Abstract
The world view of rodents is largely determined by sensation on two length scales. One is within the animal's peri-personal space. Sensorimotor control on this scale involves active movements of the nose, tongue, head, and vibrissa, along with sniffing to determine olfactory clues. The second scale involves the detection of more distant space through vision and audition; these detection processes also impact repositioning of the head, eyes, and ears. Here we focus on orofacial motor actions, primarily vibrissa-based touch but including nose twitching, head bobbing, and licking, that control sensation at short, peri-personal distances. The orofacial nuclei for control of the motor plants, as well as primary and secondary sensory nuclei associated with these motor actions, lie within the hindbrain. The current data support three themes: First, the position of the sensors is determined by the summation of two drive signals, i.e., a fast rhythmic component and an evolving orienting component. Second, the rhythmic component is coordinated across all orofacial motor actions and is phase-locked to sniffing as the animal explores. Reverse engineering reveals that the preBötzinger inspiratory complex provides the reset to the relevant premotor oscillators. Third, direct feedback from somatosensory trigeminal nuclei can rapidly alter motion of the sensors. This feedback is disynaptic and can be tuned by high-level inputs. The elucidation of synergistic coordination of orofacial motor actions to form behaviors, beyond that of a common rhythmic component, represents a work in progress that encompasses feedback through the midbrain and forebrain as well as hindbrain areas.
Keywords: Coupled oscillators, Facial nucleus, Hypoglossal nucleus, Orienting, Tongue, Vibrissa
Coordination of neuronal circuits in the brainstem is essential for exploration, navigation, feeding, social interaction, and defense. A key advantage of studying such circuitry is the concurrent access that one has to sensory input, via sensory organs, and the muscular output of motor programs. This allows brainstem circuitry to be analyzed in terms of entire sensorimotor loops. In past years, this engineering-themed approach made the analysis of brainstem circuitry a center-point of neuroscience, as highlighted by studies on the control of balance and visual stability in the vestibular and oculomotor system (Lisberger et al., 1987, Gittis and du Lac, 2006), the organization of respiratory centers (Feldman and Del Negro, 2006, Alheid and McCrimmon, 2008, Garcia et al., 2011), and the nature of nociceptive/tactile sensory pathways in the trigeminal system (Dubner and GJ Bennett, 1983).
A challenge in reverse engineering brainstem circuits concerns the identification of the circuit components that merge sets motor actions into behaviors (Berntson and Micco, 1976). Ongoing efforts to delineate such circuits combine high-resolution behavioral quantification (Kurnikova et al., 2017), simultaneous recordings of brainstem circuits dynamics, and transsynaptic viral tracing (Kleinfeld et al., 2014, Stanek 4th et al., 2014). Our particular focus is on closed sensorimotor loops, from sensor to the motor plant that controls the sensor, formed by orofacial circuits that are involved in active sensing of the nearby environment (Kleinfeld et al., 1999, 2006, Kleinfeld and Deschênes, 2011). This approach, interpreted with the analytical tools of control engineering, provides a means to reverse engineer the brainstem circuits that drive orofacial motor actions as well as coordinate these actions into holistic exploratory and orienting behaviors.
Here, we begin with a description of orofacial behavioral coordination and the underlying muscular control of relevant sensory organs (Fig. 1). These involve rhythmic motions that are tied to sniffing, as well as orienting movements, and include nose motion, head motion, and licking in addition to whisking. A high level description of the overall organizing principles for the underlying brainstem control circuits is presented (Fig. 2), followed by a synopsis on the circuitry for the coordinated rhythmic aspect of orofacial motor actions (Fig. 3). We then focus on a brainstem-centric view of the known circuitry that drives orienting behaviors, with emphasis on the vibrissae (Fig. 4) and tongue (Fig. 5), organized in terms of a progression from sensory to motor areas. Lastly, our analysis provides an introduction to the notion of nested anatomical loops across multiple levels in the brain, which is illustrated for the vibrissa system by viewing the circuitry (Fig. 4) in terms of feedback loops (Fig. 6).
Figure 1. Orofacial motor actions and their relation to the sniff cycle.
(A) Example of measurement of head position versus time. Angular velocity of the head and activation of the neck muscles is recording in the free ranging animal, along with breathing. Note rhythmic component of motion locked to breathing along, with slow deflections. Adapted from (Kurnikova et al., 2017).
(B) Example of motion of the nose in head-fixed rats captured with videography; the thermocouple records respiration. Basal breathing occurs during rest and sniffing during exploration. Adapted from (Kurnikova et al., 2017).
(C) Schematic of the view of a camera for tracking the vibrissae. Breathing was measured with a thermocouple. The time-series shows breathing (red) and the position of the left (green) and right (black) C2 vibrissa; the midpoint of whisking (magenta) was computed as the average between the upper and lower envelope of the cycle-by-cycle angle of the vibrissa. Adapted from (Kurnikova et al., 2017).
(D) Idealized time-ordered patterns of behavioral, neuronal and muscular activities associated with different phases of the respiratory rhythms. Note that the extrinsic pad retractor and protractor muscles may activate during basal respiration when the amplitude of respiration increases. Adapted from (Deschênes et al., 2016), (Kurnikova et al., 2017), and (Liao and Kleinfeld, 2016).
Figure 2. Schema for the organization of sensorimotor systems.
Each orofacial muscle in innervated by motor neurons that receive inputs from diverse sets of premotor neurons located throughout the brainstem. Direct projections from primary sensory nuclei to motor neurons mediate reflexive actions (right), whereas the rhythmic component of muscle activation is controlled by central pattern generator oscillators (left). Additional premotor populations predominantly mediate the effects of broad upstream motor and limbic controllers (central, schematized as a single projection).
Figure 3. Schematic circuit of coupled neuronal oscillators in the brainstem.
Muscles, motoneurons that control breathing, vibrissa, face, jaw, tongue, and airway along with known premotor nuclei to each of the motoneuron pools. The putative neuronal oscillators are marked with a “~”. Summarized from (Nakamura and Katakura, 1995, Travers et al., 1997, Feldman and Del Negro, 2006, Tan et al., 2010, Travers et al., 2010, Moore* et al., 2013, Takatoh et al., 2013, Molkov et al., 2017). Breathing control centers (green) project to putative premotor controllers of diverse orofacial musculature (yellow). Dashed lines are connections based on functional rather than antomical data.
Abbreviations: Acc. spinal (respiratory accessory spinal nucleus); MoV (motor trigeminal nucleus); nIRt, hIRt, and vIRt (nasal, hypoglossal, and vibrissa intermediate reticular formation, respectively); PCRt (parvocellular reticular formation); Peri-V (peri-trigeminal area). Dashed lines are correspond to connections based on indirect evidence, e.g., electrophysiological versus anatomical.
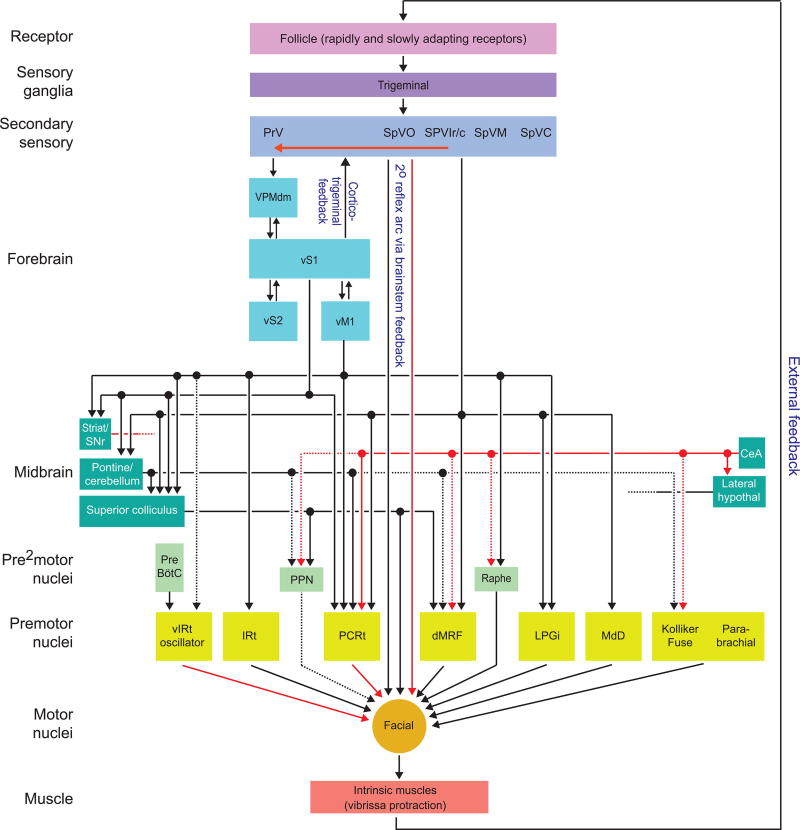
Figure 4. "Feedforward" circuit diagram for the circuit for the intrinsic muscles that drive protraction of the vibrissae.
Arrow signifies the direction of signal flow; red signifies inhibitory and black signifies excitatory connections. Compiled data as described in the text. Many feedback connections and interconnections among premotor structures have been excluded for simplicity. Dashed lines are connections based on functional rather than antomical data.
Abbreviations: PrV (principal trigeminal nucleus); SpVO (spinal subnucleus oralis); SpVIr and SpVIc (rostral and caudal divisions of spinal subnucleus interpolaris, respectively); SpVM (spinal subnucleus muralis); SpVC (spinal subnucleus caudalis); VPMdm (dorsomedial aspect of the ventral posterior medial nucleus of dorsal thalamus); Po (medial division of the posterior group nucleus); vS1 (vibrissa primary sensory cortex); vS2 (vibrissa secondary sensory cortex); vM1 (vibrissa motor cortex); Stria/SNr (striatum/substantia nigra pars reticulata); CeA (central amygdala); Pontine/cerebellum (circuit from pontine nuclei through cerebellar deep nuclei); lateral hypothal (lateral hypothalamus); PPN (pedunculopontine nucleus); Raphe (Raphe nuclei); PreBötC (preBötzinger respiratory complex); vIRt (vibrissa intermediate reticular zone); IRt (vibrissa intermediate reticular zone); PCRt (parvocellular reticular formation); dMRF (dorsal medullary reticular formation); LPGi (lateral paragigantocellular reticular formation); and MdD (dorsal medulary reticular formation).
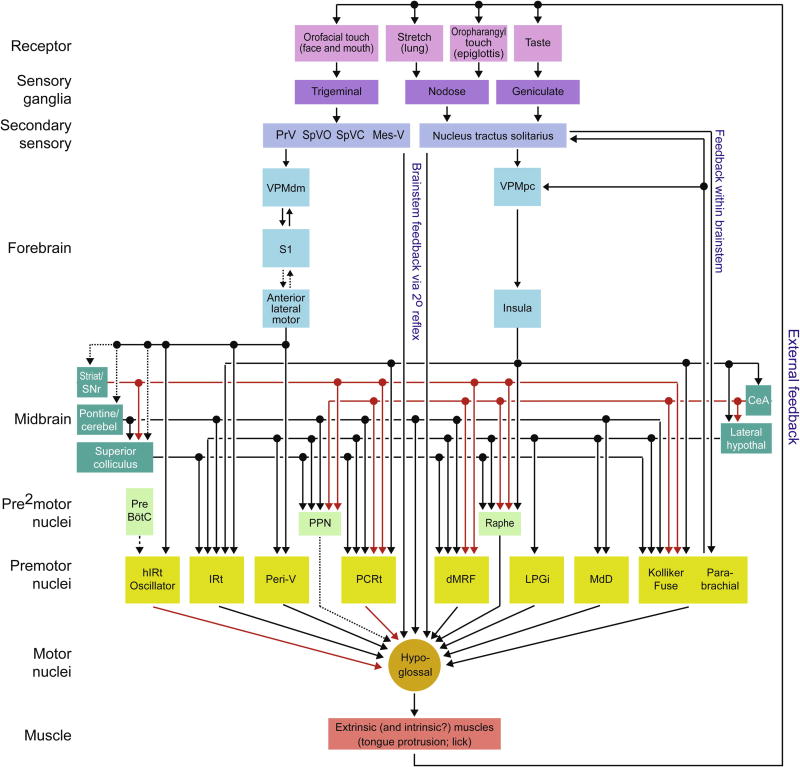
Figure 5. "Feedforward" circuit diagram for muscles of the tongue, i.e., geni- hyo-, chondro-, stylo- and patato-glossus.
Arrow signifies the direction of signal flow; red signifies inhibitory and black signifies excitatory connections. Compiled data as described in the text. Many feedback connections and interconnections among premotor structures have been excluded for simplicity. Dashed lines are connections based on functional rather than antomical data.
Abbreviations: PrV (principal trigeminal nucleus); SpVO (spinal subnucleus oralis); SpVC (spinal subnucleus caudalis); Mes-V (mesencephalic sensory nucleus); VPMdm (ventral posterior medial nucleus, dorsomedial); VPMpc (ventral posterior medial nucleus, parvicellular division); S1 (primary sensory) cortex; ALM (anterior medial motor) cortex; SNr (substantia nigra pars reticulata); CeA (central amygdala); Pontine/cerebellum (circuit from pontine nuclei through cerebellar deep nuclei); lateral hypothal (lateral hypothalamus); PPN (pedunculopontine nucleus); Raphe (Raphe nuclei); PreBötC (preBötzinger respiratory complex) hIRt (hypothalamus intermediate reticular zone); IRt (vibrissa intermediate reticular zone); Peri-V (peri-trigeminal area); PCRt (parvocellular reticular formation); dMRF (dorsal medullary reticular formation); LPGi (lateral paragigantocellular reticular formation); and MdD (dorsal medulary reticular formation).
Figure 6. Schematic circuit of the vibrissa somatosensorimotor system in terms of nested loops.
(A) Nested loops in the somatosensorimotor system. Only the pathways from the vibrissae to the brainstem and up through neocortex are shown. Black arrows indicate excitatory projections while red arrows are inhibitory projections. Adapted from Kleinfeld and Deschenes (Kleinfeld and Deschênes, 2011).
(B) Expanded diagram of first-order feedback loops involved in reflex motion of the vibrissae. Intrinsic protractor motoneurons receive both excitatory and inhibitory inputs from subnucleus SpVO, whereas nasolabialis and maxillolabialis retractor motoneurons receive excitatory input from subnucleus SpVIr neurons that also project to Po thalamus. Adapted from Bellavance et al. (Bellavance et al., 2017). The plane of the trigeminus has been tilted by ~ 30 degrees from that in part A.
Abbreviations: vS1 (vibrissa primary sensory cortex); vS2 (vibrissa secondary sensory cortex); vM1 (vibrissa motor cortex); PrV (principal trigeminal nucleus); SpVO (spinal subnucleus oralis); SpVIr and SpVIc (rostral and caudal divisions of spinal subnucleus interpolaris, respectively); SpVM (spinal subnucleus muralis); SpVC (spinal subnucleus caudalis); VPMdm (ventral posterior medial nucleus, dorsomedial); Po (medial division of the posterior group nucleus); nRt (nucleus reticularis); ZIv (ventral aspect of the zona incerta); SC (superior colliculus); vIRt (vibrissa intermediate reticular formation); NLP (nasolabialis profundus extrinsic protractor muscle); ML (maxolabialis extrinsic retrractor muscle); NL (nasolabialis extrinsic retrractor muscle); and DL (deflector nasi).
Coordination of multiple orofacial motor actions
The head of a rodent is in constant motion, bobbing from side-to-side and up-and-down, as the animal explores its peri-personal space (Fig. 1A). Further, similar mobility extends to the face itself as the nose moves from side-to-side (Fig. 1B) and the vibrissa scan back and forth (Fig. 1C). One component of this motion is a rhythmic modulation in position that is phase-locked to sniffing, the rapid aspect of breathing. This occurs with a frequency that is centered near 7 Hz in rats and 11 Hz in mice (Fig. 1D). The rhythmic component is observed in the underlying muscular control (Fig. 1D), which shows that motion of a sensor is not secondary to nearby body movement; this is illustrated for the splenius capitus muscles drive motion of the neck (Fig. 1A). In fact, the preBötzinger complex, which initiates inspiration, functions as a master oscillator that resets the premotor oscillator for whisking (Moore* et al., 2013, Deschênes et al., 2016) and is conjectured to function in a similar fashion for other orofacial rhythmic motor actions (Kleinfeld et al., 2015), including nose motion, head motion, licking, and vocalization. Thus the inspiratory phase of each sniff corresponds to a "snapshot" of multisensory sampling of the peri-personal space (Fig. 1D).
A second aspect of motor actions for orofacial sensation concerns the slow, coordinated changes in the orientation of the sensors, such as the concerted motion of the head, nose, and vibrissae toward a source of odor (Esquivelzeta Rabell et al., 2017, Kurnikova et al., 2017). It is unknown whether the coordinated movement of each sensor maximizes sensory input, such as by sweeping odorants toward the nose. Actions involving both vibrissa touch and olfaction, which includes elements of social interactions (Wolfe et al., 2011) as well as exploration and environmental disturbances (Yu et al., 2016), will lead to multimodal sensory inputs that are phase-locked to breathing. The coordination of these sensory inputs might lead to enhanced detection of external stimuli (Kleinfeld et al., 2014).
Sensorimotor network topology
Sensorimotor systems are comprised of nested loops (Kleinfeld et al., 1999, 2006, Bosman et al., 2011). The overarching loop structure consists of central and peripheral parts (Fig. 2). Through the peripheral portion of the loop, sensor movements result in changing sensory signaling. Peripheral reafference, i.e., the sensation of self-motion through the deformation of the body, as well as feedback though contact with objects in the world can directly control subsequent movements.
The central portion of sensorimotor loops comprises pathways that link sensory feedback to motor control. The most direct pathway is a reflex arc in which projections from primary sensory nuclei to the motor nucleus drive the motor plant. In parallel with reflex arcs, a multitude of other pathways mediate signal processing at many levels in the brain, including higher controllers, such as the cerebral cortex and cerebellum as we will discuss later. For muscles that participate in rhythmic motor actions, such as walking in the case of locomotion and whisking in the case of vibrissa-touch, an additional input consists of internal autonomous oscillators.
Hindbrain oscillators and coordination of orofacial motor actions
The premotor circuitry that drives rhythmic orofacial motor actions depends on several underlying oscillators. The predominant oscillator is the preBötzinger complex for inspiration (Smith et al., 1991, Feldman et al., 2013) (Fig. 3). This initiates the breathing cycle. The subsequent activation of a post-inspiratory complex, thought to be the Bötzinger complex but under re-evaluation (Anderson et al., 2016), leads to expiration. Forced expiration, as occurs during physical exertion, additionally involves the parafacial respiratory group (Molkov et al., 2017). The output from the preBötzinger includes a band of collaterals within the intermediate reticular (IRt) formation that rise toward the ventral edge of the hindbrain (Tan et al., 2010) and span multiple premotor nuclei (Moore et al., 2014). The preBötzinger output is now known to modulate the premotor whisking oscillator, denoted the vibrissa IRt (vIRt) formation (Moore* et al., 2013). The vIRt provides rhythmic inhibition of the facial motoneurons that drive the intrinsic muscles of the vibrissa mystacial pad (Deschênes et al., 2016). Intrinsic muscle motoneurons combine this rhythmic inhibitory input with sustained excitation from diverse premotor inputs that control whisker orientation and posture.
Additional pathways from the respiratory complex can control active orofacial exploration (Berg and Kleinfeld, 2003) (Fig. 3). When animals sniff, output from the preBötzinger complex appears to directly drive motoneurons of the extrinsic muscles (Deschênes et al., 2016). This results in retraction and early protraction of the mystacial pad (Hill et al., 2008, Simony et al., 2010), although the detailed relation of this phasing remains unsettled. Beyond whisking, it is conjectured that the respiratory complex entrains yet-to-be confirmed oscillators for rhythmic movements of the nose, tongue, and probably head (Kleinfeld et al., 2014) (Fig. 3). The temporal regularity of phaselocked signals, in principle, can improve the fidelity of decoding the stimuli during motor planning (Kleinfeld et al., 2014) and/or play a role in the saliency of the sensory input through further phase-locking with the hippocampal theta rhythm (Kleinfeld et al., 2016).
Other brainstem structures have a direct impact on respiration and thus rhythmic orofacial motor actions. Of note, the Kölliker-Fuse nucleus is reciprocally connected to the preBötzinger complex (Feil and Herbert, 1995, Tan et al., 2010) and plays an essential role in the control of breathing, e.g., glutamergic stimulation of the Kölliker-Fuse nucleus elicits apnea (Chamberlin and Saper, 1994). Ongoing work suggests that the Kölliker-Fuse nucleus also has a direct impact on whisking through a projection to the vibrissa region facial motor nucleus (Takatoh et al., 2013).
In addition to the preBötzinger complex, the other fundamental oscillator in the hindbrain regulates chewing (Fig. 3). Crucially, chewing appears to be incommensurate with breathing under all conditions investigated so far (McFarland and Lund, 1993, Liao and Kleinfeld, 2016). Consistent with this observation, the rhythmic motion of the tongue will coordinate with breathing during licking (Welzl and Bures, 1977), but the coordination shifts so that motion of the tongue is coherent with chewing during ingestion (Travers et al., 2010, Liao and Kleinfeld, 2016). The chewing oscillator controls muscles of the jaw and those involved with control of the airway. The strongest evidence to date appears to place the chewing oscillator in the dorsal principal trigeminal nucleus or the proximal reticular formation (Kolta et al., 2007) (Fig. 3). Chewing does not play a direct role in exploratory orofacial behaviors.
Architectonics of the vibrissa sensorimotor system
In active exploration, whisking is coordinated with other rhythmic orofacial behaviors and phase locked with sniffing (Fig. 1C). What is the underlying circuit that governs the over-all control of the motor plant for whisking and the processing of vibrissa-based touch signals? Here we focus on a "brainstem" centric map of connections that begin with trigeminal input and projection directly and indirection to the facial motoneurons for control of the intrinsic muscles (Fig. 4). Our presentation is driven by the available data and thus overemphasizes cortical loops while minimizing cerebellar loops and ignoring hippocampal loops as well as other forebrain areas such as lateral hypothalamus and the central nucleus of the amygdala. While each of the latter areas can influence vibrissa sensory and/or motor processing (Dietrich et al., 2015, Tovote et al., 2016, Han et al., 2017), the underlying circuitry is largely uncharted. We further address only the circuitry for the intrinsic muscle as the external muscles receive direct input from the respiratory complex (Deschênes et al., 2016) (Fig. 3).
Sensory plant
The vibrissa follicle-sinus complex provides the first stage for transduction of vibrissa touch as well as the motor drive for whisking through ensheathing muscle slings (Rice et al., 1986). In particular, mechanosensory transduction depends on bending of the vibrissae (Quist and Hartmann, 2012, Hires et al., 2013) and deformation of the encapsulating follicle blood sinus and epithelial specializations (Whiteley et al., 2015), i.e., the Merkel discs (Ikeda et al., 2014, Maksimovic et al., 2014), as well as specialized club endings of axons that capture the highest frequency deformations (Tonomura et al., 2015). Primary sensory axons travel via superficial or deep infraorbital branches of the trigeminal nerve (Dorfl, 1985, Rice et al., 1986). Each vibrissa follicle-sinus complex is innervated by a unique set of ~100 ganglia cells, which each express one type of mechanoreceptor and project to the periphery via one unbranched axon (Welker and Van der Loos, 1986, Li et al., 2011, Sakurai et al., 2013, Tonomura et al., 2015). Thus this first step in sensory transduction provides a high-fidelity spatial and temporal representation of vibrissa movement from individual follicles to non-overlapping subsets of parent trigeminal ganglia neurons (Jones et al., 2004), with resultant single whisker receptive fields for individual ganglion cells. Vibrissa afferents are broadly classified as rapidly adapting or slowly adapting, but their relationship to the six types of axon terminal specializations is incompletely understood (Ebara et al., 2017, Takatoh et al., 2017).
A central question is how the trigeminal system signals touch while also encoding vibrissa position. For many muscles, muscle spindle afferents travel in motor nerves and carry proprioceptive signals, yet such afferents were not identified the mystacial pad muscles or nerve fibers (Semba and Egger, 1986, Moore et al., 2015). Rather, ganglion cell responses to both artificial or awake whisking imply that trigeminal ganglion cells report self motion in addition to touch (Szwed et al., 2003, Leiser and Moxon, 2007, Severson et al., 2017). This phenomena is referred to as peripheral reafference. Physiological blockade of activity of either Merkel discs in the follicle sinus complex or their primary sensory afferents is sufficient to reduce performance of a behavioral tactile task (Ikeda et al., 2014, Maksimovic et al., 2014, Woo et al., 2014, Severson et al., 2017). These studies demonstrated that parent ganglion cells are selectively activated by “active touch”, i.e., the conjunction of whisker position within the protraction-retraction cycle and touch (Severson et al., 2017). This suggests that the activation of Merkel cells in the follicle sinus complex is a likely origin of peripheral reafference.
In addition to myelinated low-threshold mechanoreceptor input to the trigeminal ganglia, the follicles are innervated by numerous unmyelinated axons or “free nerve endings”. These axons course via the superficial sensory nerve to concentrate at the upper regions of the follicle; these unmyelinated fibers are not of sympathetic origin (Ebara et al., 2017). Unlike the mechanoreceptor afferents, little is known about the distribution or physiological properties of the parent neuron somata in the trigeminal ganglia for these afferents.
Multiple somatopic maps in the trigeminal nucleus complex
The trigeminal ganglia project into the brainstem trigeminal complex via ascending and descending central axonal branches. The trigeminal complex is comprised of six main subdivisions (Torvik, 1956, Clarke and Bowsher, 1962, Furuta et al., 2006, Matthews et al., 2015): the principal trigeminal nucleus (PrV), spinal trigeminal subnuclei pars oralis (SpVO), rostral interpolaris (SpVIr), caudal interpolaris (SpVIc), muralis (SpVM), and caudalis (SpVC). In nucleus PrV and subnucleus SpVIc (Belford and Killackey, 1979a, b, Tonomura et al., 2015), the central axons of the trigeminal ganglion cells map to structures that are well defined by cytological and histological borders, denoted “barrelettes” (Ma and Woolsey, 1984). These form topographic maps of the relative position of the mystacial vibrissa, Further, at the level of a functionally defined trigeminal nerve afferent types, such as those with terminal club endings for light touch, the central processes of individual ganglion cells are distributed across trigeminal nuclei with dense input to the nucleus PrV barrelettes and collateral projections to all other subnuclei (Tonomura et al., 2015).
Feedback among trigeminal subnuclei
A class of neurons in nucleus PrV respond to activation of multiple vibrissae, which is a departure of the single vibrissa activation patterns found for primary sensory neurons of the trigeminal ganglia (Minnery and Simons, 2003). One mechanism for generation of multi-vibrissa receptive fields is the extension of neighboring nerve afferents across barrelettes (Jacquin et al., 2015). This mechanism might complement a substrate of interneuron outputs that originate in subnucleus SpVIc and terminate with inhibitory connections in nucleus PrV (Jacquin et al., 1989, Furuta et al., 2008), as well as an excitatory projection from subnucleus SpVC to nucleus PrV (Furuta et al., 2008). The computational necessity of multi-vibrissa responses in nucleus PrV and their role in sensorimotor processing remain unclear.
Corticotrigeminal feedback
In addition to connections between trigeminal subnuclei that lie within the nucleus, trigeminal subnuclei also receive feedback via corticotrigeminal projections (Smith et al., 2015). Projections from nucleus PrV ascend to the dorsal medial aspect of the ventral posterior medial nucleus of dorsal thalamus, where they make three sets of representations (Urbain and Deschênes, 2007b); reviewed in (Deschênes et al., 2005). One of these representations, the dorsal medial aspect of the ventral posterior medial nucleus of dorsal thalamus (VPMdm), constitutes the primary afferent pathway. Neurons in VPMdm thalamus project to the middle and deep layers of vibrissa primary sensory (vS1) cortex (Shepherd et al., 2005). Crucially, this pathway carries the most salient information of vibrissa position and touch (Chiaia et al., 1991a, Chiaia et al., 1991b, Moore et al., 2015) (Fig. 4). This information is relayed to the vibrissa primary sensory (vS1) and secondary sensory (vS2) cortices.
Direct feedback connections to the caudal trigeminal spinal subnuclei arise from both vS1 (Matyas et al., 2010, Sreenivasan et al., 2015) and vS2 (Knutsen et al., 2015) cortices. The projection from vS1 cortex to spinal subnucleus SpVIc can gate the activity of PrV neurons via inhibition from subnucleus SpVIc to nucleus PrV (Furuta et al., 2010). This architecture is in the form of a classic inhibitory feedback circuit (Black, 1953) that in principle can lower the noise and increase the temporal sensitivity of the cortical response.
Premotor nuclei
We already noted that a region in the intermediate reticular zone, the vIRt, functions as the premotor oscillatory nucleus for the rhythmic component of whisking (Moore* et al., 2013, Deschênes et al., 2016). In contrast, the non-rhythmic aspects of whisking are mediated by the plethora of premotor centers in the reticular formation, spinal trigeminal subnuclei, and other brainstem premotor nuclei (Isokawa-Akesson and Komisaruk, 1987, Hattox et al., 2002). We emphasize the dual role of spinal trigeminal subnuclei as both primary sensory and premotor regions (Fig. 4). Anterograde tracing of trigeminal complex efferents identified innervation of premotor centers that were labeled from tracer injection to the facial motor nucleus (Zerari-Mailly et al., 2001). In particular, spinal trigeminal subnuclei project extensively to the dorsal medullary reticular formation, the parvocellular reticular and IRt formations, as well as others (Fig. 4). Subsequent viral tracing from muscles in the mystacial pad allowed selective identification of vibrissa-specific trigeminal outputs from subnuclei SpVO and SpVIr (Takatoh et al., 2013). These form a brainstem-level, di-synaptic reflex arc (Fig. 4). Projections from subnucleus SpVO lead to rapid, contact-induced inhibition followed by excitation to the intrinsic muscles while those from subnucleus SpVIr lead to contact-induced retraction of the mystacial pad (Bellavance et al., 2017).
Anatomical and physiological data suggest some premotor structures receive inputs primary vibrissa motor (vM1) cortex. Classical studies based on dye transport have identified vM1 cortical fibers in premotor structures including the IRt formation, parvocellular reticular, and gigantocellular reticular formations (Zerari-Mailly et al., 2001, Alloway et al., 2010). Virus tracing confirmed projections from vM1 cortex to the IRt formation (Sreenivasan et al., 2016). It will be of interest to determine whether these descending projections specifically include synapses onto premotor vIRt neurons, the site of the vibrissa oscillator.
From a functional perspective, intracellular stimulation of single layer 5 pyramidal cells in vM1 cortex evokes rhythmic whisker movements (Brecht et al., 2004b). This motor activation could arise from projections to pre-motor regions within the reticular formation or from a direct, albeit sparse projection to ventrolateral facial motor neurons (Grinevich et al., 2005, Sreenivasan et al., 2015). Alternatively, while projections from vM1 cortex to the trigeminal nuclear complex has been reported as absent (Smith et al., 2015) or limited to SpVO (Sreenivasan et al., 2015), this claim is under re-evaluation (Mercer Lindsay et al., 2016) and might provide yet another pathway from vM1 cortex to vibrissa motoneurons. Several additional structures, including the deep cerebellar nuclei and central amygdala (Hopkins and Holstege, 1978, Asanuma et al., 1983), project to brainstem regions. However, it is presently unclear whether these projections specifically target vibrissa premotor neurons.
The projections to the parvocellular reticular formation are of particular interest. While the specific relation of this region to vibrissa function has not been charted, a range of brain areas that relate to orofacial motor actions target the parvocellular reticular formation. Retrograde dye tracing identified afferents from motor cortex, as discussed above, as well as sensory cortex, deep cerebellar nuclei, substantia nigra pars reticulata, superior colliculus, the contralateral parvocellular reticular, IRt, and gigantocellular reticular formations, orofacial spinal trigeminal nuclei, and the parabrachial nucleus (Shammah-Lagnado et al., 1992). The parvocellular reticular formation is thus strategically positioned to integrate/arbitrate broad sensory and motor signals.
Midbrain motor control
The superior colliculus receives input from neurons with multi-vibrissa fields originating from trigeminal nucleus PrV and subnuclei SpVO and SpVIr, with terminals that end in the sensory intermediate layer of the colliculus. Cortico-collicular innervation to the intermediate layer originates from vS1 cortex (Zakiewicz et al., 2014, Castro-Alamancos and Favero, 2016) and vM1 cortex (Miyashita et al., 1994). The intermediate layers respond vigorously to active and passive whisker deflection when multiple vibrissae move together, but these responses rapidly depress. In contrast, the responses driven by corticocollicular inputs are weaker but more persistent. These data suggest that trigeminocollicular inputs code for novelty in the periphery while corticocollicular inputs subserve changes in sensitivity by neocortex behavioral state (Castro-Alamancos and Favero, 2016).
Electrical microstimulation of the superior colliculus produces short latency vibrissa protractions that are sustained for the duration of the stimulus (Castro-Alamancos and Keller, 2011, Stanek 4th et al., 2014). These likely reflect the direct projections from deep layers of the superior colliculus to the facial motor nucleus (Travers and Norgen, 1983, Vidal et al., 1988). However, it is unknown how the extensive local circuitry within the superior colliculus transforms vibrissa signals as they pass from intermediate sensory layers to deep motor output layers. Such circuitry might serve to coordinate vibrissa movements with broader aspects of orientation. For example, microstimulation of vibrissa units also produces coordinate movements of eyes and pinna along with vibrissae (Castro-Alamancos and Keller, 2011).
Cerebellum
As for the case of cortex and the superior colliculus, neurons in many regions of the cerebellum respond vigorously to tactile stimuli. The cerebellum receives extensive somatosensory information from cortical, brainstem, and, notably, primary afferent sources (Torvik, 1956, Jacquin et al., 1982, Mihailoff et al., 1985, Hartmann and Bower, 1998, Leergaard et al., 2000, O'Connor et al., 2002). Vibrissa stimulation is a strong driver of Purkinje cells (Shambes et al., 1978, Loewenstein et al., 2005, Bosman et al., 2010), and vibrissa somatosensory signaling has been implicated in motor planning via corticocerebellar loops (Proville et al., 2014). It remains unknown whether the cerebellum has an additional role in the control of vibrissa movements per se (Bower, 1997). Consistent with a potential motor control function, vibrissa movements are correlated with activity in deep nucleus output neurons (Lu et al., 2013), where local microstimulation has been shown evoke vibrissa movement in decerebrate rats (Cicirata et al., 1989). Projections from the cerebellum target broad areas of the reticular formation that contain orofacial premotor neurons (Cohen et al., 1958, Asanuma et al., 1983, Takatoh et al., 2013). Future efforts are needed to clarify the set of muscles and motor actions that are specifically controlled by cerebellar outputs.
Basal ganglia
The striatum receives topographically organized afferents from vM1 and vS1 cortices as well as posterior medial thalamus (Leergaard et al., 2000, Hoffer and Alloway, 2001, Smith et al., 2012). Striatal medium spiny neurons respond to vibrissa stimulation (Mowery et al., 2011), with unimodal responses in dorsal lateral regions but multisensory responses in dorsal medial regions (Reig and Silberberg, 2014). The importance of vibrissa signaling in the basal ganglia in guiding behavior has not been determined. The requirement of dopamine for normal vibrissa sensory signaling in striatum (Ketzef et al., 2017), plus the central role of the basal ganglia in reward-based sensor orientation (Hikosaka, 2007), suggests a plausible role of the substantia nigra pars reticulata, an output nucleus of the basal ganglia, in slow changes in vibrissa position. An intriguing possibility is adjustments in set-point via nigral projections to the superior colliculus.
Modulation
Cholinergic neurons in the pedunculopontine nucleus comprise a key component of the reticular activating system and innervate several brain regions implicated in vibrissa motor control (Fig. 4), including the superior colliculus, where cholinergic agonists increase the response of units to passive and active touch of whiskers (Bezdudnaya and Castro-Alamancos, 2014). The superior colliculus also projects back to the pedunculopontine nucleus (Martinez-Gonzalez et al., 2011) to form a potential feedback loop.
Of interest, the ascending output fibers of pedunculopontine nucleus project to the basal forebrain, whose activity has been previously shown to augment the responsiveness of cortical units to vibrissa (Berg et al., 2005). Selective optogenetic stimulation of pedunculopontine nucleus terminals in the basal forebrain was sufficient to elicit behavioral effects of whisking and sniffing (Lee et al., 2014). Thus cholinergic modulation by the pedunculopontine nucleus, along with serotonergic modulation by the Raphe nucleus (Hattox et al., 2003), may profoundly change the nature of whisking based on the brain state of the animal (Ganguly and Kleinfeld, 2004).
Architectonics of the lingual sensorimotor system
A broad set of orofacial behaviors depend critically on movement of the tongue. As for the case of whisking, one component of the lingual movement is phase-locked to the respiratory rhythm under licking and some behavioral contexts (Lowe and Sessle, 1973, Welzl and Bures, 1977) (Fig. 1D). Tongue movements are well integrated with facial, oral, and pharyngeal musculature. They are critical for communication, feeding, and breathing. Similar to the vibrissa movements, mammalian tongue movements arise from coordinated activation of extrinsic and intrinsic muscles groups (Sonntag, 1925, Abd-El-Malek, 1938, Lowe, 1980). The hypoglossal motor nucleus in the caudal aspect of the medulla contains the motor neurons that control both muscles groups (Lewis et al., 1971).
One type of brainstem circuit that controls tongue movements involves neuronal oscillators that transform descending and local signals into rhythmic and coordinated behaviors, i.e., licking, chewing, and swallowing (Dellow and Lund, 1971, Lowe, 1980, Nakamura and Katakura, 1995, Jean, 2001, Miller, 2002) (Fig. 5). A second type of circuit makes use of sensory feedback. Dense afferent innervation of the face, tongue, mouth, and airway provide fine somatosensory and chemosensory feedback to brainstem circuitry. Tactile signals from the oral cavity, including the tongue and teeth, are carried by trigeminal ganglia to all divisions of the trigeminal sensory complex, particularly in dorsal regions of the subnuclei (Sessle and Greenwood, 1976, Shigenaga et al., 1986a, Shigenaga et al., 1986b). In complement to trigeminal complex signaling, visceral afferent and taste signals are topographically organized in the nucleus of the tractus solitarius (Sessle, 1973, King, 2007). Taste and sensory signals from the mucous membranes of the pharynx, the posterior third of the tongue, and the tonsils are carried via the facial, glossopharyngeal, and vagus nerves (Torvik, 1956, Carleton et al., 2010). These visceral afferents are integrated in the nucleus of the tractus solitarius with secondary sensory signals from trigeminal subnuclei (Burton et al., 1979, Contreras et al., 1982, Aldes and Boone, 1985, Pinganaud et al., 1999, Zhang et al., 2001).
Feedback in lingual sensorimotor control
A large component of tongue movements arise from elementary and complex reflexes (Miller, 2002). These reflexes rely on di- and tri-synaptic pathways that link trigeminal, hypoglossal, and vagal afferents to hypoglossal motor control. In their simplest form, brainstem reflexes are controlled by a disynaptic arc in which neurons from sensory nuclei synapse onto motoneurons (Fig. 2). In particular, the trigeminal nuclei form extensive brainstem projections that include synapses directly onto hypoglossal motor neurons (Burton et al., 1979, Aldes and Boone, 1985, Pinganaud et al., 1999, Zhang et al., 2001). Within nucleus PrV, projection neurons are segregated such that neurons in the ventral part of the nucleus project to the lateral facial nucleus, while neurons in the dorsal part of nucleus PrV project to the hypoglossal motor nucleus (Pinganaud et al., 1999). In contrast, subnuclei SpVI and SpVC contain intermingled premotor neurons, including some that collateralize to both the facial and hypoglossal nuclei. This is suggestive of a locus for interaction of different orofacial motor actions within the trigeminal spinal nuclei. Lastly, a projection from the mesencephalic trigeminal neurons to the hypoglossal motor nucleus is likely to underlie the jaw-to-tongue reflex, in which jaw opening results in tongue protrusion (Zhang et al., 2001, Luo et al., 2006), which plays a critical role in maintaining airway patency (Miller, 2002).
The nucleus of the tractus solitarius sends sparse direct and numerous indirect projections to the hypoglossal motor nucleus. Direct projections arise from a caudal region in the nucleus of the tractus solitarius that receives afferents via the glossopharyngeal and vagus nerves (Torvik, 1956, Contreras et al., 1982, Borke et al., 1983, Travers and Norgen, 1983). The function of this direct projection remains unknown, whereas indirect projections from the nucleus of the tractus solitarius to the hypoglossal motor nucleus via the parabrachial sensory nucleus and medullary reticular formation play a central role in swallowing and food rejection reflexes (DiNardo and Travers, 1997, Jean, 2001, Lang, 2009).
Oscillators for rhythmic licking
As for the case of whisking, motion of the tongue is modulated in phase with respiration during breathing (Doty and Bosma, 1956, Sauerland and Mitchell, 1970, Harvold et al., 1973, Lowe and Sessle, 1973, Welzl and Bures, 1977, Wiesenfeld et al., 1977, Sawczuk and Mosier, 2001). Rhythmic licking is commonly faster than basal breathing but the onset of licking is reset by breathing (Welzl and Bures, 1977). This parallels the case of whisking and basal breathing (Moore* et al., 2013). The occurrence of licking and breathing at different rates indicates the existence of an independent licking oscillator (Travers et al., 1997, Koizumi et al., 2008, Stanek 4th et al., 2014). The one-to-one relation of licking to the sniff cycle (Liao and Kleinfeld, 2016) and the absence of licking during pharmacological block of spiking by neurons throughout the IRt formation lend support to the hypothesis that a subregion of the IRt formation, denoted the hypoglossal IRt (hIRt) formation, comprises an essential component of the licking oscillator (Travers et al., 1997, Ono et al., 1998, Chen et al., 2001) (Figs. 3 and 5).
Ingestive behaviors require precise coordination of the musculature of the jaw, face, tongue, and airway. The central role of the tongue in each aspect of feeding suggests that several oscillators and/or premotor nuclei can recruit hypoglossal motor neurons. Several populations of neurons in the IRt and parvocellular reticular formations project to multiple orofacial nuclei (Li et al., 1993, Travers et al., 2005, Stanek 4th et al., 2014) and likely underlie distinct aspects of feeding, including chewing (Nakamura and Katakura, 1995, Lund et al., 1998, Morquette et al., 2012). While tongue movements during chewing are largely in phase with jaw opening (Dellow and Lund, 1971, Morimoto and Kawamura, 1973, Lund, 1991, Liao and Kleinfeld, 2016), tongue movements also display a prominent non-rhythmic component that positions food in the mouth (Abd-El-Malek, 1955). Further, as noted earlier, the rhythmic motion of the tongue will dramatically shift from phase-locking with breathing during licking (Welzl and Bures, 1977) to locking with chewing during ingestion (Travers et al., 2010, Liao and Kleinfeld, 2016). The nature of these dynamics, as well as the control of multiple lingual muscles during licking, chewing, and swallowing, depends on unknown brainstem circuit mechanisms that enable action sequencing across different premotor nuclei.
Control of the posture of the tongue is analogous to the set-point of the vibrissa, albeit a more complicated motor act given the much greater degrees of freedom for the tongue. Tongue posture is a primary factor of pharynx patency. Synchronous tongue protrusion and inspiration improve upper airway flow by dilating the oropharynx (Blom, 1960, Lei, 1961, Lowe and Sessle, 1973, Miller and Bowman, 1974, Bartlett, 1986). The pontine respiratory group, including the parabrachial nucleus and the Kölliker-Fusse, is involved in respiratory control (Molkov et al., 2017). Stimulation of the Kölliker-Fuse activates tongue protrusion muscles (Kuna and Remmers, 1999, Yokota et al., 2011) and thus may underlie some component of respiration-lingual synchrony.
Premotor networks and descending controllers
Descending projections to brainstem circuits arise from diverse regions of the motor and limbic system (Fig. 5) and provide a plausible circuit basis for goal-directed orofacial actions.
Cerebral Cortex
The most extensively studied high-level controller of tongue movements is the motor cortex, which can evoke licking, chewing, and swallowing (Sessle, 2011). Recent work highlights the importance of the anterior lateral motor (ALM) cortex in driving movement of the tongue (Komiyama et al., 2010) and, further, supplies anatomical evidence for a direct connection from ALM cortex to the region that contains a candidate licking oscillator, the hIRt (Ono et al., 1998, Li et al., 2015). Anterior lateral motor cortex appears to be a hub that plans and executes voluntary licking under sensorimotor learning tasks (Guo et al., 2014a, 2014b, Li et al., 2015, 2016), however it is notable that licking related to food and water consumption is retained in decerebrate animals (Woods, 1964). Pyramidal tract neurons in layer 5 project to the hIRt and have activity patterns consistent with a directional motor command (Li et al., 2015). In addition to direct projections from ALM cortex to the hIRt, ALM cortex projects to the motor related, lateral sector of superior colliculus, that in turn projects to premotor neurons in the hIRt (Yasui et al., 1994). This projection might coordinate tongue movements and the general orientation or the head and face muscles, although the relative roles of the direct and indirect connections between ALM cortex and the hIRt in the control of the direction of licking remain unknown. All told, the current experimental evidence implies that ALM cortex is a major source of a motor command that initiates learned directional licking.
Basal Ganglia
The ventral-lateral portion of the striatum receives orofacial cortical afferents (McGeorge and Faull, 1987) and contains neurons that modulate their firing in relation to licking (Mittler et al., 1994). Basal ganglia output neurons in the substantia nigra pars reticulata project to diverse premotor tongue regions (Hopkins and Niessen, 1976, Schneider, 1986, von Krosigk et al., 1992, Yasui et al., 1992, Tsumori and Yasui, 1997) (Fig. 5). Further, orofacial actions can be readily evoked by perturbations to the striatum or substantia nigra pars reticulata (Delfs and Kelley, 1990, Inchul et al., 2005), although orofacial actions evoked from the basal ganglia are abnormal in form. Thus, additional approaches are needed to delineate the normal function of nigral afferents to brainstem. The substantia nigra pars reticulata strongly inhibits the lateral superior colliculus, which has been proposed to mediate the effects of the basal ganglia on orofacial actions (Redgrave et al., 1980). It remains undetermined whether the orofacial deficits that follow collicular perturbations reflect specific motor effects or are a consequence of broad sensory neglect and orienting deficits (Wang and Redgrave, 1997).
Cerebellum
Oral and perioral sensory responses are prominent in cerebellum Crus I and II (Shambes et al., 1978, Apps and Hawkes, 2009), which receive a broad spectrum of orofacial sensory inputs from the trigeminal complex (Van Ham and Yeo, 1992), primary trigeminal afferents (Jacquin et al., 1982, Jacquin and Zeigler, 1983), and potentially orofacial-based sensory reward signals from an unknown mossy fiber source (Wagner et al., 2017). Several classes of cerebellar neurons display firing patterns related to tongue movements, which suggests that the cerebellum might play an active role in tongue motor control. Purkinje cells in Crus I and II modulate activity during licking (Bryant et al., 2010) and, importantly, complex-spike-lick-responses in Purkinje cells are maintained following deafferentation of oral and perioral trigeminal sensory feedback (Welsh et al., 1995). Interneurons in the cerebellum molecular later in Crus II specifically exhibit firing patterns that correlate with licking kinematics, but not tongue position, and chemogenetic suppression of activity in this interneuron class alters tongue movements and decreases licking rates (Gaffield and Christie, 2017). In addition, output neurons in the medial deep cerebellar nucleus, i.e., the fastigial nucleus, exhibit spiking that is locked with licking (Lu et al., 2013). Stimulation of the fastigial nucleus can evoke tongue movements and complex orofacial actions (Bowman and Aldes, 1980, Berntson and Torello, 1982), and application of muscimol to the cerebellar nuclei decreases licking rate and efficiency (Bryant et al., 2010). Moreover, the neurons in the fastigial nucleus projects to diverse premotor tongue areas and directly to the hypoglossal nuclei (Cohen et al., 1958, Asanuma et al., 1983, Teune et al., 2000, Stanek 4th et al., 2014). These projections, together with the representation of both sensory and motor signals, are highly suggestive an integrative role of cerebellar circuits in sensory-guided tongue control.
Additional putative tongue control regions
Afferents to brainstem tongue premotor areas additionally arise from regions outside of the traditional somatomotor system (Hopkins and Holstege, 1978, Holstege, 1987, Van Bockstaele et al., 1989, Grofova and Keane, 1991, Shammah-Lagnado et al., 1992, Ugolini, 1995, Karimnamazi and Travers, 1998, Almeida et al., 2002). Among these are projections from the lateral hypothalamus and central amygdala (Hopkins and Holstege, 1978, Holstege, 1987), both of which have been implicated as key regulators of feeding behaviors (Kaku, 1984, Kapp et al., 1985, Schwartzbaum, 1988, Petrovich, 2011). As in the case of control of the set-point of the vibrissa, the plethora of pre- and pre2motor nuclei suggests that there are several tongue controllers, consistent with the broad diversity of tongue behaviors and evidence that many high-level areas are capable of evoking or perturbing tongue movements (Bowman and Aldes, 1980, Berntson and Torello, 1982, Kaku, 1984, Schwartzbaum, 1988, Inchul et al., 2005, Li et al., 2015).
Redrawing the anatomy to emphasize nested cortical loops and sensory feedback pathways
The anatomy of sensorimotor systems may be reworked from a sensory-to-motor flow diagrams (Figs. 4 and 5) to ones that follow the anatomy more explicity to emphasize the nested loop structure and feedback at varius circuit stages (Kleinfeld et al., 1999, 2006, Bosman et al., 2011) We do this for the vibrissa sensorimotor system (Fig. 6A), noting that similar nested loop architectures appear common in motor control circuits.
Brainstem sensory feedback loops
The most direct feedback loops in motor control are direct sensory nucleus to motor neuron loops. Such connections likely mediate automatic or innate aspects of sensorimotor behaviors. In the vibrissa system, projections from trigeminal subnuclei mediate many aspects of motor control. In particular, projections from the spinal trigeminal subnuclei to intrinsic muscle motor neurons (Nguyen and Kleinfeld, 2005, Sherman et al., 2013, Sreenivasan et al., 2015, Bellavance et al., 2017) drive protraction of the follicle (Klein and Rhoades, 1985, Hill et al., 2008, Simony et al., 2010). Neurons in subnucleus SpVO project to intrinsic muscle motor neurons and supply a touch-induced biphasic response, with fast inhibition followed by excitation (Bellavance et al., 2017) (Fig. 6B). This leads to transient decrement in the electromyogram of the mystacial pad (Kleinfeld et al., 2002) and a dip in the touch response (Deutsch et al., 2012). A class of neurons in rostral subnucleus SpVIr drive retraction of the mystacial pad (Bellavance et al., 2017) (Fig. 6B) and modulate the period of contact. These feedback signals lead to a "caressing" of an object by the vibrissa and appear to operate as a proportional-differential controller (Best, 1984), althought the computations role of such feedback in improving recognition of the environment is a matter of speculation. Local trigeminal circuitry also shapes ascending sensory processing: cells in subnucleus SpVIc inhibit neurons in nucleus PrV (leftward red arrow in brainstem row; Fig. 6A) and provide sensory feedback to spatially and temporally sharpen sensory responses (Furuta et al., 2008, Bellavance et al., 2010).
Corticothalamic loops
The monosynaptic projections within the medulla are paralleled by multiple polysynaptic pathways at the level of the hindbrain and midbrain, e.g., the superior colliculus and reticular formation, and by pathways that extend through the forebrain (Kleinfeld et al., 1999) (Fig. 6A). Projections from nucleus PrV ascend to VPMdm thalamus, form a closed loop with inhibitory cells in nucleus reticularis (nRt, red arrow in middle row; Fig. 6A), and further project to the middle and deep layers of vS1 (Shepherd et al., 2005). They cluster into columns that maintain a one-to-one relation with the spatial distribution of the vibrissae; reviewed in (Lefort et al., 2009) (top row, Fig. 6A).
The second set of asending projections emanates from trigeminal subnucleus SpVIr to the part of the posterior medial (Po) thalamis complex that borders the VPMdm thalamus. These include both direct excitatory input from subnucleus SpVIr as well as inhibitory input that comes indirectly via projections to the ventral aspect of the zona incerta (Bartho et al., 2002). The latter input is part of a forebrain loop in which activity in Po thalamus is modulated by projection neurons from vM1 cortex to zona incerta, which inactivates an inhibitory input to PO thalamus (Urbain and Deschênes, 2007a) (back-to-back red arrows in middle row; Fig. 6A). Neurons in Po thalamus project to the septa between columns and primarily form connections with dendrites on the surface and middle layers of vS1, i.e., layers 1 and 5a, in a pattern that appears complementary to that formed by input from VMPdm thalamus (top row; Fig. 6A).
Corticofugal pathways
The classically described sensory and motor regions of cortex are highly interconnected at the level of the cortex itself as well as through subcortical interactions and feedback from cortex to thalamus. The highest level feedback loop in the vibrissa system is completed by descending projections from cortex to the vibrissa motoneurons in the facial motor nucleus (Fig. 6A). The dominant pathway of cortical-induced movement of the vibrissae is from vM1 cortex (Brecht, 2004, Brecht et al., 2004a, Berg et al., 2005, Auffret et al., 2017). This involves indirect connections through the superior colliculus and other midbrain and hindbrain structures (Miyashita et al., 1994, Hattox et al., 2002), as well as a direct, albeit sparse projection (Grinevich et al., 2005) (right column, Fig. 6A). A second pathway for activation of the vibrissae involves a projection from vS1 cortex (Matyas et al., 2010) that drives cells in spinal trigeminal subnuclei that further project to the facial nucleus (Bellavance et al., 2017) (Fig. 6A). The vS1 cortical projections lead to protraction of the vibrissa, while a second, recently described projections from vS2 cortex leads to retraction of the vibrissa (Knutsen et al., 2015) so that, together, vS1 and vS2 cortices can shift the position of the vibrissa in a push-pull fashion. How vibrissa motor neurons combine parrallel premotor inputs remains poorly understood. More generally, the anatomical data suggest that motor neurons themselves might serve as arbitrators of the control of motor output from different levels in the brain, a role consistent with their electrotonically long dendrites and active currents (Nguyen et al., 2004).
Open issues on the coordination of motor actions in behaviors
The exploratory motor actions that have been quantified so far exhibit rhythmic components as well as directed movements. Our past work demonstrated that coordination of the rhythmic components by inspiratory breathing is a key element of exploratory behavior. Yet such stringent synchrony would appear to limit the behavioral repertoire of the animal, suggesting that the control structure for undiscovered stereotypic behaviors may have a more fluid strategy. In particular, are orofacial behaviors organized by brainstem circuits and gated and/or modulated under different contexts by descending controllers? Studies in which inhibitory output from the amygdala is interpreted as "releasing" different behaviors support this view (Fadok et al., 2017, Han et al., 2017, Sanford et al., 2017). Alternatively, are stereotypic behaviors coordinated and directed from outside the brainstem per se, such as in motor cortex or cerebellum? While long-duration electrical stimuli in motor cortex can lead to holistic behaviors (Graziano et al., 2002), the interpretation of such experiments remains controversial (Schwartz, 2007). Under either scenario, a key circuit-level question is how the brainstem arbitrates high-level inputs, such as from the motor cortex, the cerebellum, the amygdala, and so forth, to produce well-controlled behavior. We hope to resolve the hierarchical control structure of the vibrissa system, as a canonical sensorimotor system, in the coming years.
Highlights.
Orofacial motor actions control sensation at short peripersonal distances.
The orofacial nuclei for control of the motor plants lie within the hindbrain.
The position of the sensors is determined by rhythmic and orienting components.
The rhythmic component is phase-locked to sniffing as the animal explores.
Feedback from somatosensory trigeminal nuclei rapidly alters motion of the sensors.
Acknowledgments
We thank Ehud Ahissar, Song-Mao Liao, Nicole Mercer Lindsay, Jeffrey Moore and Haim Sompolinsky for discussions. This research was supported by grants from the Canadian Institutes of Health Research (grant MT-5877), the Howard Hughes Medical Institute, the National Institute of Mental Health (MH085499), the National Institute of Neurological Disorders and Stroke (NS058668, NS077986, NS101441 and NS0905905), the National Science Foundation (EAGER - 2144GA), and the Tourette Association of America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibiography
- Abd-El-Malek S. A contribution to the study of the movements of the tongue in animals, with special reference to the cat. Journal of Anatomy. 1938;73:15–30. [PMC free article] [PubMed] [Google Scholar]
- Abd-El-Malek S. The part played by the tongue in mastication and deglutition. Journal of Anatomy. 1955;89:250–354. [PMC free article] [PubMed] [Google Scholar]
- Aldes LD, Boone TB. Organization of projections from the principal sensory trigeminal nucleus to the hypoglossal nucleus in the rat: An experimental light and electron microscopic study with axonal tracer techniques. Experimental Brain Research. 1985;59:16–29. doi: 10.1007/BF00237661. [DOI] [PubMed] [Google Scholar]
- Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respiratory Physiology & Neurobiology. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloway KD, Smith JB, Beauchemin KJ. Quantitative analysis of the bilateral brainstem projections from the whisker and forepaw regions in rat primary motor cortex. Journal of Comparative Neurology. 2010;518:4546–4566. doi: 10.1002/cne.22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A, Cobos A, Tavares I, Lima D. Brain afferents to the medullary dorsal reticular nucleus: a retrograde and anterograde tracing study in the rat. European Journal of Neuroscience. 2002;16:81–95. doi: 10.1046/j.1460-9568.2002.02058.x. [DOI] [PubMed] [Google Scholar]
- Anderson TM, Garcia AJ, Baertsch NA, Pollak J, Bloom JC, Wei AD, Rai KG, Ramirez J-M. A novel excitatory network for the control of breathing. Nature. 2016;536:76–80. doi: 10.1038/nature18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Hawkes R. Cerebellar cortical organization: A one-map hypothesis. Nature Reviews of Neuroscience. 2009;10:670–681. doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- Asanuma C, Thach WT, Jones EG. Brainstem and spinal projections of the deep cerebellar nuclei in the monkey, with observations on the brainstem projections of the dorsal column nuclei. Brain Research. 1983;286:299–322. doi: 10.1016/0165-0173(83)90017-6. [DOI] [PubMed] [Google Scholar]
- Auffret M, Ravano VL, Rossi GMC, Hankov N, Petersen MFA, Petersen CCH. Optogenetic stimulation of cortex to map evoked whisker movements in awake head-restrained mice. Neuroscience. 2017 doi: 10.1016/j.neuroscience.2017.04.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartho P, Freund TF, Acsady L. Selective GABAergic innervation of thalamic nuclei from zona incerta. European Journal of Neuroscience. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- Bartlett D. Comprehensive Physiology. John Wiley & Sons, Inc; 1986. Upper Airway Motor Systems. [Google Scholar]
- Belford GR, Killackey HP. The development of vibrissae representation in subcortical trigeminal centers of the neonatal rat. Journal of Comparative Neurology. 1979a;188:63–74. doi: 10.1002/cne.901880106. [DOI] [PubMed] [Google Scholar]
- Belford GR, Killackey HP. Vibrissa representation in subcortical trigeminal centers of the neonatal rat. Journal of Comparative Neurology. 1979b;183:305–322. doi: 10.1002/cne.901830207. [DOI] [PubMed] [Google Scholar]
- Bellavance M-A, Demers M, Deschênes M. Feedforward inhibition determines the angular tuning of vibrissal responses in the principal trigeminal nucleus. Journal of Neuroscience. 2010;30:1057–1063. doi: 10.1523/JNEUROSCI.4805-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavance M-A, Takatoh J, Lu J, Demers M, Kleinfeld D, Wang F, Deschênes M. Parallel inhibitory and excitatory trigemino-facial feedback circuitry for reflexive vibrissa movement. Neuron. 2017;95:673–682. doi: 10.1016/j.neuron.2017.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RW, Friedman B, Schroeder LF, Kleinfeld D. Activation of nucleus basalis facilitates cortical control of a brainstem motor program. Journal of Neurophysiology. 2005;94:699–711. doi: 10.1152/jn.01125.2004. [DOI] [PubMed] [Google Scholar]
- Berg RW, Kleinfeld D. Rhythmic whisking by rat: Retraction as well as protraction of the vibrissae is under active muscular control. Journal of Neurophysiology. 2003;89:104–117. doi: 10.1152/jn.00600.2002. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Micco DJ. Organization of brainstem behavioral systems. Brain Research Bulletin. 1976;1:471–483. doi: 10.1016/0361-9230(76)90117-9. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Torello MW. The paleocerebellum and the integration of behavioral function. Physiological Psychology. 1982;10:2–12. [Google Scholar]
- Best RE. Phase-Locked Loops: Theory, Design, and Applications. New York: McGraw-Hill; 1984. [Google Scholar]
- Bezdudnaya T, Castro-Alamancos MA. Neuromodulation of whisking related neural activity in superior colliculus. Journal of Neuroscience. 2014;34:683–7695. doi: 10.1523/JNEUROSCI.0444-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black HS. Modulation Theory. New York: Van Nostrand; 1953. [Google Scholar]
- Blom S. Afferent influences on tongue muscle activity. A morphological and physiological study in the cat. Acta Physiologica Scandinavica Supplementum. 1960;49:1–97. [PubMed] [Google Scholar]
- Borke RC, Nau ME, Ringler RL. Brain-stem afferents of hypoglossal neurons in the rat. Brain Research. 1983;269:47–55. doi: 10.1016/0006-8993(83)90961-7. [DOI] [PubMed] [Google Scholar]
- Bosman LWJ, Houweling AR, Owens CB, Tanke N, Shevchouk OT, Rahmati N, Teunissen WHT, Ju C, Gong W, Koekkoek SKE, DeZeeuw CI. Anatomical pathways involved in generating and sensing rhythmic whisker movements. Frontiers in Integrative Neuroscience. 2011;5:e1. doi: 10.3389/fnint.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman LWJ, Koekkoek SEK, Shapiro J, Rijken BFM, Zandstra F, B vdE, Owens CB, Potters J-W, de Gruijl J-R, Ruigrok TJH, De Zeeuw CI. Encoding of whisker input by cerebellar Purkinje cells. Journal of Physiology. 2010;588:3757–3783. doi: 10.1113/jphysiol.2010.195180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JM. Is the cerebellum sensory for motor's sake, or motor for sensory's sake: the view from the whiskers of a rat? Progress in Brain Research. 1997;114:463–496. doi: 10.1016/s0079-6123(08)63381-6. [DOI] [PubMed] [Google Scholar]
- Bowman JP, Aldes LD. Organization of the cerebellar tongue representation in the monkey. Experimental Brain Research. 1980;39:249–259. doi: 10.1007/BF00237114. [DOI] [PubMed] [Google Scholar]
- Brecht M. What makes whiskers shake? Focus on "Current flow in vibrissa motor cortex can phase-lock with exploratory rhythmic whisking in rat". Journal of Neurophysiology. 2004;92:1265–1266. doi: 10.1152/jn.00404.2004. [DOI] [PubMed] [Google Scholar]
- Brecht M, Fee MS, Garaschuk O, Helmchen F, Margrie TW, Svoboda K, Osten P. Novel approaches to monitor and manipulate single neurons in vivo. Journal of Neuroscience. 2004a;24:9223–9227. doi: 10.1523/JNEUROSCI.3344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider Brecht M, Sakmann B, Margrie T. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature. 2004b;427:704–710. doi: 10.1038/nature02266. [DOI] [PubMed] [Google Scholar]
- Bryant JL, Boughter JD, Gong S, LeDoux MS, Heck DH. Cerebellar cortical output encodes temporal aspects of rhythmic licking movements and is necessary for normal licking frequency. European Journal of Neuroscience. 2010;32:41–52. doi: 10.1111/j.1460-9568.2010.07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Craig AD, Jr, Poulos DA, Molt JT. Efferent projections from temperature sensitive recording loci within the marginal zone of the nucleus caudalis of the spinal trigeminal complex in the cat. Jounal of Comparative Neurology. 1979;183:753–777. doi: 10.1002/cne.901830406. [DOI] [PubMed] [Google Scholar]
- Carleton A, Accolla R, Simon SA. Coding in the mammalian gustatory system. Trends in Neuroscience. 2010;33:326–334. doi: 10.1016/j.tins.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos M, Keller A. Vibrissal midbrain loops. Scholarpedia. 2011;6:7274. doi: 10.4249/scholarpedia.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Favero M. Whisker-related afferents in superior colliculus. Journal of Neurophysiology. 2016;115:2265–2279. doi: 10.1152/jn.00028.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. Journal of Neuroscience. 1994;14:500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Travers SP, Travers JB. Muscimol infusions in the brain stem reticular formation reversibly block ingestion in the awake rat. American Journal of Physiology: Regulatory, Intergrative, and Comparative Physiology. 2001;280:R1085–1094. doi: 10.1152/ajpregu.2001.280.4.R1085. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Rhoades RW, Bennett-Clark CA, Fish SE, Killackey HP. Thalamic processing of vibrissal information in the rat I. Afferent input to the medial ventral posterior and posterior nuclei. Journal of Comparative Neurology. 1991a;314:201–216. doi: 10.1002/cne.903140202. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Rhoades RW, Fish SE, Killackey HP. Thalamic processing of vibrissal information in the rat: II. Morphological and functional properties of medial ventral posterior nucleus and posterior nucleus neurons. Journal of Comparative Neurology. 1991b;314:217–236. doi: 10.1002/cne.903140203. [DOI] [PubMed] [Google Scholar]
- Cicirata F, Angaut P, Pantó MR, Serapide MF. Neocerebellar control of the motor activity: Experimental analysis in the rat. Comparative aspects. Brain Research Reviews. 1989;14:117–141. doi: 10.1016/0165-0173(89)90011-8. [DOI] [PubMed] [Google Scholar]
- Clarke WB, Bowsher D. Terminal distribution of primary afferent trigeminal fibers in the rat. Experimental Neurology. 1962;6:372–383. doi: 10.1016/0014-4886(62)90019-5. [DOI] [PubMed] [Google Scholar]
- Cohen D, Chambers WW, Sprague JM. Experimental study of the efferent projections from the cerebellar nuclei to the brainstem of the cat. Journal of Comparative Neurology. 1958;109:233–259. doi: 10.1002/cne.901090207. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: An autoradiographic study in the rat. Journal of the Autonomic Nervous System. 1982;6:303–322. doi: 10.1016/0165-1838(82)90003-0. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Kelley AE. The role of D1 and D2 dopamine receptors in oral stereotypy induced by dopaminergic stimulation of the ventrolateral striatum. Neuroscience. 1990;39:59–67. doi: 10.1016/0306-4522(90)90221-o. [DOI] [PubMed] [Google Scholar]
- Dellow PG, Lund JP. Evidence for central timing of rhythmical mastication. Journal of Neurophysiology. 1971;215:1–13. doi: 10.1113/jphysiol.1971.sp009454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes M, Takatoh J, Kurnikova A, Moore JD, Demers M, Elbaz M, Furuta T, Wang F, Kleinfeld D. Inhibition, not excitation, drives rhythmic whisking. Neuron. 2016;90:374–387. doi: 10.1016/j.neuron.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes M, Timofeeva E, Lavallée P, Dufresne E. The vibrissal system as a model of thalamic operations. Progress in Brain Research. 2005;149:31–40. doi: 10.1016/S0079-6123(05)49003-2. [DOI] [PubMed] [Google Scholar]
- Deutsch D, Pietr M, Knutsen PM, Ahissar E, Schneidman E. Fast feedback in active sensing: Touch-induced changes to whisker-object interaction. Public Library of Science ONE. 2012;7:e44272. doi: 10.1371/journal.pone.0044272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Zimmer MR, Bober J, Horvath TL. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell. 2015;160:1222–1232. doi: 10.1016/j.cell.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo LA, Travers JB. Distribution of fos-like immunoreactivity in the medullary reticular formation of the rat after gustatory elicited ingestion and rejection behaviors. Journal of Neuroscience. 1997;17:3826–3839. doi: 10.1523/JNEUROSCI.17-10-03826.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfl J. The innervation of the mystacial region of the white mouse. A topographical study. Journal of Anatomy. 1985;142:173–184. [PMC free article] [PubMed] [Google Scholar]
- Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. Journal of Neurophysiology. 1956;19:44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- Dubner R, GJ Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annual Review of Neuroscience. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- Ebara S, Furuta T, Kumamoto K. Vibrissal mechanoreceptors. Scholarpedia. 2017;12:32372. [Google Scholar]
- Esquivelzeta Rabell J, Mutlu K, Noutel J, Martin Del Olmo P, Haesler S. Spontaneous rapid odor source localization behavior requires interhemispheric communication. Current Biology. 2017;27:1542–1548. doi: 10.1016/j.cub.2017.04.027. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Müller C, Kovacevic A, Tovote P, Lüthi A. A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 2017;542:96–100. doi: 10.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- Feil K, Herbert H. Topographic organization ot spinal ana trigeminal somatosensory pathways to the rat parabrachial and Kölliker-Fuse nuclei. Journal Comparatiive Neurology. 1995;353:506–528. doi: 10.1002/cne.903530404. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: New perspectives on respiratory rhythm. Nature Reviews Neuroscience. 2006;7:232–241. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: So near, yet so far. Annual Review of Physiology. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Nakamura K, Deschenes M. Angular tuning bias of vibrissa-responsive cells in the paralemniscal pathway. Journal of Neuroscience. 2006;26:10548–10557. doi: 10.1523/JNEUROSCI.1746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Timofeeva E, Nakamura K, Okamoto-Furuta K, Togo M, Kaneko T, Deschênes M. Inhibitory gating of vibrissal inputs in the brainstem. Journal of Neuroscience. 2008;28:1789–1797. doi: 10.1523/JNEUROSCI.4627-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Urbain N, Kaneko T, Deschênes M. Corticofugal control of vibrissa-sensitive neurons in the interpolaris nucleus of the trigeminal complex. Journal of Neuroscience. 2010;30:1832–1838. doi: 10.1523/JNEUROSCI.4274-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffield MA, Christie JM. Movement rate Is encoded and influenced by widespread, coherent activity of cerebellar molecular layer interneurons. Journal of Neuroscience. 2017;37:4751–4765. doi: 10.1523/JNEUROSCI.0534-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Kleinfeld D. Goal-directed whisking behavior increases phase-locking between vibrissa movement and electrical activity in primary sensory cortex in rat. Proceedings of the National Academy of Sciences USA. 2004;101:12348–12353. doi: 10.1073/pnas.0308470101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ, Zanella S, Koch H, Doi A, Ramirez JM. Networks within networks: The neuronal control of breathing. Progress in Brain Research. 2011;188:31–50. doi: 10.1016/B978-0-444-53825-3.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Intrinsic and synaptic plasticity in the vestibular system. Current Opinion in Neurobiology. 2006;16:386–390. doi: 10.1016/j.conb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Brecht M, Osten P. Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. Journal of Neuroscience. 2005;25:8250–8258. doi: 10.1523/JNEUROSCI.2235-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grofova I, Keane S. Descending brainstem projections of the pedunculopontine tegmental nucleus in the rat. Anatomy and Embryology (Berlin) 1991;184:275–290. doi: 10.1007/BF01673262. [DOI] [PubMed] [Google Scholar]
- Guo ZV, Hires SA, Li N, O’Connor DH, Komiyama T, Ophir E, Huber D, Bonardi C, Morandell K, Gutnisky D, Peron S, Xu N-L, Cox J, Svoboda K. Procedures for behavioral experiments in head-fixed mice. Public Library of Science ONE. 2014a;9:8678. doi: 10.1371/journal.pone.0088678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZV, Li N, Huber D, Ophir E, Gutnisky D, Ting JT, Feng G, Svoboda K. Flow of cortical activity underlying a tactile decision in mice. Neuron. 2014b;81:179–194. doi: 10.1016/j.neuron.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Rangel J, M J, Motta SC, Zhang X, Perez IO, Canteras NS, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE. Integrated control of predatory hunting by the central nucleus of the amygdala. Cell. 2017;168:311–324. doi: 10.1016/j.cell.2016.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann MJ, Bower JM. Oscillatory activity in the cerebellar hemispheres of unrestrained rats. Journal of Neurophysiology. 1998;80:1598–1604. doi: 10.1152/jn.1998.80.3.1598. [DOI] [PubMed] [Google Scholar]
- Harvold EP, Vargervik K, Chierici G. Primate experiments on oral sensation and dental malocclusions. American Journal of Orthodontics. 1973;63:494–508. doi: 10.1016/0002-9416(73)90162-0. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Li Y, Keller A. Serotonin regulates rhythmic whisking. Neuron. 2003;39:343–352. doi: 10.1016/s0896-6273(03)00391-x. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Priest CA, Keller A. Functional circuitry involved in the regulation of whisker movements. Journal of Comparative Neurology. 2002;442:266–276. doi: 10.1002/cne.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. Basal ganglia mechanisms of reward-oriented eye movement. Annals of the New York Academy of Sciences. 2007;1104:229–249. doi: 10.1196/annals.1390.012. [DOI] [PubMed] [Google Scholar]
- Hill DN, Bermejo R, Zeigler HP, Kleinfeld D. Biomechanics of the vibrissa motor plant in rat: Rhythmic whisking consists of triphasic neuromuscular activity. Journal of Neuroscience. 2008;28:3438–3455. doi: 10.1523/JNEUROSCI.5008-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hires SA, Pammer L, Svoboda K, Golomb D. Tapered whiskers are required for active tactile sensation. Elife. 2013;2:e01350. doi: 10.7554/eLife.01350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer ZS, Alloway KD. Organization of corticostriatal projections from the vibrissal representations in the primary motor and somatosensory cortical areas of rodents. Journal of Comparative Neurology. 2001;439:87–103. doi: 10.1002/cne.1337. [DOI] [PubMed] [Google Scholar]
- Holstege G. Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: An HRP and autoradiographic tracing study in the cat. Journal Comparative Neurology. 1987;260:98–126. doi: 10.1002/cne.902600109. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Experimental Brain Research. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Niessen LW. Substantia nigra projections to the reticular formation, superior colliculus and central gray in the rat, cat and monkey. Neuroscience Letters. 1976;2:253–259. doi: 10.1016/0304-3940(76)90156-7. [DOI] [PubMed] [Google Scholar]
- Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell. 2014;157:664–675. doi: 10.1016/j.cell.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchul P, Amano N, Satoda T, Murata T, Kawagishi S, Yoshino K, Tanaka K. Control of oro-facio-lingual movements by the substantia nigra pars reticulata: high-frequency electrical microstimulation and GABA microinjection findings in rats. Neuroscience. 2005;134:677–689. doi: 10.1016/j.neuroscience.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Isokawa-Akesson M, Komisaruk BR. Difference in projections to the lateral and medial facial nucleus: Anatomically separate pathways for rhythmical vibrissa movement in rats. Experimental Brain Research. 1987;65:385–398. doi: 10.1007/BF00236312. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Arends JJ, Renehan WE, Waite PM, Shortland PJ. Whisker-related circuitry in the trigeminal nucleus principalis: Topographic precision. Somatosensory and Motor Research. 2015;32:8–20. doi: 10.3109/08990220.2014.937414. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Golden J, Rhoades RW. Structure-function relationships in rat brainstem subnucleus interpolaris: III. Local circuit neurons. Journal of Comparative Neurology. 1989;282:24–44. doi: 10.1002/cne.902820104. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Semba K, Rhoades RW, Egger MD. Trigeminal primary afferents project bilaterally to dorsal horn and ipsilaterally to cerebellum, reticular formation, and cuneate, solitary, supratrigeminal and vagal nuclei. Brain Research. 1982;246:285–291. doi: 10.1016/0006-8993(82)91177-5. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Zeigler HP. Trigeminal orosensation and ingestive behavior in the rat. Behavioral Neuroscience. 1983;97:62–97. doi: 10.1037//0735-7044.97.1.62. [DOI] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiological Reviews. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Jones LM, Depireux DA, Simons DJ, Keller A. Robust temporal coding in the trigeminal system. Science. 2004;204:1986–1989. doi: 10.1126/science.1097779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku T. Functional differentiation of hypoglossal motoneurons during the amygdaloid or cortically induced rhythmical jaw and tongue movements in the rat. Brain Research Bulliten. 1984;13:147–154. doi: 10.1016/0361-9230(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Schwaber JS, Driscoll PA. The organization of insular cortex projections to the amygdaloid central nucleus and autonomic regulatory nuclei of the dorsal medulla. Brain Research. 1985;360:355–360. doi: 10.1016/0006-8993(85)91254-5. [DOI] [PubMed] [Google Scholar]
- Karimnamazi H, Travers JB. Differential projections from gustatory responsive regions of the parabrachial nucleus to the medulla and forebrain. Brain Research. 1998;813:283–302. doi: 10.1016/s0006-8993(98)00951-2. [DOI] [PubMed] [Google Scholar]
- Ketzef M, Spigolon G, Johansson Y, Bonito-Oliva A, Fisone G, Silberberg G. Dopamine depletion impairs bilateral sensory processing in the striatum in a pathway-dependent manner. Neuron. 2017;94:855–865. doi: 10.1016/j.neuron.2017.05.004. [DOI] [PubMed] [Google Scholar]
- King MS. Anatomy of the rostral nucleus of the solitary tract. In: Bradley RM, editor. The Role of the Nucleus of the Solitary Tract in Gustatory Processing. Boca Raton, FL: CRC Press/Taylor & Francis; 2007. p. 158. [PubMed] [Google Scholar]
- Klein B, Rhoades R. The representation of whisker follicle intrinsic musculature in the facial motor nucleus of the rat. Journal of Comparative Neurology. 1985;232:55–69. doi: 10.1002/cne.902320106. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: Insights from the rodent vibrissa sensorimotor system. Current Opinion in Neurobiology. 2006;16:435–444. doi: 10.1016/j.conb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Berg RW, O'Connor SM. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosensory and Motor Research. 1999;16:69–88. doi: 10.1080/08990229970528. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Deschênes M. Neuronal basis for object location in the vibrissa scanning sensorimotor system. Neuron. 2011;72:455–468. doi: 10.1016/j.neuron.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Deschênes M, Ulanovsky N. Whisking, sniffing, and the hippocampal θ-rhythm: A tale of two oscillators. Public Library of Science: Biology. 2016;14:e1002385. doi: 10.1371/journal.pbio.1002385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Deschênes M, Wang F, Moore JD. More than a rhythm of life: Breathing as a binder of orofacial sensation. Nature Neurocience. 2014;15:647–651. doi: 10.1038/nn.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Moore JD, Wang F, Deschênes M. The brainstem oscillator for whisking and the case for breathing as the master clock for orofacial motor actions. Cold Spring Harbor Symposia on Quantitative Biology: Cognition. 2015;79:29–39. doi: 10.1101/sqb.2014.79.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Sachdev RNS, Merchant LM, Jarvis MR, Ebner FF. Adaptive filtering of vibrissa input in motor cortex of rat. Neuron. 2002;34:1021–1034. doi: 10.1016/s0896-6273(02)00732-8. [DOI] [PubMed] [Google Scholar]
- Knutsen PM, Mercer Lindsay N, Kleinfeld D. Society for Neuroscience Annual Meeting. Chicago: 2015. A push-pull pathway from somatosensory cortex to spinal trigeminal nuclei for motor control of the vibrissae. poster 706.08. [Google Scholar]
- Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. Journal of Neuroscience. 2008;28:2353–2365. doi: 10.1523/JNEUROSCI.3553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolta A, Brocard F, Verdier D, Lund JP. A review of burst generation by trigeminal main sensory neurons. Arch Oral Biol. 2007;52:325–328. doi: 10.1016/j.archoralbio.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O'Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Remmers JE. Premotor input to hypoglossal motoneurons from Kolliker-Fuse neurons in decerebrate cats. Respiration Physiology. 1999;117:85–95. doi: 10.1016/s0034-5687(99)00058-4. [DOI] [PubMed] [Google Scholar]
- Kurnikova A, Moore JD, Liao S-M, Deschênes M, Kleinfeld D. Coordination of orofacial motor actions into exploratory behavior by rat. Current Biology. 2017;27:1–9. doi: 10.1016/j.cub.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IM. Brain stem control of the phases of swallowing. Dysphagia. 2009;24:333–348. doi: 10.1007/s00455-009-9211-6. [DOI] [PubMed] [Google Scholar]
- Lee AM, Hoy JL, Bonci A, Wilbrecht L, Stryker MP, Niell CM. Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron. 2014;83:455–466. doi: 10.1016/j.neuron.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leergaard TB, Alloway KD, Mutic JJ, Bjaalie JG. Three-dimensional topography of corticopontine projections from rat barrel cortex: Correlations with corticostriatal organization. Journal of Neuroscience. 2000;20:8474–8484. doi: 10.1523/JNEUROSCI.20-22-08474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort S, Tomm C, Sarria J-CF, Petersen CCH. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron. 2009;61:301–316. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Lei L. [On irradiation of excitation of the respiratory center to the motor centers of the tongue muscles (respiratory contraction of the tongue)] Fiziologicheskii zhurnal SSSR imeni IM Sechenova. 1961;47:906–912. [PubMed] [Google Scholar]
- Leiser SC, Moxon KA. Responses of trigeminal ganglion neurons during natural whisking behaviors in the awake rat. Neuron. 2007;53:117–133. doi: 10.1016/j.neuron.2006.10.036. [DOI] [PubMed] [Google Scholar]
- Lewis PR, Flumerfelt BA, Shute CC. The use of cholinesterase techniques to study topographical localization in the hypoglossal nucleus of the rat. J Anat. 1971;110:203–213. [PMC free article] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, Ginty DD. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Chen T-W, Guo ZV, Gerfen CR, Svoboda K. A motor cortex circuit for motor planning and movement. Nature. 2015;519:51–56. doi: 10.1038/nature14178. [DOI] [PubMed] [Google Scholar]
- Li N, Daie K, Svoboda K, Druckmann S. Robust neuronal dynamics in premotor cortex during motor planning. Nature. 2016;532:459–464. doi: 10.1038/nature17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-Q, Takada M, Mizuno N. Premotor neurons projecting simultaneously to two ororfacial motor nuclei by sending their branched axons. A study with a fluorescent retrograde double-labeling technique in the rat. Neuroscience Letters. 1993;152:29–32. doi: 10.1016/0304-3940(93)90475-z. [DOI] [PubMed] [Google Scholar]
- Liao S-M, Kleinfeld D. Society for Neuroscience Annual Meeting. San Diego: 2016. Behaviors formed by the coordination of ingestive motor actions with breathing. poster 536.10. [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annual Review of Neurosciences. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- Loewenstein Y, Mahon S, Chadderton P, Kitamura K, Sompolinsky H, Yarom Y, Häusser M. Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nature Neuroscience. 2005;8:202–211. doi: 10.1038/nn1393. [DOI] [PubMed] [Google Scholar]
- Lowe AA. The neural regulation of tongue movements. Progress in Neurobiology. 1980;15:295–344. doi: 10.1016/0301-0082(80)90008-8. [DOI] [PubMed] [Google Scholar]
- Lowe AA, Sessle BJ. Tongue activity during respiration, jaw opening, and swallowing in cat. Canadian Journal of Physiology and Pharmacology. 1973;51:1009–1011. doi: 10.1139/y73-155. [DOI] [PubMed] [Google Scholar]
- Lu L, Cao Y, Tokita K, Heck DH, Boughter JD., Jr Medial cerebellar nuclear projections and activity patterns link cerebellar output to orofacial and respiratory behavior. Frontiers in Neural Circuits. 2013;7:56. doi: 10.3389/fncir.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JP. Mastication and its control by the brain stem. Critical Reviews in Oral Biology & Medicine. 1991;2:33–64. doi: 10.1177/10454411910020010401. [DOI] [PubMed] [Google Scholar]
- Lund JP, Kolta A, Westberg KG, Scott G. Brainstem mechanisms underlying feeding behaviors. Current Opinions of Neurobiology. 1998;8:18–24. doi: 10.1016/s0959-4388(98)80113-x. [DOI] [PubMed] [Google Scholar]
- Luo P, Zhang J, Yang R, Pendlebury W. Neuronal circuitry and synaptic organization of trigeminal proprioceptive afferents mediating tongue movement and jaw-tongue coordination via hypoglossal premotor neurons. European Journal of Neuroscience. 2006;23:3269–3283. doi: 10.1111/j.1460-9568.2006.04858.x. [DOI] [PubMed] [Google Scholar]
- Ma PM, Woolsey RA. Cytoarchitectural correlates of vibrissae in the medullary trigeminal complex of the mouse. Brain Research. 1984;306:374–379. doi: 10.1016/0006-8993(84)90390-1. [DOI] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz S, Firozi P, Woo S-H, Ranade S, Patapoutian A, E L. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509:617–621. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Frontiers in neuroanatomy. 2011;5:22. doi: 10.3389/fnana.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DW, Deschênes M, Furuta T, Moore JD, Wang F, Karten HJ, Kleinfeld D. Feedback in the brainstem: An excitatory disynaptic pathway for control of whisking. Journal of Comparative Neurology. 2015;523:921–942. doi: 10.1002/cne.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, Petersen CC. Motor control by sensory cortex. Science. 2010;330:1240–1243. doi: 10.1126/science.1195797. [DOI] [PubMed] [Google Scholar]
- McFarland DH, Lund JP. An investigation of the coupling between respiration, mastication, and swallowing in the awake rabbit. Journal of Neurophysiology. 1993;69:95–108. doi: 10.1152/jn.1993.69.1.95. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RLM. The organization and collateralization of corticostraite neurons in the motor and sensory cortex of the rat brain. Brain Research. 1987;423:318–324. doi: 10.1016/0006-8993(87)90855-9. [DOI] [PubMed] [Google Scholar]
- Mercer Lindsay N, Knutsen PM, Gibbs D, Karten HJ, Kleinfeld D. Society for Neuroscience. San Diego: 2016. Motor cortex-directed movement of the mystacial vibrissae through pre-motor neurons in the spinal trigeminal nuclei. poster 149.04. [Google Scholar]
- Mihailoff GA, Lee H, Watt CB, Yates R. Projections to the basilar pontine nuclei from face sensory and motor regions of the cerebral cortex in the rat. Journal Comparatiive Neurology. 1985;237:251–263. doi: 10.1002/cne.902370209. [DOI] [PubMed] [Google Scholar]
- Miller AJ. Oral and pharyngeal reflexes in the mammalian nervous system: Their diverse range in complexity and the pivotal role of the tongue. Critical reviews in Oral Biology and Medicine. 2002;13:409–425. doi: 10.1177/154411130201300505. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Bowman JP. Divergent synaptic influences affecting discharge patterning of genioglossus motor units. Brain Research. 1974;78:179–191. doi: 10.1016/0006-8993(74)90545-9. [DOI] [PubMed] [Google Scholar]
- Minnery BS, Simons DJ. Response properties of whisker-associated trigeminothalamic neurons in rat nucleus principalis. Journal of Neurophysiology. 2003;89:40–56. doi: 10.1152/jn.00272.2002. [DOI] [PubMed] [Google Scholar]
- Mittler T, Cho J, Peoples LL, West MO. Representation of the body in the lateral striatum of the freely moving rat: single neurons related to licking. Experimental Brain Research. 1994;98:163–167. doi: 10.1007/BF00229122. [DOI] [PubMed] [Google Scholar]
- Miyashita E, Keller A, Asanuma H. Input-output organization of the rat vibrissal motor cortex. Experimental Brain Research. 1994;99:223–232. doi: 10.1007/BF00239589. [DOI] [PubMed] [Google Scholar]
- Molkov YI, Rubin JE, Rybak IA, Smith JC. Computational models of the neural control of breathing. WIREs System Biology and Medicine. 2017:9. doi: 10.1002/wsbm.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JD, Kleinfeld D, Wang F. How the brainstem controls orofacial behaviors comprised of rhythmic actions. Trends in Neuroscience. 2014;27:370–380. doi: 10.1016/j.tins.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JD, Mercer Lindsay N, Deschênes M, Kleinfeld D. Vibrissa self-motion and touch are encoded along the same somatosensory pathway from brainstem through thalamus. Public Library of Science: Biology. 2015;13:e1002253. doi: 10.1371/journal.pbio.1002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore* JD, Deschênes* M, Furuta T, Huber D, Smear MC, Demers M, Kleinfeld D. Hierarchy of orofacial rhythms revealed through whisking and breathing. Nature. 2013;469:53–57. doi: 10.1038/nature12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto T, Kawamura Y. Properties of tongue and jaw movements elicited by stimulation of the orbital gyrus in the cat. Arch Oral Biol. 1973;18:361–372. doi: 10.1016/0003-9969(73)90160-x. [DOI] [PubMed] [Google Scholar]
- Morquette P, Lavoie R, Fhima MD, Lamoureux X, Verdier D, Kolta A. Generation of the masticatory central pattern and its modulation by sensory feedback. Progress in Neurobiology. 2012;96:340–355. doi: 10.1016/j.pneurobio.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Mowery TM, Harrold JB, Alloway KD. Repeated whisker stimulation evokes invariant neuronal responses in the dorsolateral striatum of anesthetized rats: a potential correlate of sensorimotor habits. Journal of Neurophysiology. 2011;105:2225–2238. doi: 10.1152/jn.01018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Katakura N. Generation of masticatory rhythm in the brainstem. Neuroscience Research. 1995;23:1–19. [PubMed] [Google Scholar]
- Nguyen Q-T, Kleinfeld D. Positive feedback in a brainstem tactile sensorimotor loop. Neuron. 2005;45:447–457. doi: 10.1016/j.neuron.2004.12.042. [DOI] [PubMed] [Google Scholar]
- O'Connor SM, Berg RW, Kleinfeld D. Coherent electrical activity along vibrissa sensorimotor loops during free whisking in rat. Journal of Neurophysiology. 2002;87:2137–2148. doi: 10.1152/jn.00229.2001. [DOI] [PubMed] [Google Scholar]
- Ono T, Ishiwata Y, Inaba N, Kuroda T, Nakamura Y. Modulation of the inspiratory-related activity of hypoglossal premotor neurons during ingestion and rejection in the decerebrate cat. Journal of Neurophysiology. 1998;80:48–58. doi: 10.1152/jn.1998.80.1.48. [DOI] [PubMed] [Google Scholar]
- Petrovich GD. Learning and the motivation to eat: forebrain circuitry. Physiology & Behavior. 2011;104:582–589. doi: 10.1016/j.physbeh.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinganaud G, Bernat I, Buisseret P, Buisseret-Delmas C. Trigeminal projections to hypoglossal and facial motor nuclei in the rat. Journal of Comparative Neurology. 1999;415:91–104. doi: 10.1002/(sici)1096-9861(19991206)415:1<91::aid-cne7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Proville RD, Spolidoro M, Guyon N, Dugué GP, Selimi F, Isope P, Popa D, Léna C. Cerebellum involvement in cortical sensorimotor circuits for the control of voluntary movements. Nature Neuroscience. 2014;17:1233–1239. doi: 10.1038/nn.3773. [DOI] [PubMed] [Google Scholar]
- Quist BW, Hartmann MJZ. Mechanical signals at the base of a rat vibrissa: The effect of intrinsic vibrissa curvature and implications for tactile exploration. Journal of Neurophysiology. 2012;107:2298–2312. doi: 10.1152/jn.00372.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Dean P, Donohoe TP, Pope SG. Superior colliculus lesions selectively attenuate apomorphine-induced oral stereotypy: a possible role for the nigrotectal pathway. Brain Research. 1980;196:541–546. doi: 10.1016/0006-8993(80)90422-9. [DOI] [PubMed] [Google Scholar]
- Reig R, Silberberg G. Multisensory integration in the mouse striatum. Neuron. 2014;83:1200–1212. doi: 10.1016/j.neuron.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice FL, Mance A, Munger BL. A comparative light microscopic analysis of the sensory innervation of the mystacial padIInnervation of vibrissal follicle-sinus complexes. Journal of Comparative Neurology. 1986;252:154–174. doi: 10.1002/cne.902520203. [DOI] [PubMed] [Google Scholar]
- Sakurai K, Akiyama M, Cai B, Scott A, Han B-X, Takatoh J, Sigrist M, Arber S, Wang F. The organization of submodality-specific touch afferent inputs in the vibrissa column. Cell Reports. 2013;5:87–98. doi: 10.1016/j.celrep.2013.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, Clark M, Zweifel LS. A central amygdala CRF circuit facilitates learning about weak threats. Neuron. 2017;93:164–178. doi: 10.1016/j.neuron.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerland EK, Mitchell SP. Electromyographic activity of the human genioglossus muscle in response to respiration and to positional changes of the head. Bulletin of the Los Angeles Neurological Societies. 1970;35:69–73. [PubMed] [Google Scholar]
- Sawczuk A, Mosier KM. Neural control of tongue movement with respect to respiration and swallowing. Critical Reviews in Oral Biology and Medicine. 2001;12:18–37. doi: 10.1177/10454411010120010101. [DOI] [PubMed] [Google Scholar]
- Schneider JS. Interactions between the basal ganglia, the pontine parabrachial region, and the trigeminal system in cat. Neuroscience. 1986;19:411–425. doi: 10.1016/0306-4522(86)90271-x. [DOI] [PubMed] [Google Scholar]
- Schwartz AB. Useful signals from motor cortex. Journal of Physiology (Bethesda) 2007:581–601. doi: 10.1113/jphysiol.2006.126698. 579.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbaum JS. Electrophysiology of taste, feeding and reward in lateral hypothalamus of rabbit. Physiology & Behavior. 1988;44:507–526. doi: 10.1016/0031-9384(88)90313-7. [DOI] [PubMed] [Google Scholar]
- Semba K, Egger MD. The facial "motor" nerve of the rat: Control of vibrissal movement and examination of motor and sensory components. Journal of Comparative Neurology. 1986;247:144–158. doi: 10.1002/cne.902470203. [DOI] [PubMed] [Google Scholar]
- Sessle BJ. Excitatory and inhibitory inputs to single neurones in the solitary tract nucleus and adjacent reticular formation. Brain Research. 1973;53:319–331. doi: 10.1016/0006-8993(73)90217-5. [DOI] [PubMed] [Google Scholar]
- Sessle BJ. Face sensorimotor cortex: Its role and neuroplasticity in the control of orofacial movements. Progress in Brain Research. 2011;188:71–82. doi: 10.1016/B978-0-444-53825-3.00010-3. [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Greenwood LF. Inputs to trigeminal brainstem neurones from facial, oral, tooth pulp and pharyngolaryngeal tissues: I. Responses to innocuous and noxious stimuli. Brain Research. 1976;117:211–226. doi: 10.1016/0006-8993(76)90731-9. [DOI] [PubMed] [Google Scholar]
- Severson KS, Xu D, Van de Loo M, Bai L, Ginty DD, O'Connor DH. Active touch and self-motion encoding by Merkel cell-associated afferents. Neuron. 2017;94:666–676. doi: 10.1016/j.neuron.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambes GM, Gibson JM, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain, Behavior and Evolution. 1978;15:94–140. doi: 10.1159/000123774. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Costa MS, Ricardo JA. Afferent connections of the parvocellular reticular formation: a horseradish peroxidase study in the rat. Neuroscience. 1992;50:403–425. doi: 10.1016/0306-4522(92)90433-3. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Stepanyants A, Bureau I, Chklovskii D, Svoboda K. Geometric and functional organization of cortical circuits. Nature Neuroscience. 2005;8:782–790. doi: 10.1038/nn1447. [DOI] [PubMed] [Google Scholar]
- Sherman D, Oram T, Deutsch D, Gordon G, Ahissar E, Harel S. Tactile modulation of whisking via the brainstem loop: Statechart modeling and experimental validation. Public Library of Science ONE. 2013;8:e79831. doi: 10.1371/journal.pone.0079831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga Y, Okamoto T, Nishimori T, Suemune S, Nasution ID, Chen IC, Tsuru K, Yoshida A, Tabuchi K, Hosoi M, Tsuru H. Oral and facial representation in the trigeminal principal and rostral spinal nuclei of the cat. Journal of Comparative Neurology. 1986a;244:1–18. doi: 10.1002/cne.902440102. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Suemune S, Nishimura M, Nishimori T, Sato H, Ishidori H, Yoshida A, Tsuru K, Tsuiki Y, Dateoka Y, Nasution ID, Hosoi M. Topographic representation of lower and upper teeth within the trigeminal sensory nuclei of adult cat as demonstrated by the transganglionic transport of horseradish peroxidase. Journal Comparatiive Neurology. 1986b;251:299–316. doi: 10.1002/cne.902510303. [DOI] [PubMed] [Google Scholar]
- Simony E, Bagdasarian K, Herfst L, Brecht M, Ahissar E, Golomb D. Temporal and spatial characteristics of vibrissa responses to motor commands. Journal of Neuroscience. 2010;30:8935–8952. doi: 10.1523/JNEUROSCI.0172-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JB, Mowery TM, Alloway KD. Thalamic POm projections to the dorsolateral striatum of rats: potential pathway for mediating stimulus–response associations for sensorimotor habits. Journal of Neurophysiology. 2012;108:160–174. doi: 10.1152/jn.00142.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JB, Watson GD, Alloway KD, Schwarz C, Chakrabarti S. Corticofugal projection patterns of whisker sensorimotor cortex to the sensory trigeminal nuclei. Frontiers of Neural Circuits. 2015:9. doi: 10.3389/fncir.2015.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag CF. The comparative anatomy of the tongues of the mammalia. XII. Summary, classification and phylogeny. Proceedings of the Zoological Society (London) 1925;95:701–762. [Google Scholar]
- Sreenivasan V, Esmaeili V, Kiritani T, Galan K, Crochet S, Petersen CC. Movement initiation signals in mouse whisker motor cortex. Neuron. 2016;92:1368–1382. doi: 10.1016/j.neuron.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan V, Karmakar K, Rijli FM, Petersen CC. Parallel pathways from motor and somatosensory cortex for controlling whisker movements in mice. European Journal of Neuroscience. 2015;41:354–367. doi: 10.1111/ejn.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek E, 4th, Cheng S, Takatoh J, Han BX, Wang F. Monosynaptic premotor circuit tracing reveals neural substrates for oro-motor coordination. eLife. 2014;30:e02511. doi: 10.7554/eLife.02511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwed M, Bagdasarian K, Ahissar E. Coding of vibrissal active touch. Neuron. 2003;40:621–630. doi: 10.1016/s0896-6273(03)00671-8. [DOI] [PubMed] [Google Scholar]
- Takatoh J, Nelson A, Zhou X, Bolton MM, Ehlers MD, Arenkiel BR, Mooney R, Wang F. New modules are added to vibrissal premotor circuitry with the emergence of exploratory whisking. Neuron. 2013;77:346–360. doi: 10.1016/j.neuron.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatoh J, Prevosto V, Wang F. Vibrissa sensory neurons: Linking distinct morphology to specific physiology and function. Neuroscience. 2017 doi: 10.1016/j.neuroscience.2017.06.033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Pagliardini S, Yang P, Janczewski WA, Feldman JL. Projections of preBötzinger complex neurons in adult rats. Journal of Comparative Neurology. 2010;18:1862–1878. doi: 10.1002/cne.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok TJ. Topography of cerebellar nuclear projections to the brain stem in the rat. Progress in Brain Research. 2000;124:141–172. doi: 10.1016/S0079-6123(00)24014-4. [DOI] [PubMed] [Google Scholar]
- Tonomura S, Ebara S, Bagdasarian K, Uta D, Ahissar E, Meir I, Lampl I, Kuroda D, Furuta T, Furue H, Kumamoto K. Structure-function correlations of rat trigeminal primary neurons: Emphasis on club-like endings, a vibrissal mechanoreceptor. Proceedings of the Japan Academy Series B - Physical and Biological Sciences. 2015;91:560–576. doi: 10.2183/pjab.91.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torvik A. Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures. An experimental study in the rat. Journal of Comparative Neurology. 1956;106:51–132. doi: 10.1002/cne.901060104. [DOI] [PubMed] [Google Scholar]
- Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, Wolff SB, Ramakrishnan C, Fenno L, Deisseroth K, Herry C, Arber S, Lüthi A. Midbrain circuits for defensive behaviour. Nature. 2016;534:206–212. doi: 10.1038/nature17996. [DOI] [PubMed] [Google Scholar]
- Travers JB, Dinardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neuroscience and Biobehavioral Reviews. 1997;21:631–647. doi: 10.1016/s0149-7634(96)00045-0. [DOI] [PubMed] [Google Scholar]
- Travers JB, Herman K, Travers SP. Supression of 3rd ventricular NPY-elicited feeding following medullary reticular formation infusion of muscimol. Behavior Neuroscience. 2010;124:225–233. doi: 10.1037/a0018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Norgen R. Afferent projections to the oral motor nuclei in the rat. Journal of Comparative Neurology. 1983;220:280–298. doi: 10.1002/cne.902200303. [DOI] [PubMed] [Google Scholar]
- Travers JB, Yoo JE, Chandran R, Herman K, Travers SP. Neurotransmitter phenotypes of intermediate zone reticular formation projections to the motor trigeminal and hypoglossal nuclei in the rat. Journal of Comparative Neurology. 2005;488:28–47. doi: 10.1002/cne.20604. [DOI] [PubMed] [Google Scholar]
- Tsumori T, Yasui Y. Organization of the nigro-tecto-bulbar pathway to the parvicellular reticular formation: A light- and electron-microscopic study in the rat. Experimentsl Brain Research. 1997;116:341–350. doi: 10.1007/pl00005761. [DOI] [PubMed] [Google Scholar]
- Ugolini G. Specificity of rabies virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. Journal Comparative Neurology. 1995;356:457–480. doi: 10.1002/cne.903560312. [DOI] [PubMed] [Google Scholar]
- Urbain N, Deschênes M. Motor cortex gates vibrissal responses in a thalamocortical projection pathway. Neuron. 2007a;56:714–725. doi: 10.1016/j.neuron.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Urbain N, Deschênes M. A new thalamic pathway of vibrissal information modulated by the motor cortex. Journal of Neuroscience. 2007b;27:12407–12412. doi: 10.1523/JNEUROSCI.2914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pieribone VA, Aston-Jones G. Diverse afferents converge on the nucleus paragigantocellularis in the rat ventrolateral medulla: retrograde and anterograde tracing studies. Journal Comparative Neurology. 1989;290:561–584. doi: 10.1002/cne.902900410. [DOI] [PubMed] [Google Scholar]
- Van Ham JJ, Yeo CH. Somatosensory trigeminal projections to the inferior olive, cerebellum and other precerebellar nuclei in rabbits. European Journal of Neuroscience. 1992;4:302–317. doi: 10.1111/j.1460-9568.1992.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Vidal PP, May PJ, Baker R. Synaptic organization of the tectal-facial pathways in the cat. I. Synaptic potentials following collicular stimulation. Journal of Neurophysiology. 1988;60:769–797. doi: 10.1152/jn.1988.60.2.769. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Smith Y, Bolam JP, Smith AD. Synaptic organization of GABAergic inputs from the striatum and the globus pallidus onto neurons in the substantia nigra and retrorubral field which project to the medullary reticular formation. Neuroscience. 1992;50:531–549. doi: 10.1016/0306-4522(92)90445-8. [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L. Cerebellar granule cells encode the expectation of reward. Nature. 2017;544:96–100. doi: 10.1038/nature21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Redgrave P. Microinjections of muscimol into lateral superior colliculus disrupt orienting and oral movements in the formalin model of pain. Neuroscience. 1997;81:967–988. doi: 10.1016/s0306-4522(97)00191-7. [DOI] [PubMed] [Google Scholar]
- Welker E, Van der Loos H. Quantitative correlation between barrel-field size and the sensory innervation of the whiskerpad: A comparative study in six strains of mice bred for different patterns of mystacial vibrissae. Journal of Neuroscience. 1986;6:3355–3373. doi: 10.1523/JNEUROSCI.06-11-03355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JP, Lang EJ, Suglhara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- Welzl H, Bures J. Lick-synchronized breathing in rats. Physiological Behavior. 1977;18:751–753. doi: 10.1016/0031-9384(77)90079-8. [DOI] [PubMed] [Google Scholar]
- Whiteley SJ, Knutsen PM, Matthews DM, Kleinfeld D. Deflection of a vibrissa leads to a gradient of strain across mechanoreceptors in the mystacial follicle. Journal of Neurophysiology. 2015;114:138–145. doi: 10.1152/jn.00179.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld Z, Halpern BP, Tapper DN. Licking behavior: Evidence of hypoglossal oscillator. Science. 1977;196:1122–1124. doi: 10.1126/science.558653. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Mende C, Brecht M. Social facial touch in rats. Behavior Neuroscience. 2011;125:900–910. doi: 10.1037/a0026165. [DOI] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509:622–626. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JW. Behavior of chronic decerebrate rats. Journal of Neurophysiology. 1964;27:635–644. doi: 10.1152/jn.1964.27.4.635. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Nakano K, Nakagawa Y, Kayahara T, Shiroyama T, Mizuno N. Nondopaminergic neurons in the substantia nigra project to the reticular formation around the trigeminal motor nucleus in the rat. Brain Research. 1992;585:361–366. doi: 10.1016/0006-8993(92)91237-9. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Tsumori T, Ando A, Domoto T, Kayahara T, Nakano K. Descending projections from the superior colliculus to the reticular formation around the motor trigeminal nucleus and the parvicellular reticular formation of the medulla oblongata in the rat. Brain Research. 1994;656:420–426. doi: 10.1016/0006-8993(94)91489-3. [DOI] [PubMed] [Google Scholar]
- Yokota S, Niu JG, Tsumori T, Oka T, Yasui Y. Glutamatergic Kolliker-Fuse nucleus neurons innervate hypoglossal motoneurons whose axons form the medial (protruder) branch of the hypoglossal nerve in the rat. Brain Research. 2011;1404:10–20. doi: 10.1016/j.brainres.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Yu YSW, Graff MM, Bresee CS, Man YB, Hartmann MJZ. Whiskers aid anemotaxis in rats. Science Advances. 2016;2:e1600716. doi: 10.1126/sciadv.1600716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiewicz IM, Bjaalie JB, Leergaard TB. Brain-wide map of efferent projections from rat barrel cortex. Fronteirs of NeuroInformatics. 2014;8:5. doi: 10.3389/fninf.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerari-Mailly Pinganaud G, Dauvergne C, Buisseret P, Buisseret-Delmas CJ. Trigemino-reticulo-facial and trigemino-reticulo-hypoglossal pathways in the rat. Journal of Comparative Neurology. 2001;429:80–93. doi: 10.1002/1096-9861(20000101)429:1<80::aid-cne7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Zhang J, Luo P, Pendlebury WW. Light and electron microscopic observations of a direct projection from mesencephalic trigeminal nucleus neurons to hypoglossal motoneurons in the rat. Brain Research. 2001;917:67–80. doi: 10.1016/s0006-8993(01)02911-0. [DOI] [PubMed] [Google Scholar]