Abstract
Objective
To evaluate trends in the incidence of kidney stones and characteristics associated with changes in the incidence rate over 3 decades.
Patients and methods
Adult stone formers in Olmsted County, Minnesota from January 1, 1984 to December 31, 2012 were validated and characterized by age, sex, stone composition, and imaging modality. The incidence of kidney stones per 100,000 person-years was estimated. Characteristics associated with changes in the incidence rate over time were assessed using Poisson regression models.
Results
There were 3224 confirmed symptomatic (stone seen), 606 suspected symptomatic (no seen stone), and 617 incidental asymptomatic kidney stone formers. The incidence of confirmed symptomatic kidney stone formers increased from 1984 to 2012 in men (145 to 299/100,000 person-years; incidence rate ratio per 5 years: 1.14, P<.001) and in women (51 to 217/100,000 person-years; incidence rate ratio per 5 years: 1.29, P<.001). Overall, the incidence of suspected symptomatic kidney stones did not change, but asymptomatic kidney stones increased. Utilization of computed tomography for confirmed symptomatic stones increased from 1.8% in 1984 to 77% in 2012; there was a corresponding higher increased incidence of symptomatic small stones (≤3mm) than larger stones (>3mm). Confirmed symptomatic kidney stones with documented spontaneous passage also increased. The incidence of kidney stones with unknown composition increased more than stones with known composition.
Conclusion
The incidence of both symptomatic and asymptomatic kidney stones has increased dramatically. The increased utilization of computed tomography during this period may also have improved stone detection and contributed to the increased kidney stone incidence.
Keywords: Kidney stones, incidence, population-based
Introduction
Kidney stones are a common, painful condition responsible for substantial health problems and economic costs to society. In addition to painful recurrence, kidney stone disease is a risk factor for bone fracture,1 cardiovascular disease,2–4 and chronic kidney disease.5, 6 Increasing evidence suggests that the incidence and prevalence of kidney stones is steadily increasing across the world,7, 8 especially among adolescence9, 10 and women.11–15 The factors responsible for the increased burden of kidney stones in the general population have not yet been identified. Prior studies have relied on diagnostic codes or survey questions to identify stone formers, and thus lack chart validation and clinical details, including how stones were diagnosed or whether they were causing symptoms. Granular details lacking in previous studies include stone composition, size, and location have also been lacking in prior studies.
Validation of stone formers is needed to clarify whether changes in the incidence of kidney stones are due to diagnostic factors (such as better detection of stones with improvements in imaging technology) or a true increase in stones. Identifying the type of stone formers associated with the highest increase in stone incidence could also provide insights into the underlying factors leading to an increase in incidence of kidney stones. Thus, we performed a population-based study of incident (first-time) stone formers in Olmsted County from 1984 to 2012. Our objectives were to describe trends in the incidence of kidney and bladder stones and identify any characteristics of their presentation that have changed over this time period.
Methods
Study sample
After institutional review board approval, first-time kidney or bladder stone formers who were residents of Olmsted County, Minnesota and who first presented for medical care (office, emergency room, or hospital) from January 1, 1984 to December 31, 2012 were identified using International Classification of Disease (ICD)-9 codes 592, 594, and 274.11 and the infrastructure of the Rochester Epidemiology Project,16, 17 as previously detailed.18 The comprehensive medical records of newly coded stone formers were reviewed in a random order by trained abstractors. These coded stone formers were categorized into four mutually exclusive groups in the following order. First, confirmed symptomatic kidney stone formers who were defined by the presence of both symptoms (pain or gross hematuria) and a documented stone (seen after being voided or seen on imaging to be obstructing the ureter). Second, suspected kidney stone formers who had characteristic symptoms (pain or gross hematuria) that were clinically attributed to a stone, but confirmation was lacking (i.e., imaging was deferred and the patient did not report actually seeing a voided a stone). Third, asymptomatic stone formers had a non-obstructing kidney stone detected incidentally on an imaging study done for non-stone-related purposes. Fourth, bladder stone formers only had stones in the bladder as documented by cystoscopy or imaging. Stone formers were excluded if their first stone event was prior to 1984 or to migration into Olmsted County, if they were less than 18 years of age at their first stone event, or they had no stone but some other diagnosis for their symptoms on chart review (such as musculoskeletal back pain).
Stone disease characteristics
Clinical characteristics were detailed only for the confirmed symptomatic kidney stone formers based on medical records at the time of the first stone event, including use of computed tomography (CT) for diagnosis. Diameter of the symptomatic stone was determined from radiology reports. If diameter was not reported, the radiographic images were re-examined to determine stone diameter (longest axis). If radiographic images were not available for review, the stone was considered ≤3 mm if described as “tiny” or “very small” on the report. Location of a symptomatic stone on imaging was classified as renal pelvis or lower pole, ureteropelvic junction, ureter, and ureterovesical junction. Infected stones were identified by a urinary tract infection attributed to the kidney stone with a concurrent urine pH >7.0, or if struvite was confirmed on stone analysis. Stone composition by infrared spectroscopy (if available) was categorized into mutually exclusive groups of majority calcium oxalate monohydrate (COM), majority calcium oxalate dihydrate (COD), majority hydroxyapatite, any uric acid, any brushite, or any struvite as previously described.18 We identified whether the stone event was reported to have resolved with a voided stone seen after spontaneous passage.
Statistical analyses
Incidence rates were determined by the first episode and not by any recurrent episodes. Age and sex-specific incidence rates (per 100,000 person years of risk) were calculated for each year by dividing the number of stone formers (corrected for the sampling fraction) by the estimated Olmsted County adult population as determined by the United States decennial censuses (corrected for the sampling fraction).19, 20 These rates were then standardized to the age-sex-distribution for the 2010 US census. Incidence rates for confirmed symptomatic kidney stones, suspected symptomatic kidney stones, asymptomatic kidney stones, and bladder stones were assessed overall and by sex. Incidence rates in confirmed symptomatic stone formers were also assessed in subgroups of age (18–39, 40–59, ≥60 years), stone composition, CT scan use, stone diameter ≤ 3mm, stone location on imaging, voided stone seen after spontaneous passage, and infected stones. Poisson regression was used to estimate the change in incidence rates over time. The relative change in incidence rates were reported as incidence rate ratios (IRR) per 5 years and were used for all statistical comparisons. Incidence rates in 1984 and in 2012 were also calculated based on the regression models. This allowed estimation of the absolute change in incidence rate between these two time points. Loess smoother plots with a span of 0.7 were used to graphically display changes in the incidence rate over time. Comparisons between incident stone former types were evaluated using the χ2 test for categorical variables and the ANOVA F test for continuous variables. Statistical analysis was performed using SAS®,version 9.4 and R 3.2.3.
Results
Characteristics of kidney stone formers
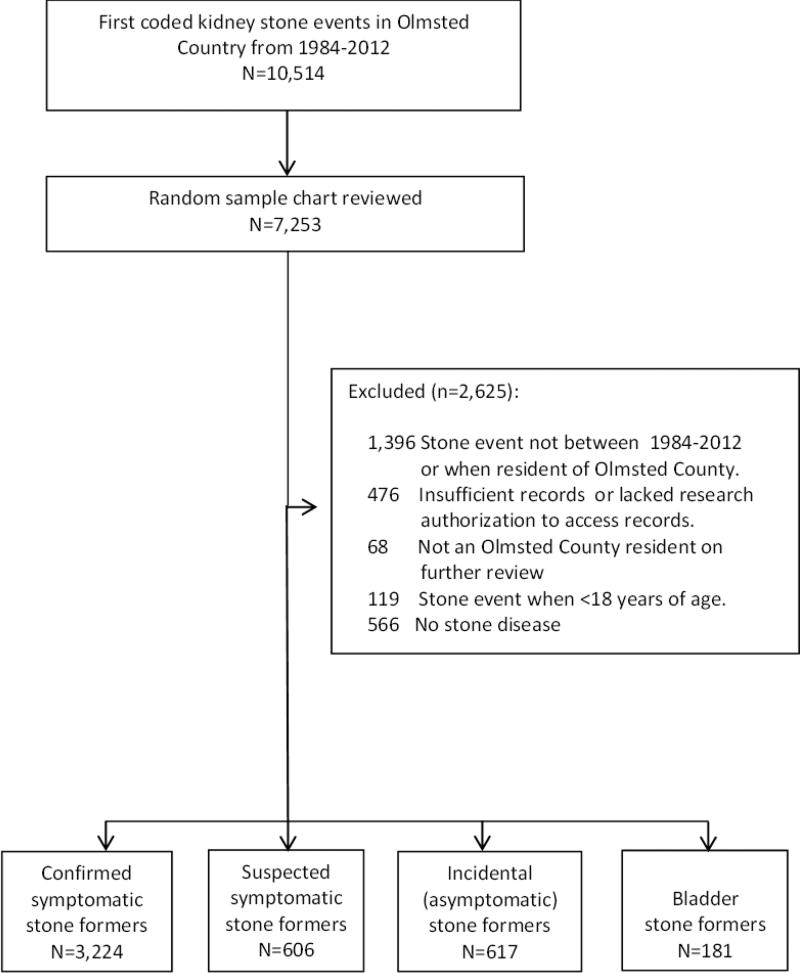
From 1984 to 2012, 10,514 adults residing in Olmsted County first received ICD-9 codes for kidney or bladder stones. A random sample of 7,253 charts were manually reviewed (Figure 1). There were 3,224 confirmed symptomatic kidney stone events, 606 suspected symptomatic kidney stone events, 617 asymptomatic kidney stones incidentally detected, and 181 bladder stones. Table 1 contains detailed characteristics of these incident stone formers. Confirmed symptomatic stone formers and suspected symptomatic stone formers had similar characteristics. Asymptomatic stone formers were older and more likely to be female, while bladder stone formers were much older and mostly men (P<.001 for all).
Figure 1.
Validation and classification of stone formers. A random sample (due to funding and time constraints) of first-time coded stone formers underwent a detailed chart review. Stone formers were categorized according to the following hierarchy: confirmed symptomatic stone formers, suspected symptomatic stone formers, asymptomatic stone formers, and bladder only stone formers.
Table 1.
Characteristics of confirmed incident stone formers in Olmsted County by type, 1984–2012
| Incident Stone Former Type | |||||
|---|---|---|---|---|---|
|
|
|||||
| Patient Characteristics | Confirmed Symptomatic Kidney (N=3,224) |
Suspected Symptomatic Kidney (N=606) |
Asymptomatic Kidney (N=617) |
Bladder (n=181) |
P-value |
| Age, y | 43.62 (15.34) | 41.47 (15.44) | 54.16 (17.54) | 67.62 (17.45) | <.001 |
| Male | 1958 (60.7%) | 311 (51.3%) | 285 (46.2%) | 154 (85.1%) | <.001 |
| Race | <.001 | ||||
| White | 2837 (88.0%) | 502 (82.8%) | 539 (87.4%) | 150 (82.9%) | |
| Non-white | 189 (5.9%) | 37 (6.1%) | 40 (6.5%) | 5 (2.8%) | |
| Unknown | 198 (6.1%) | 67 (11.1%) | 38 (6.2%) | 26 (14.4%) | |
| CT Scan at stone event | 1508 (45.7%) | n/a | n/a | n/a | - |
| Stone location on imaging | |||||
| Renal pelvis or lower pole | 276 (9%) | n/a | n/a | n/a | - |
| Ureteropelvic junction | 179 (6%) | n/a | n/a | n/a | - |
| Ureter | 1020 (32%) | n/a | n/a | n/a | - |
| Ureterovesicular junction | 879 (27%) | n/a | n/a | n/a | - |
| Stone Composition at stone event | |||||
| Unknown | 1626 (50.4%) | n/a | n/a | n/a | - |
| Known | 1,598 (49.6%) | ||||
| Calcium oxalate monohydrate | 1019 (63.8%) | n/a | n/a | n/a | - |
| Calcium oxalate dihydrate | 177 (11.1%) | n/a | n/a | n/a | - |
| Hydroxyapatite | 295 (18.5%) | n/a | n/a | n/a | - |
| Uric acid | 78 (4.9%) | n/a | n/a | n/a | - |
| Othera | 29 (1.8%) | n/a | n/a | n/a | - |
Values are mean (SD) or no. of patients (%). Comparisons were evaluated using the χ2 test for categorical variables and the ANOVA F test for continuous variables.
Other stone composition includes Brushite (n=11), Struvite (n=15), or Cystine (n=3) stones
Trends in stone incidence
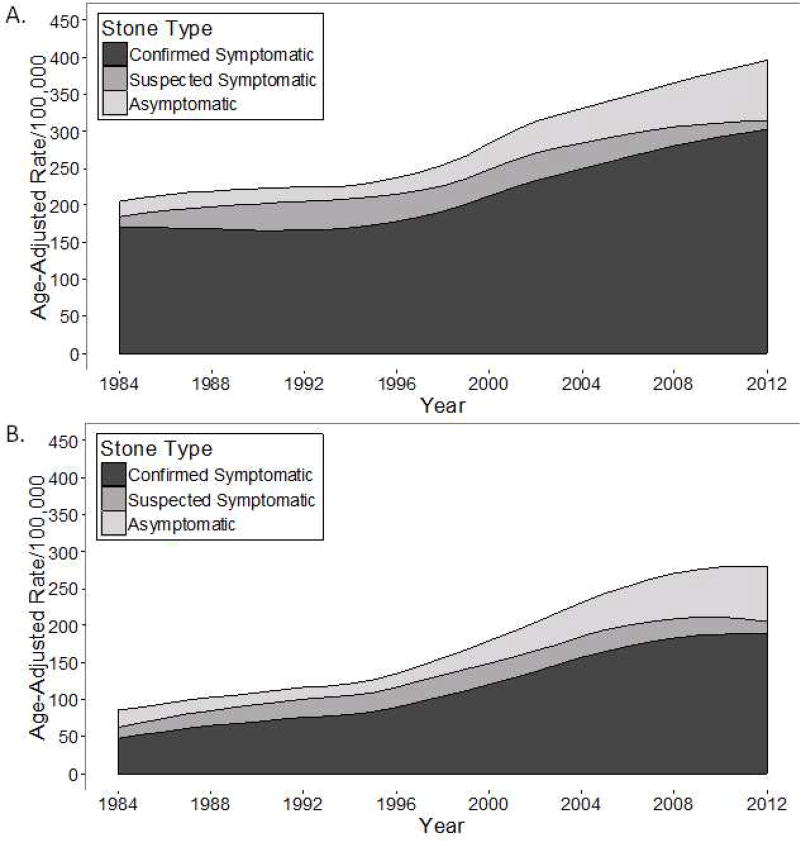
Figure 2A, B and Table 2 show trends in the age and sex-adjusted incidence rate from 1984 to 2012 for stone formers. For confirmed symptomatic kidney stone formers, the age-adjusted increase in incidence rate per 5 years was higher in women than men (IRR: 1.29 vs. 1.14, P<.001 for interaction). Because men started at a higher incidence rate in 1984, the absolute increase in incidence rate by 2012 was similar (154/100,000 person years in men and 166/100,000 person years in women). In men, the relative increase in incidence rate per 5 years was lower and then higher for ages 18–39 y to 40–59 y to ≥60 y (IRR: 1.19 vs. 1.11 vs. 1.15, P<.001 for group level interaction) In women the relative increase in incidence rate per 5 years was higher and then lower for ages 18–39 y to 40–59 y to ≥60 y (1.28 vs. 1.33 vs. 1.26, P=.003 for group level interaction). Because women ages ≥60 y started at a lower incidence rate in 1984, the absolute increase in incidence rate by 2012 was higher for ages 18–39 y (190/100,000 person years) and for ages 40–59 y (180/100,000 person years) than for ages ≥60 y (107/100,000 person years). The incidence of suspected symptomatic kidney stones was stable over time, while asymptomatic kidney stones increased over. Bladder stones were relatively rare, mostly seen in men, and the incidence decreased over time (Supplemental Figure 1).
Figure 2.
A and B. Trends in the incidence of kidney stone formers (confirmed symptomatic, suspected symptomatic, and asymptomatic) from 1984 to 2012 in Olmsted County, Minnesota among A) men and B) women.
Table 2.
Incidence rates of kidney and bladder stones in Olmsted County from 1984 to 2012, by type.
| Stone former type | IRR (95% CI) per 5- year change |
P | Estimated Incidence Rate (95% CI) in 1984a |
Estimated Incidence Rate (95% CI) in 2012a |
Absolute change in Incidence Rate from 1984–2012a |
|
|---|---|---|---|---|---|---|
| Confirmed symptomatic kidney stone | ||||||
|
| ||||||
| Overall Age-sex-adjustedb | 1.19 (1.17–1.21) | <.001 | 95 (89–102) | 254 (242–267) | 159 | |
| Men | Age-adjustedb | 1.14 (1.12–1.16) | <.001 | 145 (137–153) | 299 (286–313) | 154 |
| 18–39 years | 1.19 (1.16–1.21) | <.001 | 94 (88–100) | 243 (231–256) | 149 | |
| 40–59 years | 1.11 (1.09–1.12) | <.001 | 212 (202–221) | 375 (360–390) | 163 | |
| ≥60 years | 1.15 (1.13–1.16) | <.001 | 130 (123–137) | 277 (265–291) | 147 | |
| Women | Age-adjustedb | 1.29 (1.27–1.32) | <.001 | 51 (47–56) | 217 (205–230) | 166 |
| 18–39 yrs | 1.28 (1.26–1.31) | <.001 | 62 (58–67) | 252 (239–265) | 190 | |
| 40–59 yrs | 1.33 (1.30–1.36) | <.001 | 46 (42–50) | 226 (214–239) | 180 | |
| ≥60 yrs | 1.26 (1.22–1.29) | <.001 | 42 (38–46) | 149 (139–159) | 107 | |
|
| ||||||
| Suspected symptomatic kidney stone | ||||||
|
| ||||||
| Overall Age-sex-adjustedb | 1.00 (0.96–1.05) | .91 | 26 (23–30) | 26 (23–30) | 0 | |
| Men | Age-adjustedb | 0.96 (0.93–1.003) | .07 | 33 (29–37) | 27 (23–30) | −6 |
| Women | Age-adjustedb | 1.05 (1.002–1.10) | .04 | 21 (18–24) | 27 (23–31) | 6 |
|
| ||||||
| Asymptomatic kidney stone | ||||||
|
| ||||||
| Overall Age-sex-adjustedb | 1.36 (1.31–1.42) | <.001 | 12 (10–14) | 71 (64–79) | 59 | |
| Men | Age-adjustedb | 1.34 (1.29–1.39) | <.001 | 14 (12–16) | 73 (66–80) | 58 |
| Women | Age-adjustedb | 1.40 (1.34–1.45) | <.001 | 11 (9–13) | 73 (66–81) | 62 |
|
| ||||||
| Bladder stone | ||||||
|
| ||||||
| Overall Age-sex-adjustedb | 0.94 (0.87–1.00) | .06 | 11 (9–14) | 8 (6–10) | −3 | |
| Men | Age-adjustedb | 0.95 (0.91–1.00) | .06 | 22 (18–25) | 16 (14–20) | −6 |
| Women | Age-adjustedb | 0.86 (0.74–0.99) | .03 | 3 (2–5) | 1 (0–2) | −2 |
Incidence rate per 100,000 person years
Adjusted rates calculated using the age and sex distribution of the US 2010 census
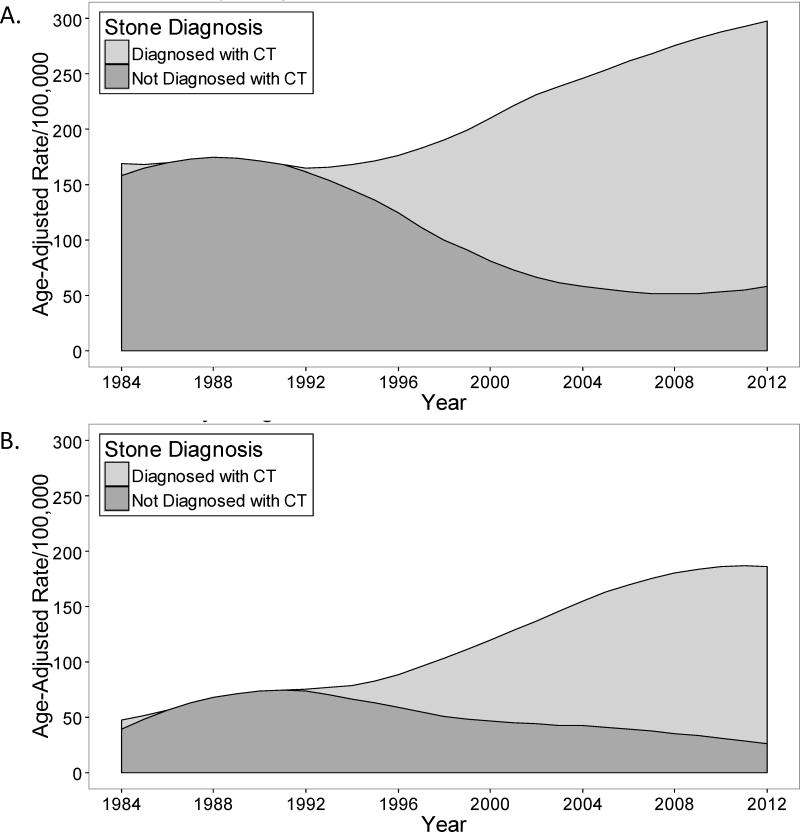
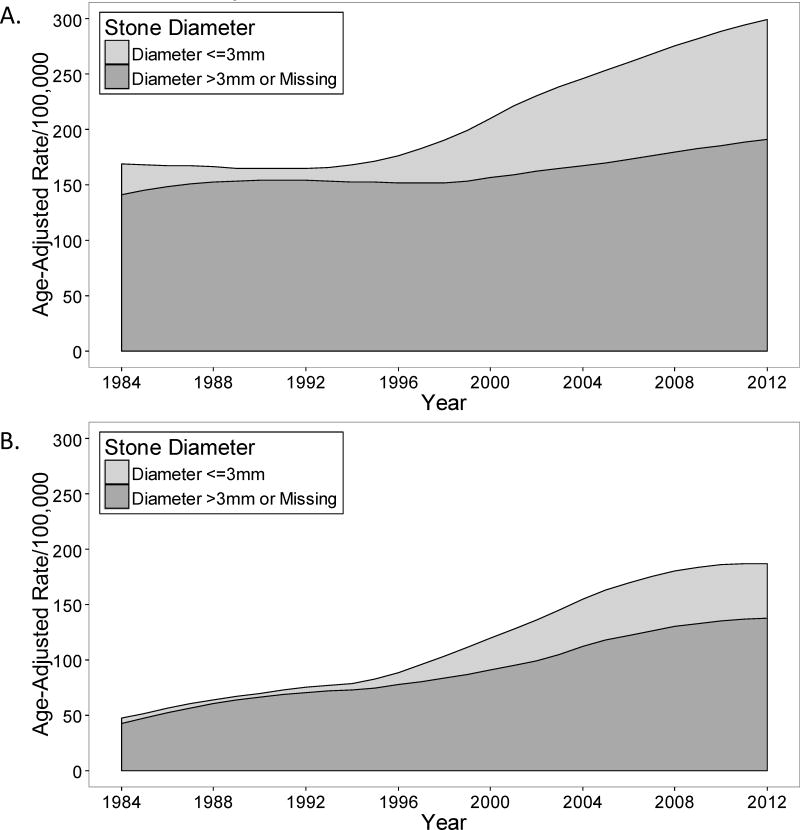
Trends in incidence of confirmed symptomatic kidney stone subgroups
Table 3 contains trends for age and sex adjusted incidence rates of subgroups of confirmed symptomatic kidney stone formers. CT was utilized to diagnose symptomatic kidney stones for 1.8% (95% CI: 0.0–5.2%) in 1984, but increased to 77% (95%CI: 69–85%) by 2012 (Figure 2C and D). A stone ≤3 mm was detected in only 8% when CT was not utilized, but in 38% when CT was utilized (P<.001). The relative increase in incidence rate per 5 years was higher for symptomatic kidney stones ≤3 mm than >3mm (IRR: 1.58 vs 1.11, P<.001 for interaction) (Figure 2E and F). The incidence rates for all symptomatic stone locations on imaging showed a relative increase over time. With a higher incidence rate in 1984, the absolute increase in incidence rate was largest with the ureter and ureterovesical junction stone locations by 2012. The incidence rate of a seen voided stone (spontaneous passage) increased over time. Infected stones were more common in women than men, and also increased over this time period (Supplemental Figure 2).
Table 3.
Incidence rates of confirmed symptomatic kidney stones in Olmsted County from 1984 to 2012, by subgroups
| Confirmed stone former subgroup | IRRa (95% CI) (per 5-year change) |
P | Estimated Incidence Rate (95% CI) in 1984b |
Estimated Incidence Rate (95% CI) in 2012b |
Absolute change in Incidence Rate from 1984–2012b |
|---|---|---|---|---|---|
| Stone Diameter | |||||
| ≤3mm | 1.58 (1.52–1.65) | <.001 | 7 (6–9) | 99 (90–109) | 92 |
| >3mm or unknown | 1.11 (1.08–1.13) | <.001 | 92 (86–99) | 162 (152–172) | 70 |
| Stone location on imaging | |||||
| Pelvic/Lower pole | 1.26 (1.19–1.34) | <.001 | 6 (5–8) | 24 (20–28) | 18 |
| Ureteropelvic junction | 1.47 (1.35–1.60) | <.001 | 2 (1–3) | 21 (17–25) | 19 |
| Ureterovesical junction | 1.29 (1.25–1.34) | <.001 | 19 (17–22) | 83 (76–91) | 64 |
| Ureter | 1.24 (1.20–1.28) | <.001 | 26 (23–30) | 90 (82–98) | 64 |
| Voided stone seen | 1.08 (1.06–1.11) | <.001 | 54 (49–59) | 85 (78–92) | 31 |
| Infected stones | 1.15 (1.03–1.28) | .01 | 2 (1–4) | 6 (4–8) | 4 |
| Stone Composition | |||||
| Unknown | 1.28 (1.25–1.31) | <.001 | 37 (34–41) | 149 (139–159) | 112 |
| COM | 1.11 (1.07–1.14) | <.001 | 38 (34–42) | 67 (61–73) | 29 |
| COD | 0.96 (0.89–1.04) | .33 | 8 (6–10) | 6 (4–8) | −2 |
| Hydroxyapatite | 1.24 (1.16–1.32) | <.001 | 6 (5–8) | 21 (18–25) | 15 |
| Uric Acid | 0.94 (0.84–1.05) | .24 | 4 (3–6) | 3 (2–4) | −1 |
Adjusted rates calculated using the age and sex distribution of the US 2010 census
Incidence rate per
Stone composition was obtained in 50% of confirmed symptomatic kidney stone formers. COM was the most common composition (64%), followed by hydroxyapatite (19%), COD (11%), and uric acid (5%) (see Table 1). Other stone compositions were too rare to be meaningfully studied. Table 3 shows the trends in the incidence for different stone compositions. The incidence rate showed a relative increase over time for kidney stones of unknown composition compared to known composition (IRR: 1.28 vs 1.10, P<.001 for interaction). Among stones with known composition, COM and hydroxyapatite stone incidence rates increased over time, whereas COD and uric acid stone incidence rates did not. The absolute increase in incidence rate was largest with stones of unknown composition.
Discussion
In this population based study, the incidence of kidney stones increased dramatically in both adult men and women from 1984 to 2012, with the largest absolute increase occurring in younger women. The incidence of suspected symptomatic kidney stones (diagnosed with symptoms and without imaging) did not increase but the incidence of asymptomatic kidney stones (diagnosed with imaging and without symptoms) did increase. The use of CT to diagnose stones increased substantially over the study period and may have increased detection of both symptomatic and asymptomatic kidney stones. The confirmed symptomatic kidney stones whose absolute change increased the most were characterized by small stones (≥3 mm), stones of unknown composition, and stones obstructing at the ureter or ureterovesical junction. These stones of unknown composition are often small distal ureteral stones (difficult to detect or distinguish from phleboliths with non-CT imaging) that spontaneously pass18 and are less likely to be captured for composition analysis.
We previously reported that the incidence of confirmed symptomatic kidney stones has recently increased among children, particularly adolescent girls.10, 11 Prior code-based studies suggested that the incidence of stone disease was increasing more in women than men.11–15 We observed that women ages 18–39 y had the highest absolute increase in the incidence of confirmed symptomatic kidney stones. Suspected symptomatic kidney stones did increase slightly in women, but not in men. The largest sex differences were observed in bladder stones, with more occurring in men due to prostatic obstruction,21 and infection stones, occurring more often in women due to recurrent urinary tract infections.22 Both these type of stone were relatively uncommon.
Improved detection of symptomatic and asymptomatic stones with modern imaging modalities (including the increased utilization of CT scans) may have caused a detection bias that has contributed to an increase in the kidney stone incidence. Imaging technology such as ultrasound and plain radiography were more widely used historically, but are known to be inferior for detecting kidney stones compared to CT scan.23, 24 One hypothesis is that these stone missed without CT scan would previously have been suspected stones, but there was no proportional decline in suspected symptomatic stones with the increase confirmed symptomatic stones. Thus, past patients with small symptomatic kidney stones that resolved with spontaneous passage may have gone undiagnosed or had a non-specific diagnosis such as back or flank pain. Concerns regarding radiation from CT scans have prompted calls for more widespread use of ultrasound rather than CT to diagnose kidney stones.25,26 A trade-off may be that more small distal ureteral stones are likely to go undiagnosed, particularly if they are not causing hydronephrosis.
The two most common types of calcium stones (COM and hydroxyapatite) have increased over time. Hypercalciuria is a shared risk factor of both calcium oxalate and hydroxyapatite stones. Dietary components known to be associated with increase urinary calcium excretion such as high salt, high animal protein and sucrose intake27–29 are increasingly consumed.30–32 Insulin resistance, associated with the ever rising obesity epidemic, can also increase intestinal absorption and urinary excretion of calcium.33 Notably, hydroxyapatite stones are more common among young women,34 the demographic group with the highest increase in the incidence rate of kidney stones.
There are some potential limitations to our study. The population is predominantly white which may limit generalizability; whites are known to have a higher risk of kidney stones than other race groups in the United States.35 Incidence rates of kidney stones may vary regionally and be higher in regions with warmer weather than Minnesota. Urine chemistries and dietary surveys were not available for study as these are not routinely obtained among first time stone formers. Finally, it is difficult to separate out detection bias from true increases in kidney stone burden since there has been a progressive increase in the use of more accurate imaging modalities for diagnosing stones from 1984 to 2012.
Conclusion
An increase in the incidence of kidney stones has occurred in both adult men and women over the past three decades. The increase has been particularly notable in young adult women and with COM and hydroxyapatite stones. However, use of better imaging modalities (particularly CT imaging) over time may have caused a detection bias that has contributed to the perceived increase in the burden of kidney stone disease. In other words, we may be now diagnosing more symptomatic and asymptomatic kidney stones that would have gone undiagnosed in the past.
Supplementary Material
Figure 3.
Trends in the incidence of confirmed symptomatic kidney stone formers from 1984 to 2012 in Olmsted County, Minnesota among (A) men and (B) women diagnosed by CT scan (CT=computed tomography).
Figure 4.
Trends in the incidence of confirmed symptomatic kidney stone formers from 1984 to 2012 in Olmsted County, Minnesota among (A) men and (B) women with stone diameter of less than 3 mm.
Acknowledgments
Support and financial disclosure:
This project was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (Mayo Clinic O’Brien Urology Research Center, DK100227 and DK83007) and made possible by the Rochester Epidemiology Project (AG034676) from the National Institutes of Health, U.S. Public Health Service. The funding sources had no role in the study design, conduct, or reporting. CHM received research support from Siemens Healthcare unrelated to this study.
Acronyms
- ICD
International Classification of Disease
- CT
computed tomography
- COM
calcium oxalate monohydrate
- COD
calcium oxalate dihydrate
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary results of this work were presented at the scientific meeting of the American Society of Nephrology (Kidney Week) held November 15–20, 2016 in Chicago, Illinois.
Disclosure: None
References
- 1.Taylor EN, Feskanich D, Paik JM, Curhan GC. Nephrolithiasis and Risk of Incident Bone Fracture. J Urol. 2016;195:1482–1486. doi: 10.1016/j.juro.2015.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheungpasitporn W, Thongprayoon C, Mao MA, O'Corragain OA, Edmonds PJ, Erickson SB. The Risk of Coronary Heart Disease in Patients with Kidney Stones: A Systematic Review and Meta-analysis. N Am J Med Sci. 2014;6:580–585. doi: 10.4103/1947-2714.145477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferraro PM, Taylor EN, Eisner BH, et al. History of kidney stones and the risk of coronary heart disease. Jama. 2013;310:408–415. doi: 10.1001/jama.2013.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rule AD, Roger VL, Melton LJ, 3rd, et al. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. 2010;21:1641–1644. doi: 10.1681/ASN.2010030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rule AD, Bergstralh EJ, Melton LJ, 3rd, Li X, Weaver AL, Lieske JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:804–811. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Zoghby ZM, Lieske JC, Foley RN, et al. Urolithiasis and the risk of ESRD. Clinical journal of the American Society of Nephrology : CJASN. 2012;7:1409–1415. doi: 10.2215/CJN.03210312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12:e86–96. [PMC free article] [PubMed] [Google Scholar]
- 8.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 9.Clayton DB, Pope JC. The increasing pediatric stone disease problem. Ther Adv Urol. 2011;3:3–12. doi: 10.1177/1756287211400491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dwyer ME, Krambeck AE, Bergstralh EJ, Milliner DS, Lieske JC, Rule AD. Temporal trends in incidence of kidney stones among children: a 25-year population based study. The Journal of urology. 2012;188:247–252. doi: 10.1016/j.juro.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieske JC, Pena de la Vega LS, Slezak JM, et al. Renal stone epidemiology in Rochester, Minnesota: an update. Kidney Int. 2006;69:760–764. doi: 10.1038/sj.ki.5000150. [DOI] [PubMed] [Google Scholar]
- 12.Scales CD, Jr, Curtis LH, Norris RD, et al. Changing gender prevalence of stone disease. J Urol. 2007;177:979–982. doi: 10.1016/j.juro.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 13.Strope SA, Wolf JS, Jr, Hollenbeck BK. Changes in gender distribution of urinary stone disease. Urology. 2010;75:543–546. 546, e541. doi: 10.1016/j.urology.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tasian GE, Ross ME, Song L, et al. Annual Incidence of Nephrolithiasis among Children and Adults in South Carolina from 1997 to 2012. Clin J Am Soc Nephrol. 2016;11:488–496. doi: 10.2215/CJN.07610715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penniston KL, McLaren ID, Greenlee RT, Nakada SY. Urolithiasis in a rural Wisconsin population from 1992 to 2008: narrowing of the male-to-female ratio. J Urol. 2011;185:1731–1736. doi: 10.1016/j.juro.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clinic proceedings. Mayo Clinic. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh P, Enders FT, Vaughan LE, et al. Stone Composition Among First-Time Symptomatic Kidney Stone Formers in the Community. Mayo Clin Proc. 2015;90:1356–1365. doi: 10.1016/j.mayocp.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao CRMJ, Rao DC. Handbook of Statistics: Epidemiology and Medical Statistics. Jordan Hill, Oxford, UK: North-Hollands Publications; 2008. [Google Scholar]
- 20.Van den Broeck JBJ. Epidemiology: Principles and Practical Guidelines. New York City, NY, USA: Springer; 2013. [Google Scholar]
- 21.Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158:481–487. doi: 10.1016/s0022-5347(01)64508-7. [DOI] [PubMed] [Google Scholar]
- 22.Steensberg J, Bartels ED, Bay-Nielsen H, Fanoe E, Hede T. Epidemiology of urinary tract diseases in general practice. Br Med J. 1969;4:390–394. doi: 10.1136/bmj.4.5680.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Passerotti C, Chow JS, Silva A, et al. Ultrasound versus computerized tomography for evaluating urolithiasis. J Urol. 2009;182:1829–1834. doi: 10.1016/j.juro.2009.03.072. [DOI] [PubMed] [Google Scholar]
- 24.Ege G, Akman H, Kuzucu K, Yildiz S. Can computed tomography scout radiography replace plain film in the evaluation of patients with acute urinary tract colic? Acta Radiol. 2004;45:469–473. doi: 10.1080/02841850410005264. [DOI] [PubMed] [Google Scholar]
- 25.Brisbane W, Bailey MR, Sorensen MD. An overview of kidney stone imaging techniques. Nat Rev Urol. 2016;13:654–662. doi: 10.1038/nrurol.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100–1110. doi: 10.1056/NEJMoa1404446. [DOI] [PubMed] [Google Scholar]
- 27.Muldowney FP, Freaney R, Moloney MF. Importance of dietary sodium in the hypercalciuria syndrome. Kidney Int. 1982;22:292–296. doi: 10.1038/ki.1982.168. [DOI] [PubMed] [Google Scholar]
- 28.Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988;66:140–146. doi: 10.1210/jcem-66-1-140. [DOI] [PubMed] [Google Scholar]
- 29.Lemann J, Jr, Piering WF, Lennon EJ. Possible role of carbohydrate-induced calciuria in calcium oxalate kidney-stone formation. N Engl J Med. 1969;280:232–237. doi: 10.1056/NEJM196901302800502. [DOI] [PubMed] [Google Scholar]
- 30.Meyer KA, Harnack LJ, Luepker RV, Zhou X, Jacobs DR, Steffen LM. Twenty-two-year population trends in sodium and potassium consumption: the Minnesota Heart Survey. J Am Heart Assoc. 2013;2:e000478. doi: 10.1161/JAHA.113.000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr. 2011;14:575–583. doi: 10.1017/S1368980010002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79:774–779. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- 33.Rumenapf G, Schmidtler J, Schwille PO. Intestinal calcium absorption during hyperinsulinemic euglycemic glucose clamp in healthy humans. Calcif Tissue Int. 1990;46:73–79. doi: 10.1007/BF02556090. [DOI] [PubMed] [Google Scholar]
- 34.Singh P, Enders FT, Vaughan LE, et al. Stone Composition Among First-Time Symptomatic Kidney Stone Formers in the Community. Mayo Clin Proc. 2015;90:1356–1365. doi: 10.1016/j.mayocp.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prochaska ML, Taylor EN, Curhan GC. Insights Into Nephrolithiasis From the Nurses' Health Studies. Am J Public Health. 2016;106:1638–1643. doi: 10.2105/AJPH.2016.303319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.