Abstract
Background
This paper aimed to evaluate the expression of REGγ and characterize its clinical significance in papillary thyroid carcinoma (PTC).
Material/Methods
In total, 54 patients with PTC who underwent partial or total thyroidectomy and cervical node dissection for PTC from February 2009 to September 2011 were retrospectively reviewed. Thyroid specimens and metastatic lymph nodes from 54 patients and normal thyroid tissues obtained from 13 volunteers were collected and analyzed. Tumor size, T-stage, and lymph nodes metastasis were recorded based on surgical pathology. Immunohistochemical (IHC) technology was performed to analyze REGγ protein expression level. Corrections between the expression of REGγ and the clinicopathological factors were analyzed.
Results
All the normal thyroid tissues were REGγ-negative. REGγ was positive in 75.9% (41/54) of PTC tissues, of which 29 cases (29/42, 69.0%) were in T1–T2 stage and 12 cases (12/12,100%) were in T3–T4 stage. Positive REGγ was found in 21 cases (21/24, 87.5%) in T1–T2 stage with lymph nodes metastasis, while 11 cases were in T3–T4 stage with metastases to lymph nodes (11/11, 100%). High level of REGγ expression was significantly correlated with T-stage (P<0.05) and lymph node metastases (P<0.05). In addition, there was no statistically significant difference between the expression of REGγ and age, sex, tumor size, or tumor multiplicity (P>0.05). Using binary logistic regression model, positive REGγ was identified as a significant independent predictor factor of lymph node metastasis in PTC.
Conclusions
High expression of REGγ seemed positively correlated with T-stage and lymph node metastasis in PTC tissues.
MeSH Keywords: Antigens, Nuclear; B-Lymphocytes; Carcinoma, Papillary
Background
Papillary thyroid carcinoma (PTC) is a well-differentiated thyroid cancer, accounting for more than 80% of all thyroid tumors [1]. Additionally, PTC encompasses several subtypes, such as the follicular variant (FV), which is characterized by a follicular pattern of growth and can be easily confused with follicular carcinomas and follicular adenomas [2]. Lymph node metastases in PTC have been demonstrated as an independent risk factor for regional recurrence and are correlated with a higher rate of distant metastasis [3]. The current treatment for PTC involves thyroid-stimulating hormone suppression therapy, surgery, and radioactive iodine (RAI) therapy [4]. However, the incidence of PTC is increasing and those patients with distant metastasis are refractory to RAI. Hence, there is an urgent need to explore new indicators for diagnosis and prognosis of PTC.
REGgamma (REGγ), also known as PA28γ, PSME3, 11sγ, or Ki antigen, was first found in the serum of systemic lupus erythematosus patients as a nuclear protein targeted by autoantibodies [5]. Although the biological roles of REGγ are not fully understood, the proteasome activator REGγ has been reported to bind and activate the 20S proteasome to stimulate the proteolytic functions of the proteasome independent of ubiquitination and ATP [6]. Mounting evidence demonstrates that REGγ is involved in cancer progression [5]. REGγ is reported to be over-expressed in many kinds of cancers, such as laryngeal carcinoma, thyroid cancer, breast cancer, and gastric cancer [7–10]. Fan et al. showed that a high level of REGγ expression was positively associated with poor clinical outcomes and prognosis of patients with breast cancer [8]. Recently, it was reported that REGγ plays a significant role in cell proliferation, cell cycle, and invasion of poorly differentiated thyroid carcinoma cells [7]. However, the clinical significance of REGγ expression in PTC has not been systematically studied.
In the present study, we retrospectively investigated the REGγ expression levels in thyroid tissues from patients with PTC with or without lymph node metastases and normal controls. Correlations between the expression levels of REGγ and clinicopathological parameters and prognosis were analyzed statistically.
Material and Methods
Human subjects
In total, 54 PTC patients with or without lymph node metastases who underwent total or subtotal thyroidectomy and neck node dissection for PTC from February 2009 to September 2011 at Xinhua Hospital, Shanghai Jiao tong University School of Medicine (Shanghai, China) were reviewed retrospectively. These patients were randomly and consecutively selected and they had no other malignant tumors or other thyroid disease. Thyroid specimens from these 54 patients were collected and established by post-operation pathology. Clinicopathological characteristics for these patients were collected and analyzed, including age, sex, tumor size, nodal status, and tumor multiplicity. Clinical staging was undertaken based on surgical and preoperative examination. Normal thyroid tissues as controls were also obtained from 13 volunteers without history of thyroid cancer, using ultrasound-guided fine-needle aspiration. Study approval was obtained from the Institutional Review Board of Shanghai Xinhua Hospital, Shanghai Jiao tong University School of Medicine (Shanghai, China).
Immunohistochemical evaluation of REGγ expression
Immunohistochemical (IHC) analysis was conducted to analyze the protein level of REGγ in PTC tissues and normal thyroid tissues. Briefly, the tissues were fixed, dehydrated, embedded in paraffin, and cut into 4-μm-thick tissue sections. Then, the sections were subjected to antigen retrieval with 0.01 M citrate buffer solution at pH 6.0 for 30 min in a 100°C water bath. After cooling to room temperature, the sections were treated with 3% H2O2 (in absolute methanol) for 10 min to block endogenous peroxidase activity. Subsequently, the sections were incubated in serum albumin (Histostain™-Plus DAB kit) at room temperature for 10 min to block non-specific staining. Pretreated tissue sections were incubated overnight at 4°C with the specific primary antibodies (rabbit polyclonal anti-REGγ antibody, 1: 300). Slides were then incubated with biotinylated anti-rabbit IgG (DAB kit) (Miaotong Shanghai biological technology co., LTD) for 10 min. After incubation with secondary horseradish peroxidase (HRP) antibody for 10 minutes, Mayer’s hematoxylin was used to counterstain sections.
Expression of REGγ was scored by assigning proportion and intensity scores, according to an immuno-reactive score (IRS) system described by Remmele and Stegner [11]. In brief, the intensity score represents the staining intensity (SI) as follows: 0=no staining, 1=faint staining, 2=moderate staining, 3=strong staining. A proportion score represented the approximate percentage of positive cells as follows: 0=no positive cells, 1=less than 5%, 2=6–10%, 3=11–50%, 4=50–100%. The final IRS, ranging from 0 to 12, was calculated by multiplying SI and PP. In this study, tumors with final IRS >5 were evaluated as positive; while those cases assigned an IRS ≤5 (negative or weak expression) were evaluated as negative. The staining intensity and the proportion of positive cells were calculated by 2 independent blinded observers using an ordinal scale [12].
Statistical analysis
Statistical analyses were performed with the use of SAS version 3.1 statistical software package (SAS Institute, Cary, NC). Student’s t-test was used for and comparing the means in continuous variables. Fisher’s exact test or Pearson’s chi-square test was used for the measurement of statistical differences in categorical variables. We performed binary logistic regression modeling to identify predictors of lymph node metastasis. Age, T-stage, and tumor multiplicity were included as covariates in the regression model. Data are presented as mean ± standard deviation (SD). All statistical tests were two-sided, with a 0.05 level of significance.
Results
Clinical pathological features in PTC patients
Total or near total thyroidectomy was performed for 52 patients (96.3%). The other 2 patients underwent lobectomy. Neck dissection for the central compartment was performed in 26 patients (48.1%), modified radical neck dissection including central neck dissection was performed in 10 patients (18.5%), and functional neck dissection was performed in 18 patients (33.4%). Tumor size, T-stage, and lymph nodes metastasis were established by post-surgical pathology. The clinicopathological characteristics of these 54 patients with PTC are listed in Table 1. These 54 patients included 14 males and 40 females with age ranging from 20 to 79 years (median, 46.0±14.0 years). T1–T2 phase, lymph node metastasis, tumor multiplicity, and positive REGγ staining occurred in 77.8% (42/54), 64.8% (35/54), 49.1% (26/53), and 75.9% (41/54), respectively. Most positive REGγ cases showed nuclear staining. The expression level of REGγ protein was different in normal thyroid tissues, PTC tissues, and metastatic lymph nodes (Table 1, Figure 1).
Table 1.
The clinicopathological characteristics in 54 patients with PTC.
| Frequency, n (%) | |
|---|---|
| Gender | |
| Male | 14 (25.9%) |
| Female | 40 (74.1%) |
| Age (years) | |
| <45 | 21 (38.9%) |
| ≥45 | 33 (61.1%) |
| T-stage | |
| T1–T2 | 42 (77.8%) |
| T3–T4 | 12 (22.2%) |
| Node metastasis | |
| Absent | 19 (35.2%) |
| Present | 35 (64.8%) |
| Tumor multiplicity (n=53) | |
| Single | 27 (50.9%) |
| Multiple | 26 (49.1%) |
| REGγ expression | |
| Negative | 13 (24.1%) |
| Positive | 41 (75.9%) |
One case had no clinical records regarding to tumor multiplicity.
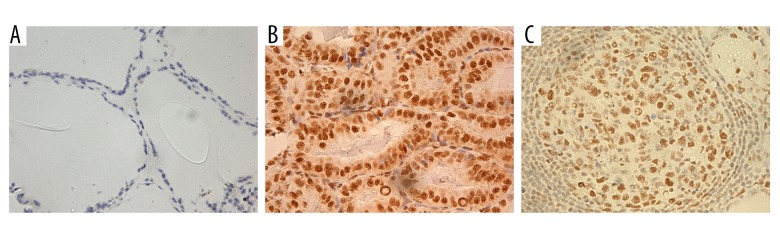
Figure 1.
Immunohistochemical (IHC) analysis of REGγ in thyroid tissues and lymph nodes (×200). (A) Negative staining of REGγ in normal thyroid tissues. (B) Positive staining of REGγ in PTC. (C) Positive staining of REGγ in metastatic lymph nodes.
The relationship between REGγ and clinicopathological findings
All the normal thyroid tissues were REGγ-negative. There were 29 REGγ-positive cases in T1–T2 (29/42, 69.0%) and 12 cases were in T3–T4 (12/12, 100%). Positive REGγ expression was more frequently found in PTC with lymph node metastasis (32/35, 91.4%). Positive REGγ was found in 21 cases (21/24, 87.5%) in T1–T2 stage with lymph nodes metastasis, while 11 cases were in T3–T4 stage (11/11, 100%). REGγ expression level was associated with lymph node metastasis (P<0.05) and T-stage (P<0.05) (Tables 2, 3). There was no statistically significance difference between the expression level of REGγ and age, sex, tumor size, or multiplicity (P>0.05) (Table 2).
Table 2.
Pathological findings according to the expression of REGγ in 54 patients with PTC.
| REGγ | |||
|---|---|---|---|
| Negative, n=13 (%) | Positive, n=41 (%) | P value | |
| Gender | |||
| Male | 3 (21.4) | 11 (78.6) | P=1.00 |
| Female | 10 (25.0) | 30 (75.0) | |
| Age (years) | |||
| <45 | 4 (19.0) | 17 (81.0) | P=0.536 |
| ≥45 | 9 (27.3) | 24 (72.7) | |
| Tumor size (mm) | 13 (1.35±0.43) | 37 (1.64±0.92) | P=0.116 |
| T-stage | |||
| T1–T2 | 13 (31.0) | 29 (69.0) | P=0.0497 |
| T3–T4 | 0 (0.00) | 12 (100) | |
| Node metastasis | |||
| Absent | 10 (52.6) | 9 (47.4) | P<0.001 |
| Present | 3 (8.6) | 32 (91.4) | |
| Multiplicity (n=53) | |||
| Single | 7 (25.9) | 20 (74.1) | P=1.00 |
| Multiple | 6 (23.1) | 20 (76.9) | |
Table 3.
Lymph nodes metastasis and the expression of REGγ in PTC with T-stage.
| REGγ | |||
|---|---|---|---|
| Negative, n=13 (%) | Positive, n=41 (%) | P value | |
| T1–T2 | |||
| No node metastasis | 10 (55,6) | 8 (44.4) | P=0.0058 |
| Node metastasis | 3 (12.5) | 21 (87.5) | |
| T3–T4 | |||
| No node metastasis | 0 (0) | 1 (100) | |
| Node metastasis | 0 (0) | 11 (100) | |
The relationship between REGγ and prognosis of patients
From binary logistic regression analysis, we found that REGγ expression was a significant independent predictor factor of lymph node metastasis of PTC (REGγ-positive: odds ratio [OR]=9.09, P=0.003, 95% confidence interval [CI]=2.1–39.28). Due to the limited number of patients, the correlations between lymph node metastasis and variables such as age, T-stage, and tumor multiplicity were not significant (age: OR=0.63, P=0.49, 95%CI=0.17–2.38; T-stage: OR=0.09, P=0.10, 95%CI=0–1.59; tumor multiplicity: OR=1.17, P=0.86, 95%CI=0.33–4.09) (Table 4).
Table 4.
Predictors for nodal metastasis in patients with PTC in binary logistic regression model.
| P value | OR | 95% CI | |
|---|---|---|---|
| REGγ (negative vs. positive) | 0.003 | 9.09 | [2.1–39.28] |
| Age (<45 vs. ≥45 years) | 0.49 | 0.63 | [0.17–2.38] |
| T-stage (T1–T2 vs. T3–T4) | 0.10 | 0.09 | [0–1.59] |
| Multiplicity (single vs. multiple | 0.81 | 1.17 | [0.33–4.09] |
Discussion
In this study, positive REGγ staining was identified in most (41/54) patients with PTC. In addition, positive REGγ expression was more frequently found in PTC patients with lymph node metastasis (32/35, 91.4%). Moreover, REGγ expression level was associated with lymph node metastasis and T-stage. REGγ expression was a significant independent predictor of lymph node metastasis of PTC patients.
REGγ is abnormally over-expressed in thyroid cancer, especially in its growth-accelerated cells, indicating that REGγ may play a role in the regulatory system for the cell cycle [13]. Zhang et al. demonstrated that the down-regulation of REGγ arrested the cell cycle, inhibited cell proliferation, and also restricted invasion of poorly differentiated thyroid carcinoma cells [7]. Moreover, studies have shown that cell proliferation and cell cycle progression were inhibited in REGγ-knockdown Drosophila, REGγ-knockout mice cells, mouse embryonic fibroblasts, breast carcinoma cells, and SW579 cells [7,14,15]. It was shown that REGγ promotes human breast ductal epithelial cells (HBL-100) to transition from G1 into S phase [16], which may trigger breast neoplasms [17,18]. Tomohisa et al. observed a significant correlation between proliferating cell nuclear antigen (PCNA) and REGγ expression [13]. As a key transition factor in cell cycle progression, PCNA is over-expressed in PTC [19]. In addition, REGγ has been demonstrated to modulate the activity of p53 and p21 proteins [20,21]. The ability of P53 to stimulate apoptosis and cell cycle arrest and strongly suppress oncogenesis is well documented [22]. p21 occupies a critical position in cell cycle regulation in many tissues [23]. Evans et al. reported on the relationship between REGγ, and p21 and p53 in human PTC tissues [24]. In the present study, we found that REGγ was over-expressed in PTC tissue specimens from most PTC patients (41/54, 75.9%). We assumed a pivotal role of REGγ in the PTC progression through affecting cell cycle and proliferation. On the other hand, most positive REGγ cases showed nuclear staining in this study, which was consistent with the finding of Okamura et al., showing that REGγ was localized within the nucleus of thyroid cancer cells [13].
The presence and the location of regional lymph node metastasis are correlated with a high risk of recurrence and distant metastases, and can affect the prognosis of PTC [25,26]. In this study, we found that REGγ expression was associated with T-stage and lymph node metastasis. Using binary logistic regression analysis, we sowed that REGγ is a significant independent predictor for lymph node metastasis. These findings indicate that the REGγ expression level may be a significant predictor factor of lymph node metastasis and T-stage in PTC patients. However, this conclusion needs validation in the future.
Our study had some limitations, mainly due to the retrospective design and the limited number of cases. However, to the best of our knowledge, the present study is the first investigation of the relationship between REGγ and pathological findings in PTC patients. Although the current study indicated that REGγ may be a predictor for lymph node metastasis and T-stage, more definitive evidence is required. Concerning the suggestion that REGγ may represent a therapeutic target in some tumors [6], further studies to uncover the role of the REGγ in PTC are required.
Conclusions
In conclusion, our results are the first to indicate that REGγ expression may be associated with lymph node metastasis and T-stage in PTC patients. The expression of REGγ has no direct correlation with the sex, age, tumor size, or tumor multiplicity of the patients, but high expression of REGγ was positively correlated with T-stage and lymph node metastasis, suggesting that REGγ is a prognostic marker of T-stage and lymph node metastasis and is also a therapeutic target for PTC.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Miftari R, Topçiu V, Nura A, Haxhibeqiri V. Management of the patient with aggressive and resistant papillary thyroid carcinoma. Med Arch. 2016;70(4):314–17. doi: 10.5455/medarh.2016.70.314-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal N, Akbani R, Aksoy BA, et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–90. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jing Y, Gong Y, Yan S, et al. Risk factors for level V lymph node metastases in solitary papillary thyroid carcinoma with clinically lateral lymph node metastases. Cancer Med. 2016;5(8):2161–68. doi: 10.1002/cam4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun R, Wang J, Li X, et al. Effect of iodine intake on p14ARF and p16INK4a expression in thyroid papillary carcinoma in rats. Med Sci Monit. 2015;21:2288–93. doi: 10.12659/MSM.893486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, Cui L, Zeng Y, et al. REGγ is associated with multiple oncogenic pathways in human cancers. BMC Cancer. 2012;12(1):1–14. doi: 10.1186/1471-2407-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao I, Liu J, Li X, Luo H. REGγ, a proteasome activator and beyond? Cell Mol Life Sci. 2008;65(24):3971–80. doi: 10.1007/s00018-008-8291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang M, Gan L, Ren GS. REGγ is a strong candidate for the regulation of cell cycle, proliferation and the invasion by poorly differentiated thyroid carcinoma cells. Braz J Med Biol Res. 2012;45(5):459–65. doi: 10.1590/S0100-879X2012007500035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan C, Yan L, Bi J, et al. High expression of REGγ is associated with metastasis and poor prognosis of patients with breast cancer. Int J Clin Exp Pathol. 2014;7(11):7834–43. [PMC free article] [PubMed] [Google Scholar]
- 9.Li LP, Cheng WB, Li H, et al. Expression of proteasome activator REGγ in human laryngeal carcinoma and associations with tumor suppressor proteins. Asian Pac J Cancer Prev. 2012;13(6):2699–703. doi: 10.7314/apjcp.2012.13.6.2699. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Tian T, Wang X, et al. Expression and clinical significance of REGγ in gastric cancer tissue and variously differentiated gastric cancer cell lines. J Cancer Res Clin Oncol. 2009;6(3):208–13. [Google Scholar]
- 11.Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue]. Pathologe. 1987;8(3):138–40. [in German] [PubMed] [Google Scholar]
- 12.Kebebew E, Peng M, Treseler PA, et al. Id1 gene expression is up-regulated in hyperplastic and neoplastic thyroid tissue and regulates growth and differentiation in thyroid cancer cells. J Clin Endocrinol Metab. 2005;89(12):6105–11. doi: 10.1210/jc.2004-1234. [DOI] [PubMed] [Google Scholar]
- 13.Okamura T, Taniguchi S, Ohkura T, et al. Abnormally high expression of proteasome activator-gamma in thyroid neoplasm. J Clin Endocrinol Metab. 2003;88(3):1374–83. doi: 10.1210/jc.2002-021413. [DOI] [PubMed] [Google Scholar]
- 14.Murata S, Kawahara H, Tohma S, et al. Growth retardation in mice lacking the proteasome activator PA28gamma. J Biol Chem. 1999;274(53):38211–15. doi: 10.1074/jbc.274.53.38211. [DOI] [PubMed] [Google Scholar]
- 15.Masson P, Lundgren J, Young P. Drosophila proteasome regulator REGγ: transcriptional activation by DNA replication-related factor DREF and evidence for a role in cell cycle progression. J Mol Biol. 2003;327(5):1001–12. doi: 10.1016/s0022-2836(03)00188-8. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Tu S, Tan J, et al. REG gamma: A potential marker in breast cancer and effect on cell cycle and proliferation of breast cancer cell. Med Oncol. 2011;28(1):31–41. doi: 10.1007/s12032-010-9546-8. [DOI] [PubMed] [Google Scholar]
- 17.Isik A, Firat D. Bilateral intra-areolar polythelia. Breast J. :2017. doi: 10.1111/tbj.12838. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Isik A, Karavas E, Peker K, et al. Male Mondor’s disease is a rare entity. Breast J. 2016;22(6):700–1. doi: 10.1111/tbj.12657. [DOI] [PubMed] [Google Scholar]
- 19.Cvejic D, Savin S, Petrovic I, et al. Galectin-3 and proliferating cell nuclear antigen (PCNA) expression in papillary thyroid carcinoma. Exp Oncol. 2005;27(3):210–14. [PubMed] [Google Scholar]
- 20.Liu J, Yu G, Zhao Y, et al. REGgamma modulates p53 activity by regulating its cellular localization. J Cell Sci. 2010;123(Pt 23):4076–84. doi: 10.1242/jcs.067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Amazit L, Long W, Lonard DM. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGγ-proteasome pathway. Mol Cell. 2007;26(6):831–42. doi: 10.1016/j.molcel.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 22.Sharpless NE, Depinho RA. p53: good cop/bad cop. Cell. 2002;110(1):9–12. doi: 10.1016/s0092-8674(02)00818-8. [DOI] [PubMed] [Google Scholar]
- 23.Weinberg WC, Denning MF. P21Waf1 control of epithelial cell cycle and cell fate. J Dent Res. 2002;13(6):453–64. doi: 10.1177/154411130201300603. [DOI] [PubMed] [Google Scholar]
- 24.Evans JJ, Crist HS, Durvesh S, et al. A comparative study of cell cycle mediator protein expression patterns in anaplastic and papillary thyroid carcinoma. Cancer Biol Ther. 2012;13(9):776–81. doi: 10.4161/cbt.20560. [DOI] [PubMed] [Google Scholar]
- 25.Wu MH, Shen WT, Gosnell J, Duh QY. Prognostic significance of extranodal extension of regional lymph node metastasis in papillary thyroid cancer. Head Neck. 2014;37(9):1336–43. doi: 10.1002/hed.23747. [DOI] [PubMed] [Google Scholar]
- 26.Min JJ, Kim TY, Kim WG, et al. Differentiating the location of cervical lymph node metastasis is very useful for estimating the risk of distant metastases in papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2014;81(4):593–99. doi: 10.1111/cen.12463. [DOI] [PubMed] [Google Scholar]