Abstract

The intermolecular [2+2] photocycloaddition of typical cyclic α,β-unsaturated enones, such as 2-cyclohexenone, with olefins was performed in moderate to good yields (42–82%) and with high enantioselectivity (82%–96% ee). An unusual substitution pattern at the chiral oxazaborolidine-AlBr3 Lewis acid complex that promotes the reaction was found to be crucial for the success of the reaction. The method was applied to the enantioselective synthesis of the monoterpene (−)-grandisol, which could be accomplished in six steps and with an overall yield of 13% starting from 3-methyl-2-cyclohexenone.
Although the intramolecular [2+2] photocycloaddition reaction of cyclic enones had been discovered as early as 1908,1 it was not before the 1960s that the first intermolecular variants of this reaction were reported.2 Over the last 40 years, the reaction has been employed as a key step in literally hundreds of natural product syntheses3 and it represents one of the most powerful transformations of organic chemistry.4 Typically, the reaction is induced by direct excitation of an enone, such as 2-cyclohexenone (1a), the triplet state (T1) of which is populated by rapid intersystem crossing (ISC) from the first excited singlet state (S1). The former state is long-lived and can be captured by a photochemically inactive alkene to generate a cyclobutane. A representative reaction is the addition of isobutene, which was employed in Corey’s landmark synthesis of racemic caryophyllene (Scheme 1).5
Scheme 1. Corey’s Landmark Synthesis of Racemic Caryophyllene with an Intermolecular [2+2] Photocycloaddition Reaction of 2-Cyclohexenone (1a) as the Key Step.
Although the olefin is commonly used in excess, the reaction is not perfectly chemo- and regioselective and an extensive purification is required to obtain product rac-2a in yields of 35–45%. The treatment with basic alumina facilitates the epimerization of the stereogenic center in α-position to the ketone carbonyl group but does not remove any undesired constitutional isomers. Most severely, there is up to this date no way to access the cyclobutane products of a typical intermolecular enone [2+2] photocycloaddition reaction in an enantioselective fashion.6,7 Indirect methods that rely on diastereoselective reactions require a major synthetic effort involving multistep sequences.8 Recently developed protocols for enantioselective intermolecular [2+2] photocycloaddition reactions9 are not applicable to unfunctionalized cyclic enones, such as 2-cyclohexenone (1a). We have now found a way to prepare cyclobutanes such as 2a from enones with high enantioselectivity and our results are disclosed in this communication.
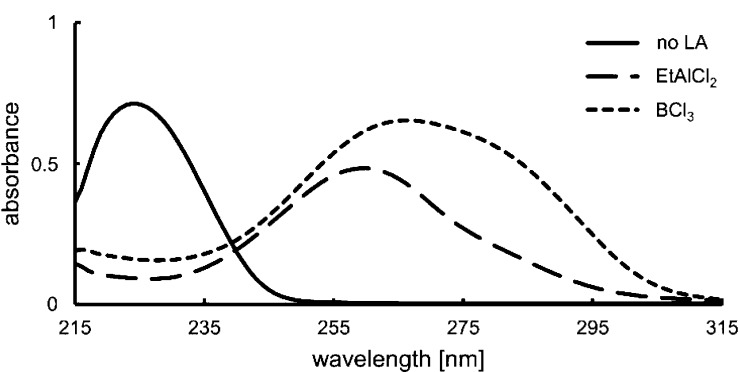
In preliminary work, we studied the UV/vis properties of 2-cyclohexenone (1a) upon treatment with Lewis acids (Figure 1). In line with previous observations,10,11 the strong ππ* absorption of the parent compound at λ = 224 nm (ε = 13 400 M–1 cm–1) was shifted bathochromically and was recorded at λ = 260 nm (ε = 9670 M–1 cm–1) with EtAlCl2 and at λ = 266 nm (ε = 13 080 M–1 cm–1) with the stronger Lewis acid BCl3. In both cases, there is still a detectable absorption of the complex at λ = 330 nm where the weak (ε = 70 M–1 cm–1) nπ* absorption of uncomplexed 2-cyclohexenone is located. Because it was expected that the AlBr3-based oxazaborolidine Lewis acids12 would exert an even stronger bathochromic shift than BCl3, it seemed possible to selectively excite the complex of cyclohexenone with a chiral Lewis acid but not the uncomplexed substrate. In this scenario, enantioselective intermolecular [2+2] photocycloaddition to an alkene should be possible as previously found for intramolecular reactions.10,13 However, several questions remained open, e.g., whether the lifetime of the complex would be sufficiently long to allow for enantioface differentiation before dissociation and whether previously used Lewis acids would provide a reasonable enantioselectivity. It was also unclear whether the photoexcited cyclohexenone would lead to a destruction of the Lewis acid and whether the known side reactions of intermolecular [2+2] photocycloaddition reactions would be enhanced. Initial results with the previously used13b Lewis acid 3a seemed to confirm the worst concerns (Table 1, entry 1). The enantioselectivity in the reaction of 1a with 2-ethylbut-1-ene under typical conditions was disappointingly low and the Lewis acid seemed unstable under the reaction conditions. Variation of Ar’ showed that the insufficient performance was not associated with this substituent but rather with the aryl group Ar (entry 2). Gratifyingly, an extensive screening of oxazaborolidines14 with different substituents Ar delivered Lewis acids with which the selectivity issues could be smoothly overcome. The superior substituent was a 2,3-dimethylphenyl group that was incorporated for example in Lewis acids 3c–3e.
Figure 1.
UV/vis spectra of 2-cyclohexenone (1a) in the absence (—) and in the presence of 20 equiv of either EtAlCl2 (− – −) or BCl3 (- - -) (c = 0.5 mM in CH2Cl2).
Table 1. Enantioselective Intermolecular [2+2] Photocycloaddition of 2-Cyclohexenone (1a) and 2-Methyl-1-butene Mediated by Chiral Lewis Acids 3.
The crude product was purified by chromatography on silica gel (eluent: pentane/Et2O = 4/1). The material was dissolved in CH2Cl2 and successively treated with basic alumina and dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate. Filtration through a short pad of silica and solvent removal delivered pure compound 2b.
The enantiomeric excess was determined by chiral GLC analysis.
The 2,3,4-trifluorophenyl derivative 3c gave already a much improved yield and enantioselectivity (entry 3) compared to 3b. The performance was further improved when employing a 2,4,6-trifluorophenyl group as Ar’ (entry 4). The last entry of the table (entry 5) illustrates that also the aryl group Ar’ has a subtle influence on the enantioselectivity but not on the regio- and chemoselectivity. Indeed, apart from the high enantioselectivity, it is notable that the yield of the [2+2] photocycloaddition significantly increased compared to the racemic reaction (see Supporting Information), which delivered a yield of only 51% for product rac-2b.
Lewis acid 3d evolved from the optimization studies as the optimal choice to promote an enantioselective intermolecular [2+2] photocycloaddition of cyclic enones 1. The Lewis acid was prepared in situ by addition of AlBr3 to the respective oxazaborolidine and it was applied to a variety of enone/olefin combinations (Table 2). The reason for the high catalyst loading of 50 mol % is the racemic background reaction that can only be suppressed if the catalyst concentration is high.10b
Table 2. Enantioselective Intermolecular [2+2] Photocycloaddition of Cyclic Enones (1) and Various Terminal Olefins to Bicyclic Products 2a.
Reactions were performed at a concentration of c = 20 mM in CH2Cl2 solution. Workup included treatment with basic alumina in CH2Cl2 (see Table 1).
The purified material was treated with dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate to remove olefinic impurities.
The reaction remained incomplete and the yield is based on enone conversion.
The Lewis acid catalyzed process enabled an enantioselective access to cyclobutane 2a, which was obtained in higher yield than racemic compound rac-2a (Scheme 1). It is known that the levorotatory enantiomer ([α]D23 = −152.8 at c = 1.4 in CHCl3) ent-2a exhibits the (1R,6R)-configuration at the two stereogenic centers of the bicyclo[4.2.0]octane skeleton.15 Product 2a was dextrorotatory ([α]D25 = +162.6 at c = 1.4 in CH2Cl2) proving unambiguously its absolute configuration as (1S,6S). The absolute configuration of the other products 2 was assigned in analogy and matches our model for the enantioface differentiation (vide infra). Product yields were moderate to good (42–82%) and the enantioselectivity was consistently high (82%–96% ee). Olefinic substrates were restricted to ethylene (products 2h, 2i, 2l, 2m, 2o, 2p) and to symmetric 1,1-disubstituted olefins in order to avoid the formation of cis/trans-diastereoisomers.16 Oxygen and chlorine substituents were tolerated (products 2e, 2f, 2g, 2n) and several cyclobutanes were prepared that had previously been employed in racemic form as starting material for natural product total syntheses.5,17 Enone substitution was varied at the β-position (products 2l, 2m, 2n, 2p) and within the six-membered ring of 2-cyclohexenone (products 2i, 2j, 2k, 2m, 2n). Five-membered substrates reacted with high enantioselectivity (products 2h, 2o) but the reaction was slower than the reaction of the respective 2-cyclohexenones.
In order to showcase the synthetic utility of the enantioselective intermolecular [2+2] photocycloaddition reaction we performed a total synthesis of (−)-grandisol (Scheme 2). Grandisol is a component of the aggregation pheromone of the cotton boll weevil (Anthonomus grandis) and has received a lot of attention by the synthetic community.18 Although many syntheses exist and some of them provided enantiomerically enriched product,19 there is no synthesis that features an enantioselective [2+2] photocycloaddition as the key step. Our synthesis follows a known bond set19f and commenced with photocycloaddition product 2l. The introduction of the olefinic double bond was accomplished by the catalytic Saegusa oxidation protocol reported by Stahl and co-workers.20 Addition of methyl lithium to enone 4 produced tertiary alcohol 5 the enantiopurity of which could be nicely increased by recrystallization. Oxidative cleavage of the double bond and further oxidation to keto acid 6 was performed with sodium periodate and RuCl3 as the catalyst. Olefination to product 7 and the reduction of the acid to the alcohol proceeded uneventfully and delivered (−)-grandisol in 96% ee. Starting from 3-methyl-2-cyclohexenone, the synthesis proceeded in only six steps with an overall yield of 13% and thus represents a concise route to the enantiopure natural product.
Scheme 2. Enantioselective Total Synthesis of (−)-Grandisol (8).
Mechanistic considerations concerning the enantioselective intermolecular [2+2] photocycloaddition, which go beyond previously established facts,10 must account for the improved performance of Lewis acid 3d compared to 3a. So far, only intramolecular Lewis acid-catalyzed [2+2] photocycloaddition reactions had been studied with substrates (dihydropyridones, β-alkoxyenones) that bear a tethered olefin in β- or γ-position. Assuming a reaction on the triplet hypersurface,21 the first bond formation step in these cases occurs rapidly in the β-position. Intermolecular trapping of photoexcited enones, such as 2-cyclohexenone (1a), in a Lewis acid complex is likely slower and it occurs with high preference in α-position (vide infra). The former fact, possibly in combination with the reactive T1 state of 1a, could be responsible for hydrogen abstraction22 in complex 1a·3a thus leading to a decomposition of the catalyst. In complex 1a·3d, the methyl groups are oriented differently (e.g., as shown in Figure 2) and not only escape hydrogen abstraction but also lead to an improved enantioface differentiation in α-position. Bond formation occurs from the Si face and establishes the first stereogenic center at the future carbon atom C1 of the bicyclo[4.2.0]octane skeleton. Circumstantial evidence for the intermediacy of 1,4-diradicals such as 9 stems from the observation that olefinic products are formed as minor impurities in the reactions with 1,1-disubstituted alkenes. They arise likely from hydrogen abstraction, which is a side reaction competing with C–C bond formation. Synthetically, the occurrence of the byproducts is a minor issue as they can be removed by treating the crude reaction mixture with dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate23 (see Tables 1 and 2).
Figure 2.

Suggested structure of 1:1 enone/Lewis acid complexes 1a·3a and 1a·3d and of putative 1,4-diradical intermediate 9.
The improved regioselectivity of the Lewis acid-catalyzed intermolecular [2+2] photocycloaddition is associated with the above-mentioned preference of α-attack, which in turn is evidenced by the exclusive formation of head-to-tail photocycloaddition products. No head-to-head products were identified in the crude product mixture while they are formed in notable amounts in the course of racemic [2+2] photocycloaddition reactions. It appears as if the Lewis acid increases the polarity of the excited state which is opposite to the ground state polarity.4 In the ground state the β-position of an enone is electrophilic, whereas in the excited state the α-position is electrophilic. In a simplistic picture this polarity reversal is due to the ππ* character of the triplet state with the half-filled π orbital lacking electron density in α-position.
In summary, the substitution pattern of the oxazaborolidine was found to have a strong influence on the outcome of the enantioselective intermolecular [2+2] photocycloaddition of enones which was mediated by an oxazaborolidine-AlBr3 complex. Catalyst 3d evolved as the catalyst of choice enabling high enantioface differentiation (82%–96% ee) and good chemoselectivity. Studies are ongoing to reveal its mode of action and to detect potential reaction intermediates.
Acknowledgments
Financial support by the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 665951 – ELICOS) is gratefully acknowledged. We thank O. Ackermann and J. Kudermann for their help with the HPLC and GLC analyses.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b01011.
The authors declare no competing financial interest.
Supplementary Material
References
- a Ciamician G.; Silber P. Ber. Dtsch. Chem. Ges. 1908, 41, 1928–1935. 10.1002/cber.19080410272. [DOI] [Google Scholar]; b Büchi G.; Goldman I. M. J. Am. Chem. Soc. 1957, 79, 4741–4748. 10.1021/ja01574a042. [DOI] [Google Scholar]
- a Schenck G. O.; Hartmann W.; Mannsfeld S.-P.; Metzner W.; Krauch C. H. Chem. Ber. 1962, 95, 1642–1647. 10.1002/cber.19620950711. [DOI] [Google Scholar]; b De Mayo P.; Takeshita H.; Sattar A. B. M. A. Proc. Chem. Soc. 1962, 119. [Google Scholar]; c Eaton P. E. J. Am. Chem. Soc. 1962, 84, 2454–2455. 10.1021/ja00871a039. [DOI] [Google Scholar]
- Reviews:; a Iriondo-Alberdi J.; Greaney M. F. Eur. J. Org. Chem. 2007, 2007, 4801–4815. 10.1002/ejoc.200700239. [DOI] [Google Scholar]; b Hoffmann N. Chem. Rev. 2008, 108, 1052–1103. 10.1021/cr0680336. [DOI] [PubMed] [Google Scholar]; c Bach T.; Hehn J. P. Angew. Chem., Int. Ed. 2011, 50, 1000–1045. 10.1002/anie.201002845. [DOI] [PubMed] [Google Scholar]; d Kärkäs M. D.; Porco J. A. Jr.; Stephenson C. R. J. Chem. Rev. 2016, 116, 9683–9747. 10.1021/acs.chemrev.5b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recent review:Poplata S.; Tröster A.; Zou Y.-Q.; Bach T. Chem. Rev. 2016, 116, 9748–9815. 10.1021/acs.chemrev.5b00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Corey E. J.; Mitra R. B.; Uda H. J. Am. Chem. Soc. 1963, 85, 362–363. 10.1021/ja00886a037. [DOI] [Google Scholar]; b Corey E. J.; Mitra R. B.; Uda H. J. Am. Chem. Soc. 1964, 86, 485–492. 10.1021/ja01057a040. [DOI] [Google Scholar]
- For reviews covering enantioselective [2+2] photocycloaddition reactions, see:; a Xu Y.; Conner M. L.; Brown M. K. Angew. Chem., Int. Ed. 2015, 54, 11918–11928. 10.1002/anie.201502815. [DOI] [PubMed] [Google Scholar]; b Brimioulle R.; Lenhart D.; Maturi M. M.; Bach T. Angew. Chem., Int. Ed. 2015, 54, 3872–3890. 10.1002/anie.201411409. [DOI] [PubMed] [Google Scholar]
- For key contributions to enantioselective catalytic photochemical reactions, see:; a Inoue Y.; Yokoyama T.; Yamasaki N.; Tai A. Nature 1989, 341, 225–226. 10.1038/341225a0. [DOI] [Google Scholar]; b Bauer A.; Westkämper F.; Grimme S.; Bach T. Nature 2005, 436, 1139–1140. 10.1038/nature03955. [DOI] [PubMed] [Google Scholar]; c Nicewicz D. A.; MacMillan D. W. C. Science 2008, 322, 77–80. 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For auxiliary-based methods, see:; a Tolbert L. M.; Ali M. B. J. Am. Chem. Soc. 1982, 104, 1742–1744. 10.1021/ja00370a053. [DOI] [Google Scholar]; b Lange G. L.; Decicco C.; Tan S. L.; Chamberlain G. Tetrahedron Lett. 1985, 26, 4707–4710. 10.1016/S0040-4039(00)94929-3. [DOI] [Google Scholar]; c Herzog H.; Koch H.; Scharf H.-D.; Runsink J. Tetrahedron 1986, 42, 3547–3558. 10.1016/S0040-4020(01)87320-8. [DOI] [Google Scholar]; d Inoue Y. Chem. Rev. 1992, 92, 741–770. 10.1021/cr00013a001. [DOI] [Google Scholar]; e Chen C.; Chang V.; Cai X.; Duesler E.; Mariano P. S. J. Am. Chem. Soc. 2001, 123, 6433–6434. 10.1021/ja010883+. [DOI] [PubMed] [Google Scholar]; f Faure S.; Piva-Le-Blanc S.; Bertrand C.; Pete J.-P.; Faure R.; Piva O. J. Org. Chem. 2002, 67, 1061–1070. 10.1021/jo001631e. [DOI] [PubMed] [Google Scholar]; g Inhülsen I.; Akiyama N.; Tsutsumi K.; Nishiyama Y.; Kakiuchi K. Highly. Tetrahedron 2013, 69, 782–790. 10.1016/j.tet.2012.10.074. [DOI] [Google Scholar]; For additional references, see ref (19).
- a Du J.; Skubi K. L.; Schultz D. M.; Yoon T. P. Science 2014, 344, 392–396. 10.1126/science.1251511. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Maturi M. M.; Bach T. Angew. Chem., Int. Ed. 2014, 53, 7661–7664. 10.1002/anie.201403885. [DOI] [PubMed] [Google Scholar]; c Blum T. R.; Miller Z. D.; Bates D. M.; Guzei I. A.; Yoon T. P. Science 2016, 354, 1391–1395. 10.1126/science.aai8228. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Tröster A.; Alonso R.; Bauer A.; Bach T. J. Am. Chem. Soc. 2016, 138, 7808–7811. 10.1021/jacs.6b03221. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Huang X.; Quinn T. R.; Harms K.; Webster R. D.; Zhang L.; Wiest O.; Meggers E. J. Am. Chem. Soc. 2017, 139, 9120–9123. 10.1021/jacs.7b04363. [DOI] [PubMed] [Google Scholar]; f Miller Z. D.; Lee B. J.; Yoon T. P. Angew. Chem., Int. Ed. 2017, 56, 11891–11895. 10.1002/anie.201706975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Brimioulle R.; Bach T. Science 2013, 342, 840–843. 10.1126/science.1244809. [DOI] [PubMed] [Google Scholar]; b Brimioulle R.; Bauer A.; Bach T. J. Am. Chem. Soc. 2015, 137, 5170–5176. 10.1021/jacs.5b01740. [DOI] [PubMed] [Google Scholar]
- For pioneering work on Lewis acid catalysis in [2+2] photocycloaddition reactions, see:; a Lewis F. D.; Howard D. K.; Oxman J. D. J. Am. Chem. Soc. 1983, 105, 3344–3345. 10.1021/ja00348a069. [DOI] [Google Scholar]; b Lewis F. D.; Barancyk S. V. J. Am. Chem. Soc. 1989, 111, 8653–8661. 10.1021/ja00205a015. [DOI] [Google Scholar]
- Review:Corey E. Angew. Chem., Int. Ed. 2009, 48, 2100–2117. 10.1002/anie.200805374. [DOI] [PubMed] [Google Scholar]
- a Guo H.; Herdtweck E.; Bach T. Angew. Chem., Int. Ed. 2010, 49, 7782–7785. 10.1002/anie.201003619. [DOI] [PubMed] [Google Scholar]; b Brimioulle R.; Bach T. Angew. Chem., Int. Ed. 2014, 53, 12921–12924. 10.1002/anie.201407832. [DOI] [PubMed] [Google Scholar]
- More than 50 different chiral oxazaborolidines were prepared and tested in combination with different Lewis acids.
- a Liu D.; Hong S.; Corey E. J. J. Am. Chem. Soc. 2006, 128, 8160–8161. 10.1021/ja063332y. [DOI] [PubMed] [Google Scholar]; b Shen R.; Corey E. J. Org. Lett. 2007, 9, 1057–1059. 10.1021/ol063092r. [DOI] [PubMed] [Google Scholar]
- Other olefins (4,4-dimethylpentene, cyclopentene) reacted also with high ee but the reactions suffered from lower selectivity.
- Examples:; a Takeda K.; Shimono Y.; Yoshii E. J. Am. Chem. Soc. 1983, 105, 563–568. 10.1021/ja00341a042. [DOI] [Google Scholar]; b El-Hachach N.; Fischbach M.; Gerke R.; Fitjer L. Tetrahedron 1999, 55, 6119–6128. 10.1016/S0040-4020(99)00258-6. [DOI] [Google Scholar]; c Ishii S.; Zhao S.; Mehta G.; Knors C. J.; Helquist P. J. Org. Chem. 2001, 66, 3449–3458. 10.1021/jo001792i. [DOI] [PubMed] [Google Scholar]
- For a review on previous photochemical approaches to grandisol, see ref (3c).
- Syntheses of (+)- and (−)-grandisol (excluding formal total syntheses):; a Hobbs P. D.; Magnus P. D. J. Am. Chem. Soc. 1976, 98, 4594–4600. 10.1021/ja00431a044. [DOI] [PubMed] [Google Scholar]; b Mori K. Tetrahedron 1978, 34, 915–920. 10.1016/0040-4020(78)88139-3. [DOI] [Google Scholar]; c Jones J. B.; Finch M. A. W.; Jakovac I. J. Can. J. Chem. 1982, 60, 2007–2011. 10.1139/v82-283. [DOI] [Google Scholar]; d Meyers A. I.; Fleming S. A. J. Am. Chem. Soc. 1986, 108, 306–307. 10.1021/ja00262a026. [DOI] [Google Scholar]; e Demuth M.; Palomer A.; Sluma H.-D.; Dey A. K.; Krüger C.; Tsay Y.-H. Angew. Chem., Int. Ed. Engl. 1986, 25, 1117–1119. 10.1002/anie.198611171. [DOI] [Google Scholar]; f Webster F. X.; Silverstein R. M. J. Org. Chem. 1986, 51, 5226–5231. 10.1021/jo00376a033. [DOI] [Google Scholar]; g Nori K.; Miake M. Tetrahedron 1987, 43, 2229–2239. 10.1016/S0040-4020(01)86806-X. [DOI] [Google Scholar]; h Mori K.; Nagano E. Liebigs Ann. Chem. 1991, 1991, 341–344. 10.1002/jlac.199119910159. [DOI] [Google Scholar]; i Hoffmann N.; Scharf H.-D. Liebigs Ann. Chem. 1991, 1991, 1273–1277. 10.1002/jlac.1991199101219. [DOI] [Google Scholar]; j Mori K.; Fukamatsu K. Liebigs Ann. Chem. 1992, 1992, 489–493. 10.1002/jlac.199219920187. [DOI] [Google Scholar]; k Alibés R.; Bourdelande J. L.; Font J. Tetrahedron Lett. 1993, 34, 7455–7458. 10.1016/S0040-4039(00)60151-X. [DOI] [Google Scholar]; l Martín T.; Rodríguez C. M.; Martín V. S. Tetrahedron: Asymmetry 1995, 6, 1151–1164. 10.1016/0957-4166(95)00141-B. [DOI] [Google Scholar]; m Langer K.; Mattay J. J. Org. Chem. 1995, 60, 7256–7266. 10.1021/jo00127a034. [DOI] [Google Scholar]; n Alibés R.; Bourdelande J. L.; Font J.; Parella T. Tetrahedron 1996, 52, 1279–1292. 10.1016/0040-4020(95)00958-2. [DOI] [Google Scholar]; o Monteiro H. J.; Zukerman-Schpector J. Tetrahedron 1996, 52, 3879–3888. 10.1016/S0040-4020(96)00175-5. [DOI] [Google Scholar]; p Hamon D. P. G.; Tuck K. L. Tetrahedron Lett. 1999, 40, 7569–7572. 10.1016/S0040-4039(99)01605-6. [DOI] [Google Scholar]; q de March P.; Figueredo M.; Font J.; Raya J. Org. Lett. 2000, 2, 163–165. 10.1021/ol991261k. [DOI] [PubMed] [Google Scholar]; r Hamon D. P. G.; Tuck K. L. J. Org. Chem. 2000, 65, 7839–7846. 10.1021/jo000853+. [DOI] [PubMed] [Google Scholar]; s de March P.; Figueredo M.; Font J.; Raya J.; Alvarez-Larena A.; Piniella J. F. J. Org. Chem. 2003, 68, 2437–2447. 10.1021/jo026705w. [DOI] [PubMed] [Google Scholar]
- Diao T.; Stahl S. S. J. Am. Chem. Soc. 2011, 133, 14566–14569. 10.1021/ja206575j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For theoretical studies on enantioselective Lewis acid-catalyzed [2+2] photocycloaddition reactions supporting the intermediacy of a triplet excited state, see:; a Wang H.; Cao X.; Chen X.; Fang W.; Dolg M. Angew. Chem., Int. Ed. 2015, 54, 14295–14298. 10.1002/anie.201505931. [DOI] [PubMed] [Google Scholar]; b Wang H.; Fang W.-H.; Chen X. J. Org. Chem. 2016, 81, 7093–7101. 10.1021/acs.joc.6b00980. [DOI] [PubMed] [Google Scholar]
- For examples of intramolecular hydrogen abstraction, see:; a Tobe Y.; Iseki T.; Kakiuchi K.; Odaira Y. Tetrahedron Lett. 1984, 25, 3895–3896. 10.1016/S0040-4039(01)91197-9. [DOI] [Google Scholar]; b Le Blanc S.; Pete J.-P.; Piva O. Tetrahedron Lett. 1992, 33, 1993–1996. 10.1016/0040-4039(92)88122-L. [DOI] [Google Scholar]
- Boger D. L., Zhang M.. Dimethyl 1,2,4,5-Tetrazine-3,6-dicarboxylate. In e-EROS Encyclopedia of Reagents for Organic Synthesis [Online]; Wiley & Sons, Posted April 15, 2001. http://onlinelibrary.wiley.com/doi/10.1002/047084289X.rd389/abstract (accessed Jan 25, 2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.