengths of infrared lights were >900 nm, above the visual range of mosquitoes (Gibson 1995). The biting rate was estimated by counting the number of engorged mosquitoes on the legs divided by the estimated number of live females for a particular night, deduced from daily mortality results.
Every morning, the mesocosms were inspected for dead mosquitoes. The dead were noticeable in contrast to the white floor and thus could be collected easily. Resting sites as well as plant leaves and plant pots (the sides and soil of which were covered with white stocking material) were also checked for dead individuals. Wing length, an approximation of body size of both sexes, and the insemination status of dead females, was recorded, because of their potential effects on behaviour. Wings were measured by ocular micrometer in a stereomicroscope; each spermatheca beneath a coverslip was inspected for sperm by compound microscope. If the number of collected females or males per day was < 20, all of them were examined. At least half of 20–35 collected bodies, and 20–25% of more than 35 bodies, were randomly selected for microscopic examination. Due to an error, wing measurements for replicate 2 were not recorded. At the end of each experiment (day 21) of the four replicates, any remaining live mosquitoes were collected by aspirator, counted, and the wing-lengths of 20–25% of them measured.
Oviposited eggs were transferred daily from the oviposition sites to a round filter paper (Whatman 1001–320, GE Healthcare Bio-Sciences, Pittsburg, PA, USA), scanned (Deskjet 1051, HP), and their numbers estimated using ImageJ (Mains, Mercer & Dobson 2008). Daily fecundity was calculated by dividing the number of eggs by the estimated number of living females. Net reproductive rate was based on the average egg count per female over 21 days.
The vectorial capacity of a mosquito population is given by the following equation:
where m is the density of mosquitoes per host (either arbitrarily held at a constant 50 per host or calculated from adult fecundity and survival data) and n is the extrinsic incubation period (an arbitrary 12 days). We calculated C using daily experimental values for a (biting rate) and g (mortality rate). Thus, for each replicate we calculated it 21 times, using daily values. This method provided the expected number of infective bites that would arise from an infective host exposed on a particular day, assuming that the estimates for mosquito mortality, biting rate, and fecundity (f) corresponded to those measured on this particular day. See Appendix S1 for further details.
Bloodless survival
To estimate the effect of plant species on mosquito survivorship in the absence of blood sources, an experiment was conducted with the plant compositions similar to the fourth replicate of the vectorial capacity experiment (Table 1) but without human-host exposure. The experiment lasted only until the survivorship in the nectar-poor mesocosm fell to approximately 10%.
Sugar accessibility
To further assess whether the flowers of P. hysterophorus affected its sugar accessibility, the mosquitoes were exposed to flowering and non-flowering plants in small (i.e., 0.3 m3) plastic cages. The shoots of potted plants were inserted into the cage through an X-slit at the bottom of each cage, and then the opening was sealed. A water wick and two black plastic cups (10 cm diameter), serving as resting sites, were included in the cage. In each of four replicates, 20–25 1-d-old males and females were separately exposed to the plants overnight for 12 h. Early the following morning, mosquitoes were collected and stored at −70 °C until they were tested for fructose by cold anthrone (Haramis & Foster, 1983; van Handel, 1972). Three replicates of a similar experiment were conducted with non-flowering Senna occidentalis L. (Fabaceae), a legume with conspicuous extra-floral nectaries, to estimate the proportion of mosquitoes that may take sugar from a sugar-rich plant under identical conditions.
Statistical analyses
Kaplan-Meier survivorship curves were constructed for males and females of each replicate. Differences in survivorship between treatments were assessed using a Cox proportional-hazard analysis in R (R Development Core Team 2014). Replicate and an interaction between replicates and treatment were included as terms in the model. The data from male and female mosquitoes were analysed separately. Mosquitoes that were still alive at the end of the experiment, as well as those that were killed accidentally during the experiment, were entered as censored data points because they had unknown times of natural death. The differences between the vectorial capacity estimates of the two treatment groups were analysed using generalized least squares. The model included treatment and time (day) as factors, as well treatment as a random term to allow for heterogeneity of variance, and incorporated an auto-regressive model of order 1 (AR-1) temporal autocorrelation structure (Zuur, Ieno, Walker, Saveliev, & Smith, 2009). To maximize the sensitivity of the tests, we used the mean of the combined four daily values of all four replicates of each treatment (n = 21).
Results
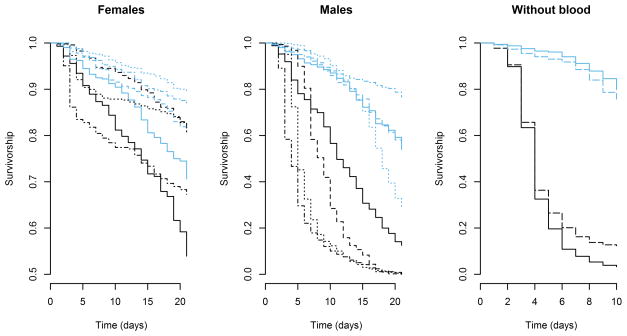
Mosquitoes of both sexes survived much longer in nectar-rich environments (Cox proportional hazard, Z = 5.307, P <0.0001) (Table 2, Figure 1). The nectar-poor community had a stronger negative effect on survival of males than on females. For both males and females, a model with an interaction term for replicate and treatment was the minimal adequate model. In the nectar-rich environment, using a parametric survival regression with a Weibull error distribution, the predicted mean survival times for females and males were 79.6 and 27.6 days, respectively. Mean survival times for females and males in the nectar-poor environment were respectively 53.3 and 9.5 days, respectively.
Table 2.
Life history trait values (daily and lifetime fecundity and daily biting and mortality rates) obtained for nectar-poor and nectar-rich mesocosms, by replicate
| Treatment | Replicate | Fecundity, β | Biting rate, a | Predicted mean lifespan of females | Net reproductive rate (Ro) at day 21 |
|---|---|---|---|---|---|
| Poor | 1 | 38.66 | 0.426 | 28.9 | 402.7 |
| 2 | 29.05 | 0.305 | 53.3 | 388.1 | |
| 3 | 42.62 | 0.362 | 80.46 | 638.7 | |
| 4 | 35.92 | 0.486 | 53.9 | 408.1 | |
| Rich | 1 | 34.89 | 0.381 | 40.9 | 565.4 |
| 2 | 39.08 | 0.371 | 53.0 | 597.2 | |
| 3 | 49.47 | 0.344 | 143.1 | 671.2 | |
| 4 | 35.59 | 0.504 | 169.3 | 509.1 |
Figure 1.
Survivorship curves for 4 replicates of female and male mosquitoes under nectar-rich and nectar-poor conditions. The blue lines represent nectar-rich environments, and the black lines nectar-poor environments. For females (left panel) and males (center panel), corresponding paired symbols represent trials from the same replication. A shorter trial (without blood) illustrated the impact of sugar on female survival in the absence of a blood source (right panel). Here, solid lines represent females, and dashed lines males
The mean daily biting rates of mosquitoes in the rich and poor environments were not significantly different (Table 2). The mean biting rates in the rich and poor mesocosms were, respectively, 0.39 (SE = 0.04) and 0.40 (SE = 0.04) per night (paired t-test = 0.22, df = 3, P = 0.84).
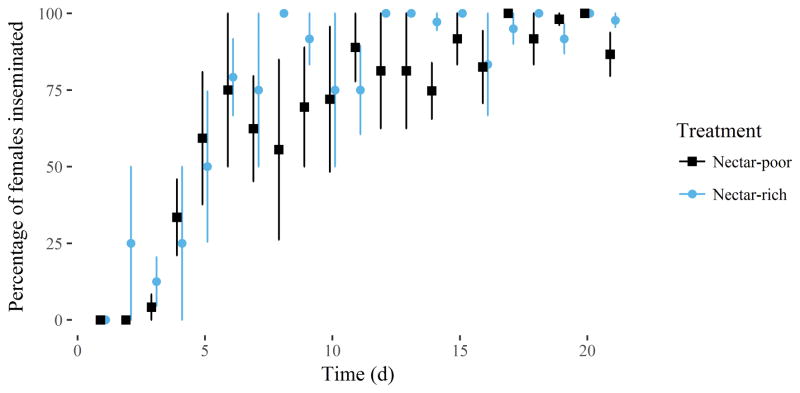
The mean insemination rates in the nectar-rich mesocosm (77.8%, SE = 4.1%) and the sugar-poor one (66.8%, SE = 4.3%) were significantly different (Figure 2) (paired t-test = 2.05, df = 83, P = 0.04). But daily fecundity in the two environments of each replicate did not differ (Table 2). Mean net replacement rates (fecundities up to day 21, Ro), on the other hand, were much greater in the nectar-rich mesocosm in every replicate, owing to the extended longevity there, averaging 101 eggs more than in the poor one by that time.
Figure 2.
Mean insemination rate of dead females (± 1 SE), collected daily from the nectar-rich (blue) and nectar-poor (black) environments
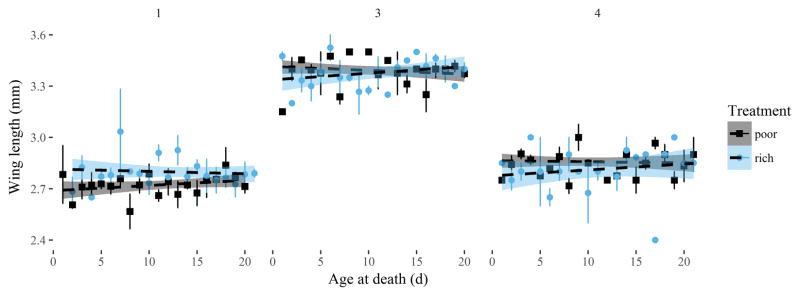
Wing lengths of females (i.e., body size) varied among replicates, despite efforts to maintain consistent rearing procedures. In replicate 1, the mean wing length of the combined dead and surviving females in the nectar-rich plant assemblage (2.80 mm, SD = 0.10 mm) was significantly larger than in the nectar-poor one (2.73 mm, SD = 0.15 mm) (t = 2.29, df = 65, P = 0.03). In replicates 3 and 4 there was no difference in female wing lengths between plant treatments. Within each replicate, there was no noticeable effect of body size on the age at death (Figure 3). The mean wing length of females that survived until the end of the experiments differed among replicates (ANOVA: F 3,212 = 364.6, P < 0.0001), following this order for the replicates within the nectar-rich treatment: 3 (3.4 mm) > 2 (3.34 mm) > 4 (2.86 mm) > 1 (2.76 mm).
Figure 3.
Mean wing length and 95% CI of female mosquitoes by their age at death (days) for replicates 1, 3, and 4
Vectorial Capacity
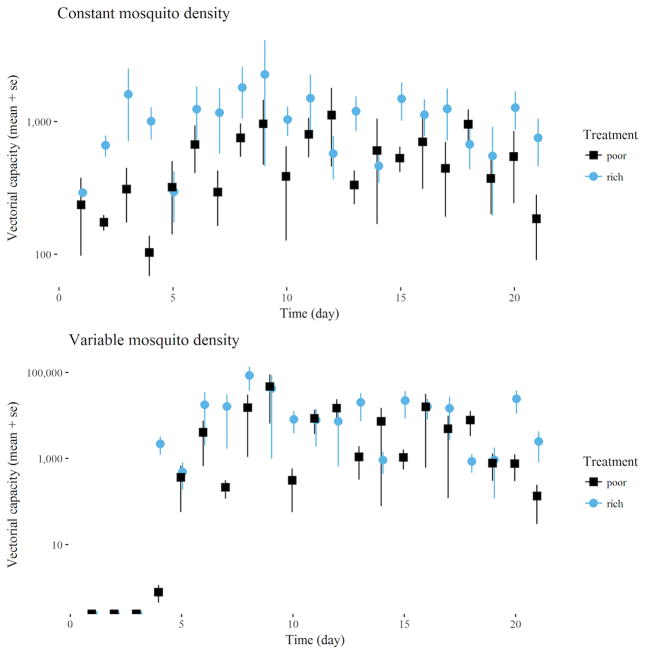
The survival, fecundity, and biting-rate estimates, above, were used to calculate vectorial capacity (C) for the nectar-rich and nectar-poor environments of each replicate (Figure 4). When we assumed a constant value of 50 for m (density), the mean (± SE) C values of the nectar-poor and nectar-rich mesocosms were 518.27 ± 60.6 and 1,089.48 ± 125.18, respectively, and this difference was statistically significant (t = 3.62, P = 0.008). When we instead calculated m as a function of female survival and fecundity, the differences were greater, yet the variances also were greater, so the C values were not statistically different (t = 1.09, P = 0.28). Nonetheless, in each of the paired replicates, C in the nectar-rich environment was greater than that in the nectar-poor environment. The mean estimates were 5,954.5 (± 2,078.3) and 14,431 (± 3,716.8) for the poor and rich mesocosms, respectively.
Figure 4.
Mean values for vectorial capacity (± 1 SE) over 4 replicates in nectar-poor and nectar-rich mesocosms, by day of the experiment
Bloodless Survival
Without blood, mosquitoes’ lives were shorter, even when sugar was abundant. In the nectar-rich environment, the predicted mean survival times (using a parametric survival regression with a Weibull error distribution) for females and males were 19.0 and 20.2 days, respectively (cf. the 79.6-day estimated female survival with blood; see above). In the nectar-poor environment, they were 4.9 and 5.7 days, respectively. The median ages at death for both sexes among mosquitoes that died during the 10-day trial were 4 and 8 days in the poor and rich environments, respectively. The effects of the plant treatment, sex, and an interaction between the two, were significant (Cox proportional hazard, P <0.001).
Sugar accessibility
After overnight exposure to flowering or flowerless P. hysterophorus, very small proportions of mosquitoes were fructose-positive: 7.2 and 1.8% of the females and males, respectively, a significant treatment difference (Z = 2, P = 0.041). When the sexes were analysed separately, the treatment differences in positivity were not significant (females: Z = 1.6, males: Z = 1.3, P > 0.05). In the presence of flowering plants, significantly more females (11.3%) than males (3.1%) were fructose positive (Z = 2.2, P = 0.027). In the absence of the flowers, the percentages dropped to 3.6 and 0% for females and males, respectively, a non-significant difference (Z = 1.3, P > 0.05). In stark contrast, after overnight exposure to S. occidentalis, 50% of females and 46.2% of males were fructose-positive. The sex difference was not significant (Z = 0.4, P > 0.05).
Discussion
The results support this study’s principal hypothesis, that the composition of African plant assemblages affects key components of A. gambiae’s vectorial capacity (C). In each of the four experimental replicates in large semi-natural enclosures, the nectar-rich assemblage always yielded a higher C. The difference observed can be attributed almost entirely to the substantially greater survival of females in the nectar-rich environments. The mean biting rates and fecundities, on the other hand, were practically the same in both environments and therefore had little or no effect. When the calculation included a vector-density factor that was dependent on combined daily female fecundity and survival, the differences in C between nectar-rich and nectar-poor plant communities were even greater, though with greater variance. One can conclude that people living among plant communities with abundant nectar-providing species will be under higher malaria-transmission pressure.
This causal connection between nectar-rich plants and a higher C is in general agreement with lab-cage experiments on A. gambiae (Gary & Foster, 2001, 2004; Straif & Beier, 1996) and with the preliminary conclusions of Gu, Müller, Schlein, Novak, and Beier (2011) and Beier, Müller, Gu, Arheart, and Schlein (2012), whose field studies on Anopheles sergentii (Theobald) compared two oases differing in available nectar. Those field studies detected an extraordinarily large difference in estimated C, with the nectar-rich site giving much higher survival and biting rates. In our earlier mesocosm study with A. gambiae (Stone et al., 2012), with access to blood each dawn, survival was better in the nectar-rich environment in three out of four replicates, but biting rate was lower, causing C to be pulled in opposite directions, creating different outcomes among replicates. We attribute the consistent results of this new study primarily to a more natural time for blood host exposure. The suitable blood-feeding time, in particular, led to biting frequencies that were more uniform and minimally affected by the availability of plant nectar.
Survival in the nectar-rich environment was consistently superior to the nectar-poor one. Most studies examining the effect of sugar on mosquito survival and its connection to blood feeding have been conducted in small laboratory cages where sugar is always easily accessible and flight is constrained. These tests have given conflicting results, indicating either that sugar-alone, sugar-plus-blood, or blood-alone regimes allowed maximum survival, depending on methods (Braks, Juliano, & Lounibos, 2006; Foster, 1995; Gary, 2005; Gary & Foster, 2001; Harrington, Edman, & Scott, 2001; Straif & Beier, 1996; Styer, Minnick, Sun, & Scott, 2007). However, both Gu et al. (2011) and Beier et al. (2012) inferred that in the nectar-rich oasis A. sergentii females lived much longer. The present study with A. gambiae further supports that result unequivocally.
The reason for the pronounced differences in survival between plant treatments, whether or not blood was available, is uncertain. We speculate that in the nectar-poor communities, sugar was insufficient to meet the flight demands required for mating, ovipositing, and all resource-foraging activities within the spacious mesocosms, this despite the energy also acquired from blood meals. This insufficiency would cause the mosquitoes to incur an energy deficit, gradually leading to early death. In the bloodless experiment, the lower survival rate, even in the nectar-rich environment, compared to the equivalent experiment in which blood was available, presents a separate issue. Apparently access to both blood and sugar provides a nutritional advantage over a sugar-only diet, which confirms the results obtained with A. gambiae in the laboratory (Gary & Foster 2001).
The similarity of biting rates in the nectar-rich and nectar-poor mesocosms was unexpected. Despite their high variability among replicates (Table 2), the average biting frequencies in the two treatments were almost the same, about 0.4 bites per female per night. This contradicts the general perception of sugar-inhibited blood feeding in mosquitoes in general, including A. gambiae, based on lab-cage studies. In those cases, females without sugar took blood more frequently from emergence onward (Gary & Foster 2001), and a higher blood-feeding frequency in the absence of sugar was found in the oldest third of female cohorts (Straif & Beier 1996). That effect was consistent with our earlier mesocosm study (Stone et al., 2012), in which there was a tendency for higher biting rates in nectar-poor environments. However, quite the opposite was reported for natural populations of A. sergentii, the interval between reproductive cycles, i.e., blood feedings, being significantly longer in the nectar-poor oasis (Gu et al. 2011). That result remains to be explained. In this new mesocosm study, any suppression of responsiveness to blood-host stimuli in A. gambiae due to nectar abundance might have been nullified by the extended opportunity for biting at the ideal time, rather than near sunrise, when host-responsiveness would be declining and shelter-seeking taking precedence. What role these factors play under the complex conditions occurring in the field is unknown.
The lack of an association between the proportion of females inseminated and their fecundity is puzzling. In the earlier mesocosm study (Stone et al., 2012), no plant-treatment difference in mean daily fecundity was detected, and also no statistical difference in net replacement rate and intrinsic rate of increase. In the present experiment, mean fecundities varied within a replicate, but again there was no overall difference in daily egg output per female between nectar-poor and nectar-rich mesocosms, despite a significant difference in the proportions inseminated. In the complete absence of sugar, a male cohort’s ability to inseminate can be severely reduced (Gary et al., 2009; Stone, Taylor, Roitberg, et al., 2009). As expected, in the present study nectar-poor plants substantially lowered male survival, and consequently the insemination rate suffered. One might therefore expect that because uninseminated females of this strain do not oviposit, fewer eggs would be laid per female per day. Yet, we failed to detect a statistically significant lower daily egg output.
In other species, including not only A. gambiae s.l., but also A. aegypti (L.) and Aedes albopictus (Skuse), laboratory-caged females are reported to attain a similar or greater intrinsic rate of increase on a human blood-only diet, despite the reduced lifespan (Braks et al., 2006; Gary, 2005; Gary & Foster, 2001; Harrington et al., 2001; Styer et al., 2007). This raises the question: If sugar either has no effect on reproductive fitness, or even works against it, why do they sugar-feed? The present results suggest the possibility that sugar’s suppression of biting frequency and daily fecundity in these lab-cage studies may be an artifact of restriction within a cage supplied with abundant sugar, and possibly also limited time for blood feeding at an appropriate time of night. To gain insight into the true fitness consequences of plant sugar on anthropophilic species in nature, the following must be taken into account: a) oviposition-site abundance, quality, and diversity, b) resulting effects of them on female oviposition behaviour and consequent offspring success in the field, and c) times when population growth is close to zero, so that more eggs laid over an extended period (Ro) may contribute more to reproductive fitness than does the theoretical intrinsic rate of increase (r). Additionally, maternal nutrition may have effects on the quality of the offspring. Differences in egg viability seemed unlikely in this study, because hatching rates of 4–6 day old eggs collected from the two environments of the fourth replicate were not conspicuously different and were at the high end of the range observed in hatching-rate studies (Ebrahimi, Shakibi & Foster 2014). Access to sugar has been shown to affect the lipid-to-protein ratio (but not total calories) in eggs of A. gambiae (Fernandes & Briegel 2005), which may translate to larval success, but this has not been investigated. And the adult offspring of females from whom sugar was withheld possibly have a lower fecundity themselves (C. M. Stone, unpubl. data).
We surmise that body size was not a confounding factor in this study of nectar’s effect. Previous laboratory studies have found that larger mosquitoes survive longer (Ameneshewa & Service 1996; Briegel 2003). Although the data did not allow us to include size in the survival analysis directly, it should be noted that the mean body size of the surviving females co-varied with survivorship between replicates, but not within them.
This study has intrinsic limitations. For example, only one person served as a blood-meal source. Humans vary in their blood composition and attractiveness to mosquitoes, so extrapolation to general effects of nectar-sources on biting behavior and fecundity must be made with caution. Another limitation is the number of replicates and the length of each one. The number of replicates performed (4) precludes a useful statistical comparison of certain outcomes (e.g., the factors in Table 2). The 3-week test period was based on other experiments, when the cohorts had largely died out by this time (Stone et al., 2012). In the current investigation, this was not the case, and estimates of longevity (Table 2) were unrealistically long, certainly for field conditions, where hazards are much greater. Had we followed cohorts still further, we would expect to see the effects of senescence (Styer et al. 2007). Thus, the predicted lifespans are likely an overestimate. This did not affect the overall conclusion, however, given that longevity is consistently greater with access to nectar-bearing plants. However, new mesocosm experiments could be designed so that they are divided into those with parameters that must be measured over lengthy periods, and into those requiring less time but greater replication.
We purposely managed temperature, humidity, mosquito dispersal, plant infestations, the presence of predators, and adult size to minimize extraneous variation. Further studies might evaluate the effect on C of each of these factors in concert with different plant species assemblages. Despite the flight opportunities in mesocosms, the blood, nectar, and oviposition-site foraging range was limited to less than 100 m3 and did not accommodate long-range translocation between blood hosts, plant hosts, and development sites. Thus, follow-up studies under field conditions will prove insightful.
Several topics deserve further attention, particularly an empirical analysis that places greater emphasis on the interactions between larval nutrition (which affects development-site productivity and adult body size) and adult nutrition. These shape both estimates of C and density-dependent population dynamics of mosquitoes, and their interaction may have great influence on local malaria epidemiology. Furthermore, as noted above, the degree of vector competence is a critical component not incorporated into C, here. In reality, both variations in sugar content and secondary metabolites of nectar, not just the inherent characteristics of the vectors, may greatly alter vector competence in natural populations (Hien et al., 2016; Kelly & Edman, 1997) and thus the efficiency of malaria transmission.
Synthesis and applications
This study’s findings lend themselves to several practical applications, both to determine how the environment affects vector-borne disease transmission intensity and to implement novel methods of integrated vector control. For example, the profound effect of plant sugar on male survival and consequent female insemination rate can be exploited to increase the effectiveness of the sterile-male technique to suppress populations. Removal of sugar-rich plants would undercut the natural male population, after which released sugar-fed sterile males would have a competitive mating advantage.
Vectorial capacity outcomes of this study with application include these two possibilities: 1) Areas at high risk for malaria transmission can be identified by considering not only development sites and rainfall patterns, but also the presence of plant communities that promote vectorial capacity. This requires field work to establish the relation between, for instance, the normalized difference vegetation index (NDVI) and nectar-feeding behaviour. It would be assisted by evidence for the connection between spatial patterns of plant species occurrence, species richness, or vegetation type (obtained either in fine-scale trials or through remote sensing, e.g., Gould 2000) and intensity of malaria transmission. 2) Favourable nectar sources can be reduced, such as eliminating nectar-rich plants and replacing them with nectar-poor ones, to reduce vector survival. This approach is particularly feasible for island-like ecosystems, such as patches of abundant vegetation around villages in otherwise arid areas, where selective removal and replacement of plant species can cause a striking reduction or elimination of a vector (Beier et al. 2012). Environmental management of this type is likely to be more complicated and costly in verdant and biodiverse expanses of the wet tropics. Furthermore, alteration of plant communities can contribute to a surge of other vectors that are more fit in the new niche (Watson 1905) or can affect the microclimate and thus change the extrinsic incubation period of Plasmodium (Afrane, Little, Lawson, Githeko, & Yan, 2008).
In large biodiverse areas, where removal of attractive nectar-rich plants is impractical, their treatment with toxic sugar around human habitations is one option. Such approaches can be used in conjunction with competing plant-based attractive toxic sugar baits (ATSB), either on peridomestic plants or in bait stations inside or near houses (Qualls et al. 2016). These integrated vector management (IVM) methods can be especially effective where transmission is intense, insecticide resistance is common, or indoor residual sprays and long-lasting insecticidal nets are insufficient to reduce malaria transmission (Brady et al. 2016).
A special case is the management of prominent invasive weeds, which can come to occupy large swaths of agricultural land, rendering them useless while perhaps simultaneously favouring malaria vectors. Several of the plants used in this study, such as P. hysterophorus, S. occidentalis, and Lantana camara are invasive to equatorial Africa and are reported to provide mosquitoes with nectar. Assessing their impact on pathogen transmission is made complicated if different populations of the same species differ in their traits in different environments. For instance, the status of P. hysterophorus as a poor provider of sugar—it appears that although the flowers do yield nectar, they do so only in very small quantities that are rapidly digested—conflicts with the results of Nyasembe et al. (2015). One possible explanation for this discrepancy is that a more nectariferous biotype exists in upland Kenya, near Nairobi; ours was from Mbita Point (Lake Victoria region), the source of our mosquito strain, where malaria is endemic. Understanding how both the spread and potential removal of invasives impact malaria transmission is a pressing concern. The present study indicates that changes in plant assemblages, due to introduction of exotic species, may indeed have a strong impact on mosquito populations and their capability to transmit pathogens. Studies performed under field conditions in a range of environments will now be required to see how predictable and consistent these outcomes are.
Supplementary Material
Appendix S1: Vectorial capacity
Acknowledgments
We thank Joan Leonard, Emily Yoders-Horn, and George Keeney for their support in the OSU Biological Sciences Greenhouse. The establishment and maintenance of the Mbita strain of A. gambiae by many personnel of the Thomas Odhiambo campus of the International Centre of Insect Physiology and Ecoloy is gratefully acknowledged. This research was supported by National Institutes of Health (NIH) grant R01-AI077722 from the National Institute of Allergy & Infectious Diseases (NIAID) to W.A.F. Its content is solely the responsibility of the authors and does not represent the official views of NIAID or NIH.
Footnotes
The following Supporting Information is available for this article online.
Authors’ contributions
B.E., B.T.J., C.M.S., and W.A.F. conceived the ideas and designed methodology; B.E., B.T.J., J.L.G., and C.M.P. collected the data; C.M.S. and B.E. analysed the data; B.E., C.M.S. and W.A.F. wrote the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Data accessibility
Data available from the Dryad Digital Repository http://dx.doi.org/10.5061/dryad.s066f (Ebrahimi et al. 2017).
References
- Afrane YA, Little TJ, Lawson BW, Githeko AK, Yan G. Deforestation and vectorial capacity of Anopheles gambiae Giles mosquitoes in malaria transmission, Kenya. Emerging Infectious Diseases. 2008;14:1533–1538. doi: 10.3201/eid1410.070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameneshewa B, Service MW. The relationship between female body size and survival rate of the malaria vector Anopheles arabiensis in Ethiopia. Medical and Veterinary Entomology. 1996;10:170–172. doi: 10.1111/j.1365-2915.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Beier JC, Müller GC, Gu W, Arheart KL, Schlein Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malaria Journal. 2012;11:1–7. doi: 10.1186/1475-2875-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briët O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CLJ, Smith DL, Hay SI, Cibulskis RE, Gething PW. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady OJ, Godfray HCJ, Tatem AJ, Gething PW, Cohen JM, McKenzie FE, Perkins TA, Reiner RC, Tusting LS, Sinka ME, Moyes CL, Eckhoff PA, Scott TW, Lindsay SW, Hay SI, Smith DL. Vectorial capacity and vector control: reconsidering sensitivity to parameters for malaria elimination. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2016;110:107–117. doi: 10.1093/trstmh/trv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks MAH, Juliano SA, Lounibos LP. Superior reproductive success on human blood without sugar is not limited to highly anthropophilic mosquito species. Medical and Veterinary Entomology. 2006;20:53–59. doi: 10.1111/j.1365-2915.2006.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel H. Physiological bases of mosquito ecology. Journal of Vector Ecology. 2003;28:1–11. [PubMed] [Google Scholar]
- Case TJ. Illustrated guide to theoretical ecology. Oxford University Press; New York: 2000. [Google Scholar]
- Dye C. The analysis of parasite transmission by bloodsucking insects. Annual Review of Entomology. 1992;37:1–19. doi: 10.1146/annurev.en.37.010192.000245. [DOI] [PubMed] [Google Scholar]
- Ebrahimi B, Jackson BT, Guseman JL, Przybylowicz CM, Stone CM, Foster WA. Data from: Alteration of plant-species assemblages can decrease the transmission potential of malaria mosquitoes. Dryad Digital Repository. 2017 doi: 10.1111/1365-2664.13001. http://dx.doi.org/10.5061/dryad.s066f. [DOI] [PMC free article] [PubMed]
- Ebrahimi B, Shakibi S, Foster WA. Delayed egg hatching of Anopheles gambiae (Diptera: Culicidae) pending water agitation. Journal of Medical Entomology. 2014;51:580–590. doi: 10.1603/me13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson HM, Read AF. Genetic and environmental determinants of malaria parasite virulence in mosquitoes. Proceedings of the Royal Society B: Biological Sciences. 2002;269:1217–1224. doi: 10.1098/rspb.2002.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes L, Briegel H. Reproductive physiology of Anopheles gambiae and Anopheles atroparvus. Journal of vector ecology. 2005;30:11. [PubMed] [Google Scholar]
- Foster WA. Mosquito sugar feeding and reproductive energetics. Annual Review of Entomology. 1995;40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- Garrett-Jones C. Prognosis for interruption of malaria transmission through assessment of the mosquito’s vectorial capacity. Nature. 1964;204:1173–1175. doi: 10.1038/2041173a0. [DOI] [PubMed] [Google Scholar]
- Gary RE. PhD Thesis. The Ohio State University; Columbus: 2005. Biology of the malaria vector Anopheles gambiae: Behavioral and reproductive components of sugar feeding. [Google Scholar]
- Gary RE, Cannon JW, III, Foster WA. Effect of sugar on male Anopheles gambiae mating performance, as modified by temperature, space, and body size. Parasites & Vectors. 2009;2:19. doi: 10.1186/1756-3305-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary RE, Foster WA. Effects of available sugar on the reproductive fitness and vectorial capacity of the malaria vector Anopheles gambiae (Diptera: Culicidae) Journal of Medical Entomology. 2001;38:22–28. doi: 10.1603/0022-2585-38.1.22. [DOI] [PubMed] [Google Scholar]
- Gary RE, Foster WA. Anopheles gambiae feeding and survival on honeydew and extra-floral nectar of peridomestic plants. Medical and Veterinary Entomology. 2004;18:102–107. doi: 10.1111/j.0269-283X.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- Gibson G. A behavioural test of the sensitivity of a nocturnal mosquito, Anopheles gambiae, to dim white, red and infra-red light. Physiological Entomology. 1995;20:224–228. [Google Scholar]
- Godfray HCJ. Mosquito ecology and control of malaria. Journal of Animal Ecology. 2013;82:15–25. doi: 10.1111/1365-2656.12003. [DOI] [PubMed] [Google Scholar]
- Gould W. Remote sensing of vegetation, plant species richness, and regional biodiversity hotspots. Ecological Applications. 2000;10:1861–1870. [Google Scholar]
- Gu W, Müller G, Schlein Y, Novak RJ, Beier JC. Natural plant sugar sources of Anopheles mosquitoes strongly impact malaria transmission potential. PLoS ONE. 2011;6:e15996. doi: 10.1371/journal.pone.0015996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramis LD, Foster WA. Visual quantification of sugar in mosquitoes using anthrone reagent. Mosquito news. 1983;43:362–364. [Google Scholar]
- Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? Journal of Medical Entomology. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- Hien DFdS, Dabiré KR, Roche B, Diabaté A, Yerbanga RS, Cohuet A, Yameogo BK, Gouagna LC, Hopkins RJ, Ouedraogo GA, Simard F, Ouedraogo JB, Ignell R, Lefevre T. Plant-mediated effects on mosquito capacity to transmit human malaria. PLOS Pathogens. 2016;12:e1005773. doi: 10.1371/journal.ppat.1005773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impoinvil DE, Kongere JO, Foster WA, Njiru BN, Killeen GF, Githure JI, Beier JC, Hassanali A, Knols BGJ. Feeding and survival of the malaria vector Anopheles gambiae on plants growing in Kenya. Medical and Veterinary Entomology. 2004;18:108–115. doi: 10.1111/j.0269-283X.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- Jackson BT, Stone CM, Ebrahimi B, Briët OJT, Foster WA. A low-cost mesocosm for the study of behaviour and reproductive potential in Afrotropical mosquito (Diptera: Culicidae) vectors of malaria. Medical and Veterinary Entomology. 2015;29:104–109. doi: 10.1111/mve.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R, Edman JD. Infection and transmission of Plasmodium gallinaceum (Eucoccidia: Plasmodidae) in Aedes aegypti (Diptera: culicidae): Effect of pre-infection sugar meals and post-infection blood meals. Journal of vector ecology. 1997;22:36–42. [PubMed] [Google Scholar]
- Lalubin F, Delédevant A, Glaizot O, Christe P. Natural malaria infection reduces starvation resistance of nutritionally stressed mosquitoes. The Journal of Animal Ecology. 2014;83:850–857. doi: 10.1111/1365-2656.12190. [DOI] [PubMed] [Google Scholar]
- Lambrechts L, Chavatte JM, Snounou G, Koella JC. Environmental influence on the genetic basis of mosquito resistance to malaria parasites. Proceedings of the Royal Society B: Biological Sciences. 2006;273:1501–1506. doi: 10.1098/rspb.2006.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains JW, Mercer DR, Dobson SL. Digital image analysis to estimate numbers of Aedes eggs oviposited in containers. Journal of the American Mosquito Control Association. 2008;24:496–501. doi: 10.2987/5740.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda H, Gouagna LC, Foster WA, Jackson RR, Beier JC, Githure JI, Hassanali A. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malaria Journal. 2007a;6:113. doi: 10.1186/1475-2875-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda H, Gouagna LC, Nyandat E, Kabiru EW, Jackson RR, Foster WA, Githure JI, Beier JC, Hassanali A. Discriminative feeding behaviour of Anopheles gambiae s.s. on endemic plants in western Kenya. Medical and Veterinary Entomology. 2007b;21:103–111. doi: 10.1111/j.1365-2915.2007.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArt SH, Koch H, Irwin RE, Adler LS. Arranging the bouquet of disease: floral traits and the transmission of plant and animal pathogens. Ecology Letters. 2014;17:624–636. doi: 10.1111/ele.12257. [DOI] [PubMed] [Google Scholar]
- Mordecai EA, Paaijmans KP, Johnson LR, Balzer C, Ben-Horin T, de Moor E, McNally A, Pawar S, Ryan SJ, Smith TC, Lafferty KD. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecology Letters. 2013;16:22–30. doi: 10.1111/ele.12015. [DOI] [PubMed] [Google Scholar]
- Nikbakhtzadeh MR, Terbot JW, II, Foster WA. Survival value and sugar access of four east African plant species attractive to a laboratory strain of sympatric Anopheles gambiae (Diptera: Culicidae) Journal of Medical Entomology. 2016;53:1105–1111. doi: 10.1093/jme/tjw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasembe VO, Cheseto X, Kaplan F, Foster WA, Teal PEA, Tumlinson JH, Borgemeister C, Torto B. The invasive American weed Parthenium hysterophorus can negatively impact malaria control in Africa. PLoS ONE. 2015;10:e0137836. doi: 10.1371/journal.pone.0137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okech BA, Gouagna LC, Kabiru EW, Beier JC, Yan G, Githure JI. Influence of age and previous diet of Anopheles gambiae on the infectivity of natural Plasmodium falciparum gametocytes from human volunteers. Journal of Insect Science. 2004:4. doi: 10.1093/jis/4.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham PE, Michael E. Modeling the effects of weather and climate change on malaria transmission. Environmental Health Perspectives. 2009;118:620–626. doi: 10.1289/ehp.0901256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualls WA, Scott-Fiorenzano J, Müller GC, Arheart KL, Beier JC, Xue RD. Evaluation and adaptation of attractive toxic sugar baits for Culex tarsalis and Culex quinquefasciatus control in the Coachella Valley, southern California. Journal of the American Mosquito Control Association. 2016;32:292–299. doi: 10.2987/16-6589.1. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: 2014. [Google Scholar]
- Reisen WK. Landscape epidemiology of vector-borne diseases. Annual Review of Entomology. 2010;55:461–483. doi: 10.1146/annurev-ento-112408-085419. [DOI] [PubMed] [Google Scholar]
- Smith DL, McKenzie FE. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malaria Journal. 2004;3:13. doi: 10.1186/1475-2875-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone CM, Jackson BT, Foster WA. Effects of plant-community composition on the vectorial capacity and fitness of the malaria mosquito Anopheles gambiae. The American Journal of Tropical Medicine and Hygiene. 2012;87:727–736. doi: 10.4269/ajtmh.2012.12-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone CM, Taylor RM, Foster WA. An effective indoor mesocosm for studying populations of Anopheles gambiae in temperate climates. Journal of the American Mosquito Control Association. 2009;25:514–516. doi: 10.2987/08-5885.1. [DOI] [PubMed] [Google Scholar]
- Stone CM, Taylor RM, Roitberg BD, Foster WA. Sugar deprivation reduces insemination of Anopheles gambiae (Diptera: Culicidae), despite daily recruitment of adults, and predicts decline in model populations. Journal of Medical Entomology. 2009;46:1327–1337. doi: 10.1603/033.046.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straif SC, Beier JC. Effects of sugar availability on the blood-feeding behavior of Anopheles gambiae (Diptera: Culicidae) Journal of Medical Entomology. 1996;33:608–612. doi: 10.1093/jmedent/33.4.608. [DOI] [PubMed] [Google Scholar]
- Styer LM, Minnick SL, Sun AK, Scott TW. Mortality and reproductive dynamics of Aedes aegypti (Diptera: Culicidae) fed human blood. Vector-Borne and Zoonotic Diseases. 2007;7:86–98. doi: 10.1089/vbz.2007.0216. [DOI] [PubMed] [Google Scholar]
- Van Handel E. The detection of nectar in mosquitoes. Mosquito News. 1972;32:458–458. [Google Scholar]
- Watson M. The effect of drainage and other measures on the malaria in Klang, Federated Malay States. J Trop Med. 1905;8:100–104. [Google Scholar]
- Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. Springer; New York, New York, NY: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Vectorial capacity