Abstract
Objective
To characterize decision-making processes and outcomes among men expressing early-treatment preferences for low-risk prostate cancer.
Methods
We conducted telephone surveys of men newly diagnosed with low-risk prostate cancer in 2012 to 2014. We analyzed subjects who had discussed prostate cancer treatment with a clinician and expressed a treatment preference. We asked about decision-making processes, including physician discussions, prostate-cancer knowledge, decision-making styles, treatment preference, and decisional conflict. We compared the responses across treatment groups with χ2 or ANOVA.
Results
Participants (n = 761) had a median age of 62; 82% were white, 45% had a college education, and 35% had no comorbidities. Surveys were conducted at a median of 25 days (range 9–100) post diagnosis. Overall, 55% preferred active surveillance (AS), 26% preferred surgery, and 19% preferred radiotherapy. Participants reported routinely considering surgery, radiotherapy, and AS. Most were aware of their low-risk status (97%) and the option for AS (96%). However, men preferring active treatment (AT) were often unaware of treatment complications, including sexual dysfunction (23%) and urinary complications (41%). Most men (63%) wanted to make their own decision after considering the doctor’s opinion, and about 90% reported being sufficiently involved in the treatment discussion. Men preferring AS had slightly more uncertainty about their decisions than those preferring AT.
Conclusions
Subjects were actively engaged in decision making and considered a range of treatments. However, we found knowledge gaps about treatment complications among those preferring AT and slightly more decisional uncertainty among those preferring AS, suggesting the need for early decision support.
Keywords: decision making, neoplasm staging, prostatectomy, prostatic neoplasms, radiotherapy, watchful waiting
1 | BACKGROUND
Prostate cancer is often indolent, and many screen-detected cancers are unlikely to ever cause clinical problems.1 Professional societies are increasingly recommending that men with low-risk localized cancers consider active surveillance (AS)—deferring active treatment (AT) but being closely monitored to be offered curative treatment if there is evidence of disease progression.2,3 Active surveillance differs from watchful waiting (WW), another observational strategy that offers only palliative treatment for cancer progression.4 While uptake of AS appears to be increasing, many men with low-risk prostate cancers continue to receive AT.5–11
Decisions about treating a low-risk cancer are complex and very sensitive to patient preferences. Patients must consider the risks of cancer progression without treatment versus the risks of complications from undergoing potentially unnecessary treatment and make a decision that best reflects their personal values. However, treatment decisions for localized prostate cancer, which encompasses low-risk cancers, are often made quickly, are not well informed, and do not reflect underlying patient preferences.12,13 We found few studies that comprehensively evaluated AS decisions in men with low-risk prostate cancer.14–17
The Patient REported outcomes for Prostate cARE (PREPARE) trial is a prospective cohort study characterizing treatment decision-making among men newly diagnosed with low-risk prostate cancer at Kaiser Permanente Northern California (KPNC).18 Kaiser Permanente Northern California has 105 urologists practicing across 18 medical centers. In developing our research questions and measures, we adapted a model from Zafar that hypothesizes roles for demographic, clinical, and decision-making factors in helping patients achieve a satisfactory treatment decision.18,19 In this report, we characterize treatment discussions, prostate-cancer knowledge, decision-making styles, treatment preferences, and decisional conflict among men who soon after diagnosis indicated a preferred method for how to treat or manage their low-risk prostate cancer.
2 | SUBJECTS AND METHODS
The PREPARE cohort was assembled by enrolling consecutive KPNC patients newly diagnosed with a low-risk prostate cancer from May 2012 to May 2014. Inclusion criteria were stage T2a or less, prostate-specific antigen (PSA) less than 10 ng/mL, and Gleason less than 7; able to provide informed consent; and English speaking. We excluded men who had already started treatment for prostate cancer, were not diagnosed from a prostate biopsy, or had a previous prostate cancer diagnosis. We restricted the current analyses to men who had discussed cancer treatment with a clinician (urologist, oncologist, or primary care provider) before the baseline interview (we also interviewed subjects at 6 and 24 months after diagnosis) and who had expressed a treatment preference (responded “yes” to “Have you decided on which treatment or management option you will choose?”). We classified treatment preference as AS/WW, radical prostatectomy, external beam radiotherapy (EBRT), brachytherapy, or hormone therapy (no one preferred this treatment). We combined AS and WW because the baseline preference could be interpreted only as indicating that the patient did not prefer an AT.
2.1 | Procedures
Each week, we identified all KPNC men with histological evidence of a new prostate cancer diagnosis. We reviewed the electronic medical record to confirm that the prebiopsy PSA level was less than 10 ng/mL and the Gleason score was less than 7. After ensuring that the urologist had informed the patient of the diagnosis, we notified the urologist of our intent to enroll the patient and gave them 1 week to indicate any disagreement. We then mailed an invitation letter to eligible men. Trained research assistants attempted to conduct the baseline telephone assessment within 30 days of the patient being informed of his prostate cancer diagnosis but continued to call up to 90 days post notification. The baseline interview required about 30 minutes, and the participants received a $20 gift card. The Kaiser Foundation Research Institute’s Institutional Review Board (IRB 00000401) approved the study.
2.2 | Measures
2.2.1 | Demographic and clinical characteristics
We obtained self-reported demographic characteristics through the telephone survey, including age, race, ethnicity, education, marital status, employment status, and income. Medical record information included diagnosis date, PSA levels, clinical stage, Gleason score, and comorbid illnesses. We used the Elixhauser Index to calculate the number of comorbid illnesses.20
2.2.2 | Treatment discussions with physicians
We asked the participants to report the physicians (by specialty) with whom they had discussed treatment, the treatments and management options their doctors had told them about, the treatments/management options they had considered, and the treatments their doctors had recommended. We also asked each participant, “Of all of the doctors with whom you have discussed your management options, which doctor have you relied on most?”
2.2.3 | Knowledge of low-risk prostate cancer and treatment options
We assessed whether men were aware of their cancer risk status and asked those responding yes to classify their risk as low, intermediate, or high. We asked the participants about their knowledge of the natural history and treatment options for low-risk prostate cancer as well as treatment side effects. Table 3 shows the knowledge questions—which were based on our previously developed scales (Cronbach alpha = 0.36).21,22 Response choices were true, false, or don’t know, with “don’t know” scored as incorrect. Given the low internal consistency, which we attribute to using a limited number of questions to address multiple domains, we do not report summary scores.
TABLE 3.
Knowledge
| All (N = 761) N (%) | Treatment Preference | |||||
|---|---|---|---|---|---|---|
| AS/WW (N = 417) N (%) | RP (N = 198) N (%) | EBRT (N = 69) N (%) | BRAC (N = 77) N (%) | P Value | ||
| Aware of risk level? | ||||||
| Yes | 741 (97.4) | 409 (98.1) | 188 (94.9) | 68 (98.6) | 76 (98.7) | .27 |
| If aware of risk level, what risk level? | <.001 | |||||
| Low | 676 (92.6) | 388 (96.5) | 159 (85.9) | 60 (89.6) | 69 (90.8) | |
| Intermediate/high | 54 (7.4) | 14 (3.5) | 26 (14.1) | 7 (10.4) | 7 (9.2) | |
| Knowledge questions (correct answer/N [%]correctly answered) | ||||||
| Usually, prostate cancer grows very quickly compared with other types of cancer. (False) | 687 (90.5) | 382 (92) | 169 (85.4) | 67 (97.1) | 69 (89.6) | .01 |
| Most men diagnosed with prostate cancer die of something other than prostate cancer. (True) | 679 (89.5) | 378 (91.1) | 171 (86.4) | 61 (88.4) | 69 (89.6) | .35 |
| Men with low-risk prostate cancer can choose to be monitored closely by their doctors, rather than receive surgery or radiation. (True) | 724 (95.5) | 413 (99.5) | 171 (86.8) | 67 (97.1) | 73 (94.8) | <.001 |
| Loss of sexual function is a common side effect of prostate cancer treatment. (True) | 597 (78.7) | 331 (79.8) | 161 (81.3) | 48 (69.6) | 57 (74.0) | .14 |
| Treatment rarely causes urinary incontinence. (False) | 470 (61.9) | 267 (64.3) | 130 (65.7) | 27 (39.1) | 46 (59.7) | .001 |
Abbreviations: AS/WW indicates active surveillance/watchful waiting; RP, radical prostatectomy; EBRT, external beam radiotherapy; BRAC, brachytherapy.
2.2.4 | Decision-making processes and outcomes
We used the Degner Control Preference Scale to assess the men’s preference for making a shared treatment decision with response options ranging from doctor-dependent (“I prefer to leave all decisions regarding treatment to my doctor”) to shared (“I prefer that my doctor and I share responsibility for deciding which treatment is best for me”) to independent (“I prefer to make the decision about which treatment I will receive.”).23 We used items from the Physician Decision Making Style scale to further characterize discussions with urologists and radiation oncologists as to whether the participant reported being encouraged by these physicians to ask questions about treatment options and recommendations and whether the participants were involved as much as they wanted in the decision-making process.24 Response options were “yes, definitely”; “yes, somewhat”; or “no.”
We used a 4-item version of the Decisional Conflict Scale (SURE test) to measure decisional certainty (Cronbach alpha = 0.71).25 Table 4 shows the item wording. Response choices were “yes” or “no,” and a higher score indicated greater certainty.
TABLE 4.
Decision-making outcomes by treatment preference
| Variable | All (N = 761) N (%) | AS/WW (N = 417) N (%) | RP (N = 198) N (%) | EBRT (N = 69) N (%) | BRAC (N = 77) N (%) | P Value |
|---|---|---|---|---|---|---|
| Sure test (% yes) | ||||||
| Do you feel sure about the best choice? | 678 (89.1) | 364 (87.3) | 179 (90.4) | 64 (92.8) | 71 (92.2) | .33 |
| Do you know the risks and benefits of each option? | 686 (90.3) | 360 (86.5) | 187 (94.4) | 66 (95.7) | 73 (94.8) | <.01 |
| Are you clear about which benefits and risks matter most to you? | 702 (92.2) | 371 (89) | 190 (96) | 66 (95.7) | 75 (97.4) | <.01 |
| Do you have enough support and advice to make a choice? | 719 (94.6) | 390 (93.8) | 190 (96) | 68 (98.6) | 71 (92.2) | .23 |
| Total score (mean, SD) | 3.7 (0.8) | 3.6 (0.9) | 3.8 (0.7) | 3.8 (0.4) | 3.8 (0.6) | <.001 |
Abbreviations: AS/WW indicates active surveillance/watchful waiting; RP, radical prostatectomy; EBRT, external beam radiotherapy; BRAC, brachytherapy.
2.3 | Statistical analyses
We assessed differences across the 4 treatment preference groups on demographic and clinical characteristics, physician discussions, knowledge, preferred decision-making involvement, satisfaction with treatment discussions, and decisional conflict. We used χ2 and Fisher exact tests for categorical variables and ANOVA for continuous variables. All tests were 2-sided. We used a Bonferroni correction for multiple comparisons, and P values less than .0011 were considered to indicate statistically significant results. When global tests were significant, we compared the responses for those preferring AS/WW versus combined responses for those preferring any AT.
3 | RESULTS
3.1 | Study subjects
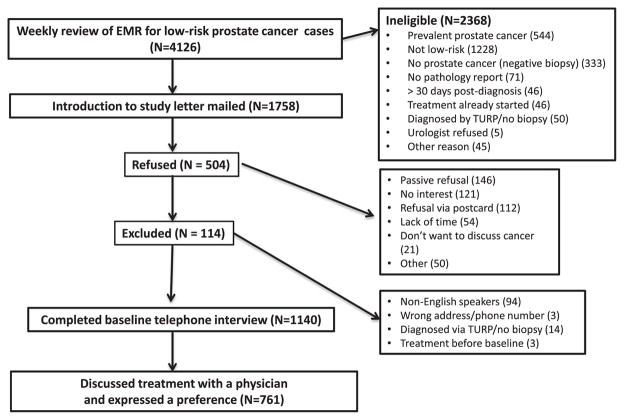
Overall, the PREPARE trial enrolled 1139 of the 1643 (69.3%) eligible men with newly diagnosed low-risk localized prostate cancer. Compared with those who declined to participate or could not be reached, the participants were more likely to be white (P < .0001). There were no significant differences on age, ethnicity, comorbidities, or PSA levels. This paper focuses on the 761 enrolled men (67%) who had discussed cancer treatment with at least 1 physician and expressed a treatment preference (Appendix Figure). We conducted interviews a median of 25 days (range 9–100) after diagnosis. The participants in this analysis significantly differed from enrollees not meeting the above criteria; the participants had a lower proportion of Hispanics, higher educational attainment, and higher incomes.
The median age was 62 years, with most reporting being non- Hispanic whites, married/in a relationship, having had at least some college education, being employed or retired, and being in good health (Table 1). The majority preferred AS/WW (54.8%) followed by radical prostatectomy (26.0%). Greater preference for AS/WW was most associated with being 70 and older; higher educational attainment; non-Hispanic ethnicity; white or other (nonblack) race; being employed or retired; and being separated, divorced, or widowed. Preferences for surgery were most associated with being younger than 60 years old, belonging to racial/ethnic minority populations, being married, not being retired, and having a high school education or less. Preferences for radiotherapy were most associated with age 60 years and older and non-Hispanic ethnicity. Comorbidity and income were not associated with treatment preference.
TABLE 1.
Participant characteristics by treatment preferencea
| Variable | All (N = 761) N (Column %) | Treatment Preference | ||||
|---|---|---|---|---|---|---|
| AS/WW (N = 417) N (Row %) | RP (N = 198) N (Row %) | EBRT (N = 69) N (Row %) | BRAC (N = 77) N (Row %) | P Value | ||
| Age in years (median, IQR) | (62, 11) | (63, 9) | (58, 11) | (66, 7) | (61, 11) | <.001 |
| Age by category | <.001 | |||||
| <50 | 51 (6.7) | 17 (33.3) | 24 (47.1) | 2 (3.9) | 8 (15.7) | |
| 50–59 | 249 (32.7) | 121 (48.6) | 91 (36.5) | 10 (4.0) | 27 (10.8) | |
| 60–69 | 373 (49) | 219 (58.75) | 78 (20.9) | 43 (11.5) | 33 (8.8) | |
| 70+ | 88 (11.6) | 60 (68.2) | 5 (5.7) | 14 (15.9) | 9 (10.2) | |
| Days from diagnosis (median, IQR) | (25, 14) | (26, 16) | (25, 14) | (25, 9) | (23, 17) | .13 |
| Race | .02 | |||||
| White | 623 (81.9) | 347 (55.7) | 150 (24.1) | 63 (10.1) | 63 (10.1) | |
| Black | 85 (11.2) | 37 (43.5) | 32 (37.6) | 5 (5.9) | 11 (12.9) | |
| Other | 53 (7) | 33 (62.3) | 16 (30.2) | 1 (1.9) | 3 (5.7) | |
| Ethnicity | .03 | |||||
| Hispanic | 93 (12.4) | 46 (49.5) | 35 (37.6) | 4 (4.3) | 8 (8.6) | |
| Non-Hispanic | 657 (87.6) | 360 (54.8) | 162 (24.7) | 66 (10.0) | 162 (24.7) | |
| Marital status | <.01 | |||||
| Married/relationship | 619 (81.4) | 330 (53.3) | 177 (28.6) | 58 (9.4) | 54 (8.7) | |
| Separated/divorced/widowed | 115 (15.1) | 71 (61.7) | 19 (16.5) | 7 (6.1) | 18 (15.7) | |
| Never married | 26 (3.4) | 15 (57.7) | 2 (7.7) | 4 (15.4) | 5 (19.2) | |
| Education | .02 | |||||
| ≤High school | 164 (21.6) | 83 (50.6) | 50 (30.5) | 13 (7.9) | 18 (11.0) | |
| Some college | 251 (33.1) | 137 (54.6) | 64 (25.5) | 26 (10.4) | 24 (9.6) | |
| College graduate | 165 (21.8) | 82 (49.7) | 41 (24.8) | 14 (8.5) | 28 (17.0) | |
| Graduate school/degree | 178 (23.5) | 112 (62.9) | 43 (24.2) | 16 (9.0) | 7 (3.9) | |
| Employment | <.001 | |||||
| Employed | 432 (57.4) | 235 (54.4) | 131 (30.3) | 25 (5.8) | 41 (9.5) | |
| Retired | 283 (37.6) | 157 (55.5) | 55 (19.4) | 41 (14.5) | 30 (10.6) | |
| Not employed/disabled | 37 (4.9) | 16 (43.2) | 12 (32.4) | 3 (8.1) | 6 (16.2) | |
| Income | .55 | |||||
| ≤$75,000 | 269 (37.9) | 144 (53.5) | 65 (24.2) | 26 (9.7) | 34 (12.6) | |
| $75,000–125,000 | 234 (100) | 134 (57.3) | 22 (9.4) | 21 (9.0) | 57 (24.4) | |
| >$125,000 | 206 (29.1) | 105 (51.0) | 66 (32.0) | 18 (8.7) | 17 (8.3) | |
| Comorbidity (Elixhauser) | .59 | |||||
| 0 | 263 (34.6) | 143 (54.4) | 72 (27.4) | 22 (8.4) | 26 (9.9) | |
| 1 | 205 (26.9) | 116 (56.6) | 56 (27.3) | 12 (5.9) | 21 (10.2) | |
| 2 | 114 (15) | 57 (50.0) | 29 (25.4) | 15 (13.2) | 13 (11.4) | |
| 3+ | 179 (23.5) | 101 (56.4) | 41 (22.9) | 20 (11.2) | 17 (9.5) | |
| PSA level | .03 | |||||
| ≤4 ng/mL | 82 (10.8) | 37 (45.1) | 31 (37.8) | 4 (4.9) | 10 (12.2) | |
| >4–10 ng/mL | 679 (89.2) | 380 (56.0) | 167 (24.6) | 65 (9.6) | 67 (9.9) | |
Number of respondents within each category may not be the same as total due to missing data.
Abbreviations: AS/WW indicates active surveillance/watchful waiting; RP, radical prostatectomy; EBRT, external beam radiotherapy; BRAC, brachytherapy; PSA, prostate-specific antigen.
3.2 | Treatment discussions with physicians
Nearly all participants had discussed treatment with a urologist; far fewer had discussions with radiation oncologists or primary care providers by the time that they were surveyed (Table 2). Surgery was the most frequently discussed treatment, followed fairly closely by EBRT, AS/WW, and then brachytherapy. Overall, most participants indicated considering each of these options. However, the preferred treatments were far more likely to have been considered than the alternatives. Hormone therapy was infrequently discussed (18.6%) or considered (9.0%).
TABLE 2.
Physician discussions by treatment preference
| Treatment Preference | ||||||
|---|---|---|---|---|---|---|
| All (N = 761) N (%) | AS/WW (N = 417) N (%) | RP (N = 198) N (%) | EBRT (N = 69) N (%) | BRAC (N = 77) N (%) | P Value | |
| Have you seen a______to discuss your prostate cancer treatment or management options? | ||||||
| Urologist | 753 (98.9) | 410 (98.3) | 198 (100) | 68 (98.6) | 77 (100) | .20 |
| Radiation oncologist | 149 (19.6) | 44 (10.6) | 34 (17.3) | 46 (66.7) | 25 (32.5) | <.001 |
| Primary care provider | 83 (10.9) | 44 (10.6) | 24 (12.1) | 9 (13) | 6 (7.8) | .69 |
| What treatment or management options have you discussed? | ||||||
| AS/WW | 643 (85.1) | 408 (98.6) | 132 (67) | 50 (73.5) | 53 (68.8) | <.001 |
| Radical prostatectomy | 722 (95.5) | 388 (93.9) | 196 (99.5) | 65 (94.2) | 73 (94.8) | .02 |
| External beam radiotherapy | 667 (88.3) | 355 (86.2) | 176 (89.3) | 69 (100) | 67 (87) | .01 |
| Brachytherapy | 602 (80.2) | 315 (77) | 155 (78.7) | 57 (83.8) | 75 (97.4) | <.001 |
| Hormone therapy | 135 (18.6) | 64 (16.2) | 40 (21.2) | 17 (25) | 14 (18.7) | .25 |
| What treatment or management options have you considered/are considering? | ||||||
| AS/WW | 550 (72.4) | 405 (97.1) | 72 (36.5) | 33 (47.8) | 40 (51.9) | <.001 |
| Radical prostatectomy | 481 (63.3) | 223 (53.6) | 197 (99.5) | 29 (42) | 32 (41.6) | <.001 |
| External beam radiotherapy | 382 (50.4) | 191 (46) | 79 (40.1) | 68 (98.6) | 44 (57.1) | <.001 |
| Brachytherapy | 386 (51) | 194 (46.7) | 75 (38.1) | 42 (60.9) | 75 (98.7) | <.001 |
| Hormone therapy | 68 (9) | 44 (10.6) | 14 (7.1) | 4 (5.8) | 6 (7.8) | .37 |
| What treatments have physicians recommended?a | ||||||
| Urologist | <.001 | |||||
| AS/WW | 225 (29.9) | 214 (52.3) | 6 (3) | 0 (0) | 5 (6.5) | |
| Active treatment | 198 (26.3) | 47 (11.5) | 87 (43.9) | 25 (36.8) | 39 (50.6) | |
| No specific treatment | 329 (43.8) | 148 (36.2) | 105 (53) | 43 (63.2) | 33 (42.9) | |
| Radiation oncologist | <.001 | |||||
| AS/WW | 13 (8.7) | 12 (27.3) | 0 (0) | 1 (2.2) | 0 (0) | |
| Active treatment | 70 (47) | 15 (34.1) | 21 (61.8) | 18 (39.1) | 16 (64) | |
| No specific treatment | 66 (44.3) | 17 (38.6) | 13 (38.2) | 27 (58.7) | 9 (36) | |
| Primary care provider | .02 | |||||
| AS/WW | 10 (14.9) | 9 (23.7) | 1 (5) | 0 (0) | 0 (0) | |
| Active treatment | 18 (26.9) | 5 (13.2) | 11 (55) | 1 (20) | 1 (25) | |
| No specific treatment | 39 (58.2) | 24 (63.2) | 8 (40) | 4 (80) | 3 (75) | |
| Of all of the doctors with whom you have discussed your management options, which doctor have you relied on most?b | <.001 | |||||
| Urologist | 181 (62.4) | 78 (69.0) | 67 (69.1) | 20 (41.7) | 16 (50.0) | |
| Radiation oncologist | 8 (2.8) | 4 (3.5) | 0 (0) | 2 (4.2) | 2 (6.3) | |
| Primary care provider | 45 (15.5) | 13 (11.5) | 7 (7.2) | 14 (29.2) | 11 (34.4) | |
Numbers are for subjects who discussed treatment with the specific provider type.
Numbers are for subjects who discussed treatment with multiple providers.
Abbreviations: AS/WW indicates active surveillance/watchful waiting; RP, radical prostatectomy; EBRT, external beam radiotherapy; BRAC, brachytherapy.
The participants reported that urologists and oncologists did not recommend a specific treatment in about 44% of treatment discussions compared with 58% of discussions with primary care providers. The participants preferring AS/WW reported that urologists were far more likely to recommend AS/WW than AT during treatment discussions, whereas men preferring surgery or radiotherapy reported that the urologists were more likely to recommend AT. The participants reported that radiation oncologists (47%) were more likely to recommend AT. Among the 38% of participants who saw more than 1 physician to discuss management options, they relied most heavily on recommendations from urologists.
3.2.1 | Knowledge of low-risk prostate cancer and treatment options
Most participants were aware of having a low-risk cancer (Table 3). Men preferring AS/WW were more likely than those preferring AT to consider themselves as low risk (96.5% vs 83.7%, P < .001). The participants usually correctly answered knowledge questions about the natural history of prostate cancer, including its indolent nature (90.5%), the higher likelihood of dying from causes other than prostate cancer (89.5%), and the option of AS (95.5%). Overall, 78.7% recognized the adverse effect of treatments on sexual function, although only 62% were aware that treatment could cause urinary incontinence. Men preferring EBRT were least likely to be aware of treatment side effects; those preferring surgery were least likely to know that prostate cancer could be slow growing and that AS is an acceptable treatment option.
3.2.2 | Decision-making processes and outcomes
When asked about decision control preferences, only 9 participants wanted to leave the final decision to physicians. Most (63.0%) preferred to make a decision after considering physician recommendations, 19.4% wanted to share decision-making responsibility, while 16.5% wanted to make their own decision. Men preferring AS/WW were more likely than those preferring AT to want to share decision responsibility (26.3% vs 11.2%, P < .001). Those preferring AT were more likely than those preferring AS to want to make their own decision (21.9% vs 12.0%, P < .001). Overall, about 94% of participants responded being definitely involved as much as they wanted in making the decision.
The overall SURE scale results showed that men preferring AS/WW were less confident in their decision than those preferring an AT (Table 4). These differences were most pronounced in the domains of feeling informed about the benefits and risks of each treatment option and in feeling clear about which benefits and risks mattered most to them. The mean SURE scale results differed significantly between men preferring AS/WW (3.6) versus all men preferring an AT (3.8), P less than .001.
4 | DISCUSSION
Men with tumors having low-risk features for progression are increasingly being advised to consider AS rather than surgery or radiotherapy, given concerns about overtreatment.2,3 We found that 55% of men diagnosed with a low-risk prostate cancer who had discussed treatment options with a physician preferred AS. Nearly all participants discussed treatment options with a urologist; among those discussing treatment with more than 1 clinician, the majority relied most heavily on the information provided by urologists. Our participants were generally aware of having an indolent cancer, although they were less knowledgeable about treatment complications. Most participants felt sufficiently engaged in decision making and confident in their preferences.
Our findings are consistent with the decision-making literature in which patients often discuss treatment only with a urologist12,26 However, studies suggest that these discussions can be flawed. A prospective study of veterans newly diagnosed with low- or intermediate-risk prostate cancer found that treatment recommendations from urologists trumped patients’ personal values, particularly regarding quality of life.14 Studies have also shown that patients with low-risk prostate cancers are not being fully informed about treatment options; this may undermine decision making because whether a urologist suggests that AS is an important determinant of selecting this option.17,27
Overall, most participants were aware of having a low-risk cancer that was not likely to progress. This finding contrasts with a study of decision making for localized prostate cancer that found that most men did not systematically process information about cancer risk.28 Our participants also reported discussing and considering each of the various treatment options, although 15% reported not discussing AS/WW and 28% reported not considering AS/WW. This represents a change compared with previous studies of men facing treatment decisions for localized prostate cancer when most considered only a single treatment, usually prostatectomy.29,30 Furthermore, our participants reported that both urologists and radiation oncologists discussed AS/WW, although urologists were more likely to recommend this option. This is important because a barrier to AS is getting conflicting messages from different specialists.31
The participants were satisfied with their discussions, generally reporting being able to ask questions and feeling well informed about the decision. However, knowledge testing indicated some important deficits. Compared with men preferring AS/WW, men preferring AT were less aware of treatment complications and the option for AS/WW. These findings suggest that men with low-risk prostate cancer, particularly those expressing early preferences for AT, might not have sufficient knowledge to make informed decisions. Nonetheless, men preferring radical prostatectomy were most likely to make their own decision, while men preferring AS/WW were most likely to engage in shared decision-making. Few participants preferred a passive role in decision making. These findings contrast with some earlier studies of men with localized prostate cancer, which found that nearly half or more would choose the treatment recommended by their physician.28,32 However, those studies were conducted before AS was widely accepted and included men who could have had higher-risk localized cancers.
Men preferring any AT were slightly more confident in their treatment decision than those preferring AS/WW. While the differences in decisional conflict measured by the SURE scale were highly significant, the effect size was only 0.25. The SURE scale items came from the Decisional Conflict Scale that considered a meaningful effect size to be 0.30.33 This suggests that our effect size, based on the subset of our cohort who expressed a treatment preference, may be of borderline clinical importance. However, we will be evaluating the SURE scale results to predict initial treatment among the entire study cohort as well as adherence among those selecting AS.
The decision processes of men preferring AS/WW are important because, while most men who eventually switch from AS to AT have some evidence of disease progression, about 20% switch due to anxiety or personal choice.34 Recent data suggest that increasing numbers of men are now entering AS5–7; however, the Prostate Cancer Research International Active Surveillance study found men reporting higher decisional conflict if the treatment decision was more physician-driven than shared.35 Decisional conflict was associated with anxiety, suggesting that initial shared decision making might reduce the proportion of men who switch in the absence of clinical progression.
The literature highlights the need to better support initial decision making by providing comprehensive, balanced information about the natural history of prostate cancer and the risks and benefits of the different treatment options.36 One approach is to use decision aids. The Cochrane review of decision aids for patients facing health treatment or screening decisions has shown that decision aids increase patient engagement in decision making, increase knowledge, reduce decisional conflict, and lead to less often wanting surgery or aggressive treatments.37 However, the effect of decision aids on the uptake of AS among men with low-risk prostate cancer is uncertain. Most studies have used decision aids presenting the option of WW, a palliative approach, rather than AS and enrolled men with higher-risk prostate cancers.38,39
4.1 | Study limitations
A limitation of our study is that we did not know the actual treatment selection or whether decision-making processes and perceptions evolved by the time of treatment selection. Additionally, the subjects excluded from this analysis had a lower socioeconomic status and were more likely to be from a minority population than eligible subjects. Nonetheless, assessing decision-making processes soon after diagnosis is important, given the rapidity in which men are learning about treatments and making decisions. Kaiser Permanente Northern California is an integrated health care system, and its insured population has higher educational levels than the general population. While these factors might limit generalizability of results, we are better able to isolate decision-making processes, given that KPNC clinicians do not have financial incentives to provide treatment.
4.2 | Clinical implications
The reported decision-making processes among men with low-risk prostate cancer who expressed an early preference for treatment met many criteria for shared decision making. The men felt actively engaged in treatment discussions, informed about various options, and were confident about their decisions. However, men preferring AT were less aware of treatment complications and the natural history of low-risk prostate cancer than men preferring AS/WW. Meanwhile, men preferring AS/WW had more decisional conflict, although the absolute difference was quite small. These findings suggest a potential role for early interventions to better support shared decision making, perhaps using validated decision aids and patient navigators.
Acknowledgments
The study was funded by the National Cancer Institute, R01 CA 155578-01. We thank the study participants for their time and efforts.
Funding information
National Cancer Institute, Grant/Award Number: R01 CA 155578-01.
APPENDIX STUDY FLOW CHART
References
- 1.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw. 2014;12:686–718. doi: 10.6004/jnccn.2014.0072. [DOI] [PubMed] [Google Scholar]
- 4.Dall’Era MA, Cooperberg MR, Chan JM, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112:1650–1659. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 5.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA. 2015;314:80–82. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 6.Weiner AB, Patel SG, Etzioni R, Eggener SE. National trends in the management of low and intermediate risk prostate cancer in the United States. J Urol. 2015;193:95–102. doi: 10.1016/j.juro.2014.07.111. [DOI] [PubMed] [Google Scholar]
- 7.Womble PR, Montie JE, Ye Z, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67:44–50. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao YH, Albertsen PC, Roberts CB, et al. Risk profiles and treatment patterns among men diagnosed as having prostate cancer and a prostate-specific antigen level below 4.0 ng/mL. Arch Intern Med. 2010;170:1256–1261. doi: 10.1001/archinternmed.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts CB, Albertsen PC, Shao YH, et al. Patterns and correlates of prostate cancer treatment in older men. Am J Med. 2011;124:235–243. doi: 10.1016/j.amjmed.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA. 2013;309:2587–2595. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeliadt SB, Ramsey SD, Penson DF, et al. Why do men choose one treatment over another?: a review of patient decision making for localized prostate cancer. Cancer. 2006;106:1865–1874. doi: 10.1002/cncr.21822. [DOI] [PubMed] [Google Scholar]
- 13.Holmes-Rovner M, Montgomery JS, Rovner DR, et al. Informed decision making: assessment of the quality of physician communication about prostate cancer diagnosis and treatment. Med Decis Making. 2015;35:999–1009. doi: 10.1177/0272989X15597226. [DOI] [PubMed] [Google Scholar]
- 14.Scherr KA, Fagerlin A, Hofer T, et al. Physician recommendations trump patient preferences in prostate cancer treatment Decisions. Med Decis Making. 2017;37:56–69. doi: 10.1177/0272989X16662841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davison BJ, Oliffe JL, Pickles T, Mroz L. Factors influencing men undertaking active surveillance for the management of low-risk prostate cancer. Oncol Nurs Forum. 2009;36:89–96. doi: 10.1188/09.ONF.89-96. [DOI] [PubMed] [Google Scholar]
- 16.Davison BJ, Breckon E. Factors influencing treatment decision making and information preferences of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87:369–374. doi: 10.1016/j.pec.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Gorin MA, Soloway CT, Eldefrawy A, Soloway MS. Factors that influence patient enrollment in active surveillance for low-risk prostate cancer. Urology. 2011;77:588–591. doi: 10.1016/j.urology.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Taylor KL, Hoffman RM, Davis KM, et al. Treatment preferences for active surveillance versus active treatment among men with low–risk prostate cancer. Cancer Epidemiol Biomarkers Prev. 2016;25:1240–1250. doi: 10.1158/1055-9965.EPI-15-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zafar SY, Alexander SC, Weinfurt KP, Schulman KA, Abernethy AP. Decision making and quality of life in the treatment of cancer: a review. Support Care Cancer. 2009;17:117–127. doi: 10.1007/s00520-008-0505-2. [DOI] [PubMed] [Google Scholar]
- 20.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Taylor KL, Davis JL, 3rd, Turner RO, et al. Educating African American men about the prostate cancer screening dilemma: a randomized intervention. Cancer Epidemiol Biomarkers Prev. 2006;15:2179–2188. doi: 10.1158/1055-9965.EPI-05-0417. [DOI] [PubMed] [Google Scholar]
- 22.Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med. 2013;173:1704–1712. doi: 10.1001/jamainternmed.2013.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29:21–43. [PubMed] [Google Scholar]
- 24.Arora NK, Weaver KE, Clayman ML, Oakley-Girvan I, Potosky AL. Physicians’ decision-making style and psychosocial outcomes among cancer survivors. Patient Educ Couns. 2009;77:404–412. doi: 10.1016/j.pec.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legare F, Kearing S, Clay K, et al. Are you SURE?: assessing patient decisional conflict with a 4-item screening test. Can Fam Physician. 2010;56:e308–e314. [PMC free article] [PubMed] [Google Scholar]
- 26.Jang TL, Bekelman JE, Liu Y, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med. 2010;170:440–450. doi: 10.1001/archinternmed.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davison BJ, Goldenberg SL. Patient acceptance of active surveillance as a treatment option for low-risk prostate cancer. BJU Int. 2011;108:1787–1793. doi: 10.1111/j.1464-410X.2011.10200.x. [DOI] [PubMed] [Google Scholar]
- 28.Steginga SK, Occhipinti S, Gardiner RA, Yaxley J, Heathcote P. Making decisions about treatment for localized prostate cancer. BJU Int. 2002;89:255–260. doi: 10.1046/j.1464-4096.2001.01741.x. [DOI] [PubMed] [Google Scholar]
- 29.Ramsey SD, Zeliadt SB, Arora NK, et al. Access to information sources and treatment considerations among men with local stage prostate cancer. Urology. 2009;74:509–515. doi: 10.1016/j.urology.2009.01.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeliadt SB, Moinpour CM, Blough DK, et al. Preliminary treatment considerations among men with newly diagnosed prostate cancer. Am J Manag Care. 2010;16:e121–e130. [PubMed] [Google Scholar]
- 31.O’Callaghan C, Dryden T, Hyatt A, et al. ‘What is this active surveillance thing?’ Men’s and partners’ reactions to treatment decision making for prostate cancer when active surveillance is the recommended treatment option. Psychooncology. 2014;23:1391–1398. doi: 10.1002/pon.3576. [DOI] [PubMed] [Google Scholar]
- 32.Davison BJ, Degner LF, Morgan TR. Information and decision-making preferences of men with prostate cancer. Oncol Nurs Forum. 1995;22:1401–1408. [PubMed] [Google Scholar]
- 33.User Manual-Decisional Conflict Scale (10-item question format) Ottawa Hospital Research Institute; 1993. [Accessed February 17, 2017]. at http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf. [Google Scholar]
- 34.Simpkin AJ, Tilling K, Martin RM, et al. Systematic review and meta- analysis of factors determining change to radical treatment in active surveillance for localized prostate cancer. Eur Urol. 2015;67:993–1005. doi: 10.1016/j.eururo.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 35.van den Bergh RC, Essink-Bot ML, Roobol MJ, et al. Anxiety and distress during active surveillance for early prostate cancer. Cancer. 2009;115:3868–3878. doi: 10.1002/cncr.24446. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman RM. Improving the communication of benefits and harms of treatment strategies: decision AIDS for localized prostate cancer treatment decisions. J Natl Cancer Inst Monog. 2012;2012:197–201. doi: 10.1093/jncimonographs/lgs023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 38.Violette PD, Agoritsas T, Alexander P, et al. Decision aids for localized prostate cancer treatment choice: systematic review and meta-analysis. CA Cancer J Clin. 2015;65:239–251. doi: 10.3322/caac.21272. [DOI] [PubMed] [Google Scholar]
- 39.Adsul P, Wray R, Spradling K, Darwish O, Weaver N, Siddiqui S. Systematic review of decision aids for newly diagnosed patients with prostate cancer making treatment decisions. J Urol. 2015;194:1247–1252. doi: 10.1016/j.juro.2015.05.093. [DOI] [PubMed] [Google Scholar]