Abstract
Background
Attentional difficulties often first present in preschool years, but it is unknown whether they result from concurrent brain developmental problems or whether normal developmental processes uncover alterations in brain development from an earlier age. This study addresses this issue, examining the relationship between an infant’s sensory gating of auditory evoked potentials and parent-reported behavior at 40 months of age.
Methods
P50 sensory gating, an auditory evoked potential measure reflective of inhibitory processes in the brain, was measured in 50 infants around 70 days of age. Parents, using the Child Behavior Checklist, reported on the child’s behavior at 40 months of age.
Results
Controlling for gender, infants with diminished sensory gating had higher externalizing scores (F=4.17, ndf=1, ddf=46, p=.047), as well as higher scores in the specific domains of attention (F=5.23, ndf=1, ddf=46, p=.027) and anxious/depressed (F=5.36, ndf=1, ddf=46, p=.025).
Conclusions
Diminished infant P50 sensory gating predicts attention related symptoms 3 years later, at 40 months of age. These results support the hypothesis that preschool attentional dysfunction is related, at least in part, to alterations in brain development that begin years prior to symptom onset.
Keywords: attention, evoked potentials, infant, preschool, sensory gating
Background
As children reach preschool age, problems with attention-driven behavior may emerge and interfere with the child’s ability to function. This initial preschool presentation of attention-driven behavior impairments often presages other behavior problems such as oppositional behavior and aggression (Duchesne, Larose, Vitaro, & Tremblay, 2010; Wahlstedt, Thorell, & Bohlin, 2008; Farmer & Bierman, 2002; Caspi, Henry, McGee, Moffitt, & Silva, 1995), and mood and anxiety disturbance (Duchesne et al., 2010; Wahlstedt et al., 2008). While preschool years are often the youngest age at which attention-driven behavioral deficits can be clinically identified, it is unknown whether the brain dysfunction associated with these behavioral deficits also develops in the preschool years. The initial clinical presentation of many neuropsychiatric impairments may be not due solely to concurrent brain changes, but instead may represent the uncovering of a brain developmental aberration which preceded symptom onset by years (Ross, Kisley, & Tregellas, 2005; Murray & Lewis, 1987; Censits, Ragland, Gur, & Gur, 1997; Rapoport, Addington, Frangou, & Psych, 2005; Galera et al., 2011; Poelmans, Pauls, Buitelaar, & Franke, 2011). Thus, it is possible that preschool emergence of attention-driven behavioral deficits may be related to aberrant brain development at a younger age. This report explores this issue by utilizing a biomarker of infant brain development, P50 sensory gating, to assess whether preschool attention-driven behavioral impairments are predicted by infant brain dysfunction.
P50 sensory gating utilizes an auditory evoked potential paradigm to measure inhibitory processes in the brain. In the P50 sensory gating paradigm, auditory evoked potentials to two successive auditory stimuli, in this case 2 “clicks” are compared. Most healthy adults show an attenuated response to the second stimulus reflecting the ability of the brain to unconsciously block reception of irrelevant repetitive stimuli (Adler, Pachtman, Franks, & Freedman, 1982; Siegel, Waldo, Milner, Adler, & Freedman, 1984; Suzuki & Azuma, 1977). Sensory gating is often considered an automatic and involuntary first step in the attentional process (Potter, Summerfelt, Gold, & Buchanan, 2006; Jerger, Biggins, & Fein, 1992; Lijffijt et al., 2009; Braff & Light, 2004; Boutros, Belger, Campbell, DΓÇÖSouza, & Krystal, 1999), and, in adults, sensory gating deficits are associated with neurocognitive and behavioral problems in attention (Adler et al., 1982; Potter et al., 2006; Paus, 1989; Chen & Faraone, 2000; Freedman et al., 1987; Wan, Friedman, Boutros, & Crawford, 2008). Further emphasizing the association between P50 sensory gating deficits and attentional dysfunction, P50 sensory gating is impaired in a number of psychiatric disorders characterized by attentional dysfunction, including schizophrenia (Adler et al., 1982), bipolar disorder (Martin et al., 2007), attention deficit-hyperactivity disorder (Olincy, Ross, Harris, & Freedman, 1999), lower IQ autism (Orekhova et al., 2008), post-traumatic stress disorder (Stewart & White, 2008; Ghisolfi et al., 2004; Neylan et al., 1999), panic disorder (Ghisolfi et al., 2006) and Parkinson’s Disease (Abel, Friedman, Jesberger, Malki, & Meltzer, 1991).
Recently, P50 sensory gating has been described in early infancy (Suzuki & Azuma, 1977; Kisley, Polk, Ross, Levisohn, & Freedman, 2003) with stable performance from infancy to 4 years of age (Gillow, Hunter, & Ross, 2010). Having a parent with psychosis as a presumed increased genetic vulnerability or a mother with anxiety, and/or prenatal exposure to nicotine or other illicit substances as possible environmental risk factors are well-established larger effect size risk factors for later attention-driven behavioral impairment. Each of these risk factors is associated with impaired infant P50 sensory gating, with effects identifiable in even relatively small samples (Hunter, Kisley, McCarthy, Freedman, & Ross, 2011; Hunter et al., 2012).
Thus, at least some of the same prenatal factors are associated with both impaired infant P50 sensory gating and later attentional dysfunction. This manuscript reports on an attempt to bridge the gap, addressing whether impaired infant P50 sensory gating predicts later behavioral symptoms. Such a relationship would suggest that preschool emergence of attention-driven behavioral deficits, like many other neuropsychiatric symptoms, are neurodevelopmental in nature, where onset of clinical symptoms represents, at least in part, an uncovering of aberrant brain growth from an earlier stage of development.
Method
Participants
Infants and their families were initially recruited from the Denver metropolitan area through a state birth registry as part of a study focusing on the impact of maternal factors on infant psychophysiology (Hunter et al., 2011). Exclusionary criteria included known birth defect, chromosomal abnormality, infant major neurological disorder, infant visual or auditory sensory disorder, or maternal non-nicotine substance use disorder active during pregnancy. Ninety-three (93) infants, born between January 2007 and February 2009 completed an auditory evoked potential recording with an age adjusted for gestational age at birth of 70.6 days (s.d 30.9 days). Fifty infants (54%) had a parent complete the Child Behavior Checklist (mean ± s.d child age at time of CBCL report: 40.9±0.7 months). The infants who later had parental report of behavior at 40 months did not differ from those lacking later parental report on gender, gestational age at birth, mother’s age at birth, mothers years of education, race/ethnicity, maternal education level, or frequency at living with both biological parents (all p values >.11).
Demographic information for the 50 infants is summarized in Table 1. Infants with robust sensory gating were more likely than infants with diminished sensory gating to be female (65% versus 33%; χ2=5.13 (d.f. = 1), p=.024), but did not differ in gestational age at birth, age at P50 recording, race and ethnicity, or whether they lived with both biological parents. Gender was included in all subsequent group comparisons.
Table 1.
Demographic information for infants with robust and diminished sensory gating. All values are number of subjects (percentage) or mean ± s.d.
| Robust infant sensory gating N=26 |
Diminished infant sensory gating N=24 |
Statistic | p | |
|---|---|---|---|---|
|
| ||||
| Age at P50 (days±s.d.) | 81.8±34.26 | 64.0±29.5 | t=1.96 (d.f. = 48) | .056 |
|
| ||||
| Gestational Age at birth (days±s.d.) | 272.9±13.1 | 273.0±11.9 | t=.02 (d.f. = 48) | .98 |
|
| ||||
| Number female (%) | 17 (65%) | 8 (33%) | χ2=5.13 (d.f. = 1) | .024 |
|
| ||||
| Number of each race/ethnicity (%) | χ2=1.57 (d.f. = 2) | .46 | ||
| Caucasian Non-Hispanic | 16 (62%) | 12 (50%) | ||
| Caucasian Hispanic | 7 (27%) | 6 (25%) | ||
| Other/Mixed | 3 (12%) | 6 (25%) | ||
|
| ||||
| Lives with both biological parents | 23 (89%) | 19 (79%) | χ2=.80 (d.f. = 1) | .37 |
|
| ||||
| Auditory evoked potentials | ||||
| Latency±s.d. of P50 response to… | ||||
| First Stimulus (ms) | 73.0±17.9 | 70.0±13.8 | t=.64 (d.f. = 47) | .53 |
| Second Stimulus (ms) | 73.2±16.5 | 71.0±14.3 | t=.52 (d.f. = 47) | .62 |
| Amplitude±s.d. of P50 response to… | ||||
| First Stimulus (μV) | 2.23±1.07 | 2.36±1.19 | t=.48 (d.f. = 48) | .67 |
| Second Stimulus (μV) | 0.50±0.36 | 1.55±0.81 | t=11.75 (d.f. = 48) | <.001 |
| P50 sensory gating ratio | .22±.12 | .69±.25 | ᵜ | ᵜ |
variable used to sort into groups, so statistical test not performed
Procedures
Informed parental consent was obtained and monitored by a local institutional review board.
P50 sensory gating
The methods used for the infant P50 sensory gating have been previously described (Suzuki & Azuma, 1977). Briefly, 50 ms auditory stimuli (clicks) were presented in pairs with an interstimulus interval of 500 ms and an interpair interval of 10 seconds. This interstimulus interval has been established among other intervals to be the amount of time in which sensory gating is the most active (Nagamoto, Adler, Waldo, Griffith, & Freedman, 1991; Dolu, Süer, & Çigdem, 2001). Auditory evoked potentials from the vertex were recorded during active sleep which is the infant equivalent of REM sleep. Active sleep was identified by combined elelectroencephalogram, electrooculography, submental electromyography, behavioral criteria (e.g. eyes closed) and respiratory pattern (Anders, Emde, & Parmelee, 1971). From the identified active sleep periods, the first 15-min period (approximately 85 stimulus pairs) was retained and used for further analyses. Single-trial-evoked potentials were extracted from 100 ms before each click to 200 ms following each click. Trials, in which the signal on the recording of any of these identified periods exceeded 75mV, were excluded from further analysis. The average waveforms computed from these single trials were bandpass filtered between 10 and 50 Hz, to accentuate middle latency components.
For each participant, the amplitude and latency of the largest positive peak between 50 and 150 ms after a click, preceded by a negative trough, was determined (Figure 1). The auditory evoked response in infants occurs about 70 ms after the stimulus rather than the 50 ms after the stimulus generally seen in adult populations. However, to keep terminology consistent with adult literature, the wave is termed P50 even in the infant subject. P50 sensory gating was measured by dividing the average amplitude of P50 evoked by the second click by the average amplitude evoked by the first click. A P50 ratio closer to 0 is indicative of robust suppression (gating), where as a ratio closer to 1 is indicative of diminished sensory gating.
Figure 1.
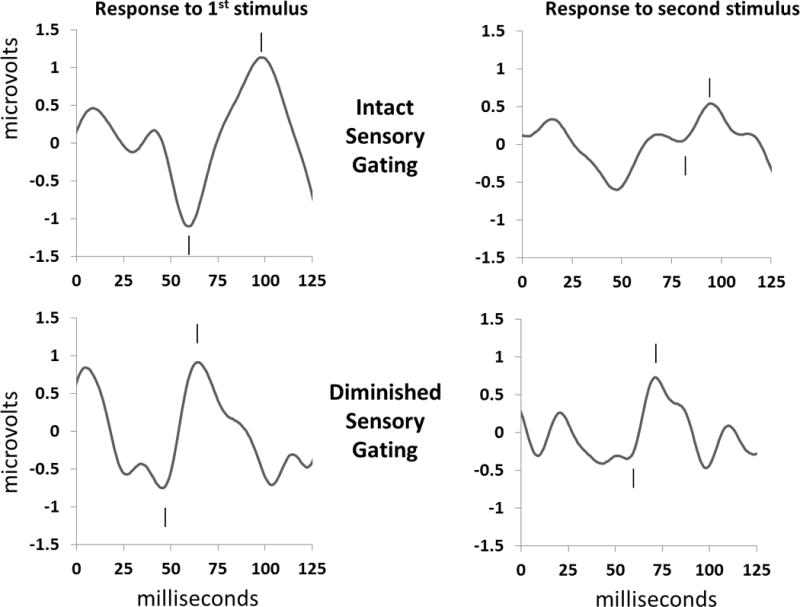
Representative individual examples of P50 sensory gating responses during active sleep. Clicks are presented 500 ms apart; the P50 response is noted by hashmarks. The positive P50 peak (hashmark above) was measured relative to the preceding negative trough (hashmark below). (A) An example of robust sensory gating in a male infant with an age, adjusted for gestational age at birth, of 51 days. Note that the response to the second stimulus (on the right) is suppressed in comparison to the response to the first stimulus (on the left) for a P50 sensory gating ratio of 0.22. (B) An example of diminished sensory gating in a male infant with an age, adjusted for gestational age at birth, of 58 days. This infant’s P50 response to the second stimulus (on the right) is closer in amplitude to that for the response to the first stimulus (on the left), demonstrating diminished response suppression with a sensory gating ratio = 0.65.
Parent Report of Child Behavior
The Child Behavior Checklist (CBCL 1.5-5) is one broadly validated parental report tool to help parse out problematic behaviors (Achenbach & Rescorla, 2000). The CBCL consists of 99 items for the parent to report on child behavior. Each question is scored on a scale from 0–2 with scores summed across items. The CBCL produces 7 subscales; two domain scores—externalizing behavior which is the sum of 2 subscales and internalizing behavior consisting of the sum of 4 subscales—and an overall Total Problems Score. These classical subscales are based on factor analysis and have internal reliability ranging from acceptable to excellent (Cronbach’s α’s of .66-.95), although somatic complaints may be less reliable in some populations (Tan, Dedrick, & Marfo, 2007). In addition, items can be regrouped into DSMIV-oriented scales, including affective problems, anxiety problems, pervasive developmental problems, attention deficit-hyperactivity problems, and oppositional defiant problems. For 49 of the 50 children (98%), the CBCL was completed by the mother.
Results
Infant P50 sensory gating
Ratios have more variance than the underlying amplitude measurements. Dichotomization of P50 values into robust and diminished sensory gating decreases this variance and has been used to detect genetic and treatment effects. Children were split into two groups: P50 sensory gating ratios ≤ 0.4 considered robust sensory gating and scores > 0.4 indicating diminished sensory gating. This division into robust and diminished P50 sensory gating is similar to values often used in adults, where a sensory gating ratios of <0.4 is considered normal.12
For the group as a whole, mean P50 amplitude in response to the second stimulus (1.00±0.80 μV) was significantly lower (paired t=9.88, d.f. =49, p<.001) than response to the first stimulus (2.29±1.12 μV) demonstrating the presence of sensory gating. Mean ± standard deviation for P50 sensory gating ratios was 0.44±0.31, consistent with reported infant values (Hunter et al., 2011; Hunter et al., 2012). Those infants with robust sensory gating were older than infants with diminished sensory gating although the difference did not reach significance and no relationship between age and P50 sensory gating values has previously been identified (Suzuki & Azuma, 1977). Those infants with robust sensory gating did not significantly differ from those with diminished sensory gating in latency of responses to the first or second sound or in the amplitude of response to the first sound (Table 1). The difference in ratios was due to infants with robust sensory gating having lower amplitude of P50 response to the second sound relative to infants with diminished sensory gating (Students t=11.75, d.f. = 48, p<.001).
40-month-old Child Behavior
Results from the Child Behavior Checklist (CBCL) are summarized in Table 2. As expected from a normally developing population, the modal score for the majority of subscales was the lowest possible score ‘0,’ reflecting a strong floor effect with the instrument. For each scale, zero to four percent of children had scores in the clinically affected range, consistent with what has been found with other populations recruited from non-clinical sources (Tan et al., 2007).
Table 2.
Measures of child behavior from parent-report utilizing the Child Behavior Checklist and the impact of gender and infant sensory gating ability. Nonparametric analyses of covariance were applied to each 40-month-old behavior score after a rank transformation38, 39 to estimate the association with gender and infant P50 sensory gating. All analyses of covariance have the same degrees of freedom: ndf=1, ddf=46.
| Raw Score for | Mode | Median | Mean | % subjects in clinical range | gender | Infant P50 sensory gating | ||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | |||||
| Total problems | 12 | 20.5 | 24.34 | 4 | 3.63 | .06* | 3.31 | .08* |
| Externalizing | 1 | 10 | 10.72 | 2 | 2.34 | .13 | 4.17 | .047** |
| Attention | 0 | 2 | 2.08 | 0 | 6.27 | .016** | 5.23 | .027** |
| Aggression | 8 | 8 | 8.16 | 0 | 1.10 | .30 | 2.93 | .09* |
| Internalizing | 2 | 4.5 | 5.98 | 2 | 0.91 | .35 | 1.55 | .22 |
| Emotionality | 0 | 1 | 1.70 | 2 | 0.17 | .68 | .21 | .65 |
| Anxious/depressed | 0 | 1 | 1.72 | 2 | 0.00 | .96 | 5.36 | .025** |
| Somatic | 0 | 1 | 1.70 | 2 | 0.15 | .70 | 0.14 | .72 |
| Withdrawn | 0 | 0 | 0.84 | 0 | 0.61 | .48 | .53 | .47 |
| Other | ||||||||
| Sleep | 0 | 2 | 2.26 | 2 | 1.25 | .27 | .52 | .48 |
| DSM-Oriented Scales | ||||||||
| Affective | 0 | 1 | 1.60 | 2 | 0.52 | .48 | 1.48 | .23 |
| Anxiety | 0 | 1 | 2.12 | 0 | 1.12 | .30 | 5.64 | .022** |
| Pervasive Developmental | 2 | 2 | 2.24 | 4 | 0.20 | .66 | 1.06 | .31 |
| Attention Deficit-Hyperactivity | 1 | 3.5 | 3.94 | 2 | 3.28 | .07* | 5.40 | .025** |
| Oppositional Defiant | 3 | 3 | 2.94 | 4 | 0.65 | .43 | 3.94 | .053* |
p<.10
p<.05
Relationship Between Infant P50 Sensory Gating and 40-month-old Behavior Scores
Due the non-normal distribution of CBCL scores, a non-parametric (distribution-free) analysis of covariance was applied to the 40-month-old behavior scores after a rank transformation (Conover & Iman, 1982) to estimate the association with infant P50 sensory gating after adjusting for gender (Table 2). It has been shown that such a parametric analysis of covariance of the ranks is extremely well approximated by normal theory software after transforming the dependent variable to ranks (Zeng, Pan, MaWhinney, Barron, & Zerbe, 2011).
Male children had higher ranking on attentional symptoms, with a trend towards an increased ranking on total problems. No effects of gender were identified on any other scale (all p values >.13). The impact of sensory gating was more wide-spread. Infants with diminished sensory gating were, 3 years later, ranked significantly higher (Figure 2) on attention (F=5.23, ndf=1, ddf=46, p=.027), anxious/depressed (F=5.36, ndf=1, ddf=46, p=.025), and externalizing symptoms (F=4.17, ndf=1, ddf=46, p=.047); and ranked significantly higher on the DSM-Oriented scales of Anxiety Disorders (F=5.64, ndf=1, ddf=46, p=.022) and Attention Deficit-Hyperactivity Disorders (F=5.40, ndf=1, ddf=46, p=.025), with a strong trend toward higher ranking on Oppositional Defiant Disorders (F=3.94, ndf=1, ddf=46, p=.053). There was also a non-significant trend for infants with diminished sensory gating to be later ranked higher on Aggression Symptoms (F=2.93, ndf=1, ddf=46, p=.09) and Total Problems (F=3.31, ndf=1, ddf=46, p=.08).
Figure 2.

Distribution of parent-report symptoms for 40-month-old preschoolers who had robust and diminished P50 sensory gating based on assessment in infancy. Parent-report preschool scores are based on the Child Behavior Checklist. A significant main effect for gender is identified for attention symptoms (F=6.27, ndf=1, ddf=46, p=.016), so attention symptoms are separated by gender. There is a significant effect of robust versus diminished infant P50 sensory gating for A) the domain of externalizing symptoms (F=4.17, ndf=1, ddf=46, p=.047), the specific subscales B) anxiety/depressed subscales (F=5.36, ndf=1, ddf=46, p=.025) and for (C and D) attention (F=5.23, ndf=1, ddf=46, p=.027). There was also a significant effect on robust versus diminished P50 sensory gating for Child Behavior Checklist DSM categories of E) anxiety (F=5.64, ndf=1, ddf=46, p=.022) and G) attention-deficit hyperactivity problems (F=5.40, ndf=1, ddf=46, p=.025), as well as a notable trend for F) oppositional defiant problems (F=3.94, ndf=1, ddf=46, p=.053). All statistical results are nonparametric, based on a rank transformation for Child Behavior Checklist results.
Discussion
Infants with diminished sensory gating deficit ranked higher, at 40 months of age, on parent-reported problems in attention and anxiety/depression, in the overall externalizing symptoms domain, and in DSMIV-oriented scales of anxiety disorders and attention deficit-hyperactivity disorders. Infants with diminished sensory gating were later higher rated for oppositional defiant symptoms although the difference did not reach statistical significance. Externalizing problems in early childhood have been linked to continuing problems with attention and behavior as children develop (Hill, Degnan, Calkins, & Keane, 2006; Bellanti & Bierman, 2000; Campbell, Pierce, March, Ewing, & Szumowski, 1994; Olson, Schilling, & Bates, 1999; Mesman & Koot, 2001; Fischer, Rolf, Hasazi, & Cummings, 1984; Lavigne et al., 1998), including higher risk for oppositional defiant disorder, conduct disorder and attention deficit-hyperactivity disorder (Swaab-Barneveld et al., 2000). Preschool externalizing symptoms also predict later internalizing symptoms, perhaps because children with early externalizing symptoms may have a hard time forming relationships with peers and this rejection may later lead to internalizing symptoms like anxiety and depression (Mesman, Bongers, & Koot, 2001). Internalizing and externalizing symptoms detected in early preschool account for most of the variance in predicting preadolescent psychopathology with the only other main contributor being physical health problems (Mesman & Koot, 2001). Similarly, adolescents and adults with major psychiatric diagnoses often report that they exhibited symptoms like problems with attention or aggression when young (Biederman et al., 1995; Busch et al., 2002; Banaschewski et al., 2005; Hollis, 1995; Doerfler, Connor, & Toscano, 2011; Harty, Miller, Newcorn, & Halperin, 2009). Thus, diminished infant sensory gating may not only suggest increased behavior problems in preschool years, but may also be predictive of increased risk for later childhood, adolescent, and even adult psychopathology.
P50 sensory gating is reflective of the ability to subconsciously filter out irrelevant auditory information and is fully developed by early infancy (Kisley et al., 2003), stable over time (Hunter, Corral, Ponicsan, & Ross, 2008; Gillow et al., 2010), and, in adults, correlated with attentional function (Cullum et al., 1993; Kisley, Noecker, & Guinther, 2004). The finding reported here that diminished infant P50 sensory gating is associated, 3 years later, with an elevation of attention-driven dysfunctional behavior support the hypothesis that symptoms of inattention, are, at least in part, due to abnormalities in brain development detectable in infancy.
P50 sensory gating is a psychophysiological measure of primarily inhibition involving the hippocampus and cortex (Freedman et al., 1994). However, this inhibitory process is also part of the physiology of other cortical brain regions and is critical for multiple cognitive processes including working memory (prefrontal cortex), somatosensory specificity (somatosensory cortex), associative fear learning (auditory cortex), and recognition memory (hippocampus) (Lewis, Melchitzky, & Burgos, 2002; Pouille, Marin-Burgin, Adesnik, Atallah, & Scanziani, 2009; Staiger, Zuschratter, Luhmann, & Schubert, 2009; Letzkus et al., 2011; Wiebe & Stäubli, 2001). Factors which disturb normal development of cerebral inhibition in the hippocampus are likely to also increase risk for less-than-optimal development of cerebral inhibition, and related cognitive processes, in other brain regions. This “comorbidity” of abnormal brain development may contribute to the relationship found in this study between infant P50 sensory gating and anxiety at 40 months of age.
This study is limited by a lack of data related to environmental factors between infancy and 40-months-of age. It is possible that there may be some maternal factor which effects both infant early sensory gating performance and later behavior without there being a direct relationship between the two. In addition, the relatively small sample size limits power to include maternal variables, such as education level or socio-economic status, in the analysis. The limited number of 40-month-old children with clinically important symptoms also raises questions about generalizability of results into clinical populations. Future work with larger sample sizes and which includes post-infant maternal assessments may help clarify these issues.
In summary, impaired sensory gating is predictive of parent-reported problems with attention, anxiety and externalizing problems at preschool age. The identification of infant brain changes associated with later onset of symptoms suggests that attentional dysfunction may, at least in part, be due to alterations in brain development which occur years prior to clinical presentation. Identification of early brain changes may be useful in identifying infants who would benefit from primary prevention interventions.
Key Points.
Preschool age is the earliest that attentional symptoms that may interfere with the child’s ability to function can be clinically identified.
Diminished infant P50 sensory gating predicts attention and related symptoms 3 years later, at 40 months of age.
Diminished infant sensory gating suggest not only increased behavior problems in preschool years, but may also be predictive of increased risk for later childhood, adolescent, and even adult psychopathology.
Preschool attentional dysfunction is related, at least in part, to alterations in brain development that begin years prior to symptom onset
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (MH56539, MH068582, and MH086383), by an American Academy of Child and Adolescent Psychiatry Elaine Schlosser Lewis award (AH), and by the Institute for Children’s Mental Disorders. A special thanks to all the families who participated in this project.
Dr. Zerbe has equity interest in Abbott Laboratories, Johnson and Johnson Pharmaceuticals, Merck Pharmaceuticals, and Pfizer. Dr. Zerbe also has a contract with Merck Pharmaceuticals as a statistician in a study of potential benefits of a booster dose of vaccine for varicella zoster. Drs Hunter, Wagner, Zerbe and Ross receive salary support from NIH-funded grants.
Abbreviations
- ADHD
Attention-Deficit Hyperactivity Disorder
- CBCL
Child Behavior Checklist
Footnotes
Disclosures: All other authors have no conflicts to disclose.
Contributor Information
Dr Amanda K Hutchison, Department of Psychiatry, School of Medicine University of Colorado Denver, United States.
Dr Sharon K Hunter, Department of Psychiatry, School of Medicine University of Colorado Denver, United States.
Dr Brandie Wagner, Department of Biostatistics and Informatics, Colorado School of Public Health, University of Colorado Denver.
Dr Elizabeth Calvin, Department of Psychiatry, School of Medicine University of Colorado Denver, United States.
Dr Gary O Zerbe, Department of Biostatistics and Informatics, Colorado School of Public Health, University of Colorado Denver.
Dr Randal G Ross, Department of Psychiatry, School of Medicine University of Colorado Denver, United States.
Reference List
- Abel L, Friedman L, Jesberger J, Malki A, Meltzer HY. Quantitative assessment of smooth pursuit gain and catch-up saccades in schizophrenia and affective disorders. Biological Psychiatry. 1991;29:1063–1072. doi: 10.1016/0006-3223(91)90248-k. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla L. Manual for the ASEBA preschool forms & profiles: an integrated system of multi-informant assessment. Burlington, VT: ASEBA; 2000. [Google Scholar]
- Adler LE, Pachtman E, Franks R, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biological Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Anders T, Emde R, Parmelee A. A manual of standardized terminology, techniques and criteria for scoring of states of sleep and wakefulness in newborn infants. Los Angeles: UCLA Brain Information Service NINDS Neurological Information Network; 1971. [Google Scholar]
- Banaschewski T, Hollis C, Oosterlaan J, Roeyers H, Rubia K, Willcutt E, et al. Towards an understanding of unique and shared pathways in the psychopathophysiology of ADHD. Developmental Science. 2005;8:132–140. doi: 10.1111/j.1467-7687.2005.00400.x. [DOI] [PubMed] [Google Scholar]
- Bellanti CJ, Bierman KL. Disentangling the impact of low cognitive ability and inattention on social behavior and peer relationships. Conduct Problems Prevention Re search Group. Journal of Clinical Child Psychology. 2000;29:66–75. doi: 10.1207/S15374424jccp2901_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Mick E, Spencer T, Wilens T, Kiely K, et al. High risk for attention deficit hyperactivity disorder among children of parents with childhood onset of the disorder: a pilot study. American Journal of Psychiatry. 1995;152:431–435. doi: 10.1176/ajp.152.3.431. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Belger A, Campbell D, DΓÇÖSouza C, Krystal J. Comparison of four components of sensory gating in schizophrenia and normal subjects: a preliminary report. Psychiatry Research. 1999;88:119–130. doi: 10.1016/s0165-1781(99)00074-8. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology. 2004;174:75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- Busch B, Biederman J, Cohen LG, Sayer JM, Monuteaux MC, Mick E, et al. Correlates of ADHD among children in pediatric and psychiatric clinics. Psychiatric Services. 2002;53:1103–1111. doi: 10.1176/appi.ps.53.9.1103. [DOI] [PubMed] [Google Scholar]
- Campbell SB, Pierce EW, March CL, Ewing LJ, Szumowski EK. Hard-to-manage preschool boys: symptomatic behavior across contexts and time. Child Development. 1994;65:836–851. [PubMed] [Google Scholar]
- Caspi A, Henry B, McGee RO, Moffitt TE, Silva PA. Temperamental Origins of Child and Adolescent Behavior Problems: From Age Three to Age Fifteen. Child Development. 1995;66:55–68. doi: 10.1111/j.1467-8624.1995.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Censits DM, Ragland JD, Gur RC, Gur RE. Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: a longitudinal study. Schizophrenia Research. 1997;24:289–298. doi: 10.1016/s0920-9964(96)00091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Faraone SV. Sustained attention deficits as markers of genetic susceptibility to schizophrenia. American Journal of Medical Genetics. 2000;97:52–57. doi: 10.1002/(sici)1096-8628(200021)97:1<52::aid-ajmg7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics. 1982;38:715–724. [PubMed] [Google Scholar]
- Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, et al. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophrenia Research. 1993;10:131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- Doerfler LA, Connor DF, Toscano PF. Aggression, ADHD symptoms, and dysphoria in children and adolescents diagnosed with bipolar disorder and ADHD. Journal of Affective Disorders. 2011;131:312–319. doi: 10.1016/j.jad.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Dolu N, Süer C, Çigdem Õ. A comparison of the different interpair intervals in the conditioningΓÇôtesting P50 paradigms. International Journal of Psychophysiology. 2001;41:265–270. doi: 10.1016/s0167-8760(01)00134-9. [DOI] [PubMed] [Google Scholar]
- Duchesne S, Larose S, Vitaro F, Tremblay RE. Trajectories of anxiety in a population sample of children: Clarifying the role of children’s behavioral characteristics and maternal parenting. Development and Psychopathology. 2010;22:361–373. doi: 10.1017/S0954579410000118. [DOI] [PubMed] [Google Scholar]
- Farmer J, Bierman KL. Predictors and Consequences of Aggressive-WIthdrawn Problem Profiles in Early Grade School. Journal of Clinical Child and Adolescent Psychology. 2002;31:299. doi: 10.1207/S15374424JCCP3103_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Rolf JE, Hasazi JE, Cummings L. Follow-up of a preschool epidemiological sample: cross-age continuities and predictions of later adjustment with internalizing and externalizing dimensions of behavior. Child Development. 1984;55:137–150. [PubMed] [Google Scholar]
- Freedman R, Adler LE, Gerhardt GA, Waldo M, Baker N, Rose GM, et al. Neurobiological studies of sensory gating in schizophrenia. Schizophrenia Bulletin. 1987;13:669–678. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Bickford P, Byerley W, Coon H, Cullum CM, et al. Schizophrenia and Nicotinic Receptors. Harvard Review of Psychiatry. 1994;2:179–192. doi: 10.3109/10673229409017136. [DOI] [PubMed] [Google Scholar]
- Galera C, Cote SM, Bouvard MP, Pingault JB, Melchior M, Michel G, et al. Early Risk Factors for Hyperactivity-Impulsivity and Inattention Trajectories From Age 17 Months to 8 Years. Archives of General Psychiatry. 2011;68:1267–1275. doi: 10.1001/archgenpsychiatry.2011.138. [DOI] [PubMed] [Google Scholar]
- Ghisolfi ES, Heldt E, Zanardo AP, Strimitzer IM, Prokopiuk AS, Becker J, et al. P50 sensory gating in panic disorder. Journal of Psychiatric Research. 2006;40:535–540. doi: 10.1016/j.jpsychires.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Ghisolfi ES, Margis R, Becker J, Zanardo AP, Strimitzer IM, Lara DR. Impaired P50 sensory gating in post-traumatic stress disorder secondary to urban violence. International Journal of Psychophysiology. 2004;51:209–214. doi: 10.1016/j.ijpsycho.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Gillow S, Hunter S, Ross R. Stability of P50 sensory gating in preschoolers. Journal of Investigative Medicine. 2010;58(1):154–155. [Google Scholar]
- Harty SC, Miller CJ, Newcorn JH, Halperin JM. Adolescents with childhood ADHD and comorbid disruptive behavior disorders: aggression, anger, and hostility. Child Psychiatry and Human Development. 2009;40:85–97. doi: 10.1007/s10578-008-0110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AL, Degnan KA, Calkins SD, Keane SP. Profiles of externalizing behavior problems for boys and girls across preschool: the roles of emotion regulation and inattention. Developmental Psychology. 2006;42:913–928. doi: 10.1037/0012-1649.42.5.913. [DOI] [PubMed] [Google Scholar]
- Hollis C. Child and adolescent (juvenile onset) schizophrenia. A case control study of premorbid developmental impairments. British Journal of Psychiatry. 1995;166:489–495. doi: 10.1192/bjp.166.4.489. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Corral N, Ponicsan H, Ross RG. Reliability of p50 auditory sensory gating measures in infants. Neuroreport. 2008;19:79–82. doi: 10.1097/WNR.0b013e3282f35823. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Kisley MA, McCarthy L, Freedman R, Ross RG. Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: Parental psychosis, maternal depression, and nicotine use. Schizophrenia Bulletin. 2011;37:1200–1208. doi: 10.1093/schbul/sbq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Mendoza JH, D’Anna KL, Zerbe GO, McCarthy L, Hoffman C, et al. Antidepressants may mitigate the effects of prenatal maternal anxiety on infant auditory sensory gating. American Journal of Psychiatry. 2012;169:616–624. doi: 10.1176/appi.ajp.2012.11091365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerger K, Biggins C, Fein G. P50 suppression is not affected by attentional manipulations. Biological Psychiatry. 1992;31:365–377. doi: 10.1016/0006-3223(92)90230-w. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Noecker TL, Guinther PM. Comparison of sensory gating to mismatch negativity and self-reported perceptual phenomena in healthy adults. Psychophysiology. 2004;41:604–612. doi: 10.1111/j.1469-8986.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Polk SD, Ross RG, Levisohn PM, Freedman R. Early postnatal development of sensory gating. Neuroreport. 2003;14:693–697. doi: 10.1097/00001756-200304150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne JV, Arend R, Rosenbaum D, Binns HJ, Christoffel KK, Gibbons RD. Psychiatric disorders with onset in the preschool years: I. Stability of diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:1246–1254. doi: 10.1097/00004583-199812000-00007. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SBE, Meyer EMM, Tovote P, Courtin J, Herry C, et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Burgos GG. Specificity in the functional architecture of primate prefrontal cortex. Journal of Neurocytology. 2002;31:265–276. doi: 10.1023/a:1024174026286. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Lane SD, Meier SL, Boutros NN, Burroughs S, Steinberg JL, et al. P50, N100, and P200 sensory gating: relationships with behavioral inhibition, attention, and working memory. Psychophysiology. 2009;46:1059–1068. doi: 10.1111/j.1469-8986.2009.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LF, Mei-Hua H, Ross RG, Zerbe G, Freedman R, Olincy A. Physiology of schizophrenia, bipolar disorder and schizoaffective disorder. American Journal of Psychiatry. 2007;164:1900–1906. doi: 10.1176/appi.ajp.2007.06010017. [DOI] [PubMed] [Google Scholar]
- Mesman J, Bongers IL, Koot HM. Preschool developmental pathways to preadolescent internalizing and externalizing problems. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42:679–689. [PubMed] [Google Scholar]
- Mesman J, Koot HM. Early preschool predictors of preadolescent internalizing and externalizing DSM-IV diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1029–1036. doi: 10.1097/00004583-200109000-00011. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? British Medical Journal Clinical Research Edition. 1987;295:681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Waldo MC, Griffith J, Freedman R. Gating of auditory response in schizophrenics and normal controls: Effects of recording site and stimulation interval on the P50 wave. Schizophrenia Research. 1991;4:31–40. doi: 10.1016/0920-9964(91)90007-e. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Fletcher DJ, Lenoci M, Mcallin K, WEiss DS, Schoenfeld FB, et al. Sensory gating in chronic posttraumatic stress disorder: reduced auditory P50 suppression in combat veterans. Biological Psychiatry. 1999;46:1656–1664. doi: 10.1016/s0006-3223(99)00047-5. [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Harris JG, Freedman R. Neurophysiological studies of the P50 auditory evoked potential in adult attention deficit disorder: comparison with schizophrenia. Schizophrenia Research. 1999;36:257. doi: 10.1016/s0006-3223(00)00239-0. [DOI] [PubMed] [Google Scholar]
- Olson SL, Schilling EM, Bates JE. Measurement of impulsivity: construct coherence, longitudinal stability, and relationship with externalizing problems in middle childhood and adolescence. Journal of Abnormal Child Psychology. 1999;27:151–165. doi: 10.1023/a:1021915615677. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofyev AO, Nygren G, Gillberg C, Elam M. Sensory gating in young children with autism: Relation to age, IQ, and EEG gamma oscillations. Neuroscience Letters. 2008;434:218–223. doi: 10.1016/j.neulet.2008.01.066. [DOI] [PubMed] [Google Scholar]
- Paus T. The development of sustained attention in children might be related to the maturation of frontal cortical functions. Acta Neurobiologiae Experimentalis (Warszawa) 1989;49:51–55. [PubMed] [Google Scholar]
- Poelmans G, Pauls DL, Buitelaar JK, Franke B. Integrated Genome-Wide Association Study Findings: Identification of a Neurodevelopmental Network for Attention Deficit Hyperactivity Disorder. American Journal of Psychiatry. 2011;168:365–377. doi: 10.1176/appi.ajp.2010.10070948. [DOI] [PubMed] [Google Scholar]
- Potter D, Summerfelt A, Gold J, Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophrenia Bulletin. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Marin-Burgin A, Adesnik H, Atallah BV, Scanziani M. Input normalization by global feedforward inhibition expands cortical dynamic range. Nature Neuroscience. 2009;12:1577–1585. doi: 10.1038/nn.2441. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MRC. The neurodevelopmental model of schizophrenia: update 2005. Molecular Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Ross RG, Kisley MA, Tregellas JR. Neurophysiological measures help define genetic etiology, isolate brain dysfunction, and confirm the neurodevelopmental hypothesis of schizophrenia. In: Findling RL, Schulz C, editors. Juvenile-Onset Schizophrenia: Assessment, Neurobiology, and Treatment. Baltimore, Maryland: Johns Hopkins University Press; 2005. pp. 148–173. [Google Scholar]
- Siegel C, Waldo M, Milner G, Adler LE, Freedman R. Deficits in sensory gating in schizophrenic patients and their relatives. Evidence obtained with auditory evoked responses. Archives of General Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- Staiger J, Zuschratter W, Luhmann H, Schubert D. Local circuits targeting parvalbumin-containing interneurons in layer IV of rat barrel cortex. Brain Structure and Function. 2009;214:1–13. doi: 10.1007/s00429-009-0225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart LP, White PM. Sensory filtering phenomenology in PTSD. Depression and Anxiety. 2008;25:38–45. doi: 10.1002/da.20255. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Azuma M. Prefrontal neuronal activity during gazing at a light spot in the monkey. Brain Research. 1977;126:497–508. doi: 10.1016/0006-8993(77)90600-x. [DOI] [PubMed] [Google Scholar]
- Swaab-Barneveld H, de Sonneville L, Cohen-Kettenis P, Gielen A, Buitelaar J, Van Engeland H. Visual sustained attention in a child psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:651–659. doi: 10.1097/00004583-200005000-00020. [DOI] [PubMed] [Google Scholar]
- Tan TX, Dedrick RF, Marfo K. Factor Structure and Clinical Implications of Child Behavior Checklist/1.5ΓÇô5 Ratings in a Sample of Girls Adopted from China. Journal of Pediatric Psychology. 2007;32:807–818. doi: 10.1093/jpepsy/jsm025. [DOI] [PubMed] [Google Scholar]
- Wahlstedt C, Thorell LB, Bohlin G. ADHD Symptoms and Executive Function Impairment: Early Predictors of Later Behavioral Problems. Developmental Neuropsychology. 2008;33:160–178. doi: 10.1080/87565640701884253. [DOI] [PubMed] [Google Scholar]
- Wan L, Friedman BH, Boutros NN, Crawford HJ. P50 sensory gating and attentional performance. International Journal of Psychophysiology. 2008;67:91–100. doi: 10.1016/j.ijpsycho.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe SP, Stäubli UV. Recognition Memory Correlates of Hippocampal Theta Cells. The Journal of Neuroscience. 2001;21:3955–3967. doi: 10.1523/JNEUROSCI.21-11-03955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Pan Z, MaWhinney S, Barron AE, Zerbe GO. A note on permutation tests for multivariate general linear model. The American Statistician. 2011;65:31–36. [Google Scholar]


