Abstract
Significance: Eicosanoids are endogenous lipid mediators that play important roles in brain function and disease. Acute brain injury such as that which occurs in stroke and traumatic brain injury increases the formation of eicosanoids, which, in turn, exacerbate or diminish injury. In chronic neurodegenerative diseases such as Alzheimer's disease and vascular dementia (VD), eicosanoid synthetic and metabolizing enzymes are altered, disrupting the balance between neuroprotective and neurotoxic eicosanoids.
Recent Advances: Human and experimental studies have established the opposing roles of hydroxy- and epoxyeicosanoids and their potential utility as diagnostic biomarkers and therapeutic targets in neural injury.
Critical Issues: A gap in knowledge remains in understanding the cellular and molecular mechanisms underlying the neurovascular actions of specific eicosanoids, such as specific isomers of epoxyeicosatrienoic (EETs) and hydroxyeicosatetraenoic acids (HETEs).
Future Directions: EETs and HETEs exert their actions on brain cells by targeting multiple mechanisms, which include surface G-protein coupled receptors. The identification of high-affinity receptors for EETs and HETEs and their cellular localization in the brain will be a breakthrough in our understanding of these eicosanoids as mediators of cell-cell communications and contributors to brain development, function, and disease. Antioxid. Redox Signal. 28, 987–1007.
Keywords: : P450 eicosanoids, ROS, brain injury, neuroprotection, epoxyeicosatrienoic, hydroxyeicosatetraenoic acids
Introduction
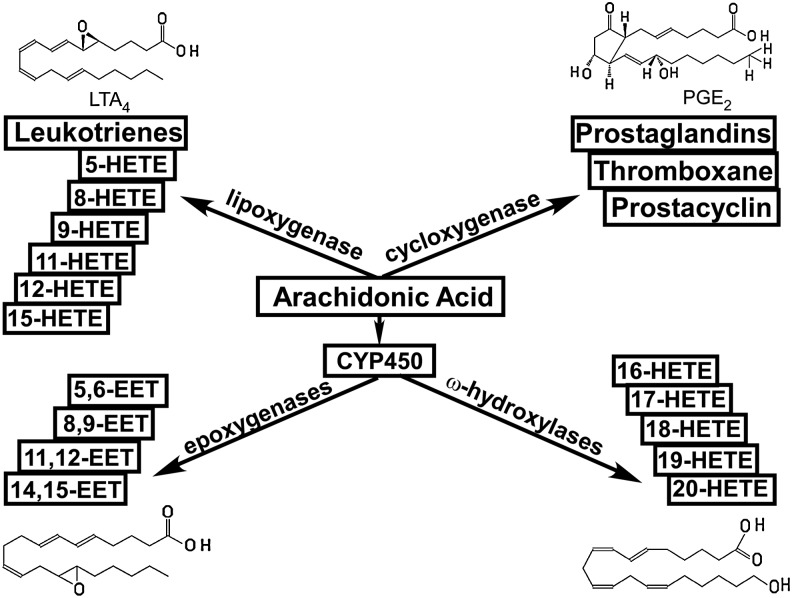
Eicosanoids play important roles in normal homeostasis and tissue injury (140). They are synthesized from arachidonic acid (AA) via one of the three pathways, namely, the cyclooxygenase (COX), lipoxygenase (LOX), and heme-containing cytochrome P450 (P450) pathways (35, 55). As depicted in Figure 1, prostaglandins, thromboxane, and prostacyclin, collectively known as prostanoids, are generated via the COX pathway. Midchain hydroxyl derivatives (5-, 8-, 9-, 11-, 12-, and 15-HETE) and leukotrienes are generated via the LOX pathway. The production of epoxyeicosatrienoic acids (EETs) and ω-terminal hydroxyeicosatetraenoic acids (HETEs) (16-, 17-, 18-, 19-, and 20-HETE) is catalyzed by members of the cytochrome P450 family where P450 epoxygenases form EETs and ω-hydroxylases form HETEs (35, 55).
FIG. 1.
A brief overview of the metabolic pathways for eicosanoid synthesis. Represented structures are those of LTA4, PGE2, 14,15-EET, and 20-HETE. EETs, epoxyeicosatrienoic acids; HETE, hydroxyeicosatetraenoic acids.
Recognition of the biological importance of eicosanoids has evolved over the past several decades. The importance of prostaglandins in various physiological functions has been recognized and extensively studied since the mid-1930s (184); the physiological and pathophysiological roles of leukotrienes and lipoxins were introduced in the late 1970s (152, 160); whereas the roles of EETs and HETEs were not recognized until the early 1980s (34). More recently, non-enzymatically formed isoprostanes and non-AA products of COX, LOX, and P450 metabolites, such as resolvins, protectins, and maresins, were discovered and implicated in tissue injury and resolution of inflammation (124, 159).
Prostanoids and leukotrienes are involved in a multitude of normal and pathological conditions, such as vascular reactivity, platelet aggregation, cell maturation, and inflammation (45). The diverse roles of these prostanoids and leukotrienes are mediated, in part, by a family of membrane-bound G-protein coupled receptors (GPCRs) that can activate distinct intracellular signaling pathways that are triggered by ligand binding to the receptors (45). Extensive data in the literature suggest that EETs and HETEs may also exert their effects via membrane-bound receptors (38, 79, 185, 192, 198), although their corresponding putative receptors are largely unknown.
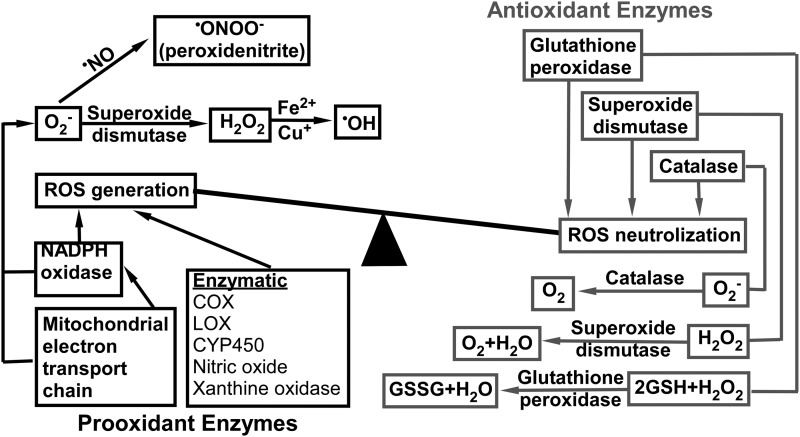
There is a duality and reciprocity to the roles that P450 eicosanoids play in brain injury. As a general rule, epoxygenase products are neuroprotective, whereas hydroxylase products are neurotoxic. Furthermore, the conversion of AA to eicosanoids by COX-, LOX-, and P450 enzyme-catalyzed reactions generates reactive oxygen species (ROS) such as superoxide anion (O•−) and hydroxyl radical (OH•) (63, 64). Under normal physiological conditions, the generation of free radicals is balanced by the capacity of cellular anti-oxidant systems (63, 64). The low level of ROS serves as an intracellular signal and can be beneficial, but at high concentrations, ROS causes lipid peroxidation, DNA damage, and protein modifications (111). As summarized in Figure 2, in general, the level of cellular ROS is determined by the balance between its production and neutralization.
FIG. 2.
ROS generation and scavenging systems. ROS, reactive oxygen species.
ROS is produced by the mitochondrial electron transport chain and enzymatic reactions utilizing oxygen, whereas they are neutralized by intracellular scavengers such as antioxidant enzymes and antioxidants (111). The redox balance in cells may be disturbed due to a rapid increase in prooxidant mechanisms and a limited antioxidant capacity of cells, resulting in a lag in the antioxidant response (63). In disease states, such as ischemia and inflammation, inflammatory cytokines and ROS increase the activity of phospholipase A2 (PLA2), leading to a higher PLA2-catalyzed release of AA from cell membranes (102).
As mentioned earlier, at a higher rate of synthesis, AA metabolizers, in turn, generate ROS, worsening the redox environment and exacerbating tissue injury (82, 146, 189). Furthermore, as discussed later, eicosanoids themselves can augment or suppress oxidative stress. These observations demonstrate the reciprocal relationship between eicosanoids and ROS. The current review summarizes recent data on the interplay between P450 eicosanoids and ROS in the context of brain injury caused by stroke, traumatic brain injury (TBI), and neurodegenerative diseases such as Alzheimer's disease (AD) and aging-related vascular cognitive impairment (VCI). We synthesize these findings to highlight the dual role of P450 enzymes and their metabolites in brain disease as pro- and anti-inflammatory, and pro- and anti-oxidant and neuroprotection.
Selectivity of regio- and stereoisomers
As mentioned earlier, EETs and HETEs act in opposite manners under normal physiological conditions and disease states. For example, HETEs tend to induce vasoconstriction whereas EETs tend to be vasodilators (57, 69, 131, 158, 197, 206, 217). HETEs augment ROS production, whereas EETs reduce ROS production (82, 146, 189). HETEs promote inflammation, whereas EETs suppress inflammation (41, 48, 97, 129). However, these generalizations ignore the unique and tissue-specific roles that different regio- and stereoisomers of EETs and HETEs play under different conditions (61, 73, 129, 137, 167, 176). For example, levels of 5-, 8-, 12-, and 15-HETE and 5,6- and 8,9-EET but not 11,12- or 14,15-EET increase after intestinal ischemia induced by superior mesenteric artery occlusion (73). In a vascular inflammation model, 11,12-EET inhibited the expression of vascular cell adhesion molecule-1 (VCAM-1) induced by tumor necrosis factor-a (TNF-a), in contrast to 14,15-EET, which had no effect on VCAM-1 expression (129).
Evidence suggests that different HETEs regio- and stereoisomers also have different efficacies, and their effects are tissue- and organ specific. For example, 12(S)-HETE induced a more profound constriction than 12(R)-HETE and 15-HETE in isolated rat kidney afferent arterioles (206), whereas 15-HETE induced vasoconstriction in rat internal carotid arteries (217); 20-HETE has been recognized as a potent vasoconstrictor in multiple vascular beds (69, 70, 207). In rat cerebral arteries, 20-HETE formation correlated with an increasing intraluminal pressure, and an increasing 20-HETE concentration dose dependently reduced the diameter of isolated rat cerebral arteries (69). This pressure-dependent vasoconstriction, known as myogenic vasoconstriction, is the hallmark of autoregulation of blood flow in the brain, heart, and kidney (69). Nevertheless, even for 20-HETE, its effect seems to be tissue specific. For example, in rat basilar arteries, 20-HETE produces dilation that is inhibited by indomethacin, suggesting the conversion of 20-HETE by COX to secondary products (58). In isolated mouse aortic rings, 19-HETE induces vasodilation by activating the prostacyclin receptor (179). Later, we discuss possible explanations for the tissue selectivity of different EETs and HETEs and their regio- and stereoisomer-specific effects.
Synthetic enzymes
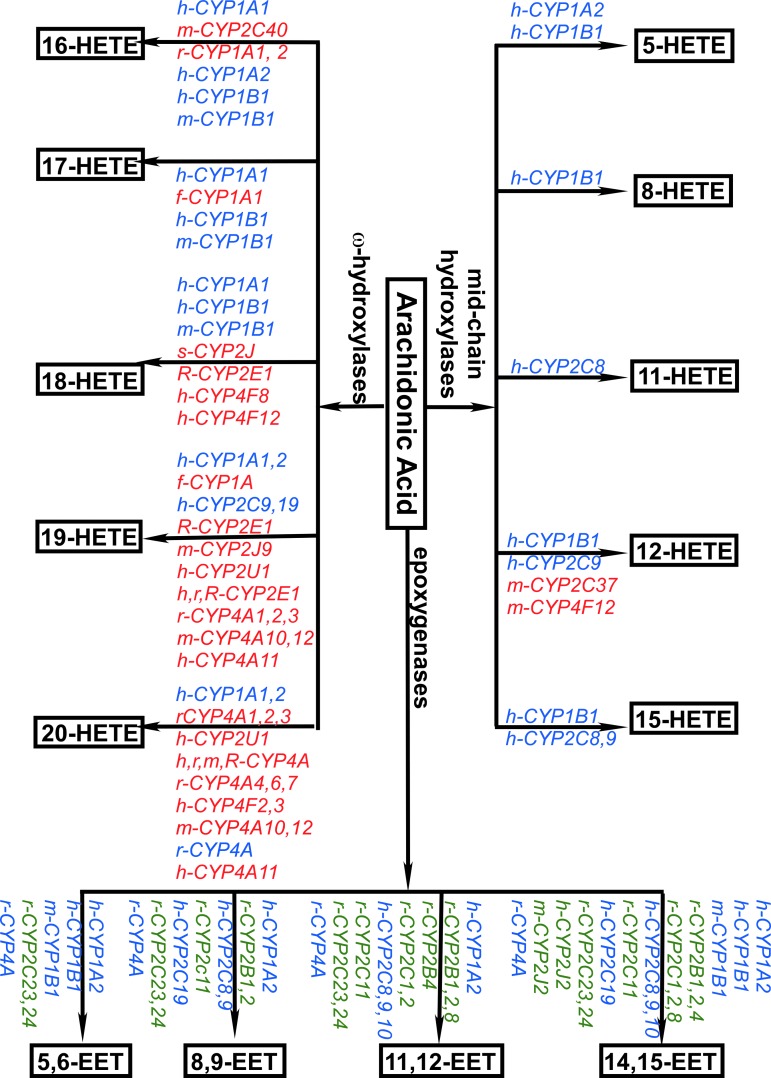
In addition to LOX-catalyzed production of midchain HETEs (35, 55, 135), HETEs and EETs are produced by members of the cytochrome 450 enzymes superfamily (Fig. 3) (8, 29, 34, 40, 46, 55, 72, 96, 108, 148, 210). Among the panel of enzymes discovered, several are selective for ω-terminal HETEs regioisomers (labeled in red). Rat CYP2C11 (r-CYP2C11) selectively produces EETs (labeled in green). In addition, the activation of several enzymes (labeled in blue) catalyzes the production of both HETEs and EETs, although the efficiencies for HETEs versus EETs production by these enzymes differ. For example, human CYP1A1 (h-CYP1A1) is more efficient in synthesizing terminal HETEs than midchain HETEs and EETs; h-CYP1A2 is more efficient in synthesizing EETs; h-CYP1B1 is more efficient in synthesizing midchain HETEs; and mouse CYP1B1 (m-CYP1B1) is more efficient in synthesizing EETs (40).
FIG. 3.
Specificity of major P450 enzymes involved in eicosanoid production. Labels in red indicate that the enzyme only catalyzes the production of HETEs regioisomers. Labels in blue indicate that the enzyme catalyzes the production of both EETs and HETEs regioisomers. Labels in green indicate that the enzyme only catalyzes the production of EETs regioisomers. For clarity, cytochrome P450 enzyme names are preceded by the first letter of the species that they are found in, where h = human, m = mouse, r = rat, f = fish, R = rabbit, and s = sheep.
Local concentrations of HETEs and EETs
In addition to synthetic enzyme activation, local concentrations of HETEs and EETs are affected by additional factors. First, differential incorporation of HETEs and EETs into phospholipids can affect their bioavailability (20, 24, 59, 98, 165, 183). For example, the level of lipid-bound 14,15-EET in rat plasma is more than three times higher than the level of 8,9-EET and approximately 20% more than 11,12-EET (98). In human umbilical artery smooth muscle cells, 70% of (3H)5-HETE was incorporated into cell lipids, whereas only 12% of 12-HETE and 8% of 15-HETE were incorporated into smooth muscle cell lipids over 20 h of incubation (24).
Second, the conversion of HETEs and EETs into secondary products could change their bioavailability. For example, EETs are converted into dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolases (sEH) in vascular cells (59, 121, 183, 211), and HETEs can be converted into secondary products such as 20-hydroxyendoperoxides, 20-COOH-AA, and 20-OH-PGH2, which are products of 20-HETE (44, 58, 158). The secondary products may sometimes be active and exert additional effects that may differ from those induced by the primary products. For example, in pre-constricted bovine coronary artery rings, 14,15-DHET induced vasorelaxation of the artery rings, although with less potency than 14,15-EET (31). The vasodilatory effects of other DHETs such as 8,9-, 11,12-, and 14,15-DHET have also been observed in isolated human coronary arterioles where they induce vasodilation by activating BKCa channels (103). The secondary products produced from 20-HETE such as 20-hydroxyendoperoxides, 20-COOH-AA, and 20-OH-PGH2 displayed vasoconstrictive properties (158). Interestingly, as mentioned earlier, in mouse basilar arteries, both 20-COOH-AA and 20-HETE induced vasodilation (58).
Third, cell-specific release of HETEs and EETs could, in addition, modulate the local concentration and bioavailability of these compounds. Earlier studies indicated that HETEs were produced in platelets and neutrophils (149, 180). However, later studies demonstrated that they are also produced by vascular smooth muscle cells (VSMCs) (70, 125, 157, 219); 14,15-EET is produced by endothelial cells (ECs) and astrocytes (8, 32, 68, 128) but exerts its effect on VSMCs, inflammatory cells, and neurons (57, 93, 131, 197).
Direct modulation of membrane protein functions
Incorporation of HETEs and EETs into phospholipids (20, 24, 59, 98, 165, 183) suggests that they may modify the function of membrane proteins directly in the lipid bilayer. For example, in HEK cells expressing the transient receptor potential cation channel subfamily V member 4 (TRPV4) channel, which is a Ca2+-permeable non-selective cation channel (143), 500 nM 5,6-EET opens TRPV4 channels directly as demonstrated by both whole-cell and inside-out single-channel recordings (186). To a lesser extent, 8,9-EET also increased intracellular Ca2+ in HEK cells expressing TRPV4, whereas 11,12- and 14,15-EET had no effect on TRPV4 channel function (186); 5,6-EET increased intracellular Ca2+ in ECs isolated from mouse aorta (186). In contrast, both 5,6-EET and 14,15-EET were shown to increase endothelial permeability in rat and mouse lungs where the expression of TRPV4 was confirmed in bronchiolar epithelium as well as in smooth muscle cells (SMCs) in human and rat extra-alveolar vessels (10). In this case, however, it is not clear whether the effects of 5,6-EET and 14,15-EET were due to direct modulation of channel activity since their effects were measured 45 min after treatment (10). In another study, 3 μM 11,12-EET induced TRPV4-like cation current in VSMCs as well as vasodilation in mesenteric arteries of the wild-type (WT) mouse. These effects were absent from TRPV4 knockout mice and the effect of 11,12-EET was fully reversed by the potent, non-specific TRPV4 blocker, ruthenium red (54).
Several other channels are also modulated by EETs and HETEs. For example, 20-HETE induced dose-dependent increases in blood pressure, coronary perfusion pressure (isolated Langendorff), and pressure-induced constriction of resistance arteries and these effects were attenuated in TRPV1 knockout mice or after treatment with the TRPV1 antagonist RP67580 (25). In cultured rat brain astrocytes, exogenously applied 20-HETE (100–300 nM) significantly reduced the activity of two types of Ca2+-activated potassium channels (KCa), with unitary conductances of 71 pS and 161 pS (72). Also, 20–125 nM EETs decreased the open probability of porcine cardiac L-type Ca2+ channels that were reconstituted in lipid bilayers (36). On the other hand, 3 μM 14,15-EET increased the opening probability but not single-channel conductance of large-conductance Ca2+-activated K+ (BKCa) channels that were assayed in inside-out patches obtained from rat pituitary GH(3) cells (194). In freshly isolated vascular muscle cells from cat cerebral arteries, 5 μM 8,9-EET or 11,12-EET increased the open probability of a 98-pS K+ channel that was recorded in the cell-attached mode (71).
Receptor targets for HETEs and EETs
High-affinity receptors
Besides the impact of local production, their incorporation into membrane lipids, and their secondary metabolism, the effects of HETEs and EETs also depend on the availability of their putative membrane receptors as well as on modes of ligand-receptor interaction and the signal transduction pathways activated by receptor-ligand engagement. Due to the low level of EETs and HETEs produced endogenously (6, 76, 163), their effects are likely to be mediated by high-affinity receptors under normal physiological conditions. Multiple ligand binding studies suggest the existence of a high-affinity receptor for 14,15-EET (37, 192, 198), and this putative 14,15-EET receptor exerts its vasodilator effect via the Gs-coupled signaling pathway, leading to increased intracellular cAMP (107, 113, 192, 193). To date, such high-affinity receptors have not been identified, although a 47 kDa membrane protein was found to bind to a 14,15-EET analog with a high affinity (37). In addition, a high-affinity receptor for 11,12-EET was also suggested by the following studies. In isolated rat small renal arteries, a nanomolar concentration of 11(R),12(S)-EET, but not 11(S),12(R)-EET or 14,15-EET, increased the activity of K+ channels in cell-attached patches obtained from VSMCs (218). However, this stimulatory effect was absent in excised inside-out or outside-out patches (218). A similar observation was made in cultured astrocytes where exogenous 11,12-EET (100 nM) only increased the NPo of both the 71 pS and 161 pS KCa channels in cell-attached but not in excised inside-out patches (196). Subsequently, it was demonstrated in isolated patches from VSMCs of small bovine coronary arteries that the stimulatory effect of a nanomolar concentration of 11,12-EET on the large conductance KCa channel requires the presence of Gsα, suggesting that 11,12-EET may interact with a Gs-coupled membrane receptor (107).
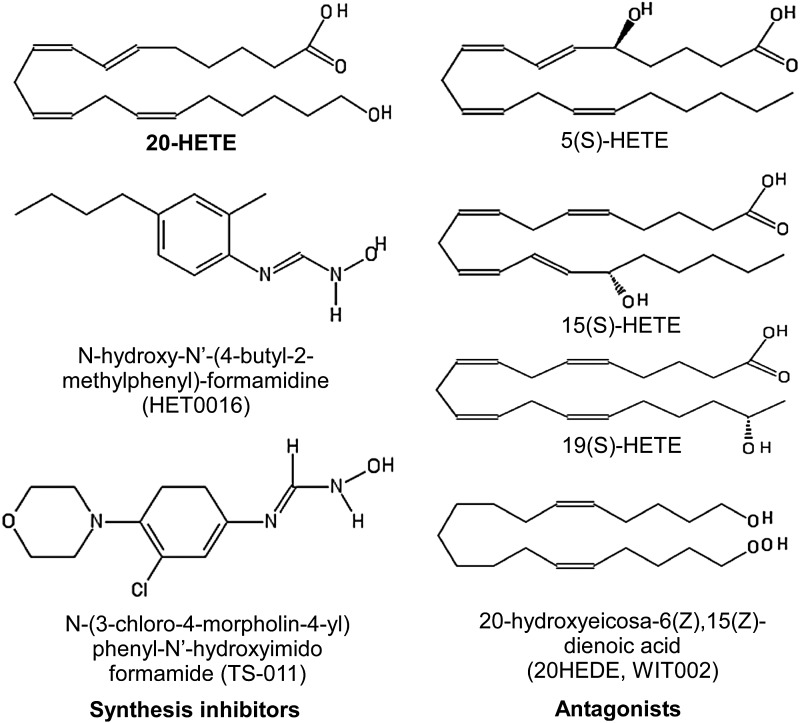
For HETEs isomers, GPR31 was identified as a high-affinity receptor for 12(S)-HETE (79). Similarly, a high-affinity binding site for 12-HETE was found in the human epidermal cell line (78), but it is unknown whether the receptor observed in this study is GPR31. The existence of a high-affinity 15-HETE receptor was demonstrated by the high-affinity specific binding of (3H)15-HETE in cultured PT-18 mast/basophil cells (142, 185). A high-affinity receptor for 20-HETE has also been suggested by functional readouts induced by nanomolar concentrations. For example, in rat middle cerebral arteries, 20-HETE induced a dose-dependent reduction in vessel diameter in the nanomolar range. Interestingly, this constrictive effect was blocked by 1 μM 15-HETE (69). This acute, dose-dependent constriction induced by 20-HETE is consistent with activation of a Gq-coupled receptor. The case for a putative 20-HETE receptor is also strengthened by the discovery of several 20-HETE antagonists such as 5(S)-HETE, 15(S)-HETE, 19(S)-HETE, and 6(Z),15(Z)-2-HEDE based on structure-activity studies (9).
Low-affinity receptors
We have recently identified several low-affinity receptors that were stimulated by 1 μM 14,15-EET (113). One of these low-affinity receptors, PTGER2, has been shown to mediate the effect of 14,15-EET in isolated rat mesenteric arteries in the micromolar range (197); 14,15-EET and other EETs regioisomers were also suggested to be agonists for a peroxisome proliferator-activated receptor (PPARγ) with micromolar efficacies based on the competitive bindings of EETs regioisomers toward the recombinant protein of the PPARγ ligand-binding domain (114). In addition, 14,15-EET has been suggested as an antagonist for thromboxane-prostanoid receptor (TP) based on the observation that 14,15-EET-inhibited TP agonist (U46619) induced vasoconstriction but not that induced by other non-TP agonists such as phenylephrine, endothelin-1 (19); 14,15-EET exhibited competitive binding toward TP in cultured CHO cells expressing TP with a Ki of 3.3 μM, which is nearly six times lower than the Ki toward PTGER2 determined in the same assay (19).
A low-affinity receptor for 19-HETE was identified as the prostacyclin receptor (179). Interestingly, in HEK cells overexpressing TP, 1 μM 12-HETE inhibited U46619-induced increase in intracellular Ca2+ and displaced specific binding toward SQ29548, thus acting as an antagonist for TP (162); 15-HETE was implicated as a TP agonist based on the observation that 15-HETE-induced constriction was prevented by SQ29548 in rabbit aortic rings (176). Furthermore, 12(S)-HETE and 15(S)-HETE have been implicated as PPARγ receptor agonists based on the observation that they inhibited ischemia-induced inflammation by activating PPARγ receptor, and their effects were reversed by the PPARγ antagonist GW9662 (167). In addition, 20-HETE has been suggested as an agonist for TP based on the observation that it induced contraction of isolated rat aortic rings or rat kidney arteries, both of which were abolished by pretreatment with SQ29548 (13, 56). However, this observation was in contrast to results from a study in dog renal arteries where the constriction induced by 20-HETE was not blocked by pretreatment with SQ29548 (116).
The apparent antagonistic nature of 15-HETE toward 20-HETE raises an interesting question as to whether 20-HETE exhibits a similar antagonistic function toward the putative 15-HETE receptor (185), and whether different HETEs regioisomers serve as agonists and antagonists for other putative regioisomer-specific receptors. In Table 1, we summarize the regioisomers of HETEs and EETs that are either associated with known receptors or their interaction with the corresponding putative receptors has been implicated by biochemical and pharmacological studies.
Table 1.
Summary of Potential Hydroxyeicosatetraenoic Acids and Epoxyeicosatrienoic Acids Receptors
| Regioisomer | Receptor ID | Affinity | Ref. |
|---|---|---|---|
| 12-HETE | GPR31 | High | 79 |
| 12(S)-HETE | PPARγ | Low | 167 |
| 15-HETE | ? | High | 142,185 |
| 15-HETE | TP | Low | 19,176 |
| 15(S)-HETE | PPARγ | Low | 167 |
| 19-HETE | PTGIR | Low | 179 |
| 20-HETE | TP | Low | 13,19,56 |
| 11,12-EET | ? | High | 107 |
| 14,15-EET | ? | High | 37,38,192,198 |
| 14,15-EET | PPARγ | Low | 114 |
| 14,15-EET | Prostaglandin receptors | Low | 113,197 |
High affinity refers to effective concentrations <1 μM. Low affinity refers to effective concentrations ≤1 μM.
? indicates the existence of a receptor, although identity has not been confirmed.
Prostaglandin receptors: PTGER2, PTGER4, PTGDR, PTGFR, and PTGER3IV.
EETs, epoxyeicosatrienoic acids; HETE, hydroxyeicosatetraenoic acids; PPARγ, peroxisome proliferator-activated receptor γ; PTGIR, prostacyclin receptor; TP, thromboxane-prostanoid receptor.
Mechanisms of putative HETEs and EETs receptor activation
It is reasonable to expect that a certain level of specificity for the action of regioisomers of HETEs and EETs might be achieved based on the factors mentioned earlier, such as semi-selective CYP450 enzyme-catalyzed production of eicosanoids, differential incorporation of regioisomers into phospholipids, conversion of HETEs and EETs into secondary products, and cell-dependent receptor distribution. However, the next level determining the selectivity of HETEs and EETs is likely dictated by the modes of ligand-receptor interactions and the activation or inhibition of signaling pathways resulting from such interactions. Accumulating evidence indicates that TP interacts with multiple HETEs and EETs regioisomers to modulate vasoreactivity (13, 19, 56, 176) whereas PPARγ interacts with multiple HETEs and EETs regioisomers to modulate inflammation (129, 167).
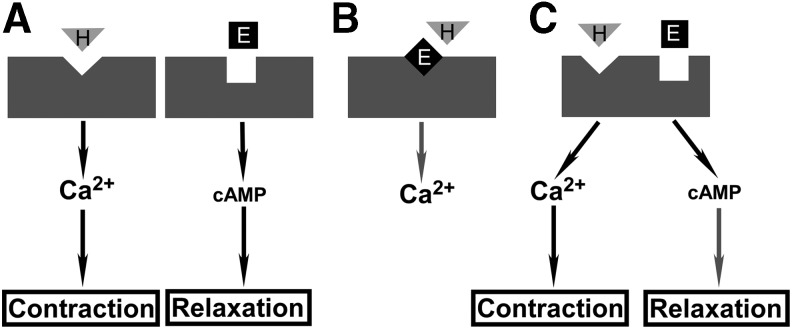
Data in the literature point to three potential modes of ligand-receptor interactions (Fig. 4). These modes of action are not necessarily exclusive of each other. Mode A represents a simple ligand binding-induced activation of putative receptors for HETEs and EETs, respectively. Data in the literature indicate that HETEs stimulate an increase in intracellular Ca2+ whereas EETs stimulate an increase in intracellular cAMP (70, 107, 113, 192, 193, 206, 207). These simple mechanisms, while reasonable, may not provide the most efficient control to balance the opposing effects of HETEs and EETs as they must rely on the upstream events to control the local concentrations of these molecules and receptor distribution to achieve a fine balance between the opposing actions of these two classes of molecules.
FIG. 4.
Potential modes of activation of HETEs and EETs receptors. Vascular reactivity is used as an example to depict potential ligand-receptor interactions and the subsequent effects on vascular reactivity that are dictated by the activation of different intracellular signaling pathways. (A) Mode A, in which HETEs and EETs bind to separate receptors to increase intracellular Ca2+ or cAMP, respectively. (B) Mode B, in which an EET acts as an antagonist for HETE. (C) Mode C, in which HETEs and EETs bind to the same receptor but at different sites to increase intracellular Ca2+ or cAMP, respectively. Gray downward triangle represents HETEs. Black square represents EETs. Black arrows indicate an increase or activation, whereas gray arrows indicate a decrease or inhibition in the downstream targets.
Additional control, however, could be exerted by the actions of antagonists or partial agonists. For example, 15-HETE blocks 20-HETE-induced vasoconstriction in rat middle cerebral arteries (69) whereas 5-, 15-, and 19-HETE are demonstrated to block 20-HETE-induced vasoconstriction in rat renal arterioles (9).
Mode B depicts a dual-ligand interaction mechanism where HETEs and EETs compete for a single binding site on a single receptor. This scheme is proposed based on the observation that 20-HETE and 15-HETE are TP agonists (13, 56, 176) whereas 14,15-EET is an antagonist for TP (19), although it has not been shown experimentally whether 14,15-EET can actually block the agonist effect of 15- or 20-HETE on TP. Nevertheless, this type of shared binding site may pose a limitation on the binding affinity as the ligand coordination site has to be able to accommodate the binding of ligands that not only are similar, to some extent, but also differ structurally (Fig. 1), thus limiting the efficacy of the agonists or antagonists. It is not surprising then that the interactions between HETEs and EETs with TP tend to be of a low affinity (13, 19, 56, 176).
Mode C is proposed based on the general observation that HETEs and EETs tend to exert opposing effects in various tissues and conditions. It is curious to see whether they can bind to the same receptor but at separate binding sites to activate different signaling pathways. An example for this model is the orphan GPCR, GPR17, which was found to be responsive to two distinct unrelated ligands (43). GPR17 belongs to the rhodopsin family of GPCRs within the purin receptor cluster, along with purinergic receptors (P2Y) and cysteinyl leukotriene (cysLT) receptor (43, 65). The natural ligands for P2Y receptors are extracellular nucleotides (1, 27), whereas the natural ligand for cysLT receptor is cysLT (23). GPR17 was activated by both nucleotide and cysLT, leading to a decrease in cAMP and a transient increase in intracellular Ca2+ (43). The advantages of this mode of action are fast dynamic control by the relative concentrations of EETs versus HETEs, and having separate binding sites tailored for different ligands to achieve high-affinity binding for both ligands, thus enhancing the overall efficacy of the receptor.
HETEs, EETs, and ROS in Stroke
Ischemic stroke
Ischemic stroke is characterized by blood clots inside cerebral blood vessels that block blood flow to the brain. The sudden loss of blood supply deprives brain cells from essential oxygen and nutrients and triggers a cascade of cellular events that, if protracted, result in cell death. The most notable event is the generation of ROS (63, 130, 134), which mostly occurs during reperfusion, presumably due to the rapid increase in prooxidant mechanisms and the limited antioxidative capacity of cells (63, 111).
Although great progress has been made in understanding the roles of EETs and HETEs and their interplay with ROS in ischemia, the detailed mechanisms are still not fully characterized. Ischemic injury increases the activity of PLA2 (11), which hydrolyzes membrane phospholipids to liberate AA (17). An increase in AA promotes the subsequent production of HETEs and EETs along with other eicosanoids and the generation of ROS (82, 146). The activation of PLA2 after ischemia is, in part, attributed to increased glutamate release from neuronal cells (136), which has also been shown to stimulate AA release in mouse hippocampal slices and cultured mouse brain astrocytes (150, 164).
HETEs in ischemic stroke
The important role that HETEs play in ischemia is supported by their increased production after ischemia (12, 138, 167, 174). The specific regioisomer produced depends on the location, severity of injury, and method of induction of injury, among other factors. For example, in human plasma of patients who suffered from ischemic stroke and those with Parkinson's disease, the levels of 5(S)-, 12(S)-, 15(S)-, and 20-HETEs were increased, suggesting a role for both LOX and P450 hydroxylase metabolites (105). In another study, a significant positive correlation between lesion size and plasma 20-HETE was observed in patients suffering from ischemic stroke (190).
In a mouse bilateral occlusion model of transient global ischemia, 12/15-LOX upregulation correlated with injury, and 12/15-LOX pharmacological inhibition and gene deletion were protective against focal and global cerebral ischemia (182, 205). Similarly, in the mouse middle cerebral artery occlusion (MCAO) model, inhibition of 20-HETE synthesis reduced infarct volume and blocked the decrease in cerebral blood flow (CBF) during reperfusion (119).
In decapitation-induced global ischemia of the rat brain, levels of 5-HETE and 12-HETE increase along with increases in thromboxane B2 (TXB2) and ROS, suggesting activation of LOX and COX pathways (60). In the isolated perfused rat brain, levels of 5- and 15-HETE increased during brief ischemia, but levels normalized within 30 min of reperfusion, suggesting transient activation of the LOX pathway (174). In contrast, PGF2 alpha, PGE2, PGD2, 6-keto-PGF1α, and TXB2 did not change during ischemia, but increased during reperfusion, suggesting activation of the COX pathway by reoxygenation (174).
In the rat, 72 h after focal cerebral ischemia induced by MCAO, when brain edema was at its maximum, levels of 5-, 9-, 11-, and 15-HETEs increased, suggesting a role for LOX metabolites in brain edema (12, 181). In the anesthetized ischemic rat brain, the levels of 12(S)- and 15(S)-HETE increased along with increased expression of 12/15-LOX, predominantly in neurons (167). Interestingly, exogenous 12(S)- and 15(S)-HETE in this study were shown to be protective and anti-inflammatory, in part via PPARγ activation (167); 12/15-LOX total brain protein level increased significantly along with the level of 12-HETE after ischemia-reperfusion in rats (83).
This apparent contradiction in the effects of LOX metabolites may be related to the differences in mechanisms employed by endogenous and exogenous HETEs. For example, exogenous 15-HETE acts as an antagonist for 20-HETE in rat middle cerebral arteries to block 20-HETE-induced vasoconstriction (69) but it induces vasoconstriction in isolated rabbit aortic rings, and this vasoconstrictive effect was blocked by the TP antagonist, suggesting an interaction with TP (176). High concentrations of HETEs applied exogenously are more likely to induce their effect via both low- and high-affinity receptors whereas the low concentrations of HETEs produced endogenously might only interact with high-affinity receptors.
Genetic factors also contribute to the level of HETEs in ischemic stroke. For example, variations of the gene encoding h-CYP2C8, h-CYP4A11, and EPHX2 are significantly higher in patients who developed neurologic deterioration after acute stroke, which also coincided with an increased plasma 20-HETE concentration and a decreased level of EETs (204). Several mutations in the genes encoding h-CYP4F2 and h-CYP4A11, which catalyzed the production of 20-HETE, increased the risk of ischemic stroke, cerebral infarction in patients, especially in men (47, 62, 66).
In hypertensive stroke-prone rats, ischemic stroke-induced elevation of 20-HETE is associated with an increase in the expression of r-CYP4A protein, r-CYP4A1, and r-CYP4A8 mRNA (53). Gene interaction between the loci of h-CYP4A11 and h-CYP4F2 increases the level of plasma 20-HETE and the risk for ischemic stroke (110). In addition, variations of the gene ALOX5AP are associated with patients with ischemic stroke (49, 50, 88, 95, 115). This gene encodes the nuclear membrane-associated 5-LOX–activating protein required for the activation of 5-LOX and the production of 5-HETE (133).
The development of specific 20-HETE synthesis inhibitors, including N-hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine (HET0016) (123) and N-(3-chloro-4-morpholin-4-yl) phenyl-N′-hydroxyimido formamide (TS-011) (122), played a major role in advancing our understanding of the role of 20-HETE in ischemia. HET0016 and TS-011 were originally shown to be more selective toward 20-HETE over 11,12-EET in isolated rat kidney microsomes (122, 123). The selectivity to other HETE regioisomers was later demonstrated in studies where HET0016 was shown to block the formation of 20-HETE but not 12-HETE in brain cortical tissue (138). TS-011 selectively blocked the formation of 20-HETE in cerebral arteries, with no effect on the production of 12-HETE, 11,12-EET, or 14,15-EET (171). Furthermore, in rats challenged with transient MCAO, pre-treatment with TS-011 significantly reduced the level of 20-HETE but not the levels of 5-, 12-, and 15-HETE (145), demonstrating its selectivity toward 20-HETE production over other regioisomers. HET0016 and TS-011 also selectively inhibited the formation of 20-HETE in rat renal microsomes and inhibited the synthesis of 20-HETE by recombinant h-CYP4A11, h-CYP4F2, or h-CYP4F3 (122).
Despite the fact that multiple HETE regioisomers have been reported to be altered by ischemia (12, 60, 137, 163, 167, 174), 20-HETE has been recognized as a major player in ischemic injury (47, 53, 132, 145, 146, 172). Recently, it was suggested to be an independent predictor of neurological deterioration in acute minor ischemic stroke (203). Earlier studies indicated that inhibition of 20-HETE production by HET0016 and TS-011 was effective in reducing ischemic brain damage, increasing CBF (53, 122, 145, 171) and improving neurological and functional outcomes (172). It was initially believed that increased 20-HETE production after ischemia may cause brain damage by reducing blood flow due to its vasoconstrictive properties (119, 138). However, a later study showed that inhibition of 20-HETE by HET0016 did not alter the diameter of pial arteries (33). Several other studies also failed to observe a difference in CBF between vehicle-treated and 20-HETE inhibitor-treated animals; 20-HETE inhibition, however, did reduce infarct size and neuronal damage, perhaps through modulating other signaling pathways (145, 200). For example, it was recently shown that in neurons the expression of m-CYP4A/4F-derived enzymes was significantly increased after oxygen-glucose deprivation (OGD) and inhibition of 20-HETE synthesis or antagonizing 20-HETE increased cell survival (212).
In addition to synthesis inhibitors, the identification of 20-HETE antagonists such as 5-HETE, 15-HETE, 19-HETE, and 6(Z),15(Z)-2-HEDE, also known as 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (WIT002) (9, 207), provided important tools for investigating the physiological function of 20-HETE. However, in the absence of a known putative receptor for 20-HETE, data using presumed 20-HETE antagonists should be interpreted with care. As discussed later, an antagonist for 14,15-EET (14,15-EEZE) that was initially identified by using similar structure-activity studies (67) was later found to have other activity besides being an antagonist for 14,15-EET (85, 113). In Figure 5, we compiled the known structures of 20-HETE along with its synthesis inhibitors and antagonists.
FIG. 5.
Structures of 20-HETE (top left) and its synthesis inhibitors (lower left) and antagonists (right) reported in the literature.
EETs in ischemic stroke
EETs are abundantly produced in the brain (8). Cells producing EETs in the brain include astrocytes, ECs, and neurons (8, 32, 68, 91, 92, 94, 128). EETs are rapidly hydrated to their corresponding vicinal diols (DHETs) by sEH (59, 121, 183, 211). Therefore, the reported cellular/plasma concentrations of EETs are generally low (6, 76, 98), and data on stroke-induced changes in EETs concentration are scarce. In one group of patients in Australia who suffered from acute ischemic stroke (<96 h), the plasma concentration of EETs was elevated to about 240 pM, which was more than double the value seen in control patients. The levels of 20-HETE and ROS were also increased in these patients, and plasma 20-HETE and EETs were attenuated 30 days after the stroke in a subset of patients (190).
Several studies demonstrated that EETs are protective in ischemic brain injury. In particular, the beneficial role of 14,15-EET has been elucidated by using genetic and pharmacological manipulations of CYP450 enzymes and sEH, which utilize 14,15-EET as their preferred substrate over other EETs regioisomers (121, 211). The first suggestion that EETs are protective in stroke came from preconditioning studies where protection by brief ischemia against subsequent prolonged ischemia in the rat (7) was accompanied by an increased expression of r-CYP2C11 epoxygenase (6, 8), suggesting that upregulation of EETs synthesis may serve as an endogenous protective mechanism in stroke (7).
We had previously identified astrocytes as the cellular source of expression of r-CYP2C11 in the rat brain (8). The mechanism of upregulation of CYP2C11 in astrocytes may be linked to either hypoxia-inducible factor 1α (HIF-1α) or glutamate. For example, we have shown that hypoxic preconditioning induces r-CYP2C11 mRNA expression in primary cultured astrocytes via HIF-1α (112). We have also shown that expression of r-CYP2C11 and EETs synthesis in primary cultured astrocytes are stimulated by glutamate (6), a major excitatory neurotransmitter that is significantly elevated in the ischemic brain. In cultured hippocampal astrocytes, brief hypoxia also increased the expression of r-CYP2C11 and the production of EETs (196).
In addition to astrocytic CYP450 isoforms, the human endothelial h-CYP2J2 and h-CYP2C8 isoforms also protect against stroke-related brain injury (96, 108). Interestingly, the protective effects of m-CYP2J2 and m-CYP2C8 are gender dependent, as demonstrated in a genetically engineered mouse model (Tie2-CYP2J2) where human h-CYP2J2 is specifically expressed in ECs (96, 108). Male Tie2-CYP2J2 mice sustain a greater reduction in infarct size, increased CBF, and reduced neuronal death and ROS production (96, 108). Overexpression of endothelial h-CYP2C8 isoform reduced infarct size, but by a different mechanism than that used by h-CYP2J2 where h-CYP2J2 exerts its protective effect by enhancing blood flow whereas h-CYP2C8 exerts its protective effect by suppressing inflammation (96).
Overall, elevating the level of 14,15-EET by inhibiting sEH, genetically deleting the gene encoding sEH, or exogenously administering 14,15-EET all protect against cerebral ischemic injury. In contrast to EETs synthetic enzymes, which are expressed in cells responding to changes in the surrounding environment (astrocytes and ECs), sEH seems to be expressed in target cells, such as neurons. For example, in the mouse brain, sEH is predominantly expressed in neuronal cell bodies and processes, and, to a lesser extent, in cerebral blood vessels (214). Pharmacological inhibition of sEH reduced infarct size after MCAO, and the protective effect was lost when EETs synthesis was also inhibited (214), suggesting that protection by sEH inactivation is specifically linked to EETs.
Similarly, deletion of the gene encoding sEH protected against stroke-induced brain damage. In the sEH knockout (sEHKO) mice, infarct size and neurological deficit were significantly smaller after MCAO compared with WT control mice (215), which was also associated with higher CBF in sEHKO mice. The total plasma concentration of sEH metabolite 14,15-DHET was significantly lower in sEHKO mice relative to controls, indicating a reduction in the conversion of 14,15-EET by sEH (215). In a co-culture of astrocytes and neurons, inhibition of sEH increased neuronal survival after OGD (216). Mutations in the sEH gene, designated ephx2, also affect its activity and alter the rate of neuronal survival, as seen in rat primary cortical neuronal culture transduced with human EPHX2 mutant proteins. Transduction of neuronal cells with these mutant proteins resulted in a variable rate of cell death induced by OGD, which was, in part, related to how the enzyme's hydrolase activity is affected, whereas pharmacological inhibition of sEH prevented the increase in cell death induced by transduction with WT sEH, indicating a protective role for EETs (100).
The mechanisms of protection by EETs in ischemic stroke are linked to changes in CBF, vasodilation, neuroprotection, promotion of angiogenesis, suppression of platelet aggregation, oxidative stress, and post-ischemic inflammation (90). Less clear are the molecular entities or pathways targeted by 14,15-EET to protect against ischemic stroke. As mentioned earlier, a specific receptor has been proposed to mediate the actions of 14,15-EET, but whether this putative receptor is involved here is less clear. This is especially true since the genetic and pharmacological methods mentioned earlier are all likely to increase the level of EETs above its normal physiological level. In cultured astrocytes, the concentration of EETs secreted into culture media was reported in the micromolar range (216).
Exogenous EETs treatment at concentrations between 250 nM and 10 μM before ischemic injury was found to be protective against ischemic brain injury (108, 215). In this range, not only the putative high-affinity receptor but also low-affinity receptors could potentially participate, which maybe important therapeutically (100, 112, 141, 175, 178). One such receptor is PTGER2 which is activated by 14,15-EET (113, 197), and activation of PTGER2 in cultured rat hippocampal slices by PGE2 and its selective agonist butaprost was neuroprotective against toxicity induced by glutamate receptor agonist NMDA. PTGER2 agonists were also protective against neuronal cell death induced by OGD (120). In addition, genetic deletion of the PTGER2 increased infarct volume after MCAO/reperfusion-induced transient cerebral ischemia in mice (120).
As with 20-HETE, for example, a presumed antagonist for 14,15-EET (14,15-EEZE) identified based on similar structure-activity studies (67) was found to exhibit agonist and partial agonist activities in the bovine coronary artery (26, 67) and mouse mesenteric arteries (85), which may be related to activation of PGE2 receptors. In support of the latter idea, we have recently shown that 14,15-EEZE behaved as an agonist or a partial agonist for PTGER2 using an exogenous expression system. In Xenopus oocytes coexpressing PTGER2 and CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) chloride channel, which served as a cAMP sensor because channel activation is dependent on the elevation of intracellular cAMP, exposure to 1 μM 14,15-EET or 14,15-EEZE resulted in a robust increase in chloride current, demonstrating the activation of PTGER2 by both compounds, especially given that these compounds had no effect on the conductance in the absence of PTGER2 expressed (113).
Interplay between EETs, HETEs, and ROS in ischemic stroke
Because of their reactive chemical nature, ROS generation results in damage to multiple cell components, including DNA alteration, lipid peroxidation (14, 86, 191), and protein modifications (64). DNA damage introduces mutations and alters normal protein synthesis. Modification of proteins by ROS generates diverse chemical species at the protein side chains, leading to a variety of functional modifications (64). Most notably, an ROS sensor has been identified as an ROS-activated, nonselective calcium permeable cation channel (transient receptor potential M2 [TRPM2]) (84). TRPM2 (also known as LTRPC2) is broadly expressed in neuronal cells, myocytes, β cells, and immune cells (195). It is activated by an H2O2-induced increase in NAD+ that binds directly to TRPM2 at the intracellular domain, which opens the channel and allows extracellular Ca2+ to rush into the cell in a voltage-independent manner, and Ca2+ overload resulting from TRPM2 activation leads to cell death (84). Not surprisingly, TRPM2 is found to be detrimental in transient cerebral ischemia (5, 202).
20-HETE production contributes to ROS generation. In brain slices obtained from the rat neocortex or hippocampus, increases in 20-HETE correlated with increases in ROS production (82, 146). In isolated rat brain slices, 1 μM 20-HETE induced a rapid increase in superoxide that reached a plateau within 3 min (82), and ROS production was reduced by pretreatment with 20-HETE synthesis inhibitor (82, 146). On the other hand, exogenously administered 14,15-EET opposes ROS production in primary cultures of cortical neurons. Pre-exposure to 20 nM 14,15-EET prevented the generation of ROS after OGD (189). This is, in part, linked to 14,15-EET-induced phosphorylation of Akt (p-Akt) (141), which participates in multiple cellular functions, including cell survival (2, 151).
The role of Akt in mediating the protective effect of EETs in ischemia was further confirmed by a recent study using the co-culture of primary astrocytes and neurons (216). That study made several interesting observations. First, it was observed that inhibition of sEH increased secretion of vascular endothelial growth factor (VEGF) by astrocytes into the culture media after OGD, and this increase was blocked by 14,15-EEZE. Second, exogenous 14,15-EET also increased VEGF release after OGD. Third, the addition of astrocyte-conditioned culture medium collected after OGD to neurons increased their survival. Fourth, the stimulation of VEGF receptor-2 in neurons by astrocyte- and OGD-conditioned medium increased p-Akt, suggesting that 14,15-EET secreted from astrocytes after OGD is involved in neuronal protection and it exerts its action via p-Akt (216).
One potential common mechanism linking HETEs and EETs with ROS is the TP, which is regulated by the two classes of P450 eicosanoids in opposite directions (13, 19, 56, 176). Activation of TP stimulates ROS production in isolated rabbit renal afferent arterioles and the mouse chronic kidney disease model (156, 188). Furthermore, a thromboxane A2 receptor antagonist, ONO-3708, has been shown to increase the survival rate of dogs after complete global brain ischemia, suggesting a detrimental role of TP activation in ischemia (170). Together, these observations suggest a potential role for TP in mediating the protective effect of 14,15-EET and the detrimental effect of 20-HETE in ischemia. These results do not necessarily exclude the potential involvement of other putative receptors for EETs and HETEs. Although 12-HETE has been shown to be an antagonist for TP (162), the inhibitory effect of 12-HETE on ROS production in ischemia has not been reported.
Hemorrhagic stroke
Hemorrhagic stroke is characterized by damage to brain cells due to blood vessel rupture and subsequent bleeding. The most common cause is aneurysm rupture, which leads to subarachnoid hemorrhage (SAH), and its most serious complication is cerebral vasospasm and the associated delayed cerebral ischemia (DCI) (137, 163). As with ischemic stroke, although both HETEs and EETs are implicated in hemorrhagic stroke, the specific regioisomer involved depends on the specific disease condition. In human SAH patients, 5-HETE was increased in cerebrospinal fluid (CSF), which coincided with the occurrence of cerebral vasospasm, suggesting a role for lipid peroxidation in the pathogenesis of chronic vasospasm after SAH (155, 168). In another study with two patients who suffered from SAH and developed cerebral vasospasm, levels of 12- and 20-HETE in CSF were significantly higher than those seen in matched controls who suffered SAH but did not develop vasospasm (137). Similarly, in another study, the level of 20-HETE increased immediately after SAH and by day 10 decreased to a level comparable to that seen in patients who did not develop DCI (163). After aneurysmal SAH, an increased CSF 20-HETE level was associated with unfavorable outcomes, including DCI and clinical neurologic deterioration (52).
20-HETE is also a key player in experimental SAH models. In a rat SAH model, the concentration of CSF 20-HETE increased more than 10-fold after SAH induced by injecting arterial blood into the cisterna magna, and inhibition of 20-HETE formation reduced the initial fall in CBF (99). Interestingly, along with an increase in the level of 20-HETE, SAH also increased the level of 5-hydroxytryptamine-1B (5-HT) in CSF (30). Furthermore, an intracisternal injection of 5-HT increased the level of CSF 20-HETE level and reduced CBF, whereas inhibition of 20-HETE synthesis prevented 5-HT-induced fall in CBF, suggesting an interaction between 20-HETE and 5-HT receptor after SAH (30).
In addition, HET0016 blocked the production of 20-HETE by r-CYP4A11, r-CYP4F2, and r-CYP4F3 in a concentration-dependent manner and administration of HET0016 after SAH induction returned CBF to the control level within 30 min whereas CBF of vehicle-treated rats remained at 30% below the control over the 2-h course of the experiment (99). The level of 20-HETE also increased in delayed vasospasm after SAH in a dog model where dual injections of blood into the cisterna magna on days 1 and 4 consistently induced delayed vasospasm (80). A decrease in the basilar artery diameter 3 days after the second injection of blood is accompanied by an increase in 20-HETE (80). Furthermore, acute inhibition of 20-HETE formation by TS-011 increased the diameter of the basilar artery, and chronic administration of TS-011 attenuated the development of delayed vasospasm, suggesting an important role of 20-HETE in SAH and subsequent injuries (80).
Among the regioisomers of EETs, only 14,15-EET has been implicated in SAH. In SAH patients, the level of 14,15-EET in CSF increased at 10 days after SAH, regardless of whether they develop DCI (163). Genetic variability of h-CYP2C8, h-CYP2J2, and h-CYP2C9 among patients with aneurysmal SAH was associated with different clinical outcomes depending on the level of EETs in CSF (51). In addition, genetic polymorphisms of sEH have also been linked to neurological outcome and survival after aneurysmal SAH (118).
ROS plays a critical role in SAH (199). ROS concentration increased in the primary cortical neuron SAH model after exposure to fresh blood obtained from veins of mice, which coincided with neuronal cell death (106). In a rat intracerebral hemorrhage model, a 10-min infusion with heparinized blood into the right caudate nucleus in cerebrum induced a significant increase in ROS production (77).
HETEs, EETs, and ROS in TBI
TBI refers to brain injury induced by external mechanical forces, which is characterized by primary mechanical damage as well as by secondary injury resulting from the initial injury (61, 144). The secondary injury is believed to largely result from glutamate-induced excitotoxicity, ischemia, and oxidative damage (187).
It has been demonstrated that the levels of 5- and 12-HETE dramatically increased in patients after TBI (61). Similarly, in a rat TBI model, EETs and HETEs were released within 24 h after TBI. In this study, the level of EETs was nearly fourfold higher than that of HETEs (74). However, in Ephx2-KO mice, only 8,9-EET level was increased due to TBI and these mice showed improved motor coordination (166). Furthermore, TBI induces an increase in ROS in both human and animal models (18, 39, 81, 101, 117, 173, 187) and the severity of injury correlated with the degree of oxidative stress (173). In animal TBI models, the generation of ROS leads to oxidation of the delayed rectifier potassium channel (KCNB1) and TRPM2, causing neuronal damage and apoptosis in the hippocampus, in addition to the induction of lipid peroxidation (208, 209).
Although ischemia is one of the major secondary injuries resulting from TBI (187), the roles of 20-HETE and 14,15-EET in TBI-induced secondary injury are not well described and the interplay between HETEs, EETs, and ROS is not well characterized in TBI.
HETES, EETS, and ROS in Neurodegenerative Diseases
Alzheimer's disease
AD is a chronic age-related neurodegenerative disorder that is associated with progressive cognitive impairment and memory loss (4). It is characterized by an overproduction and excessive accumulation of amyloid beta (Aβ), a 40–42 amino-acid-long peptide, and formation of neurofibrillary tangles resulting from hyper-phosphorylation of tau, a microtubule stabilizing protein (4, 22).
Although recent work suggest that tau is a better marker for tracking dementia status and a better predictor for cognitive performance than Aβ (22), extensive literature suggests that Aβ not only induces accumulation of ROS but also causes extensive cellular damage and modification of multiple targets, including lipid peroxidation, protein oxidation, and DNA and reactive nitrogen species (RNS) oxidation (28). Unfortunately, it was recently reported that an IgG1 monoclonal anti-Aβ antibody, known as solanezumab, did not provide a significant improvement in cognitive decline in patients (147). Failure of this drug only heightens the need to further understand the molecular and cellular mechanisms related to AD and to develop alternative therapies to treat AD.
ROS is an important player in AD. As a major intracellular organelle for oxygen-dependent ATP production, mitochondria stand as a major source for ROS. Not surprisingly, mitochondrial dysfunction in both neurons and astrocytes (3) plays an important role in AD (87). In co-culture of hippocampal neurons and astrocytes, mitochondrial membrane potential in astrocytes was altered by Aβ but not in neurons (3). Aβ-induced decrease in mitochondrial membrane potential causes an increase in the generation of ROS in astrocytes due to the activation of NADPH oxidase (3), which catalyzes one-electron reduction of oxygen to generate superoxide (O2−) (15).
Considering that astrocytes also produce EETs, which are neuroprotective (8), the potential effect of EETs in AD was recently explored. In microsomes isolated from the rat cerebral cortex, Aβ reduced the concentrations of 14,15-EET and 11,12-EET in a region- and cell-specific manner (153, 154). Incubation with soluble Aβ led to a decrease in 14,15-EET production in the cerebrum, but not in the cerebellum, and Aβ had no effect on the production of 11,12-EET in these regions (153). A more detailed analysis showed that Aβ treatment decreased 14,15-EET production in astrocytes and neurons, but the production of 11,12-EET was affected by Aβ only in astrocytes (153). In the cortex and hippocampus, on the other hand, the production of 11,12-EET, but not 14,15-EET, was reduced by Aβ treatment (153).
Furthermore, blocking endogenous EETs production by an epoxygenase inhibitor aggravated the effect of Aβ on astrocyte mitochondrial dysfunction whereas pretreatment of primary hippocampal astrocyte culture with exogenous 11,12-EET or 14,15-EET for 24 h prevented Aβ-induced mitochondrial dysfunction (154). Although the molecular mechanisms underlining the protective effects of 11,12-EET and 14,15-EET are unknown, these results suggest that 11,12-EET, 14,15-EET, and other EETs may have therapeutic potential against Aβ−induced mitochondria dysfunction.
Other regioisomers have also been implicated in AD, with no consistent patterns of change in different studies. In AD patients, the levels of 12-HETE and 15-HETE were elevated (139, 201). In an AD mouse model, although the level of 12-HETE was elevated, the level of 15-HETE was found to be lower in 10 month-old AD mice than in the controls (169). In primary cultures of rat cortical neurons, 12-HETE was believed to be involved in inducing cell death because exposure to exogenous 12-HETE but not 5-HETE increased apoptosis (104). In contrast, in cultured human neuro-2A neuroblastoma cells expressing Aβ precursor, 5-HETE increases Aβ by activating cAMP-response element binding protein (CREB) (42). Factors such as species, cell types, and experimental procedures could potentially explain the differences observed among different studies. Stimulation of CREB by 5-HETE in the Aβ−expressing cell line is of particular interest because it suggests that Gs-coupled signaling might mediate the effect of 5-HETE.
Overall, the molecular and cellular mechanisms underlining the effects of EETs and HETEs in AD are not well understood. It is of interest to note that TP receptor is expressed in the brain (21) and activation of TP by isoprostanes, a marker for in vivo oxidative stress, increased the levels of amyloid precursor protein (APP) and Aβ production in a mouse AD model (161). Because both EETs and HETEs regioisomers can interact with TP, their effects to modulate Aβ production could potentially be mediated via their actions on TP.
Vascular cognitive impairment
VCI is the second most common cause of dementia after AD. It is defined as cognitive impairment due to vascular injury, with or without clinical stroke (75). VCI is a microvascular disease that is caused, in part, by microscopic bleeding or poor microvascular blood flow in the brain (177). The most severe form of VCI is vascular dementia (VD) (75). The mechanisms underlying VD are poorly understood, with no specific treatments currently available to prevent or treat VD and associated VCI. Because of the interdependence of cells within the neurovascular unit, damage to vascular cells will inevitably affect the health and function of neuronal cells (89).
Both HETEs and EETs have been implicated in VCI, supported by their dominant role in regulation of the microvasculation. Human autopsy brains with VCI had an increased ratio of DHETs to EETs, suggesting upregulation of sEH (126). The upregulation was localized by immunohistochemistry to the microvascular endothelium. In a separate study in mice, we found that high-fat diet-induced type 2 diabetes leads to impaired spatial memory (assessed by the Morris water maze) and upregulates cerebrovascular sEH expression in WT mice, suggesting that diabetes, a known risk factor for VCI, may, in part, be responsible for endothelial upregulation of sEH observed in the postmortem human VCI brain (220). sEH upregulation could potentially contribute to neuronal injury by causing a state of chronic hypoperfusion. In the mouse, chronic hypoferfusion can be induced by unilateral common carotid artery occlusion, which, we have shown, impairs cognition (221). In addition to decreases in EETs, 5-HETE, 12-HETE, 18-HETE, and 20-HETE were increased in VCI patients (126). VCI is also associated with increased free radicals (16). Several natural compounds such as Gastrodin, Betaine, and Puerarin have been shown to neutralize ROS and reduce the cognitive dysfunction in experimental VCI (109, 127, 213).
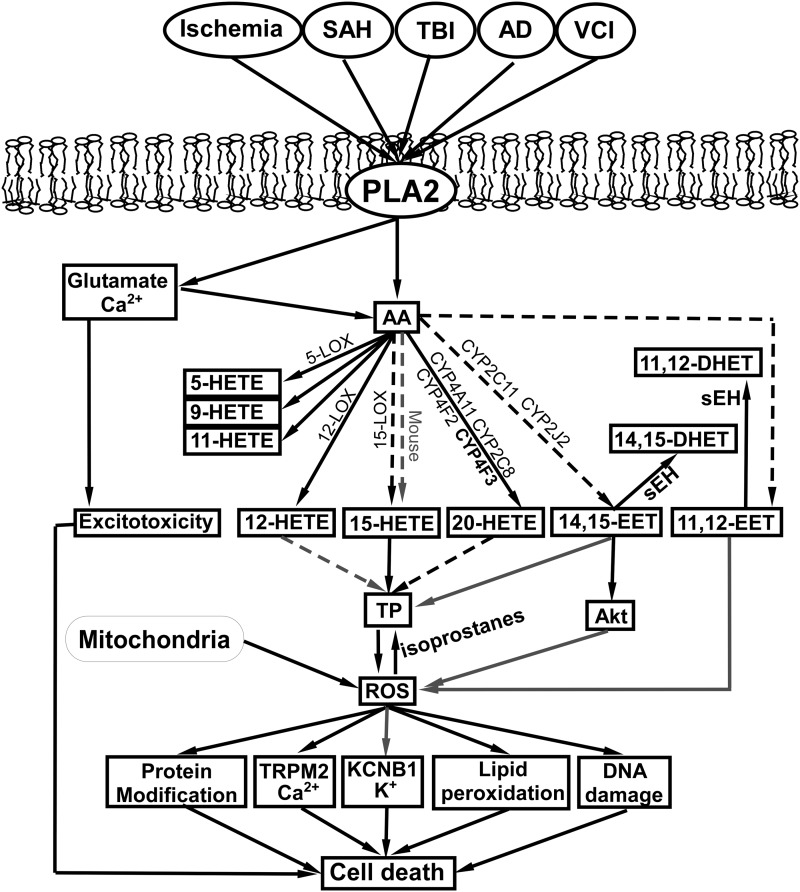
In Figure 6, we summarize potential interactions between ROS, HETEs, and EETs, along with enzymes that determine the production and conversion of HETEs and EETs in brain diseases and injuries discussed in this review. We also summarized the patterns of changes in the levels of HETEs and EETs in brain diseases/injury and protection in Table 2.
FIG. 6.
A brief overview on the interplay between ROS, HETEs, and EETs in brain diseases/injury based on data from multiple studies by multiple groups. Causative relationships were drawn based on at least one published result. Black arrows refer to increases, gray arrows indicate decreases of the targets, and dashed arrows indicate conflicting results.
Table 2.
Changes of Eicosanoid Levels in Acute and Chronic Brain Disease
| Animal model | Human | ||||||
|---|---|---|---|---|---|---|---|
| Disease injury | Protection | Ref. | Disease injury | Protection | Ref. | ||
| LOX | 5-HETE | ↑ (IS) ↑ (TBI) |
↓ (IS) | 12c, 60, 74r, 174r, 181r | ↑ (IS) | 50, 61, 88, 95, 105, 115, 155, 168 | |
| ↑ (SAH) | |||||||
| ↑ (TBI) | |||||||
| 8-HETE | ↑ (IS) | ↓ (IS) | 74r, 181r | ||||
| 9-HETE | ↑ (IS) | ↓ (IS) | 12c, 74r, 181r | ||||
| ↑ (TBI) | |||||||
| 11-HETE | ↑ (IS) | ↓ (IS) | 12c, 74r, 181r | ||||
| ↑ (TBI) | |||||||
| 12-HETE | ↑ (IS) | ↓ (IS) | 74r, 82m, 83r, 99d, 167r, 169m, 205m, 181r | ↑ (IS) | 61, 105, 137, 139, 201 | ||
| ↑ (TBI) | ↑ (SAH) | ||||||
| ↑ (AD) | ↑ (TBI) | ||||||
| ↑ (AD) | |||||||
| 15-HETE | ↑ (IS) | ↓ (IS) | 12c, 74r, 83r, 167r, 169m, 174r, 181r, 182m, 205m | ↑ (IS) ↑ (AD) |
105, 139, 201 | ||
| ↑ (TBI) | |||||||
| ↓ (AD) | |||||||
| P450 | 17-HETE | ↑ (AD) | 169m | ||||
| 18-HETE | ↑ (AD) | 169m | |||||
| 19-HETE | ↑ (TBI) | ↓ (IS) | 74r | ||||
| 20-HETE | ↑ (IS) | ↓ (IS) ↓ (SAH) |
30r, 51m, 53r, 74r, 80d, 99d, 146r, 119m, 122r, 132r, 138r, 145, 171r, 172r, 207r | ↑ (IS) | 47, 52, 62, 66, 99, 105, 110, 122, 137, 163, 190, 203, 204 | ||
| ↑ (SAH) | ↑ (SAH) | ||||||
| ↑ (TBI) | ↑ (VCI) | ||||||
| P450 | 5,6-EET | ↓ (AD) | ↑ (IS) | 7r, 74r, 214m | ↑ (IS) ↓ (VCI) |
118, 126, 190 | |
| ↑ (TIS) | |||||||
| ↑ (TBI) | |||||||
| 8,9-EET | ↓ (AD) | ↑ (IS) | 7r, 51m, 52m, 74r, 166m, 214m | ↑ (IS) ↓ (VCI) |
118, 126, 190 | ||
| ↑ (TIS) | |||||||
| ↑ (TBI) | |||||||
| 11,12-EET | ↓ (AD) | ↑ (IS) | 7r, 51m, 52m, 74r, 153r, 154r, 214m | ↑ (IS) ↓ (VCI) |
118, 126, 190 | ||
| ↑ (TIS) | |||||||
| ↑ (TBI) | |||||||
| ↑ (AD) | |||||||
| 14,15-EET | ↓ (AD) | ↑ (IS) | 7r, 51m, 52m, 74r, 100m, 214m–216r, 153, 154r | ↑ (IS) ↓ (SAH) ↓ (VCI) |
↑ (SAH) | 51, 100, 118, 126, 163, 190 | |
| ↑ (TIS) | |||||||
| ↑ (OGD) | |||||||
| ↑ (TBI) | |||||||
| ↑ (AD) | |||||||
When reported results did not specify regioisomers, an increase in HETEs or EETs is assumed to include all regioisomers listed in the table. Superscripts next to the references identify the animal species used in the cited studies, where c = cat, d = dog, m = mouse, and r = rat.
AD, Alzheimer's disease; IS, ischemic stroke; LOX, lipoxygenases; OGD, oxygen-glucose deprivation; SAH, subarachnoid hemorrhage; TBI, traumatic brain injury; TIS, transient ischemic stroke; VCI, vascular cognitive impairment.
Conclusions and Future Directions
This review is aimed at highlighting the dual role of P450 enzymes and their metabolites in brain diseases and at providing an update on the molecular and cellular mechanisms underlying the effects of EETs and HETEs in various brain pathological conditions. In general, the two classes of P450 eicosanoids, EETs and HETEs, exert opposite effects on neuronal survival, and two potential convergence points for their antagonistic actions are ROS generation and TP activation (13, 19, 56, 176). Even though the interactions between EETs and HETEs with TP are of a low affinity (13, 19, 56), eicosanoid levels under pathological conditions and pharmacological interventions reach sufficiently high tissue concentrations to engage TP. The identification and role of any potential high-affinity receptors for EETs and HETEs remain elusive and limit our understanding of the roles of EETs and HETEs and their interplay with ROS in various disease states.
Abbreviations Used
- 5-HT
5-hydroxytryptamine-1B
- AA
arachidonic acid
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- Aβ
amyloid beta
- BKCa
large-conductance Ca2+-activated K+ channels
- CBF
cerebral blood flow
- CFTR
Cystic Fibrosis Transmembrane Conductance Regulator
- COX
cyclooxygenases
- CREB
cAMP-response element-binding protein
- CSF
cerebrospinal fluid
- cysLT
cysteinyl leukotriene
- DCI
delayed cerebral ischemia
- DHETs
dihydroxyeicosatrienoic acids
- ECs
endothelial cells
- EETs
epoxyeicosatrienoic acids
- GPCR
G-protein coupled receptor
- HET0016
N-hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine
- HETEs
hydroxyeicosatetraenoic acid
- KCa
Ca2+-activated potassium channels
- KCNB1
delayed rectifier potassium channel
- LOX
lipoxygenases
- MCAO
middle cerebral artery occlusion
- OGD
oxygen-glucose deprivation
- P2Y
purinergic receptors
- P450
cytochrome P450
- PLA2
phospholipase A2
- PPARγ
peroxisome proliferator-activated receptor γ
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SAH
subarachnoid hemorrhage
- sEH
soluble epoxide hydrolases
- SMCs
smooth muscle cells
- TBI
traumatic brain injury
- TNF-a
tumor necrosis factor-a
- TP
thromboxane-prostanoid receptor
- TRPM2
transient receptor potential M2
- TRPV4
transient receptor potential cation channel subfamily V member 4
- TS-011
N-(3-chloro-4-morpholin-4-yl) phenyl-N′-hydroxyimido formamide
- VCAM-1
vascular cell adhesion molecule-1
- VCI
vascular cognitive impairment
- VD
vascular dementia
- VEGF
vascular endothelial growth factor
- VSMCs
vascular smooth muscle cells
- WIT002
20-hydroxyeicosa-6(Z),15(Z)-dienoic acid
- WT
wild type
Acknowledgments
This work was supported by KCVI and NIH grants NS44313, NS070837, and AG043857 (Alkayed), and AHA GRNT31380022 (Alkayed).
References
- 1.Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, and Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci 24: 52–55, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abeyrathna P. and Su Y. The critical role of Akt in cardiovascular function. Vascul Pharmacol 74: 38–48, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramov AY, Canevari L, and Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci 24: 565–575, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adibhatla RM. and Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 12: 125–169, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Alim I, Teves L, Li R, Mori Y, and Tymianski M. Modulation of NMDAR subunit expression by TRPM2 channels regulates neuronal vulnerability to ischemic cell death. J Neurosci 33: 17264–17277, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, and Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke 28: 1066–1072, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Alkayed NJ, Goyagi T, Joh HD, Klaus J, Harder DR, Traystman RJ, and Hurn PD. Neuroprotection and P450 2C11 upregulation after experimental transient ischemic attack. Stroke 33: 1677–1684, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, and Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke 27: 971–979, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Alonso-Galicia M, Falck JR, Reddy KM, and Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol 277: F790–F796, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, and Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 99: 988–995, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arai K, Ikegaya Y, Nakatani Y, Kudo I, Nishiyama N, and Matsuki N. Phospholipase A2 mediates ischemic injury in the hippocampus: a regional difference of neuronal vulnerability. Eur J Neurosci 13: 2319–2323, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Asano T, Koide T, Gotoh O, Joshita H, Hanamura T, Shigeno T, and Takakura K. The role of free radicals and eicosanoids in the pathogenetic mechanism underlying ischemic brain edema. Mol Chem Neuropathol 10: 101–133, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Askari B, Bell-Quilley CP, Fulton D, Quilley J, and McGiff JC. Analysis of eicosanoid mediation of the renal functional effects of hyperchloremia. J Pharmacol Exp Ther 282: 101–107, 1997 [PubMed] [Google Scholar]
- 14.Ayala A, Munoz MF, and Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014: 360438, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babior BM. NADPH oxidase: an update. Blood 93: 1464–1476, 1999 [PubMed] [Google Scholar]
- 16.Back SA, Kroenke CD, Sherman LS, Lawrence G, Gong X, Taber EN, Sonnen JA, Larson EB, and Montine TJ. White matter lesions defined by diffusion tensor imaging in older adults. Ann Neurol 70: 465–476, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balsinde J, Winstead MV, and Dennis EA. Phospholipase A(2) regulation of arachidonic acid mobilization. FEBS Lett 531: 2–6, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Bayir H, Marion DW, Puccio AM, Wisniewski SR, Janesko KL, Clark RS, and Kochanek PM. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J Neurotrauma 21: 1–8, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, and Douglas SA. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. J Pharmacol Exp Ther 328: 231–239, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Bernstrom K, Kayganich K, Murphy RC, and Fitzpatrick FA. Incorporation and distribution of epoxyeicosatrienoic acids into cellular phospholipids. J Biol Chem 267: 3686–3690, 1992 [PubMed] [Google Scholar]
- 21.Borg C, Lim CT, Yeomans DC, Dieter JP, Komiotis D, Anderson EG, and Le Breton GC. Purification of rat brain, rabbit aorta, and human platelet thromboxane A2/prostaglandin H2 receptors by immunoaffinity chromatography employing anti-peptide and anti-receptor antibodies. J Biol Chem 269: 6109–6116, 1994 [PubMed] [Google Scholar]
- 22.Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TL, and Ances BM. Tau and Abeta imaging, CSF measures, and cognition in Alzheimer's disease. Sci Transl Med 8: 338ra366, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brink C, Dahlen SE, Drazen J, Evans JF, Hay DW, Nicosia S, Serhan CN, Shimizu T, and Yokomizo T. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev 55: 195–227, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Brinkman HJ, van Buul-Wortelboer MF, and van Mourik JA. Selective conversion and esterification of monohydroxyeicosatetraenoic acids by human vascular smooth muscle cells: relevance to smooth muscle cell proliferation. Exp Cell Res 192: 87–92, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Bubb KJ, Wen H, Panayiotou CM, Finsterbusch M, Khan FJ, Chan MV, Priestley JV, Baker MD, and Ahluwalia A. Activation of neuronal transient receptor potential vanilloid 1 channel underlies 20-hydroxyeicosatetraenoic acid-induced vasoactivity: role for protein kinase A. Hypertension 62: 426–433, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Bukhari IA, Gauthier KM, Jagadeesh SG, Sangras B, Falck JR, and Campbell WB. 14,15-Dihydroxy-eicosa-5(Z)-enoic acid selectively inhibits 14,15-epoxyeicosatrienoic acid-induced relaxations in bovine coronary arteries. J Pharmacol Exp Ther 336: 47–55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burnstock G. and Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240: 31–304, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Butterfield DA, Drake J, Pocernich C, and Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol Med 7: 548–554, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Bylund J, Ericsson J, and Oliw EH. Analysis of cytochrome P450 metabolites of arachidonic and linoleic acids by liquid chromatography-mass spectrometry with ion trap MS. Anal Biochem 265: 55–68, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Cambj-Sapunar L, Yu M, Harder DR, and Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke 34: 1269–1275, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Campbell WB, Deeter C, Gauthier KM, Ingraham RH, Falck JR, and Li PL. 14,15-Dihydroxyeicosatrienoic acid relaxes bovine coronary arteries by activation of K(Ca) channels. Am J Physiol Heart Circ Physiol 282: H1656–H1664, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Campbell WB, Gebremedhin D, Pratt PF, and Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Cao S, Wang LC, Kwansa H, Roman RJ, Harder DR, and Koehler RC. Endothelin rather than 20-HETE contributes to loss of pial arteriolar dilation during focal cerebral ischemia with and without polymeric hemoglobin transfusion. Am J Physiol Regul Integr Comp Physiol 296: R1412–R1418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capdevila JH. and Falck JR. Biochemical and molecular characteristics of the cytochrome P450 arachidonic acid monooxygenase. Prostaglandins Other Lipid Mediat 62: 271–292, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Capdevila JH, Falck JR, and Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res 41: 163–181, 2000 [PubMed] [Google Scholar]
- 36.Chen J, Capdevila JH, Zeldin DC, and Rosenberg RL. Inhibition of cardiac L-type calcium channels by epoxyeicosatrienoic acids. Mol Pharmacol 55: 288–295, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Falck JR, Manthati VL, Jat JL, and Campbell WB. 20-Iodo-14,15-epoxyeicosa-8(Z)-enoyl-3-azidophenylsulfonamide: photoaffinity labeling of a 14,15-epoxyeicosatrienoic acid receptor. Biochemistry 50: 3840–3848, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Falck JR, Tuniki VR, and Campbell WB. 20-125Iodo-14,15-epoxyeicosa-5(Z)-enoic acid: a high-affinity radioligand used to characterize the epoxyeicosatrienoic acid antagonist binding site. J Pharmacol Exp Ther 331: 1137–1145, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho HJ, Sajja VS, Vandevord PJ, and Lee YW. Blast induces oxidative stress, inflammation, neuronal loss and subsequent short-term memory impairment in rats. Neuroscience 253: 9–20, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, and Schenkman JB. Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1b1. Drug Metab Dispos 32: 840–847, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Christmas P. Role of cytochrome P450 s in Inflammation. Adv Pharmacol 74: 163–192, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Chu J. and Pratico D. 5-lipoxygenase as an endogenous modulator of amyloid beta formation in vivo. Ann Neurol 69: 34–46, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, and Abbracchio MP. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J 25: 4615–4627, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins XH, Harmon SD, Kaduce TL, Berst KB, Fang X, Moore SA, Raju TV, Falck JR, Weintraub NL, Duester G, Plapp BV, and Spector AA. Omega-oxidation of 20-hydroxyeicosatetraenoic acid (20-HETE) in cerebral microvascular smooth muscle and endothelium by alcohol dehydrogenase 4. J Biol Chem 280: 33157–33164, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Cornejo-Garcia JA, Perkins JR, Jurado-Escobar R, Garcia-Martin E, Agundez JA, Viguera E, Perez-Sanchez N, and Blanca-Lopez N. Pharmacogenomics of prostaglandin and leukotriene receptors. Front Pharmacol 7: 316, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daikh BE, Lasker JM, Raucy JL, and Koop DR. Regio- and stereoselective epoxidation of arachidonic acid by human cytochromes P450 2C8 and 2C9. J Pharmacol Exp Ther 271: 1427–1433, 1994 [PubMed] [Google Scholar]
- 47.Deng S, Zhu G, Liu F, Zhang H, Qin X, Li L, and Zhiyi H. CYP4F2 gene V433 M polymorphism is associated with ischemic stroke in the male Northern Chinese Han population. Prog Neuropsychopharmacol Biol Psychiatry 34: 664–668, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Deng Y, Theken KN, and Lee CR. Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J Mol Cell Cardiol 48: 331–341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diakite B, Hamzi K, Hmimech W, and Nadifi S. Genetic polymorphisms of T-1131C APOA5 and ALOX5AP SG13S114 with the susceptibility of ischaemic stroke in Morocco. J Genet 95: 303–309, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Domingues-Montanari S, Fernandez-Cadenas I, del Rio-Espinola A, Corbeto N, Krug T, Manso H, Gouveia L, Sobral J, Mendioroz M, Fernandez-Morales J, Alvarez-Sabin J, Ribo M, Rubiera M, Obach V, Marti-Fabregas J, Freijo M, Serena J, Ferro JM, Vicente AM, Oliveira SA, and Montaner J. Association of a genetic variant in the ALOX5AP with higher risk of ischemic stroke: a case-control, meta-analysis and functional study. Cerebrovasc Dis 29: 528–537, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Donnelly MK, Conley YP, Crago EA, Ren D, Sherwood PR, Balzer JR, and Poloyac SM. Genetic markers in the EET metabolic pathway are associated with outcomes in patients with aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 35: 267–276, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donnelly MK, Crago EA, Conley YP, Balzer JR, Ren D, Ducruet AF, Kochanek PM, Sherwood PR, and Poloyac SM. 20-HETE is associated with unfavorable outcomes in subarachnoid hemorrhage patients. J Cereb Blood Flow Metab 35: 1515–1522, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, and Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295: H2455–H2465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, and Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol 297: H1096–H1102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edin ML, Cheng J, Gruzdev A, Hoopes SL, and Zeldin DC. Cytochrome P450 Structure, Mechanism, and Biochemistry, edited by Ortiz de Montellano PR. Switzerland: Springer International Publishing, 2015 [Google Scholar]
- 56.Escalante B, Sessa WC, Falck JR, Yadagiri P, and Schwartzman ML. Vasoactivity of 20-hydroxyeicosatetraenoic acid is dependent on metabolism by cyclooxygenase. J Pharmacol Exp Ther 248: 229–232, 1989 [PubMed] [Google Scholar]
- 57.Falck JR, Krishna UM, Reddy YK, Kumar PS, Reddy KM, Hittner SB, Deeter C, Sharma KK, Gauthier KM, and Campbell WB. Comparison of vasodilatory properties of 14,15-EET analogs: structural requirements for dilation. Am J Physiol Heart Circ Physiol 284: H337–H349, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Fang X, Faraci FM, Kaduce TL, Harmon S, Modrick ML, Hu S, Moore SA, Falck JR, Weintraub NL, and Spector AA. 20-Hydroxyeicosatetraenoic acid is a potent dilator of mouse basilar artery: role of cyclooxygenase. Am J Physiol Heart Circ Physiol 291: H2301–H2307, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Fang X, VanRollins M, Kaduce TL, and Spector AA. Epoxyeicosatrienoic acid metabolism in arterial smooth muscle cells. J Lipid Res 36: 1236–1246, 1995 [PubMed] [Google Scholar]
- 60.Farias SE, Basselin M, Chang L, Heidenreich KA, Rapoport SI, and Murphy RC. Formation of eicosanoids, E2/D2 isoprostanes, and docosanoids following decapitation-induced ischemia, measured in high-energy-microwaved rat brain. J Lipid Res 49: 1990–2000, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farias SE, Heidenreich KA, Wohlauer MV, Murphy RC, and Moore EE. Lipid mediators in cerebral spinal fluid of traumatic brain injured patients. J Trauma 71: 1211–1218, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Fava C, Montagnana M, Almgren P, Rosberg L, Lippi G, Hedblad B, Engstrom G, Berglund G, Minuz P, and Melander O. The V433 M variant of the CYP4F2 is associated with ischemic stroke in male Swedes beyond its effect on blood pressure. Hypertension 52: 373–380, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J 4: 2587–2597, 1990 [PubMed] [Google Scholar]
- 64.Forman HJ, Torres M, and Fukuto J. Redox signaling. Mol Cell Biochem 234–235: 49–62, 2002 [PubMed] [Google Scholar]
- 65.Fredriksson R, Lagerstrom MC, Lundin LG, and Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63: 1256–1272, 2003 [DOI] [PubMed] [Google Scholar]
- 66.Fu Z, Nakayama T, Sato N, Izumi Y, Kasamaki Y, Shindo A, Ohta M, Soma M, Aoi N, Sato M, Matsumoto K, Ozawa Y, and Ma Y. Haplotype-based case study of human CYP4A11 gene and cerebral infarction in Japanese subject. Endocrine 33: 215–222, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, and Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res 90: 1028–1036, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Gauthier KM, Edwards EM, Falck JR, Reddy DS, and Campbell WB. 14,15-epoxyeicosatrienoic acid represents a transferable endothelium-dependent relaxing factor in bovine coronary arteries. Hypertension 45: 666–671, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, and Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res 87: 60–65, 2000 [DOI] [PubMed] [Google Scholar]
- 70.Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, and Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol 507 (Pt 3): 771–781, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, and Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol 263: H519–H525, 1992 [DOI] [PubMed] [Google Scholar]
- 72.Gebremedhin D, Zhang DX, Carver KA, Rau N, Rarick KR, Roman RJ, and Harder DR. Expression of CYP 4A omega-hydroxylase and formation of 20-hydroxyeicosatetreanoic acid (20-HETE) in cultured rat brain astrocytes. Prostaglandins Other Lipid Mediat 124: 16–26, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gobbetti T, Le Faouder P, Bertrand J, Dubourdeau M, Barocelli E, Cenac N, and Vergnolle N. Polyunsaturated fatty acid metabolism signature in ischemia differs from reperfusion in mouse intestine. PLoS One 8: e75581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gopez JJ, Yue H, Vasudevan R, Malik AS, Fogelsanger LN, Lewis S, Panikashvili D, Shohami E, Jansen SA, Narayan RK, and Strauss KI. Cyclooxygenase-2-specific inhibitor improves functional outcomes, provides neuroprotection, and reduces inflammation in a rat model of traumatic brain injury. Neurosurgery 56: 590–604, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, and Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42: 2672–2713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goulitquer S, Dreano Y, Berthou F, Corcos L, and Lucas D. Determination of epoxyeicosatrienoic acids in human red blood cells and plasma by GC/MS in the NICI mode. J Chromatogr B Analyt Technol Biomed Life Sci 876: 83–88, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Grienberger HJ, Pillai DR, Schlachetzki F, Gruber M, and Dittmar MS. Detection of free radicals by isolated perfusion of the rat brain following hemorrhagic stroke: a novel approach to cerebrovascular biomarker research. Exp Brain Res 206: 311–317, 2010 [DOI] [PubMed] [Google Scholar]
- 78.Gross E, Ruzicka T, von Restorff B, Stolz W, and Klotz KN. High-affinity binding and lack of growth-promoting activity of 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE) in a human epidermal cell line. J Invest Dermatol 94: 446–451, 1990 [DOI] [PubMed] [Google Scholar]
- 79.Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly AK, Hsu A, Zhou S, Maddipati KR, Liu J, Joshi S, Tucker SC, Lee MJ, and Honn KV. Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J Biol Chem 286: 33832–33840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hacein-Bey L, Harder DR, Meier HT, Varelas PN, Miyata N, Lauer KK, Cusick JF, and Roman RJ. Reversal of delayed vasospasm by TS-011 in the dual hemorrhage dog model of subarachnoid hemorrhage. AJNR Am J Neuroradiol 27: 1350–1354, 2006 [PMC free article] [PubMed] [Google Scholar]
- 81.Hall ED, Yonkers PA, Andrus PK, Cox JW, and Anderson DK. Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury. J Neurotrauma 9 Suppl 2: S425–S442, 1992 [PubMed] [Google Scholar]
- 82.Hama-Tomioka K, Kinoshita H, Azma T, Nakahata K, Matsuda N, Hatakeyama N, Kikuchi H, and Hatano Y. The role of 20-hydroxyeicosatetraenoic acid in cerebral arteriolar constriction and the inhibitory effect of propofol. Anesth Analg 109: 1935–1942, 2009 [DOI] [PubMed] [Google Scholar]
- 83.Han J, Sun L, Xu Y, Liang H, and Cheng Y. Activation of PPARgamma by 12/15-lipoxygenase during cerebral ischemia-reperfusion injury. Int J Mol Med 35: 195–201, 2015 [DOI] [PubMed] [Google Scholar]
- 84.Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, and Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9: 163–173, 2002 [DOI] [PubMed] [Google Scholar]
- 85.Harrington LS, Falck JR, and Mitchell JA. Not so EEZE: the ‘EDHF’ antagonist 14, 15 epoxyeicosa-5(Z)-enoic acid has vasodilator properties in mesenteric arteries. Eur J Pharmacol 506: 165–168, 2004 [DOI] [PubMed] [Google Scholar]
- 86.Hauck AK. and Bernlohr DA. Oxidative stress and lipotoxicity. J Lipid Res 57: 1976–1986, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hauptmann S, Keil U, Scherping I, Bonert A, Eckert A, and Muller WE. Mitochondrial dysfunction in sporadic and genetic Alzheimer's disease. Exp Gerontol 41: 668–673, 2006 [DOI] [PubMed] [Google Scholar]
- 88.Helgadottir A, Gretarsdottir S, St. Clair D, Manolescu A, Cheung J, Thorleifsson G, Pasdar A, Grant SF, Whalley LJ, Hakonarson H, Thorsteinsdottir U, Kong A, Gulcher J, Stefansson K, and MacLeod MJ. Association between the gene encoding 5-lipoxygenase-activating protein and stroke replicated in a Scottish population. Am J Hum Genet 76: 505–509, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iadecola C. The pathobiology of vascular dementia. Neuron 80: 844–866, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iliff JJ. and Alkayed NJ. Soluble epoxide hydrolase inhibition: targeting multiple mechanisms of ischemic brain injury with a single agent. Future Neurol 4: 179–199, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iliff JJ, Close LN, Selden NR, and Alkayed NJ. A novel role for P450 eicosanoids in the neurogenic control of cerebral blood flow in the rat. Exp Physiol 92: 653–658, 2007 [DOI] [PubMed] [Google Scholar]
- 92.Iliff JJ, Fairbanks SL, Balkowiec A, and Alkayed NJ. Epoxyeicosatrienoic acids are endogenous regulators of vasoactive neuropeptide release from trigeminal ganglion neurons. J Neurochem 115: 1530–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iliff JJ, Jia J, Nelson J, Goyagi T, Klaus J, and Alkayed NJ. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat 91: 68–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iliff JJ, Wang R, Zeldin DC, and Alkayed NJ. Epoxyeicosanoids as mediators of neurogenic vasodilation in cerebral vessels. Am J Physiol Heart Circ Physiol 296: H1352–H1363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ji R, Jia J, Ma X, Wu J, Zhang Y, and Xu L. Genetic variants in the promoter region of the ALOX5AP gene and susceptibility of ischemic stroke. Cerebrovasc Dis 32: 261–268, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Jia J, Davis CM, Zhang W, Edin ML, Jouihan S, Jia T, Bradbury JA, Graves JP, DeGraff LM, Lee CR, Ronnekleiv O, Wang R, Xu Y, Zeldin DC, and Alkayed NJ. Sex- and isoform-specific mechanism of neuroprotection by transgenic expression of P450 epoxygenase in vascular endothelium. Exp Neurol 279: 75–85, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kantarci A. and Van Dyke TE. lipoxins in chronic inflammation. Crit Rev Oral Biol Med 14: 4–12, 2003 [DOI] [PubMed] [Google Scholar]
- 98.Karara A, Wei S, Spady D, Swift L, Capdevila JH, and Falck JR. Arachidonic acid epoxygenase: structural characterization and quantification of epoxyeicosatrienoates in plasma. Biochem Biophys Res Commun 182: 1320–1325, 1992 [DOI] [PubMed] [Google Scholar]
- 99.Kehl F, Cambj-Sapunar L, Maier KG, Miyata N, Kametani S, Okamoto H, Hudetz AG, Schulte ML, Zagorac D, Harder DR, and Roman RJ. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. Am J Physiol Heart Circ Physiol 282: H1556–H1565, 2002 [DOI] [PubMed] [Google Scholar]
- 100.Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, and Alkayed NJ. Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci 27: 4642–4649, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kontos HA. and Wei EP. Superoxide production in experimental brain injury. J Neurosurg 64: 803–807, 1986 [DOI] [PubMed] [Google Scholar]
- 102.Korbecki J, Baranowska-Bosiacka I, Gutowska I, and Chlubek D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J Physiol Pharmacol 64: 409–421, 2013 [PubMed] [Google Scholar]
- 103.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, and Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol 290: H491–H499, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lebeau A, Terro F, Rostene W, and Pelaprat D. Blockade of 12-lipoxygenase expression protects cortical neurons from apoptosis induced by beta-amyloid peptide. Cell Death Differ 11: 875–884, 2004 [DOI] [PubMed] [Google Scholar]
- 105.Lee CY, Seet RC, Huang SH, Long LH, and Halliwell B. Different patterns of oxidized lipid products in plasma and urine of dengue fever, stroke, and Parkinson's disease patients: cautions in the use of biomarkers of oxidative stress. Antioxid Redox Signal 11: 407–420, 2009 [DOI] [PubMed] [Google Scholar]
- 106.Li M, Wang W, Mai H, Zhang X, Wang J, Gao Y, Wang Y, Deng G, Gao L, Zhou S, Chen Q, and Wang X. Methazolamide improves neurological behavior by inhibition of neuron apoptosis in subarachnoid hemorrhage mice. Sci Rep 6: 35055, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li PL. and Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ Res 80: 877–884, 1997 [DOI] [PubMed] [Google Scholar]
- 108.Li R, Xu X, Chen C, Yu X, Edin ML, Degraff LM, Lee CR, Zeldin DC, and Wang DW. Cytochrome P450 2J2 is protective against global cerebral ischemia in transgenic mice. Prostaglandins Other Lipid Mediat 99: 68–78, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li Y. and Zhang Z. Gastrodin improves cognitive dysfunction and decreases oxidative stress in vascular dementia rats induced by chronic ischemia. Int J Clin Exp Pathol 8: 14099–14109, 2015 [PMC free article] [PubMed] [Google Scholar]
- 110.Liao D, Yi X, Zhang B, Zhou Q, and Lin J. Interaction between CYP4F2 rs2108622 and CPY4A11 rs9333025 variants is significantly correlated with susceptibility to ischemic stroke and 20-hydroxyeicosatetraenoic acid level. Genet Test Mol Biomarkers 20: 223–228, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lipina C. and Hundal HS. Modulation of cellular redox homeostasis by the endocannabinoid system. Open Biol 6: 150276, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu M. and Alkayed NJ. Hypoxic preconditioning and tolerance via hypoxia inducible factor (HIF) 1alpha-linked induction of P450 2C11 epoxygenase in astrocytes. J Cereb Blood Flow Metab 25: 939–948, 2005 [DOI] [PubMed] [Google Scholar]
- 113.Liu X, Qian ZY, Xie F, Fan W, Nelson JW, Xiao X, Kaul S, Barnes AP, and Alkayed NJ. Functional screening for G protein-coupled receptor targets of 14,15-epoxyeicosatrienoic acid. Prostaglandins Other Lipid Mediat 132: 31–40, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, Hammock BD, and Shyy JY. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci U S A 102: 16747–16752, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lohmussaar E, Gschwendtner A, Mueller JC, Org T, Wichmann E, Hamann G, Meitinger T, and Dichgans M. ALOX5AP gene and the PDE4D gene in a central European population of stroke patients. Stroke 36: 731–736, 2005 [DOI] [PubMed] [Google Scholar]
- 116.Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BS, Harder DR, and Roman RJ. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res 72: 126–136, 1993 [DOI] [PubMed] [Google Scholar]
- 117.Marklund N, Clausen F, Lewander T, and Hillered L. Monitoring of reactive oxygen species production after traumatic brain injury in rats with microdialysis and the 4-hydroxybenzoic acid trapping method. J Neurotrauma 18: 1217–1227, 2001 [DOI] [PubMed] [Google Scholar]
- 118.Martini RP, Ward J, Siler DA, Eastman JM, Nelson JW, Borkar RN, Alkayed NJ, Dogan A, and Cetas JS. Genetic variation in soluble epoxide hydrolase: association with outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg 121: 1359–1366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marumo T, Eto K, Wake H, Omura T, and Nabekura J. The inhibitor of 20-HETE synthesis, TS-011, improves cerebral microcirculatory autoregulation impaired by middle cerebral artery occlusion in mice. Br J Pharmacol 161: 1391–1402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, and Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci 24: 257–268, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McGee J. and Fitzpatrick F. Enzymatic hydration of leukotriene A4. Purification and characterization of a novel epoxide hydrolase from human erythrocytes. J Biol Chem 260: 12832–12837, 1985 [PubMed] [Google Scholar]
- 122.Miyata N, Seki T, Tanaka Y, Omura T, Taniguchi K, Doi M, Bandou K, Kametani S, Sato M, Okuyama S, Cambj-Sapunar L, Harder DR, and Roman RJ. Beneficial effects of a new 20-hydroxyeicosatetraenoic acid synthesis inhibitor, TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N′-hydroxyimido formamide], on hemorrhagic and ischemic stroke. J Pharmacol Exp Ther 314: 77–85, 2005 [DOI] [PubMed] [Google Scholar]
- 123.Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, and Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol 133: 325–329, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morrow JD, Harris TM, and Roberts LJ., 2nd Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem 184: 1–10, 1990 [DOI] [PubMed] [Google Scholar]
- 125.Natarajan R, Gu JL, Rossi J, Gonzales N, Lanting L, Xu L, and Nadler J. Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc Natl Acad Sci U S A 90: 4947–4951, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nelson JW, Young JM, Borkar RN, Woltjer RL, Quinn JF, Silbert LC, Grafe MR, and Alkayed NJ. Role of soluble epoxide hydrolase in age-related vascular cognitive decline. Prostaglandins Other Lipid Mediat 113–115: 30–37, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nie C, Nie H, Zhao Y, Wu J, and Zhang X. Betaine reverses the memory impairments in a chronic cerebral hypoperfusion rat model. Neurosci Lett 615: 9–14, 2016 [DOI] [PubMed] [Google Scholar]
- 128.Nithipatikom K, Pratt PF, and Campbell WB. Determination of EETs using microbore liquid chromatography with fluorescence detection. Am J Physiol Heart Circ Physiol 279: H857–H862, 2000 [DOI] [PubMed] [Google Scholar]
- 129.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, and Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, and Floyd RA. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A 87: 5144–5147, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oltman CL, Weintraub NL, VanRollins M, and Dellsperger KC. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res 83: 932–939, 1998 [DOI] [PubMed] [Google Scholar]
- 132.Omura T, Tanaka Y, Miyata N, Koizumi C, Sakurai T, Fukasawa M, Hachiuma K, Minagawa T, Susumu T, Yoshida S, Nakaike S, Okuyama S, Harder DR, and Roman RJ. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke 37: 1307–1313, 2006 [DOI] [PubMed] [Google Scholar]
- 133.Peters-Golden M. and Brock TG. 5-lipoxygenase and FLAP. Prostaglandins Leukot Essent Fatty Acids 69: 99–109, 2003 [DOI] [PubMed] [Google Scholar]
- 134.Peters O, Back T, Lindauer U, Busch C, Megow D, Dreier J, and Dirnagl U. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 18: 196–205, 1998 [DOI] [PubMed] [Google Scholar]
- 135.Phillis JW, Horrocks LA, and Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Rev 52: 201–243, 2006 [DOI] [PubMed] [Google Scholar]
- 136.Phillis JW. and O'Regan MH. Mechanisms of glutamate and aspartate release in the ischemic rat cerebral cortex. Brain Res 730: 150–164, 1996 [DOI] [PubMed] [Google Scholar]
- 137.Poloyac SM, Reynolds RB, Yonas H, and Kerr ME. Identification and quantification of the hydroxyeicosatetraenoic acids, 20-HETE and 12-HETE, in the cerebrospinal fluid after subarachnoid hemorrhage. J Neurosci Methods 144: 257–263, 2005 [DOI] [PubMed] [Google Scholar]
- 138.Poloyac SM, Zhang Y, Bies RR, Kochanek PM, and Graham SH. Protective effect of the 20-HETE inhibitor HET0016 on brain damage after temporary focal ischemia. J Cereb Blood Flow Metab 26: 1551–1561, 2006 [DOI] [PubMed] [Google Scholar]
- 139.Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, and Lee VM. 12/15-lipoxygenase is increased in Alzheimer's disease: possible involvement in brain oxidative stress. Am J Pathol 164: 1655–1662, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Puppolo M, Varma D, and Jansen SA. A review of analytical methods for eicosanoids in brain tissue. J Chromatogr B Analyt Technol Biomed Life Sci 964: 50–64, 2014 [DOI] [PubMed] [Google Scholar]
- 141.Qu YY, Yuan MY, Liu Y, Xiao XJ, and Zhu YL. The protective effect of epoxyeicosatrienoic acids on cerebral ischemia/reperfusion injury is associated with PI3K/Akt pathway and ATP-sensitive potassium channels. Neurochem Res 40: 1–14, 2015 [DOI] [PubMed] [Google Scholar]
- 142.Rabier M, Crastes de Paulet A, and Damon M. Evidence for specific binding of 15 hydroxyeicosatetraenoic acid to pituitary rat cells. Biochem Biophys Res Commun 152: 1179–1184, 1988 [DOI] [PubMed] [Google Scholar]
- 143.Ramsey IS, Delling M, and Clapham DE. An introduction to TRP channels. Annu Rev Physiol 68: 619–647, 2006 [DOI] [PubMed] [Google Scholar]
- 144.Ray SK, Dixon CE, and Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol 17: 1137–1152, 2002 [DOI] [PubMed] [Google Scholar]
- 145.Renic M, Klaus JA, Omura T, Kawashima N, Onishi M, Miyata N, Koehler RC, Harder DR, and Roman RJ. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab 29: 629–639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Renic M, Kumar SN, Gebremedhin D, Florence MA, Gerges NZ, Falck JR, Harder DR, and Roman RJ. Protective effect of 20-HETE inhibition in a model of oxygen-glucose deprivation in hippocampal slice cultures. Am J Physiol Heart Circ Physiol 302: H1285–H1293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roher AE, Maarouf CL, Kokjohn TA, Belden C, Serrano G, Sabbagh MS, and Beach TG. Chemical and neuropathological analyses of an Alzheimer's disease patient treated with solanezumab. Am J Neurodegener Dis 5: 158–170, 2016 [PMC free article] [PubMed] [Google Scholar]
- 148.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 149.Rosolowsky M, Falck JR, and Campbell WB. Metabolism of arachidonic acid by canine polymorphonuclear leukocytes synthesis of lipoxygenase and omega-oxidized metabolites. Biochim Biophys Acta 1300: 143–150, 1996 [DOI] [PubMed] [Google Scholar]
- 150.Saitoh H, Namatame Y, Hirano A, and Sugawara M. An excised patch membrane sensor for arachidonic acid released in mouse hippocampal slices under stimulation of L-glutamate. Anal Biochem 329: 163–172, 2004 [DOI] [PubMed] [Google Scholar]
- 151.Salinas M, Martin D, Alvarez A, and Cuadrado A. Akt1/PKBalpha protects PC12 cells against the parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium and reduces the levels of oxygen-free radicals. Mol Cell Neurosci 17: 67–77, 2001 [DOI] [PubMed] [Google Scholar]
- 152.Samuelsson B, Borgeat P, Hammarstrom S, and Murphy RC. Introduction of a nomenclature: leukotrienes. Prostaglandins 17: 785–787, 1979 [DOI] [PubMed] [Google Scholar]
- 153.Sarkar P, Narayanan J, and Harder DR. Differential effect of amyloid beta on the cytochrome P450 epoxygenase activity in rat brain. Neuroscience 194: 241–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sarkar P, Zaja I, Bienengraeber M, Rarick KR, Terashvili M, Canfield S, Falck JR, and Harder DR. Epoxyeicosatrienoic acids pretreatment improves amyloid beta-induced mitochondrial dysfunction in cultured rat hippocampal astrocytes. Am J Physiol Heart Circ Physiol 306: H475–H484, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sasaki T, Asano T, Takakura K, Sano K, Nakamura T, Suzuki N, Imabayashi S, and Ishikawa Y. [Cerebral vasospasm and lipid peroxidation—lipid peroxides in the cerebrospinal fluid after subarachnoid hemorrhage]. No To Shinkei 34: 1191–1196, 1982 [PubMed] [Google Scholar]
- 156.Schnackenberg CG, Welch WJ, and Wilcox CS. TP receptor-mediated vasoconstriction in microperfused afferent arterioles: roles of O(2)(-) and NO. Am J Physiol Renal Physiol 279: F302–F308, 2000 [DOI] [PubMed] [Google Scholar]
- 157.Schulz R, Krueger C, Manickavel V, Steele JA, and Cook DA. Production of 15-HETE by cultured smooth muscle cells from cerebral artery. Pharmacology 46: 211–223, 1993 [DOI] [PubMed] [Google Scholar]
- 158.Schwartzman ML, Falck JR, Yadagiri P, and Escalante B. Metabolism of 20-hydroxyeicosatetraenoic acid by cyclooxygenase. Formation and identification of novel endothelium-dependent vasoconstrictor metabolites. J Biol Chem 264: 11658–11662, 1989 [PubMed] [Google Scholar]
- 159.Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J 31: 1273–1288, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Serhan CN, Hamberg M, and Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A 81: 5335–5339, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shineman DW, Zhang B, Leight SN, Pratico D, and Lee VM. Thromboxane receptor activation mediates isoprostane-induced increases in amyloid pathology in Tg2576 mice. J Neurosci 28: 4785–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Siangjong L, Gauthier KM, Pfister SL, Smyth EM, and Campbell WB. Endothelial 12(S)-HETE vasorelaxation is mediated by thromboxane receptor inhibition in mouse mesenteric arteries. Am J Physiol Heart Circ Physiol 304: H382–H392, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Siler DA, Martini RP, Ward JP, Nelson JW, Borkar RN, Zuloaga KL, Liu JJ, Fairbanks SL, Raskin JS, Anderson VC, Dogan A, Wang RK, Alkayed NJ, and Cetas JS. Protective role of p450 epoxyeicosanoids in subarachnoid hemorrhage. Neurocrit Care 22: 306–319, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stella N, Tence M, Glowinski J, and Premont J. Glutamate-evoked release of arachidonic acid from mouse brain astrocytes. J Neurosci 14: 568–575, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stenson WF. and Parker CW. Metabolism of arachidonic acid in ionophore-stimulated neutrophils. Esterification of a hydroxylated metabolite into phospholipids. J Clin Invest 64: 1457–1465, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Strauss KI, Gruzdev A, and Zeldin DC. Altered behavioral phenotypes in soluble epoxide hydrolase knockout mice: effects of traumatic brain injury. Prostaglandins Other Lipid Mediat 104–105: 18–24, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sun L, Xu YW, Han J, Liang H, Wang N, and Cheng Y. 12/15-Lipoxygenase metabolites of arachidonic acid activate PPARgamma: a possible neuroprotective effect in ischemic brain. J Lipid Res 56: 502–514, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Suzuki N, Nakamura T, Imabayashi S, Ishikawa Y, Sasaki T, and Asano T. Identification of 5-hydroxy eicosatetraenoic acid in cerebrospinal fluid after subarachnoid hemorrhage. J Neurochem 41: 1186–1189, 1983 [DOI] [PubMed] [Google Scholar]
- 169.Tajima Y, Ishikawa M, Maekawa K, Murayama M, Senoo Y, Nishimaki-Mogami T, Nakanishi H, Ikeda K, Arita M, Taguchi R, Okuno A, Mikawa R, Niida S, Takikawa O, and Saito Y. Lipidomic analysis of brain tissues and plasma in a mouse model expressing mutated human amyloid precursor protein/tau for Alzheimer's disease. Lipids Health Dis 12: 68, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Takahashi M, Iwatsuki N, Ono K, Tazima T, Koga Y, and Hashimoto Y. The effects of a thromboxane A2 receptor antagonist on neurologic recovery after 15 min complete global brain ischemia in dogs. J Anesth 5: 73–78, 1991 [DOI] [PubMed] [Google Scholar]
- 171.Takeuchi K, Renic M, Bohman QC, Harder DR, Miyata N, and Roman RJ. Reversal of delayed vasospasm by an inhibitor of the synthesis of 20-HETE. Am J Physiol Heart Circ Physiol 289: H2203–H2211, 2005 [DOI] [PubMed] [Google Scholar]
- 172.Tanaka Y, Omura T, Fukasawa M, Horiuchi N, Miyata N, Minagawa T, Yoshida S, and Nakaike S. Continuous inhibition of 20-HETE synthesis by TS-011 improves neurological and functional outcomes after transient focal cerebral ischemia in rats. Neurosci Res 59: 475–480, 2007 [DOI] [PubMed] [Google Scholar]
- 173.Tavazzi B, Signoretti S, Lazzarino G, Amorini AM, Delfini R, Cimatti M, Marmarou A, and Vagnozzi R. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery 56: 582–589; discussion 582–589, 2005 [DOI] [PubMed] [Google Scholar]
- 174.Tegtmeier F, Weber C, Heister U, Haker I, Scheller D, Nikolov R, and Holler M. Eicosanoids in rat brain during ischemia and reperfusion—correlation to DC depolarization. J Cereb Blood Flow Metab 10: 358–364, 1990 [DOI] [PubMed] [Google Scholar]
- 175.Terashvili M, Sarkar P, Nostrand MV, Falck JR, and Harder DR. The protective effect of astrocyte-derived 14,15-epoxyeicosatrienoic acid on hydrogen peroxide-induced cell injury in astrocyte-dopaminergic neuronal cell line co-culture. Neuroscience 223: 68–76, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tesfamariam B, Brown ML, and Cohen RA. 15-Hydroxyeicosatetraenoic acid and diabetic endothelial dysfunction in rabbit aorta. J Cardiovasc Pharmacol 25: 748–755, 1995 [DOI] [PubMed] [Google Scholar]
- 177.Thies W. and Bleiler L. 2013 Alzheimer's disease facts and figures. Alzheimers Dement 9: 208–245, 2013 [DOI] [PubMed] [Google Scholar]
- 178.Tse MM, Aboutabl ME, Althurwi HN, Elshenawy OH, Abdelhamid G, and El-Kadi AO. Cytochrome P450 epoxygenase metabolite, 14,15-EET, protects against isoproterenol-induced cellular hypertrophy in H9c2 rat cell line. Vascul Pharmacol 58: 363–373, 2013 [DOI] [PubMed] [Google Scholar]
- 179.Tunaru S, Chennupati R, Nusing RM, and Offermanns S. Arachidonic acid metabolite 19(S)-HETE induces vasorelaxation and platelet inhibition by activating prostacyclin (IP) receptor. PLoS One 11: e0163633, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Turner SR, Tainer JA, and Lynn WS. Biogenesis of chemotactic molecules by the arachidonate lipoxygenase system of platelets. Nature 257: 680–681, 1975 [DOI] [PubMed] [Google Scholar]
- 181.Usui M, Asano T, and Takakura K. Identification and quantitative analysis of hydroxy-eicosatetraenoic acids in rat brains exposed to regional ischemia. Stroke 18: 490–494, 1987 [DOI] [PubMed] [Google Scholar]
- 182.van Leyen K, Kim HY, Lee SR, Jin G, Arai K, and Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke 37: 3014–3018, 2006 [DOI] [PubMed] [Google Scholar]
- 183.VanRollins M, Kaduce TL, Knapp HR, and Spector AA. 14,15-Epoxyeicosatrienoic acid metabolism in endothelial cells. J Lipid Res 34: 1931–1942, 1993 [PubMed] [Google Scholar]
- 184.von Euler US. On the specific vaso-dilating and plain muscle stimulating substances from accessory genital glands in man and certain animals (prostaglandin and vesiglandin). J Physiol 88: 213–234, 1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vonakis BM. and Vanderhoek JY. 15-Hydroxyeicosatetraenoic acid (15-HETE) receptors. Involvement in the 15-HETE-induced stimulation of the cryptic 5-lipoxygenase in PT-18 mast/basophil cells. J Biol Chem 267: 23625–23631, 1992 [PubMed] [Google Scholar]
- 186.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, and Nilius B. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res 97: 908–915, 2005 [DOI] [PubMed] [Google Scholar]
- 187.Wagner AK, Bayir H, Ren D, Puccio A, Zafonte RD, and Kochanek PM. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J Neurotrauma 21: 125–136, 2004 [DOI] [PubMed] [Google Scholar]
- 188.Wang C, Luo Z, Kohan D, Wellstein A, Jose PA, Welch WJ, Wilcox CS, and Wang D. Thromboxane prostanoid receptors enhance contractions, endothelin-1, and oxidative stress in microvessels from mice with chronic kidney disease. Hypertension 65: 1055–1063, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang L, Chen M, Yuan L, Xiang Y, Zheng R, and Zhu S. 14,15-EET promotes mitochondrial biogenesis and protects cortical neurons against oxygen/glucose deprivation-induced apoptosis. Biochem Biophys Res Commun 450: 604–609, 2014 [DOI] [PubMed] [Google Scholar]
- 190.Ward NC, Croft KD, Blacker D, Hankey GJ, Barden A, Mori TA, Puddey IB, and Beer CD. Cytochrome P450 metabolites of arachidonic acid are elevated in stroke patients compared with healthy controls. Clin Sci (Lond) 121: 501–507, 2011 [DOI] [PubMed] [Google Scholar]
- 191.Watanabe Y, Cohen RA, and Matsui R. Redox regulation of ischemic angiogenesis—another aspect of reactive oxygen species. Circ J 80: 1278–1284, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wong PY, Lai PS, and Falck JR. Mechanism and signal transduction of 14 (R), 15 (S)-epoxyeicosatrienoic acid (14,15-EET) binding in guinea pig monocytes. Prostaglandins Other Lipid Mediat 62: 321–333, 2000 [DOI] [PubMed] [Google Scholar]
- 193.Wong PY, Lai PS, Shen SY, Belosludtsev YY, and Falck JR. Post-receptor signal transduction and regulation of 14(R),15(S)-epoxyeicosatrienoic acid (14,15-EET) binding in U-937 cells. J Lipid Mediat Cell Signal 16: 155–169, 1997 [DOI] [PubMed] [Google Scholar]
- 194.Wu SN, Li HF, and Chiang HT. Actions of epoxyeicosatrienoic acid on large-conductance Ca(2+)-activated K(+) channels in pituitary GH(3) cells. Biochem Pharmacol 60: 251–262, 2000 [DOI] [PubMed] [Google Scholar]
- 195.Yamamoto S. and Shimizu S. Targeting TRPM2 in ROS-coupled diseases. Pharmaceuticals (Basel) 9: 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yamaura K, Gebremedhin D, Zhang C, Narayanan J, Hoefert K, Jacobs ER, Koehler RC, and Harder DR. Contribution of epoxyeicosatrienoic acids to the hypoxia-induced activation of Ca2+-activated K+ channel current in cultured rat hippocampal astrocytes. Neuroscience 143: 703–716 [DOI] [PubMed] [Google Scholar]
- 197.Yang C, Kwan YW, Au AL, Poon CC, Zhang Q, Chan SW, Lee SM, and Leung GP. 14,15-Epoxyeicosatrienoic acid induces vasorelaxation through the prostaglandin EP(2) receptors in rat mesenteric artery. Prostaglandins Other Lipid Mediat 93: 44–51, 2010 [DOI] [PubMed] [Google Scholar]
- 198.Yang W, Tuniki VR, Anjaiah S, Falck JR, Hillard CJ, and Campbell WB. Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20-125i-14,15-epoxyeicosa-8(Z)-enoic acid. J Pharmacol Exp Ther 324: 1019–1027, 2008 [DOI] [PubMed] [Google Scholar]
- 199.Yang Y, Chen S, and Zhang J. The updated role of oxidative stress in subarachnoid hemorrhage. Curr Drug Deliv 14: 832–842, 2017 [DOI] [PubMed] [Google Scholar]
- 200.Yang ZJ, Carter EL, Kibler KK, Kwansa H, Crafa DA, Martin LJ, Roman RJ, Harder DR, and Koehler RC. Attenuation of neonatal ischemic brain damage using a 20-HETE synthesis inhibitor. J Neurochem 121: 168–179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yao Y, Clark CM, Trojanowski JQ, Lee VM, and Pratico D. Elevation of 12/15 lipoxygenase products in AD and mild cognitive impairment. Ann Neurol 58: 623–626, 2005 [DOI] [PubMed] [Google Scholar]
- 202.Ye M, Yang W, Ainscough JF, Hu XP, Li X, Sedo A, Zhang XH, Zhang X, Chen Z, Li XM, Beech DJ, Sivaprasadarao A, Luo JH, and Jiang LH. TRPM2 channel deficiency prevents delayed cytosolic Zn2+ accumulation and CA1 pyramidal neuronal death after transient global ischemia. Cell Death Dis 5: e1541, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yi X, Han Z, Zhou Q, Lin J, and Liu P. 20-Hydroxyeicosatetraenoic acid as a predictor of neurological deterioration in acute minor ischemic stroke. Stroke 47: 3045–3047, 2016 [DOI] [PubMed] [Google Scholar]
- 204.Yi X, Lin J, Wang C, and Zhou Q. CYP genetic variants, CYP metabolite levels, and neurologic deterioration in acute ischemic stroke in Chinese population. J Stroke Cerebrovasc Dis 26: 969–978, 2017 [DOI] [PubMed] [Google Scholar]
- 205.Yigitkanli K, Zheng Y, Pekcec A, Lo EH, and van Leyen K. Increased 12/15-lipoxygenase leads to widespread brain injury following global cerebral ischemia. Transl Stroke Res 8: 194–202, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yiu SS, Zhao X, Inscho EW, and Imig JD. 12-Hydroxyeicosatetraenoic acid participates in angiotensin II afferent arteriolar vasoconstriction by activating L-type calcium channels. J Lipid Res 44: 2391–2399, 2003 [DOI] [PubMed] [Google Scholar]
- 207.Yu M, Cambj-Sapunar L, Kehl F, Maier KG, Takeuchi K, Miyata N, Ishimoto T, Reddy LM, Falck JR, Gebremedhin D, Harder DR, and Roman RJ. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. Eur J Pharmacol 486: 297–306, 2004 [DOI] [PubMed] [Google Scholar]
- 208.Yu W, Parakramaweera R, Teng S, Gowda M, Sharad Y, Thakker-Varia S, Alder J, and Sesti F. Oxidation of KCNB1 potassium channels causes neurotoxicity and cognitive impairment in a mouse model of traumatic brain injury. J Neurosci 36: 11084–11096, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yuruker V, Naziroglu M, and Senol N. Reduction in traumatic brain injury-induced oxidative stress, apoptosis, and calcium entry in rat hippocampus by melatonin: possible involvement of TRPM2 channels. Metab Brain Dis 30: 223–231, 2015 [DOI] [PubMed] [Google Scholar]
- 210.Zeldin DC, DuBois RN, Falck JR, and Capdevila JH. Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch Biochem Biophys 322: 76–86, 1995 [DOI] [PubMed] [Google Scholar]
- 211.Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, and Capdevila JH. Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J Biol Chem 268: 6402–6407, 1993 [PubMed] [Google Scholar]
- 212.Zhang H, Falck JR, Roman RJ, Harder DR, Koehler RC, and Yang ZJ. Upregulation of 20-HETE synthetic cytochrome P450 isoforms by oxygen-glucose deprivation in cortical neurons. Cell Mol Neurobiol 37: 1279–1286, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang J, Guo W, Tian B, Sun M, Li H, Zhou L, and Liu X. Puerarin attenuates cognitive dysfunction and oxidative stress in vascular dementia rats induced by chronic ischemia. Int J Clin Exp Pathol 8: 4695–4704, 2015 [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, and Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab 27: 1931–1940, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, and Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke 39: 2073–2078, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang Y, Hong G, Lee KS, Hammock BD, Gebremedhin D, Harder DR, Koehler RC, and Sapirstein A. Inhibition of soluble epoxide hydrolase augments astrocyte release of vascular endothelial growth factor and neuronal recovery after oxygen-glucose deprivation. J Neurochem 140: 814–825, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhu Y, Chen L, Liu W, Wang W, Zhu D, and Zhu Y. Hypoxia-induced 15-HETE enhances the constriction of internal carotid arteries by down-regulating potassium channels. J Neurol Sci 295: 92–96, 2010 [DOI] [PubMed] [Google Scholar]
- 218.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, and Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am J Physiol 270: F822–F832, 1996 [DOI] [PubMed] [Google Scholar]
- 219.Zou M. and Anges C. Cell-cell interaction of macrophages and vascular smooth muscle cells in the synthesis of leukotriene b(4). Mediators Inflamm 3: 297–302, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zuloaga KL, Johnson LA, Roese NE, Marzulla T, Zhang W, Nie X, Alkayed FN, Hong C, Grafe MR, Pike MM, Raber J, and Alkayed NJ. High fat diet-induced diabetes in mice exacerbates cognitive deficit due to chronic hypoperfusion. J Cereb Blood Flow Metab 36: 1257–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zuloaga KL, Zhang W, Yeiser LA, Stewart B, Kukino A, Nie X, Roese NE, Grafe MR, Pike MM, Raber J, and Alkayed NJ. Neurobehavioral and imaging correlates of hippocampal atrophy in a mouse model of vascular cognitive impairment. Transl Stroke Res 6: 390–398, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]