Abstract
Background
Sleep and olfaction are both critical physiological processes that tend to worsen with age. Decline in olfaction can be an early indicator of neurodegenerative diseases whereas poor sleep quality is associated with reduced physical and mental health. Given associations with aging-related health declines, we explored whether variations in sleep were associated with olfactory function among older adults.
Methods
We assessed the relationship between sleep characteristics and olfaction among 354 community-dwelling older adults. Olfaction was measured using a validated field and survey research tool. Sleep characteristics were measured using wrist actigraphy and self-report of sleep problems. We fit structural equation models of latent constructs of olfaction based on olfactory task items and let this be a function of each sleep characteristic.
Results
Actigraph sleep quality measures were associated with odor identification, but not with odor sensitivity. Longer duration sleepers had worse odor sensitivity compared to medium (5 to 8 hours) sleepers but sleep duration was not associated with odor identification. Reported sleep problems and reported usual duration were not associated with olfaction.
Conclusions
Diminished sleep quality was associated with reduced capacity to identify odors. Determining whether this is a causal association will require further study and longitudinal data.
Keywords: Olfaction, sleep, actigraphy, aging
Introduction
Olfaction is critical for human health and is involved in psychosocial functioning, nutrition, social ties, memory, emotion, mood, and overall well-being [1]–[3]. Olfactory dysfunction can be harmful via decreased nutritional status, worse emotional and physical well-being, increased depressive symptoms, and social isolation [4]–[8].
Olfaction has been shown to progressively decline after age 57 [9], [10], with up to 80% of those over age 80 showing impairment [11]. Olfactory dysfunction is an early indicator of neurodegenerative diseases including Alzheimer’s, Parkinson’s, and Huntington’s disease [11] and also predicts mortality among cognitively intact older adults [12]–[15]. Olfactory impairment is also more prevalent among men, smokers, African-Americans, Hispanics, those with lower SES and lower cognition scores, and stroke victims [13], [16], [17]. As olfaction is an indicator of neural degeneration, aging, and death, determining the underlying mechanisms of these relationships is important to understanding health trajectories.
Like olfaction, sleep is a physiological process crucial to brain health. Older adults experience increased sleep disturbances including more wake after sleep onset (WASO), greater sleep fragmentation, and poorer self-reported sleep quality including insomnia symptoms[18], [19]. Deep sleep and REM sleep also decline with age [20]. Though findings are inconsistent about sleep duration and mortality (see Kurina et al., 2013 [21]), sleep disturbances have consistently been associated with chronic disease, overall physical and mental health, cognitive function, and mortality [18], [22]–[26].
Given the relationships of olfactory decline and sleep with aging-related health problems, we sought to explore whether variations in sleep were associated with olfactory dysfunction among older adults.
Previous studies have investigated the relationship between sleep and olfaction in humans, including olfactory function and memory consolidation of odors during sleep [27]–[30] and memory consolidation during sleep using olfactory cues [31]–[34]. However less attention has been given to whether variations in sleep are associated with olfactory function more generally. Two experimental studies found that sleep deprivation was associated with worse ability to identify odors among adults [27], [35], consistent with neuroimaging studies showing that sleep deprivation leads to decreases in the cerebral metabolic rate for glucose in the prefrontal cortex, including the orbitofrontal region which is highly involved in olfactory processing [36]. However, the relationship between sleep and olfactory function has not been assessed in a population setting among older adults, the group that experiences increased prevalence of olfactory dysfunction and disordered sleep.
The current analysis examines the relationship between actigraph and self-reported measures of sleep duration and quality with objectively-assessed olfactory function among a subsample of a national study of older adults.
Methods
Study Sample
The National Social Life, Health, and Aging Project (NSHAP) is a nationally representative sample of community-dwelling older adults born between 1920 and 1947 and their consenting spouses, regardless of their age. Survey data and biomeasures were collected via in-person at-home interviews.
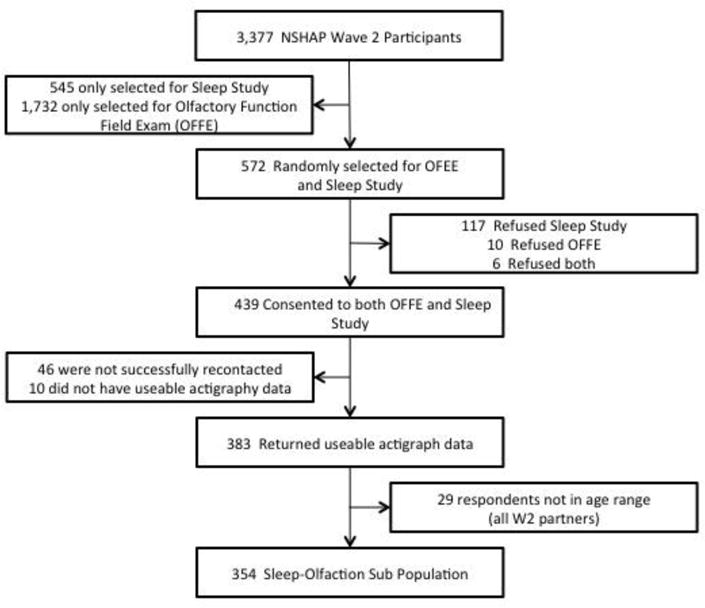
In Wave 2 (2010/2011), the NSHAP population was divided into six subgroups to allocate participants to additional modules. [37] Four subgroups (n=2,304) were selected to receive the Olfactory Function Field Exam (OFFE) which was developed to measure olfactory function in survey research [38]. Two subgroups (n=1,117) were selected to participate in the sleep module. A total of 572 individuals were selected to receive both the OFFE and the sleep module. There were 354 participants aged 62-90 who consented to both modules and provided complete data (see Figure 1).
Figure 1.
Measures
Olfaction
The OFFE includes measures of odor identification and sensitivity. Odor identification was assessed using a field survey version of a validated test [39]. Participants were presented with five odor filled felt tip pens and were instructed to identify each odor from a selection of four word/picture options. The number of correctly identified odors yielded a score from 0 to 5.
The odor sensitivity module of the OFFE assessed participants’ capacities to detect n-butanol, a common testing odorant. Participants were presented with three felt tip pens and asked to select the one pen that contained n-butanol. Six sets with increasing concentrations of n-butanol in one pen were presented. Scores of 0-6 represent how many concentrations were correctly detected. This screening test is reliable and highly correlated (r = 0.92) with psychophysical olfactory sensitivity testing among older adults [40]. A detailed protocol of olfactory data collection, including interviewer training, has been described elsewhere [41].
Sleep
Sleep characteristics were collected via self-report and wrist actigraphy. Actigraphs (Actiwatch Spectrum model, Phillips Respironics) were worn by study participants for a 72-hour period. Participants were asked to push an event marker each night when they started trying to sleep and when they awoke. The event markers, activity counts, and ambient light data (recorded by the actigraph) were used to manually determine the main rest interval for each 24-hour period. Actiwatch software calculated sleep metrics from the pattern of activity counts within each rest interval. We used metrics frequently derived from actigraphy: total sleep duration – the sum of 15-second epochs scored as sleep during the main sleep interval; wake after sleep onset (WASO) –total minutes awake during the main sleep interval; and fragmentation – an index of restlessness expressed as a percentage. Averages of each were calculated from the three nights. A detailed protocol of sleep data collection, including quality control measures, has been described elsewhere [42].
Self-reported sleep duration was assessed via a question asking how many hours participants slept each night. Sleep problems similar to insomnia symptoms (i.e., frequency of trouble falling asleep, trouble waking, waking too early, and restorative sleep) were combined to create a troubled sleep scale. The scale ranges from 0 to 8, with higher scores indicating more insomnia symptoms, as described in detail elsewhere [43].
Cognitive function
Cognitive function was measured using the Montreal Cognitive Assessment (MoCA) [44] adapted for survey administration (MoCA-SA) [45], [46]. The MoCA was developed to assess mild cognitive impairment (MCI) across key cognitive domains and was shown to have a 90% sensitivity in detecting clinically diagnosed MCI [44]. The instrument is more sensitive to variation in cognitive function than screeners designed to identify severe impairment, such as the Mini-Mental State Exam. The MoCA-SA is highly correlated (r=0.973) with the full MoCA [45], [46].
Additional covariates
Demographic and control variables included age, gender, body mass index (BMI), race/ethnicity (white, Hispanic, black, other), current smoking, depressive symptoms, medications, and a comorbidity index. BMI was calculated from direct measures (kg/m2). Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression scale, which assesses frequency of depressive feelings over the past week. Medications were physically brought to the interviewers and recorded. We include indicators for current usage of antidepressants and hypnotics. Comorbidities were summarized using a modified version of the Charlson Comorbidity Index (CCI) [47], as implemented in NSHAP [48].
Statistical Analysis
To assess the relationship between sleep characteristics and odor identification, we fit a generalized structural equation model to the five observed olfactory identification items assuming a single latent construct of odor identification through a logistic regression (i.e., a 1-parameter item-response model). This model was then extended by specifying odor identification to be a function of each sleep characteristic separately, first only adjusting for age and sex and then adjusting for additional covariates. Due to evidence in the literature of a U-shaped relationship between sleep time and some health outcomes, we also tested a three-level categorical variable for sleep time [21], [49]. The beta coefficient of the main independent variable (the particular sleep characteristic) represents the average change in performance on the odor identification construct, for each additional unit change of the sleep characteristic. The same analysis was performed for odor sensitivity.
As a secondary analysis, we assessed potential mediation of the relationship between sleep characteristics and odor identification via cognition. To assess potential mediation, we augmented the structural equation models to allow us to consider possible mediating effects of cognition using the widely implemented product method [50].
All analyses took into account the study design and sampling weights to account for probabilities of selection and nonresponse [51]. All data were analyzed using Stata Version 13.0 (StataCorp LP, College Station, TX).
Results
Table 1 summarizes the demographic, health, and sleep characteristics of the olfaction-sleep sub-sample. Actigraph total sleep time averaged 7.2 hours, WASO averaged 37.5 minutes, and the fragmentation index averaged 14.2%. Average self-reported sleep was 7.4 hours and the average troubled sleep scale was 2.7. Just over half were able to identify all five odors (51.4%), however only 7.1% were able to detect the weakest concentration of n-butanol. The distribution of covariates in the olfaction-sleep sub-sample was generally similar to the larger study population (see Table S1).
Table 1.
Demographics of the NSHAP Wave 2 Olfaction-Sleep subsample (N=354)
| Characteristic | Weighted Value | N |
|---|---|---|
| Age, mean (sd) | 71.7 (7.5) | 354 |
| Female | 53.6% | 354 |
| Race | 354 | |
| White | 82.3% | |
| African American | 6.2% | |
| Hispanic | 6.3% | |
| Other | 5.2% | |
| Modified Charlson Comorbidity | 354 | |
| 0 | 48.1% | |
| 1 | 25.4% | |
| 2 | 14.0% | |
| 3+ | 12.4% | |
| Montreal Cognitive Assessment – Survey Adapted, 0-20 scale, mean (sd) | 14.2 (3.5) | 354 |
| Body Mass Index, mean (sd) | 29.1 (5.50) | 345 |
| Current Smoker | 13.6% | 354 |
| CES-D1, mean (sd) | 7.3 (3.3) | 354 |
| Medication Usage | ||
| Antidepressants | 17.4% | 334 |
| Sleep Aids | 8.7% | 334 |
| Actigraph-Measured Sleep | 354 | |
| WASO2 (minutes), mean (sd) | 37.5 (22.6) | |
| Total Sleep Time (hours), mean (sd) | 7.2 (1.4) | |
| Less than 5 hours, % | 5.5 % | |
| 5 to 8 hours, % | 70.2% | |
| More than 8 hours, % | 24.3% | |
| Fragmentation (%) | 14.2 (5.9) | |
| Self-Reported Sleep Duration (hours), mean (sd) | 7.4 (1.3) | 287 |
| Troubled Sleep Scale3, mean (sd) | 2.7 (2.1) | 354 |
| Olfactory Function Field Exam | ||
| Odor Identification (score)4 | 354 | |
| 0 | 2.9% | |
| 1 | 1.3% | |
| 2 | 6.0% | |
| 3 | 7.8% | |
| 4 | 30.7% | |
| 5 | 51.3% | |
| Odor Sensitivity (score)5 | 354 | |
| 0 | 7.1% | |
| 1 | 8.5% | |
| 2 | 12.4% | |
| 3 | 15.9% | |
| 4 | 23.8% | |
| 5 | 25.3% | |
| 6 | 7.1% |
CES-D (range:0-22): Center for Epidemiologic Study Depression Scale
Wake after sleep onset
Troubled Sleep Scale (range:0-8) is a combined metric (0 = Never/rarely, 1 = Sometimes, 2 = Most of the time) from four questions: feeling rested in the morning, trouble falling asleep, trouble waking during the night and trouble waking too early
Odor identification (range: 0-5) is measured via correct identification of five odorants: rose, leather, orange, fish, and peppermint.
Odor sensitivity (range: 0-6) is assessed by participants’ capacities to detect n-butanol.
Table 2 presents the coefficients for the sleep characteristics in the age and sex adjusted (Model 1) and fully adjusted (Model 2) structural equation models for both odor identification and odor sensitivity. The outcome for each is the latent variable construct of the particular odor scale, where a higher score indicates better performance. In the age and sex adjusted models, both WASO and fragmentation were significantly inversely associated with odor identification. In the fully adjusted models, each additional minute of WASO was associated with a 0.013 worse odor identification score (p<0.01), and each additional percent of fragmentation associated with a 0.039 worse odor identification score (p=0.09).
Table 2.
Associations between sleep characteristics and odor identification and olfactory sensitivity among NSHAP Wave 2 olfaction-sleep sub-sample (N=345)
| Model 11 | Model 22 | |||||
|---|---|---|---|---|---|---|
| Odor Identification | ||||||
| Variable | Beta coefficient | 95% CI | p-value | Beta coefficient | 95% CI | p-value |
| WASO | −0.013 | (−0.022, −0.003) | <0.01 | −0.013 | (−0.022, −0.004) | <0.01 |
| Fragmentation | −0.043 | (−0.083, −0.003) | 0.04 | −0.039 | (−0.084, 0.006) | 0.09 |
| Duration – hours (continuous) | 0.027 | (−0.111, 0.164) | 0.70 | 0.005 | (−0.156, 0.167) | 0.95 |
| Duration (categorical) | ||||||
| <5 hours | −0.291 | (−0.829, 0.771) | 0.94 | 0.383 | (−0.773, 1.539) | 0.51 |
| 5-8 hours | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| >8 hours | 0.080 | (−0.325, 0.484) | 0.69 | 0.293 | (−0.142, 0.727) | 0.18 |
| Self-Reported Duration – hours | −0.130 | (−0.323, 0.063) | 0.38 | −0.154 | (−0.396, 0.088) | 0.21 |
| Troubled Sleep | 0.010 | (−0.095, 0.114) | 0.85 | 0.064 | (−0.065, 0.194) | 0.32 |
| Olfactory Sensitivity | ||||||
| WASO | −0.003 | (−0.008, 0.003) | 0.39 | −0.003 | (−0.009, 0.004) | 0.42 |
| Fragmentation | 0.003 | (−0.027, 0.032) | 0.84 | 0.000 | (−0.030, 0.030) | 0.99 |
| Duration – hours (continuous) | −0.180 | (−0.290, −0.70) | <0.01 | −0.152 | (−0.275, −0.029) | 0.02 |
| Duration (categorical) | ||||||
| <5 hours | 0.214 | (−0.388, 0.816) | 0.49 | 0.104 | (−0.728, 0.936) | 0.80 |
| 5-8 hours | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| >8 hours | −0.532 | (−0.991, −0.072) | 0.02 | −0.426 | (−0.897, 0.044) | 0.08 |
| Self-Reported Duration – hours | −0.107 | (−0.216, 0.002) | 0.05 | −0.074 | (−0.204, 0.055) | 0.25 |
| Troubled Sleep | −0.022 | (−0.103, 0.059) | 0.59 | −0.010 | (−0.077, 0.096) | 0.82 |
Models are adjusted for age and gender
Models are further adjusted for race/ethnicity, BMI, a modified Charlson Comorbidity Index, Center for Epidemiologic Studies Depression Scale (CES-D), sleep medication and antidepressant usage, and current smoking status.
Neither the troubled sleep scale, self-reported sleep duration, nor actigraph total sleep time were associated with odor identification in adjusted models. Modeled as a categorical variable, there was no evidence of a U-shaped relationship between actigraph-measured total sleep time and odor identification.
Neither WASO, fragmentation, self-reported sleep duration, nor the troubled sleep scale score were associated with odor sensitivity. Modeled as a continuous variable, actigraph total sleep time was inversely associated with odor sensitivity; each additional hour of sleep time was associated with a 0.152 worse odor sensitivity score (p=0.02). When modeled as a categorical variable, long sleepers (subjects averaging eight hours or more per night) were less able to detect odors compared to medium (5 to 8 hours) sleepers, with marginal significance (β=-0.426, p=0.08)
We assessed potential mediation by cognition between both WASO and fragmentation with odor identification (Table 3). The indirect association represents the portion of the association between the sleep measure and odor identification that is mediated by cognition. Only small portions of the relationships for WASO and fragmentation with odor identification were mediated by cognition (-0.002, p=0.14 and -0.006, p=0.22, respectively).
Table 3.
Mediation of the associations between WASO and fragmentation and odor identification by cognition in NSHAP Wave 2 olfaction-sleep subsample
| Beta Coefficient | 95% CI | p-value | |
|---|---|---|---|
| WASO | |||
| A: Association with cognition | −0.034 | (−0.056, −0.012) | <0.01 |
| B: Association of cognition with Odor ID (adjusting for WASO) | 0.063 | (−0.006, 0.131) | 0.07 |
| Indirect association of WASO with Odor ID through cognition | −0.002 | (−0.005, 0.001) | 0.14 |
| Direct association of WASO on Odor ID (not mediated through cognition) | 0.011 | (−0.020, −0.002) | 0.01 |
| Fragmentation | |||
| A: Association with cognition | −0.083 | (−0.152, −0.015) | 0.02 |
| B: Association of cognition with Odor ID (adjusting for fragmentation) | 0.073 | (0.007, 0.140) | 0.03 |
| Indirect association of Fragmentation with Odor ID through cognition | −0.006 | (−0.012, 0.003) | 0.22 |
| Direct association of Fragmentation on Odor ID (not mediated through cognition) | −0.033 | (−0.014, 0.002) | 0.15 |
Discussion
In our assessment of sleep and olfaction in community-dwelling older adults, we found a positive association between two actigraph measures of sleep disruption and worse ability to correctly identify odors, after adjusting for demographics and comorbidities. We did not find an association between insomnia symptoms or sleep duration, measured either by self-report of actigraphy, and ability to identify odors. We also did not find an association between sleep disruption or insomnia symptoms and odor sensitivity. However, we did observe an unexpected inverse association between total sleep time (measured with actigraphy) and odor sensitivity. When considered as a categorical variable, we found that the relationship was primarily driven by poorer odor sensitivity among long sleepers (8 or more hours per night). As a possible mechanism, we considered models controlling for reported neurological conditions (Parkinson’s, Alzheimer’s, and stroke), but found no difference in the estimated association. We also considered day-time napping behavior, but did not find that this confounded the observed associations. While the mechanism of the relationship between longer actigraph-measured sleep time and odor sensitivity remains unclear, there have been many reports of worse health outcomes among long sleepers [52].
We believe that this is the first study to assess the relationship between sleep and olfaction in a population of community-dwelling older adults and the first to include objective measures of both. Actigraph estimates of sleep characteristics avoid potential biases of survey sleep questions and are practical to implement in the field [53]. Similarly, the OFFE includes a validated measure of odor identification [39] and a novel survey-modified measure of odor sensitivity [40], [54]. Although only a minority of NSHAP participants received both modularized measures, we did find several significant, and heretofore unobserved, associations.
Our study did have some important limitations. First, the data are cross-sectional, preventing the observation of temporal associations between disrupted sleep, olfaction, and cognition. Second, actigraphy cannot measure dimensions of sleep that may be salient for odor identification or sensitivity, such as sleep architecture. Third, only 354 of 3,196 age-eligible Wave 2 NSHAP participants have both actigraphy and olfaction data, and a larger sample may be needed to detect associations with some of the sleep measures. Finally, NSHAP participants were not asked specifically about sleep apnea, although they were given an opportunity to list additional medical conditions. Only one participant in this sub-sample reported sleep apnea. There is likely to be both unreported and undiagnosed sleep apnea in the sample population, and we were unable to assess the role of sleep apnea in these associations.
Given prior evidence that poor sleep could be a risk factor for cognitive impairment [24], [25], [55] and that odor identification requires both detection and recognition (a cognitive function) [56], [57], we explored whether the observed relationship between poor sleep quality and worse odor identification was mediated by cognition. Unexpectedly, it was not. It may be that the MoCA-SA does not adequately capture the cognitive components relevant to this pathway. Factor analysis has demonstrated that while the full MoCA captures more variability in MCI than screeners such as the Mini-Mental State Exam [58], it may not accurately identify domain-specific areas of cognitive impairment [59]. While impaired olfaction has been associated with global cognition, it has also been associated specifically with perceptual speed and episodic memory [60]. Thus, it is possible that sleep disruption does diminish capacity to identify odors through a cognitive pathway that is not well measured by the MoCA-SA. However, sleep disruption may affect olfactory processing centers independently of cognition. This alternative would suggest that the difference between the relationship of sleep to odor identification versus odor sensitivity is not related to cognition.
Our study is broadly consistent with animal models on the role of sleep in the consolidation of odor memory. Barnes and Wilson found that manipulation via restriction of slow wave sleep in rats impaired memory consolidation relative to odor recognition [61]. Fragmentation and WASO may reflect lack of slow wave sleep. There are experimental studies of sleep restriction: Prehn-Kristensen et al. and Killgore and McBride both found that sleep deprivation caused reduced capacity to recognize odors by adults [27], [35]. Our findings differ in that we found an association for lack of sleep consolidation (not sleep duration) and odor identification. Also, we are examining routine sleep variation in the population rather than experimentally manipulated sleep.
Further study is needed to understand whether poor sleep quality leads to olfactory decline or vice-versa, or whether underlying physiologic processes explain both, inducing the observed correlation between the two. Longitudinal data would help answer these questions. If sleep disruption is an early indicator of olfactory dysfunction, it will be important to consider whether modifications to sleep routines aimed at improving sleep quality could delay the onset of other aging-related health outcomes.
Supplementary Material
Acknowledgments
Funding:
This work was supported by R01AG042164 from the National Institute of Aging and the Basic Behavioral and Social Sciences Research Opportunity Network (OppNet) at the National Institutes of Health. The core data collection in the National Social Life and Aging Project (NSHAP) was supported by R01 AG021487 and R01 AG033903 from the National Institute of Aging at the National Institutes of Health. The ancillary sleep data collection for NSHAP received additional support from Phillips Respironics and the Health and Retirement Survey.
References
- 1.Doucet S, Soussignan R, Sagot P, Schaal B. The secretion of Areolar (Montgomery’s) glands from lactating women elicits selective, unconditional responses in neonates. PLoS One. 2009;4(10) doi: 10.1371/journal.pone.0007579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacob S, Garcia S, Hayreh D, McClintock MK. Psychological effects of musky compounds: comparison of androstadienone with androstenol and muscone. Horm Behav. 2002;42(3):274–283. doi: 10.1006/hbeh.2002.1826. [DOI] [PubMed] [Google Scholar]
- 3.Patel RM, Pinto JM. Olfaction: anatomy, physiology, and disease. Clini Anat. 2014;27(1):54–60. doi: 10.1002/ca.22338. [DOI] [PubMed] [Google Scholar]
- 4.Kim WY, Hur M, Cho MS, Lee HS. Effect of olfactory function on nutritional status of Korean elderly women. Nutr Res. 2003;23(6):723–34. [Google Scholar]
- 5.Toussaint N, de Roon M, van Campen JPCM, Kremer S, Boesveldt S. Loss of olfactory function and nutritional status in vital older adults and geriatric patients. Chem Senses. 2015;40(3):197–203. doi: 10.1093/chemse/bju113. [DOI] [PubMed] [Google Scholar]
- 6.Boesveldt S, Lindau ST, McClintock MK, Hummel T, Lundström JN. Gustatory and olfactory dysfunction in older adults: a national probability study. Rhinology. 2011;49(3):324–330. doi: 10.4193/rhino10.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sivam A, Wroblewski KE, Alkorta-Aranburu G, Barnes LL, Wilson RS, Bennett DA, Pinto JM. Olfactory dysfunction in older adults is associated with feelings of depression and loneliness. Chem Senses. 2016;41(4):293–9. doi: 10.1093/chemse/bjv088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller A, Malaspina D. Hidden consequences of olfactory dysfunction: a patient report series. BMC Ear, Nose, Throat Disord. 2013;13(1):8. doi: 10.1186/1472-6815-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, Mcclintock MK. The rate of age-related olfactory decline among the general population of older U.S. adults. J Geront A Biol Sci Med Sci. 2015;70(11):1435–41. doi: 10.1093/gerona/glv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doty RL, Reyes PF, Gregor T. Presence of both odor an detection deficits in Alzheimer’s disease. Brain Res Bull. 1987;18(5):597–600. doi: 10.1016/0361-9230(87)90129-8. [DOI] [PubMed] [Google Scholar]
- 11.Attems J, Walker L, Jellinger KA. Olfaction and Aging: A Mini-Review. Gerontology. 2015;61(6):485–90. doi: 10.1159/000381619. [DOI] [PubMed] [Google Scholar]
- 12.Wilson RS, Yu L, Bennett DA. Odor identification and mortality in old age. Chem Senses. 2011;36(1):63–7. doi: 10.1093/chemse/bjq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto JM, Schumm LP, Wroblewski KE, Kern DW, Mcclintock MK. Racial disparities in olfactory loss among older adults in the United States. J Geront A Biol Sci Med Sci. 2014;69(3):323–9. doi: 10.1093/gerona/glt063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devanand DP, Lee S, Manly J, Andrews H, Schupf N, Masurkar A, Stern Y, Mayeux R, Doty RL. Olfactory identification deficits and increased mortality in the community. Ann Neurol. 2015;78(3):401–411. doi: 10.1002/ana.24447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schubert CR, Fischer ME, Pinto AA, Klein BEK, Klein R, Tweed TS, Cruickshanks KJ. Sensory Impairments and Risk of Mortality in Older Adults. J Geront A Biol Sci Med Sci. 2016 doi: 10.1093/gerona/glw036. vol. Epub 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman HJ, Rawal S, Li C-M, Duffy VB. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev Endocr Metab Disord. 2016 doi: 10.1007/s11154-016-9364-1. vol. Epub 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy C, Schubert CR, Cruickshanks KJ, Klein BEK, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307–12. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 18.Schubert CR, Cruickshanks KJ, Dalton DS, Klein BEK, Klein R, Nondahl DM. Prevalence of sleep problems and quality of life in an older population. Sleep. 2002;25(8):889–93. [PubMed] [Google Scholar]
- 19.Floyd JA, Medler SM, Ager JW, Janisse JJ. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Heal. 2000;23(2):106–17. doi: 10.1002/(sici)1098-240x(200004)23:2<106::aid-nur3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 21.Kurina LM, McClintock MK, Chen J-H, Waite LJ, Thisted RA, Lauderdale DS. Sleep duration and all-cause mortality: a critical review of measurement and associations. Ann Epidemiol. 2013 Jun;23(6):361–70. doi: 10.1016/j.annepidem.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rod NH, Vahtera J, Westerlund H, Kivimaki M, Zins M, Goldberg M, Lange T. Sleep disturbances and cause-specific mortality: results from the GAZEL cohort study. Am J Epidemiol. 2011;173(3):300–9. doi: 10.1093/aje/kwq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An C, Yu L, Wang L, Jin G, Song M, Zhu Q, Jia H, Liu K, Wang M, Wang X. Association between sleep characteristics and mild cognitive impairment in elderly people. Neurophysiology. 2014;46(1):88–94. [Google Scholar]
- 24.Spira AP, Chen-Edinboro LP, Wu MN, Yaffe K. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry. 2014;27(6):478–83. doi: 10.1097/YCO.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, Fink HA, Stone KL. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Geront A Biol Sci Med Sci. 2006;61(4):405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 26.Knutson KL, Van Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, Lauderdale D. Association between sleep and blood pressure in mid life: The CARDIA Sleep Study. Arch Intern Med. 2009;169(11):1055–61. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prehn-Kristensen A, Lotzkat K, Bauhofer E, Wiesner CD, Baving L. Sleep supports memory of odors in adults but not in children. PLoS One. 2015;10(9):1–14. doi: 10.1371/journal.pone.0139069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes DC, Wilson DA. Slow-wave sleep-imposed replay modulates both strength and precision of memory. J Neurosci. 2014;34(15):5134–42. doi: 10.1523/JNEUROSCI.5274-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badia P, Wesensten N, Lammers W, Culpepper J, Harsh J. Responsiveness to olfactory stimuli presented in sleep. Physiol Behav. 1990;48(1):89–90. doi: 10.1016/0031-9384(90)90266-7. [DOI] [PubMed] [Google Scholar]
- 30.Stuck BA, Stieber K, Frey S, Freiburg C, Hörmann K, Maurer JT, Hummel T. Arousal responses to olfactory or trigeminal stimulation during sleep. Sleep. 2007;30(4):506–10. doi: 10.1093/sleep/30.4.506. [DOI] [PubMed] [Google Scholar]
- 31.Rasch B, Sutton J. About sleep’s role in memory. Psychologist. 2014;27(5):320–323. [Google Scholar]
- 32.Rasch B, Büchel C, Gais S, Born J. Odor Cues During Slow-Wave Sleep Prompts Declarative Memory Consolidatoin. Science (80-) 2007 Mar;315:1426–1429. doi: 10.1126/science.1138581. no. [DOI] [PubMed] [Google Scholar]
- 33.Diekelmann S, Biggel S, Rasch B, Born J. Offline consolidation of memory varies with time in slow wave sleep and can be accelerated by cuing memory reactivations. Neurobiol Learn Mem. 2012;98(2):103–111. doi: 10.1016/j.nlm.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Diekelmann S, Büchel C, Born J, Rasch B. Labile or stable: Opposing consequences for memory when reactivated during waking and sleep. Neuroforum. 2011;17(2):74–76. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- 35.Killgore WDS, McBride SA. Odor identification accuracy declines following 24 h of sleep deprivation. J Sleep Res. 2006;15(2):111–6. doi: 10.1111/j.1365-2869.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 36.Thomas ML, Sing HC, Belenky G, Holcomb HH, Mayberg HS, Dannals RF, Wagner HN, Thorne DR, Popp KA, Rowland LM, Welsh AB, Balwinski SM, Redmond DP. Neural basis of alertness and cognitive performance impairments during sleepiness II: effects of 48 and 72 h of sleep deprivation on waking human regional brain activity. Thalamus Relat Syst. 2003;2(3):199–229. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 37.O’Doherty K, Jaszczak A, Hoffmann JN, You HM, Kern DW, Pagel K, McPhillips J, Schumm LP, Dale W, Huang ES, Mcclintock MK. Survey field methods for expanded biospecimen and biomeasure collection in NSHAP Wave 2. J Geront B Phychol Sci Soc Sci. 2014;69(Suppl 2):S27–37. doi: 10.1093/geronb/gbu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern DW, Wroblewski KE, Schumm LP, Pinto JM, Mcclintock MK. Field survey measures of olfaction: The Olfactory Function Field Exam (OFFE) Field methods. 2014;26(4):421–34. doi: 10.1177/1525822X14547499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mueller C, Renner B. A new procedure for the short screening of olfactory function using five items from the ‘Sniffin’ Sticks’ identification test kit. Am J Rhinol. 2006;20(1):113–6. [PubMed] [Google Scholar]
- 40.Kern DW, Schumm LP, Wroblewski KE, Pinto JM, Hummel T, McClintock MK. Olfactory Thresholds of the U.S. Population of Home-Dwelling Older Adults: Development and Validation of a Short, Reliable Measure. PLoS One. 2015;10(3):e0118589. doi: 10.1371/journal.pone.0118589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinto JM, Kern DW, Wroblewski KE, Chen RC, Schumm LP, Mcclintock MK. Sensory Function: Insights From Wave 2 of the National Social Life, Health, and Aging Project. Journals Gerontol Ser B Psychol Sci Soc Sci. 2014;69(810):144–153. doi: 10.1093/geronb/gbu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauderdale DS, Schumm LP, Kurina LM, McClintock M, Thisted RA, Chen J-H, Waite L. Assessment of sleep in the National Social Life, Health, and Aging Project. J Geront B Phychol Sci Soc Sci. 2014;69(Suppl 2):S125–33. doi: 10.1093/geronb/gbu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J-H, Waite L, Kurina LM, Thisted RA, Mcclintock MK, Lauderdale D. Insomnia symptoms and actigraph-estimated sleep characteristics in a nationally representative sample of older adults. J Geront A Biol Sci Med Sci. 2015;70(2):185–92. doi: 10.1093/gerona/glu144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 45.Shega JW, Sunkara PD, Kotwal AA, Kern DW, Henning SL, Mcclintock MK, Schumm P, Waite LJ, Dale W. Measuring cognition: The Chicago Cognitive Function Measure in the National Social Life, Health and Aging Project, Wave 2. J Geront B Phychol Sci Soc Sci. 2014;69(Suppl 2):S166–76. doi: 10.1093/geronb/gbu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotwal AA, Schumm LP, Kern DW, Mcclintock MK, Waite LJ, Shega JW, Huisingh-Scheetz MJ, Dale W. Evaluation of a brief survey instrument for assessing subtle differences in cognitive function among older adults. Alzheimer Dis Assoc Disord. 2015;29(4):317–24. doi: 10.1097/WAD.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 48.Vasilopoulos T, Kotwal AA, Huisingh-Scheetz MJ, Waite LJ, Mcclintock MK, Dale W. Comorbidity and chronic conditions in the National Social Life, Health and Aging Project (NSHAP), Wave 2. J Geront B Phychol Sci Soc Sci. 2014;69(Suppl 2):S154–65. doi: 10.1093/geronb/gbu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 51.O’Muircheartaigh C, English N, Pedlow S, Kwok PK. Sample design, sample augmentation, and estimation for Wave 2 of the NSHAP. J Geront B Phychol Sci Soc Sci. 2014;69(Suppl 2):S15–26. doi: 10.1093/geronb/gbu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramos AR, Tarraf W, Daviglus M, Davis S, Gallo LC, Mossavar-Rahmani Y, Penedo FJ, Redline S, Rundek T, Sacco RL, Sotres-Alvarez D, Wright CB, Zee PC, Gonzalez HM. Sleep duration and neurocognitive function in the Hispanic Community Study/Study of Latinos. Sleep. 2016;39(10):1843–51. doi: 10.5665/sleep.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 54.Kern DW, Wroblewski KE, Schumm LP, Pinto JM, Chen RC, McClintock MK. Olfactory function in Wave 2 of the National Social Life, Health, and Aging Project. J Geront B Phychol Sci Soc Sci. 2014;69(Suppl 2):S134–43. doi: 10.1093/geronb/gbu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, Laffan A, Stone KL. Association of Sleep Characteristics and Cognition in Older Community-Dwelling Men: the MrOS Sleep Study. Sleep. 2011;34(10):1347–1356. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devanand DP, Tabert MH, Cuasay K, Manly JJ, Schupf N, Brickman AM, Andrews H, Brown TR, DeCarli C, Mayeux R. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging. 2010;31(9):1593–600. doi: 10.1016/j.neurobiolaging.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swan GE, Carmelli D. Impaired olfaction predicts cognitive decline in nondemented older adults. Neuroepidemiology. 2002;21(2):58–67. doi: 10.1159/000048618. [DOI] [PubMed] [Google Scholar]
- 58.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 59.Coen RF, Robertson DA, Kenny RA, King-Kallimanis BL. Strengths and limitations of the MoCA for assessing cognitive functioning. J Geriatr Psychiatry Neur. 2016;29(1):18–24. doi: 10.1177/0891988715598236. [DOI] [PubMed] [Google Scholar]
- 60.Wilson RS, Arnold SE, Tang Y, Bennett DA. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology. 2006;26(2):61–67. doi: 10.1159/000090250. [DOI] [PubMed] [Google Scholar]
- 61.Barnes DC, Wilson DA. Sleep and olfactory cortical plasticity. Front Behav Neurosci. 2015;8(134) doi: 10.3389/fnbeh.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


