Abstract
Purpose of review
The treatment of pain in patients with cirrhosis is complicated by unpredictable hepatic drug metabolism and a higher risk of adverse drug reactions. We aimed to conduct a scoping review regarding pain management in cirrhosis.
Recent findings
Despite the high prevalence of pain in patients with cirrhosis, there is little literature to guide the management of pain in this population. Complex pain syndromes and disease-specific pain etiologies exist are common in patients with cirrhosis. There are numerous contraindications and limitations when considering pharmacotherapy for analgesia in cirrhosis, specifically with non-steroidal anti-inflammatory drugs (NSAIDS) and opioid medications. Non-pharmacologic therapies for pain have not been specifically assessed in this population.
Summary
As with other populations, a multi-dimensional treatment approach to pain with a focus on physical, behavioral, procedural and pharmacologic treatment is recommended when caring for patients with cirrhosis and pain. However, more research is needed to evaluate opioid-sparing and non-pharmacologic analgesia in this population.
Keywords: pain, cirrhosis, analgesia, chronic liver disease
Introduction
Pain is common in patients with liver disease and is difficult to manage. Pain has been found in up to 82% of patients with cirrhosis and is chronic in over half of patients [1•–3]. Patients with more advanced liver disease have an increased prevalence of pain and this is associated with associated sleep and mood symptoms as well as a high risk of pain-related disability and opioid use [1,4–6•]. Despite the high prevalence of pain and its adverse consequences, there is limited guidance on the management on pain in cirrhosis. Given the importance of this topic to patients with cirrhosis, we aimed to conduct a scoping review on the topic of pain management and cirrhosis. We here discuss the assessment and management of pain in patents with cirrhosis with a focus on both non-pharmacologic and pharmacologic treatments and an overall goal of developing a multi-dimensional treatment approach.
Research Methods
A scoping review was done using a MEDLINE search with initial focus on literature published in the past five years addressing overall pain management and evaluation of pain in patients with chronic liver disease. Using a title focused search for “pain” or “analgesics” or “analgesia” or “fibromyalgia” AND “cirrhosis” or “cirrhotic” or “liver” or “hepatic” a total of 122 studies were identified. After critical appraisal and exclusion of studies focused on acute post-operative pain management of hepatic resection or liver transplantation, a total of 10 studies were identified[1,4–12•]. These studies were predominantly descriptive. Given the limited recent data, subsequent MEDLINE searches were done for individual treatment modalities, and historical studies beyond five years were evaluated as needed to complete our review.
The Assessment of Pain in Patients with Cirrhosis
General Principles
The workup of pain is easily overlooked in hepatology clinic given the competing issues requiring attention of the clinician. An evaluation for pain can be accomplished via standard screening assessments, such as numeric or visual analog scales, review of systems or medical interviews based on patient chief complaint. Once identified, the next step is to determine the nature of the pain, including location, quality, and duration and perform a physical examination to assess underlying etiology.
Location
The location of pain guides examination, workup, and treatment. Pain that is localized can be treated with local modalities such as injections or topical treatments and is often easier to address than widespread pain. Often patients with cirrhosis have widespread pain that requires consideration of more systemic processes including inflammation [6].
Quality
Generally pain can be divided into nociceptive and neuropathic types. Nociceptive pain relates to tissue injury and can be classified into somatic and visceral subtypes. Somatic nociceptive pain arises from bone and muscle and is typically localized, while visceral nociceptive pain can be more diffuse. Neuropathic pain is generally burning and tingling in quality. Other characteristics that should be considered include the frequency of symptoms and relieving and aggravating factors.
Duration
Pain is typically considered to be chronic when it lasts 12 weeks. The approaches to acute and chronic pain differ, so it is critical to assess duration.
Pain related disability and quality of life
Assessing the impact of pain on the patient’s function and quality of life is critical to determining the urgency and intensity of treatment. A three-question validated tool, the PEG (Pain, Enjoyment, General activity), can be used. This simple assessment asks about the average pain intensity, pain interference with enjoyment of life, and pain interference with general activity over the past week, all on a scale of 0–10. The 3 scales are averaged for a score from 0–10. The PEG can be administered repeatedly over time to assess for changes in pain function and disability [13,14].
Depression and Anxiety
Depression and/or anxiety often co-occur with chronic pain. Psychiatric disorders are a risk factor for the development of chronic pain but can also be exacerbated by pain-related impairment [15,16]. Depression and anxiety are also implicated in worse clinical outcomes and quality of life in patients with cirrhosis independent of pain [17,18]. Effective treatment of these psychiatric disorders can reduce pain-related impairment in general populations, although this has not been specifically studied in patients with liver disease [19].
Substance Use Disorders (SUDs)
Opioid use disorder has been associated with lower pain tolerance [20]. Acute pain has also been associated with increase in relapse to substance use. Thus, understanding the patient’s SUD status, including prior history and current use, is essential to devising an appropriate pain treatment plan [21–23•].
Specific Considerations in Patients with Cirrhosis
The majority of patients with liver disease reporting pain describe abdominal pain; however, a large proportion of patients also report pain in the lower back, large joints, and diffusely [1•]. As non-alcoholic fatty liver disease (NAFLD) increases in prevalence, it may overlap with other common non-hepatic painful conditions, such as osteoarthritis related to the common etiological factor of obesity. In addition to the common causes of pain in the general population, cirrhosis itself may exacerbate common non-hepatic painful conditions. For example, cirrhosis-related gynecomastia may cause mastalgia. Other examples of cirrhosis-related pain include:
Abdominal Pain
The high prevalence of abdominal pain in patients with cirrhosis is likely related to several factors including ascites, hepatic capsular distension and splenomegaly [1•,4,24]. Spontaneous bacterial peritonitis commonly presents with abdominal pain such that any patient with pain and ascites should have a diagnostic paracentesis [25]. Massive splenomegaly in the setting of cirrhosis has been associated with pain that in some extreme cases has even led to partial splenic embolization for symptom control [26]. Another example of cirrhosis-associated abdominal pain is the visceral hypersensitivity described in patients with HCV [27].
Widespread pain
Cirrhosis is a pro-inflammatory state and the same cytokines associated with cirrhosis are also associated with pain. A fibromyalgia-like syndrome has been found in both HCV and non-HCV related liver disease, which may be related to this systemic inflammation [6]. Thus addressing the liver disease and psychiatric comorbidities with similar pro-inflammatory cytokine profiles is a key component of pain management.
The Management of Pain in Patients with Cirrhosis
General Principles
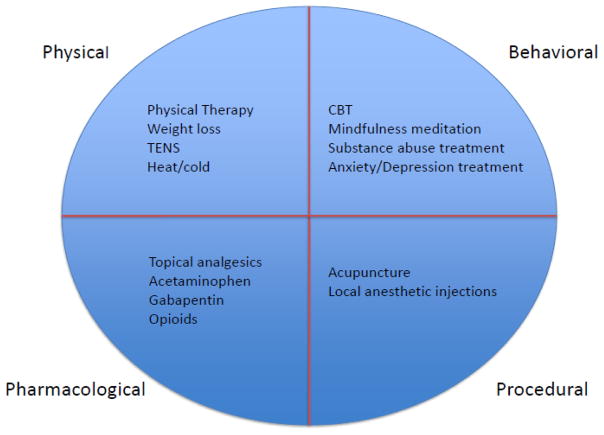
Similar to treatment of pain in any patient, the pain management plan for patients with cirrhosis should focus on functional outcomes, with particular attention to the most bothersome symptoms for the patient. For severe chronic pain, the treatment plan should be individualized with consideration of physical, behavioral, pharmacological and procedural approaches based on disease stage and etiology of pain [Figure 1]. The treatment of chronic pain can be difficult and is best accomplished with a multidisciplinary approach including behavioral health, palliative care or chronic pain specialists.
Figure 1.
Pain Management in Cirrhosis: Multi-dimensional Treatment Approach
Non-pharmacologic pain management
In general, a biopsychosocial approach to pain includes non-pharmacologic options, treatment of underlying psychiatric comorbidities, and an individualized approach. Non-pharmacologic interventions may include a range of options from simple heat and cold therapy and weight loss to more formal treatments such as physical therapy, transcutaneous electrical nerve stimulation (TENS), massage therapy, acupuncture, acupressure, cognitive behavioral therapy, hypnosis and meditation. These modalities have all been described in the palliative care literature as having beneficial effects in the treatment of cancer-related pain [28]. However, there are not data regarding these modalities in patients with cirrhosis specifically. The comparative effectiveness and risks and benefits of these modalities of pain management may differ for this subpopulation and by severity of liver disease.
Physical Treatment
Physical therapy (PT)
PT serves a clear role in rehabilitation of acute injuries but can also aid in the management of pain related to chronic conditions like HIV [29]. Sarcopenia and poor physical function in patients with cirrhosis are associated with poor outcomes and PT may help both pain and these associated conditions [30]. Psychologically-based PT, which is more commonly-practiced outside of the US, may be particularly useful in patients with comorbid depression and anxiety [31] and merits further study in patients with cirrhosis.
Weight loss
Studies have consistently shown that weight loss for obese patients improves pain as much as medication [32,33]. Physical activity, which is a part of many weight loss regimens, is also key for maintaining function and muscle strength. Weight loss has the added benefit of treating NAFLD but may not be advisable in patients in the more catabolic phase of cirrhosis.
Behavioral Treatment
Cognitive behavioral therapy (CBT)
CBT has been shown to improve pain-related outcomes in general populations and is being actively tailored to manage pain in patients with HIV [34]. CBT is appealing for patients with cirrhosis because behavioral therapy also may address comorbid substance use, depression, and anxiety, which are also associated with pain and poor outcomes in this population [1•].
Mindfulness
A recent large randomized trial showed significant long-term pain reduction in adults with chronic back pain who participated in a mindfulness meditation program [35]. These results were corroborated by a meta-analysis in 2017 that showed a small but significant reduction in pain and depression and improvement in quality of life in patients with chronic pain [36•]. Although not specifically studied in patients with cirrhosis, there is little downside to this approach, though patients with active encephalopathy may not be able to participate in behavioral treatments.
Invasive Procedures
Acupuncture
Acupuncture, including sham acupuncture, has been associated with decreased pain [37]. Although generally considered safe, a systematic review found a wide range of bleeding complications (0.03–38%) such as ecchymosis and hematoma. The disparate risk estimates highlight the need to proceed with caution with expert practitioners, particularly in patients with coagulopathies [38].
Analgesic injections
Nerve blocks and local analgesic injections can positively impact pain in the general population. They should be considered on an individual basis by a pain management specialist, with attention to the increased bleeding risk in advanced cirrhosis.
Pharmacologic pain management
The pharmacological approach to pain management is particularly challenging in patients with cirrhosis due to altered and often unpredictable drug metabolism. The difficulty of pharmacotherapy further supports a more holistic approach to pain in this population. In treating chronic pain, clinicians need to be aware of the potential for addiction with specific medications. This is particularly true of opioid medications and in patients with a history of SUD [23].
Topical Pharmacotherapy
Lidocaine 5% topical patch has been used with success in the treatment of both neuropathic as well as acute and chronic somatic pain syndromes and is a low risk, well tolerated treatment worth consideration in cirrhosis [39,40]. Topical nonsteroidal anti-inflammatory drugs (NSAIDs) have also proven successful in treating muscular skeletal pain in both the acute and chronic settings and have limited systemic side effects when compared to oral NSAIDs [41,42]. However, the safety profile of these medications is unclear in patients with cirrhosis, and this should be evaluated given the dangers of systemic NSAIDS in this population. A systematic review described a moderate efficacy for topical capsaicin in the treatment of both musculoskeletal and neuropathic pain (with the caveat that a topical burning sensation may limit use in some patients) [43].
Systemic pharmacotherapy
The liver is responsible for metabolizing the majority of drugs. Drug metabolism is affected not only by the intrinsic hepatocyte function, but also by hepatic blood flow, drug binding by plasma proteins, and biliary excretion [8,44,45], all which can be affected by cirrhosis. Unlike renal disease where the creatinine clearance provides an accurate reflection of renal function, there is no analogous measure of hepatocyte metabolic function that can be used to predict hepatic metabolism of specific drugs. While the MELD score and Child-Pugh classification predict survival, they are imprecise at predicting dose adjustments given the complex and varied nature of drug metabolism [46,47].
In addition to intrinsic hepatocyte function, other factors affect hepatic metabolism in the context of cirrhosis. Cirrhosis frequently leads to spontaneous portosystemic shunting and sometimes necessitates the intentional creation of a transjugular intrahepatic portosystemic shunt. These shunts further alter hepatic blood flow and can decrease the clearance of drugs relying on first-pass metabolism, leading to higher bioavailability [48]. Furthermore, for highly protein-bound drugs, the decreased albumin production in cirrhosis alters metabolism and elimination. This is compounded by frequent increase in extracellular fluid from edema and ascites that can lead to a significantly increased volume of drug distribution [49].
Hepatic drug metabolism relies largely on oxidation-reduction reactions catalyzed by the cytochrome P450 (CYP) enzymes. These reactions require oxygen and are sensitive to the relative hypoxia induced by shunting [50]. Although P450 isoforms may function relatively well with mild liver disease, a decrease in metabolic activity of all isoforms has been observed in the setting of severe liver disease [51]. Metabolism by glucuronidation seems to be less affected by cirrhosis than oxidation-reduction reactions, possibly secondary to a compensatory increase in extrahepatic metabolism or upregulation of glucuronidation enzymes [52]. Lastly, patients with progressive liver disease are at risk for related renal dysfunction and are thus vulnerable to alterations in renal metabolite excretion. Thus the metabolism of medications in the context of cirrhosis is complex. Predicting the overall effects of the disease on drug metabolism requires an understanding of the metabolism of individual drugs as well as an assessment of each patient’s renal function, volume of distribution, and presence of portosystemic shunting. Future research should aim to create models to predict safe dosing of medications for individual patients.
Acetaminophen (Paracetamol)
Although commonly used by the general public for analgesia, acetaminophen is frequently avoided in patients with liver disease out of fear for its well-known often-fatal hepatotoxicity in high doses. However, although limited data exist regarding chronic daily acetaminophen, a reduced dose of 2g/day is generally considered safe in patients with cirrhosis [8,44,45]. Acetaminophen is predominantly metabolized by glucuronidation and sulfation with 5% undergoing oxidation by CYP to the hepatotoxic compound N-acetyl-p-benzoquinone imine (NAPQI). NAPQI requires glutathione for detoxification which can be depleted in the setting of chronic alcohol use but has been found to be relatively preserved in patients with cirrhosis [53]. Supporting this is a double-blind study in which 20 patients with chronic liver disease (of undocumented severity) were treated with acetaminophen 4g/day or placebo for 13 days followed by crossover to the alternative treatment for 13 days with no resultant significant changes in laboratory tests [54]. Thus low-dose acetaminophen (≤2gm/day) is considered to be a first-line analgesic for patients with cirrhosis.
Nonsteroidal Anti-inflammatory Drugs (NSAIDS)
Given perceived concerns about acetaminophen use, patients with cirrhosis are often given NSAIDS as a “less toxic” alternative, when in fact NSAIDS are likely more dangerous in this population. NSAIDs are predominantly metabolized by CYP enzymes and are heavily protein-bound. Patients with cirrhosis rely on renal prostaglandin activity to maintain adequate renal perfusion, but NSAIDs inhibit prostaglandin production, which leads to decreased GFR, rise in creatinine and decreased natriuresis [55,56]. Furthermore, NSAIDs increase the risk of bleeding via inhibition of thrombaxane A2 production by platelets. One case-control study found an association between NSAID use and variceal bleeding (OR=2.9) [57]. Given these significant risks, NSAIDS are contraindicated in patients with cirrhosis. Patients with cirrhosis should be educated about safe and unsafe over-the-counter analgesia since there is often confusion among both providers and patients regarding the relative safety and danger of these medications.
Opioids
The well-publicized dangers of opioid use in the general population may be magnified in the context of cirrhosis [58••,59]. Chronic liver disease is a risk factor for prescription opioid overdose and toxicity and opioid use has been associated with adverse transplant outcomes [60–63•]. A history of substance abuse is common in patients with cirrhosis and prescription opioids can lead to addiction, particularly among those with history of addiction. In general opioids should be avoided to the extent possible in this population. One notable exception is the hospice/end-of-life setting, where the risk-benefit profile of these medications is changed.
Opioids have well-established side effects of respiratory suppression, sedation and constipation, which can lead to new or worsening hepatic encephalopathy if not managed carefully [64•]. Given the established relationship between opioids and encephalopathy, which may be in part mediated by constipation, it is reasonable to provide lactulose and/or rifaximin to all patients taking opioids[45].
When prescribing opioids, it should be in conjunction with the guidance of a chronic pain and/or palliative care specialist. When prescribing opioids for any patient, the CDC recommends a short duration of <7 days for acute pain since longer prescriptions are rarely needed and increase the addictive potential [58]. The decision to use opioids for the treatment of chronic pain is difficult and should only be done when non-opioid treatment options have been exhausted and the potential gain in patient function and quality of life is felt to outweigh the significant risks of long-term opioids.
The “safe” limits of opioids have yet to be established in the context of cirrhosis and are likely even lower than the general population. Doses >50 MME per day have been associated with increased overdose risk in general population [65]. However, pre-transplant opioid doses as low as 10 MME per day, were associated with significant increases in post-transplant mortality in one study [61•]. Sustained release opioids should be avoided because of the risk of drug accumulation in cirrhosis. Similarly, due to the risk of acetaminophen toxicity with combination pills, combination opioid-acetaminophen pills should be avoided in patients with cirrhosis. The metabolism and data regarding the different opioid types are outlined below.
Oxycodone
Oxycodone is metabolized by CYP to the active metabolite oxymorphone. This reliance on metabolism to the active metabolite may again reduce its analgesic efficacy in the context of cirrhosis [66]. Given the fluctuations in drug concentration, increased half-life and unpredictable blood levels of oxycodone in patients with liver disease, it is not the drug of choice in patients with cirrhosis [44,67].
Morphine
Morphine does not rely on CYP metabolism but undergoes significant first-pass metabolism via glucuronidation. Glucuronidation is affected by decreased hepatic blood flow leading to increased oral bioavailability in the setting of impaired hepatic function [68]. One neurotoxic metabolite of morphine, normorphine, can precipitate seizures when renal clearance is decreased and thus morphine should not be used in patients with renal dysfunction [69].
Hydromorphone
Unlike the aforementioned opioids, hydromorphone does not require metabolism to an active analgesic metabolite. Similar to morphine, hydromorphone undergoes high first-pass metabolism via glucuronidation, which is again decreased in the setting of cirrhosis leading to a higher oral bioavailability [70]. In a pharmacokinetic study conducted in patients with moderate hepatic impairment, even though the maximum drug concentration was increased after a single dose of hydromorphone, reduced hepatic function did not have any effect on the elimination of hydromorphone [70]. The metabolites of hydromorphone are generally considered to have minimal neurotoxicity and it is frequently the opioid of choice in the context of renal and hepatic dysfunction [45].
Fentanyl
Fentanyl is metabolized by CYP with a high rate of hepatic extraction suggesting that clearance would be decreased by decreased hepatic blood flow [71]. However, when studied in patients with well-compensated cirrhosis and preserved hepatic blood flow, the pharmacokinetics of a single intravenous dose in these patients were similar to those of healthy controls [72]. These results cannot be extrapolated to decompensated cirrhosis or transdermal use. The manufacture label states there is a change in fentanyl patch area under the curve (AUC) from 35% in controls to 73% with liver disease and as a result the patch should be avoided in “severe hepatic impairment” [73].
Tramadol
Metabolized by CYP to an active metabolite that has a low affinity for opioid receptors, tramadol has been historically considered to be less sedating and addictive then other opioids [74]. In the setting of cirrhosis, the metabolism of tramadol can be decreased as liver disease progresses, which may reduce the formation of the active metabolite and decrease the analgesic effects in a manner similar to patients with poor intrinsic metabolizing ability [75,76]. Tramadol lowers the seizure threshold and should not be used in patients with a seizure disorder or in combination with selective serotonin reuptake inhibitors, anticonvulsants or tricyclic antidepressants given the risk of serotonin syndrome [77]. More research is needed to establish the safety of tramadol in patients with cirrhosis.
Buprenorphine
Metabolized by CYP to an active metabolite that is eliminated via glucuronidation with biliary excretion again necessitates dose reduction and slow up titration of buprenorphine in the setting of chronic liver disease [78]. The active metabolite is a weak mu-opioid receptor agonist but with high binding affinity. This property can cause displacement of other circulating opioid agonists and has led to combination formulations with a theoretical decreased risk of overdose and adverse effects [79]. A recent review concluded that bupreinorphine has similar efficacy for pain reduction when compared to other opioids but further study is needed in general populations [80]. This medication requires hepatic metabolism and more data are needed in patients with cirrhosis.
Methadone
Methadone is a synthetic opioid with a long half-life. It is frequently used in the treatment of opioid use disorders but is also used for chronic pain. Methadone is metabolized by the CYP system and limited studies suggest that drug clearance is not altered by liver disease or renal impairment and thus dose adjustment is not theoretically necessary [81,82]. However, while this made methadone an attractive analgesic for patients with liver disease in the past, its long half-life can lead to accumulation over time which has decreased its use for pain management in the context of liver disease.
Anticonvulsants
Anticonvulsants including gabapentin and pregabalin serve a prominent role in the treatment of neuropathic pain. Gabapentin and pregabalin bind to voltage gated calcium channels in the CNS and undergo minimal hepatic metabolism with unchanged drug excretion from the kidney [83]. Gabapentin has no convincing reports of significant hepatotoxicity, and is subsequently been a first-line agent for the treatment of neuropathic pain with a maximum does of 3600mg/day assuming normal renal function [84]. Pregabalin does have rare reports of idiosyncratic liver injury and should thus be used as a second line agent [85]. While carbamazepine is used for neuropathic pain, hepatotoxicity and drug reaction with eosinophilia and systemic symptoms (DRESS) has been well described with this drug and its use in cirrhotic patients should be avoided [86]. Carbamazepine can also induce bone marrow suppression, which is also already a concern among patients with cirrhosis. Gabapentin and pregabalin are both reasonable first line medications for the treatment of neuropathic pain. However, they can cause sedation and have addictive potential so the doses should again be slowly up-titrated with preferential dosing before bed.
Antidepressants
Neuropathic pain can be difficult to treat and antidepressants have also been frequently used with moderate success. Nearly all classes of antidepressants have been implicated in idiosyncratic drug-induced liver injury to some extent, although this is a rare clinical outcome [87]. Tricyclic antidepressants (TCAs) are commonly used for the treatment of neuropathic pain and are metabolized by CYP enzymes and rely on renal elimination and as such drug accumulation can be seen in progressive liver disease. Nortriptyline and desipramine are preferred to amitriptyline, imipramine and doxepin as they appear to have less anti-cholinergic and sedating effects. However, all TCAs have the potential to induce or exacerbate encephalopathy and have fatal overdose potential so their use is generally not recommended in the context of liver disease [88].
Serotonin-norepinephrine reuptake inhibitors (SNRIs) including venlafaxine and duloxetine have also been used in the treatment of neuropathic pain but are likely not good options for patients with cirrhosis. Duloxetine carries a manufacture warning of hepatotoxicity given its implication in numerous cases of drug-induced liver injury and is not recommended in patients with chronic liver disease [89]. Venlafaxine relies heavily on hepatic CYP metabolism necessitating significant dose reduction if it is used [90].
Selective serotonin reuptake inhibitors (SSRIs) are not recommended for the treatment of neuropathic pain in general populations as they have a lower efficacy compared with TCAs and also pose an increased risk of GI bleeding [91,92]. However, given the risks of TCAs in this population, SSRIs are often used, though more work is needed to determine the effectiveness of SSRIs for chronic pain in this population.
Cannabis
While there is growing interest regarding the efficacy of cannabis for treating pain in general populations, the safety profile of cannabis in cirrhosis requires more research. For example, an initial cross-sectional study suggested an association between daily cannabis use and liver fibrosis [93]. More recently, however, a prospective cohort study of 690 HIV-HCV co-infected patients without liver fibrosis at baseline found no association between self-reported cannabis use and subsequent development of hepatic fibrosis, although the median follow-up was limited at 2.7 years [94]. Certainly cannabis use poses difficulties in a patient population with a high prevalence of addiction, and variable transplant policies around marijuana and transplantation exist. Additionally, most studies of the medicinal efficacy of cannabinoids assessed pharmaceutical medications dronabinol and nabilone, not smoked cannabis [95]. More research on the effects and proper patient selection is needed before medicinal cannabis can be recommended.
Conclusions and Recommendations
Pain is common in cirrhotic patients and can vary in location and presentation. We recommend that an assessment of pain symptoms, comorbid conditions, function and disability be a routine part of clinical care, recognizing that this can be challenging when faced with competing needs to address the many medical consequences of cirrhosis.
All pain treatment should progress in a step-wise fashion with an increasing focus on non-pharmacologic low-risk treatment interventions. Attention to comorbid conditions such as depression, anxiety and substance abuse represent separate but exceedingly important treatment targets in a multi-dimensional treatment approach (Figure 1) [96].
In terms of pharmacotherapy, NSAIDS and other hepatotoxic medications should be avoided. Opioid use should be limited and avoided to the extent possible, given both the lack of efficacy in the context of chronic non-malignant pain and the potential of opioids to causing encephalopathy, addiction and overdose. If opioids are used, it is critical to maintain the lowest possible dose with the goal to use opioid-containing medications for <7 days if at all. Sustained release oral preparations and combination medications should be avoided. The data on the safety of transdermal fentanyl in cirrhosis suggests that it should be avoided. Hydromorphone may be a reasonable first line opioid agent given its short half-life, lack of hepatic metabolism to an active analgesic metabolite and absence of neurotoxic metabolites. Lactulose should be started empirically to avoid constipation and prevent hepatic encephalopathy when using psychoactive medications. Overall, opioids are to be avoided for chronic management of pain in this population and other approaches should be used. More research is urgently needed to guide the management of pain in patients with cirrhosis.
Directions for Future Research
Future research should address non-pharmacologic treatment of pain for patients with cirrhosis with a focus on a multi-dimentional model of care. More work is also needed to understand the comparative effectiveness and risks associated with the use of various analgesic medications in this population. Safety thresholds for different opioids should be established for patients with cirrhosis in order to help decrease the risks of opioids for those already taking them. Implementation research should focus on increasing the uptake of opioid-sparing medications and approaches. Personalized medicine approaches can be considered to predict effectiveness and safety of medications.
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Conflict of Interest
Shari Rogal reports grants from Gilead Sciences, outside of the submitted work.
Matthew Klinge, Tami Coppler, Jane M. Liebschutz, Mohannad Dugum, Ajay Wassan, and Andrea DiMartini each declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1•.Rogal SS, Bielefeldt K, Wasan AD, Lotrich FE, Zickmund S, Szigethy E, DiMartini AF. Inflammation, psychiatric symptoms, and opioid use are associated with pain and disability in patients with cirrhosis. Clinical Gastroenterology and Hepatology. 2015 May 31;13(5):1009–16. doi: 10.1016/j.cgh.2014.10.029. Prospective study highlighting the prevalence of pain and opioid use among patients with cirrhosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehead AJ, Dobscha SK, Morasco BJ, Ruimy S, Bussell C, Hauser P. Pain, substance use disorders and opioid analgesic prescription patterns in veterans with hepatitis C. Journal of pain and symptom management. 2008 Jul 31;36(1):39–45. doi: 10.1016/j.jpainsymman.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Silberbogen AK, Janke EA, Hebenstreit C. A closer look at pain and hepatitis C: preliminary data from a veteran population. Journal of rehabilitation research and development. 2007 Feb 15;44(2):231. doi: 10.1682/jrrd.2006.05.0053. [DOI] [PubMed] [Google Scholar]
- 4.Rogal SS, Winger D, Bielefeldt K, Szigethy E. Pain and opioid use in chronic liver disease. Digestive diseases and sciences. 2013 Oct 1;58(10):2976–85. doi: 10.1007/s10620-013-2638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Imani F, Motavaf M, Safari S, Alavian SM. The therapeutic use of analgesics in patients with liver cirrhosis: a literature review and evidence-based recommendations. Hepatitis monthly. 2014 Oct;14(10) doi: 10.5812/hepatmon.23539. Recent review of pain management in cirrhosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogal SS, Bielefeldt K, Wasan AD, Szigethy E, Lotrich F, DiMartini AF. Fibromyalgia symptoms and cirrhosis. Digestive diseases and sciences. 2015 May 1;60(5):1482–9. doi: 10.1007/s10620-014-3453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen L, Leo MC, Chang MF, Zucker BL, Sasaki A. Pain and self-care behaviours in adult patients with end-stage liver disease: a longitudinal description. Journal of palliative care. 2014 Mar 1;30(1):32–40. [PMC free article] [PubMed] [Google Scholar]
- 8•.Dwyer JP, Jayasekera C, Nicoll A. Analgesia for the cirrhotic patient: a literature review and recommendations. Journal of gastroenterology and hepatology. 2014 Jul 1;29(7):1356–60. doi: 10.1111/jgh.12560. Recent review of pain management in cirrhosis. [DOI] [PubMed] [Google Scholar]
- 9.Brecher DB, West TL. Pain management in a patient with renal and hepatic dysfunction. Journal of palliative medicine. 2014 Feb 1;17(2):249–52. doi: 10.1089/jpm.2013.0151. [DOI] [PubMed] [Google Scholar]
- 10.Perry CJ. Which analgesics are appropriate in patients with liver dysfunction? Journal of the American Academy of Physician Assistants. 2013 Nov 1;26(11):16–8. doi: 10.1097/01.JAA.0000436292.16894.2b. [DOI] [PubMed] [Google Scholar]
- 11.Loftis JM, Hauser P. Pain and opioid use in chronic liver disease: optimal treatment must address the mental health care needs of the patient. Digestive diseases and sciences. 2013;58(10):2753–5. doi: 10.1007/s10620-013-2809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogal SS, Winger D, Bielefeldt K, Rollman BL, Szigethy E. Healthcare utilization in chronic liver disease: the importance of pain and prescription opioid use. Liver International. 2013 Nov 1;33(10):1497–503. doi: 10.1111/liv.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, Asch SM, Kroenke K. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. Journal of general internal medicine. 2009 Jun 1;24(6):733–8. doi: 10.1007/s11606-009-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kean J, Monahan PO, Kroenke K, Wu J, Yu Z, Stump TE, Krebs EE. Comparative responsiveness of the PROMIS Pain Interference short forms, Brief Pain Inventory, PEG, and SF-36 Bodily Pain subscale. Medical care. 2016 Apr 1;54(4):414–21. doi: 10.1097/MLR.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Archives of internal medicine. 2003 Nov 10;163(20):2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 16.McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004 Sep 30;111(1):77–83. doi: 10.1016/j.pain.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Gayowski T, Wagener MM, Marino IR. Depression in patients with cirrhosis (impact on outcome) Digestive diseases and sciences. 1997 Jul 1;42(7):1421–7. doi: 10.1023/a:1018898106656. [DOI] [PubMed] [Google Scholar]
- 18.Nardelli S, Pentassuglio I, Pasquale C, Ridola L, Moscucci F, Merli M, Mina C, Marianetti M, Fratino M, Izzo C, Merkel C. Depression, anxiety and alexithymia symptoms are major determinants of health related quality of life (HRQoL) in cirrhotic patients. Metabolic brain disease. 2013 Jun 1;28(2):239–43. doi: 10.1007/s11011-012-9364-0. [DOI] [PubMed] [Google Scholar]
- 19.Lin EH, Katon W, Von Korff M, Tang L, Williams JW, Jr, Kroenke K, Hunkeler E, Harpole L, Hegel M, Arean P, Hoffing M. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. Jama. 2003 Nov 12;290(18):2428–9. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- 20.Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug and alcohol dependence. 2001 Jul 1;63(2):139–46. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 21.Alford DP, Compton P, Samet JH. Acute Pain Management for Patients Receiving Maintenance Methadone or Buprenorphine TherapyAcute Pain Management for Patients Receiving OAT. Annals of Internal Medicine. 2006 Jan 17;144(2):127–34. doi: 10.7326/0003-4819-144-2-200601170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karasz A, Zallman L, Berg K, Gourevitch M, Selwyn P, Arnstein J. The experience of chronic severe pain in patients undergoing methadone maintenance treatment. Journal of pain and symptom management. 2004 Nov 30;28(5):517–25. doi: 10.1016/j.jpainsymman.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 23•.Liebschutz J, Beers D, Lange A. Managing chronic pain in patients with opioid dependence. Current treatment options in psychiatry. 2014 Jun 1;1(2):204–23. doi: 10.1007/s40501-014-0015-4. Reviews the management of pain in patients with opioid dependence, which is particularly relevant in patients with cirrhosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riley TR, III, Koch K. Characteristics of upper abdominal pain in those with chronic liver disease. Dig Dis Sci. 2003;48:1914–1918. doi: 10.1023/a:1026149732756. [DOI] [PubMed] [Google Scholar]
- 25.Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013 Apr 1;57(4):1651–3. doi: 10.1002/hep.26359. [DOI] [PubMed] [Google Scholar]
- 26.Hadduck TA, McWilliams JP. Partial splenic artery embolization in cirrhotic patients. World journal of radiology. 2014 May 28;6(5):160. doi: 10.4329/wjr.v6.i5.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fouad YM, Makhlouf MM, Khalaf H, Mostafa Z, Abdel Raheem E, Meneasi W. Is irritable bowel syndrome associated with chronic hepatitis C? J Gastroenterol Hepatol. 2010;25:1285–1288. doi: 10.1111/j.1440-1746.2010.06311.x. [DOI] [PubMed] [Google Scholar]
- 28.Pan CX, Morrison RS, Ness J, Fugh-Berman A, Leipzig RM. Complementary and alternative medicine in the management of pain, dyspnea, and nausea and vomiting near the end of life: a systematic review. Journal of pain and symptom management. 2000 Nov 30;20(5):374–87. doi: 10.1016/s0885-3924(00)00190-1. [DOI] [PubMed] [Google Scholar]
- 29.Mgbemena O, Westfall AO, Ritchie CS, Hicks J, Raper JL, Overton ET, Norton WE, Merlin JS. Preliminary outcomes of a pilot physical therapy program for HIV-infected patients with chronic pain. AIDS care. 2015 Feb 1;27(2):244–7. doi: 10.1080/09540121.2014.940272. [DOI] [PubMed] [Google Scholar]
- 30.Montano–Loza AJ, Meza–Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clinical Gastroenterology and Hepatology. 2012 Feb 29;10(2):166–73. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 31.Foster NE, Delitto A. Embedding psychosocial perspectives within clinical management of low back pain: integration of psychosocially informed management principles into physical therapist practice—challenges and opportunities. Physical therapy. 2011 May 1;91(5):790–803. doi: 10.2522/ptj.20100326. [DOI] [PubMed] [Google Scholar]
- 32.Okifuji A, Hare BD. The association between chronic pain and obesity. Journal of pain research. 2015;8:399. doi: 10.2147/JPR.S55598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, Ettinger WH, Pahor M, Williamson JD. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis & Rheumatology. 2004 May 1;50(5):1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 34.Lamb SE, Lall RS, Hansen Z, Castelnuovo E, Withers EJ, Nichols VP, Griffiths F, Potter R, Szczepura A, Underwood M. A multicentered randomised controlled trial of a primary care-based cognitive behavioural programme for low back pain: the back skills training (BeST) trial. Health Technology Assessment. 2010;14(41):1–281. doi: 10.3310/hta14410. [DOI] [PubMed] [Google Scholar]
- 35.Morone NE, Greco CM, Moore CG, Rollman BL, Lane B, Morrow LA, Glynn NW, Weiner DK. A mind-body program for older adults with chronic low back pain: a randomized clinical trial. JAMA internal medicine. 2016 Mar 1;176(3):329–37. doi: 10.1001/jamainternmed.2015.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Hilton L, Hempel S, Ewing BA, Apaydin E, Xenakis L, Newberry S, Colaiaco B, Maher AR, Shanman RM, Sorbero ME, Maglione MA. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Annals of Behavioral Medicine. 2016 Sep 22;51(2):199–213. doi: 10.1007/s12160-016-9844-2. Recent review of mindfulness for pain management. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ernst E, White AR. Prospective studies of the safety of acupuncture: a systematic review. The American journal of medicine. 2001 Apr 15;110(6):481–5. doi: 10.1016/s0002-9343(01)00651-9. [DOI] [PubMed] [Google Scholar]
- 38.Vickers AJ, Cronin AM, Maschino AC, Lewith G, MacPherson H, Foster NE, Sherman KJ, Witt CM, Linde K Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: individual patient data meta-analysis. Archives of internal medicine. 2012 Oct 22;172(19):1444–53. doi: 10.1001/archinternmed.2012.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meier T, Wasner G, Faust M, Kuntzer T, Ochsner F, Hueppe M, Bogousslavsky J, Baron R. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain. 2003 Nov 30;106(1):151–8. doi: 10.1016/s0304-3959(03)00317-8. [DOI] [PubMed] [Google Scholar]
- 40.Gimbel J, Linn R, Hale M, Nicholson B. Lidocaine patch treatment in patients with low back pain: results of an open-label, nonrandomized pilot study. American journal of therapeutics. 2005 Jul 1;12(4):311–9. doi: 10.1097/01.mjt.0000164828.57392.ba. [DOI] [PubMed] [Google Scholar]
- 41.Massey T, Derry S, Moore RA, McQuay HJ. Topical NSAIDs for acute pain in adults. Cochrane Database Syst Rev. 2010 Jan;1:6. doi: 10.1002/14651858.CD007402.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Derry S, Moore RA, Rabbie R. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2012 Jan;1:9. doi: 10.1002/14651858.CD007400.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. Bmj. 2004 Apr 22;328(7446):991. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosilkovska M, Walder B, Besson M, Daali Y, Desmeules J. Analgesics in patients with hepatic impairment. Drugs. 2012 Aug 1;72(12):1645–69. doi: 10.2165/11635500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clinic Proceedings; 2010 May 31; Elsevier; pp. 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christensen E, Schlichting P, Fauerholdt L, Gluud C, Andersen PK, Juhl E, Poulsen H, Tygstrup N. Prognostic Value of Child-Turcotte Criteria in Medically Treated Cirrhosis. Hepatology. 1984 May 1;4(3):430–5. doi: 10.1002/hep.1840040313. [DOI] [PubMed] [Google Scholar]
- 47.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim W. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001 Feb 1;33(2):464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 48.Blaschke TF, Rubin PC. Hepatic first-pass metabolism in liver disease. Clinical Pharmacokinetics. 1979 Dec 1;4(6):423–32. doi: 10.2165/00003088-197904060-00002. [DOI] [PubMed] [Google Scholar]
- 49.Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. European journal of clinical pharmacology. 2008 Dec 1;64(12):1147. doi: 10.1007/s00228-008-0553-z. [DOI] [PubMed] [Google Scholar]
- 50.Morgan DJ, McLean AJ. Therapeutic implications of impaired hepatic oxygen diffusion in chronic liver disease. Hepatology. 1991 Dec 1;14(6):1280–2. [PubMed] [Google Scholar]
- 51.Frye RF, Zgheib NK, Matzke GR, Chaves-Gnecco D, Rabinovitz M, Shaikh OS, Branch RA. Liver disease selectively modulates cytochrome P450–mediated metabolism. Clinical Pharmacology & Therapeutics. 2006 Sep 1;80(3):235–45. doi: 10.1016/j.clpt.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Mazoit JX, Sandouk P, Scherrmann JM, Roche A. Extrahepatic metabolism of morphine occurs in humans. Clinical Pharmacology & Therapeutics. 1990 Dec 1;48(6):613–8. doi: 10.1038/clpt.1990.203. [DOI] [PubMed] [Google Scholar]
- 53.Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. American journal of therapeutics. 2005 Mar 1;12(2):133–41. doi: 10.1097/01.mjt.0000140216.40700.95. [DOI] [PubMed] [Google Scholar]
- 54.Benson GD. Acetaminophen in chronic liver disease. Clinical Pharmacology & Therapeutics. 1983 Jan 1;33(1):95–101. doi: 10.1038/clpt.1983.14. [DOI] [PubMed] [Google Scholar]
- 55.Wong F, Massie D, Hsu P, Dudley F. Indomethacin-induced renal dysfunction in patients with well-compensated cirrhosis. Gastroenterology. 1993 Mar 31;104(3):869–76. doi: 10.1016/0016-5085(93)91024-c. [DOI] [PubMed] [Google Scholar]
- 56.Clària J, Kent JD, López-Parra M, Escolar G, Ruiz-del-Arbol L, Ginès P, Jiménez W, Vucelic B, Arroyo V. Effects of celecoxib and naproxen on renal function in nonazotemic patients with cirrhosis and ascites. Hepatology. 2005 Mar 1;41(3):579–87. doi: 10.1002/hep.20595. [DOI] [PubMed] [Google Scholar]
- 57.De Ledinghen V, Heresbach D, Fourdan O, Bernard P, Liebaert-Bories MP, Nousbaum JB, Gourlaouen A, Becker MC, Ribard D, Ingrand P, Silvain C. Anti-inflammatory drugs and variceal bleeding: a case-control study. Gut. 1999 Feb 1;44(2):270–3. doi: 10.1136/gut.44.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep. 2016;65(RR-1):1–49. doi: 10.15585/mmwr.rr6501e1. DOI: http://dx.doi.org/10.15585/mmwr.rr6501e1https://www.cdc.gov/Other/disclaimer.html. Current guidelines for opioid prescribing for chronic pain for general population. [DOI] [PubMed] [Google Scholar]
- 59.Rudd RA, Aleshire N, Zibbell JE, Matthew Gladden R. Increases in drug and opioid overdose deaths—United States, 2000–2014. American Journal of Transplantation. 2016 Apr 1;16(4):1323–7. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 60•.Zedler B, Xie L, Wang L, Joyce A, Vick C, Kariburyo F, Rajan P, Baser O, Murrelle L. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain medicine. 2014 Nov 1;15(11):1911–29. doi: 10.1111/pme.12480. Demonstrates that liver disease is associated with more opioid-related adverse events. [DOI] [PubMed] [Google Scholar]
- 61•.Randall HB, Alhamad T, Schnitzler MA, Zhang Z, Ford-Glanton S, Axelrod DA, Segev DL, Kasiske BL, Hess GP, Yuan H, Ouseph R. Survival implications of opioid use before and after liver transplantation. Liver Transplantation. 2017 Mar 1;23(3):305–14. doi: 10.1002/lt.24714. Finds a dose-dependent association between pre-transplant opioid use and post-transplant mortality. [DOI] [PubMed] [Google Scholar]
- 62•.Rogal S, Mankaney G, Udawatta V, Good CB, Chinman M, Zickmund S, Bielefeldt K, Jonassaint N, Jazwinski A, Shaikh O, Hughes C. Association between opioid use and readmission following liver transplantation. Clinical transplantation. 2016 Oct 1;30(10):1222–9. doi: 10.1111/ctr.12806. Describes an association between opioid use and post-transplant readmission. [DOI] [PubMed] [Google Scholar]
- 63.Rogal S, Dew MA, DiMartini A. High-dose opioid use and liver transplantation: An underestimated problem? Liver Transplantation. 2017 Mar 1;23(3):285–7. doi: 10.1002/lt.24731. [DOI] [PubMed] [Google Scholar]
- 64•.Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB, Ganapathy D, Fagan A, Sikaroodi M, Bajaj JS. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Alimentary pharmacology & therapeutics. 2017 Jan 1;45(2):319–31. doi: 10.1111/apt.13858. Demonstrates that opioid use is associated with admission in patients with cirrhosis and posits one involved mechanism. [DOI] [PubMed] [Google Scholar]
- 65.Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, Blow FC. Association between opioid prescribing patterns and opioid overdose-related deaths. Jama. 2011 Apr 6;305(13):1315–21. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 66.Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC, Rossier MF, Hochstrasser D, Dayer P, Desmeules JA. Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. British journal of pharmacology. 2010 Jun 1;160(4):919–30. doi: 10.1111/j.1476-5381.2010.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oshlack B, Chasin M, Minogue JJ, Kaiko RF, inventors; Euro-Celtique SA, assignee. Controlled release oxycodone compositions. 5,656,295. United States patent US. 1997 Aug 12;
- 68.Crotty B, Watson KJ, Desmond PV, Mashford ML, Wood LJ, Colman J, Dudley FJ. Hepatic extraction of morphine is impaired in cirrhosis. European journal of clinical pharmacology. 1989 Sep 1;36(5):501–6. doi: 10.1007/BF00558076. [DOI] [PubMed] [Google Scholar]
- 69.Tegeder I, Lötsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clinical pharmacokinetics. 1999 Jul 1;37(1):17–40. doi: 10.2165/00003088-199937010-00002. [DOI] [PubMed] [Google Scholar]
- 70.Durnin CO, Hind ID, Ghani SP, Yates DB, Molz KH. Pharmacokinetics of oral immediate-release hydromorphone (Dilaudid IR) in subjects with moderate hepatic impairment. Proceedings of the Western Pharmacology Society. 2001;44:83–84. [PubMed] [Google Scholar]
- 71.Jin SJ, Jung JY, Noh MH, Lee SH, Lee EK, Choi BM, Song MH, Noh GJ. The Population Pharmacokinetics of Fentanyl in Patients Undergoing Living-Donor Liver Transplantation. Clinical Pharmacology & Therapeutics. 2011 Sep 1;90(3):423–31. doi: 10.1038/clpt.2011.133. [DOI] [PubMed] [Google Scholar]
- 72.Haberer JP, Schoeffler P, Couderc E, Duvaldestin P. Fentanyl pharmacokinetics in anaesthetized patients with cirrhosis. BJA: British Journal of Anaesthesia. 1982 Dec 1;54(12):1267–70. doi: 10.1093/bja/54.12.1267. [DOI] [PubMed] [Google Scholar]
- 73.Durogesic (Fentanyl Transdermal System) for transdermal administration [package insert on the Internet] Janseen Pharmaceuticals. 2016 revised 3/2017 [cited 12/2017]. Available from: https://www.janssenmd.com/pdf/duragesic/duragesic_pi.pdf.
- 74.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clinical pharmacokinetics. 2004 Nov 1;43(13):879–923. doi: 10.2165/00003088-200443130-00004. [DOI] [PubMed] [Google Scholar]
- 75.Lee CR, McTavish D, Sorkin EM. Tramadol. Drugs. 1993 Aug 1;46(2):313–40. doi: 10.2165/00003495-199346020-00008. [DOI] [PubMed] [Google Scholar]
- 76.Stamer UM, Lehnen K, Höthker F, Bayerer B, Wolf S, Hoeft A, Stuber F. Impact of CYP2D6 genotype on postoperative tramadol analgesia. Pain. 2003 Sep 30;105(1):231–8. doi: 10.1016/s0304-3959(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 77.Sansone RA, Sansone LA. Tramadol: seizures, serotonin syndrome, and coadministered antidepressants. Psychiatry (Edgmont) 2009 Apr;6(4):17. [PMC free article] [PubMed] [Google Scholar]
- 78.Cone EJ, Gorodetzky CW, Yousefnejad DA, Buchwald WF, Johnson RE. The metabolism and excretion of buprenorphine in humans. Drug Metabolism and Disposition. 1984 Sep 1;12(5):577–81. [PubMed] [Google Scholar]
- 79.Johnson RE, Fudala PJ, Payne R. Buprenorphine: considerations for pain management. Journal of pain and symptom management. 2005 Mar 31;29(3):297–326. doi: 10.1016/j.jpainsymman.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 80.Buprenorphine for Chronic Pain: A Review of the Clinical Effectiveness [online] Canadian Agency for Drugs and Technologies in Health; 2017. Jan 6, [PubMed] [Google Scholar]
- 81.Novick DM, Kreek MJ, Fanizza AM, Yancovitz SR, Gelb AM, Stenger RJ. Methadone disposition in patients with chronic liver disease. Clinical Pharmacology & Therapeutics. 1981 Sep 1;30(3):353–62. doi: 10.1038/clpt.1981.172. [DOI] [PubMed] [Google Scholar]
- 82.Novick DM, Kreek MJ, Ams PA, Lau LL, Yancovitz SR, Gelb AM. Effect of severe alcoholic liver disease on the disposition of methadone in maintenance patients. Alcoholism: Clinical and Experimental Research. 1985 Jul 1;9(4):349–54. doi: 10.1111/j.1530-0277.1985.tb05558.x. [DOI] [PubMed] [Google Scholar]
- 83.Vollmer KO, Von Hodenberg A, Kölle EU. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Arzneimittel-Forschung. 1986 May;36(5):830–9. [PubMed] [Google Scholar]
- 84.Gabapentin [Internet] Livertox.nlm.nih.gov. 2017 [cited 4 August 2017]. Available from: https://livertox.nlm.nih.gov//Gabapentin.htm.
- 85.Einarsdottir S, Björnsson E. Pregabalin as a probable cause of acute liver injury. European journal of gastroenterology & hepatology. 2008 Oct 1;20(10):1049. doi: 10.1097/MEG.0b013e328300c978. [DOI] [PubMed] [Google Scholar]
- 86.Syn WK, Naisbitt DJ, Holt AP, Pirmohamed M, Mutimer DJ. Carbamazepine-induced acute liver failure as part of the DRESS syndrome. International journal of clinical practice. 2005 Aug 1;59(8):988–91. doi: 10.1111/j.1368-5031.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- 87.Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury: a review for clinicians. American Journal of Psychiatry. 2014 Apr;171(4):404–15. doi: 10.1176/appi.ajp.2013.13050709. [DOI] [PubMed] [Google Scholar]
- 88.Kerr GW, McGuffie AC, Wilkie S. Tricyclic antidepressant overdose: a review. Emergency Medicine Journal. 2001 Jul 1;18(4):236–41. doi: 10.1136/emj.18.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vuppalanchi R, Hayashi PH, Chalasani N, Fontana RJ, Bonkovsky H, Saxena R, Kleiner D, Hoofnagle JH. Duloxetine hepatotoxicity: a case-series from the drug-induced liver injury network. Alimentary pharmacology & therapeutics. 2010 Nov 1;32(9):1174–83. doi: 10.1111/j.1365-2036.2010.04449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holliday SM, Benfield P. Venlafaxine. Drugs. 1995 Feb 1;49(2):280–94. doi: 10.2165/00003495-199549020-00010. [DOI] [PubMed] [Google Scholar]
- 91.Dworkin RH, O’connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, Kent JL, Krane EJ, LeBel AA, Levy RM, Mackey SC. Mayo Clinic Proceedings. 3. Vol. 85. Elsevier; 2010. Mar 31, Recommendations for the pharmacological management of neuropathic pain: an overview and literature update; pp. S3–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anglin R, Yuan Y, Moayyedi P, Tse F, Armstrong D, Leontiadis GI. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. The American journal of gastroenterology. 2014 Jun 1;109(6):811. doi: 10.1038/ajg.2014.82. [DOI] [PubMed] [Google Scholar]
- 93.Hézode C, Roudot-Thoraval F, Nguyen S, Grenard P, Julien B, Zafrani ES, Pawlostky JM, Dhumeaux D, Lotersztajn S, Mallat A. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005 Jul 1;42(1):63–71. doi: 10.1002/hep.20733. [DOI] [PubMed] [Google Scholar]
- 94.Brunet L, Moodie EE, Rollet K, Cooper C, Walmsley S, Potter M, Klein MB Canadian Co-infection Cohort Investigators. Marijuana smoking does not accelerate progression of liver disease in HIV–hepatitis C coinfection: a longitudinal cohort analysis. Clinical Infectious Diseases. 2013 Jun 28;57(5):663–70. doi: 10.1093/cid/cit378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilkinson ST, D’Souza DC. Problems with the medicalization of marijuana. Jama. 2014 Jun 18;311(23):2377–8. doi: 10.1001/jama.2014.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Figure modified from “Boston University School of Medicine SCOPE of Pain” presentation slide.