Abstract
During the past few decades, supercritical fluid (SCF) has emerged as an effective alternative for many traditional pharmaceutical manufacturing processes. Operating active pharmaceutical ingredients (APIs) alone or in combination with various biodegradable polymeric carriers in high-pressure conditions provides enhanced features with respect to their physical properties such as bioavailability enhancement, is of relevance to the application of SCF in the pharmaceutical industry. Herein, recent advances in drug delivery systems manufactured using the SCF technology are reviewed. We provide a brief description of the history, principle, and various preparation methods involved in the SCF technology. Next, we aim to give a brief overview, which provides an emphasis and discussion of recent reports using supercritical carbon dioxide (SC-CO2) for fabrication of polymeric carriers, for applications in areas related to drug delivery, tissue engineering, bio-imaging, and other biomedical applications. We finally summarize with perspectives.
Keywords: bioavailability enhancement, biomedical applications, drug delivery, polymeric carriers, supercritical fluids, tissue engineering
1. Introduction
The supercritical fluid (SCF) technology is perhaps one of the most renowned high-pressure techniques so far to obtain products with better performances. This technology has been commercially used for many years in the pharmaceutical, textile, and food industries.[1] The reasons behind the wide adoption of this technology are not only due to its environmentally benign nature in various processes but also because of its economically promising character.[2] While many conventional pharmaceutical products rely on the use of organic solvents,[3] the SCF technology, by contrast, takes advantage of benign solvents such as CO2 and water to replace the organic solvents, therefore serving as an alternative in synthesizing delivery systems.
Drug delivery relies on various formulations and strategies for transporting pharmaceutically active compounds to achieve desired therapeutic effects.[4] However, it has been associated with challenges related to solubility and diffusion.[5] Regarding this, the biopharmaceutical classification system (BCS) has clearly categorized all drugs, and specific formulations based on their physical and chemical properties as well as their pharmacokinetics and pharmacodynamics.[5a,6] New chemical entities also suffer from poor solubility and stability and require frequent administration.[7] To overcome these issues, excipients such as biodegradable polymers are utilized in many pharmaceutical formulations to encapsulate the core materials such as active pharmaceutical ingredients (APIs) or drugs, which coalesce to form matrices/frameworks.[7b,8] Particles synthesized via micronization can be administered through various routes such as intravenous, intramuscular, pulmonary, and others, in addition to oral delivery.[8a] Furthermore, the use of particles helps to protect the sensitive agents from the harsh environments in the body.[7b,9]
According to a formulator anticipation, the ideal drug delivery system should carry a high payload to the desired site and release in a controlled manner, which reduce the frequency of dosage.[6,9c] In few instances, chemical modification of drug or attachment to macromolecules such as polymers or polysaccharides to increase the drug encapsulation efficiency may be chosen to accomplish the design.[4a,7b,10] More often, sustained delivery of drugs from encapsulating polymeric particles such as those micro- or nanoscale in size also improves the efficacy and reduces the undesired side effects.[11] These advancements have integrated materials science with drug delivery to deal with controlling parameters (such as morphology) and manufacturing process of particle formation.[5b] However, conventional particle fabrication technologies have a few limitations, such as thermal and chemical degradation of drugs, the use of large amounts of organic solvents, broad particle size distribution, and solvent residues in the end products.[8b,12] To overcome these limitations, an efficient production platform applying particle engineering at industrial scale is necessary.[7b,13] To this end, the compressed/pressurized fluid bottom-up technique utilizing supercritical solvents has largely addressed the challenges. This technology precipitates micro-[14] as well as nano-sized particles[15] with large surface areas, controlled uniform particle sizes, and smooth surfaces,[5b,14,16] which eventually augment drug bioavailability.
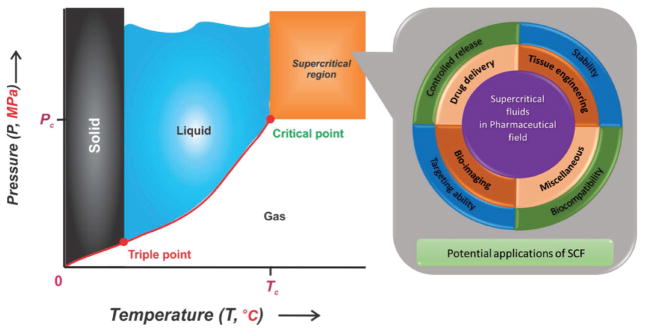
Recently, the application of the SCF technology has attracted interest of many researchers as it is non-toxic, non-flammable, non-reactive, economical, and non-polluting.[17] This green technology has a potentially high impact in the pharmaceutical field to overcome the curbs of various conventional methods such as spray-drying and others.[12,18] Indeed, SCF exists as a single phase beyond critical conditions (i.e., temperature and pressure). Furthermore, the physical properties (density, diffusivity, and viscosity) of the SCF are intermediate between liquid and gas and can be easily manipulated by adjusting the temperature and pressure during operations.[3,6,16c,17] In general, CO2 is the most used supercritical solvent and is recognized as safe by the United States Food and Drug Administration (US-FDA) in the pharmaceutical processing,[19] i.e., drug delivery applications, because of its low toxicity, low cohesive energy, as well as low density. Supercritical CO2 (SC-CO2) is a cost-effective solvent operated at mild conditions (critical temperature (Tc = 31.1 °C) and low critical pressure (Pc = 7.38 MPa)) at gaseous standard state under ambient circumstances (Figure 1). It has been extensively used as a solvent, anti-solvent, and plasticizer for synthesis, modification, and purification of both natural and synthetic polymers.[17] Moreover, other SCFs include water[20] and solvents such as acetone, CO2/ethanol (EtOH) mixture, chlorodifluoromethane, diethyl ether, nitrous oxide, propane, and trifluoromethane operated at their respective supercritical conditions.[20e,21] Recent literature have already reported the properties including solubility and critical parameters of several commonly used supercritical solvents.[22]
Figure 1.
Schematic representation of CO2 phase diagram elucidating CO2 existence as various phases along with the supercritical phase beyond the critical point (Tc = 31.1 °C, Pc = 7.38 MPa) (Left). Graphic illustration elucidating the potential applications of SCF (Right).
SCF is dense, but can be highly compressible, where the solvation power and pressure changes merely result in density alteration at the critical point.[3] The high diffusivity of SCF results in the ease of penetration of CO2 into the polymers, and the fluctuation in diffusivity determines the supercritical status of the fluid with the non-homogenous distribution of molecules in the space.[13b,17] In the SCF-assisted process, the mechanism lying behind the particle formation or crystallization is the attainment of a high degree of supersaturation state of the material in non-equilibrium conditions of temperature and pressure, which leads to nucleation, crystal growth, and eventually agglomeration.[13b] The control over the critical conditions during operation is highly advantageous over conventional process. Since it is operated at extreme conditions using pressurized gasses and solvents, this process usually requires safety rules and stringent regulations such as Current-Good Manufacturing Practices (cGMP) to control the mechanical and chemical hazards.[3]
Despite many reviews[1,2,3,5b,8b,9a,12,13,17,18b,20d,e,23] published on the potential of the SCF technology, very few focused on the use of it for fabrication of particulate delivery systems.[1,3,5b,23b,e] Few reports focused only on carriers intended for one of the drug delivery routes or the SCF process of particle formation, and critical analysis on a theoretical basis.[23d,h,p] Herewith, we present a perspective on an extensive survey of the past two decades on the use of the SCF technology in drug delivery and related biomedical applications.
2. Supercritical Processes for Particle Formation
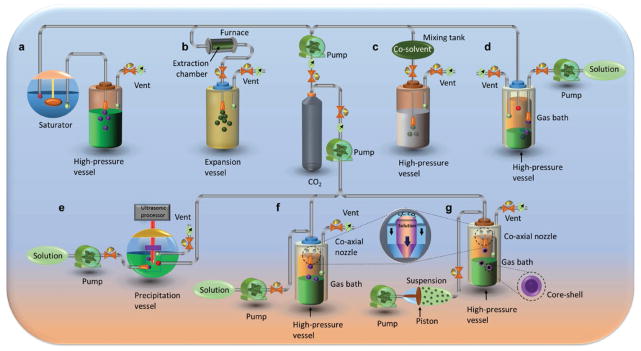
Conventional methods (i.e., mechanical or chemical) for synthesizing polymeric particles have several limitations, such as heterogeneous particle size distribution, particle damage by shear forces, and others.[24] Indeed, utilizing the SCF technology in pharmaceutical manufacturing and processing, the internal obstacles caused by conventional approaches can be minimized. Further, this technology has probably become the most sustainable process for addressing environmental concerns.[21a,23c,d] The first report of the SCF technology was from Hannay et al. in 1879,[25] following which variants in the technology have been developed to fabricate drug delivery vehicles. Different processes (Figure 2) of particle formation are categorized based on the behavior of SCF as solute (particle formation from gas-saturated solutions (PGSS)); solvent (rapid expansion of supercritical solutions (RESS));[26] anti-solvent (supercritical anti-solvent (SAS),[27] gaseous anti-solvent (GAS),[28] aerosol solvent extraction system (ASES),[21a] precipitation with compressed anti-solvent (PCA),[29] supercritical anti-solvent with enhanced mass transfer (SAS-EM),[30] solution enhanced dispersion by supercritical fluids (SEDS),[31] suspension-enhanced dispersion by supercritical fluids (SpEDS)[32]); and others such as depressurization of an expanded liquid organic solution (DELOS)[1a,33] and the supercritical-assisted atomization (SAA) process, etc.[34] SCF acts as a re-precipitation aid for rapid and uniform nucleation of solute in all the above-mentioned methods of fine particle formation. The performance efficiency of this technology is based on proper solvent selection and by adjusting critical parameters (temperature and pressure) during operations.[12] Herewith, we discuss these various supercritical processes involving particle formation in brief.
Figure 2.
Conceptual representation of various processes of particle formation using SC-CO2. a) Particle formation from gas saturated solutions (PGSS). b) Rapid expansion of supercritical solutions (RESS). c) Rapid expansion of a supercritical solution into a liquid solvent (RESOLV). d) Precipitation with compressed anti-solvent (PCA)/Aerosol solvent extraction system (ASES). e) Supercritical antisolvent with enhanced mass transfer (SAS-EM). f) Solution enhanced dispersion by supercritical fluids (SEDS). g) Suspension enhanced dispersion by supercritical fluids (SpEDS).
2.1. PGSS Process
SCF acts as a solute in the PGSS method (Figure 2a).[35] The SC-CO2 is compressed and dissolved in a molten polymer after autoclave treatment, where the solution expands and becomes cooled by the Joule-Thomson effect.[21a] Microparticles are formed when operated at a relatively low pressure.[21a] This approach is advantageous over other SCF techniques since it uses low volumes of SCF.[21a,35] However, the application of this process is limited due to particle agglomeration and nozzle blockage.[23f]
2.2. RESS Process
In a RESS process, SCF acts as a solute carrier, and this solution is expanded adiabatically leading to a rapid drop in temperature and pressure and further generation of small-sized particles after spraying through a nozzle (Figure 2b).[21a,26,36] In designing this process, the solubility of the material plays a crucial role in particle formation and processing since most of the pharmaceutical substances such as polymers, drugs, and high-molecular weight proteins are polar in nature. In few instances, small amounts of organic solvents are added to improve the affinity of polar drug molecules.[23b] RESS is the simplest and an efficient method in the SCF technology, but it is limited in its application because of its relatively high cost and poor solubility of polymers in non-polar SC-CO2. High amounts of SC-CO2 are preferred at industrial scale to address this issue.[24] Further, the advancements in the RESS process have been made to overcome certain limitations. One of them is the RESS process in an aqueous solution containing a surfactant or other reducing agents known as the rapid expansion of a supercritical solution into a liquid solvent (RESOLV) process (Figure 2c),[21b] where the SCF is expanded into a liquid medium. This modified process inhibits the particle agglomeration in the expansion jet.[37] The other modified process is the rapid expansion of a supercritical solution with solid co-solvent (RESS-SC), which results in smaller-sized particles. During synthesis, the added co-solvent improves the solubility of the APIs to a greater extent by avoiding superficial contact between particles, which increases the surface area of exposure to SCF and eventually, lyophilization can remove the co-solvent.[38] Despite its advancements, RESS still has certain limitations that are surpassed by the altered SCF behavior as anti-solvent in the reaction vessel.
2.3. SAS Process
The SAS process is proposed to process the molecules with poor solubility in SCF. This process predominantly utilizes an organic solvent such as acetone, dichloromethane (DCM), and dimethyl sulfoxide (DMSO), to dissolve the materials, where SCF behaves as a non-solvent to solute/API.[6] During the process, the mixture expands to supersaturation and results in fast nucleation, demonstrating the high mass transfer ratio due to the low viscosity and high diffusivity of SCF.[6] The outcome of this process utterly depends on the order of addition of solvent, SCF, and other substrates. Additionally, factors such as temperature, pressure, chemical composition of solute (drug, polymer), as well as organic solvent are required to be optimized. SAS has gained better drug loading than the RESS process, enabling the formation of fine particles.[23b] Other SAS processes comprise of GAS, which is based on the recrystallization of SCF insoluble solute and has a flexibility of choosing organic solvent to improve the solubility. This process has less operational problems compared to the conventional SAS method and is easy to scale-up in the manufacturing.[28] Recent advancements in SAS micronization techniques include, i.e., expanded liquid anti-solvent (ELS)[39] and the supercritical-assisted injection in a liquid anti-solvent (SAILA) methods;[40] however, deep analyses on these processes yet remain to be reported. ELS is operated using SCF and an organic solvent at expanding liquidity conditions.[39] The other modified SAS techniques include ASES,[21a] SCF-assisted extraction of emulsions (SFEE),[41] SAS-EM,[30] SEDS,[14,42] and SpEDS.[32]
2.4. ASES Process
Particle generation in ASES happens to be favorable at high anti-solvent-to-solvent ratio after spraying the drug/polymer solution into SCF via an atomization device. Further, the mass transfer of SCF depends on atomization efficiency, while solvent mass transfer relies on dispersing and mixing of SCF and organic solvent. This process is not suitable to load high amounts of drugs due to their usually high affinity towards organic solvent, which eventually reduces the loading amount in polymer after organic solvent extraction.[23b] Furthermore, a slight modification of ASES, known as the PCA manufacturing process (Figure 2d) effectively produces particles with a narrow size distribution.[23p] This process has been reported as a single-step technique operated to precipitate proteins.[23p]
2.5. SFEE Process
SFEE has emerged as a modified SAS process to encapsulate poorly water-soluble drugs. SCF interacts with the emulsion droplets and extracts the organic solvents/oily phase and leads to rapid precipitation of microparticles.[43] The advancement is to minimize the separation of the solid phase and agglomeration of particles during the SAS process.
2.6. SAS-EM Process
SAS-EM is an advanced SAS process to overcome the existing limitations of the SAS process.[30] The modification of the technique is that it utilizes a vibrating ultrasonic processor to atomize the solution jet into micro-droplets (Figure 2e). This processing method yields high turbulence, which enhances the mixing operation and subsequently the mass transfer and generates smaller-sized particles.[44]
2.7. SEDS Process
SEDS is another important process of SAS technique operated at a lesser drying time and increased mass transfer rates, which minimize the ASES process limitations. In a typical SEDS process (Figure 2f), the dispersed components are sprayed through a specially designed co-axial nozzle to control the particle morphology.[23b] Mass transfer of SCF into the sprayed droplet determines the particle formation by the rate of solvent transfer into SCF phase. A high mass transfer allows a faster nucleation and results in smaller particle sizes with less agglomeration.[23p] In fact, the polymer processing using organic solvents is highly accessible with this process because of solubility problems. Moreover, the continuous SEDS operation has extended the shelf life of polymeric materials. Water-soluble compounds can also be dealt with by introducing organic solvent through a co-axial three-compartment nozzle.[45]
2.8. SpEDS Process
SpEDS is an advancement of the SEDS process to overcome its processing damage issues.[32a] The apparatus and operation of both processes are almost similar, but SpEDS has an auxiliary injector setup to effectively pump the loaded suspension (Figure 2g).[32a] This process is designed to obtain core-shell structured microparticles with higher drug encapsulation efficiency and longer sustained drug release property compared to other SCF-assisted co-precipitation processes.[46]
2.9. Others
DELOS is another process of operation utilizing traces of organic solvent for the precipitation of particles.[23h] In this process, SCF acts as a co-solvent and is suitable for thermo-sensitive substances over various SAS methods. Other advantages of this process include minimum CO2 consumption and easier scalability of micronization of drugs.[33b]
Other important processes include the SAA process[34] and the CO2-assisted nebulization with a bubble dryer (CAN-BD) process[47] for aerosolization of particles, where SCF assists the nebulization for processing. Despite the similarity, CAN-BD requires no sophisticated setup for processing and is more suitable for thermolabile substances. An SAA approach resembles the DELOS process except that SAA uses water during operation. Moreover, by introducing a hydrodynamic cavitation mixer, the hybridized supercritical-assisted atomization-hydrodynamic cavitation mixer (SAA-HCM) was developed to improve the mixing operation during synthesis.[48] Several other methods such as microemulsion method, drying medium in the sol-gel process, and metal particles in SCF as the reaction medium, among others, are under practice as well.[49]
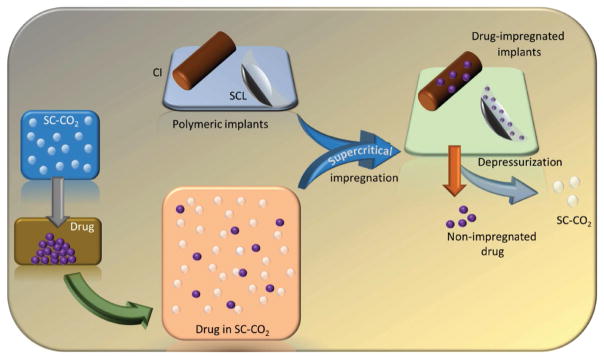
Drugs are also successfully encapsulated using the supercritical solvent impregnation (SSI) process involving a series of steps.[50] Initially, the drug is dissolved/saturated in SCF and then mixed with the polymer, and further optimization of operation parameters at the time of impregnation results in higher drug loading efficiency. Co-solvency is also used to support better drug impregnation; however, a better understanding is necessary.[9a]
SCF-assisted spray-drying (SASD) process is another method developed as a valid alternative technique to the conventional spray-drying (SD) process and SAS process for the preparation of nanoparticles. Herewith, SCF plays multiple roles such as co-solvent and partially miscible solvent, and and in addition, as a pneumatic agent to generate fine particles through atomization.[51] In another case, the SCF process is hybridized with a conventional method of particle formation results in SCF processing (SCP), which acts as an alternative to solvent evaporation method for manufacturing very tiny particles.[52] SCF-expansion depressurization (SFED) process is another innovative technique, which has shown great potential in micronization of water-soluble drugs, where SCF acts neither as solvent nor anti-solvent; however, when SCF is in contact with ternary phase mixture, it starts dissolving in the solution.[53]
The supercritical hydrothermal process is another process of the SCF technology, which is treated as an extension of the conventional hydrothermal technology operating just near or above the supercritical temperature of water.[20e,54] One of the advantages of this process during particle formation is the higher reaction rates, which lead to fast nucleation and result in small-sized particles.[54] Adschiri et al. pioneered this technique for the preparation of metal oxide nanomaterials for bio-imaging applications.[20e]
All these methods and their properties have been utilized for the production of the pure drug and composite (polymer-drug) particles for different deliveries, with the added advantages of impurity separation, selective precipitation, and control of crystalline forms. Basic safety hazards and precautionary measures should be strictly followed while operating SCF equipment.
3. Drug Delivery
Solid dosage forms are of primary choice for drug formulations and are mostly administered through the oral route.[3,7b] However, they face manufacturing hurdles such as physical instability during particle formation.[4a] Moreover, traditional methods undergo a multi-step process to manufacture dosage forms. Increased demand of pharmaceutical industries in developing new approaches for drug delivery, has evidenced the SCF technology as an alternative[55] for the syntheses of micro-[35,56] as well as nano-size particles[20d,e,57] with homogenous size distribution and high-performance.[21a] Indeed, this technology has been used to manufacture various formulations of different drug categories such as those treating anesthesia,[58] antibiotics,[53,59] asthma,[60] cancer,[61] central nervous system,[62] cardiovascular system,[31a] diabetes,[28] diuretics,[63] inflammation (steroidal[64] and non-steroidal anti-inflammatory agents[65]), lipid-lowering,[66] among others, administered through oral,[67] intravenous,[41] ophthalmic,[68] pulmonary,[47] transdermal,[61c] and polymeric implants for sustained delivery.[69] Revercheon et al. have highlighted the production of various nanoconstructs such as nanoparticles,[70] nanofibers,[71] nanowires,[72] nanotubes,[73] and other nanostructured materials using supercritical-based techniques.[20d] In addition, Adschiri et al. explored the adaptive properties of SCF for the synthesis of advanced nanomaterials include carbon nanotubes (CNTs), fullerenes, magnetic particles, quantum dots, phosphors, nanocomposites (e.g., peptide/hydroxyapatite (HAp)), and gold nanoshells for drug delivery and other biomedical applications such as imaging, sensing, and cancer theranostics.[20e]
The SCF processing of drugs improves bioavailability with also a significant increase in the surface area of the particles after micronization. However, the solubility of the drug in SCF and size of the particles depend on the density of the fluid and the pre-expansion concentration of the solute, respectively.[71] Eventually, to improve the drug dissolution characteristics, the amorphous form of poorly water-soluble drugs and their uniformly sized particles are feasible, because of their higher surface area exposure to solvent. The high surface area increases dissolution rate and subsequently, results in higher efficacy and lower dosage requirement for administration.[74]
3.1. Polymeric Carriers for Controlled Drug Release
To improve the fate and performance of a drug,[75] a protein,[76] or a vaccine,[77] a suitable carrier is required to change its delivery pattern.[72,78] Recently, controlled delivery using various polymers has garnered increasing interest, which prolongs the drug effect by maintaining the levels in the therapeutic window. In addition, targeted delivery such as magnetically directed[30,79] or using targeting ligands[61b,80] is anticipated to make APIs available at the desired sites with minimum adverse effects.[7b,61b,c] Several efforts are being made to address various challenging issues in preparing delivery systems by tailoring the morphology of polymeric carriers. Impurities-free biodegradable polymers are the best choice due to their versatility for encapsulation and efficiency in delivering various pharmaceutical agents in the body to a specific site in a controlled fashion. Spray-drying, emulsions, ionic gelation, phase separation, polyelectrolyte complexation, and SCF-assisted precipitation are the most preferred processes for polymeric micro-/nano-encapsulation.[23l] The adaptive properties of SCF are quite promising, and this green technology has emerged for particle design with low residual solvent in the product. Polymer selection is crucial based on its solubility in SCF and other solvents to overcome its separation during the manufacturing process. Further, the physical properties of polymer and operation conditions should be optimized to generate particles with different desired morphologies.[1]
As depicted in Table 1, many APIs have been encapsulated in biodegradable polymers to synthesize different sizes of particles for controlled delivery.[26,65a,b,81] Generally, the drug encapsulation in the polymeric shell involves sequential steps as follows. In the case of non-polar or low-molecular weight drugs, the API is initially soluble in SCF. Subsequently, the polymer is added for impregnation. Eventually, the SCF is separated to remove the free drug, followed by the extraction of organic solvent, if used. In few cases, multiple drugs were also impregnated through this technology.[62b] In fact, large molecules such as proteins are difficult to be absorbed when administered through non-invasive routes and are usually compromised by using absorption enhancers. The SCF technology has created a new path in fabricating sustain delivery carriers for the transport of large molecules with structural stability.[18a,24,82] Eventually, this strategy has surpassed the drawbacks of conventional processes such as solubility and recrystallization issues.
Table 1.
Polymeric drug delivery systems synthesized using various methods of preparation with the SCF technology.
| Pharmaceutical compound | SCF Process | Polymer | Solvent | Purpose | Reference |
|---|---|---|---|---|---|
| 2,6-Dimethyl-8-(2-ethyl-6-methylbenzylamino)-3-hydroxymethylimidazo-[1,2-a] pyridine mesylate | SEDS | Eudragit® E100, Mannitol | Acetone, DMSO, MeOH | Bioavailability enhancement | [42a] |
| 5-Aminosalicylic Acid | SEDS | Eudragit® S100 | Acetone, DMSO | Bioavailability enhancement | [190] |
| 5-Fluorouracil | SEDS | PLA | DCM | Controlled release | [61a] |
| PLA, Silica | DCM | [191] | |||
| PEG, PLA–PEG | Acetone, DCM, Water | [192] | |||
| SAS + SSI | PLA | EtOH | [18a] | ||
| SCP | PLGA | - | [85b] | ||
| PLA | EtOH | [193] | |||
| 17α-Methyltestosterone | SAS | PCL, PLA | DCM | Controlled release | [27] |
| α-Tocopheryl acetate | SSI | MCM-41-type silica | EtOH, Toluene | Controlled release | [194] |
| β-Carotene | SFEE | PCL | DCM | Controlled release | [195] |
| SEDS | PHBV | - | [196] | ||
| β-Estradiol | SCP | PLGA | - | Controlled release | [85b] |
| PVP | EtOH | [197] | |||
| β-Sitosterol | RESS | - | Trifluoromethane | Bioavailability enhancement | [81d] |
| Acetaminophen | RESS-N | EC, PEG, PEG-PPG-PEG, PLA, PMMA | EtOH | Controlled release | [81b] |
| SAS | Eudragit® RL100 | Acetone | [75] | ||
| Adefovir dipivoxil | RESS | - | - | Bioavailability enhancement | [13c] |
| Ampicillin | SAA | - | Acetone, MeOH | Bioavailability enhancement | [34a] |
| SAS | - | DMSO, EtOH, NMP | [198] | ||
| SAS | EC | DCM, DMSO | Controlled release | [199] | |
| SAA | HPMC | Phosphate buffers | [200] | ||
| Amoxicillin | SAS | - | NMP | Injectable | [83a] |
| - | DMSO, EtOH | [201] | |||
| SCF-PI | PMMA | Acetone, DMSO | Controlled release | [202] | |
| PVDF–HFP | Acetone | [203] | |||
| SEDS | Chitosan | - | Bioavailability enhancement | [204] | |
| Arbutine | SAS | - | EtOH | Topical administration | [205] |
| Artemisinin | RESS | PVP-K25 | DCM | Oral delivery | [81a] |
| - | - | Bioavailability enhancement | [206] | ||
| SCP | Silica, Sodium alginate, and Starch aerogels | EtOH, Isopropanol | [207] | ||
| Aspirin | RESS-N | PEG | EtOH | Controlled release | [81b] |
| SCF-MIP | P(DEGDMA) | [101] | |||
| Avidin | SCP | PLA | – | Controlled release | [149] |
| Azacytidine | SAS | PLA | DCM, DMSO | Controlled release | [208] |
| Baicalein | SEDS | – | Acetone, DMSO, EtOH | Bioavailability enhancement | [209] |
| Bevacizumab | SCF-PQT | PLA, PLGA | DCM | Controlled release | [50] |
| BSA | SEE-C | PCL, PLA, PVA | Acetone, Glycerol, Water | Controlled size | [210] |
| SSI | Chitosan, PNIPAAm | Acetic acid | Stimuli-responsive release | [76] | |
| PGSS | Chitosan, PEG, PLGA | – | Controlled release gastroretentive system | [67] | |
| SAA | PLA | Chloroform | Stability improvement | [211] | |
| Bupivacaine HCl | SAS | PLA, PLGA | DCM, EtOH | Controlled release | [91] |
| Calcitonin (Salmon) | SASD | Chitosan glutamate | EtOH, Water | Bioavailability enhancement | [51] |
| Camptothecin | SAS | Dextran | DMSO | Targeted delivery | [80] |
| Carbamazepine | SSI | PVP | – | Controlled release | [98a] |
| GAS | PEG | Acetone | Bioavailability enhancement | [212] | |
| SCP | Gelucire, PVP-K30, TPGS | MeOH | [52] | ||
| Celicoxib | SEDS | PLGA | n-Butyl acetate | Injectable | [31a] |
| Cefuroxime axetil | SEDS | HPMC, PVP-K30 | DCM, EtOH | Bioavailability enhancement | [213] |
| Chelerythrine | SEDS-PA | – | MeOH | Bioavailability enhancement | [214] |
| Chlorohexidine diacetate | SSI | PEMA, THFMA | – | Polymeric foam | [215] |
| Cilostazol | SAS | – | DCM | Bioavailability enhancement | [216] |
| Cholesterol | SSI | PCL, PMMA | – | Controlled release | [217] |
| Cu2(indomethacin)4DMF2 | ASES, GAS | – | DMF | Bioavailability enhancement | [21c] |
| Curcumin | SEDS | – | Acetone | Bioavailability enhancement | [218] |
| SF | HFIP | [219] | |||
| Dexamethasone | SAA | – | Acetone, MeOH | Bioavailability enhancement | [34a] |
| Diclofenac sodium | SFEE | PLGA, PVA | EA, Water | Injectable | [41] |
| Dihydroquercetin | SCP | Starch aerogels | EtOH | Oral delivery | [207] |
| Dithranol | SCP | Silica aerogels | ACN, MeOH | Oral delivery | [220] |
| Docetaxol | SCP | PVP-K17 | Myristyl alcohol | Bioavailability enhancement | [221] |
| Erlotinib HCl | SAS | – | MeOH | Bioavailability enhancement | [74] |
| HPMC-phthalate | DMSO | [222] | |||
| Felodipine | PGSS | PEG-4000 | – | Bioavailability enhancement | [35] |
| SAS | HPMC | DCM, EtOH | [223] | ||
| Fenofibrate | PGSS | PEG-4000 | – | Bioavailability enhancement | [35] |
| RESOLV | Alginate, HPMC, PLGA | – | [37] | ||
| SCP | Silica matrix | – | [224] | ||
| Fumed silica | – | [225] | |||
| Silica SBA-15 | – | [226] | |||
| Fenprofen | RESS | – | – | Bioavailability enhancement | [227] |
| Fluconazole | SAS | – | Acetone, DCM, EtOH | Bioavailability enhancement | [228] |
| Flufenamic acid | SCF-MIP | Methacrylic acid, NIPAAm cross-linked EGDMA | – | Controlled release | [102] |
| Fulvestrant | SAS | – | EA | Bioavailability enhancement | [74] |
| Furosemide | SAS | PVP | Acetone, EtOH, MeOH | Bioavailability enhancement | [63] |
| Gentamycin | PCA | PLA | DCM | Controlled release | [29] |
| PVM-MA | Acetone | [85a] | |||
| PLGA | Acetone | Intracellular targeting | [229] | ||
| Griseofulvin | RESS | – | Trifluoromethane | Bioavailability enhancement | [81d] |
| RESS-SC | – | DCM, Menthol | [38] | ||
| SFEE | PVA, PVP | DCM, EA | [230] | ||
| SCP | Silica aerogels | ACN, MeOH | Oral delivery | [220] | |
| Human growth hormone | SEDS | – | Isopropanol | Bioavailability enhancement | [231] |
| Hydrocortisone | SEDS | PCL, PLA, PLGA | Acetone, DCM, EA, Hexane, Isopropanol | Controlled release | [95] |
| SFEE | PLGA | DMSO, EA, EtOH | [232] | ||
| Ibuprofen | SSI | PVP | EtOH | Controlled release | [65a] |
| PMMA, PVP | EtOH, Toluene | [233] | |||
| Chitosan, PNIPAAm | Acetic acid | Stimuli-responsive release | [76] | ||
| Gelatin, Silica | Acetone | Controlled release | [234] | ||
| SCFS | PCL, Starch | Menthol | [235] | ||
| RESOLV | PEG, PVA, PVP | – | [70a] | ||
| SCP | PLA | Chloroform | [145a] | ||
| PGSS | Gelucire, Glyceryl Monostearate, Pluronic F127 | Water | [236] | ||
| SCF-PI | CA | Acetone | [134b] | ||
| SCP | PCL | – | [237] | ||
| PEG, PVP | EtOH | [98b] | |||
| MCM-41-type silica | – | [238] | |||
| Silica, Sodium alginate, and Starch aerogels | EtOH | Oral delivery | [207] | ||
| Immunoglobulin G (IgG) | SAS | – | EtOH | Biopharmaceutical powders | [239] |
| Indinavir | SAS | – | Acetone | Bioavailability enhancement | [240] |
| Indomethacin | SAS, SAS-EM | Eudragit® RS 100, Magnetite, PLGA, PMMA | DCM | Magnetically responsive drug release | [30] |
| SFEE | Eudragit RS®, PLGA | EA | Controlled release | [96b] | |
| SEDS | PLA, PLGA | DCM | [14] | ||
| Iron oxide, PLA | DCM | Magnetically responsive drug release | [241] | ||
| SSI | Chitosan | – | Controlled release | [81c] | |
| HPMC | – | [242] | |||
| PLA, PLA-PEG, PLGA | Acetone, Water | [243] | |||
| Insulin | SAS | PLA | DCM, DMSO | Subcutaneous delivery | [130] |
| GAS | PEG, PLA | DMSO | Controlled release | [131] | |
| PEG, PLA | DCM, DMSO | [244] | |||
| SFEE | PLGA | – | [245] | ||
| SCP | Chitosan, PAA, PEO | – | Bioavailability enhancement | [246] | |
| Itraconazole | SCP | HPMC, PVP | Cetyl alcohol | Bioavailability enhancement | [247] |
| Ketoprofen | SSI | PVP-K10 | – | Bioavailability enhancement | [248] |
| Gelatin, Silica | Acetone | Controlled release | [234] | ||
| PGSS | Gelucire, PEG | – | [65c] | ||
| SCF-IP, SSI | PVP | – | Oral delivery | [115a] | |
| SFEE | PLGA | EA | Bioavailability enhancement | [249] | |
| Eudragit RS®, PLGA | Controlled release | [96b] | |||
| SCFD | Alginate | EtOH | Oral delivery | [250] | |
| SCP | PLGA | – | Controlled release | [251] | |
| Silica aerogels | ACN, MeOH | Oral delivery | [220] | ||
| Levothyroxine sodium | GAS, ARISE | – | EtOH | Bioavailability enhancement | [252] |
| Lipase | RESS-N | PEG, PEG-PPG-PEG, PLA, PLGA, PMMA | Acetone, EtOH, MeOH, Propanol, Toluene | Bioavailability enhancement | [87] |
| Loratidine | SCP | Silica, and Starch aerogels | EtOH, Isopropanol | Oral delivery | [207] |
| Lovastatin | RESS | PLA | - | Bioavailability enhancement | [55] |
| Lutein | SEDS | Zein | Acetone, DMSO | Controlled release | [253] |
| Lysozyme | SFEE | PLGA | DMSO, EA | Bioavailability enhancement | [254] |
| RESS-N | PEG, PEG-PPG-PEG, PLA, PLGA, PMMA | EtOH, Toluene | [87] | ||
| ASES | PLA | DMSO | [255] | ||
| PCA | PLA, PLGA | DCM | [92] | ||
| SEDS | PEG, PLA | DMSO | [256] | ||
| Mangiferin | SAS | – | Acetone, DMSO, EtOH, NMP | Bioavailability enhancement | [257] |
| Megestrol acetate | SAS | HPMC, PVP, PEG | DCM, EtOH | Bioavailability enhancement | [258] |
| Methotrexate | SpEDS | Iron oxide, SF | DCM, HFIP | Transdermal delivery | [61c] |
| PEG, PLA | Acetone, DMSO | Controlled release | [32a] | ||
| Iron oxide, PEG, PLA | DCM | Targeted Controlled release | [46] | ||
| SEDS | – | DMSO | Bioavailability enhancement | [259] | |
| Miconazole | SCP | Silica aerogels | ACN, MeOH | Bioavailability enhancement | [220] |
| Minocycline | SAS | – | EtOH | Bioavailability enhancement | [260] |
| Morphine | SEDS | PLA-PEG-PLA | DCM, MeOH | Controlled release | [261] |
| PLA | DCM, EtOH, Water | [262] | |||
| Naloxone | PCA | PLA | DCM | Controlled release | [29] |
| Naphthalene | SSI | PMMA | – | Controlled release | [263] |
| Naproxen | SAS | EC, Methyl cellulose | DCM, DMSO | Oral delivery | [264] |
| RESS | PLA | – | Bioavailability enhancement | [65b] | |
| SCP | Ethylene-vinyl-acetate, Eudragit® E100 | DCM | Transdermal controlled delivery | [99] | |
| Naltrexone | PCA | PLA | DCM | Controlled release | [29] |
| Niclosamid | SCP | Silica aerogels | ACN, MeOH | Oral delivery | [220] |
| Nifedipine | PGSS | PEG-4000 | – | Bioavailability enhancement | [35] |
| Nilotinib | SAS | HPMC-phthalate, | Acetone, Chloroform, DMSO | Bioavailability enhancement | [222] |
| Nimesulide | SAS | – | Acetone, Chloroform, DCM | Bioavailability enhancement | [265] |
| SSI | PDMS | – | Controlled release | [266] | |
| Olanzapine | RESS, RESSAS | HPMC, PEG | – | Oral delivery | [267] |
| Oxaliplatin | SCP | – | Myristyl alcohol | Oral delivery | [268] |
| Oxeglitazar | SAS | PEG, Poloxamer 188 and 407, PVP-K17 | Chloroform, DCM, DMSO, EtOH, NMP, THF | Oral delivery | [25] |
| pDNA | SCF foaming | Chitosan, PLGA | – | Controlled release | [156] |
| Paclitaxel | SSI | PLA | EtOH | Controlled release | [269] |
| SEDS | PEG-PLA | DCM | Targeted delivery | [61b] | |
| PLA, PLGA | DCM | Controlled release | [270] | ||
| GAS, SCF-PI | Basil seeds mucilage | DMSO, EtOH | Bioavailability enhancement | [271] | |
| RESOLV | PVP | – | [272] | ||
| Phenytoin | RESS-SC | – | Menthol | Bioavailability enhancement | [62a] |
| Piroxicam | SAILA | PLA, PLGA | – | Controlled release | [40b] |
| SSI | PDMS | – | [266] | ||
| SFEE | PLGA, PVA | EA, Water | Injectable | [41] | |
| PCA | PVP | – | Bioavailability enhancement | [273] | |
| Progesterone | SCP | PEG, Gelucire 44/14 TPGS | – | Transdermal delivery | [274] |
| PGSS | – | Bioavailability enhancement | [275] | ||
| Puerarin | SEDS | PLA | DCM | Bioavailability enhancement | [15,276] |
| Quercetin | SFEE | Pluronic L64, Soybean lecithin | EA | Bioavailability enhancement | [43] |
| Retinyl acetate | SFEE | PLGA | Acetone | Sustained release | [277] |
| Retinyl palmitate | RESOLV | PLA | – | Bioavailability enhancement | [278] |
| Rhodamine | SFEE | PLA | EA | Controlled release | [279] |
| Rifabutin | SCP | Silica, Sodium alginate, and Starch aerogels | EtOH, Isopropanol | Oral delivery | [207] |
| RNA | SEDS | PEG, PLA | DCM | Transdermal controlled delivery | [280] |
| Salbutamol | SCFD | Chitosan | EtOH | Controlled release | [281] |
| Salicylic acid | SCF-MIP | P(DEGDMA) | EtOH | Controlled release | [101] |
| SSI | Alumina, Amberlite, Silica gel | - | [62b] | ||
| Silymarin | SEDS | Phospholipids | DCM, EtOH | Bioavailability enhancement | [282] |
| Simvastatin | RESS | - | Trifluoromethane | Bioavailability enhancement | [66] |
| Sulfamethizole | SAS | - | Acetone, DMF | Ultrasound application | [283] |
| Sulfathiazole | SAS | - | Acetone, MeOH | Bioavailability enhancement | [284] |
| Terfenadine | SCP | Silica aerogels | ACN, MeOH | Oral delivery | [220] |
| Tetanus toxoid | SCP | PLA | - | Sustained release | [77] |
| Theophylline | SAS | HPMC | - | Controlled release | [285] |
| RESS-N | EC, PEG | EtOH | [81b] | ||
| Timolol maleate | SSI | Chitosan derivatives | EtOH | Ophthalmic delivery | [142c] |
| PCL, PCL/POE, PCL/PEVA | THF | Controlled release | [141] | ||
| Tolfenamic acid | SAS | - | Acetone, EA | Bioavailability enhancement | [286] |
| Triclabenzadol | SAA | - | Acetone, MeOH | Bioavailability enhancement | [34a] |
| Triflusal | SCP | PMMA | Acetone | Controlled release | [287] |
| PMMA, Silica | - | Bioavailability enhancement | [70b] | ||
| SSI | Gelatin, Silica | Acetone | Controlled release | [234] | |
| Trypsin | SAA-HCM | Chitosan | Water | Controlled release | [82] |
| Vitamins (2-methyl-1, 4-naphthquinone (vitamin K3) cholecalciferol (vitamin D3)) | SSI | Sodium alginate aerogels | EtOH | Controlled release | [288] |
| Zidovudine | SAS | PLA | DCM, EtOH | Bioavailability enhancement | [289] |
Abbreviations: 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), Acetonitrile (ACN), Aerosol solvent extraction system (ASES), Atomized rapid injection solvent extraction process (ARISE), Bovine serum albumin (BSA), Cellulose acetate (CA), Continuous-Supercritical emulsions extraction (SEE-C), D-alpha tocopheryl polyethylene glycol-1000 succinate (TPGS), Dichloromethane (DCM), Dimethylformamide (DMF), Dimethylsulfoxide (DMSO), Ethanol (EtOH), Ethyl acetate (EA), Ethyl cellulose (EC), Ethylene glycol dimethacrylate (EGDMA), Gas anti-solvent (GAS), Hydroxypropyl methylcellulose (HPMC), Methanol (MeOH), Mobil composition of matter (MCM), N-methylpyrrolidone (NMP), Particle formation from gas saturated solutions (PGSS), Poly(3-hydroxybutyrate-co-3-hydroxy valerate) (PHBV), Polycaprolactone (PCL), Polydimethylsiloxanes (PDMS), Poly(lactide-co-glycolide) (PLGA), Polyethylene glycol (PEG), Poly(ethyl methacrylate)/tetrahydrofurfuryl methacrylate (PEMA/THFMA), Poly(ethylene-vinyl acetate) (PEVA), Polyethylene oxide (PEO), Polylactic acid (PLA), Poly(methyl methacrylate) (PMMA), Poly(diethylene glycol dimethacrylate) P(DEGDMA), Poly(methyl vinyl ether-co-maleic anhydride) (PVM-MA), Poly(N-isopropylacrylamide) (PNIPAAm) Poly(oxyethylene) (POE), Polypropylene glycol (PPG), Poly-vinyl alcohol (PVA), Poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF–HFP), Polyvinylpyrrolidone (PVP), Precipitation with Compressed anti-solvent (PCA), Rapid expansion of a supercritical solution into a liquid solvent (RESOLV), Rapid expansion of supercritical solutions (RESS), Rapid expansion of supercritical solutions into aqueous solution (RESSAS), Rapid expansion from supercritical solution with a non-solvent (RESS-N), Rapid expansion of supercritical solution with solid co-solvent (RESS-SC), Santa Barbara Amorphous (SBA), Silk fibroin (SF), Solution-enhanced dispersion by supercritical fluids (SEDS), Solution-enhanced dispersion by supercritical fluids-prefilming atomization (SEDS-PA), Supercritical-assisted injection in a liquid anti-solvent (SAILA), Supercritical anti-solvent (SAS), Supercritical anti-solvent with enhanced mass transfer (SAS-EM), Supercritical-assisted atomization (SAA), Supercritical-assisted atomization-hydrodynamic cavitation mixer (SAA-HCM), SCF-assisted molecular imprinting (SCF-MIP), SCF-assisted spray drying (SASD), SCF-drying (SCFD), SCF-assisted extraction of emulsions (SFEE), SCF-inkjet printing (SCF-IP), SCF-phase inversion process (SCF-PI), SCF-pressure-quench technology (SCF-PQT), SCF-assisted processing (SCP), SCF-assisted sintering (SCFS), Supercritical solvent impregnation (SSI), Suspension-enhanced dispersion by supercritical fluids (SpEDS), Tetrahydrofuran (THF).
In general, drugs with poor oral bioavailability, i.e., either with low solubility or low intestinal permeability, are opted to improve by optimizing the conditions of the manufacturing process.[4a,5b] Numerous studies with respective to delivery systems using the SCF technology are compiled and tabulated focusing on the relevant parameters related to the purpose of delivery and method of preparation (Table 1). Few of the studies have reported the micronization of APIs alone[81d,83] and few were impregnated in various water-soluble polymeric matrices such as polyvinylpyrrolidone (PVP), starch derivatives, modified cellulose, and some other synthetic polymers.[1,3,5b,23b,e,61b,84]
Polymers are highly advantageous as carriers because of easy drug impregnation, ability to carry a high payload of drugs, biocompatibility, biodegradability, and sustainability of drug release from the matrix.[7b,85] Polymers are chosen based on polarity and thermosensitivity. The key feature is the polymer solubility in SCF, which favors the reduction of polymer melting temperature and subsequently decreased viscosity[86] guided by the molecular structure, i.e., functional group, and its morphology. Polymers usually have low solubility in SCF. However, the increase in pressure or temperature results in enhancement of their solubility.[23b] Notably, few polymers are soluble only in the organic solvents, whose industrial applicability is limited.[87] To this end, phase mixing in a combination of solvents can be pursued to overcome this issue.[88]
During the supercritical process, polymeric particle formation undergoes sequential steps: initially, nucleation phase, which results in the formation of the polymeric nucleus and then followed by growth phase yielding desired particles.[13b] In fact, altered operating conditions (i.e., temperature, pressure, and solution flow rate) result in different shapes of polymeric composites such as microspheres and microfibers. The various shapes with altered critical conditions result in changes in the polymeric physical properties such as reduction in melting point and solubility improvement in relatively high-pressure systems.[89]
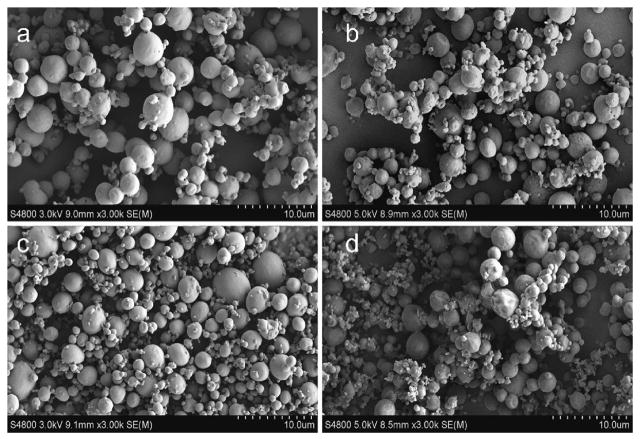
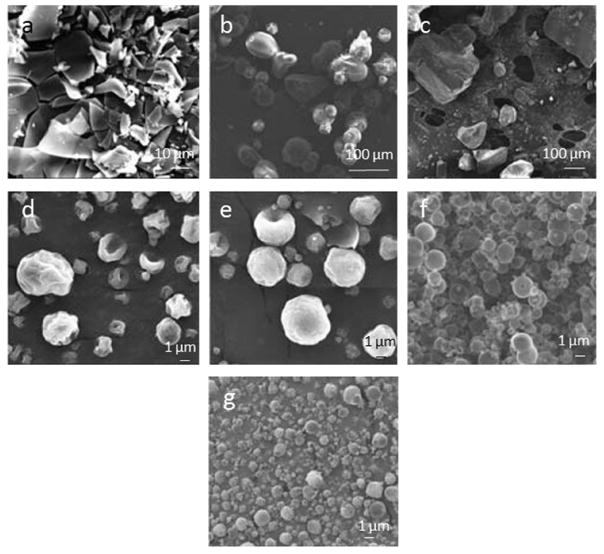
Most of the microencapsulated systems are prepared using biodegradable polylactic acid (PLA),[84b,90] since it is suitable for controlling delivery of short half-life drugs or highly potent drugs due to its long in vivo degradation time. For example, paclitaxel (PTX) was loaded in a synthetic biodegradable diblock copolymer poly(ethylene glycol)-PLA (PEG-PLA), and subsequently conjugated with folic acid (FA) in the PEG terminal ends to form FA-PEG-PLA complex (Figure 3). The hydrophobic drug-loaded microparticles for use as the tumor-targeted delivery system (PTX-FA-PEG-PLA) were produced via the SEDS process, which resulted in a spherical shape and presented significantly higher cellular uptake than FA-free carriers (PTX-PEG-PLA) in the tumor tissue. This study and others demonstrate that the semi-crystalline and amorphous forms of PLA, and amorphous poly(lactide-co-glycolide) (PLGA) are all suitable in the SCF technology for particle formation.[61b,84a]
Figure 3.
SEM images showing microparticles prepared by the SEDS process. a) FA-PEG-PLA, b) PTX-loaded FA-PEG-PLA, c) PEG-PLA, and d) PTX-loaded PEG-PLA particles. Reproduced with permission.[61b] Copyright 2015, Springer.
Among all the SCF methods for particle formation, the SAS process is one of the most efficient to produce microparticles with desired morphologies, where SCF acts as the anti-solvent, and the alternative organic solvent is used to dissolve the polymer. Further, the SCF miscibility with organic solvent leads to its expansion and subsequent reduction in density and the solvation capacity. These consequences enable supersaturation, solute nucleation, and particle formation.[8b] Different linear polyesters of lactic acid (e.g., PLA) and their co-polymers with polyvinyl alcohol (PVA) such as PVA-PLA and PVA-PLGA are processed through the SAS method.[8b,91] Solvent selection plays a crucial role in polymer dissolution and mutual miscibility with SCF.
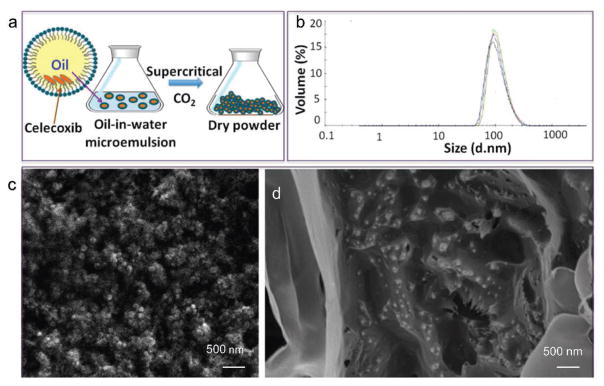
In addition to micro-size particles, SCF can generate nanometric domains with improved therapeutic efficacy. In a case, Zare et al. prepared nanometric domains of celecoxib by SC-CO2 extraction from a volatile oil-in-water microemulsion (Figure 4a). Celecoxib and PLGA (lactide:glycolide = 75:25) were dissolved in the dispersed phase (n-butyl acetate); concomitant extraction yielded solid powder composed of spherical nanoparticles with an average size of 110 nm (Figure 4b and c). In addition, these polymeric nanocarriers were dispersed in an injectable crosslinked hydrogel (Figure 4d) composed of biocompatible and biodegradable polymers, PVA (Mw = 89–98 KDa, 10 wt.%) and PVP (Mw ≈ 40 KDa, 3 wt.%). Celecoxib was capable of inducing angiogenesis in normally perfused and ischemic organs, and the intravenously administered nanoparticle formulation profoundly worked for improving therapeutic angiogenesis.[31a]
Figure 4.
Celecoxib nanoparticles formation and characterization. a) Schematic representation of nanoparticle formation process. b) Particle size measurements in water by dynamic light scattering (DLS). c) SEM image of nanoparticles in powder. d) SEM image of nanoparticles embedded in hydrogel (dried). Reproduced with permission.[31a] Copyright 2015, American Chemical Society.
The SCF technology is rather the alternative to treat/encapsulate large molecules such as proteins due to its promising operating conditions. Polymeric carriers such as biodegradable PLGA microspheres are usually chosen to encapsulate and deliver because of their sensitivity and relatively fast degradation behavior.[17,23b,31a] This impregnation of proteins surpasses limitations such as agglomeration and limited solubility of proteins in organic solvents.[92] Most of the proteins processed as inhalation powders for therapeutic delivery are prepared using SAS[93] and its associated processes.[94]
In addition, polymeric particle formation in PGSS is easier after solubilizing SCF in polymers (i.e., PEG and polyethylene (PE)) through rapid depressurization and their phase-separation by pressure alteration. Polymer plasticization upon SCF treatment results in substantial reduction in its viscosity and results in smaller particles and allows effective immobilization of drugs.[8b] This reduction in particle size demonstrates the effective dissolution of particles and exhibits controlled release of drugs from the hydrophilic polymer matrix. Out of all processing techniques operated to synthesize drug delivery vehicles using the SCF technology, PGSS holds several advantages over others, such as no organic solvent requirement for solubilizing polymers, and eventually no extraction process for solvent removal.[17]
In general, the SCF technology is operated using any one of the supercritical solvents available. Few instances, combination of SCFs are preferred to minimize severe flocculation of polymeric microparticles due to plasticizing effect of residual SCF and this approach also results in fine-sized particles.[95] Eventually, these green solvents are also utilized to extract the residual organic solvent at <100-psi pressures from the conventional preparations, which enables increasing the particle porosity.[96]
During particle fabrication, the SCF technology offers many advantages over other processes such as colloid chemistry, microfluidics, spray-drying, and electrospray. Unlike these conventional processes, the SCF technology does not rely on the use of organic solvents. This green technology precipitates micro- and nano-sized particles with narrow size distribution by altering critical conditions (i.e., temperature and pressure) and flow rate of SCF and others.[5b,20d,21a,23r] The SCF possesses unique properties such as its solvating power, anti-solvent effect, and large compressibility.[3] The SCF technology provides many ways of particle fabrication (See Section 2) such as rapidly exceeding the saturation point of a substrate and rapid depressurization, among others.[1,21a,23e] These SCF processes yield smaller-sized particles usually faster than other techniques such as those based on microfluidics. The polymeric particles fabricated by the SCF processes can be completely dispersed in most cases, unlike various colloidal particles that rely on certain interaction forces such as electrostatic, steric, and van der Waals forces to disperse.[97] Another advantage of the SCF technology is that it allows single-step fabrication of particles that are difficult to obtain by traditional techniques.
Despite the efficiency of SCF in producing particulate delivery systems, poor solubility of polar substrates (i.e., drugs and polymers) in SCF has remained as a challenge.[98] Several alternatives can overcome this issue, such as pre-mixing of substrates and usage of organic solvent. Pre-mixing involves the mixing of all the substrates, i.e., drug and polymer, as well as other excipients, before the SCF treatment.[98] In addition, the drug is dissolved in an organic solvent before pressurizing CO2. Further, the resultant solution is supplied to a polymer, which facilitates the impregnation of drug in the swollen polymer.[99] Eventually, the organic solvent traces can be removed post-fabrication, which results in unaltered surface properties of the particles.[17]
In addition to carrier design, the SCF technology is also used to synthesize certain biodegradable polymers by rapid depressurization at the end of polymerization.[100] Indeed, the SAS process is preferred to co-precipitate desired molecules of interest and is beneficial over other conventional methods. The plasticization phenomena of polymers in compressed SCF has a great impact on their physical and mechanical properties, which alters glass transition temperature of the polymer and allows to design advanced materials.[23g] Molecular imprinting (MIP) is one such method to synthesize polymers using various polymerization mechanisms. It has enormous applicability in different fields, which has also inspired the pharmaceutical field to design controlled release systems with high encapsulation efficiency.[101] This technique is very precise and easy to tailor the polymer because monomer is chosen based on the requirement to obtain the desired polymer.[68] Copolymer MIP process is also applied in the manufacturing process to attain control over the therapeutic release and the nature of interaction confinement during self-assembly to direct the drug release, which depends on molecular recognition of functional monomers.[102]
In addition to polymers, cyclodextrins (CD) are the most used pharmaceutical delivery vehicles to produce solid-state inclusion complexes.[3,103] Inclusion approach alters the physicochemical properties such as solubility and dissolution rate to enhance the bioavailability of poorly soluble drugs.[103f] The specific interactions present between the host and guest molecules direct the release rate of the drug.[104] Several conventional methods in the past were used to prepare the CD complexes, which were time-consuming multi-stage processes and resulted in traces of organic solvents after preparation.[103f] The SCF technology is probably the most suitable method for the preparation of inclusion complexes using various CDs (Table 2). Following the solubilization of the drug in SCF, inclusion of the drug is possible by partitioning the dissolved drug with the SCF phase and hydrophobic CD cavity and establishes the molecular interactions such as hydrogen bonding.[103e] The SCF technology has remained as the most advantageous method of preparation over traditional methods to load large molecules in CD complexes.[103g,h]
Table 2.
Inclusion complexes of various cyclodextrins synthesized using various methods of preparation with the SCF technology.
| Pharmaceutical compound | SCF Process | CD | Solvent | Purpose | Reference |
|---|---|---|---|---|---|
| Benzocaine | SCI | β-CD | EtOH | Bioavailability enhancement | [58] |
| Budesonide | SEDS | γ-CD | EtOH | Bioavailability enhancement | [103g,h] |
| SCI | HP-β-CD | - | [103e] | ||
| Bupivacaine HCl | SCP | β-CD | EtOH | Bioavailability enhancement | [58] |
| Curcumin | ARISE | HP-β-CD | Acetone, EtOH | Inhalation powders | [290] |
| Econazole | SCI | β-CD | - | Bioavailability enhancement | [291] |
| Eflucimibe | SAS | γ-CD | DMSO | Bioavailability enhancement | [292] |
| Fluconazole | SCI | β-CD | - | Bioavailability enhancement | [291] |
| Flurbiprofen | SCP | Trimethyl-β-CD | EtOH | Bioavailability enhancement | [293] |
| Ibuprofen | SCI | Methyl-β-CD | - | Bioavailability enhancement | [103d] |
| β-CD | - | [103f,294] | |||
| Imazalil | SCI | β-CD | - | Bioavailability enhancement | [295] |
| Indomethacin | SCI | HP-β-CD | - | Bioavailability enhancement | [103e] |
| Methyl-β-CD | - | [296] | |||
| Itraconazole | ASES | HP-β-CD | DCM, EtOH | Bioavailability enhancement | [297] |
| SCI | β-CD | - | [291,298] | ||
| Ketoprofen | SCI | Methyl-β-CD | - | Bioavailability enhancement | [103b] |
| Mepivacaine | SCI | β-CD | EtOH | Bioavailability enhancement | [58] |
| Miconazole | SCI | β-CD | - | Bioavailability enhancement | [299] |
| HP-γ-CD | - | [300] | |||
| Naproxen | ASES | HP-β-CD, Methyl-β-CD | Acetone, DMSO, EtOH | Pulmonary delivery | [103c] |
| SCP | Trimethyl-β-CD | EtOH | Bioavailability enhancement | [293] | |
| SCI | β-CD | - | [301] | ||
| Olanzapine | SCI | Methyl-β-CD | - | Bioavailability enhancement | [104] |
| Piroxicam | SCI | β-CD | - | Bioavailability enhancement | [299,302] |
| Acetone, EtOH | [303] | ||||
| Quercetin | PGSS | HP-β-CD | - | Bioavailability enhancement | [304] |
| Simvastatin | SAS | HP-β-CD | DCM, EtOH | Bioavailability enhancement | [305] |
| Tocopherol | PGSS | HP-β-CD | - | Bioavailability enhancement | [304] |
Abbreviations: Aerosol solvent extraction system (ASES), Atomized rapid injection solvent extraction process (ARISE), Cyclodextrin (CD), Dichloromethane (DCM), Dimethylsulfoxide (DMSO), Ethanol (EtOH), Hydroxypropyl-β-cyclodextrin (HP-β-CD), Particle formation from gas saturated solutions (PGSS), Polyvinylpyrrolidone (PVP), Solution-enhanced dispersion by supercritical fluids (SEDS), Anti-solvent (SAS), SCF-assisted processing (SCP), Supercritical inclusion (SCI) method.
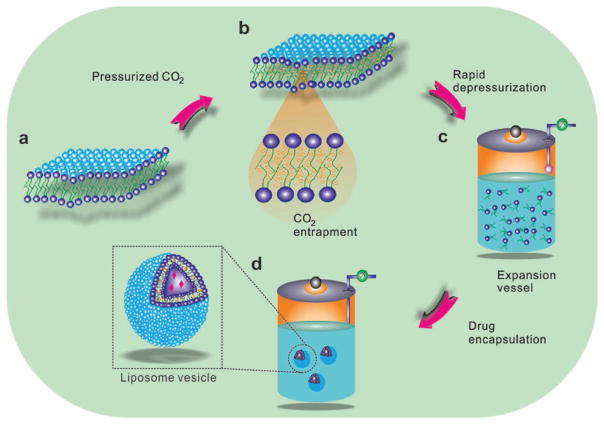
Liposomes are the versatile delivery systems formed by the colloidal association of amphiphilic lipid substances,[23i,106] which are suitable for delivering both hydrophilic and hydrophobic drugs[107] as well as proteins.[108] Most of the genes are delivered using adenoviral or liposomal vectors, in addition to non-viral vectors known as polymers.[109] SCF has received considerable attention as a green alternative in liposome scale-up because conventional processes require large amounts of organic solvents.[3,105,106b,110] The SCF-treated liposomes resulted in higher encapsulation efficiency[3,106b,108] than the conventional liposome preparation methods.[111] Two ways have been used to prepare liposomes, one of them is performed by mixing all components, i.e., phospholipids, SCF, and organic solvent together followed by decompression, and the other is by mixing phospholipids, SCF, and organic solvent and pumping the mixture into an aqueous phase.[23]
Zhao et al. prepared liposomes utilizing modified supercritical process involving the equilibration of phospholipid suspension in water with the high-pressure CO2 and the CO2-expanded liquid phase at a constant depressurization rate. The mechanism behind the liposome yield is simultaneous pressurization and depressurization phenomena, which leads to a dispersing effect of CO2 in phospholipids and released upon depressurization.[105] Eventually, due to hydrophobic interactions, phospholipids aggregate to yield spherical bilayers (Figure 5). This is similar to conventional homogenization process; however, the heat is removed from the phospholipid suspension, resulting in a temperature drop and cooling effect in the liposome suspension.[105] These lead to nano-sized particles at elevated pressure while higher depressurization rates contribute to enhanced uniformity.
Figure 5.
Schematic mechanism of liposome formation by the modified supercritical method. a) Phospholipid curvatures present at ambient condition, b) formation of expanded phospholipid bilayers after pressurization and equilibration with CO2, c) formation of an instantaneous dispersion of discrete phospholipid molecules during depressurization and release of CO2, and d) formation of liposome vesicle due to hydrophobic interactions after depressurization. Reproduced with permission.[105] Copyright 2015, Elsevier.
Lipid delivery vehicles manufactured through the SCF technology (Table 3) possess different physicochemical properties with high stability and narrow particle size distribution.[106b] The single-step continuous mode is under operation to improve the process,[112] which has no interference in encapsulation efficiency and no loss of entrapped drug. High drug entrapment efficiency in liposomes is yielded when they are prepared from unsaturated phospholipids.[113] This approach was later utilized to develop various drug formulations using SCF after optimization of formulation variables.[114] Although much research has been done in preparing liposomes through the SCF technology, the scalability and industrial implementation of these processes are expensive.[105,106b]
Table 3.
Liposomes as drug delivery carriers synthesized using various methods of preparation with the SCF technology.
| Pharmaceutical compound | SCF Process | Lipid | Solvent | Purpose | Reference |
|---|---|---|---|---|---|
| BSA | SuperLip | Phospholipids | EtOH | Controlled size | [108,306] |
| DELOS | PEG, Phospholipids | - | Targeted delivery | [33b] | |
| Cyclosporin A | SAS | Cholesterol, Phospholipids | EtOH | Stability improvement | [111] |
| SCP | Phospholipids | - | Ocular delivery | [307] | |
| Docetaxol | SAS | Cholesterol, PEG, Phospholipids | Chloroform, MeOH | Controlled release | [114] |
| Human growth hormone | SAA | PEG, Phospholipids, Tristearin | DMSO | Oral delivery | [308] |
| Insulin | SAA | PEG, Phospholipids | DMSO | Oral delivery | [308,309] |
| Ketoprofen | PGSS | Glycerolipids | - | Controlled release | [310] |
| Miconazole | ASES | Phospholipids | DCM, MeOH | Pulmonary delivery | [311] |
| Ribonuclease A | PGSS | PEG, Phospholipids | DCM, DMSO | Controlled release | [312] |
| Silymarin | SEDS | Phospholipids | DCM, EtOH | Bioavailability enhancement | [282] |
| Theophylline | PGSS | Hydrogenated palm oil | - | Controlled release | [313] |
Abbreviations: Aerosol solvent extraction system (ASES), Bovine serum albumin (BSA), Depressurization of an expanded liquid organic solution (DELOS), Dichloromethane (DCM), Dimethylsulfoxide (DMSO), Ethanol (EtOH), Methanol (MeOH), Particle formation from gas saturated solutions (PGSS), Polyethylene glycol (PEG), Solution-enhanced dispersion by supercritical fluids (SEDS), Supercritical anti-solvent (SAS), Supercritical-assisted atomization (SAA), Supercritical-assisted liposome formation (SuperLip), SCF-assisted processing (SCP).
3.2. Mechanisms of Drug Release
Drug delivery systems generally consist of a drug encapsulated within a biocompatible polymeric matrix, which is intended to release the drug through various mechanisms in the body. The release mechanism depends on a few factors such as the type of polymer used and the method of preparation of any formulation.[4a,6] These factors influence successful encapsulation of drug in the polymeric matrix or a micro-reservoir either in a laboratory or during scale-up, while other factors such as pH of the target environment also play a crucial role in its release.[4a] The incorporated drug can be released through two major ways of diffusion and burst-out phenomena.[6] In a diffusion process, the high affinity or specific interactions between the encapsulated drug and the polymeric matrix are weakened and the polymeric matrix becomes porous and subsequently releases the drug in a controlled fashion. The other way is the burst release, where the drug in the polymeric matrix is weakly bound and after exchange of the surrounding fluid it results in burst-out release of almost the entire drug cargo. The burst-out phenomena can be achieved through pH-/temperature-sensitive polymers used for encapsulation.[76] In addition, surface-adsorbed drug molecules during co-precipitation process may result in their immediate release. Currently, drug delivery vehicles have been designed to contain different payload of single/multiple drugs in a formulation possessing various release characteristics such as initial burst release from the outer layer and sustained release pattern from the inner layer within the same vehicle.[23c] Others include micro-reservoir-like vehicles, where the mode of release is modulated through a microchannel array.[115]
3.3. Pulmonary Delivery
Pulmonary delivery of drugs has become an attractive target in healthcare as the lung is suitable for absorption of many drugs due to its high surface area,[116] receipt of the entire cardiac output, low enzyme activity,[23a] and lack of the first-pass metabolism.[117] This non-invasive route is now used for the administration of various drugs and proteins for systemic as well as localized therapy through pressurized metered-dose inhalers (MDIs), nebulizers, and dry powder inhalers (DPIs) containing very tiny particles (<7 μm) for efficient deposition in lungs.[51,118] These formulations should provide excellent aerodynamic performance with suitable electrostatic charge and morphology, as well as long shelf-life stability and high deposition rate.[23j] In addition to aerodynamic performance, inter-particulate interactions are also considered, which significantly depends on the particle dispersibility to define the overall particle size distribution and deposition after inhalation.[23j] These aerosol formulations are produced widely by traditional ways, such as milling or spray drying;[51] however, the end product of these methods has few limitations such as remnants of solvents and sensitive molecules (proteins, and genes) encapsulated are susceptible to degradation.[51] In addition, selection of suitable excipients also remains a challenge for formulating sustained-release pulmonary delivery systems.[119]
Researchers have applied the SCF technology using CO2 and other solvents to synthesize fine particles of drugs,[51,59a,60,64,83,103c,120] proteins,[28,93b,c,121] and genes[122] for inhalation delivery (Table 4). Many inhalation formulations are produced at a high yield using a single-step SCF process, which can fine-tune the particle morphology, size, and charge after optimizing of all parameters.[23a] The ease of modulations has resulted in product with excellent aerodynamic performance as well as pulmonary deposition after nebulization.[23f] Particle size plays a crucial role in sustained pulmonary delivery, where larger-sized particles face difficulty in attaining bronchial penetration,[123] and small-sized particles are prone to alveolar macrophages uptake.[124] The SAS-EM technique is optimum to improve the particle size distribution in sub-micron range using the ultrasonic frequency vibrations.[93a]
Table 4.
Drug delivery systems intended for pulmonary route of administration synthesized using various methods of preparation with the SCF technology.
| Pharmaceutical compound | SCF Process | Polymer | Solvent | Purpose | Reference |
|---|---|---|---|---|---|
| 5-Fluorouracil | SAS | α-Lactose monohydrate | Acetone, DCM, EtOH, MeOH | Inhalation powders | [314] |
| Amphotericin B | CAN-BD | – | EtOH | Pulmonary delivery | [47] |
| Albuterol sulfate | SEDS | α-Lactose monohydrate | DCM, MeOH | Inhalation powders | [315] |
| Amoxicillin | SAS | – | NMP | Inhalation powders | [83a] |
| – | DMSO, EtOH | [201] | |||
| – | DMSO, NMP | Pulmonary delivery | [120a] | ||
| Beclomethasone-17, 21-dipropionate | GAS | – | Acetone, EtOH, MeOH | Pulmonary delivery | [316] |
| ASES | – | DCM, MeOH | Pulmonary delivery | [64] | |
| Betamethasone-17-valerate | ASES | – | DCM, MeOH | Pulmonary delivery | [64] |
| BSA | ASES | PLA-PLGA | DCM, MeOH, TFE | Pulmonary delivery | [317] |
| SAA-HCM | – | Water | [48] | ||
| Budesonide | ASES | – | DCM | Inhalation powders | [83b] |
| – | DCM, MeOH | Pulmonary delivery | [64] | ||
| SEDS | – | Acetone, MeOH | Inhalation powders | [318] | |
| α-Lactose monohydrate | Acetone | [315] | |||
| Calcitonin (Salmon) | SASD | Chitosan glutamate | EtOH, Water | Nasal powders | [51] |
| Celicoxib | SCF-PQT | PLGA | – | Sustained release | [124] |
| Cyclosporin A | RESS, PGSS | – | – | Inhalation powders | [319] |
| Curcumin | ARISE | HP-β-CD, PVP | Acetone, EtOH | Inhalation powders | [290] |
| Dexamethasone | ASES | – | DCM, MeOH | Pulmonary delivery | [64] |
| Flunisolide | ASES | – | DCM, MeOH | Pulmonary delivery | [64] |
| SEDS | – | Acetone, MeOH | Inhalation powders | [318b] | |
| Fluticasone-17-propionate | ASES | Heptafluoropropane-227 | DCM | MDI | [120c] |
| – | DCM, MeOH | Pulmonary delivery | [64] | ||
| Hydrocortisone | SEDS | – | Acetone, MeOH | Inhalation powders | [320] |
| Insulin | SAS | Mannitol | DMSO | Pulmonary delivery | [93b] |
| SCFD | Trimethyl chitosan, Dextran | [321] | |||
| SAS | – | HFIP | Inhalation powders | [121a] | |
| GAS | – | DMF, DMSO | [28] | ||
| Ipratropium bromide | ASES | – | DMF, EtOH | Pulmonary delivery | [129,322] |
| Ketoprofen | SFEE | Starch | EtOH | Aerogels | [128] |
| Levofloxacin hydrochloride | SAA-HCM | – | MeOH | Pulmonary delivery | [323] |
| Lysozyme | SAS | – | DMSO | Aerosol delivery | [93c] |
| PCA | PLA | DCM | Pulmonary delivery | [324] | |
| SEDS | – | DMSO | [94] | ||
| SAA-HCM | – | EtOH, Water | [325] | ||
| SAS-EM | – | DMSO | Controlled release | [93a] | |
| Miconazole | ASES | Cholesterol, Phospolipids, Poloxamer 407 | DCM, MeOH | Pulmonary delivery | [311] |
| Nalmefene | SAS | – | EtOH | Pulmonary delivery | [21a,326] |
| Naproxen | ASES | HP-β-CD, Methyl-β-CD | Acetone, DMSO, EtOH | Pulmonary delivery | [103c] |
| CAN-BD | – | EtOH | [47] | ||
| Nicotinic acid | SEDS | – | MeOH | Pulmonary delivery | [327] |
| pDNA | SEDS | Mannitol | Isopropanol, Water | Inhalation powders | [122] |
| SCP | Chitosan, Mannitol | EtOH, Water | [109,132] | ||
| SFEE | PLGA | EA, Water | Pulmonary delivery | [328] | |
| Prednisolone | ASES | – | DCM, MeOH | Pulmonary delivery | [64] |
| Rifampicin | SAS | PLA | DCM | Inhalation powders | [59a] |
| – | DMSO | [120g] | |||
| SAA | – | MeOH | [329] | ||
| RNA | SCP | Chitosan, Mannitol | EtOH | Inhalation powders | [119] |
| Salbutamol | SAS | – | DMSO, MeOH, EtOH | Pulmonary delivery | [60] |
| SCP | – | Menthol | Inhalation powders | [125,330] | |
| Salmeterol xinafoate | SEDS | – | Acetone, MeOH, THF | Inhalation powders | [331] |
| Terbutaline | SAA | – | Water | Aerosol delivery | [120d] |
| ASES | – | DMF, EtOH | Pulmonary delivery | [129] | |
| SEDS | α-Lactose monohydrate | EtOH, MeOH, Water | Inhalation powders | [120e] | |
| Tetracycline | SAS | – | NMP | Pulmonary delivery | [120f] |
| SFED | – | EtOH, Water | Inhalation powders | [53] | |
| SAA | – | Water | [329] | ||
| Triamcinolone acetonide | ASES | – | DCM, MeOH | Inhalation powders | [64] |
Abbreviations: 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), 2,2,2-Trifluoroethanol (TFE), Aerosol solvent extraction system (ASES), Atomized rapid injection solvent extraction process (ARISE), Bovine serum albumin (BSA), Carbon dioxide-assisted nebulization with a bubble dryer (CAN-BD), Cyclodextrin (CD), Dichloromethane (DCM), Dimeth-ylformamide (DMF), Dimethylsulfoxide (DMSO), Ethanol (EtOH), Ethyl acetate (EA), Gas anti-solvent crystallization technique (GAS), Hydroxypropyl-β-cyclodextrin (HP-β-CD), Metered dose inhaler (MDI), Methanol (MeOH), N-methylpyrrolidone (NMP), Particle formation from gas saturated solutions (PGSS), Poly(lactide-co-glycolide) (PLGA), Polylactic acid (PLA), Polyvinylpyrrolidone (PVP), Precipitation with Compressed anti-solvent (PCA), Rapid expansion of supercritical solutions (RESS), Solution-enhanced dispersion by supercritical fluids (SEDS), Supercritical anti-solvent (SAS), Supercritical anti-solvent with enhanced mass transfer (SAS-EM), Supercritical-assisted atomization (SAA), Supercritical-assisted atomization-hydrodynamic cavitation mixer (SAA-HCM), SCF-assisted spray drying (SASD), SCF-assisted drying (SCFD), SCF-expansion depressurization (SFED), SCF-assisted extraction of emulsions (SFEE), SCF-pressure-quench technology (SCF-PQT), SCF-assisted processing (SCP), Tetrahydrofuran (THF).
DPIs are one of the most used inhalation formulations, and their clinical performance completely depends on the inspiratory flow of a patient.[17,23a,f,p] DPIs prepared using SCF are less susceptible to flow patterns than others. Further, the formulation is tolerant to physical stress, but the particles can break down upon inhalation.[125] During the processing of proteins for inhalation formulation, conditions should be optimized in the SCF technology to yield the end product with no significant change in protein conformation.[23a,126] In addition, poorly soluble proteins are also facilitated to increase their solubility in the supercritical atmosphere, suggesting the new path for bioavailability improvement in formulating protein powders. Most of the protein inhalation formulations are prepared by the SAS process, where SCF acts as an anti-solvent and an organic solvent is chosen for protein precipitation.[28,93b,c,121a] DMSO is one the most commonly used organic solvents to disperse proteins, because of its ability of expansion with pressure.[93b,127] DMSO usually disrupts the protein conformation; however, proteins such as lysosome and trypsin refold on rehydration of inhaled powders.[23a] The resultant protein product is very fine, uniform, and discrete particles (<4 μm in size) with relatively insensitive morphology and no loss of bioactivity at the varied conditions of temperature and pressure. Similar to SAS, the SFEE process also results in the formation of uniform crystalline drug particles for inhalation formulation and helpful in coating of microparticles to prevent agglomeration, which is a serious consequence in conventional coating techniques.[128]
In a comparative study involving powder formulation of salmon calcitonin (sCT) from both the conventional SD and the innovative SASD methods to investigate the role of CO2 in the particle formation process,[51] Various formulations were designed with both the methods utilizing stabilizer (inulin, trehalose) and absorption enhancers (chitosan, sodium taurocholate, β-CD) (Figure 6). Particle size distribution of the SASD process was in a nanometric range, whereas SD resulted in >2 μm in size on average in all the formulations, and failed to detect the size of pure sCT particles accurately by zetasizer due to its poor dispersibility. The particle formation in SASD involves the origination of secondary droplets from the first droplets, which are generated by atomization through a nozzle. These secondary particles are the resultant droplets due to the rapid expansion of CO2 within the primary droplets (decompressive atomization). In vivo absorption capacity of SASD-processed sCT in rats showed much higher nasal absorption than unprocessed and SD-processed sCT, demonstrating that the preparation of fine nasal powder with a proper absorption enhancer using the SCF process could be a promising approach.[51]
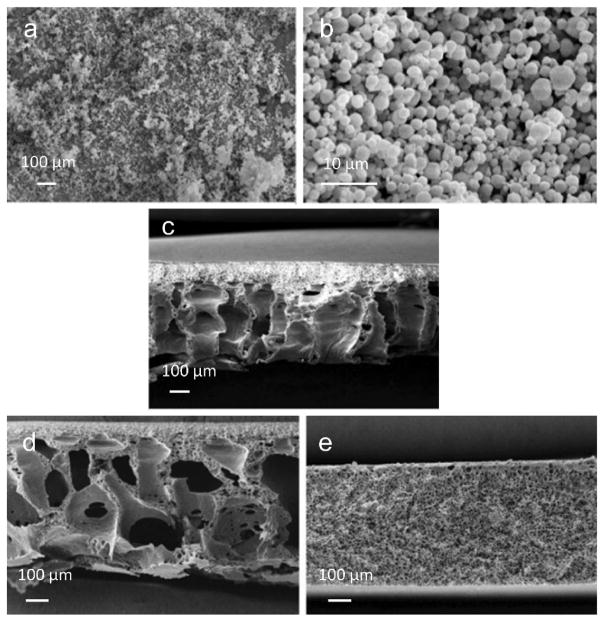
Figure 6.
SEM images of (a) raw salmon calcitonin, (b) raw inulin, (c) raw trehalose, formulations prepared by (d,e) SD method, and (f, g) SASD method. Reproduced with permission.[51] Copyright 2015, Elsevier.
Optimization of the physical parameters for better performance of inhalation powders is still under development.[129] Few alternatives were discussed to improve the performance, for example, different constituents (polymer and drug) in a formulation have different solubility rate, which can be addressed by optimizing the amounts and solvent admixture preparation.[130] Furthermore, the addition of lower-molecular weight PEG can enhance the drug release because of its dissolution and concomitant pore formation.[131] Moreover, albumin addition can affect the particle morphology, which results in aerosolization improvement and consistency in lung deposition.[129]
Genes have also been formulated using the SCF technology (Table 4) for better stability during preparation, storage, and delivery[109,122,132] via the pulmonary route to avoid degradation in blood, and reduce the dosage.[23a,109] Eventually, the SCF technology is anticipated to be successful in fabricating various drug, protein, and gene formulations for pulmonary delivery.
3.4. Transdermal Delivery
The transdermal delivery has been a major route of drug administration in pharmaceutics, due to advantages such as pain-free self-administration, minimal frequency of dosing, and avoidance of hepatic first-pass metabolism by escaping from various metabolic enzymes.[3,133] Nevertheless, it is yet to be an alternative to oral or parenteral delivery since it is limited to delivery of drugs with narrow therapeutic ranges.[133] Many synthetic polymers such as PE, PLA, PLGA, poly(urethanes) (PU), polycaprolactone (PCL), poly(acrylonitrile) (PAN), silicone rubbers, and natural polymers (such as cellulose, chitosan, alginate, collagen, and gelatin) are utilized to prepare these topical delivery systems.[133,134] Although polymeric carriers have shown potential in delivering drugs, the penetrating ability of the therapeutic molecules is still limited. Further, this delivery system has progressed with very few active strategies to deliver therapeutic moieties and cosmetic application[135] for enhanced percutaneous permeation studies including electroporation,[136] iontophoresis,[137] microneedle,[138] and ultrasound pretreatment[139] and others.[133] However, these methods have their own limitations such as inflammation in the microneedles technique and skin rupture due to electric field associated devices such as electroporation.[61c]
Bioactive molecule impregnation using SCF holds several advantages over conventional processing systems such as high drug diffusivity into a matrix, high solubility and plasticizing effect, reduction of residual solvent, and uniform particle size distribution.[140] Chen et al. proposed a synergistic approach through the combination of methotrexate-loaded silk fibroin (SF) magnetic nanoparticles using the SpEDS process with stationary/alternating magnetic fields to achieve transdermal drug delivery.[61c] Upon supersaturation, the SF polymer precipitated on methotrexate-deposited Fe3O4 nanoparticles, and eventually, the permeation flux of drug was significantly enhanced under the influence of an applied magnetic field (Figure 7). This altered stationary and alternating magnetic field acted as the massage-like effect on the skin, which would significantly broaden the application of transdermal drug-delivery systems. Further advances in its manufacturing process are under progress.[61c]
Figure 7.
The experimental setup of permeation studies. a) Diffusion cell with the stationary magnetic field. b) Diffusion cell with the alternating magnetic field. c) Diffusion cell with stationary/alternating magnetic fields. Reproduced with permission.[61c] Copyright 2015, Dove Press.
3.5. Polymeric Implants
Despite its efficacy, the systemic administration is not the first choice of treatment for all types of ailments, since few of them depend on the intensity or type of the treatment, physical properties, pharmacokinetics, as well as toxicity profile of the drug and patient compliance.[69] Drug administration using medical implants with improved results has partially overcome the limitations associated with systemic routes of drug administration.[69] Implants are the medical devices pre-loaded with APIs and surgically mounted in the body for long-term therapy to avoid post-operative complications.[23c] Several traditional manufacturing processes have been developed to produce drug-eluting implants such as hot-melt extrusion and solvent casting.[23c] However, these suffer from few limitations such as high processing temperature and large amounts of organic solvent utilization. To minimize these drawbacks, the SC-CO2-assisted impregnation process has garnered strong attention in manufacturing the drug-eluting implants.[141] The SC-CO2-assisted impregnation process takes the advantages such as good solvating power and high diffusivity properties of SCF.[17] In this process, SC-CO2 is first injected into a reactor to solubilize the drug and then CO2 plus drug are allowed to contact polymer for impregnation of drug (Figure 8). Various factors such as solubility of the drug in CO2, absorption of SCF into the polymer, and affinity between drug and polymer influence the drug loading process may affect the properties of the final products.[23c] Numerous APIs (drugs, genes, or proteins) have been packed into various biocompatible polymeric reservoirs (PLA, PLGA, PCL, poly(methyl methacrylate) (PMMA), and others), chitosan derivatives, and silicone-based copolymers (Table 5).[68,142] These implants have considerable mechanical strengths and have been envisioned for various biomedical applications such as ophthalmic and other implantable reservoirs to increase the bioavailability of the drugs.[68,69,141–143]
Figure 8.
Graphical illustration of drug impregnation into polymeric implants (SCL-Soft contact lens, CI- Conjunctival implants) by using SCF technology.
Table 5.
Implantable drug delivery systems synthesized using various methods of preparation with the SCF technology.
| Pharmaceutical compound | SCF Process | Polymer | Solvent | Purpose | Reference |
|---|---|---|---|---|---|
| Acetazolamide | SSI | Silicone-based hydrogels | EtOH, Water | Ophthalmic delivery | [143a] |
| Cefuroxime sodium | SSI | PMMA | EtOH | Ophthalmic delivery | [142a] |
| Ciprofloxacin | SSI | P-HEMA | EtOH | Extended ocular delivery | [143b] |
| Dexamethasone | SSI, SFE | SCL | EtOH | Extended ocular delivery | [68] |
| SSI | P-HEMA | - | Ophthalmic delivery | [143b] | |
| Flurbiprofen | SSI | SCL | EtOH | Ophthalmic delivery | [143c] |
| SSI, SFE | SCL | - | Extended ocular delivery | [68] | |
| SSI | Chitosan derivatives | - | Ophthalmic delivery | [142c] | |
| SCP | P(MMA-EHA-EGDMA) | - | [143d] | ||
| Ibuprofen | SSI, SFE | SCL | EtOH | Extended ocular delivery | [68] |
| Ketoprofen | SCP | Alginate, Gelatin | EtOH | Ureteral stents | [155] |
| Norfloxacin | SSI | HEMA, BEM | - | Ophthalmic delivery | [142b] |
| Paclitaxel | SCF foaming | PLGA | DCM | Post-surgical implants | [332] |
| Roxithromycin | SSI | PLA | DCM | Polymeric implants | [69] |
| Timolol maleate | SSI | SCL | EtOH | Ophthalmic delivery | [143c] |
| Chitosan derivatives | - | [142c] | |||
| Silicone-based hydrogels | - | [143a] | |||
| PCL, PCL/POE, PCL/PEVA | THF | Conjunctival implants | [141] |
Abbreviations: 2-butoxyethyl methacrylate (BEM), 2-hydroxyethyl methacrylate (HEMA), Dichloromethane (DCM), Dimethylsulfoxide (DMSO), Ethanol (EtOH), Poly(2-hydroxyethyl methacrylate) (P-HEMA), Polycaprolactone (PCL), Poly(lactide-co-glycolide) (PLGA), Poly(ethylene-vinyl acetate) (PEVA), Polylactic acid (PLA), Poly(co-ethyl hexyl acrylate-methylmethacrylate-co-ethylene glycol-dimethacrylate) (P(MMA-EHC-EGDMA)), Poly(methyl methacrylate) (PMMA), Poly(oxyethylene) (POE), Soft contact lenses (SCL), SCF-assisted processing (SCP), Supercritical freeze extraction (SFE), Supercritical solvent impregnation (SSI), Tetrahydrofuran (THF).
The SC-CO2-assisted impregnation process has been used to prepare drug-eluting implants by impregnating drugs into polymeric matrices.[23c] Among the studies that investigated the SCF-assisted impregnation process, few of them were highlighted specific to their applications. The SC-CO2-assisted impregnation process has been applied widely in preparing ocular devices such as lenses (intraocular lenses (IOL) and soft contact lenses (SCL)) as well as conjunctival implants to create implantable drug reservoirs (Figure 8).[68,142a,b,143] These reservoirs are applied to extend the residence time of the drugs in the aqueous humor, which enhance the bioavailability of the drugs.[23c] In addition, the drug loading efficiency through the SCF impregnation process is higher than the conventional aqueous soaking process due to the higher solubility of drugs in SCF compared to water.[143c] Recently, polymeric blends for implantable drug delivery were designed to create degradable subconjunctival implants, at which in vitro release experiments have shown both initial burst release for first 8 hours and progressive controlled release for a month.[141]
In addition to drug-eluting implants, few patents have been reported based on the SC-CO2-assisted impregnation process in preparation of catheters and stents with antibacterial and anti-fungal drugs.[23c] Another promising application of this SCF process can be expected in the development of polymeric endoprostheses such as hip and knee prosthesis.[23c]
4. Tissue Engineering
Tissue engineering has attracted significant attention since the conception of the field due to the increase in the demand for organ replacement therapies and a shortage of donor organs.[144] This field integrates various disciplines, including but are not limited to chemistry, material science, engineering, and biology for the generation of functional tissue substitutes.[145] In addition, the tremendous progress in the past few decades has evidenced the advancements of various methods in generating three-dimensional (3D) porous scaffolds.[144] The use of these scaffolds oftentimes constitutes an important pre-requisite of tissue engineering, which are essential to repair/improve the control over the microenvironment for cell and tissue growth.[44,144,146]
Various techniques such as solvent casting–particle leaching, freeze-drying–particle leaching, thermally induced phase separation, foaming, self-assembly, compression molding, extrusion, electrospinning, sacrificial templating, and injection molding are also available to produce 3D porous scaffolds.[17,23t,144,147] However, the applications associated with some of these approaches have been limited due to the need for large amounts of organic solvents utilized during the preparation of scaffolds in many cases.[23n,147] These organic solvents may damage bioactive molecules such as growth factors during the fabrication procedure, while the residues may also subsequently affect the cells and the surrounding tissues.[33a] In addition, the solvent residues might result in the inflammatory responses after implantation.[148] Some other techniques such as self-assembly are not able to control of internal scaffold structure and achieving the microarchitecture is challenging.[147] The bioactive molecules such as proteins incorporated by an adsorption-associated freeze-drying process are remained on the surface of the scaffolds, and their release is rapid upon delivery.[8b,149] To this end, the SCF technology has attracted the attention in designing porous polymeric scaffolds, overcoming the above-mentioned disadvantages of the conventional scaffold fabrication methods. The SCF processes involve the interaction of highly dense gas with polymers, which enables precise control over the porous morphology by tuning the operation conditions and produces scaffolds upon depressurization.[33a,150] Moreover, SCF-assisted preparations lead to generation of stable scaffolds such that the structure remains unchanged during drying and the incorporated bioactive molecules such as proteins are distributed homogenous throughout the scaffold.[8b,149,151] The only minor disadvantage is that the end-products may possess denser surfaces with less interconnected porosity than the internal space, which usually has a high porosity with interconnected and open pores. However, the surface can be subsequently removed to expose the interior before application.[152]
Out of all SCFs operated, SC-CO2 is the most suitable solvent to generate porous polymeric scaffolds, since it holds strong interactions with the carbonyl groups of polymers such as PLA and PLGA.[153] Gas-foaming, and phase-inversion are the mostly used SCF techniques to prepare tissue-engineered scaffolds. In the gas-foaming method, SCF plasticizes the glassy biomaterials after saturation and results in foaming of the polymers for the formation of the porous scaffolds and sponges[154] with normal porosity. Polymers from natural (alginate,[155] chitosan[154b,156]) and synthetic (poly(methyl vinyl ether-co-maleic anhydride) (PVM-MA),[85a,154a] PLA,[152] PLGA[157]) origin and bioceramics such as HAp[158] act as a template to promote tissue growth.[23t,33a,159] This foaming of biodegradable polymers has numerous advantageous[160] and applicable in various fields of tissue engineering such as periodontal regeneration, bone formation, cartilage development, repair of nasal and auricular malformations, as artificial corneas, in ligament replacement, in tumors, and in tendon repair.[17,33a,140,161] In addition, natural deep eutectic solvents also used as enhancers in producing polymeric foams in the SCF technology.[162] In the SCF-assisted phase-inversion process, SCF acts as a non-solvent, which after in contact with polymer solution results in polymeric scaffolds through phase separation.[163] In this case, altered critical operating conditions can tailor the properties of SCF, which result in subsequent changes in the morphology and size of the porous scaffolds.[33a] After phase separation, the organic solvent is removed by flushing SCF.
Another SCF process for tissue engineering applications is the emulsion-templating method, which is effective in preparing hydrophilic porous polymeric scaffolds.[164] The pores are generated after removal of the internal phase from the oil-in-water emulsion. This platform is highly advantageous in processing heat-sensitive biomolecules over conventional approaches due to the ability to use low amounts of solvents if necessary and operation at low temperature. Herewith, SC-CO2 acts as the internal phase, which can generate well-organized interconnected pores in surfactant-polymer complex, further increase in the internal phase and surfactant concentration leads to increase in porosity and open, interconnected pores, respectively.[164] In another way, proteins are incorporated by dispersing in water and polymer in the oil phase followed by their saturation with SC-CO2, and subsequent depressurization results in porous scaffolds for controlled release of protein.[165] The double-emulsion method is preferred for creating homogenous matrix to encapsulate multiple proteins in polymeric scaffolds.[166]
Polymeric foams generated at both supercritical as well as subcritical temperatures are chosen to sinter biological moieties such as cells based on the crystalline behavior of the polymer.[33a,167] Initially, the selected polymer is subjected to compressed CO2 treatment at a constant temperature and pressure until it attains a stable swollen state. Subsequently, the pressure and/or temperature are altered, leading to phase separation and eventually pore generation. In general, different porosity range in the polymeric scaffolds is utilized for different biomedical applications.[168] For generation of pores and better interconnectivity, elutable porogens such as sodium chloride, sucrose, or other porogens with low decomposition temperature such as ammonium carbonate are added during the preparation of porous scaffold.[151] Few studies have also reported significant pore interconnectivity without using any porogen. For example, the cartilage repair was performed using poly(ethyl methacrylate)/tetrahydrofurfuryl methacrylate (PEMA/THFMA) polymeric scaffold, which possessed a significant pore interconnectivity to culture chondrocytes and to form a 3D environment for tissue generation without any porogen.[169] In addition, post-processing of solvent-free ultrasound technique also improved the interconnectivity of pores generated from gas-foamed scaffolds with increase in pore size.[170] Further, mechanical properties of the scaffolds were altered by changing the SCF processing conditions and utilized for various tissue engineering applications.[171]
More often, polymer content variation results in the change in morphology of pores yielding heterogeneous pores.[134b] In addition, various drugs have also been impregnated into polymers using SCF for the self-healing process in tissue engineering and regeneration.[134a] Interestingly, Cardea et al. demonstrated the SCF-assisted phase-inversion process for the preparation of ibuprofen-loaded cellulose acetate (CA) structures at a short processing time (Figure 9).[134b] In this study, CA-loaded structures were different with increasing polymer concentration in the starting solution. At the lower concentration, it yielded microparticles (Figure 9a,b) to a bicontinuous one with macrovoids at moderate concentration (Figure 9c,d), and to a cellular one at an extreme higher concentration (Figure 9e). Nevertheless, the ibuprofen presence had no effect on the void formation.[134b] In addition, from the drug release point of view, the pore size of the CA structures was indirectly proportional to the pressure applied, where the high pressure resulted in small pore size and eventually controlled the drug release and vice versa.
Figure 9.
CA structures loaded with ibuprofen at 10% w/w, obtained at 250 bars and 35 °C, starting from different polymer concentrations. a–b) 5% w/w, c) 10% w/w, d) 15% w/w, and e) 20% w/w. Reproduced with permission.[134b] Copyright 2016, Elsevier.
A work from Duarte et al. addressed the preparation of bio-compatible polymeric scaffolds made of blends of natural and synthetic polymer using the SCF technology, which mimic the functions of extracellular matrix (ECM).[172] These biocompatible scaffolds are available in the form of the 3D porous matrix, non-fibrous matrix, polymeric hydrogel, or porous micro-sphere, which enables cell attachment throughout the space in the matrix and promotes its integration with the host tissue with a potential of rapid angiogenesis facilitated by cell migration and nutrient transfer.[153a] These scaffolds prepared by the SCF-based technology tend to show biological acceptance and function as a temporary support for the tissue regeneration by preserving the ability of cells to proliferate, as well as controlling cell function, growth, reorganization, and possibly neovas cularization.[33a,158,173] Apart from cell attachment, these porous scaffolds prepared by SCF processes are also utilized to release precise amounts of guest species[23t] i.e., essential growth factors such as vascular endothelial growth factor (VEGF),[174] transforming growth factor-beta 3 (TGF-β3),[175] basic fibroblast growth factor (bFGF),[176] and others.[177] All these preparations by homogeneously incorporating various signaling moieties promote cell infiltration, adhesion, migration, expansion, and differentiation.[33a,178]
5. Bio-Imaging
Bio-imaging allows visualization of biological structures as well as functional analysis often taking advantage of contrast agents.[20e] Since tracing of a targeted system is a prolonged course, inorganic constructs are most preferred to organic molecules because of their long biological half-lives. For example, phosphor-containing nanoparticles such as those made of rare earth phosphates and semiconductor nanoparticles such as quantum dots have attracted attention, since their optical emission wavelengths can be conveniently controlled by compositions and particle sizes.[20e,179] Inorganic metal oxides have also garnered potential interest for use as contrast agents and others, whose application potential is dictated by their surface, particle size, and shape.[20e] The supercritical hydrothermal process has been used for the generation of metal oxide nanoparticles such as those of zinc oxide (ZnO), iron oxide (Fe3O4), titanium oxide (TiO2), gadolinium vanadate (GdVO4), and others, for bio-imaging purposes. Surface modification is preferred either by immobilizing various organic or heat-labile carriers for better performance.[20e,180]
Out of various metal oxides obtained by the SCF technology, magnetic (iron oxide) nanocarriers have profoundly attracted the attention in numerous biomedical applications such as magnetic resonance imaging (MRI) contrast agents[20e] and targeted drug delivery purposes.[30,61c,79] Although in its infancy, SCF-assisted nanoparticle preparation is anticipated to possess widespread biomedical applications.
6. Miscellaneous Applications
Apart from the generation of drug delivery carriers and 3D porous scaffolds for various biomedical applications, the SCF technology can also be utilized in other applications during pharmaceutical processing such as coating of delivery systems, sterilization of products, and extraction of solvents and active gradients. In this section, we present a brief overview on these aspects.
The process of coating of any drug delivery system is to mask its unpleasant taste or odor, to control the delivery site, and to protect the formulation both physically and chemically.[181] In a conventional process, the coating material is dissolved in either water or an organic solvent and is eventually sprayed over the particles. This procedure has its own disadvantages such as long drying time, chances of agglomeration, limited solubility of coating substance, toxicity and inflammability of the residual organic solvents.[182] The organic solvent-free SCF technology thus has the capability to coat various APIs to improve the hydrophobicity of certain moisture-sensitive biodegradable materials.[181] A fluidized bed coating process has been applied based on a multi-step RESS process through optimization of various parameters such as temperature, pressure, and solidification of coating material on the surface, which play key roles in an effective coating strategy.[181] This process is highly advantageous to coat sensitive materials such as proteins.[183]
Another application of SCF is sterilization, which is already in practice in the food, pharmaceutical, and biotechnological industries where contamination is a major concern.[184] Since sterilization is a preliminary concern of these fields, it is mandated to ensure that every component such as glassware, raw materials, and final products is free from contamination. In addition to traditional methods of sterilization, SCF has been utilized to inactivate a wide variety of microbes and their spores providing suitable conditions (14–21 MPa, 30–45 °C for 0.6–4 h). The inactivation of microbes involves a series of mechanisms in disrupting the integrity of the cells. Unique mass transfer property of SCF creates an ability to enter the cells by extracting cell wall lipids, further damaging cell membrane and inducing cytoplasmic pH change and metabolic enzyme inactivation intracellularly.[184]
SCF leads to the efficient green synthesis of biodegradable polymers, ideal for pharmaceutical applications. In few instances, high productivity of polymers has been evidenced by using various metal catalysts and organic solvents.[100b] Extraction of these co-solvents, however, is critical during the processing of the porous scaffolds for subsequent applications in drug delivery or tissue engineering due to that the trace amounts can significantly affect the tissue response and mechanical properties of the scaffolds. SCF is advantageous in removing the traces relatively quicker than the traditional vacuum drying approach with no significant alteration in the scaffold architectures.
In addition, pre-cleaning of scaffolds using SCF may also minimize the adverse responses as well as to improve the bio-compatibility. This pre-treatment does not alter any biochemical or biomechanical properties of the scaffolds, and would thus improve their integration with the surrounding tissues post-implantation through relieved immunological responses.[33a] Recently, single-step supercritical defatting and sterilization of human bone allograft powder has also been proposed.[185]
The SCF technology is also used to extract bioactive ingredients such as polyphenols, terpenoids from the natural products and is potentially more advantageous over conventional methods, which usually involve the usage of organic solvents.[186] The advancement of the SCF-assisted extraction processes and examples discussing the pharmacological benefits of the active moieties were compiled by da Silva et al.[187] These mild operating conditions provided by SCF are more appropriate to recover various sensitive ingredients.[188] Further, the hybridization of impregnation to the supercritical extraction process resulted in the high encapsulation efficiency of extracted active moieties.[188] So far, SCF extraction of solvent is under practice in various fields such as food processing, agriculture, and others.[189]
7. Conclusions and Remarks
In summary, this critical review has highlighted and discussed various SCF processes in producing controlled drug release carriers such as micro- as well as nano-sized polymeric particles, liposomes and CDs for the application focusing oral, and pulmonary and transdermal routes of administration. These polymeric carriers produced by the eco-friendly SCF technology are advantageous over those fabricated by conventional methods of preparation with high efficiency. In addition, we also gave an overview of SCF-assisted preparation of products intended for related biomedical applications such as tissue engineering and bio-imaging, among others.
Despite its success at laboratory scale, the applications of the SCF technology at industrial scale is still in infancy due to the lack of fundamental studies accurately describing the phase behavior of the multi-component mixtures including biodegradable complex compounds. Recently, steps have been taken for commercialization of these processes for pharmaceutical application. Pierre Fabre CDMO Supercritical Fluids, a France-based SC-CO2 GMP unit for pharmaceutical applications, has been performing pre-formulation studies of various APIs through patented processes such as Formulcoat, Fomulplex, and Formuldisp. However, no established marketed product manufactured by the SCF technology is yet available to date.
We anticipate that integrating SCF with a conventional process such as spray drying or others may result in the advancement of the carriers. It may open a new paradigm in the field of pharmaceutical science to reduce the complexity of manufacturing process and a better understanding of the product behavioral characteristics and performance. It is anticipated that with further optimization, the SCF technology can create novel opportunities not only in laboratory research but also for the industrial processing of delivery systems in the future.
Acknowledgments
A.Z.C. acknowledges financial support from National Natural Science Foundation of China (U1605225, 31570974 and 31470927), Public Science and Technology Research Funds Projects of Ocean (201505029), and Promotion Program for Young and Middle-aged Teacher in Science and Technology Research of Huaqiao University (ZQN-PY107). Y.S.Z. acknowledges the National Cancer Institute of the National Institutes of Health Pathway to Independence Award (K99CA201603) and the Lush Prize. R.K.K. acknowledges financial support from Huaqiao University (Project No. 16BS803). The copyright line for this article was changed on August 23rd after original online publication.
Biographies

Ranjith K. Kankala is an Assistant Professor in the Institute of Biomaterials and Tissue Engineering at Huaqiao University, P. R. China. He received his B.S. and M.S. degrees in Pharmaceutical Sciences from Kakatiya University, India in 2009 and 2012, respectively, and earned his Ph.D. degree in 2016 in the field of nano-medicine from the National Dong Hwa University, Taiwan. His expertise relates to the preparation of cutting-edge multifunctional nanocomposites and engineered inorganic nano-/micro- hybrid systems with optimized properties for innovative biomedical applications.

Yu Shrike Zhang received his B.Eng. degree in biomedical engineering in 2008 from Southeast University, P. R. China and Ph.D. degree in biomedical engineering in 2013 from Georgia Institute of Technology. He is currently an Instructor of Medicine and Associate Bioengineer in the Division of Engineering in Medicine at the Brigham and Women’s Hospital, Harvard Medical School. His research interests include biomaterials, bioprinting, organs-on-chips, medical devices, biomedical imaging, and bio-sensing, with a focus on innovating medical engineering technologies to recreate functional biomimetic tissues.

Ai-Zheng Chen received his Ph.D. in Biomedical Engineering from Sichuan University in 2007. After completing the postdoctoral research at The Hong Kong Polytechnic University, he joined Huaqiao University, where he is now a professor at College of Chemical Engineering and Director of Institute of Biomaterials and Tissue Engineering. He was a visiting research professor for one year in Prof. Ali Khademhosseini Laboratory at Harvard Medical School. His research interests include supercritical fluid technology, biomaterials, and tissue engineering.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/adhm.201700433.
Contributor Information
Dr. Ranjith Kumar Kankala, College of Chemical Engineering, Huaqiao University, Xiamen 361021, P. R. China. Institute of Biomaterials and Tissue Engineering, Huaqiao University, Xiamen 361021, P. R. China. Fujian Provincial Key Laboratory of Biochemical Technology, Xiamen 361021, P. R. China
Dr. Yu Shrike Zhang, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA
Prof. Shi-Bin Wang, College of Chemical Engineering, Huaqiao University, Xiamen 361021, P. R. China. Institute of Biomaterials and Tissue Engineering, Huaqiao University, Xiamen 361021, P. R. China. Fujian Provincial Key Laboratory of Biochemical Technology, Xiamen 361021, P. R. China
Prof. Chia-Hung Lee, Department of Life Science and Institute of Biotechnology, National Dong Hwa University, Hualien 97401, Taiwan
Prof. Ai-Zheng Chen, College of Chemical Engineering, Huaqiao University, Xiamen 361021, P. R. China. Institute of Biomaterials and Tissue Engineering, Huaqiao University, Xiamen 361021, P. R. China. Fujian Provincial Key Laboratory of Biochemical Technology, Xiamen 361021, P. R. China. Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA
References
- 1.Kompella UB, Koushik K. Crit Rev Ther Drug Carrier Syst. 2001;18:173. [PubMed] [Google Scholar]
- 2.Hauthal WH. Chemosphere. 2001;43:123. doi: 10.1016/s0045-6535(00)00332-5. [DOI] [PubMed] [Google Scholar]
- 3.Pasquali I, Bettini R. Int J Pharm. 2008;364:176. doi: 10.1016/j.ijpharm.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 4.a) Langer R. Science. 1990;249:1527. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]; b) De Jong WH, Borm PJ. Int J Nanomedicine. 2008;3:133. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Dahan A, Miller JM. AAPS J. 2012;14:244. doi: 10.1208/s12248-012-9337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ginty PJ, Whitaker MJ, Shakesheff KM, Howdle SM. Mater Today. 2005;8:42. [Google Scholar]
- 6.Kalani M, Yunus R. Int J Nanomedicine. 2011;6:1429. doi: 10.2147/IJN.S19021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Savjani KT, Gajjar AK, Savjani JK. ISRN Pharmaceutics. 2012;2012:195727. doi: 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Chem Rev. 1999;99:3181. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 8.a) Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Annu Rev Chem Biomol Eng. 2010;1:149. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yeo SD, Kiran E. J Supercrit Fluids. 2005;34:287. [Google Scholar]
- 9.a) Cocero MJ, Martín Á, Mattea F, Varona S. J Supercrit Fluids. 2009;47:546. [Google Scholar]; b) Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Angew Chem Int Ed. 2014;53:12320. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]; c) Kankala RK, Kuthati Y, Sie HW, Shih HY, Lue SI, Kankala S, Jeng CC, Deng JP, Weng CF, Liu CL, Lee CH. J Colloid Interface Sci. 2015;458:217. doi: 10.1016/j.jcis.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 10.Kankala RK, Tsai PY, Kuthati Y, Wei PR, Liu CL, Lee CH. J Mater Chem B. 2017;5:1507. doi: 10.1039/c6tb03146c. [DOI] [PubMed] [Google Scholar]
- 11.Gref R, Minamitake Y, Peracchia M, Trubetskoy V, Torchilin V, Langer R. Science. 1994;263:1600. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 12.Vemavarapu C, Mollan MJ, Lodaya M, Needham TE. Int J Pharm. 2005;292:1. doi: 10.1016/j.ijpharm.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 13.a) Elizondo E, Veciana J, Ventosa N. Nanomedicine. 2012;7:1391. doi: 10.2217/nnm.12.110. [DOI] [PubMed] [Google Scholar]; b) Fahim TK, Zaidul ISM, Abu Bakar MR, Salim UM, Awang MB, Sahena F, Jalal KCA, Sharif KM, Sohrab MH. Chem Eng Process. 2014;86:47. [Google Scholar]; c) Yim JH, Kim WS, Lim JS. J Supercrit Fluids. 2013;82:168. [Google Scholar]
- 14.Kang Y, Wu J, Yin G, Huang Z, Yao Y, Liao X, Chen A, Pu X, Liao L. Eur J Pharm Biopharm. 2008;70:85. doi: 10.1016/j.ejpb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Chen A-Z, Li Y, Chau F-T, Lau T-Y, Hu J-Y, Zhao Z, Mok DK-w. Acta Biomater. 2009;5:2913. doi: 10.1016/j.actbio.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 16.a) Wang Y, Dave RN, Pfeffer R. J Supercrit Fluids. 2004;28:85. [Google Scholar]; b) Fages J, Lochard H, Letourneau JJ, Sauceau M, Rodier E. Powder Technol. 2004;141:219. [Google Scholar]; c) Wu K, Li J. J Supercrit Fluids. 2008;46:211. [Google Scholar]
- 17.Davies OR, Lewis AL, Whitaker MJ, Tai H, Shakesheff KM, Howdle SM. Adv Drug Del Rev. 2008;60:373. doi: 10.1016/j.addr.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 18.a) Zhan S, Chen C, Zhao Q, Wang W, Liu Z. Ind Eng Chem Res. 2013;52:2852. [Google Scholar]; b) Wais U, Jackson AW, He T, Zhang H. Nanoscale. 2016;8:1746. doi: 10.1039/c5nr07161e. [DOI] [PubMed] [Google Scholar]
- 19.Djerafi R, Masmoudi Y, Crampon C, Meniai A, Badens E. J Supercrit Fluids. 2015;105:92. [Google Scholar]
- 20.a) Goodey J, Min Ok K, Broussard J, Hofmann C, Escobedo FV, Halasyamani PS. J Solid State Chem. 2003;175:3. [Google Scholar]; b) Hayashi H, Torii K. J Mater Chem. 2002;12:3671. [Google Scholar]; c) Li G, Smith RL, Jr, Inomata H, Arai K. Mater Lett. 2002;53:175. [Google Scholar]; d) Reverchon E, Adami R. J Supercrit Fluids. 2006;37:1. [Google Scholar]; e) Byrappa K, Ohara S, Adschiri T. Adv Drug Del Rev. 2008;60:299. doi: 10.1016/j.addr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 21.a) Hakuta Y, Hayashi H, Arai K. Curr Opin Solid State Mater Sci. 2003;7:341. [Google Scholar]; b) Meziani MJ, Rollins HW, Allard LF, Sun YP. J Phys Chem B. 2002;106:11178. [Google Scholar]; c) Warwick B, Dehghani F, Foster NR, Biffin JR, Regtop HL. Ind Eng Chem Res. 2002;41:1993. [Google Scholar]; d) Kröber H, Teipel U. J Supercrit Fluids. 2002;22:229. [Google Scholar]
- 22.Perry RH, Green DW. Perry’s Chemical Engineers’ Handbook. McGraw-Hill Professional; New York, USA: 1997. [Google Scholar]
- 23.a) Okamoto H, Danjo K. Adv Drug Del Rev. 2008;60:433. doi: 10.1016/j.addr.2007.02.002. [DOI] [PubMed] [Google Scholar]; b) Mishima K. Adv Drug Del Rev. 2008;60:411. doi: 10.1016/j.addr.2007.02.003. [DOI] [PubMed] [Google Scholar]; c) Champeau M, Thomassin JM, Tassaing T, Jérôme C. J Control Release. 2015;209:248. doi: 10.1016/j.jconrel.2015.05.002. [DOI] [PubMed] [Google Scholar]; d) Sharif KM, Rahman MM, Azmir J, Mohamed A, Jahurul MHA, Sahena F, Zaidul ISM. J Food Eng. 2014;124:105. [Google Scholar]; e) Pathak P, Meziani MJ, Sun YP. Expert Opin Drug Deliv. 2005;2:747. doi: 10.1517/17425247.2.4.747. [DOI] [PubMed] [Google Scholar]; f) Pilcer G, Amighi K. Int J Pharm. 2010;392:1. doi: 10.1016/j.ijpharm.2010.03.017. [DOI] [PubMed] [Google Scholar]; g) Boyère C, Jérôme C, Debuigne A. Eur Polym J. 2014;61:45. [Google Scholar]; h) Martín A, Cocero MJ. Adv Drug Del Rev. 2008;60:339. doi: 10.1016/j.addr.2007.06.019. [DOI] [PubMed] [Google Scholar]; i) Patil YP, Jadhav S. Chem Phys Lipids. 2014;177:8. doi: 10.1016/j.chemphyslip.2013.10.011. [DOI] [PubMed] [Google Scholar]; j) Yongda S. Curr Pharm Des. 2015;21:2516. doi: 10.2174/1381612821666150416100201. [DOI] [PubMed] [Google Scholar]; k) Pratik S, Harpreet S, Dharmendra S, Waseem M, Navnit S, Kislalioglu MS. Curr Drug Del. 2012;9:269. [Google Scholar]; l) Lima AC, Sher P, Mano JF. Expert Opin Drug Deliv. 2012;9:231. doi: 10.1517/17425247.2012.652614. [DOI] [PubMed] [Google Scholar]; m) Naylor A, Lewis AL, Ilium L. Ther Deliv. 2011;2:1551. doi: 10.4155/tde.11.125. [DOI] [PubMed] [Google Scholar]; n) Duarte ARC, Mano JF, Reis RL. J Bioact Compatible Polym. 2009;24:385. [Google Scholar]; o) Garg T, Goyal AK. Expert Opin Drug Deliv. 2014;11:767. doi: 10.1517/17425247.2014.891014. [DOI] [PubMed] [Google Scholar]; p) Shoyele SA, Cawthorne S. Adv Drug Del Rev. 2006;58:1009. doi: 10.1016/j.addr.2006.07.010. [DOI] [PubMed] [Google Scholar]; q) Zhang A, Zhang Q, Bai H, Li L, Li J. Chem Soc Rev. 2014;43:6938. doi: 10.1039/c4cs00100a. [DOI] [PubMed] [Google Scholar]; r) Esfandiari N. J Supercrit Fluids. 2015;100:129. doi: 10.1016/j.supflu.2022.105539. [DOI] [PMC free article] [PubMed] [Google Scholar]; s) Chattopadhyay P, Shekunov BY, Yim D, Cipolla D, Boyd B, Farr S. Adv Drug Del Rev. 2007;59:444. doi: 10.1016/j.addr.2007.04.010. [DOI] [PubMed] [Google Scholar]; t) Bhamidipati M, Scurto AM, Detamore MS. Tissue Eng Part B Rev. 2013;19:221. doi: 10.1089/ten.teb.2012.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]; u) Campardelli R, Baldino L, Reverchon E. J Supercrit Fluids. 2015;101:193. [Google Scholar]; v) Pasquali I, Bettini R, Giordano F. Adv Drug Del Rev. 2008;60:399. doi: 10.1016/j.addr.2007.08.030. [DOI] [PubMed] [Google Scholar]; w) Moribe K, Tozuka Y, Yamamoto K. Adv Drug Del Rev. 2008;60:328. doi: 10.1016/j.addr.2007.03.023. [DOI] [PubMed] [Google Scholar]; x) Kiran E. J Supercrit Fluids. 2016;110:126. [Google Scholar]
- 24.Chen AZ, Zhao Z, Wang SB, Li Y, Zhao C, Liu YG. J Supercrit Fluids. 2011;59:92. [Google Scholar]
- 25.Majerik V, Charbit G, Badens E, Horváth G, Szokonya L, Bosc N, Teillaud E. J Supercrit Fluids. 2007;40:101. [Google Scholar]
- 26.Gosselin PM, Thibert R, Preda M, McMullen JN. Int J Pharm. 2003;252:225. doi: 10.1016/s0378-5173(02)00649-x. [DOI] [PubMed] [Google Scholar]
- 27.Sacchetin PSC, Setti RF, Ve Rosa PdT, Moraes ÂM. Mater Sci Eng, C. 2016;58:870. doi: 10.1016/j.msec.2015.09.071. [DOI] [PubMed] [Google Scholar]
- 28.Yeo SD, Lim GB, Debendetti PG, Bernstein H. Biotechnol Bioeng. 1993;41:341. doi: 10.1002/bit.260410308. [DOI] [PubMed] [Google Scholar]
- 29.Falk R, Randolph TW, Meyer JD, Kelly RM, Manning MC. J Control Release. 1997;44:77. [Google Scholar]
- 30.Chattopadhyay P, Gupta RB. Ind Eng Chem Res. 2002;41:6049. [Google Scholar]
- 31.a) Margulis K, Neofytou EA, Beygui RE, Zare RN. ACS Nano. 2015;9:9416. doi: 10.1021/acsnano.5b04137. [DOI] [PubMed] [Google Scholar]; b) Chen AZ, Wang GY, Wang SB, Feng JG, Liu YG, Kang YQ. Materials. 2012;5:1841. [Google Scholar]
- 32.a) Chen AZ, Wang GY, Wang SB, Li L, Liu YG, Zhao C. Int J Nanomedicine. 2012;7:3013. doi: 10.2147/IJN.S32662. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chen AZ, Lin XF, Wang SB, Li L, Liu YG, Ye L, Wang GY. Toxicol Lett. 2012;212:75. doi: 10.1016/j.toxlet.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 33.a) García-González CA, Concheiro A, Alvarez-Lorenzo C. Bioconjugate Chem. 2015;26:1159. doi: 10.1021/bc5005922. [DOI] [PubMed] [Google Scholar]; b) Cabrera I, Elizondo E, Esteban O, Corchero JL, Melgarejo M, Pulido D, Córdoba A, Moreno E, Unzueta U, Vazquez E, Abasolo I, Schwartz S, Villaverde A, Albericio F, Royo M, García-Parajo MF, Ventosa N, Veciana J. Nano Lett. 2013;13:3766. doi: 10.1021/nl4017072. [DOI] [PubMed] [Google Scholar]
- 34.a) Reverchon E. Ind Eng Chem Res. 2002;41:2405. [Google Scholar]; b) Shen YB, Du Z, Wang Q, Guan YX, Yao SJ. Powder Technol. 2014;254:416. [Google Scholar]
- 35.Kerč J, Srčič S, Knez Ž, Senčar-Božič P. Int J Pharm. 1999;182:33. doi: 10.1016/s0378-5173(99)00063-0. [DOI] [PubMed] [Google Scholar]
- 36.Kayrak D, Akman U, Hortaçsu Ö. J Supercrit Fluids. 2003;26:17. [Google Scholar]
- 37.Dalvi SV, Azad MA, Dave R. Powder Technol. 2013;236:75. [Google Scholar]
- 38.Thakur R, Gupta RB. Ind Eng Chem Res. 2005;44:7380. [Google Scholar]
- 39.Prosapio V, Reverchon E, De Marco I. Powder Technol. 2016;292:140. [Google Scholar]
- 40.a) Campardelli R, Adami R, Della Porta G, Reverchon E. Chem Eng J. 2012;192:246. [Google Scholar]; b) Campardelli R, Oleandro E, Reverchon E. Powder Technol. 2016;287:12. [Google Scholar]
- 41.Della Porta G, Falco N, Reverchon E. J Pharm Sci. 2010;99:1484. doi: 10.1002/jps.21920. [DOI] [PubMed] [Google Scholar]
- 42.a) Juppo AM, Boissier C, Khoo C. Int J Pharm. 2003;250:385. doi: 10.1016/s0378-5173(02)00577-x. [DOI] [PubMed] [Google Scholar]; b) Hooton JC, German CS, Allen S, Davies MC, Roberts CJ, Tendler SJ, Williams P. Pharm Res. 2003;20:508. doi: 10.1023/a:1022684911383. [DOI] [PubMed] [Google Scholar]; c) Chen AZ, Wang GY, Wang SB, Feng JG, Liu YG, Kang YQ. Materials. 2012;5 [Google Scholar]
- 43.Lévai G, Martín Á, de Paz E, Rodríguez-Rojo S, Cocero MJ. J Supercrit Fluids. 2015;100:34. [Google Scholar]
- 44.Langer R, Vacanti JP. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 45.Palakodaty S, York P, Pritchard J. Pharm Res. 1998;15:1835. doi: 10.1023/a:1011949805156. [DOI] [PubMed] [Google Scholar]
- 46.Chen AZ, Li L, Wang SB, Lin XF, Liu YG, Zhao C, Wang GY, Zhao Z. J Supercrit Fluids. 2012;67:139. [Google Scholar]
- 47.Sievers RE, Huang ETS, Villa JA, Engling G, Brauer PR. J Supercrit Fluids. 2003;26:9. [Google Scholar]
- 48.Wang Q, Guan YX, Yao SJ, Zhu ZQ. J Supercrit Fluids. 2011;56:97. [Google Scholar]
- 49.McLeod MC, McHenry RS, Beckman EJ, Roberts CB. J Phys Chem B. 2003;107:2693. [Google Scholar]
- 50.Yandrapu SK, Upadhyay AK, Petrash JM, Kompella UB. Mol Pharm. 2013;10:4676. doi: 10.1021/mp400487f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho W, Kim MS, Jung MS, Park J, Cha KH, Kim JS, Park HJ, Alhalaweh A, Velaga SP, Hwang SJ. Int J Pharm. 2015;478:288. doi: 10.1016/j.ijpharm.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 52.Sethia S, Squillante E. Int J Pharm. 2004;272:1. doi: 10.1016/j.ijpharm.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 53.Zhiyi L, Jingzhi J, Xuewu L, Yuanjing X, Shunxuan Z, Jian W. Chem Eng Process. 2008;47:1311. [Google Scholar]
- 54.Adschiri T, Hakuta Y, Sue K, Arai K. J Nanopart Res. 2001;3:227. [Google Scholar]
- 55.Tom JW, Lim G-B, Debenedetti PG, Prud’homme RK. Supercritical Fluid Engineering Science. Vol. 514. American Chemical Society; 1992. p. 238. Ch 19. [Google Scholar]
- 56.Chen A, Dang T, Wang S, Tang N, Liu Y, Wu W. Science China-Life Sciences. 2014;57:698. doi: 10.1007/s11427-014-4680-8. [DOI] [PubMed] [Google Scholar]
- 57.a) Zhao Z, Chen A, Li Y, Hu J, Liu X, Li J, Zhang Y, Li G, Zheng Z. J Nanopart Res. 2012:14. [Google Scholar]; b) Zhao Z, Li Y, Chen A-Z, Zheng Z-J, Hu J-Y, Li J-S, Li G. Ind Eng Chem Res. 2013;52:3752. [Google Scholar]
- 58.Al-Marzouqi A, Jobe B, Corti G, Cirri M, Mura P. J Incl Phenom Macrocycl Chem. 2007;57:223. [Google Scholar]
- 59.a) Patomchaiviwat V, Paeratakul O, Kulvanich P. AAPS Pharm Sci Tech. 2008;9:1119. doi: 10.1208/s12249-008-9152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Reverchon E, De Marco I. Powder Technol. 2006;164:139. [Google Scholar]
- 60.Reverchon E, Della Porta G, Pallado P. Powder Technol. 2001;114:17. [Google Scholar]
- 61.a) Chen A-Z, Pu X-M, Kang Y-Q, Liao L, Yao Y-D, Yin G-F. Macromol Rapid Commun. 2006;27:1254. [Google Scholar]; b) Huang X, Zhang Y, Yin G, Pu X, Liao X, Huang Z, Chen X, Yao Y. J Mater Sci Mater Med. 2015;26:95. doi: 10.1007/s10856-015-5447-x. [DOI] [PubMed] [Google Scholar]; c) Chen AZ, Chen LQ, Wang SB, Wang YQ, Zha JZ. Int J Nanomedicine. 2015;10:4639. doi: 10.2147/IJN.S85999. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Xie M, Fan D, Chen Y, Zhao Z, He X, Li G, Chen A, Wu X, Li J, Li Z, Hunt JA, Li Y, Lan P. Biomaterials. 2016;103:33. doi: 10.1016/j.biomaterials.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 62.a) Thakur R, Gupta RB. Int J Pharm. 2006;308:190. doi: 10.1016/j.ijpharm.2005.11.005. [DOI] [PubMed] [Google Scholar]; b) Domingo C, García-Carmona J, Fanovich MA, Llibre J, Rodríguez-Clemente R. J Supercrit Fluids. 2001;21:147. [Google Scholar]
- 63.De Zordi N, Moneghini M, Kikic I, Grassi M, Del Rio Castillo AE, Solinas D, Bolger MB. Eur J Pharm Biopharm. 2012;81:131. doi: 10.1016/j.ejpb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Steckel H, Thies J, Müller BW. Int J Pharm. 1997;152:99. [Google Scholar]
- 65.a) Kazarian SG, Martirosyan GG. Int J Pharm. 2002;232:81. doi: 10.1016/s0378-5173(01)00905-x. [DOI] [PubMed] [Google Scholar]; b) Kim JH, Paxton TE, Tomasko DL. Biotechnol Progr. 1996;12:650. [Google Scholar]; c) Gonçalves VSS, Rodríguez-Rojo S, Matias AA, Nunes AVM, Nogueira ID, Nunes D, Fortunato E, de Matos APA, Cocero MJ, Duarte CMM. Int J Pharm. 2015;478:9. doi: 10.1016/j.ijpharm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Fattahi A, Karimi-Sabet J, Keshavarz A, Golzary A, Rafiee-Tehrani M, Dorkoosh FA. J Supercrit Fluids. 2016;107:469. [Google Scholar]
- 67.Casettari L, Castagnino E, Stolnik S, Lewis A, Howdle SM, Illum L. Pharm Res. 2011;28:1668. doi: 10.1007/s11095-011-0403-z. [DOI] [PubMed] [Google Scholar]
- 68.Yañez F, Martikainen L, Braga MEM, Alvarez-Lorenzo C, Concheiro A, Duarte CMM, Gil MH, de Sousa HC. Acta Biomater. 2011;7:1019. doi: 10.1016/j.actbio.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Yu JP, Guan YX, Yao SJ, Zhu ZQ. Ind Eng Chem Res. 2011;50:13813. [Google Scholar]
- 70.a) Pathak P, Meziani MJ, Desai T, Sun YP. J Supercrit Fluids. 2006;37:279. [Google Scholar]; b) Murillo-Cremaes N, López-Periago AM, Saurina J, Roig A, Domingo C. J Supercrit Fluids. 2013;73:34. [Google Scholar]
- 71.Baldelli A, Boraey MA, Nobes DS, Vehring R. Mol Pharm. 2015;12:2562. doi: 10.1021/mp500758s. [DOI] [PubMed] [Google Scholar]
- 72.Debenedetti PG, Tom JW, Sang-Do Y, Gio-Bin L. J Control Release. 1993;24:27. [Google Scholar]
- 73.Calderon Moreno JM, Yoshimura M. J Am Chem Soc. 2001;123:741. doi: 10.1021/ja003008h. [DOI] [PubMed] [Google Scholar]
- 74.Tien YC, Su CS, Lien LH, Chen YP. J Supercrit Fluids. 2010;55:292. [Google Scholar]
- 75.Chong GH, Yunus R, Choong TSY, Abdullah N, Spotar SY. J Supercrit Fluids. 2011;60:69. [Google Scholar]
- 76.Temtem M, Barroso T, Casimiro T, Mano JF, Aguiar-Ricardo A. J Supercrit Fluids. 2012;66:398. [Google Scholar]
- 77.Baxendale AJ, van Hooff P, Durrant LG, Spendlove I, Howdle SM, Woods HM, Whitaker MJ, Davies OR, Naylor A, Lewis AL, Illum L. Int J Pharm. 2011;413:147. doi: 10.1016/j.ijpharm.2011.04.053. [DOI] [PubMed] [Google Scholar]
- 78.a) Zhao Z, Li Y, Zhang Y, Chen A-Z, Li G, Zhang J, Xie M-B. Powder Technol. 2014;268:118. [Google Scholar]; b) Chen AZ, Kang YQ, Wang SB, Tang N, Su XQ. J Mater Chem B. 2015;3:6439. doi: 10.1039/c5tb00715a. [DOI] [PubMed] [Google Scholar]
- 79.Fuchigami T, Kawamura R, Kitamoto Y, Nakagawa M, Namiki Y. Biomaterials. 2012;33:1682. doi: 10.1016/j.biomaterials.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 80.Zhao X, Wang D, Zu Y, Jiang R, Zhao D, Li Y, Zu B, Sun Z, Zhang Q. J Control Release. 2011;152:e90. doi: 10.1016/j.jconrel.2011.08.143. [DOI] [PubMed] [Google Scholar]
- 81.a) Van Nijlen T, Brennan K, Van den Mooter G, Blaton N, Kinget R, Augustijns P. Int J Pharm. 2003;254:173. doi: 10.1016/s0378-5173(03)00009-7. [DOI] [PubMed] [Google Scholar]; b) Matsuyama K, Mishima K, Hayashi KI, Ishikawa H, Matsuyama H, Harada T. J Appl Polym Sci. 2003;89:742. [Google Scholar]; c) Gong K, Darr JA, Rehman IU. Int J Pharm. 2006;315:93. doi: 10.1016/j.ijpharm.2006.02.030. [DOI] [PubMed] [Google Scholar]; d) Türk M, Hils P, Helfgen B, Schaber K, Martin HJ, Wahl MA. J Supercrit Fluids. 2002;22:75. [Google Scholar]
- 82.Shen YB, Guan YX, Yao SJ. Int J Pharm. 2015;489:226. doi: 10.1016/j.ijpharm.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 83.a) Reverchon E, De Marco I, Caputo G, Della Porta G. J Supercrit Fluids. 2003;26:1. [Google Scholar]; b) Steckel H, Pichert L, Müller BW. Eur J Pharm Biopharm. 2004;57:507. doi: 10.1016/j.ejpb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 84.a) Montes A, Gordillo MD, Pereyra C, Martínez de la Ossa EJ. J Supercrit Fluids. 2013;81:236. [Google Scholar]; b) Chen AZ, Pu XM, Kang YQ, Liao L, Yao YD, Yin GF. J Mater Sci Mater Med. 2007;18:2339. doi: 10.1007/s10856-007-3173-8. [DOI] [PubMed] [Google Scholar]
- 85.a) Elizondo E, Sala S, Imbuluzqueta E, Gonzalez D, Blanco-Prieto MJ, Gamazo C, Ventosa N, Veciana J. Pharm Res. 2011;28:309. doi: 10.1007/s11095-010-0248-x. [DOI] [PubMed] [Google Scholar]; b) Guney O, Akgerman A. AlChE J. 2002;48:856. [Google Scholar]
- 86.Tomasko DL, Li H, Liu D, Han X, Wingert MJ, Lee LJ, Koelling KW. Ind Eng Chem Res. 2003;42:6431. [Google Scholar]
- 87.Mishima K, Matsuyama K, Tanabe D, Yamauchi S, Young TJ, Johnston KP. AlChE J. 2000;46:857. [Google Scholar]
- 88.De Marco I, Prosapio V, Cice F, Reverchon E. J Supercrit Fluids. 2013;83:153. [Google Scholar]
- 89.Pasquali I, Comi L, Pucciarelli F, Bettini R. Int J Pharm. 2008;356:76. doi: 10.1016/j.ijpharm.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 90.Kang YQ, Zhao C, Chen AZ, Wang SB, Liu YG, Wu WG, Su XQ. Materials. 2013;6:3571. doi: 10.3390/ma6083571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee S, Kim MS, Kim JS, Park HJ, Woo JS, Lee BC, Hwang SJ. J Microencapsul. 2006;23:741. doi: 10.1080/09687860600945552. [DOI] [PubMed] [Google Scholar]
- 92.Young TJ, Johnston KP, Mishima K, Tanaka H. J Pharm Sci. 1999;88:640. doi: 10.1021/js980237h. [DOI] [PubMed] [Google Scholar]
- 93.a) Chattopadhyay P, Gupta RB. AlChE J. 2002;48:235. [Google Scholar]; b) Todo H, Iida K, Okamoto H, Danjo K. J Pharm Sci. 92:2475. doi: 10.1002/jps.10497. [DOI] [PubMed] [Google Scholar]; c) Winters MA, Knutson BL, Debenedetti PG, Sparks HG, Przybycien TM, Stevenson CL, Prestrelski SJ. J Pharm Sci. 85:586. doi: 10.1021/js950482q. [DOI] [PubMed] [Google Scholar]
- 94.Moshashaee S, Bisrat M, Forbes RT, Nyqvist H, York P. Eur J Pharm Sci. 2000;11:239. doi: 10.1016/s0928-0987(00)00108-1. [DOI] [PubMed] [Google Scholar]
- 95.Ghaderi R, Artursson P, Carlfors J. Eur J Pharm Sci. 2000;10:1. doi: 10.1016/s0928-0987(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 96.a) Herberger J, Murphy K, Munyakazi L, Cordia J, Westhaus E. J Control Release. 2003;90:181. doi: 10.1016/s0168-3659(03)00152-4. [DOI] [PubMed] [Google Scholar]; b) Chattopadhyay P, Huff R, Shekunov BY. J Pharm Sci. 2006;95:667. doi: 10.1002/jps.20555. [DOI] [PubMed] [Google Scholar]
- 97.Luc B. J Phys: Condens Matter. 2000;12:R549. [Google Scholar]
- 98.a) Ugaonkar S, Needham TE, Bothun GD. Int J Pharm. 2011;403:96. doi: 10.1016/j.ijpharm.2010.10.031. [DOI] [PubMed] [Google Scholar]; b) Labuschagne PW, Kazarian SG, Sadiku RE. J Supercrit Fluids. 2011;57:190. [Google Scholar]
- 99.Argemí A, Ellis JL, Saurina J, Tomasko DL. J Pharm Sci. 2011;100:992. doi: 10.1002/jps.22346. [DOI] [PubMed] [Google Scholar]
- 100.a) Shieh YT, Zhao C, Wang TL, Yang CH. J Supercrit Fluids. 2014;91:1. [Google Scholar]; b) Comim Rosso SR, Bianchin E, de Oliveira D, Oliveira JV, Ferreira SRS. J Supercrit Fluids. 2013;79:133. [Google Scholar]
- 101.Duarte ARC, Casimiro T, Aguiar-Ricardo A, Simplício AL, Duarte CMM. J Supercrit Fluids. 2006;39:102. [Google Scholar]
- 102.da Silva MS, Nobrega FL, Aguiar-Ricardo A, Cabrita EJ, Casimiro T. J Supercrit Fluids. 2011;58:150. [Google Scholar]
- 103.a) Van Hees T, Piel G, Evrard B, Otte X, Thunus L, Delattre L. Pharm Res. 1999;16:1864. doi: 10.1023/a:1018955410414. [DOI] [PubMed] [Google Scholar]; b) Banchero M, Ronchetti S, Manna L. J Chem. 2013;2013:8. [Google Scholar]; c) Mammucari R, Dehghani F, Foster NR. Pharm Res. 2006;23:429. doi: 10.1007/s11095-005-9094-7. [DOI] [PubMed] [Google Scholar]; d) Charoenchaitrakool M, Dehghani F, Foster NR. Int J Pharm. 2002;239:103. doi: 10.1016/s0378-5173(02)00078-9. [DOI] [PubMed] [Google Scholar]; e) Bandi N, Wei W, Roberts CB, Kotra LP, Kompella UB. Eur J Pharm Sci. 2004;23:159. doi: 10.1016/j.ejps.2004.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Hussein K, Türk M, Wahl MA. Eur J Pharm Sci. 2008;33:306. doi: 10.1016/j.ejps.2008.01.003. [DOI] [PubMed] [Google Scholar]; g) Toropainen T, Heikkila T, Leppanen J, Matilainen L, Velaga S, Jarho P, Carlfors J, Lehto VP, Jarvinen T, Jarvinen K. Pharm Res. 2007;24:1058. doi: 10.1007/s11095-006-9227-7. [DOI] [PubMed] [Google Scholar]; h) Toropainen T, Velaga S, Heikkilä T, Matilainen L, Jarho P, Carlfors J, Lehto VP, Järvinen T, Järvinen K. J Pharm Sci. 2006;95:2235. doi: 10.1002/jps.20702. [DOI] [PubMed] [Google Scholar]
- 104.Rudrangi SRS, Trivedi V, Mitchell JC, Wicks SR, Alexander BD. Int J Pharm. 2015;494:408. doi: 10.1016/j.ijpharm.2015.08.062. [DOI] [PubMed] [Google Scholar]
- 105.Zhao L, Temelli F. J Supercrit Fluids. 2015;100:110. [Google Scholar]
- 106.a) Kankala RK, Kuthati Y, Liu CL, Lee CH. RSC Adv. 2015;5:42666. [Google Scholar]; b) Santo IE, Pedro AS, Fialho R, Cabral-Albuquerque E. Nanoscale Res Lett. 2013;8:386. doi: 10.1186/1556-276X-8-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pedro AS, Villa SD, Caliceti P, d Melo SABV, Albuquerque EC, Bertucco A, Salmaso S. J Supercrit Fluids. 2016;107:534. [Google Scholar]
- 108.Campardelli R, Espirito Santo I, Albuquerque EC, de Melo SV, Della Porta G, Reverchon E. J Supercrit Fluids. 2016;107:163. [Google Scholar]
- 109.Okamoto H, Sakakura Y, Shiraki K, Oka K, Nishida S, Todo H, Iida K, Danjo K. Int J Pharm. 2005;290:73. doi: 10.1016/j.ijpharm.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 110.Magnan C, Badens E, Commenges N, Charbit G. J Supercrit Fluids. 2000;19:69. [Google Scholar]
- 111.Karn PR, Cho W, Park HJ, Park JS, Hwang SJ. Int J Nano-medicine. 2013;8:365. doi: 10.2147/IJN.S39025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lesoin L, Crampon C, Boutin O, Badens E. J Supercrit Fluids. 2011;60:51. [Google Scholar]
- 113.Sakai H, Gotoh T, Imura T, Sakai K, Otake K, Abe M. J Oleo Sci. 2008;57:613. doi: 10.5650/jos.57.613. [DOI] [PubMed] [Google Scholar]
- 114.Naik S, Patel D, Surti N, Misra A. J Supercrit Fluids. 2010;54:110. [Google Scholar]
- 115.a) Marizza P, Keller SS, Müllertz A, Boisen A. J Control Release. 2014;173:1. doi: 10.1016/j.jconrel.2013.09.022. [DOI] [PubMed] [Google Scholar]; b) Stevenson CL, Santini JT, Jr, Langer R. Adv Drug Del Rev. 2012;64:1590. doi: 10.1016/j.addr.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patton JS, Trinchero P, Platz RM. J Control Release. 1994;28:79. [Google Scholar]
- 117.Lipworth BJ. Br J Clin Pharmacol. 1996;42:697. doi: 10.1046/j.1365-2125.1996.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoshida H, Okumura K, Hori R, Anmo T, Yamaguchi H. J Pharm Sci. 1979;68:670. doi: 10.1002/jps.2600680550. [DOI] [PubMed] [Google Scholar]
- 119.Okuda T, Kito D, Oiwa A, Fukushima M, Hira D, Okamoto H. Biol Pharm Bull. 2013;36:1183. doi: 10.1248/bpb.b13-00167. [DOI] [PubMed] [Google Scholar]
- 120.a) Reverchon E, Della Porta G, Falivene MG. J Supercrit Fluids. 2000;17:239. [Google Scholar]; b) Reverchon E, Spada A. Powder Technol. 2004;141:100. [Google Scholar]; c) Steckel H, Müller BW. Int J Pharm. 1998;173:25. doi: 10.1016/j.ijpharm.2009.05.008. [DOI] [PubMed] [Google Scholar]; d) Reverchon E, Della Porta G. Int J Pharm. 2003;258:1. doi: 10.1016/s0378-5173(03)00024-3. [DOI] [PubMed] [Google Scholar]; e) Rehman M, Shekunov BY, York P, Lechuga-Ballesteros D, Miller DP, Tan T, Colthorpe P. Eur J Pharm Sci. 2004;22:1. doi: 10.1016/j.ejps.2004.02.001. [DOI] [PubMed] [Google Scholar]; f) Reverchon E, Della Porta G. Powder Technol. 1999;106:23. [Google Scholar]; g) Reverchon E, De Marco I, Della Porta G. Int J Pharm. 2002;243:83. doi: 10.1016/s0378-5173(02)00261-2. [DOI] [PubMed] [Google Scholar]
- 121.a) Snavely WK, Subramaniam B, Rajewski RA, Defelippis MR. J Pharm Sci. 2026;91 doi: 10.1002/jps.10193. [DOI] [PubMed] [Google Scholar]; b Chen A-Z, Tang N, Wang S-B, Kang Y-Q, Song H-F. J Supercrit Fluids. 2015;101:117. [Google Scholar]
- 122.Tservistas M, Levy MS, Lo-Yim MYA, O’Kennedy RD, York P, Humphrey GO, Hoare M. Biotechnol Bioeng. 2001;72:12. doi: 10.1002/1097-0290(20010105)72:1<12::aid-bit2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 123.Vijayaraghavan M, Stolnik S, Howdle SM, Illum L. Int J Pharm. 2013;441:580. doi: 10.1016/j.ijpharm.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 124.Dhanda DS, Tyagi P, Mirvish SS, Kompella UB. J Control Release. 2013;168:239. doi: 10.1016/j.jconrel.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 125.Hira D, Okuda T, Ichihashi M, Kojima H, Okamoto H. Chem Pharm Bull. 2012;60:334. doi: 10.1248/cpb.60.334. [DOI] [PubMed] [Google Scholar]
- 126.Randolph TW, Clark DS, Blanch HW, Prausnitz JM. Science. 1988;239:387. doi: 10.1126/science.239.4838.387. [DOI] [PubMed] [Google Scholar]
- 127.Winters MA, Frankel DZ, Debenedetti PG, Carey J, Devaney M, Przybycien TM. Biotechnol Bioeng. 1999;62:247. doi: 10.1002/(sici)1097-0290(19990205)62:3<247::aid-bit1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 128.García-González CA, Smirnova I. J Supercrit Fluids. 2013;79:152. [Google Scholar]
- 129.Kim YH, Sioutas C, Fine P, Shing KS. Powder Technol. 2008;182:354. [Google Scholar]
- 130.Elvassore N, Bertucco A, Caliceti P. Ind Eng Chem Res. 2001;40:795. [Google Scholar]
- 131.Elvassore N, Bertucco A, Caliceti P. J Pharm Sci. 2001;90:1628. doi: 10.1002/jps.1113. [DOI] [PubMed] [Google Scholar]
- 132.Okamoto H, Nishida S, Todo H, Sakakura Y, Iida K, Danjo K. J Pharm Sci. 2003;92:371. doi: 10.1002/jps.10285. [DOI] [PubMed] [Google Scholar]
- 133.Prausnitz MR, Langer R. Nat Biotechnol. 2008;26:1261. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.a) Dias AMA, Braga MEM, Seabra IJ, Ferreira P, Gil MH, de Sousa HC. Int J Pharm. 2011;408:9. doi: 10.1016/j.ijpharm.2011.01.063. [DOI] [PubMed] [Google Scholar]; b) Cardea S, Scognamiglio M, Reverchon E. Mater Sci Eng, C. 2016;59:480. doi: 10.1016/j.msec.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 135.García-González CA, Sd Sousa AR, Argemí A, Periago AL, Saurina J, Duarte CMM, Domingo C. Int J Pharm. 2009;382:296. doi: 10.1016/j.ijpharm.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 136.Denet AR, Vanbever R, Preat V. Adv Drug Del Rev. 2004;56:659. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 137.Mayes S, Ferrone M. Ann Pharmacother. 2006;40:2178. doi: 10.1345/aph.1H135. [DOI] [PubMed] [Google Scholar]
- 138.Sivamani RK, Liepmann D, Maibach HI. Expert Opin Drug Deliv. 2007;4:19. doi: 10.1517/17425247.4.1.19. [DOI] [PubMed] [Google Scholar]
- 139.Park EJ, Werner J, Smith NB. Pharm Res. 2007;24:1396. doi: 10.1007/s11095-007-9306-4. [DOI] [PubMed] [Google Scholar]
- 140.Duarte ARC, Mano JF, Reis RL. Eur Polym J. 2009;45:141. [Google Scholar]
- 141.Natu MV, Gil MH, de Sousa HC. J Supercrit Fluids. 2008;47:93. [Google Scholar]
- 142.a) Masmoudi Y, Ben Azzouk L, Forzano O, Andre JM, Badens E. J Supercrit Fluids. 2011;60:98. [Google Scholar]; b) González-Chomón C, Braga MEM, de Sousa HC, Concheiro A, Alvarez-Lorenzo C. Eur J Pharm Biopharm. 2012;82:383. doi: 10.1016/j.ejpb.2012.07.007. [DOI] [PubMed] [Google Scholar]; c) Braga MEM, Pato MTV, Silva HSRC, Ferreira EI, Gil MH, Duarte CMM, de Sousa HC. J Supercrit Fluids. 2008;44:245. [Google Scholar]
- 143.a) Costa VP, Braga MEM, Duarte CMM, Alvarez-Lorenzo C, Concheiro A, Gil MH, de Sousa HC. J Supercrit Fluids. 2010;53:165. [Google Scholar]; b) Bouledjouidja A, Masmoudi Y, Sergent M, Trivedi V, Meniai A, Badens E. Int J Pharm. 2016;500:85. doi: 10.1016/j.ijpharm.2016.01.016. [DOI] [PubMed] [Google Scholar]; c) Costa VP, Braga MEM, Guerra JP, Duarte ARC, Duarte CMM, Leite EOB, Gil MH, de Sousa HC. J Supercrit Fluids. 2010;52:306. [Google Scholar]; d) Ana Rita CD, Ana Luisa S, Arlette V-G, Pascale S-P, Patricia C, Gil MH, Herminio CdS, Catarina MMD. Curr Drug Del. 2008;5:102. [Google Scholar]
- 144.Khademhosseini A, Langer R. Nat Protoc. 2016;11:1775. doi: 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- 145.a) Cardea S, Baldino L, Scognamiglio M, Reverchon E. J Mater Sci - Mater Med. 2014;25:989. doi: 10.1007/s10856-013-5130-z. [DOI] [PubMed] [Google Scholar]; b) Annabi N, Fathi A, Mithieux SM, Weiss AS, Dehghani F. J Supercrit Fluids. 2011;59:157. [Google Scholar]
- 146.Langer R, Vacanti JP, Vacanti CA, Atala A, Freed LE, Vunjak-Novakovic G. Tissue Eng. 1995;1:151. doi: 10.1089/ten.1995.1.151. [DOI] [PubMed] [Google Scholar]
- 147.Nichol JW, Khademhosseini A. Soft Matter. 2009;5:1312. doi: 10.1039/b814285h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thavornyutikarn B, Chantarapanich N, Sitthiseripratip K, Thouas GA, Chen Q. Progress in biomaterials. 2014;3:61. doi: 10.1007/s40204-014-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Watson MS, Whitaker MJ, Howdle SM, Shakesheff KM. Adv Mater. 2002;14:1802. [Google Scholar]
- 150.Dehghani F, Annabi N, Valtchev P, Mithieux SM, Weiss AS, Kazarian SG, Tay FH. Biomacromolecules. 2008;9:1100. doi: 10.1021/bm700891b. [DOI] [PubMed] [Google Scholar]
- 151.Deng A, Chen A, Wang S, Li Y, Liu Y, Cheng X, Zhao Z, Lin D. J Supercrit Fluids. 2013;77:110. [Google Scholar]
- 152.Yang D-Z, Chen A-Z, Wang S-B, Li Y, Tang X-L, Wu Y-J. Biomed Mater. 2015;10:035015. doi: 10.1088/1748-6041/10/3/035015. [DOI] [PubMed] [Google Scholar]
- 153.a) Sheridan MH, Shea LD, Peters MC, Mooney DJ. J Control Release. 2000;64:91. doi: 10.1016/s0168-3659(99)00138-8. [DOI] [PubMed] [Google Scholar]; b) Cabezas LI, Gracia I, García MT, de Lucas A, Rodríguez JF. J Supercrit Fluids. 2013;80:1. [Google Scholar]; c) Chen BQ, Kankala RK, Chen AZ, Yang DZ, Cheng XX, Jiang NN, Zhu K, Wang SB. Int J Nanomedicine. 2017;12:1877. doi: 10.2147/IJN.S129526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.a) Song HF, Chen AZ, Wang SB, Kang YQ, Ye SF, Liu YG, Wu WG. Materials. 2014:7. doi: 10.3390/ma7042459. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Song H-F, Chen A-Z, Wang S-B, Kang Y-Q, Ye S-F, Liu Y-G, Wu W-G. Materials. 2014;7:2459. doi: 10.3390/ma7042459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Barros AA, Oliveira C, Reis RL, Lima E, Duarte ARC. Int J Pharm. 2015;495:651. doi: 10.1016/j.ijpharm.2015.08.040. [DOI] [PubMed] [Google Scholar]
- 156.Nie H, Lee LY, Tong H, Wang C-H. J Control Release. 2008;129:207. doi: 10.1016/j.jconrel.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 157.Cabezas LI, Fernández V, Mazarro R, Gracia I, de Lucas A, Rodríguez JF. J Supercrit Fluids. 2012;63:155. [Google Scholar]
- 158.Baldino L, Naddeo F, Cardea S, Naddeo A, Reverchon E. J Mech Behav Biomed Mater. 2015;51:225. doi: 10.1016/j.jmbbm.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 159.Chen BQ, Kankala RK, Chen AZ, Yang DZ, Cheng XX, Jiang NN, Zhu K, Wang SB. Int J Nanomedicine. 2017;12:1877. doi: 10.2147/IJN.S129526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kiran E. J Supercrit Fluids. 2010;54:308. [Google Scholar]
- 161.a) Singh M, Sandhu B, Scurto A, Berkland C, Detamore MS. Acta Biomater. 2010;6:137. doi: 10.1016/j.actbio.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) van der Pol U, Mathieu L, Zeiter S, Bourban PE, Zambelli PY, Pearce SG, Boure LP, Pioletti DP. Acta Biomater. 2010;6:3755. doi: 10.1016/j.actbio.2010.03.028. [DOI] [PubMed] [Google Scholar]; c) Garg T, Singh O, Arora S, Murthy R. Crit Rev Ther Drug Carrier Syst. 2012;29:1. doi: 10.1615/critrevtherdrugcarriersyst.v29.i1.10. [DOI] [PubMed] [Google Scholar]
- 162.Martins M, Aroso IM, Reis RL, Duarte ARC, Craveiro R, Paiva A. AlChE J. 2014;60:3701. [Google Scholar]
- 163.Duarte ARC, Mano JF, Reis RL. Acta Biomater. 2009;5:2054. doi: 10.1016/j.actbio.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 164.Butler R, Davies CM, Cooper AI. Adv Mater. 2001;13:1459. [Google Scholar]
- 165.Hile DD, Amirpour ML, Akgerman A, Pishko MV. J Control Release. 2000;66:177. doi: 10.1016/s0168-3659(99)00268-0. [DOI] [PubMed] [Google Scholar]
- 166.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Nat Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 167.Ma T, Zhang YS, Chen A-Z, Ju J, Gu C-W, Kankala RK, Wang S-B. J Supercrit Fluids. 2017;120:43. [Google Scholar]
- 168.Barry JJA, Gidda HS, Scotchford CA, Howdle SM. Bioma-terials. 2004;25:3559. doi: 10.1016/j.biomaterials.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 169.Barry JJA, Nazhat SN, Rose FRAJ, Hainsworth AH, Scotchford CA, Howdle SM. J Mater Chem. 2005;15:4881. [Google Scholar]
- 170.Wang X, Li W, Kumar V. Biomaterials. 2006;27:1924. doi: 10.1016/j.biomaterials.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 171.Mathieu LM, Mueller TL, Bourban P-E, Pioletti DP, Müller R, Månson J-AE. Biomaterials. 2006;27:905. doi: 10.1016/j.biomaterials.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 172.Duarte ARC, Mano JF, Reis RL. J Supercrit Fluids. 2010;54:282. [Google Scholar]
- 173.Silva SS, Duarte ARC, Mano JF, Reis RL. Green Chem. 2013;15:3252. [Google Scholar]
- 174.Sheridan MH, Shea LD, Peters MC, Mooney DJ. J Control Release. 2000;64:91. doi: 10.1016/s0168-3659(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 175.Kim SH, Kim SH, Jung Y. J Control Release. 2015;206:101. doi: 10.1016/j.jconrel.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 176.Park KE, Kim BS, Kim MH, You HK, Lee J, Park WH. Polymer. 2015;76:8. [Google Scholar]
- 177.Diaz-Gomez L, Concheiro A, Alvarez-Lorenzo C, García-González CA. Carbohydr Polym. 2016;142:282. doi: 10.1016/j.carbpol.2016.01.051. [DOI] [PubMed] [Google Scholar]
- 178.Yang XB, Whitaker MJ, Sebald W, Clarke N, Howdle SM, Shakesheff KM, Oreffo RO. Tissue Eng. 2004;10:1037. doi: 10.1089/ten.2004.10.1037. [DOI] [PubMed] [Google Scholar]
- 179.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Nat Mater. 2005;4:435. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 180.Li L, Beniash E, Zubarev ER, Xiang W, Rabatic BM, Zhang G, Stupp SI. Nat Mater. 2003;2:689. doi: 10.1038/nmat983. [DOI] [PubMed] [Google Scholar]
- 181.Tsutsumi A, Nakamoto S, Mineo T, Yoshida K. Powder Technol. 1995;85:275. [Google Scholar]
- 182.Iley WJ. Powder Technol. 1991;65:441. [Google Scholar]
- 183.Rosenkranz K, Kasper MM, Werther J, Brunner G. J Supercrit Fluids. 2008;46:351. [Google Scholar]
- 184.Dillow AK, Dehghani F, Hrkach JS, Foster NR, Langer R. Proc Natl Acad Sci USA. 1999;96:10344. doi: 10.1073/pnas.96.18.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chang L, Chen Y-J, Chen Y-P, Chen C-T, Yu W-H. Formosan Journal of Musculoskeletal Disorders. 2011;2:55. [Google Scholar]
- 186.Fanovich MA, Ivanovic J, Misic D, Alvarez MV, Jaeger P, Zizovic I, Eggers R. J Supercrit Fluids. 2013;78:42. [Google Scholar]
- 187.da Silva RPFF, Rocha-Santos TAP, Duarte AC. Trends Anal Chem. 2016;76:40. [Google Scholar]
- 188.Machado FRS, Jr, Reis DF, Boschetto DL, Burkert JFM, Ferreira SRS, Oliveira JV, Burkert CAV. Ind Crops Prod. 2014;54:17. [Google Scholar]
- 189.Koegler WS, Patrick C, Cima MJ, Griffith LG. J Biomed Mater Res. 2002;63:567. doi: 10.1002/jbm.10209. [DOI] [PubMed] [Google Scholar]
- 190.Hu D, Liu L, Chen W, Li S, Zhao Y. Int J Mol Sci. 2012;13:6454. doi: 10.3390/ijms13056454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen A-Z, Li Y, Chen D, Hu J-Y. J Mater Sci Mater Med. 2009;20:751. doi: 10.1007/s10856-008-3633-9. [DOI] [PubMed] [Google Scholar]
- 192.Zhang C, Li G, Wang Y, Cui F, Zhang J, Huang Q. Int J Pharm. 2012;436:272. doi: 10.1016/j.ijpharm.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 193.Zhan S, Zhao Q, Chen S, Wang J, Liu Z, Chen C. J Chem Eng Data. 2014;59:1158. [Google Scholar]
- 194.Belhadj-Ahmed F, Badens E, Llewellyn P, Denoyel R, Charbit G. J Supercrit Fluids. 2009;51:278. [Google Scholar]
- 195.de Paz E, Rodríguez S, Kluge J, Martín Á, Mazzotti M, Cocero MJ. J Supercrit Fluids. 2013;84:105. [Google Scholar]
- 196.a) Priamo WL, de Cezaro AM, Benetti SC, Oliveira JV, Ferreira SRS. J Supercrit Fluids. 2011;56:137. [Google Scholar]; b) Priamo WL, de Cezaro AM, Ferreira SRS, Oliveira JV. J Supercrit Fluids. 2010;54:103. [Google Scholar]; c) Franceschi E, De Cesaro AM, Feiten M, Ferreira SRS, Dariva C, Kunita MH, Rubira AF, Muniz EC, Corazza ML, Oliveira JV. J Supercrit Fluids. 2008;47:259. [Google Scholar]; d) Franceschi E, De Cesaro AM, Ferreira SRS, Vladimir Oliveira J. J Food Eng. 2009;95:656. [Google Scholar]
- 197.Bush JR, Akgerman A, Hall KR. J Supercrit Fluids. 2007;41:311. [Google Scholar]
- 198.Tenorio A, Gordillo MD, Pereyra C, de la Ossa EJM. J Supercrit Fluids. 2007;40:308. [Google Scholar]
- 199.Montes A, Gordillo MD, Pereyra C, Martínez de la Ossa EJ. J Supercrit Fluids. 2012;63:92. [Google Scholar]
- 200.Reverchon E, Lamberti G, Antonacci A. J Supercrit Fluids. 2008;46:185. [Google Scholar]
- 201.Kalogiannis CG, Pavlidou E, Panayiotou CG. Ind Eng Chem Res. 2005;44:9339. [Google Scholar]
- 202.Reverchon E, Cardea S, Rappo ES. J Membr Sci. 2006;273:97. [Google Scholar]
- 203.Cardea S, Sessa M, Reverchon E. Ind Eng Chem Res. 2010;49:2783. [Google Scholar]
- 204.Patel J, Patil P. J Microencapsul. 2012;29:398. doi: 10.3109/02652048.2012.655329. [DOI] [PubMed] [Google Scholar]
- 205.Park CI, Shin MS, Kim H. Korean J Chem Eng. 2008;25:581. [Google Scholar]
- 206.Yu H, Zhao X, Zu Y, Zhang X, Zu B, Zhang X. Int J Mol Sci. 2012;13:5060. doi: 10.3390/ijms13045060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lovskaya DD, Lebedev AE, Menshutina NV. J Supercrit Fluids. 2015;106:115. [Google Scholar]
- 208.Argemí A, Vega A, Subra-Paternault P, Saurina J. J Pharm Biomed Anal. 2009;50:847. doi: 10.1016/j.jpba.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 209.Yan T, Cheng Y, Wang Z, Huang D, Miao H, Zhang Y. J Supercrit Fluids. 2015;104:177. [Google Scholar]
- 210.Della Porta G, Campardelli R, Reverchon E. J Supercrit Fluids. 2013;76:67. [Google Scholar]
- 211.Adami R, Liparoti S, Reverchon E. Chem Eng J. 2011;173:55. [Google Scholar]
- 212.Moneghini M, Kikic I, Voinovich D, Perissutti B, Filipović-Grčić J. Int J Pharm. 2001;222:129. doi: 10.1016/s0378-5173(01)00711-6. [DOI] [PubMed] [Google Scholar]
- 213.Jun SW, Kim M-S, Jo GH, Lee S, Woo JS, Park J-S, Hwang S-J. J Pharm Pharmacol. 2005;57:1529. doi: 10.1211/jpp.57.12.0003. [DOI] [PubMed] [Google Scholar]
- 214.Hong HL, Suo QL, Li FW, Wei XH, Zhang JB. Chem Eng Technol. 2008;31:1051. [Google Scholar]
- 215.Gong K, Braden M, Patel MP, Rehman IU, Zhang Z, Darr JA. J Pharm Sci. 2007;96:2048. doi: 10.1002/jps.20850. [DOI] [PubMed] [Google Scholar]
- 216.Kim M-S, Lee S, Park J-S, Woo J-S, Hwang S-J. Powder Technol. 2007;177:64. [Google Scholar]
- 217.Elvira C, Fanovich A, Fernández M, Fraile J, San Román J, Domingo C. J Control Release. 2004;99:231. doi: 10.1016/j.jconrel.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 218.a) Xie M, Fan D, Zhao Z, Li Z, Li G, Chen Y, He X, Chen A, Li J, Lin X, Zhi M, Li Y, Lan P. Int J Pharm. 2015;496:732. doi: 10.1016/j.ijpharm.2015.11.016. [DOI] [PubMed] [Google Scholar]; b) Zhao Z, Xie M, Li Y, Chen A, Li G, Zhang J, Hu H, Wang X, Li S. Int J Nanomedicine. 2015;10:3171. doi: 10.2147/IJN.S80434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.a) Xie MB, Li Y, Zhao Z, Chen AZ, Li JS, Hu JY, Li G, Li Z. J Supercrit Fluids. 2015;103:1. [Google Scholar]; b) Xie M, Li Y, Zhao Z, Chen A, Li J, Li Z, Li G, Lin X. Mater Lett. 2016;167:175. [Google Scholar]
- 220.Smirnova I, Suttiruengwong S, Arlt W. J Non-Cryst Solids. 2004;350:54. [Google Scholar]
- 221.Choi J, Ko E, Chung HK, Lee JH, Ju EJ, Lim HK, Park I, Kim KS, Lee JH, Son WC, Lee JS, Jung J, Jeong SY, Song SY, Choi EK. Int J Nanomedicine. 2015;10:6121. doi: 10.2147/IJN.S88375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jesson G, Brisander M, Andersson P, Demirbuker M, Derand H, Lennernas H, Malmsten M. Pharm Res. 2014;31:694. doi: 10.1007/s11095-013-1191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Won D-H, Kim M-S, Lee S, Park J-S, Hwang S-J. Int J Pharm. 2005;301:199. doi: 10.1016/j.ijpharm.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 224.Bouledjouidja A, Masmoudi Y, Van Speybroeck M, Schueller L, Badens E. Int J Pharm. 2016;499:1. doi: 10.1016/j.ijpharm.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 225.Sanganwar GP, Gupta RB. Int J Pharm. 2008;360:213. doi: 10.1016/j.ijpharm.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 226.Ahern RJ, Hanrahan JP, Tobin JM, Ryan KB, Crean AM. Eur J Pharm Sci. 2013;50:400. doi: 10.1016/j.ejps.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 227.Zeinolabedini Hezave A, Esmaeilzadeh F. J Dispersion Sci Technol. 2012;33:1106. [Google Scholar]
- 228.Park HJ, Kim M-S, Lee S, Kim J-S, Woo J-S, Park J-S, Hwang S-J. Int J Pharm. 2007;328:152. doi: 10.1016/j.ijpharm.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 229.Imbuluzqueta E, Elizondo E, Gamazo C, Moreno-Calvo E, Veciana J, Ventosa N, Blanco-Prieto MJ. Acta Biomater. 2011;7:1599. doi: 10.1016/j.actbio.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 230.Shekunov BY, Chattopadhyay P, Seitzinger J, Huff R. Pharm Res. 2006;23:196. doi: 10.1007/s11095-005-8635-4. [DOI] [PubMed] [Google Scholar]
- 231.Velaga SP, Carlfors J. Drug Dev Ind Pharm. 2005;31:135. doi: 10.1081/ddc-200047368. [DOI] [PubMed] [Google Scholar]
- 232.Falco N, Reverchon E, Della Porta G. Int J Pharm. 2013;441:589. doi: 10.1016/j.ijpharm.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 233.Hussain YA, Grant CS. J Supercrit Fluids. 2012;71:127. [Google Scholar]
- 234.Veres P, López-Periago AM, Lázár I, Saurina J, Domingo C. Int J Pharm. 2015;496:360. doi: 10.1016/j.ijpharm.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 235.Aroso IM, Craveiro R, Rocha Â, Dionísio M, Barreiros S, Reis RL, Paiva A, Duarte ARC. Int J Pharm. 2015;492:73. doi: 10.1016/j.ijpharm.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 236.Fraile M, Martín ÿ, Deodato D, Rodriguez-Rojo S, Nogueira ID, Simplício AL, Cocero MJ, Duarte CMM. J Supercrit Fluids. 2013;81:226. [Google Scholar]
- 237.Roshan Y, Raffaella M, Neil RF. Journal of Physics: Conference Series. 2010;215:012087. [Google Scholar]
- 238.Li-hong W, Xin C, Hui X, Li-li Z, Jing H, Mei-juan Z, Jie L, Yi L, Jin-wen L, Wei Z, Gang C. Int J Pharm. 2013;454:135. doi: 10.1016/j.ijpharm.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 239.Nesta DP, Elliott JS, Warr JP. Biotechnol Bioeng. 2000;67:457. [PubMed] [Google Scholar]
- 240.Imperiale JC, Bevilacqua G, de Rosa PT, Sosnik A. Drug Dev Ind Pharm. 2014;40:1607. doi: 10.3109/03639045.2013.838581. [DOI] [PubMed] [Google Scholar]
- 241.Chen A-Z, Kang Y-Q, Pu X-M, Yin G-F, Li Y, Hu J-Y. J Colloid Interface Sci. 2009;330:317. doi: 10.1016/j.jcis.2008.10.085. [DOI] [PubMed] [Google Scholar]
- 242.Gong K, Rehman IU, Darr JA. J Pharm Biomed Anal. 2008;48:1112. doi: 10.1016/j.jpba.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 243.Liu H, Finn N, Yates MZ. Langmuir. 2005;21:379. doi: 10.1021/la047934b. [DOI] [PubMed] [Google Scholar]
- 244.Caliceti P, Salmaso S, Elvassore N, Bertucco A. J Control Release. 2004;94:195. doi: 10.1016/j.jconrel.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 245.Della Porta G, Falco N, Giordano E, Reverchon E. J Biomater Sci Polym Ed. 2013;24:1831. doi: 10.1080/09205063.2013.807457. [DOI] [PubMed] [Google Scholar]
- 246.Zia H, Dondeti P, Needham TE. Particul Sci Technol. 1997;15:273. [Google Scholar]
- 247.Park JW, Yun JM, Lee ES, Youn YS, Kim KS, Oh YT, Oh KT. Arch Pharmacal Res. 2013;36:1369. doi: 10.1007/s12272-013-0187-2. [DOI] [PubMed] [Google Scholar]
- 248.Marizza P, Pontoni L, Rindzevicius T, Alopaeus JF, Su K, Zeitler JA, Keller SS, Kikic I, Moneghini M, De Zordi N, Solinas D, Cortesi A, Boisen A. J Supercrit Fluids. 2016;107:145. [Google Scholar]
- 249.Kluge J, Fusaro F, Mazzotti M, Muhrer G. J Supercrit Fluids. 2009;50:336. [Google Scholar]
- 250.Del Gaudio P, Auriemma G, Mencherini T, Porta GD, Reverchon E, Aquino RP. J Pharm Sci. 2013;102:185. doi: 10.1002/jps.23361. [DOI] [PubMed] [Google Scholar]
- 251.Weinstein RD, Muske KR, Martin S-A, Schaeber DD. Ind Eng Chem Res. 2010;49:7281. [Google Scholar]
- 252.de Melo SABV, Danh LT, Mammucari R, Foster NR. J Supercrit Fluids. 2014;93:112. [Google Scholar]
- 253.Hu D, Lin C, Liu L, Li S, Zhao Y. J Food Eng. 2012;109:545. [Google Scholar]
- 254.Kluge J, Fusaro F, Casas N, Mazzotti M, Muhrer G. J Supercrit Fluids. 2009;50:327. [Google Scholar]
- 255.Tu LS, Dehghani F, Foster NR. Powder Technol. 2002;126:134. [Google Scholar]
- 256.Chen A-Z, Pu X-M, Yin G-F, Zhao C, Wang S-B, Liu Y-G, Wang G-Y, Kang Y-Q. J Appl Polym Sci. 2012;125:3175. [Google Scholar]
- 257.Montes A, Wehner L, Pereyra C, Md l Ossa EJ. J Supercrit Fluids. 2016;112:44. [Google Scholar]
- 258.Ha ES, Kim JS, Baek IH, Yoo JW, Jung Y, Moon HR, Kim MS. Drug Des Devel Ther. 2015;9:4269. doi: 10.2147/DDDT.S90706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chen A-Z, Li L, Wang S-B, Zhao C, Liu Y-G, Wang G-Y, Zhao Z. J Supercrit Fluids. 2012;67:7. [Google Scholar]
- 260.a) Cardoso MAT, Monteiro GA, Cardoso JP, Prazeres TJV, Figueiredo JMF, Martinho JMG, Cabral JMS, Palavra AMF. J Supercrit Fluids. 2008;44:238. [Google Scholar]; b) Tavares Cardoso MA, Geraldes V, Cabral JMS, Palavra AMF. J Supercrit Fluids. 2008;46:71. [Google Scholar]
- 261.Chen F, Yin G, Liao X, Yang Y, Huang Z, Gu J, Yao Y, Chen X, Gao H. J Mater Sci Mater Med. 2013;24:1693. doi: 10.1007/s10856-013-4926-1. [DOI] [PubMed] [Google Scholar]
- 262.Zhang Y-Z, Liao X-M, Yin G-F, Yuan P, Huang Z-B, Gu J-W, Yao Y-D, Chen X-C. Powder Technol. 2012;221:343. [Google Scholar]
- 263.Üzer S, Akman U, Hortaçsu Ö. J Supercrit Fluids. 2006;38:119. [Google Scholar]
- 264.Duarte ARC, Costa MS, Simplício AL, Cardoso MM, Duarte CMM. Int J Pharm. 2006;308:168. doi: 10.1016/j.ijpharm.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 265.Moneghini M, Perissutti B, Vecchione F, Kikic I, Alessi P, Cortesi A, Princivalle F. Curr Drug Del. 2007;4:241. doi: 10.2174/156720107781023901. [DOI] [PubMed] [Google Scholar]
- 266.Alessi P, Ireneo K, Angelo C, Alessia F, Mariarosa M. J Supercrit Fluids. 2003;27:309. [Google Scholar]
- 267.Paisana MC, Müllers KC, Wahl MA, Pinto JF. J Supercrit Fluids. 2016;109:124. [Google Scholar]
- 268.Lee SJ, Kim YH, Lee SH, Hahn M. Pharm Dev Technol. 2012;17:212. doi: 10.3109/10837450.2010.531735. [DOI] [PubMed] [Google Scholar]
- 269.Yoda S, Sato K, Oyama HT. RSC Adv. 2011;1:156. [Google Scholar]
- 270.Kang Y, Yin G, Ouyang P, Huang Z, Yao Y, Liao X, Chen A, Pu X. J Colloid Interface Sci. 2008;322:87. doi: 10.1016/j.jcis.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 271.Akbari I, Ghoreishi SM, Habibi N. J Supercrit Fluids. 2014;94:182. [Google Scholar]
- 272.Pathak P, Prasad GL, Meziani MJ, Joudeh AA, Sun Y-P. Langmuir. 2007;23:2674. doi: 10.1021/la062739d. [DOI] [PubMed] [Google Scholar]
- 273.Wu K, Li J, Wang W, Winstead DA. J Pharm Sci. 2009;98:2422. doi: 10.1002/jps.21598. [DOI] [PubMed] [Google Scholar]
- 274.Falconer JR, Wen J, Zargar-Shoshtari S, Chen JJ, Farid M, El Maghraby GM, Alany RG. Pharm Dev Technol. 2014;19:238. doi: 10.3109/10837450.2013.769570. [DOI] [PubMed] [Google Scholar]
- 275.Falconer JR, Wen J, Zargar-Shoshtari S, Chen JJ, Farid M, Tallon SJ, Alany RG. Drug Dev Ind Pharm. 2014;40:458. doi: 10.3109/03639045.2013.768630. [DOI] [PubMed] [Google Scholar]
- 276.Chen A-Z, Li Y, Chau F-T, Lau T-Y, Hu J-Y, Zhao Z, Mok DK-w. J Supercrit Fluids. 2009;49:394. [Google Scholar]
- 277.Porta GD, Campardelli R, Falco N, Reverchon E. J Pharm Sci. 2011;100:4357. doi: 10.1002/jps.22647. [DOI] [PubMed] [Google Scholar]
- 278.Sane A, Limtrakul J. J Supercrit Fluids. 2009;51:230. [Google Scholar]
- 279.Campardelli R, Della Porta G, Gomez L, Irusta S, Reverchon E, Santamaria J. J Mater Chem B. 2014;2:409. doi: 10.1039/c3tb21099e. [DOI] [PubMed] [Google Scholar]
- 280.Jacobson GB, Gonzalez-Gonzalez E, Spitler R, Shinde R, Leake D, Kaspar RL, Contag CH, Zare RN. J Pharm Sci. 2010;99:4261. doi: 10.1002/jps.22147. [DOI] [PubMed] [Google Scholar]
- 281.Obaidat RM, Tashtoush BM, Bayan MF, Al Bustami RT, Alnaief M. AAPS Pharm Sci Tech. 2015;16:1235. doi: 10.1208/s12249-015-0312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yang G, Zhao Y, Zhang Y, Dang B, Liu Y, Feng N. Int J Nano-medicine. 2015;10:6633. doi: 10.2147/IJN.S92665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yeo S-D, Lee J-C. J Supercrit Fluids. 2004;30:315. [Google Scholar]
- 284.Careno S, Boutin O, Badens E. J Cryst Growth. 2012;342:34. [Google Scholar]
- 285.Moneghini M, Perissutti B, Kikic I, Grassi M, Cortesi A, Princivalle F. Drug Dev Ind Pharm. 2006;32:39. doi: 10.1080/03639040500388037. [DOI] [PubMed] [Google Scholar]
- 286.Chen H-H, Su C-S, Liu J-J, Sheu M-T. J Supercrit Fluids. 2015;101:17. [Google Scholar]
- 287.Argemí A, López-Periago A, Domingo C, Saurina J. J Pharm Biomed Anal. 2008;46:456. doi: 10.1016/j.jpba.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 288.Pantić M, Knez Ž, Novak Z. J Non-Cryst Solids. 2016;432:519. [Google Scholar]
- 289.Yoshida VM, Balcao VM, Vila MM, Oliveira JM, Junior, Aranha N, Chaud MV, Gremiao MP. J Pharm Sci. 2015;104:1691. doi: 10.1002/jps.24377. [DOI] [PubMed] [Google Scholar]
- 290.Kurniawansyah F, Duong HTT, Luu TD, Mammucari R, Vittorio O, Boyer C, Foster N. Chem Eng J. 2015;279:799. [Google Scholar]
- 291.Al-Marzouqi AH, Elwy HM, Shehadi I, Adem A. J Pharm Biomed Anal. 2009;49:227. doi: 10.1016/j.jpba.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 292.Rodier E, Lochard H, Sauceau M, Letourneau J-J, Freiss B, Fages J. Eur J Pharm Sci. 2005;26:184. doi: 10.1016/j.ejps.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 293.Moribe K, Fujito T, Tozuka Y, Yamamoto K. J Incl Phenom Macrocycl Chem. 2007;57:289. [Google Scholar]
- 294.a) Türk M, Upper G, Steurenthaler M, Hussein K, Wahl MA. J Supercrit Fluids. 2007;39:435. [Google Scholar]; b) Hussein K, Türk M, Wahl MA. Pharm Res. 2007;24:585. doi: 10.1007/s11095-006-9177-0. [DOI] [PubMed] [Google Scholar]
- 295.Lai S, Locci E, Piras A, Porcedda S, Lai A, Marongiu B. Carbohydr Res. 2003;338:2227. doi: 10.1016/s0008-6215(03)00358-6. [DOI] [PubMed] [Google Scholar]
- 296.Rudrangi SRS, Bhomia R, Trivedi V, Vine GJ, Mitchell JC, Alexander BD, Wicks SR. Int J Pharm. 2015;479:381. doi: 10.1016/j.ijpharm.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 297.Lee S-Y, Jung I-I, Kim J-K, Lim G-B, Ryu J-H. J Supercrit Fluids. 2008;44:400. [Google Scholar]
- 298.Hassan HA, Al-Marzouqi AH, Jobe B, Hamza AA, Ramadan GA. J Pharm Biomed Anal. 2007;45:243. doi: 10.1016/j.jpba.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 299.Van Hees T, Barillaro V, Piel G, Bertholet P, De Hassonville S, Evrard B, Delattre L. J Incl Phenom Macrocycl Chem. 2002;44:271. [Google Scholar]
- 300.a) Barillaro V, Evrard B, Delattre L, Piel G. AAPS J. 2005;7:E149. doi: 10.1208/aapsj070116. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Barillaro V, Bertholet P, Henry de Hassonville S, Ziemon E, Evrard B, Delattre L, Piel G. J Pharm Pharm Sci. 2004;7:378. [PubMed] [Google Scholar]
- 301.Junco S, Casimiro T, Ribeiro N, Nunes Da Ponte M, Cabral Marques HM. J Incl Phenom Macrocycl Chem. 2002;44:69. [Google Scholar]
- 302.Van Hees T, Piel G, Evrard B, Otte X, Thunus L, Delattre L. Pharm Res. 1999;16:1864. doi: 10.1023/a:1018955410414. [DOI] [PubMed] [Google Scholar]
- 303.Grandelli HE, Hassler JC, Whittington A, Kiran E. J Supercrit Fluids. 2012;71:19. [Google Scholar]
- 304.Nunes AV, Rodriguez-Rojo S, Almeida AP, Matias AA, Rego D, Simplicio AL, Bronze MR, Cocero MJ, Duarte CMM. J Control Release. 2010;148:e11. doi: 10.1016/j.jconrel.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 305.Jun SW, Kim MS, Kim JS, Park HJ, Lee S, Woo JS, Hwang SJ. Eur J Pharm Biopharm. 2007;66:413. doi: 10.1016/j.ejpb.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 306.Santo IE, Campardelli R, Albuquerque EC, de Melo SV, Della Porta G, Reverchon E. Chem Eng J. 2014;249:153. [Google Scholar]
- 307.Karn PR, Kim HD, Kang H, Sun BK, Jin SE, Hwang SJ. Int J Nanomedicine. 2014;9:3791. doi: 10.2147/IJN.S65601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Salmaso S, Bersani S, Elvassore N, Bertucco A, Caliceti P. Int J Pharm. 2009;379:51. doi: 10.1016/j.ijpharm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 309.Salmaso S, Elvassore N, Bertucco A, Caliceti P. J Pharm Sci. 2009;98:640. doi: 10.1002/jps.21434. [DOI] [PubMed] [Google Scholar]
- 310.Gonçalves VSS, Matias AA, Rodríguez-Rojo S, Nogueira ID, Duarte CMM. Int J Pharm. 2015;495:302. doi: 10.1016/j.ijpharm.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 311.Kunastitchai S, Pichert L, Sarisuta N, Müller BW. Int J Pharm. 2006;316:93. doi: 10.1016/j.ijpharm.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 312.Vezzù K, Borin D, Bertucco A, Bersani S, Salmaso S, Caliceti P. J Supercrit Fluids. 2010;54:328. [Google Scholar]
- 313.Rodrigues M, Peiriço N, Matos H, Gomes de Azevedo E, Lobato MR, Almeida AJ. J Supercrit Fluids. 2004;29:175. [Google Scholar]
- 314.Kalantarian P, Najafabadi AR, Haririan I, Vatanara A, Yamini Y, Darabi M, Gilani K. Int J Nanomedicine. 2010;5:763. doi: 10.2147/IJN.S12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Schiavone H, Palakodaty S, Clark A, York P, Tzannis ST. Int J Pharm. 2004;281:55. doi: 10.1016/j.ijpharm.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 316.Bakhbakhi Y, Charpentier PA, Rohani S. Int J Pharm. 2006;309:71. doi: 10.1016/j.ijpharm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 317.Engwicht A, Girreser U, Müller BW. Int J Pharm. 1999;185:61. doi: 10.1016/s0378-5173(99)00127-1. [DOI] [PubMed] [Google Scholar]
- 318.a) Velaga SP, Bergh S, Carlfors J. Eur J Pharm Sci. 2004;21:501. doi: 10.1016/j.ejps.2003.11.006. [DOI] [PubMed] [Google Scholar]; b) Velaga SP, Berger R, Carlfors J. Pharm Res. 2002;19:1564. doi: 10.1023/a:1020477204512. [DOI] [PubMed] [Google Scholar]
- 319.Tandya A, Dehghani F, Foster NR. J Supercrit Fluids. 2006;37:272. [Google Scholar]
- 320.Velaga SP, Ghaderi R, Carlfors J. Int J Pharm. 2002;231:155. doi: 10.1016/s0378-5173(01)00870-5. [DOI] [PubMed] [Google Scholar]
- 321.a) Amidi M, Pellikaan HC, de Boer AH, Crommelin DJ, Hennink WE, Jiskoot W. Eur J Pharm Biopharm. 2008;68:191. doi: 10.1016/j.ejpb.2007.05.007. [DOI] [PubMed] [Google Scholar]; b) Amidi M, Krudys KM, Snel CJ, Crommelin DJA, Della Pasqua OE, Hennink WE, Jiskoot W. J Control Release. 2008;127:257. doi: 10.1016/j.jconrel.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 322.Kim YH, Shing KS. Powder Technol. 2008;182:25. [Google Scholar]
- 323.Cai M-Q, Guan Y-X, Yao S-J, Zhu Z-Q. J Supercrit Fluids. 2008;43:524. [Google Scholar]
- 324.Kang Y-Q, Zhao C, Chen A-Z, Wang S-B, Liu Y-G, Wu W-G, Su X-Q. Materials. 2013;6:3571. doi: 10.3390/ma6083571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Du Z, Guan Y-X, Yao S-J, Zhu Z-Q. Int J Pharm. 2011;421:258. doi: 10.1016/j.ijpharm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 326.Adami R, Reverchon E, Järvenpää E, Huopalahti R. Powder Technol. 2008;182:105. [Google Scholar]
- 327.Rehman M, Shekunov BY, York P, Colthorpe P. J Pharm Sci. 2001;90:1570. doi: 10.1002/jps.1107. [DOI] [PubMed] [Google Scholar]
- 328.Mayo AS, Ambati BK, Kompella UB. Int J Pharm. 2010;387:278. doi: 10.1016/j.ijpharm.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Reverchon E, Della Porta G. J Supercrit Fluids. 2003;26:243. [Google Scholar]
- 330.Fa S, Muhammad SA, Langrish T, Tang P, Adi H, Chan H-K, Kazarian SG, Dehghani F. Int J Pharm. 2010;388:114. doi: 10.1016/j.ijpharm.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 331.Shekunov BY, Feeley JC, Chow AHL, Tong HHY, York P. J Aerosol Sci. 2003;34:553. [Google Scholar]
- 332.a) Ong BYS, Ranganath SH, Lee LY, Lu F, Lee HS, Sahinidis NV, Wang CH. Biomaterials. 2009;30:3189. doi: 10.1016/j.biomaterials.2009.02.030. [DOI] [PubMed] [Google Scholar]; b) Lee LY, Ranganath SH, Fu Y, Zheng JL, Lee HS, Wang CH, Smith KA. Chem Eng Sci. 2009;64:4341. [Google Scholar]