Abstract
In 7 patients with chronic heart failure (CHF) and 6 controls we examined: a) resting and post-exercise muscle vascular endothelial growth factor (VEGF) mRNA levels, and b) their relationship with muscle structure and function. Muscle biopsies were taken after 30 min of single leg knee-extensor exercise at 50% of maximum work rate (50% WRmax) from both the exercised and rested legs. Muscle blood flow (Q. ) and O2 uptake V̇O2 were measured during exercise. Resting VEGF mRNA levels were not different between patients and controls and both groups upregulated VEGF mRNA equally in response to acute exercise. Patients had lower Q., V̇O2 and mitochondrial density, but similar capillarity and fiber area. These findings reveal a normal basal level of muscle VEGF mRNA, its appropriate upregulation in response to acute exercise and, despite increased vascular resistance during exercise, a normal skeletal muscle vascular structure in patients with CHF.
Keywords: CHF, muscle, acute exercise, VEGF, morphology
INTRODUCTION
Although chronic heart failure (CHF) is considered to be predominantly a centrally limiting disease, a variety of skeletal muscle alterations, including muscle atrophy, alterations in fiber type, reduced oxidative capacity, reduced mitochondrial enzymes, and decreased mitochondrial volume density, have all been associated with this pathology (Drexler et al. 1992; Harrington et al. 1997; Mancini et al. 1992; Massie et al. 1996). While at the peripheral vascular level, CHF has been associated with greater sympathetic vasoconstrictor tone (Magnusson et al. 1997), decreased capillary-to-fiber ratio, reduced capillary density and smaller capillary diameter (Duscha et al. 1999; LeJemtel et al. 1986; Sullivan et al. 1989). These findings indicate that in addition to the inability of the heart to appropriately increase its pumping capacity during exercise, the skeletal muscle and its vasculature may also contribute to the exercise intolerance associated with CHF.
As skeletal muscle O2 diffusional conductance is positively related to the number of capillaries per muscle fiber (Hepple et al. 2000), it is likely that angiogenesis plays an important role in the facilitation of O2 transport to skeletal muscle mitochondria and thereby may influence maximal muscle oxygen uptake (V̇O2MAX) (Hogan et al. 1989; Richardson et al. 1995; Richardson et al. 1994). Vascular endothelial growth factor (VEGF) plays a major role in the regulation of the angiogenic process (Leung et al. 1989; Wilting et al. 1993). Previous investigations have documented that VEGF mRNA is up-regulated in human skeletal muscle in response to a single bout of dynamic exercise (Gustafsson et al. 1999; Richardson et al. 1999) and this angiogenic signal is attenuated when exercise training improves capillarity and O2 diffusional conductance (Richardson et al. 2000). Thus, teleologically VEGF regulation seems to parallel normal skeletal muscle structure and function, but little is known about skeletal muscle VEGF and CHF.
Several studies have examined VEGF levels and angiogenesis in CHF, but only plasma VEGF levels have been studied with equivocal results (Abraham et al. 2000; Arakawa et al. 2003; Chin et al. 2002; De Boer et al. 2002). To our knowledge, only one investigation has examined muscle VEGF mRNA at rest both before and after exercise training, however this study lacked healthy controls, did not examine the response to an acute exercise challenge or the relationship to vascularity (Gustafsson et al. 2001). Therefore, the upregulation of VEGF mRNA in response to acute exercise and even basal levels of muscle VEGF mRNA in patients with CHF in relation to healthy controls remains unknown. Similar to the present investigation the previous work by Gustafsson et al. (Gustafsson et al. 2001) employed the single-leg knee-extensor (KE) exercise paradigm, which not only avoids overshadowing muscle function with cardiac limitation but allows muscle sampling of both the rested and exercised leg on the same occasion (Richardson et al. 2000).
Thus, the chief purpose of the present study was to test the following hypotheses: a) resting skeletal muscle VEGF mRNA levels will be reduced in CHF patients compared with controls and b) the upregulation of skeletal muscle VEGF mRNA associated with acute exercise will be attenuated in patients with CHF compared to controls. Additionally, this study was designed to examine the relationship between existing muscle structure and function and both resting VEGF mRNA levels and the VEGF mRNA response to acute exercise. Therefore, it was also hypothesized c) that there would be evidence of significant relationships between basal and exercise-induced VEGF mRNA and muscle structure (e.g. capillarity) and function (e.g. blood flow), which would differ amongst CHF patients and control subjects.
MATERIALS AND METHODS
Subjects
Seven male CHF patients and six healthy age, sex, and activity matched (questionnaire and interview) control subjects gave written informed consent to participate in this study, which was approved by the Human Subjects Protection Program, University of California, San Diego. Their physical and anthropometric characteristics are presented in Table 1. All the CHF patients were clinically stable with symptoms compatible with New York Heart Association (NYHA) functional class II-III. Mean left ventricular ejection fraction in the CHF patients was 25 ± 3%. Patient medications were not altered during the study, with the exception of beta blockers that were withheld for 48 h prior to each experimental day. All subjects were screened to rule out any smoking history or systemic diseases such as peripheral vascular disease, diabetes, chronic-obstructive pulmonary disease, hypercholesterolemia and hypertension. The investigation conforms to the principles outlined in the declaration of Helsinki.
Table 1.
Characteristics of controls and patients with CHF
| controls | CHF | |
|---|---|---|
| Age (yrs) | 50±3 | 54±2 |
| Height (cm) | 176±1 | 180±2 |
| Body Mass (kg) | 82±7 | 99±6 |
| Quadriceps muscle mass (kg) | 2.1±0.2 | 2.2±0.2 |
| NYHA class | - | II-III |
| Peak Pulm. Cycle V̇O2 (l/min) | 2.34±0.30 | 1.66±0.21* |
| Peak Pulm. Cycle V̇O2 (ml/kg/min) | 29.1±3.2 | 16.8±2.0* |
| Maximum cycle work rate (W) | 170±23 | 101±13* |
| Peak cycling leg V̇O2 (ml/min) | 479±35 | 336±36* |
| Medications (fraction of users) | ||
| Digoxin | 0/6 | 7/7 |
| Diuretics | 0/6 | 7/7 |
| Long-acting nitrates | 0/6 | 6/7 |
| Statins | 0/6 | 5/7 |
| Aspirin | 0/6 | 5/7 |
| β-Blockers | 0/6 | 4/7 |
| Warfarin | 0/6 | 3/7 |
| ACE inhibitors | 0/6 | 5/7 |
| Ca2+ channel blockers | 0/6 | 5/7 |
CHF, chronic heart failure; ACE, angiotensin-converting enzyme.
Anthropometric data and severity of disease (NYHA class) in controls and CHF patients. Work rate and pulmonary V̇O2 at maximum cycle ergometric exercise, together with peak leg V̇O2 at maximum KE exercise, are reported. The type of medications taken by CHF patients is also listed. Data are expressed as mean ± SE;
P<0.05.
Experimental Design
To determine KE maximum work rate (WRmax), both CHF patients and controls performed an 8–12 min graded KE exercise test to exhaustion. Several days later, CHF patients and controls reported again to the laboratory and after a 5 min warm-up performed 30 min of KE extensor exercise at 50% of WRmax. Both the resting and exercised legs were biopsied within one hour after the exercise stimulus. During KE studies, skeletal muscle blood flow and muscle V̇O2 were measured. To achieve this, two catheters (radial artery and left femoral vein) and a thermocouple (left femoral vein) were emplaced using a sterile technique to facilitate blood gas and blood flow measurements and O2 transport calculations, as previously reported (Richardson et al. 1993).
Exercise apparatus
The KE ergometer was utilized to produce the acute exercise stimulus, as previously described (Richardson et al. 1995). During exercise, the contraction rate was maintained at 60 repetitions per minute.
Muscle biopsy
All biopsies were taken from the vastus lateralis muscle at an approximate depth of 3–5 cm, 15 cm proximal to the knee and slightly distal to the ventral midline of the muscle using a Bergstrom needle, as described previously (Bergstrom 1975). The muscle samples from each biopsy were either immediately frozen in liquid nitrogen and stored at −80°C for subsequent histochemical analysis or immersion-fixed in glutaraldehyde fixative and later processed for electron microscopy and morphometry.
Histochemistry
Thick transverse sections of 8 mm were cut at −24°C on a cryostat (Jung-Reichert Cryocut 1800) and stained for fiber types I and II, and capillaries (Rosenblatt et al. 1987).
Electron microscopy
The glutaraldehyde-fixed samples were processed for electron microscopy, as described previously (Mathieu-Costello 1987). Electron micrographs for morphometry were taken on 70 mm films with a Zeiss 10 electron microscope.
Morphometry
The relative cross-sectional area and number of types I and II fibers were estimated by point-counting on a light microscope (250x) using an eyepiece square grid test A100 (Weibel 1979). Capillary density (i.e. capillary number per fiber cross-sectional area), capillary-to-fiber ratio (i.e. capillary number per fiber number), number of capillaries around a fiber (NCAF) and fiber cross-sectional area were measured by point-counting with a light microscope (400x). The volume density of mitochondria per volume of muscle fiber was estimated by point-counting (490x).
Competitive RT-PCR analysis of VEGF mRNA levels
The relative VEGF mRNA levels in skeletal muscle from healthy and CHF patients were measured by competitive RT-PCR analysis according to the method of Zachar et al (1993). A Polaroid photograph of the gel was quantified by computer densitometry (GelPro Analyzer, Media Cybernetics, Silver Springs, MD). VEGF mRNA signals were then expressed as the ratio of VEGF target DNA/internal standard. It should be noted that VEGF protein expression was not assessed due to the differing time course of VEGF mRNA and VEGF protein expression (1 vs 4 hrs, respectively), as this would have required an additional biopsy and was not performed for ethical reasons.
Vascular and metabolic measurements
The continuous infusion thermodilution approach was used to measure blood flow during exercise, as previously described (Richardson et al. 1993). Femoral arterial and venous blood pressures were continuously monitored at heart level by pressure transducers (model PX-MK099, Baxter). Mean arterial pressure (MAP) and mean venous blood pressures (MVP) were computed by the integration of each pressure curve. Leg vascular resistance (LVR) was calculated as (MAP–MVP)/muscle blood flow. Before each blood flow measurement, 3–4 ml samples of arterial and femoral venous blood were withdrawn from the catheters anaerobically to measure lactate concentration [La], PO2, PCO2, pH, O2 saturation, and hemoglobin concentration ([Hb]). All measurements were made on an IL 1306 blood gas analyzer and IL 482 CO-oximeter (Instrumentation Laboratories, Lexington, MA.). Arterial and venous O2 concentrations were calculated as [1.39 ml O2 × [Hb] g/100 ml × O2 saturation (%)] + [0.003 ml O2/100 ml of blood × PO2 (mmHg)]. Arterial-venous O2 concentration difference was calculated from the difference in radial artery and femoral venous O2 concentration measurements. Muscle V̇O2 was calculated as the product of blood flow and arterial-venous O2 difference, while Q. O2 was calculated as the product of blood flow and arterial O2 concentration.
Norepinephrine spillover
Plasma epinephrine and norepinephrine (NE) were assayed in duplicate by the method of Kennedy and Zeigler (1990). The rate of NE spillover was determined as described previously (Savard et al. 1989) using the following equation:
where Cv and Ca are the plasma NE concentrations in the femoral vein and radial artery, respectively. Ee is the fractional extraction of epinephrine, and LPF is the leg plasma flow, determined from leg Q. O2 and hematocrit. This approach utilizes the high correlation between NE and epinephrine extraction and avoids the potential problem of recirculation (Savard et al. 1989), if a titrated isotope of NE had been used to measure NE extraction directly.
Statistical analysis
Rested and acutely exercised muscle VEGF signals were quantitatively assessed using densitometry (normalized by the internal standard) and differences were assessed by two-way ANOVA for repeated measures. Differences between groups and conditions were then identified using a Newman-Keuls post-hoc analysis. Functional and morphometric measurements in CHF patients and controls were compared using unpaired Student t-test. Despite the relatively small sample size, a priori power calculations, based upon our previously published assessments of the acute VEGF mRNA response to exercise and muscle morphometry (Richardson et al. 1999; Richardson et al. 2000), revealed adequate power (> 0.7) in the major variables of interest. Statistical significance was accepted if P<0.05. All data are reported as mean ± SE.
RESULTS
Muscle metabolic and vascular response to acute exercise
Single-leg KE WRmax was 40±2 W for controls and 30±3 W for patients with CHF (P<0.05). Therefore, the 50% of WRmax exercise stimulus was, in absolute terms, significantly lower for the patients with CHF and thus resulted in a lower muscle blood flow and muscle V. O2 (P<0.05). The patients with CHF exhibited a 44% higher LVR than the controls. Complete O2 transport and metabolic data are presented in Table 3.
Table 3.
Vascular and metabolic response to knee-extensor exercise at 50% of maximum work rate (50% WRmax).
| controls | CHF | |
|---|---|---|
| 50% WRmax (W) | 20±3 | 15±4 |
| V̇O2 leg (ml/min) | 313±28 | 255±33* |
| Q. leg (ml/min) | 2450±223 | 1827±243* |
| Q. O2 (ml/min) | 460±41 | 338±46* |
| Hb (g/dl) | 13.9±0.3 | 13.3±0.4 |
| SaO2 (%) | 96 ± 1 | 96 ± 1 |
| CaO2 (ml/100ml) | 18.8±0.3 | 18.4±0.3 |
| CvO2 (ml/100ml) | 5.9±0.5 | 4.2±0.6 |
| PaO2 (mmHg) | 99.9±3 | 95.0±5 |
| PvO2(mmHg) | 23±1 | 19±1 |
| LVR (mmHg/L/min) | 41±6 | 59±8* |
| MAP (mmHg) | 121 ± 11 | 108 ± 5 |
| MAP-MVP (mmHg) | 100.0±11.5 | 97.7±7.5 |
| Arterial Lactate (mMol) | 2.1±0.4 | 1.6±0.2 |
| Ne Spillover (nM·min−1·kg−1) | 0.59±0.09 | 2.47±0.35* |
CHF, chronic heart failure; 50% WRmax, 50% of maximum work rate; V̇O2 leg, leg oxygen uptake; Q. leg, leg blood flow; CaO2, arterial O2 concentration; CvO2, venous O2 concentration; PaO2, arterial O2 partial pressure; PvO2, venous O2 partial pressure; LVR, leg vascular resistance; MAP, mean arterial pressure; MVP, mean venous pressure; Ne, norepinephrine. Data are expressed as mean ± SE;
P<0.05 (controls vs CHF).
VEGF mRNA at rest and response to acute exercise
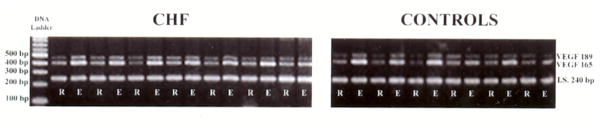
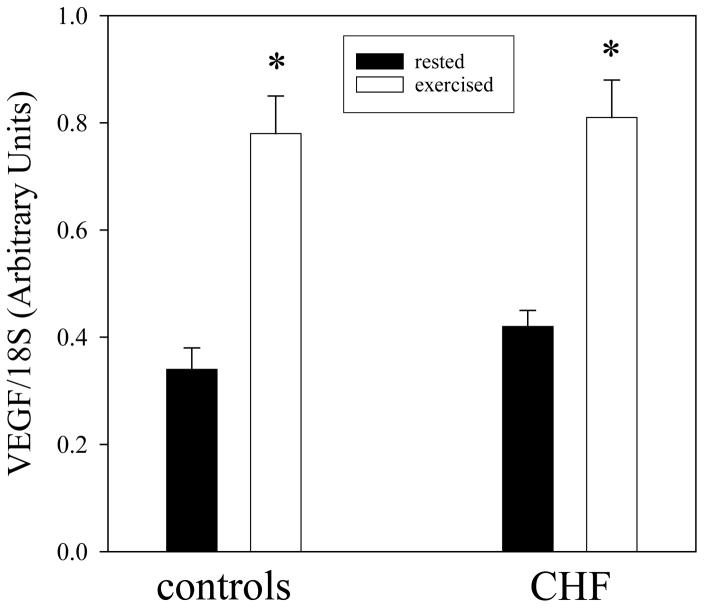
The VEGF mRNA gels for all patients with CHF and controls are presented in Figure 1. In the rested leg VEGF mRNA levels were similar in both CHF patients and healthy controls (0.42±0.03 and 0.33±0.04, respectively; P>0.05) (figure 2). One hour following acute KE exercise, both groups revealed a significant increase in VEGF mRNA levels in the vastus lateralis muscle (0.81±0.07 and 0.78±0.07 in CHF and controls, respectively; figure 2). This increase in VEGF mRNA abundance, as a consequence of acute exercise, was similar in both CHF patients and controls. Consequently, the VEGF mRNA exercise-to-rest ratio was not different between CHF patients and controls (2.0±0.2 and 2.5±0.5, respectively; figure 2).
Figure 1.
Rest (R) and exercise (E) VEGF mRNA expression in CHF patients and controls.
Figure 2.
The expression of VEGF mRNA in rested (black bars) and exercised (30 min at 50% WRmax, white bars) skeletal muscle of controls and patients with CHF. Note that the patients with CHF and the controls have a similar basal and acute exercise-induced VEGF response.
Muscle structure
Muscle fiber cross-sectional area was not different between the two groups. The patients with CHF did not reveal altered capillarity compared to controls when expressed as capillary density, capillary-to-fiber ratio or NCAF. However, the volume density of mitochondria was 22% lower (P<0.05) in patients with CHF than in controls.
DISCUSSION
Currently the role of VEGF in the regulation of skeletal muscle vascular structure in patients with CHF has not been well characterized. This study has provided four novel findings in this area of research, 1) resting skeletal muscle VEGF mRNA levels in patients with CHF do not differ from well matched healthy controls, 2) in the same subjects, the assessment of skeletal muscle capillarity revealed similar vascular structure in the patients with CHF and controls, 3) acute exercise induced a similar increase in skeletal muscle VEGF mRNA in CHF patients and controls and 4) evidence of similar structural and structural functional relationships shared by both patients and controls. Taken together, the multiple similarities between patients and controls question the concept of a VEGF-mediated impact of CHF on skeletal muscle capillarity and angiogenesis in response to physical activity.
Basal VEGF mRNA and skeletal muscle capillarity
The present interest in basal levels of VEGF has been fueled by the finding that in mice the knockout of all three isoforms, VEGF120, VEGF168, VEGF188, or its receptors (Flk-1 and Flt-1) results in embryonic lethality (Ferrara et al. 1996; Fong et al. 1995; Shalaby et al. 1995). Therefore, it was not until recently that the specific role of basal VEGF integrity in skeletal muscle was documented in adult mice by the viral delivery of cre recombinase to regions of muscle containing a floxed VEGF gene resulting in the deletion of exon 3 and the local inactivation of all three VEGF isoforms (Tang et al. 2004). Within 8 weeks these infected regions of muscle experienced a 70% reduction in capillary-to-fiber ratio, highlighting the importance of VEGF as an essential survival factor for muscle capillarity. The recent development of a lifelong skeletal muscle VEGF deficient mouse model (Olfert et al. 2009), further supports the role of VEGF in adult skeletal muscle, with these animals exhibiting significant reductions in both skeletal muscle capillarity and exercise capacity.
Although there are several studies that have reported capillary rarefaction in patients with CHF and the possible link between this structural difference and the regulation of skeletal muscle angiogenesis by VEGF, the data in this particular area are sparse (Gustafsson et al. 2001; Kraus et al. 1999; Testa et al. 2000). In 1999 preliminary data, published only in abstract form, by Kraus et al. (Kraus et al. 1999) revealed a low VEGF level in the skeletal muscle of patients with CHF compared with controls. While in 2001, Gustafsson et al. (Gustafsson et al. 2001) assessed basal levels of VEGF mRNA and protein and revealed a proportional increase in both due to exercise training, however this study and the work of Testa et al. (2000) lacked a healthy control group which limits its interpretation in this arena. In the present study basal VEGF mRNA levels in the rested skeletal muscle of the patients with CHF did not differ from the levels of healthy age- and activity-matched controls, suggesting the novel finding of normal VEGF levels in patients with CHF at rest. This conclusion is additionally supported by the morphometric assessment of capillarity density, the capillary-to-fiber ratio and NCAF in the same subjects, none of which revealed a capillarity difference between the patients and healthy controls. It should be noted, that although VEGF protein levels were not assessed in the present study, previous findings (discussed earlier) have documented proportional changes in VEGF mRNA and protein expression in this population (Gustafsson et al. 2001).
VEGF response to acute exercise
To our knowledge, there are has not been a single study that has examined the effect of acute exercise on VEGF mRNA levels in patients with CHF. In a prior study of this population, utilizing the same KE exercise modality to maximize the impact of skeletal muscle movement and minimize the role of the failing heart, it was documented that both resting VEGF mRNA and protein concentrations increased in response to one-leg KE training (Gustafsson et al. 2001). This was interpreted as an evidence for molecular plasticity and a potentially intact VEGF response to exercise that could be beneficial to this population when encouraged to exercise. However, although cutting edge, this study did not assess the acute response to exercise, assess muscle structure, or include a healthy control group. In the present study, the patients with CHF exhibited a similar VEGF mRNA response to acute exercise as did the healthy controls (figures 1 and 2). These data reveal the exciting and clinically relevant observation that the sparking capacity of acute exercise to ignite the process of new capillary growth and maintenance of existing vessels is still intact in the skeletal muscle of patients with CHF.
Muscle morphometry
CHF is a clinical syndrome in which several severe abnormalities are often cited in peripheral skeletal muscle, suggested to be responsible for exercise intolerance and other morbidities associated with this disease. However, in the current patients with CHF the normal morphometric findings are internally consistent with similar resting levels of skeletal muscle VEGF mRNA. Specifically, in addition to no significant fiber type differences, three measures of capillarity, capillary density, capillary-to-fiber ratio, and NCAF, revealed similar vascular structure in the patients and controls (table 2). Additionally, the same VEGF mRNA response to exercise in CHF patients and controls also supports these morphometric findings. Indeed, this is an independent evidence of the preserved capacity to signal the maintenance and the potential to realize new capillary formation in response to an acute increase in activity (figures 1 and 2). Indeed, the only morphometric difference between CHF patients and controls was at the subcellular level with the volume density of mitochondria in CHF patients being 22% lower than in controls (table 2, figure 3). However, here too the patients revealed a similar relationship with capillarity as did the controls.
Table 2.
Morphometric characteristics of skeletal muscle in controls and in patients with CHF.
| controls | CHF | |
|---|---|---|
| Capillary density (capillaries/mm2) | 432 ± 25 | 415 ± 46 |
| NCAF | 3.8 ± 0.1 | 3.6 ± 0.3 |
| Capillary-to-fiber ratio | 1.57±0.10 | 1.46±0.14 |
| Fiber area (μ2) | 4086±625 | 3366±199 |
| Mitochondria volume density (%) | 4.5±0.4 | 3.5±0.4* |
| Area type I fibers (%) | 41±3.8 | 35±3.9 |
| Area type II fibers (%) | 59±3.8 | 65±3.9 |
CHF, chronic heart failure; NCAF, number of capillaries around a fiber.
P<0.05
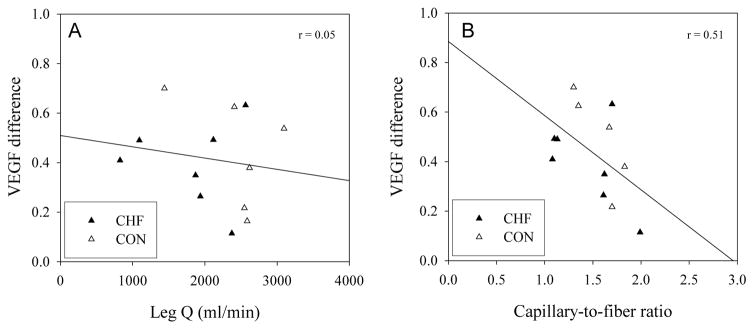
Figure 3.
Evidence of a poor relationship between leg blood flow (Q. ) achieved during the KE exercise stimulus and the VEGF response to exercise (VEGF difference) (panel A), and an inverse relationship between capillary-to-fiber ratio and VEGF difference in response to acute exercise (panel B).
Muscle metabolic and vascular response to acute exercise
A unique feature of this study was that the physiological response to the KE exercise stimulus utilized to upregulate VEGF mRNA was well documented in terms of the metabolic and vascular responses (table 3). An initial important observation was that maximal WR and leg V̇O2 were significantly lower in the patients with CHF than in controls. Guided by the ethos that exercise training adaptations are stimulated by relative and not absolute WR, this meant that the selection of a relative percentage of WRmax would likely yield a lower metabolic and vascular challenge for the patients than the controls and indeed this was the case. Leg muscle blood flow and leg V̇O2 at 50% of WRmax in CHF patients were 27% and 18% lower, respectively, than the matched controls (P<0.05). However, despite this difference in absolute metabolic and vascular stress, the same relative challenge (50% WRmax), the VEGF mRNA response was not different between CHF patients and controls (figures 1 and 2).
The other striking difference between the physiological response to the exercise stimulus between CHF patients and controls was the 44% greater LVR in the patients. This phenomenon, likely the consequence of elevated muscle sympathetic nerve activity (MSNA) (Magnusson et al. 1997), has been previously documented in this population during cycle exercise in which it has been supposed that the elevated skeletal muscle NE spillover was an attempt to minimize peripheral vasodilation due to the limited pumping capacity of the heart (Magnusson et al. 1997). However, it has previously been suggested that the adoption of the KE exercise model and the use of a small muscle mass avoid this response in patients with CHF compared with controls, but the increased MSNA is again seen if two legs perform KE exercise (Magnusson et al. 1997). In the current patients with CHF this did not appear to be the case, based upon their much greater NE spillover than healthy controls during only single leg KE exercise (table 3). As some authors have proposed that both exercise-induced increases in blood flow and tissue stretch, which elevates shear stress, wall tension and basement membrane stretching, particularly important stimuli for angiogenesis in skeletal muscle (Egginton et al. 1998; Hudlicka 1998; Zhou et al. 1998), the possibility that the patients with CHF experience a greater stimulus in this respect is a possibility. However, it is interesting to note that, whether the patients were considered alone or in combination with the controls, there was no discernible relationship between skeletal muscle blood flow or NE spillover (r = 0.05) and the VEGF mRNA levels increase due to the acute exercise challenge (figure 4).
Figure 4.
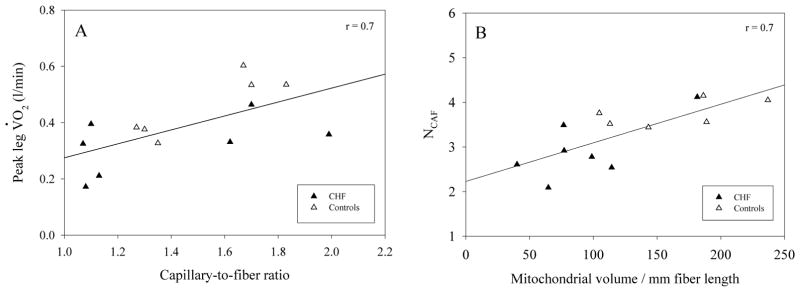
The similarly strong relationship between capillary-to-fiber ratio and peak leg oxygen consumption (V̇O2, panel A) and mitochondrial volume and NCAF (panel B). The shared relationship between patients with CHF and controls reveals the preservation of normal structure and structure function relationships in the patients.
Physical activity versus pathophysiology
The goal of the present study was to recruit healthy controls who were not only matched for age and stature, but also for physical activity level and thus all control subjects who partook in this study would be considered relatively inactive compared to the normal healthy population. It is possible that in the current study the multiple similarities between the patients and the controls, such as capillarity and VEGF mRNA levels, were a function of the focus upon activity level in the subject matching process. Additionally, there are several normal and potentially activity-dependent structural and structural/functional relationships that are shared by both CHF patients and healthy controls.
Specifically, as illustrated in figure 4A, with the inclusion of both patients and controls there is a relatively strong relationship between capillary-to-fiber ratio and peak leg V̇O2 measured during KE exercise. This suggests that, in line with current thinking (Hepple et al. 2000), muscle capillarity plays an important role in determining O2 flux to muscle during exercise and the current patients with CHF appear to have a similar relationship to the current healthy controls. A similar theme is supported by the equally strong relationship between mitochondrial volume, which was on average significantly attenuated in the patients with CHF (table 1), and the NCAF (figure 4B). This relationship, again equally shared by the healthy controls, suggests that muscle, whether from healthy controls or patients with CHF, exhibits a strong link between structural metabolic capacity (mitochondria) and the structural O2 transport capacity (capillaries).
Summary
In terms of VEGF mRNA, both the basal levels and response to exercise in the patients with CHF were not different from the controls and this was supported by similar structural and structural/functional relationships shared by both groups. Only reduced mitochondrial content and an elevated peripheral vascular resistance during the submaximal KE exercise challenge separated the CHF patients from the controls. It is concluded that VEGF upregulation and capillarity are unlikely to be responsible for the attenuated exercise capacity in the present group of patients with CHF, but decreased skeletal muscle mitochondrial content and increased peripheral vascular resistance may play a role in this symptom. However, care must be taken in interpreting these results, as muscle biopsies may not be completely representative of the entire muscle. Moreover, patients with CHF present with many different phenotypes, as in many other chronic diseases, thus the participants in the current study may not represent the entire CHF population as a whole.
Acknowledgments
FUNDING
This work was supported by National Institutes of Health grants from National Heart, Lung, and Blood Institute [Grant number HL-17731]; and the Sam and Rose Stein Institute for Research on Aging, and Tobacco-Related Disease Research Program [Grant number 15RT-0100].
The authors thank the patients and subjects for their committed participation in this study. At the time of the study, Prof. Esposito was on leave from University of Brescia, Italy. He was partly supported by “Centro per lo Studio del Trattamento dello Scompenso Cardiaco”, University of Brescia.
Footnotes
CONFLICT OF INTERESTS
None declared.
References
- Abraham D, Hofbauer R, Schafer R, Blumer R, Paulus P, Miksovsky A, Traxler H, Kocher A, Aharinejad S. Selective downregulation of VEGF-A(165), VEGF-R(1), and decreased capillary density in patients with dilative but not ischemic cardiomyopathy. Circ Res. 2000;87:644–7. doi: 10.1161/01.res.87.8.644. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Ikeda U, Hojo Y, Ueno S, Nonaka-Sarukawa M, Yamamoto K, Shimada K. Decreased serum vascular endothelial growth factor concentrations in patients with congestive heart failure. Heart. 2003;89:207–8. doi: 10.1136/heart.89.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–16. [PubMed] [Google Scholar]
- Chin BS, Chung NA, Gibbs CR, Blann AD, Lip GY. Vascular endothelial growth factor and soluble P-selectin in acute and chronic congestive heart failure. Am J Cardiol. 2002;90:1258–60. doi: 10.1016/s0002-9149(02)02848-5. [DOI] [PubMed] [Google Scholar]
- De Boer RA, Henning RH, Tio RA, Pinto YM, Brouwer RM, Ploeg RJ, Bohm M, Van Gilst WH, Van Veldhuisen DJ. Identification of a specific pattern of downregulation in expression of isoforms of vascular endothelial growth factor in dilated cardiomyopathy. Heart. 2002;88:412–4. doi: 10.1136/heart.88.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of Skeletal Muscle in Chronic Heart Failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II-III chronic heart failure independent of other peripheral alterations. Journal of the American College of Cardiology. 1999;33:1956–63. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Egginton S, Hudlicka O, Brown MD, Walter H, Weiss JB, Bate A. Capillary growth in relation to blood flow and performance in overloaded rat skeletal muscle. J Appl Physiol. 1998;85:2025–32. doi: 10.1152/jappl.1998.85.6.2025. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Gustafsson T, Bodin K, Sylven C, Gordon A, Tyni-Lenne R, Jansson E. Increased expression of VEGF following exercise training in patients with heart failure. Eur J Clin Invest. 2001;31:362–6. doi: 10.1046/j.1365-2362.2001.00816.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson T, Puntschart A, Kaijser L, Jansson E, Sundberg CJ. Exercise-induced expression of angiogenesis-related transcription and growth factors in human skeletal muscle. Am J Physiol. 1999;276:H679–85. doi: 10.1152/ajpheart.1999.276.2.H679. [DOI] [PubMed] [Google Scholar]
- Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, Coats AJ. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. Journal of the American College of Cardiology. 1997;30:1758–64. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Hogan MC, Stary C, Bebout DE, Mathieu-Costello O, Wagner PD. Structural basis of muscle O(2) diffusing capacity: evidence from muscle function in situ. J Appl Physiol. 2000;88:560–6. doi: 10.1152/jappl.2000.88.2.560. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Roca J, West JB, Wagner PD. Dissociation of maximal O2 uptake from O2 delivery in canine gastrocnemius in situ. J Appl Physiol. 1989;66:1219–26. doi: 10.1152/jappl.1989.66.3.1219. [DOI] [PubMed] [Google Scholar]
- Hudlicka O. Is physiological angiogenesis in skeletal muscle regulated by changes in microcirculation? Microcirculation. 1998;5:5–23. [PubMed] [Google Scholar]
- Kennedy B, Ziegler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–53. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- Kraus WE, Deuscha BD, Thompson MA, Pippen AM, Keteyian SJ, Sullivan MJ. Vascular endothelial growth factor level in skeletal muscle are reduced in patients with congestive heart failure (abstract) Circulation. 1999;100:246. [Google Scholar]
- LeJemtel TH, Maskin CS, Lucido D, Chadwick BJ. Failure to augment maximal limb blood flow in response to one-leg versus two-leg exercise in patients with severe heart failure. Circulation. 1986;74:245–51. doi: 10.1161/01.cir.74.2.245. [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Magnusson G, Kaijser L, Sylvén C, Karlberg KE, Isberg B, Saltin B. Peak skeletal muscle perfusion is maintained in patients with chronic heart failure when only a small muscle mass is exercised. Cardiovascular Research. 1997;33:297–306. doi: 10.1016/s0008-6363(96)00249-0. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of Skeletal Muscle Atrophy to Exercise Intolerance and Altered Muscle Metabolism in Heart Failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- Massie BM, Simonini A, Sahgal P, Wells L, Dudley GA. Relation of systemic and local muscle exercise capacity to skeletal muscle characteristics in men with congestive heart failure. Journal of the American College of Cardiology. 1996;27:140–5. doi: 10.1016/0735-1097(95)00416-5. [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O. Capillary tortuosity and degree of contraction or extension of skeletal muscles. Microvasc Res. 1987;33:98–117. doi: 10.1016/0026-2862(87)90010-0. [DOI] [PubMed] [Google Scholar]
- Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol. 2009;587:1755–67. doi: 10.1113/jphysiol.2008.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Knight DR, Poole DC, Kurdak SS, Hogan MC, Grassi B, Wagner PD. Determinants of maximal exercise V̇O2 during single leg knee-extensor exercise in humans. Am J Physiol. 1995;268:H1453–61. doi: 10.1152/ajpheart.1995.268.4.H1453. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol. 1993;75:1911–6. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Wagner PD. Red blood cell transit time in man: theoretical effects of capillary density. Adv Exp Med Biol. 1994;361:521–32. doi: 10.1007/978-1-4615-1875-4_91. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Wagner H, Mudaliar SR, Henry R, Noyszewski EA, Wagner PD. Human VEGF gene expression in skeletal muscle: effect of acute normoxic and hypoxic exercise. Am J Physiol. 1999;277:H2247–52. doi: 10.1152/ajpheart.1999.277.6.H2247. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Wagner H, Mudaliar SR, Saucedo E, Henry R, Wagner PD. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279:H772–8. doi: 10.1152/ajpheart.2000.279.2.H772. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Kuzon WM, Jr, Plyley MJ, Pynn BR, McKee NH. A histochemical method for the simultaneous demonstration of capillaries and fiber type in skeletal muscle. Stain Technol. 1987;62:85–92. doi: 10.3109/10520298709107973. [DOI] [PubMed] [Google Scholar]
- Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise in humans: role of muscle mass. Am J Physiol. 1989;257:H1812–8. doi: 10.1152/ajpheart.1989.257.6.H1812. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–6. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–81. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics. 2004;18:63–9. doi: 10.1152/physiolgenomics.00023.2004. [DOI] [PubMed] [Google Scholar]
- Testa M, Ennezat PV, Vikstrom KL, Demopoulos L, Gentilucci M, Loperfido F, Fanelli R, Kitsis RN, Leinwand LA, LeJemtel TH. Modulation of vascular endothelial gene expression by physical training in patients with chronic heart failure. Ital Heart J. 2000;1:426–30. [PubMed] [Google Scholar]
- Weibel E. Practical methods for biological morphometry. Academic Press; London-New York-Torronto: 1979. [Google Scholar]
- Wilting J, Christ B, Bokeloh M, Weich HA. In vivo effects of vascular endothelial growth factor on the chicken chorioallantoic membrane. Cell Tissue Res. 1993;274:163–72. doi: 10.1007/BF00327997. [DOI] [PubMed] [Google Scholar]
- Zachar V, Thomas RA, Goustin AS. Absolute quantification of target DNA: a simple competitive PCR for efficient analysis of multiple samples. Nucleic Acids Res. 1993;21:2017–8. doi: 10.1093/nar/21.8.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou AL, Egginton S, Brown MD, Hudlicka O. Capillary growth in overloaded, hypertrophic adult rat skeletal muscle: an ultrastructural study. Anat Rec. 1998;252:49–63. doi: 10.1002/(SICI)1097-0185(199809)252:1<49::AID-AR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]