Abstract
We present an 8-year-old male with metastatic alveolar rhabdomyosarcoma (ARMS) who developed precipitous cardiopulmonary collapse with severe tumor lysis syndrome (TLS) 48 hr after initiation of chemotherapy. Despite no detectable pulmonary metastases, acute hypoxemic respiratory failure developed, requiring extracorporeal membrane oxygenation (ECMO). Although TLS has been reported in disseminated ARMS, this singular case of life-threatening respiratory deterioration developing after initiation of chemotherapy presented unique therapeutic dilemmas. We review the clinical aspects of this case, including possible mechanisms of respiratory failure, and discuss the role of ECMO utilization in pediatric oncology.
Keywords: alveolar rhabdomyosarcoma, ECMO, pediatric cancer, tumor lysis syndrome
INTRODUCTION
Alveolar rhabdomyosarcoma (ARMS) is a rare pediatric tumor that portends a poor prognosis when metastatic.[1] Intensive treatment with chemotherapy, radiation, and surgery is essential for optimal outcome.[1] Therefore, many patients experience severe complications secondary to toxicities. Childhood cancer patients frequently require the use of supportive modalities in the intensive care unit (ICU); however, the role of extracorporeal membrane oxygenation (ECMO) in these patients has not been extensively assessed. We present a case of ARMS with unique complications of chemotherapy that prompted consideration of the use of ECMO.
RESULTS
This is the case of an 8-year-old male who presented initially with pancytopenia. Bone marrow (BM) biopsy revealed replacement of hematopoietic elements with atypical cells, which stained positive for myogenin and desmin (Fig. 1). Fluorescence in situ hybridization revealed FOXO1 rearrangement, with t(2;13)(q36;q14) on karyotypic analysis, characteristic of ARMS. 18Fluoro-2-deoxyglucose positron emission tomography (FDG-PET) demonstrated a soft tissue lesion posterior to the L5 vertebral body, with the uptake of FDG diffusely in the BM and no apparent lung metastases (Fig. 2).
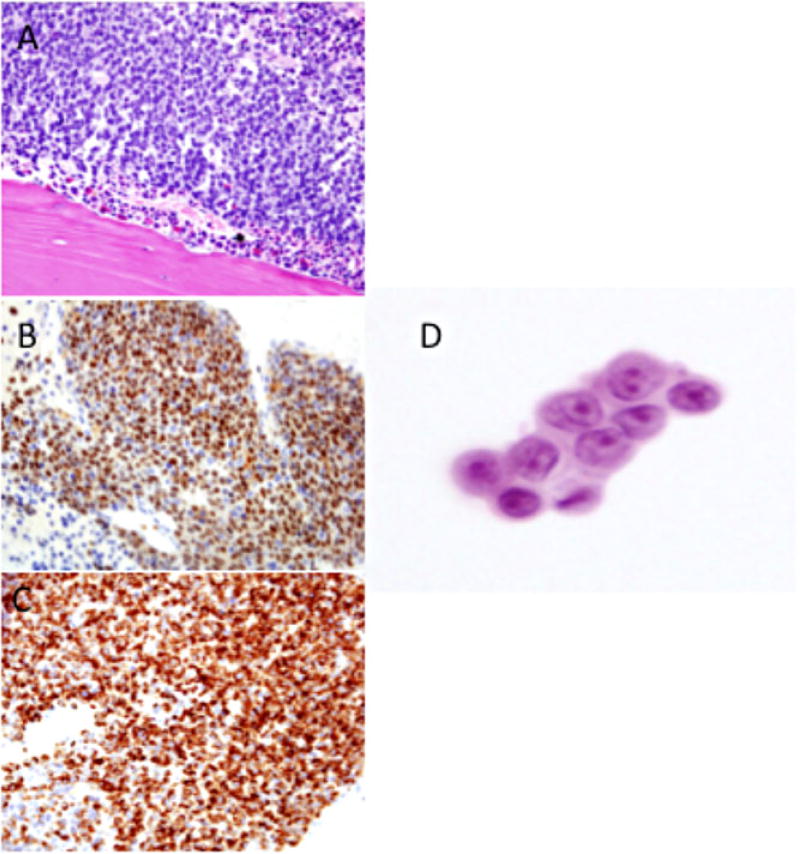
Fig. 1.
Bone marrow biopsy demonstrating replacement of hematopoietic elements with (A) atypical cells that stain positive for (B) myogenin and (C) desmin. (D) Samples from tracheal aspirates and chest tube output were notable for small, round cells with atypical nuclei.
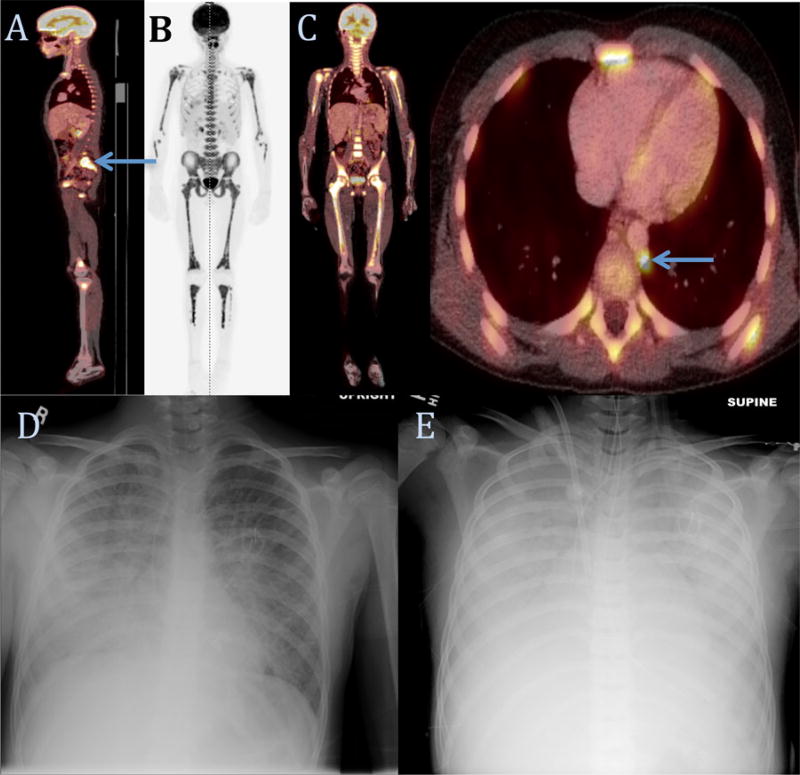
Fig. 2.
FDG-PET imaging (A) demonstrated right-sided enhancing paravertebral lesion. Bone scan (B) and FDG-PET (C) concerning for diffuse bone marrow and paraaortic lymph node uptake. Chest films on initial presentation (D) and after cannulation onto ECMO (E), which demonstrate progression of bilateral hazy opacities to near complete opacification of bilateral lung fields.
Chemotherapy with vincristine and irinotecan were given on therapy day 1, and irinotecan alone on day 2. On day 3, nausea and dyspnea developed. Emergent evaluation revealed an ill appearing dyspneic child. Serum laboratory values were consistent with tumor lysis syndrome (TLS): urate 14.5 mg/dl, lactate dehydrogenase 24,740 units/l, creatinine 1.1 mg/dl, phosphorous 8.9 mg/dl, potassium 4.75 mmol/l, and lactate 6.9 mmol/l. He received a dose of recombinant urate oxidase. Chest X-ray revealed bilateral lung field opacities. Noninvasive ventilation was initiated and the patient was transferred to the ICU.
Over the subsequent 16 hr, worsening hypoxia and hypercarbia necessitated intubation. Repeat chest X-ray showed worsening pleural effusions (Fig. 2), and chest tubes were placed. Pulse-less ventricular tachycardia occurred during a prolonged hypoxemic episode. Spontaneous return of circulation was achieved after 2 minutes of resuscitation. Given that the patient had single organ failure and the possibility of prolongation of life with chemotherapy, the decision was made to initiate ECMO. Cannulation to veno-venous (VV) ECMO was complicated by multiple cardiac arrests requiring cardiopulmonary resuscitation. While on VV-ECMO, there continued to be profound hypotension and an echocardiogram demonstrated right ventricular strain concerning for pulmonary hypertension. This prompted cannulation to veno-arterial (VA) ECMO in order to completely bypass the cardiopulmonary circuit and provide improved arterial perfusion pressures. Acute kidney injury and anuria developed requiring hemodialysis. After 48 hr, pulmonary compliance improved and there was markedly improved lung aeration on X-ray. Samples from chest tube and tracheal aspirates were sent for pathology review, with atypical cells not consistent with lung parenchyma discovered (Fig. 1). After a second dose of recombinant urate oxidase, urate levels remained in normal range.
Due to concern for possible recurrence of pulmonary dysfunction with further chemotherapy, the patient remained on VA-ECMO during resumption of irinotecan. VA-ECMO was discontinued after 5 days. The patient was successfully extubated 2 weeks after initial presentation and hemodialysis was discontinued. At the time of this report, he is in week 30 of treatment for ARMS, without apparent neurologic sequelae and no further unanticipated complications. Disease reassessment at treatment week 15 showed no evidence of disease on FDG-PET or within the BM.
DISCUSSION
This patient experienced acute respiratory failure with profuse pulmonary edema and TLS immediately after initiation of chemotherapy for metastatic ARMS. This was an unanticipated complication as chemotherapy for this disease is typically well tolerated. Diffuse BM involvement and TLS have been reported in metastatic ARMS.[2] Indeed, this tumor has been reported to occasionally behave in a “leukemic” fashion.[3] However, the degree of TLS observed in the current case following two doses of chemotherapy is rare even among rapidly mitotic hematologic malignancies. We speculate this tumor had a rapid rate of mitosis, given the evidence of widespread, synchronized cell death that occurred with initiation of chemotherapy. Additionally, diffuse BM distribution may have allowed rapid access of chemotherapy drugs to tumor cells, with cell death kinetics different from that which would occur in the setting of a predominantly solid tumor.
The cause of massive pulmonary edema that occurred upon initiation of chemotherapy is unclear, as this is not a hallmark of TLS. Local effect of vincristine and irinotecan on undetected ARMS within the lungs is one possible explanation. Although initial staging evaluation did not identify pulmonary disease on PET/CT scan, atypical cells consistent with ARMS were recovered from pleural samples. It is conceivable that death of miliary pulmonary metastatic disease resulted in local lung tissue disruption leading to interstitial edema. Idiosyncratic reaction to the specific chemotherapy agents was also considered, but the drugs were tolerated on repeated administration both while on ECMO and subsequently.
When to offer ECMO in patients with cancer is controversial and there are no standardized criteria. Decision makers must balance factors including disease prognosis, reversibility of acute illness, morbidity associated with treatments, and appropriate use of resources.[4,5] ECMO use in pediatric oncology patients has been reported in the setting of high-dose chemotherapy with autologous stem cell rescue,[6] cardiopulmonary arrest due to TLS,[7] and with mediastinal masses at risk of triggering respiratory or cardiovascular collapse.[8]
A meta-analysis of adult patients who received ECMO reported an overall in-hospital mortality of 54%,[9] but pediatric ECMO patients have lower reported in-hospital mortality rates ranging from 27 to 43%.[10,11] Poorer outcomes have been reported in ECMO patients with cancer. Adult patients with cancer receiving ECMO have reported in-hospital mortality rates of 70%.[12] Similarly, retrospective studies of pediatric ECMO patients have demonstrated higher mortality rates in patients who are immunosuppressed and/or on chemotherapy (65–70%) compared to other pediatric patients (~43%).[11, 13–15] A report on pediatric stem cell transplant recipients who received ECMO similarly had increased mortality rate of 79%.[16] Pediatric oncology patients on ECMO also experienced increased rates of hemorrhagic, cardiovascular, and renal complications as compared to nononcologic patients. Despite concerns for short- and long-term mortality/complications, 95% of centers indicated they would consider use of ECMO in pediatric malignancy.[15] Critically ill oncology patients with an optimistic medium-term disease prognosis, few comorbidities, and high potential for reversibility of the acute condition should be considered candidates for a trial of ECMO therapy. The 5-year survival of metastatic ARMS is approximately 20%, but approximately 65% of such patients are alive at 2 years with modern treatment.[17] Therefore, in our case, it was decided that the potential reversibility of single organ failure and the possibility of life prolongation with chemotherapy outweighed the risks of ECMO.
In summary, acute pulmonary edema developed following chemotherapy initiation in this patient with metastatic ARMS. This is a previously unreported complication of ARMS treatment that may have been related to undetected pulmonary metastatic disease. The severe TLS in this patient with BM involvement of ARMS should prompt consideration of inpatient monitoring during initiation of chemotherapy in such patients.
Uncertain or poor long-term prognosis for pediatric oncology patients has led to ambiguity regarding when to offer ECMO. Additionally, critically ill pediatric oncology patients on ECMO have higher mortality and complication rates than nononcology patients. How practitioners assess prognosis, individual disease characteristics, prolongation of life, patient/parent wishes, and reversibility of acute illness remains an evolving topic worthy of further investigation. Our case indicates that careful selection can lead to successful outcomes.
Abbreviations
- ARMS
alveolar rhabdomyosarcoma
- BM
bone marrow
- ECMO
extracorporeal membrane oxygenation
- FDG-PET
18Fluoro-2-deoxyglucose positron emission tomography
- ICU
intensive care unit
- TLS
tumor lysis syndrome
- VA
veno-arterial
- VV
veno-venous
Footnotes
Conflict of interest: The authors deny any affiliations to their knowledge that have a direct interest, particularly a financial, in the subject matter discussed.
References
- 1.Hawkins DA, Gupta AA, Rudzinski E. What’s new in the biology and treatment of pediatric rhabdomyosarcoma? Curr Opin Pediatr. 2014;26:50–56. doi: 10.1097/MOP.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bien E, Maciejka-Kapuscinska L, Niedzwiecki M, Stefanowicz J, Szolkiewicz A, Krawczyk M, Maldyk J, Izycka-Swieszewska E, Tokarska B, Balcerska A. Childhood rhabdomyosarcoma metastatic to bone marrow presenting with disseminated intravascular coagulation and acute tumour lysis syndrome: Review of the literature apropos of two cases. Clin Exp Metastasis. 2010;27:399–407. doi: 10.1007/s10585-010-9335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shinkoda Y, Nagatoshi Y, Fukano R, Nishiyama K, Okamura J. Rhabdomyosarcoma masquerading as acute leukemia. Pediatr Blood Cancer. 2009;52:286–287. doi: 10.1002/pbc.21783. [DOI] [PubMed] [Google Scholar]
- 4.Custer JR. The evolution of patient selection criteria and indications for extracorporeal life support in pediatric cardiopulmonary failure. Organogenesis. 2022;7:13–22. doi: 10.4161/org.7.1.14024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lantos JD, Frader J. Extracorporeal membrane oxygenation and the ethics of clinical research in pediatrics. N Engl J Med. 1990;323:409–413. doi: 10.1056/NEJM199008093230610. [DOI] [PubMed] [Google Scholar]
- 6.Wolfson RK, Kahana MD, Nachman JB, Lantos J. Extracorporeal membrane oxygenation after stem cell transplant: Clinical decision-making in the absence of evidence. Pediatr Crit Care Med. 2005;6:200–203. doi: 10.1097/01.PCC.0000155635.02240.9C. [DOI] [PubMed] [Google Scholar]
- 7.Huang M, Owen E, Myers S, Raj A. Cardiopulmonary failure requiring ECMO bypass resulting from leukemia cell lysis in a patient with childhood acute myelomonocytic leukemia. Case Rep Hematol. 2015;2015:640528. doi: 10.1155/2015/640528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wickiser JE, Thompson M, Leavey PJ, Quinn CT, Garcia NM, Aquino VM. Extracorporeal membrane oxygenation (ECMO) initiation without intubation in two children with mediastinal malignancy. Pediatr Blood Cancer. 2007;49:751–754. doi: 10.1002/pbc.20741. [DOI] [PubMed] [Google Scholar]
- 9.Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, Patroniti N, Antonelli M, Pesenti A, Pappalardo F. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15:172–178. [PubMed] [Google Scholar]
- 10.Gray BW, Haft JW, Hirsch JC, Annich GM, Hirschl RB, Bartlett RH. Extracorporeal life support: Experience with 2,000 patients. ASAIO J. 2015;61:2–7. doi: 10.1097/MAT.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. 2011;39:364–370. doi: 10.1097/CCM.0b013e3181fb7b35. [DOI] [PubMed] [Google Scholar]
- 12.Gow KW, Lao OB, Leong T, Fortenberry JD. Extracorporeal life support for adults with malignancy and respiratory or cardiac failure: The extracorporeal life support experience. Am J Surg. 2010;199:669–675. doi: 10.1016/j.amjsurg.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Masiakos PT, Islam S, Doody DP, Schnitzer JJ, Ryan DP. Extracorporeal membrane oxygenation for non-neonatal acute respiratory failure. Arch Surg. 1999;134:375–380. doi: 10.1001/archsurg.134.4.375. [DOI] [PubMed] [Google Scholar]
- 14.Gupta M, Shanley TP, Moler F. Extracorporeal life support for severe respiratory failure in children with immune compromised conditions. Pediatr Crit Care Med. 2008;9:380–385. doi: 10.1097/PCC.0b013e318172d54d. [DOI] [PubMed] [Google Scholar]
- 15.Gow KW, Wulkan ML, Heiss KF, Haight AE, Heard ML, Rycus P, Fortenberry JD. Extracorporeal membrane oxygenation for support of children after hematopoietic stem cell transplantation: The extracorporeal life support organization experience. J Pediatr Surg. 2006;41:662–667. doi: 10.1016/j.jpedsurg.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Gow KW, Heiss KF, Wulkan ML, Katzenstein HM, Rosenberg ES, Heard ML, Rycus PT, Fortenberry JD. Extracorporeal life support for support of children with malignancy and respiratory or cardiac failure: The extracorporeal life support experience. Am J Surg. 2010;199:669–675. doi: 10.1016/j.amjsurg.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Rodeberg DA, Garcia-Henriquez N, Lyden ER, Davicioni E, Parham DM, Skapek SX, Hayes-Jordan AA, Donaldson SS, Brown KL, Triche TJ, Meyer WH, Hawkins DS. Prognostic significance and tumor biology of regional lymph node disease in patients with rhabdomyosarcoma: A report from the Children’s Oncology Group. J Clin Oncol. 2011;29:1304–1311. doi: 10.1200/JCO.2010.29.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]