Abstract
The actions of bioactive lipids and reactive oxygen species (ROS) are usually coupled, and their interplay is a common and important mechanism mediating tissue injury, inflammation, and other pathologies. Understanding the interplay of ROS and lipid mediators will extend horizons for researchers to clarify the pathogenesis of different diseases and help identify therapeutic targets for treatment of these diseases. Some bioactive lipids are converted into oxidized lipids during cell or tissue oxidative stress such as isoprostanes and isoketals, which are even more bioactive than their precursors. Moreover, many enzymes that produce lipid mediators such as prostaglandin H synthases, lipoxygenases, and cytochrome P450 isoforms may catalyze the production of ROS. Bioactive lipids—lysophospholipids, sphingolipids, or deposited lipids in cells—are shown to stimulate redox enzymes to produce ROS. In addition, a lipid-channel-ROS axis in different organelles may be associated with the crosstalk of ROS and bioactive lipids. This Forum focuses on the crosstalk of ROS with sphingolipids, P450 eicosanoids, lysophospholipids, and deposited plasma lipids and related novel signaling pathway in their reciprocal actions, which is expected to provide novel insights into the pathogenesis of different diseases associated with the participation of these lipid mediators. It is imperative to further understand the molecular mechanism mediating the crosstalk of ROS with specific lipid mediators and to develop more effective therapeutic strategies to target the interplay of ROS and lipid mediators for treatment specific to different organ diseases. Antioxid. Redox Signal. 28, 911–915.
Keywords: : lipid mediators, ROS, ceramide, sphingolipids, lysophospholipids, eicosanoids
Lipid mediators are referred to as an array of bioactive lipids that are endogenously produced from different cells or tissues in response to a variety of physiological and pathological stimuli. Over the past 20 years, these bioactive lipids have taken center stage in research on the physiological regulation of cell and organ function and on the pathophysiology of different diseases. There is substantial evidence that various bioactive lipids are importantly implicated in the development of many human diseases and thus they are widely accepted as therapeutic targets for treatment and prevention of these diseases.
These bioactive lipids are often categorized into three different classes, that is, (i) arachidonic acid (AA)-derived eicosanoids such as prostaglandins (PGs), thromboxanes (Txs), and leukotrienes; (ii) lysophospholipids and their derivatives such as lysophosphatidic acid (LPA), sphingolipids, and endocannabinoids; (iii) ω-3 polyunsaturated fatty acid metabolites such as resolvins and protectins (6). Recent studies have indicated that locally produced or circulating fatty acids with different chain lengths may have bioactive effects even without further metabolism, including short-, medium-, and long-chain fatty acids. Studies on microbiome found that some short-chain fatty acids from gut microbiota are critically involved in the development of different diseases such as atherosclerosis, hypertension, obesity, and diabetes mellitus. These fatty acids may be another possible class of lipid mediators.
Bioactive lipids act to produce physiological regulation or pathogenic effects often in association with redox signaling and regulatory pathways. It has been reported that some bioactive lipids can be converted into oxidized lipids during cell or tissue oxidative stress such as isoprostanes and isoketals, which are even more bioactive than their precursor lipid mediators. In contrast, many enzymes that produce lipid mediators such as prostaglandin H synthases, lipoxygenases, and cytochrome P450 (CYP450) isoforms may catalyze the production of lipid-derived radicals in different cells or tissues. Evidence is also accumulating that bioactive lipids may stimulate redox enzymes to produce free radicals or to scavenge reactive oxygen species (ROS). In many biological activities, the roles of bioactive lipids and free radicals or ROS are usually coupled, and their interplay is a common and important mechanism during tissue injury, inflammation, and other related pathological changes.
This Forum summarizes current research results about the crosstalk of ROS with sphingolipids, P450 eicosanoids, lysophospholipids, and deposited or converted active plasma lipids, which will provide novel insights into the molecular mechanisms and pathogenesis of different diseases.
ROS as a Messenger for the Actions of Lipid Mediators
It has been well known that ROS play an important role as a messenger to mediate or regulate cell–cell communication and intracellular signal transduction. ROS including the oxygen radicals such as superoxide (O2•−), hydroxyl (OH−), peroxyl (RO2), alkoxyl (RO•), and hydroperoxyl (HO2•) as well as oxygen nonradicals such as hydrogen peroxide (H2O2), hypochlorous acid (HOCl), ozone (O3), singlet oxygen (ΔgO2), and peroxynitrite (ONOO−), all are oxygen derivatives and biologically reactive to oxidize or reduce other molecules in living cells or tissues, thereby changing cellular activity and organ function (3). Many bioactive substances produced endogenously or administrated exogenously such as autocrines, paracrines, hormones, and various chemical metabolites or agents may exert their action by targeting ROS, and under such conditions ROS as a messenger to either mediate or modulate the activity of these bioactive substances to regulate cell and organ functions. Lipid mediators are mostly considered as autocrines or paracrines, which also use ROS as a signaling molecule to exert their role.
Eicosonoids from the metabolism of AA have been extensively studied and their actions are involved in the interaction with ROS. It is generally accepted that the level of cellular ROS is determined by the balance between their production and neutralization, which may be stimulated or inhibited by different eicosanoids depending upon physiological or pathological status of cells or organs. The control of the redox balance in cells is extremely important, which not only ensures normal cellular signaling but also exerts protective role in cell defense against pathogenic factors such as pathogen-associated molecular patterns or damage-associated molecular patterns. Under pathological conditions such as ischemia, inflammation, and metabolic disturbance, both enhanced AA metabolizing process and associated metabolites can increase ROS production, leading to derangement of the redox balance in cells, which may exacerbate cell or tissue injury. Some eicosanoids may interact with ROS to form more powerful injury factors such as isoprostanes from PGs and isoketals from AA, and these ROS products of lipid mediators may importantly participate in the development of different diseases such as hypertension, atherosclerosis, Alzheimer disease, and others. In contrast, some eicosanoids may enhance cellular antioxidative system or even interact with ROS to exert protective role during pathological conditions.
A good example is the role of CYP450 metabolites of AA, which may show dual effects under certain pathological processes such as ischemic brain injury. It has been reported that increased CYP450 product of AA, 20-hydroxyeicosatetraenoic acid (HETE), increases ROS production, but enhanced production of CYP405 metabolite, 14,15-epoxyeicosatrienoic acid (EET), reduces ROS level in neurons. The Tx-prostanoid receptor (TP) may mediate the action of HETEs and EETs on ROS production. Activation of TP stimulates ROS production in many different cells and tissues, and HETEs may activate, but EETs inhibit TP activity to control ROS production (5).
In addition to eicosnoids, lysophospholipids (LPLs) and their derivatives have also been reported to work through ROS. It has been reported that binding of LPLs to G-protein coupled receptor alters transcriptional regulatory events and thereby regulates generation of ROS, which may be an important contributing mechanism to tissue oxidation and inflammatory injury induced by increased LPLs. Endogenous LPA and phosphatidic acids are produced in many mammalian cells, and they mediate or regulate cell function such as apoptosis in an ROS-dependent manner. It is generally accepted that ROS may mediate the action of LPA to stimulate extracellular-signal-regulated kinase and alpha serine/threonine-protein kinase phosphorylation and nuclear factor-kappa beta activities, leading to changes in cell proliferation, differentiation, and growth. In addition, LPA was reported to activate the small GTPase and thereby enhance nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, stimulating ROS production. LPA receptor blocker Ki16425 or cellular knockdown of LPA1 decreased oxidant generation and blocked translocation of Rac1 to plasma membrane (7).
Another family of LPLs is sphingolipid, ceramide (CER), and its product sphingosine (SPH) and sphingosine-1-phosphate (S1P). The cross-talk between these sphingolipids and redox signaling has recently been known to be an important mechanism in the physiological regulation of cell function or in the development of a number of organ injuries or diseases. There is accumulating evidence that sphingolipids including CER, SPH, and S1P have the ability to change cellular redox homeostasis through regulation of NADPH oxidase activity, mitochondrial integrity, nitric oxide synthase (NOS) activity, or antioxidant enzyme system. Among these sphingolipids, CER has been reported to activate various ROS-generating enzymes such as NADPH oxidase, xanthine oxidase, uncoupled NOS, and enzymes in the mitochondrial respiratory chain. CER as an important fusigen can mediate the fusion of small membrane raft (MR) domains, which leads to the formation of CER-enriched membrane platforms clustering and aggregating subunits of NADPH oxidase. This MR redox signaling platform (MRRSP) is characterized by NADPH oxidase subunits assembling and enzyme activation. This MRRSP driven by CER is considered as a critical mechanism in mediating transmembrane signaling and thereby as an important pathogenic event during different diseases such as inflammatory diseases, atherosclerosis, hypertension, and chronic kidney disease (1).
Moreover, CER and its producing enzyme have been shown to mediate cellular apoptosis and necrosis via tumor necrosis factor α-induced excessive ROS. Another CER-producing enzyme neutral sphingomyelinase (NSM) is confirmed to regulate ROS release upon mycobacterial infection, which may allow intracellular survival of mycobacteria during the development of tuberculosis. It has been indicated that increased production and release of ROS by NSM activation may suppress autophagy in infected macrophages, allowing the pathogen to survive within macrophages and leading to sustained mycobacterial infection (9). This CER or its producing enzyme-mediated effect linking to ROS was also observed in Staphylococcus aureus infection. In this regard, CD44 as a molecule that mediates the infection in macrophages can be activated by S. aureus, which enhances ROS production resulting in CER release, clustering of CD44 in CER-enriched membrane platforms, and S. aureus internalization and killing. In acid sphingomyelinase (ASMase)-deficient macrophages, fusion of phagosomes with lysosomes was reduced, which resulted in reduced intracellular killing of S. aureus and consequent replication of internalized S. aureus causing severe pneumonia. It is clear that ROS not only act as a killer of bacteria or mycobacteria in phagocytes but also as a regulator of CER production that controls their internalization into host cells (4).
Evidence is increasing that SPH within cells interacts with redox signaling pathway to exert regulatory actions. It has been reported that SPH and sphingoid base-dihydrosphingosine reduce O2•− production via inhibition of protein kinase C (PKC) in intact neutrophils. SPH can inhibit NADPH oxidase activity by suppression of translocation of p47phox subunit for assembling of the active enzyme complex to produce O2•− or ROS. Furthermore, SPH and its CER analogues (N-acetyl sphingosine and N-hexanoyl sphingosine) also reduce tissue ROS level by their O2•− scavenging effects. It is suggested that SPH may have antioxidant action in cells and tissues. However, SPH metabolite, S1P, has been reported to stimulate ROS production in different cells via activation of NADPH oxidase, which induces lipids peroxidation and bioactive aldehydes accumulation. In the heart, cardiac fibrosis is enhanced by S1P-induced local oxidative stress. This S1P-induced increase in ROS production or tissue oxidative stress is associated with enhancement of p47phox translocation, which may be mediated by a PKCd/PYK2 signaling pathway (1).
Recent studies have indicated that abnormal accumulation of lipids is not only the consequence of tissue injury or organ disease, but also mediates cell or organ damage. It is now referred to as lipotoxicity, which is caused by defects or insufficiency of β-oxidation and consequent aggregation of multiple toxic lipid intermediates in nonadipose tissues. These toxic lipid products promote excess ROS generation to induce pathological changes in tissues such as renal tissues or cells. It is proposed that ROS may be one of the critical mechanisms by which lipotoxicity and associated cell or tissue injuries occur. There is evidence that intracellular lipid aggregation alone is not adequate to produce its toxic and pathogenic actions, but other factors such as lower oxidation capability, enhanced ROS production, and increased lipid peroxidation may synergistically work together to produce tissue injury, leading to lipotoxicity (8).
Lipid Mediators as a Target for the Actions of ROS
The opposite direction of ROS–lipid mediators cross-talk is related to the regulation of lipid mediator production and metabolism by ROS and associated functional changes. It has been shown that ROS may control the expression and activity of enzyme that produces or metabolizes lipid mediators and thereby exerts their regulatory action on cellular activity and organ functions. Earlier studies have found that biochemical events responsible for ROS generation promote AA release from its phospholipid stores in renal mesangial cells. Dexamethasone-induced reduction of H2O2 generation was attributed to a decrease in AA availability. In addition, ROS may mediate vascular responses at least partially due to stimulation of prostanoid production.
By biochemical analysis, the addition of enzymatically generated ROS to incubation media was shown to cause a dose-dependent increase in the production of PGE2, PGF2a, 6-keto-PGF1a, and Tx B2 in some cell types such as renal glomerular cells, which was due to the generation of H2O2. 14C-AA-labeled experiments also demonstrated that ROS, in particular H2O2, increased PG synthesis, which was associated with enhanced AA availability. This AA availability enhancement may be related to increased Ca2+ flux and subsequent activation of phospholipase A2 (PLA2) activity. In contrast, excessive ROS were also found to suppress PGE2 generation, reducing the protective effects of this PG in face of pathological challenges. In some disease states such as ischemia and inflammation, increased inflammatory cytokines and ROS may increase the activity of PLA2, resulting in enhanced release of AA from cell membranes and activating CYP450 metabolism of AA to produce HETEs-induced pathological changes (5).
Previous studies have also indicated that ROS play a role in the regulation of LPLs metabolism. For example, endogenous LPA production has also been reported to be associated with ROS-dependent cellular activities. In particular, ROS stimulated under pathological conditions such as diabetes may increase the production of LPL metabolites such as LPA and lysophosphatidic cholines (7). With respect to sphingolipids metabolism, ROS have also been shown to exert their regulatory or modulatory roles. A good example for ROS-mediated regulation of CER metabolism or signaling is the MRRSP formation in endothelial cell membrane, which is characterized by ROS regulation of CER production via sphingomyelinases (SMase) activity-dependent zinc and related C-terminal cysteine modification. Interaction of zinc with ROS changes microenvironment for SMase activity to produce CER. In addition, a feed-forward amplifying mechanism also exists in the formation of such MRRSP. This mechanism relates to the production of ROS within MRRSP that further increases MRs clustering and thus amplifies the whole process. In this feed-forwarding amplification of signals by ROS, the formation of ASMase dimers due to modification of the free C-terminal cysteine is crucial to the enhancement of its enzymatic activity.
Recently, ROS have been reported to induce acid ceramidase (CDase) degradation and thereby reduce SPH production. Action of ROS on acid CDase may be due to cathepsin B activation. There is also accumulating evidence that the S1P production or its biological actions are reciprocally regulated by redox molecules. In vascular smooth muscle cells, low concentration of H2O2 and ox-low-density lipoproteins increased S1P generation, leading to smooth muscle cell proliferation. It has been accepted that ROS generated from different resources such as MRRSP and mitochondrial respiratory chain may activate sphingosine kinase 1 to generate S1P, which may mediate intracellular signaling independent of ROS, thereby regulating the cellular activity or organ function (1).
In mechanistic studies on lipid-channel-ROS axis, it has been demonstrated that ion channels are a target of modulation by lipids and lipid mediators. In different cells, ion channel function changes may determine production of ROS, in particular, in mitochondria and lysosomes. Reciprocally, ROS may modulate intracellular channel activity to trigger a feedback control of cell function. Excessive production ROS has been indicated to be harmful to the cellular lipids or lipid mediators, which may produce oxidized forms of these cellular lipid constituents that ultimately affect ion channel function. This ion channel-mediated ROS regulation of lipid metabolism and the action of lipid mediators may also support the view that lipid mediators can serve as a target for the action of ROS in a variety of regulatory activities under physiological and pathological conditions (2).
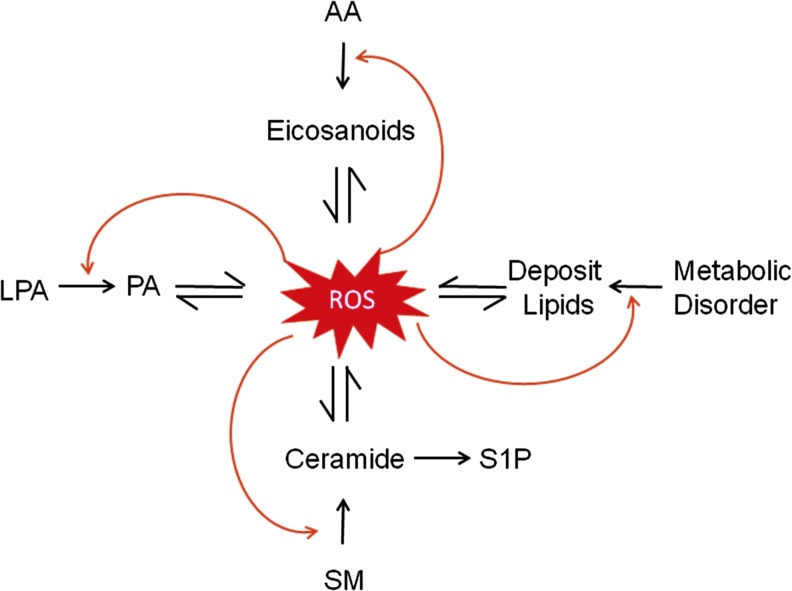
Figure 1 shows the topics that this Forum deals with. The cross-talk of ROS with lipid mediators is ubiquitous and reciprocal in many cell types under physiological and pathological conditions. Lipid mediators produced from different metabolic pathways such as metabolism of AA, LPLs, and derived deposited lipids in cells or tissues, more or less, exert their action via ROS-mediated redox signaling. Some actions may be injurious, but others are possibly protective depending upon cell or tissue status. Reciprocally, the production or release of lipid mediators from almost all metabolic pathways may be controlled or modulated by ROS. This ROS regulation of bioactive lipid mediators also importantly contributes to the control of cell function and the development of different diseases (Fig. 1). Undoubtedly, understanding the interplay of ROS with lipid mediators will extend horizons for researchers in this field to explore the pathogenesis of different diseases and may help identify therapeutic targets for effective treatment of these diseases. Such disease-oriented review or discussion of the crosstalk between ROS and lipid mediators can be found in individual articles of this Forum in great detail.
FIG. 1.
Crosstalk of ROS and lipid mediators from the metabolism of AA, LPLs, and SM or deposited lipids due to metabolic disorder or lipotoxicity. AA, arachidonic acid; LPL, lysophospholipid; ROS, reactive oxygen species; SM, sphingomyelin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Abbreviations Used
- AA
arachidonic acid
- ASM
acid sphingomyelinase
- CDase
ceramidase
- CER
ceramide
- CYP450
cytochrome P450
- EET
epoxyeicosatrienoic acid
- H2O2
hydrogen peroxide
- HETE
hydroxyeicosatetraenoic acid
- LPA
lysophosphatidic acid
- LPL
lysophospholipid
- MR
membrane raft
- MRRSP
membrane raft redox signaling platform
- NADPH
nicotinamide adenine dinucleotide phosphate
- NOS
nitric oxide synthase
- NSM
neutral sphingomyelinase
- PG
prostaglandin
- PKC
protein kinase C
- PLA2
phospholipase A2
- ROS
reactive oxygen species
- S1P
sphingosine-1-phosphate
- SMase
sphingomyelinases
- SPH
sphingosine
- TP
Tx-prostanoid receptor
- Tx
thromboxane
Acknowledgments
Many studies cited in this editorial from our laboratory were supported by grants HL57244, HL075316, DK54927, and HL091464 (to P.L.L.) from the National Institutes of Health and grants HG 1697/2-2 and GU 335/33-1 (to E.G.) of Deutsche Forschungsgemeinschaft (DFG).
References
- 1.Bhat OM, Yuan X, Li G, Lee R, and Li PL. Sphingolipids and redox signaling in renal regulation and diseases. Antioxid Redox Signal [Epub ahead of print]; DOI: 10.1089/ars.2017.7129, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brini M, Leanza L, and Szabo I. Lipid-mediated modulation of intracellular ion channels and redox state: physiopathological implications. Antioxid Redox Signal 28: 949–972, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Jin S, Zhou F, Katirai F, and Li PL. Lipid raft redox signaling: molecular mechanisms in health and disease. Antioxid Redox Signal 15: 1043–1083, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Wu Y, Orian-Rousseau V, Zhang Y, Gulbins E, and Grassmé H. Regulation of Staphylococcus aureus infection of macrophages by CD44, reactive oxygen species and acid sphingomyelinase. Antioxid Redox Signal 28: 916–934, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Davis CM, and Alkayed NJ. P450 eicosanoids and ROS interplay in brain injury and neuroprotection. Antioxid Redox Signal 28: 987–1007, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami M. Lipid mediators in life science. Exp Anim 60: 7–20, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Shao Y, Nanayakkara G, Cheng J, Cueto R, Yang WY, Park JY, Wang H, and Yang X. Lysophospholipids and their receptors in tissue oxidative and inflammatory injury as conditional DAMP. Antioxid Redox Signal 28: 973–986, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su H, Wan C, Lei CT, Zhang CY, Ye C, Tang H, Qiu Y, and Zhang C. Lipid deposition in kidney diseases: interplay among redox, lipid mediators and renal impairment. Antioxid Redox Signal 28: 1027–1043, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Gulbins E, and Grassmé H. The crosstalk between sphingomyelinases and ROS in mycobacterial infection. Antioxid Redox Signal 28: 935–948, 2018 [DOI] [PubMed] [Google Scholar]