Abstract
Background
The impact of surgical history on graft outcomes in patients with functionally univentricular hearts (UH) is not well understood. We compared graft outcomes after heart transplantation in children with UH between patients transplanted without prior cardiac surgery (Group A) and patients transplanted after prior cardiac surgery (Group B).
Methods
We reviewed all patients transplanted for UH at our institution from 1990–2009. Differences in the probability of acute rejection (AR), incidence of graft vasculopathy (GV), and incidence of death or retransplantation were compared between Group A and Group B. Student’s t-test, Mann-Whitney U test, the log-rank test, logistic regression, and Cox proportional hazards modeling were used as appropriate.
Results
There were 180 patients with UH transplanted during the study period; 105 in Group A and 75 in Group B (median age 84 days [IQR 47–120] vs. 584 [IQR 168–2956], respectively; p<0.001). The odds of AR were higher in Group B (OR 2.7, 95% CI 1.3–5.4). Univariable risks of GV and graft loss were lower in Group A (p=0.034 and p=0.003, respectively). Median graft survival was 18 years in Group A vs. 8 years in Group B. There was a higher risk of graft loss after 5 years post-transplant in Group B patients that were ≥1 year old at time of transplant(p<0.001).
Conclusions
Heart transplantation without prior cardiac surgery in patients with UH was associated with better graft survival and lower probability of AR. The effect of age is complex and time dependent, with age affecting outcomes after 5 years.
Keywords: cardiac transplantation, graft loss, risk factors, single ventricle
INTRODUCTION
Cardiac transplantation in infancy is used as primary treatment for hypoplastic left heart syndrome (HLHS) and other forms of functionally univentricular hearts (UH). Long term survival is excellent, with median survival of >18 years.(1–4) As the results of staged surgical palliation improved and donor availability remained a challenge, the majority of infants with UH were treated with surgical palliation rather than transplantation.(5,6) However, a significant number of UH surgical palliations fail, requiring cardiac transplantation to be performed in children with a history of prior cardiac surgeries.(7–11) Single and multicenter evaluations of children undergoing cardiac transplantation with and without prior surgery have shown mixed outcomes.(12,13) However, few studies have evaluated the effect of prior cardiac surgery on outcomes after transplantation specifically in patients with UH.(14) Investigation of this subgroup is relevant as surgical palliation outcomes are not remarkably better than those for primary transplantation.(15–17) In order to determine if cardiac surgery prior to transplantation in patients with UH is a risk factor for graft loss, we evaluated its effect on graft outcomes in our large, single center population of patients with UH.
METHODS
This was a retrospective chart review of patients with UH who underwent cardiac transplant between 1990–2009 at our institution. All patients who underwent heart transplantation during the study period were enrolled in consecutive fashion. Era 1 and Era 2 were defined as 1990–1999 and 2000–2009, respectively. Those patients without cardiac surgery prior to transplantation were defined as Group A and those transplanted after prior cardiac surgery were defined as Group B. Clinical data included demographics, donor and recipient weight, diagnosis, age at transplantation, ventricular morphology, the presence of a restrictive atrial communication, need for mechanical circulatory support (MCS) and/or mechanical ventilation prior to transplantation, date of transplantation, and date of graft loss or last known follow-up. Race was defined as Black, Caucasian, Hispanic, or other (Asian, Arab, mixed ethnicity, and unspecified).
Laboratory data at the time of transplantation included creatinine, human leukocyte antigen (HLA) panel reactive anti-body (PRA), and retrospective recipient-donor crossmatch results. Glomerular filtration rate (GFR) was estimated using the Schwartz equation.(18) PRA testing was performed by testing recipient serum for complement-mediated lytic activity in the presence of anti-human immunoglobulin and dithiothreitol, by enzyme linked immunosorbent assay, or by the Luminex test, depending on the era of transplantation. A PRA of ≥10% was considered positive. Retrospective crossmatching was performed by mixing recipient serum with donor lymphoid tissue, excluding the presence of autoantibodies by autologous serum crossmatch with recipient B- and T-cells and the donor-recipient crossmatch by IgG was considered positive if there was evidence of B- or T-cell cytotoxicity in the presence of dithiothreitol. CMV serology prior to transplantation was not included in the analysis due to the large number of infants with maternal anti-CMV IgG.
Graft outcomes included the number of acute rejection (AR) episodes, the diagnosis of graft vasculopathy (GV), the need for re-transplantation, or death. AR was defined as any event that led to an augmentation of immunosuppression, usually with steroids or anti-lymphocyte therapy, as defined by the Pediatric Heart Transplant Study group (PHTS).(19) GV was defined by a maximal intimal thickness by intravascular ultrasound of ≥ 0.3 mm or when there was evidence of stenosis or distal pruning by angiography. Graft loss was defined as death or re-transplantation. Immunosuppression and rejection surveillance protocols have been previously reported by our center and were largely unchanged throughout the study period except that anti-lymphocyte globulin was used for induction until anti-thymocyte globulin was approved by the FDA in 1991. (20)
Statistical Analysis
It was our hypothesis that the risk of graft loss would be higher in Group B. The primary outcome for this study was graft loss, as defined above. Student’s t-test, chi square test, Mann-Whitney U test, and logistic regression were used as appropriate. Kaplan-Meier survival curves and the log rank test were performed to determine the incidence of GV and graft loss. Univariable Cox proportional hazards modeling was performed to identify potential pre-transplant risk factors for graft loss, including age ≥1 year, sex, race, era of transplantation, donor/recipient weight ratio, number of pre-transplant cardiac surgeries, PRA>10%, graft ischemic time, UNOS waitlist time, GFR, pre-transplant need for mechanical ventilation, and pre-transplant inotropic support. UNOS listing status was not included in the analysis because of the different age based criteria for determining listing status. Patients with listing status of 1A and 1B were considered status 1 for the purpose of descriptive statistics. Univariable analysis of incidence of GV was compared between patients with regard to prior surgery, age ≥1 yr, male sex, era of surgery 2000–2009, non-white race, and PRA >10%.
We also examined the impact of prior surgery and age by re-classifying the patients, both over the entire follow-up period and during the intervals of 0–5 years post-transplant and >5 years post-transplant, using Kaplan-Meier plots, log-rank tests and Cox’s proportional hazards models to estimate hazard ratios. Patients were classified according to <1 year of age at transplant in Group A, < 1 year of age at transplant in Group B and ≥ 1 year of age at transplant in Group B. Values of p < 0.05 were considered statistically significant.
RESULTS
Study Population
Between 1990 and 2009, 180 patients with UH underwent a first heart transplantation. There were 105 patients in Group A and 75 in Group B. Of those in Group B, 36% had one surgery, 32% had 2 surgeries, 24% had 3 surgeries, and 8% had 4 or more surgeries. Surgeries performed included 24 pulmonary artery bandings, 37 systemic-pulmonary artery shunts, 9 stage I palliations (modified Norwood procedure or Damus-Kay-Stansel), 26 stage II palliations, 21 stage III (Fontan), 3 Fontan revisions, and one Blaylock-Hanlon septectomy. Of the 9 patients in our study that had undergone stage I palliation, 6 went on to stage II and two went on to Fontan palliation prior to transplantation. Coarctation repair in borderline left ventricles was performed in 9 patients, 6 of which had coinciding PA band placement.
The overall median time from transplant to last follow-up or graft loss was 6.6 years [IQR 2.0–11.5]. The median time from transplant to last follow-up or graft loss in Group A and Group B was 7.8 yr [IQR 2.6–11.7] and 5.0 yr [IQR 1.6–10.6], respectively (p=0.13). The median number of pre-transplant surgeries in Group B was 2 [IQR 1–3]. The median number of surgeries for Group B in era 1 was lower than in era 2 (2 [IQR 1–3] vs. 3 [IQR 1–3], respectively; p=0.004). Racial distribution was Black (3%), Caucasian (68%), Hispanic (17%), unknown (6 %), and other (5%).
Patients’ characteristics are shown in Table 1. Median age at transplant in Group A was 84 days [IQR 47–120] vs. 584 [IQR 168–2956] in Group B (p<0.001). Group A had significantly more patients <1 year of age at the time of transplant (98% vs. 43%, p<0.001). There were only 2 patients ≥1 year of age in Group A. There was a significantly higher proportion of patients with a single right ventricle in Group A and a significantly higher proportion of patients with a single left ventricle in Group B (Table 1). There was no difference in the median number of surgeries in Group B based on ventricular morphology (p=0.24). Overall, patients with a single right ventricle were significantly younger than patients with a single left ventricle (median age at transplant 0.26 yr [IQR 0.16–0.48] vs. 3.8 yr [IQR 0.39–10.8], respectively; p<0.001). When analyzed within group B only, patients in Group B with a single right ventricle also were significantly younger than patients with a single left ventricle (median age at transplant 1.1 yr [IQR 0.47–3.8] vs. 4.4 yr [IQR 0.45–13.5], respectively; p=0.046). A PRA>10% was present in 6 patients in Group A and 6 patients in Group B, with 4 of the patients in Group B having undergone 3 or more prior surgeries. There was only one positive retrospective crossmatch out of 148 patients with available crossmatch data. Only 3 patients had a PRA > 50%. There were no patients with a PRA>10% that had a positive retrospective crossmatch. There was no difference in the proportion of patients in the two groups with a PRA>10% based on ventricular morphology (p=0.15). Previous surgeries in patients with a PRA>10% included one stage I palliation, 3 aortopulmonary shunts, two stage II palliations, and 4 Fontans.
Table 1.
Patient Characteristics
| Characteristics | Total, N=180 | Group A, N=105 | Group B, N=75 | P Value |
|---|---|---|---|---|
| Median Age (days) [IQR] | 120 [65–383] | 84 [47–120] | 584 [168–2956] | ‡ p<0.001 |
|
| ||||
| Age < 1 year | 135 | 103 | 32 | † p<0.001 |
| Age ≥ 1 Year | 45 | 2 | 43 | |
|
| ||||
| Male | 114 | 71 | 43 | ns |
| Female | 66 | 34 | 32 | |
|
| ||||
| Single Right Ventricle | 138 | 99 | 39 | † p<0.001 |
| Single Left Ventricle* | 41 | 6 | 35 | |
|
| ||||
| UNOS Status 1 | 142 | 98 | 44 | † p<0.001 |
| UNOS Status 2 | 27 | 0 | 27 | |
|
| ||||
| Era 1990–1999 | 93 | 53 | 40 | ns |
| Era 2000–2009 | 87 | 52 | 35 | |
|
| ||||
| Ischemic Time (min) | 257 +/− 71 | 257 +/− 72 | 256 +/− 79 | ns |
|
| ||||
| Waitlist time (days [IQR]) | 74 [33–115] | 72 [35–110] | 79 [33–125] | ns |
|
| ||||
| Median GFR (ml/min/m2 [IQR]) | 67.5 | 56.7 [39.3–74.1] | 94.3 [62.7–125.9] | ‡ p<0.001 |
Ventricular morphology was indeterminate in 1
P value for difference in proportions between Group A and Group B by the χ2 test.
P value for difference in median between Group A and Group B by the Mann-Whitney U test.
Group A = No Prior Cardiac Surgery
Group B = Had Prior Cardiac Surgery
Acute Graft Rejection
There were AR data available for 174 patients. There was at least one AR episode in 60% of Group A and in 80% of Group B (OR 2.7, 95% CI 1.3–5.4). The odds of AR in the first year after transplant were higher in Group B (OR 2.4, 95% CI 1.3–4.4) and in patients ≥1 year of age at the time of transplant (OR 2.7, 95% CI 1.3–5.4) in univariable analysis.
Graft Loss
Graft loss occurred in 76 patients (42%). Overall graft survival was 86% at 1 year, 72% at 5 years, 59% at 10 years, and 44% at 15 years. Causes of graft loss are shown in Table 2. The incidence of GV was lower in Group A than Group B at ten years (40% vs. 46 %, respectively; p=0.03). Graft survival was higher in Group A compared to Group B (86% vs. 87% at one year, 76% vs. 66% at 5 years, and 68% vs. 46% at 10 years, respectively, p=0.002; Figure 1). Median graft survival was 18 years in Group A and 8 years in Group B. Re-transplantation was required in 7 in Group A and 8 in Group B (p=0.35). Univariable hazards for graft loss are shown in Table 3. MCS and a positive retrospective donor specific crossmatch were not included in the analysis as there were only two patients in the entire cohort on MCS at the time of transplant and there was only one patient that had a positive retrospective crossmatch, out of 148 patients with available crossmatch data. Univariable risk of graft loss was significantly higher in Group B. Using Group A as a reference, patients with 1–2 prior surgeries (HR 1.82, 95% CI 1.10–3.02) and patients with ≥3 prior surgeries (HR 2.43, 95% CI 1.27–2.64) had increasingly higher risks of graft loss. There was no significant univariable association between graft loss and age ≥1 year, sex, era of transplantation, ventricular morphology, GFR, the presence of a restrictive atrial septal communication, race, pre-transplant inotropic support, donor-to-recipient weight ratio, PRA >10%, mechanical ventilation prior to transplantation, ischemic time, or waitlist time (Table 3). When re-transplantation was removed as an endpoint, there was higher overall incidence of death in Group B (p=0.016).
Table 2.
Causes of Graft Loss (N=76).
| Cause of Graft Loss | Total N (%) |
Group A N (%) |
Group B N (%) |
|---|---|---|---|
| Graft Vasculopathy | 10 (5.6) | 5 (14.3) | 5 (12.2) |
| Acute Rejection | 26 (14.4) | 13 (37.1) | 13 (31.7) |
| Chronic Graft Failure | 7 (3.9) | 1 (2.9) | 6 (14.6) |
| Primary Graft Failure | 7 (3.9) | 5 (14.3) | 2 (4.9) |
| Infection | 4 (2.2) | 2 (5.7) | 2 (4.9) |
| Hemorrhage | 3 (1.7) | 1 (2.9) | 2 (4.9) |
| Other: Multi-Organ System Failure, CVA, PTLD | 12 (6.7) | 5 (14.3) | 7 (17.1) |
| Unknown | 7 (3.9) | 3 (8.6) | 4 (9.8) |
|
| |||
| Total | 76 (42.2) | 35 (19.4) | 41 (22.8) |
Figure 1.
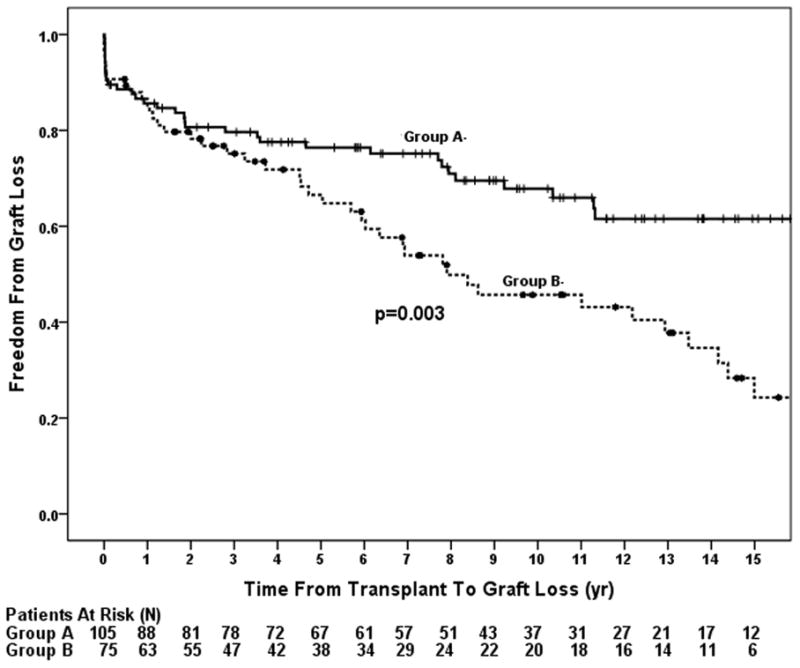
Post-transplant primary graft survival (freedom from death or re-transplantation) comparing patients with (Group B) and without (Group A) surgery prior to transplantation. Patients with prior cardiac surgery had a higher incidence of graft loss compared to those without prior surgery.
Table 3.
Univariable Cox Proportional Hazard Modeling of Risk Factors for Graft Loss
| Pre-Tx Characteristics, N (%) | Model N | Events | HR | 95% Confidence Interval | p Value |
|---|---|---|---|---|---|
| Any Surgery, 75 (41.7) | 180 | 76 | 1.98 | 1.25–3.13 | 0.004 |
| Number of Surgeries | 180 | 76 | 1.26 | 1.06–1.50 | 0.009 |
| Age ≥ 1 Yr, 45 (25) | 180 | 76 | 1.59 | 0.97–2.61 | ns |
| Male Sex, 114 (63) | 180 | 76 | 0.64 | 0.40–1.02 | ns |
| Era 2000–2009*, 87 (48.3) | 180 | 76 | 0.98 | 0.59–1.61 | ns |
| Left Ventricular Morphology† | 179 | 75 | 1.22 | 0.73–2.06 | ns |
| GFR (Schwartz) | 170 | 69 | 1 | 0.998–1.004 | ns |
| Restrictive Atrial Septum, 44 (24) | 165 | 68 | 0.99 | 0.57–1.72 | ns |
| Non-White Race, 46 (26) | 170 | 69 | 1.23 | 0.72–2.11 | ns |
| Inotropic Support, 22 (12) | 170 | 69 | 1.52 | 0.75–3.08 | ns |
| Donor: Recipient Weight | 168 | 67 | 0.97 | 0.66–1.42 | ns |
| PRA >10%, 12 (6.7) | 156 | 60 | 1.45 | 0.52–4.06 | ns |
| Mechanical Ventilation, 10 (5.6) | 169 | 69 | 1.51 | 0.55–4.2 | ns |
| Ischemic time | 171 | 71 | 1 | 0.997–1.003 | ns |
| Waitlist Time | 180 | 75 | 1.00 | 0.999–1.003 | ns |
Reference is Era 1990–1999
Reference is Right Ventricular Morphology
When classifying the data according to age and prior cardiac surgery, patients <1 year of age in Group A had the best graft survival (p=0.017, Figure 2a). Graft loss was more than twice as likely to occur in those ≥1 year of age in Group B (HR=2.06, p=0.009). There was a higher risk of graft loss in those <1 year of age in Group B (HR=1.75, p=0.054, Table 4) compared with those <1 year in Group A. The odds of AR were also significantly higher in those ≥1 year of age in Group B compared to those <1 year of age in Group A (OR 3.87, 95% CI 1.49–10.06, p=0.005) but not in those <1 year in Group B. Analyzing the data in separate post-transplant intervals revealed no significant difference in graft survival between groups in the first 5 years post-transplantation (p=0.36, Figure 2b). However, among those who survived to 5 years post-transplant, patients ≥1 year of age at the time of transplant in Group B had a 6 fold higher incidence of graft loss compared to those <1 year of age in Group A (HR 6.48, 95% CI 2.63–15.92, p<0.001; Figure 2c, Table 4).
Figure 2.
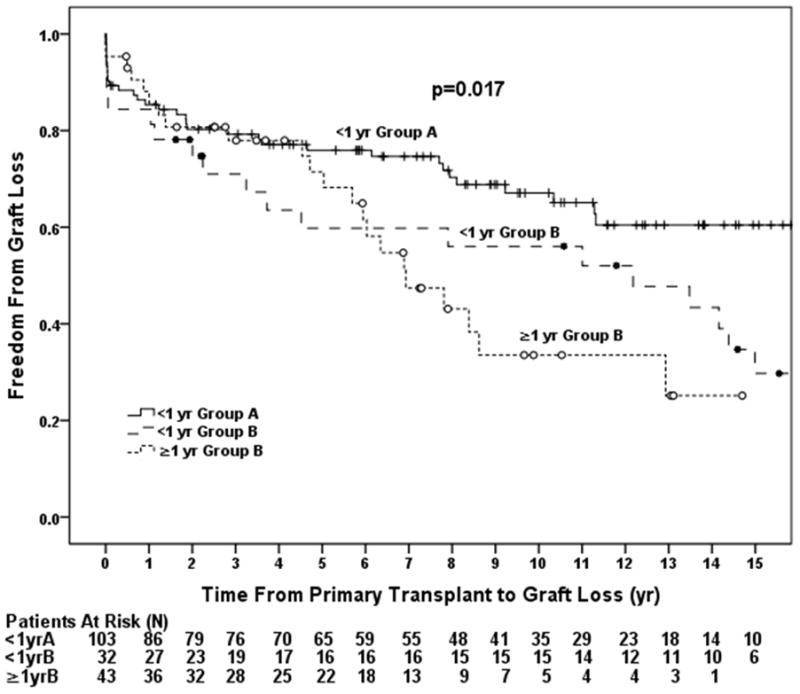
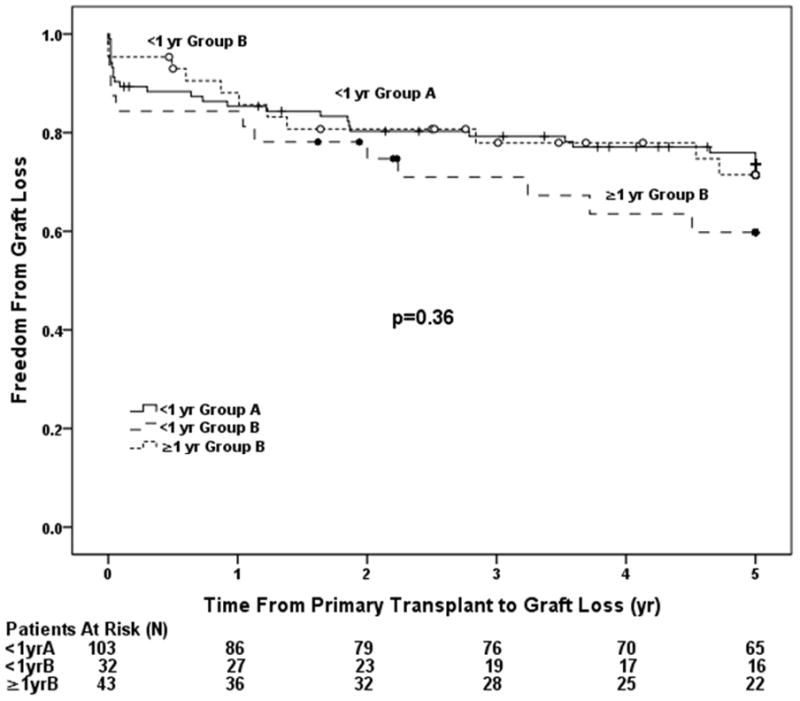
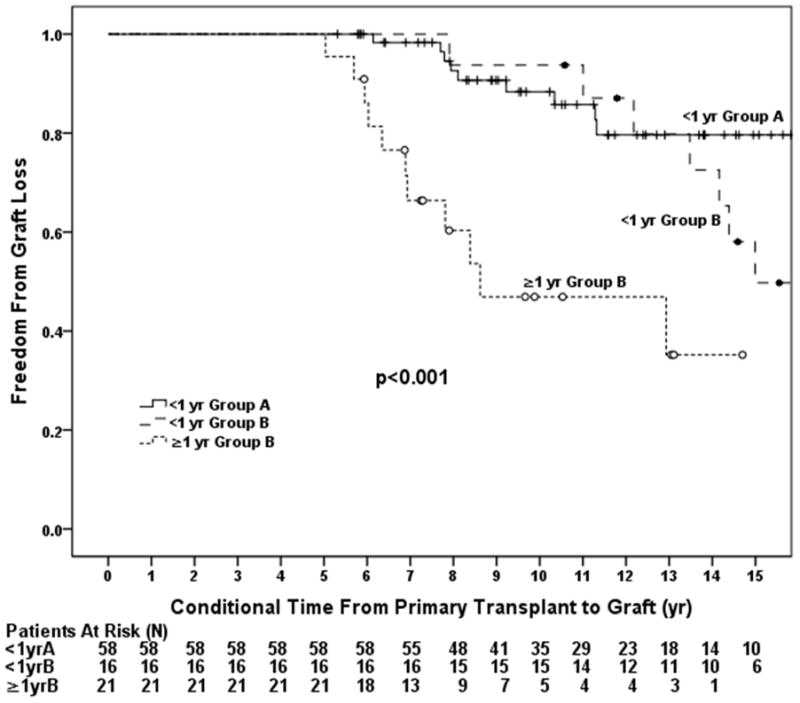
Groups were analyzed based on age and the presence of prior surgery and included patients < 1 year of age in Group A, < 1 year of age in Group B, and ≥ 1 year of age in Group B. A) Overall incidence of graft loss was higher in patients with prior cardiac surgery and was highest in those with a history of prior surgery transplanted outside of infancy. B) Incidence of graft loss in the first 5 years post-transplant. There was no difference in graft loss between the three groups through 5 years. C) Incidence of graft loss in patients that survived 5 years post-transplant. Patients in Group B the highest incidence of graft loss compared to those in Group A.
Table 4.
Cox Proportional Hazard Modeling of Risk Factors for Graft Loss
| Variable | N (Events) | HR | 95% Confidence Interval | P Value |
|---|---|---|---|---|
| Overall Survival | 178 (75) | |||
| • Age <1 yr Group A | 103 | |||
| • Age <1 yr Group B | 32 | 1.75 | 0.99–3.10 | 0.053 |
| • Age ≥1 yr Group B | 43 | 2.06 | 1.20–3.56 | 0.009 |
| 5 Year Contingent Graft Survival | 103 (28) | |||
| • Age <1 yr Group A | 65 | |||
| • Age <1 yr Group B | 16 | 1.90 | 0.70–5.15 | 0.21 |
| • Age ≥1 yr Group B | 22 | 6.48 | 2.63–15.92 | <0.001 |
Effect of Era on Graft Outcomes
Table 1 shows the proportion of patients in Groups A and B transplanted in Eras 1 and 2. In Group B, there were 22 infants transplanted in Era 1 and 10 infants transplanted in Era 2. All patients that had undergone stage 1 palliation prior to transplantation were transplanted in Era 2. There was no difference in the proportion of patients with a PRA >10% between eras and there was no significant difference between eras in the odds of AR (OR 0.56, 95% CI 0.29–1.07). There were no univariable differences between eras for the risk of GV (HR 1.29, 95% CI 0.69–2.42) or graft loss (HR 0.98, 95% CI 0.59–1.61 Table 3).
DISCUSSION
In this single center retrospective analysis comparing outcomes of patients with UH undergoing cardiac transplantation, we found that surgery prior to transplantation was a significant risk factor for graft loss following transplantation. The most striking difference was found late, with 10 and 15 year graft survival of 68% and 57%, respectively in Group A and 46 % and 24%, respectively, in Group B (Figure 1). Half of patients in Group A were still alive 18 years post transplant while half of the patients in Group B had suffered graft loss by 8 years post-transplant. The interaction between a history of prior surgery and age at transplantation is complex. While surgery prior to transplantation seems to be the most important risk factor (Figure 1 and Table 3), transplantation outside of infancy is also likely affecting outcomes, as demonstrated by our analysis in separate post-transplant intervals (Figure 2). There was no significant difference in early graft survival between infants vs. non-infants in Group B through 5 years, however, late graft loss accelerates for non-infants in Group B so that by 10 years there is a significant difference in graft survival between infants and non-infants in Group B (Figures 2a and 2c; Table 3). We also found that the overall improved graft survival between Group A and infants in Group B was of borderline statistical significance (HR 1.75, 95% CI 0.99–3.10, p=0.053; Table 4). Since there were only 32 infants in Group B we suspect that we were underpowered to determine a difference in graft survival between Group A and infants in Group B. We speculate that with more infant patients in Group B we would have found a statistically significant difference between the two groups.
The overall graft survival of 86% at one year and 72% at 5 years is comparable to other series of patients transplanted primarily for single ventricle physiology.(17, 21) However, in contrast to prior reports, survival for both Group A and Group B were nearly identical throughout the first year post-transplant, signifying that peri-operative deaths did not play a role in outcome differences.(11,12, 14) A study published by the PHTS showed that infants transplanted without prior surgery for HLHS had better early survival compared to infants with HLHS and prior surgery.(14) Comparison of late outcomes between the two studies are limited by the shorter duration of follow-up in the PHTS study and differences in outcomes occurring later than 9 years following transplant could not be evaluated.(14) The reasons for less early mortality in our center’s population (87% 1 year graft survival in Group B vs. 70% 1 year overall survival in the PHTS study) may be related to differences in ventricular morphology and/or clinical condition at the time of transplantation between the groups with prior surgery in the two studies. In a single center study, Dionigi and colleagues compared 154 patients with primary transplantation for HLHS and 160 patients transplanted after failed surgical palliation for complex congenital heart disease, 58 of whom had palliated UH, and found no difference in long term survival between the two groups.(13) Graft outcomes in Group A were comparable to the Dionigi cardiomyopathy group but better than the un-operated infant HLHS group at one year, while graft outcomes in Group B were slightly worse than those of the Dionigi complex congenital heart disease group, which included a large proportion of patients with biventricular physiology. A smaller study by Jacobs et. al. comparing outcomes of primary heart transplantation in infants with HLHS vs. rescue transplantation for failed surgical palliation in HLHS found that graft outcomes at 5 years were worse in the palliated group with borderline significance, however, this study included only 8 patients transplanted after surgical palliation, which limits potential for valid comparisons.(9) The 10 year survival of 46% in Group B was similar to that of patients reported by Chen, et al with complex congenital heart disease requiring pulmonary artery reconstruction at the time of transplant, which is not surprising since nearly all of our Group B required some form of pulmonary artery reconstruction.(8) AR was less likely in Group A, which is consistent with prior studies and likely played a significant role in Group A’s better long term outcomes.(22) Whether Group A was less likely to have AR due to a more plastic immune system as a result of their young age, a lack of a previous sensitizing surgery, or a combination thereof, remains unclear.
Ventricular morphology was not associated with graft loss in univariate analysis. This finding could have been influenced by the larger proportion of patients in Group A (75%) and the relatively few patients (9) that had undergone a Norwood procedure, which is supported by a study from the PHTS database that found a previous Norwood procedure was highly associated with the presence and degree of HLA sensitization and that those with a PRA >50% were at highest risk of poor outcomes.(23) The small number of patients that underwent Norwood palliation and the large proportion of infant transplants in our cohort likely explains why we found only 7% of our population to have an elevated PRA. Only one of the 9 patients that underwent Norwood palliation developed an elevated PRA, which may have been influenced by three Norwood patients that were less than a year of age at the time of transplant. We found no difference in HLA sensitization based on ventricular morphology, which we speculate is due to the younger age of patients in our cohort with a single right ventricle.
Regardless of the reason for better outcomes in Group A, transplantation following palliative surgery is associated with worse long term graft survival. The identification of prior cardiac surgery as a predictor of graft loss after transplant has implications for the management of children with UH. Group A median graft survival was 18 years vs. 8 years in Group B. Therefore, most infants transplanted without prior cardiac surgery for UH will experience graft loss at a much older age than most previously palliated transplant recipients. Limited donor supply makes it unrealistic to transplant all infants with single ventricle physiology, which is reflected by the American Heart Association guideline recommendations that heart transplantation is not a feasible standard for any specific congenital heart lesion.(24) Since some patients with staged palliation of UH live well into their adult years, better predictors of early surgical palliation failure, early ventricular failure and interstage mortality are needed at the time of diagnosis in neonates with UH.(10, 25 ) No one strategy for treatment of UH is ideal for all patients. While some may point to waitlist mortality as the driving force in the decision to perform surgical palliation, outcomes from the time of stage one palliation must be taken into account as well. Risk factors that were found to be predictive of adverse outcomes following stage one palliation in intermediate term follow-up of the Single Ventricle Reconstruction Trial included obstructed pulmonary venous return, a lower right ventricular fractional area of change, a genetic syndrome, lower socioeconomic status, non-HLHS diagnosis, lower gestational age, and pre-Norwood surgery.(26) Consideration should be given to these data when determining a treatment strategy for an individual patient. Long-term follow-up and secondary analyses are still needed to determine risk factors for death or transplantation prior to stage one palliation in order to determine which children with UH will have acceptable long term survival. Genetic data collected at the time of enrollment may be of benefit in this endeavor as well.(27)
Limitations
There were several limitations to this study. This was a retrospective study. Our single center outcomes may not reflect the outcomes of the pediatric transplant population as a whole. We were also limited by the nature of the patient population. The inability to perform multivariable Cox proportional hazards modeling was due to a selection bias in the cohort as only two patients older than one year of age did not have surgery prior to transplantation. This selection bias is unavoidable in the analysis of these two populations, since patients listed for primary transplantation without surgical palliation were likely to be transplanted or die on the waiting list prior to one year of age.(16, 21) We could not, therefore, fully determine whether age at the time of transplantation or history of prior cardiac surgery were independently responsible for the differences in long term outcomes since very few patients are able to live past a year of life without palliation or transplantation. Also, it is important to note that this study analyzed patients with UH from the time of transplantation and did not study outcomes from the time of listing or outcomes of surgical palliation from the time of the first surgery. Therefore, one cannot conclude from this study whether or not an initial strategy of palliation or transplantation achieves better overall outcomes.
Conclusions
Patients with UH who have had surgery prior to undergoing transplantation have a higher risk of graft loss than those without prior surgery, with the largest effect on graft loss seen late in those with a history of prior surgery. The interaction between surgical history and age at transplantation is complex and time dependent, with age affecting outcomes after 5 years.
Acknowledgments
Sources of Funding
The study was supported in part by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views
This work was presented at the International Society for Heart and Lung Transplantation Conference on April 13th, 2011.
ABBREVIATIONS
- UH
functionally univentricular heart(s)
- GV
Graft vasculopathy
- AR
Acute rejection
- PRA
Panel reactive antibody
- CMV
Cytomegalovirus
- MCS
Mechanical circulatory support
- GFR
Glomerular Filtration Rate
- HLHS
Hypoplastic left heart syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Backer CL, Idriss FS, Zales VR, Mavroudis C. Cardiac transplantation for hypoplastic left heart syndrome: a modified technique [see comment] Ann Thorac Surg. 1990;50:894–8. doi: 10.1016/0003-4975(90)91115-r. [DOI] [PubMed] [Google Scholar]
- 2.Chiavarelli M, Gundry SR, Razzouk AJ, Bailey LL. Cardiac transplantation for infants with hypoplastic left-heart syndrome. JAMA. 1993;270:2944–7. [PubMed] [Google Scholar]
- 3.Mayer JE., Jr Cardiac transplantation for neonates with hypoplastic left heart syndrome. Ann Thorac Surg. 1990;50:864–5. doi: 10.1016/0003-4975(90)91109-o. [DOI] [PubMed] [Google Scholar]
- 4.Kirk R, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Fourteenth Pediatric Heart Transplantation Report--2011. J Heart Lung Transplant. 2011;30:1095–103. doi: 10.1016/j.healun.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Bove EL, Lloyd TR. Staged reconstruction for hypoplastic left heart syndrome. Contemporary results. Ann Surg. 1996;224:387–94. doi: 10.1097/00000658-199609000-00015. discussion 394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kern JH, Hayes CJ, et al. Survival and risk factor analysis for the Norwood procedure for hypoplastic left heart syndrome. Am J Cardiol. 1997;80:170–174. doi: 10.1016/s0002-9149(97)00313-5. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein D, Naftel D, Chin C, et al. Outcome of Listing for Cardiac Transplantation for Failed Fontan: A Multi-Institutional Study. Circulation. 2006;114:273–280. doi: 10.1161/CIRCULATIONAHA.105.548016. [DOI] [PubMed] [Google Scholar]
- 8.Chen JM, Davies RR, Mital SR, et al. Trends and outcomes in transplantation for complex congenital heart disease: 1984 to 2004. Ann Thorac Surg. 2004;78:1352–61. doi: 10.1016/j.athoracsur.2004.04.012. discussion 1352-61. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs JP, Quintessenza JA, Chai PJ, et al. Rescue cardiac transplantation for failing staged palliation in patients with hypoplastic left heart syndrome. Cardiol Young. 2006;16:556–62. doi: 10.1017/S1047951106001223. [DOI] [PubMed] [Google Scholar]
- 10.Khairy P, Fernandes SM, Mayer JE, Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 11.Lamour JM, Kanter KR, Naftel DC, et al. The effect of age, diagnosis, and previous surgery in children and adults undergoing heart transplantation for congenital heart disease. J Am Coll Cardiol. 2009;54:160–5. doi: 10.1016/j.jacc.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Canter C, Naftel D, Caldwell R, et al. Survival and Risk Factors for Death After Cardiac Transplantation in Infants: A Multi-institutional Study. Circulation. 1997;96:227–231. doi: 10.1161/01.cir.96.1.227. [DOI] [PubMed] [Google Scholar]
- 13.Dionigi B, Razzouk AJ, Hasaniya NW, et al. Late outcomes of pediatric heart transplantation are independent of pre-transplant diagnosis and prior cardiac surgical intervention. J Heart Lung Transplant. 2008;27:1090–5. doi: 10.1016/j.healun.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Everitt MD, Boyle GJ, et al. Early survival after heart transplant in young infants is lowest after failed single-ventricle palliation: A multi-institutional study. J Heart Lung Transplant. 2012 Feb 8; doi: 10.1016/j.healun.2011.12.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Kirk R, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirteenth official pediatric heart transplantation report--2010. The Journal of Heart and Lung Transplantation. 2010;29:1119–1128. doi: 10.1016/j.healun.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guleserian KJ, Schechtman KB, Zheng J, et al. Outcomes after listing for primary transplantation for infants with unoperated-on non-hypoplastic left heart syndrome congenital heart disease: A multi-institutional study. J Heart Lung Transplant. 2011;30:1023–1032. doi: 10.1016/j.healun.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–90. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 19.Chin C, Naftel D, Pahl E, Shankel T, Clark ML, Gamberg P, et al. Cardiac retransplantation in pediatrics: a multi-institutional study. J Heart Lung Transplant. 2006;25:1420–4. doi: 10.1016/j.healun.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Karamichalis JM, Miyamoto SD, Campbell DN, et al. Pediatric cardiac retransplant: Differing patterns of primary graft failure by age at first transplant. J Thorac Cardiovasc Surg. 2011;141(1):223–30. doi: 10.1016/j.jtcvs.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Chrisant MR, Naftel DC, Drummond-Webb J, et al. Fate of infants with hypoplastic left heart syndrome listed for cardiac transplantation: a multicenter study. J Heart Lung Transplant. 2005;24:576–82. doi: 10.1016/j.healun.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim JE, Sweet SC, et al. Rejection is reduced in thoracic organ recipients when transplanted in the first year of life. J Heart Lung Transplant. 2002;21(3):311–318. doi: 10.1016/s1053-2498(01)00395-3. [DOI] [PubMed] [Google Scholar]
- 23.Mahle WT, Tresler MA, et al. Allosensitization and outcomes in pediatric heart transplantation. J Heart Lung Transplant. 2011;30:1221–1227. doi: 10.1016/j.healun.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Canter CE, Shaddy RE, et al. Indications for Heart Transplantation in Pediatric Heart Disease. Circulation. 2007;115(5):658–676. doi: 10.1161/CIRCULATIONAHA.106.180449. [DOI] [PubMed] [Google Scholar]
- 25.Altmann K, Printz BF, Solowiejczky DE, Gersony WM, Quaegebeur J, Apfel HD. Two-dimensional echocardiographic assessment of right ventricular function as a predictor of outcome in hypoplastic left heart syndrome. Am J Cardiol. 2000;86:964–8. doi: 10.1016/s0002-9149(00)01131-0. [DOI] [PubMed] [Google Scholar]
- 26.Tweddell JS, Sleeper LA, Ohye RG, et al. Intermediate-term mortality and cardiac transplantation in infants with single-ventricle lesions: Risk factors and their interaction with shunt type. J Thorac Cardiovasc Surg. 2012 Feb 14; doi: 10.1016/j.jtcvs.2012.01.016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mital S, Chung WK, et al. Renin-angiotensin-aldosterone genotype influences ventricular remodeling in infants with single ventricle. Circulation. 2011;123(21):2353–2362. doi: 10.1161/CIRCULATIONAHA.110.004341. [DOI] [PMC free article] [PubMed] [Google Scholar]


