Abstract
This article is a continuation of the “Do You Know Your Guidelines” series, initiated by the Education committee of the American Head and Neck Society. Treatment guidelines for advanced head and neck squamous cell carcinoma are reviewed here, including the critical roles of radiation, chemotherapy and the recent application of immunotherapy agents. We will be limiting this discussion to include cancers of the oral cavity, oropharynx, hypopharynx, and larynx. It should be noted that much of the article pertains to HPV-negative oropharynx cancer where applicable, as HPV-positive oropharyngeal squamous carcinoma carries a different natural history, different prognosis, and now different staging criteria. Additionally, the article will not include information on nasopharyngeal or sinus cancers, as these latter topics are covered in separate “Do You Know Your Guidelines?” installments and these diagnoses carry somewhat different approaches to diagnosis and management that diverge from the focus of this paper.
INDEXING KEY WORDS: head and neck cancer, advanced head and neck cancer, National Comprehensive Cancer Network, management, guidelines as topic
Introduction
Approximately two-thirds of patients with head and neck squamous cell carcinoma (HNSCC) present with advanced stage disease. It is therefore essential to follow current treatment guidelines for patients with advanced or unresectable disease to optimize patient outcomes1. This review will outline the latest National Comprehensive Cancer Network (NCCN) guidelines versions 1.20172 regarding advanced HNSCC and, where appropriate, will provide a brief summary of the current literature supporting these guidelines, detailing recent advances in therapy. Although this discussion focuses on advanced disease in most sites of HNSCC and general treatment principles are applicable in most cases, it should be noted that Human Papillomavirus (HPV) associated oropharyngeal cancers have a more favorable prognosis3–6 and will be staged differently compared to HPV-negative cancers in accordance with the American Joint Committee of Cancer (AJCC) 8th edition7–9. Nevertheless, treatment strategies remain the same despite HPV status. Further research is necessary to determine the safety and efficacy of altering the management for patients with HPV positive HNSCC with respect to the new staging. The following article discusses current guidelines for management of advanced HNSCC.
How is “Advanced Disease” Defined in HNSCC?
“Advanced disease” as it relates to HNSCC can be defined several ways, depending on the context. This review focuses on four clinical settings where HNSCC disease progression can be considered ‘advanced’ – (1) postoperative “high-risk” HNSCC, (2) potentially resectable locoregional disease, (3) locoregionally unresectable disease, and (4) distant-metastatic HNSCC. The management of recurrent HNSCC will also be discussed briefly, though this subject is a topic of a separate article in the series. Two clinical features have been generally agreed upon to define ‘high-risk, resected disease’: when extranodal extension (ENE) is present, and second, the presence of disease at the surgical margins of resection10–20. Newly diagnosed unresectable disease is defined generally as T4b classified (stage group IVB) tumors according to AJCC TNM staging system21. “Unresectable” tumors are typically those that cannot be removed without causing unacceptable morbidity, such as tumors with dense involvement of the cervical vertebrae, brachial plexus, deep muscles of the neck, base of skull or carotid artery. Subsequently, we will discuss treatment of patients with distant metastatic HNSCC and finally, we will review guidelines for treatment of HNSCC tumors that persist or recur following attempted primary treatment.
What Are the Guidelines for Treatment of High-Risk Resectable HNSCC?
After surgical resection with curative intent, adjuvant therapy with postoperative radiation (PORT) to the local and regional sites of disease is the standard of care for patients with AJCC stage III or IV HNSCC - that is for patients with T3 or T4 local disease and/or for patients with stage N2a or greater nodal metastases2. As stated above, the presence of ENE and positive surgical margins are generally accepted as indicators of disease that is at “high-risk” of recurring both locoregionally or distantly. After resection, additional treatment is indicated for this high-risk group to reduce these risks. Moreover, adjuvant radiation is also recommended if patients have significant perineural or lymphovascular invasion identified on pathologic evaluation2.
For patients with positive surgical margins, if additional resection can be carried out with a high likelihood of achieving a complete surgical resection without long-term complications, then re-resection is recommended2. For patients with positive margins that cannot be cleared surgically, and/or for patients with ENE, postoperative adjuvant radiation (POCRT) with concurrent cisplatin treatment is indicated2. The evidence for the use of POCRT compared to radiotherapy (RT) alone derives from two large phase III trials, the Radiation Therapy Oncology Group (RTOG (#9501)) study17 and the European Organisation for Research and Treatment of Cancer (EORTC (#22931)) study18, detailed below.
Though each trial had similar aims, the definition of high-risk disease and inclusion criteria differed between these two studies. The EORTC (#22931) trial included not only patients with T1/T2 and N0/N1 disease with the above noted high-risk features, but also enrolled patients without high-risk pathological findings18. Patients were included if they had T3/T4 tumors regardless of nodal involvement (except T3N0 of the larynx) with negative margins, or T1/T2 tumors with N2/N3 disease. For this trial, high-risk features comprised of the following: ENE, positive resection margins, perineural involvement, or vascular tumor embolism. The RTOG (#9501) trial selectively included patients with defined high-risk features, including histologic evidence of invasion of two or more regional lymph nodes, ENE, and microscopically involved mucosal margins of resection17. Both studies concluded that the addition of cisplatin to postoperative radiation benefited patients with high-risk HNSCC. The EORTC (#22931) study demonstrated significant improvement in both progression-free survival and overall survival (OS) in the combined-therapy group. In the RTOG (#9501) study, locoregional control was improved and disease-free survival was significantly longer after combined-therapy; however, OS did not differ significantly between the two study groups.
Post-operative chemoradiation carries a high burden of toxicity and potential morbidity to patients. Therefore, to determine which patients would maximally benefit from receiving POCRT, a meta-analysis of EORTC (#22931) and RTOG (#9501) was published19). This meta-analysis concluded that patients with nodal ENE and/or positive surgical margins derived a significant OS benefit from combined therapy over radiation alone. When neither of these risk factors was present, there was no significant survival advantage observed as a result of POCRT compared to PORT alone. Furthermore, in a long-term follow-up to RTOG (#9501), at a median time of 9.4 years, the only significant difference in locoregional control and disease-free progression was detected in the subgroup of patients with ENE and/or positive surgical margins20. Therefore, current guidelines recommend POCRT for patients with ENE or positive surgical margins2.
What are the Treatment Guidelines for Newly Diagnosed T4b, Unresectable Disease?
Locoregionally advanced unresectable HNSCC carries an unfavorable prognosis, and effective treatment options often carry high toxicity and morbidity. Management of this disease is best provided by an interdisciplinary team which includes individuals from the disciplines of head and neck surgical oncology, radiation oncology, medical oncology, nutrition support, speech and language pathology, physical medicine and rehabilitation, and palliative care. It is imperative to have quality of life discussions with the patient while establishing realistic expectations. Generally, enrollment in clinical trials is preferred, if possible2. The NCCN guidelines base treatment options on an individual’s performance status (PS) as defined by the Eastern Cooperative Oncology Group (ECOG, Table 1).
Table 1.
Eastern Cooperative Oncology Group Performance Status
Performance status scale for assessing how disease impacts patients daily living abilities as published by Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology.1982; 5:649-55. In the public domain.
| Grade | ECOG Performance Status Definition |
|---|---|
| 0 | Fully active, able to carry on all pre-disease performance without restriction |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work |
| 2 | Ambulatory and capable of all selfcare but unable to carry out any work activities; up and about more than 50% of waking hours |
| 3 | Capable of only limited selfcare; confined to bed or chair more than 50% of waking hours |
| 4 | Completely disabled; cannot carry on any selfcare; totally confined to bed or chair |
| 5 | Dead |
For patients with an ECOG PS of 0 or 1 the recommendations include concurrent systemic therapy (cisplatin is the preferred agent) and radiation, or induction chemotherapy followed by either RT alone or RT and additional systemic therapy. The controversy regarding the additional benefit that induction chemotherapy offers to these patients is discussed below. Patients with an ECOG PS of 2 should undergo definitive RT with or without concurrent systemic therapy, depending on the perceived ability of the patient to tolerate chemotherapy. With an ECOG PS of 3, goals shift toward palliative treatment either with RT, single agent systemic therapy, or supportive care alone to manage symptoms and provide comfort2. Such patients with severe functional decline are treated in a comparable manner as those with incurable recurrent or metastatic disease, which is discussed later in this review.
Concurrent cisplatin-based chemoradiotherapy remains one of the more substantially supported options for managing unresectable advanced HNSCC or for patients who cannot tolerate surgery. A standard treatment approach uses high-dose cisplatin on days 1, 22, and 43 during RT and has been found to be effective and easy to administer, and a low dose, weekly administration schedule of cisplatin is also employed2. Limited information is available regarding outcome differences between alternative chemotherapy regimens. Cetuximab, as a single agent with RT, is a recommended alternative2.
Cetuximab, a recombinant chimeric anti-EGFR (epidermal growth factor receptor) monoclonal antibody, has been shown to be effective in the treatment of HNSCC2,22. EGFR is expressed in roughly 90% of all HNSCC tumors and elevated levels of EGFR protein expression is associated with decreased survival23–27. Results from several clinical trials have established the activity of cetuximab in the treatment of HNSCC, including in combination with cisplatin and cisplatin/5-fluorouracil22,28–35. The landmark phase III trial by Bonner et al.29 supporting cetuximab, demonstrated increased disease-free survival and OS for patients with locoregionally advanced HNSCC treated with cetixumab plus RT versus RT alone.
Alternative radiosensitizing chemotherapeutic combinations recommended for treatment of unresectable disease include carboplatin/5-fluorouracil36,37, 5-fluorouracil/hydroxyurea38, cisplatin/paclitaxel38, cisplatin/5-fluorouracil39, or carboplatin/paclitaxel40. Unfortunately, there is a lack of quality studies comparing differences between these individual systemic therapies plus RT with respect to one another, and therefore, there is no established superiority of one regimen over others.
Evidence behind the recommendation for use of concurrent chemotherapy and radiation derives from many randomized trials41–47 and several meta-analyses48–52. The more significant of these, the large Meta-Analysis of Chemotherapy on Head and Neck Cancer (MACH-NC), was recently updated in 201649–52. The recent update in 201652 analyzed 94 trials (18,934 patients) comparing local RT alone to RT plus concurrent/alternating chemotherapy, or induction chemotherapy followed by concurrent chemoradiotherapy (CRT). The addition of chemotherapy to RT led to improved OS versus RT alone, but this benefit was limited to concomitant CRT, but not induction chemotherapy, translating into a 5-year and 10-year absolute survival benefit of 6.5% and 3.5%, respectively.
Nevertheless, potential benefits of induction chemotherapy remain unclear. Induction chemotherapy may allow for significant reduction in the rate of distant metastasis according to results of the 2009 MACH-NC update51. Additional rationale behind induction chemotherapy include possible increase in the efficacy of subsequent chemoradiotherapy resulting from its down-staging effects on the gross tumor volume and that it may act as a test of tumor radiosensitivity53. These potential advantages have driven continued active research focusing on the utility of induction chemotherapy in HNSCC53–57. A triplet chemotherapy regimen (cisplatin plus, 5-fluorouracil, and a taxane - TPF) revealed improved responses over cisplatin/5-fluorouracil58–64, and for doectaxel containing regimens59, improved overall survival, which has led to USFDA approval of docetaxel for HNSCC and further exploration of TPF as an induction regimen.
Despite this recent emphasis on triplet chemotherapy as an induction strategy, the current literature has not yielded consistent results in favor of this approach. If induction chemotherapy followed by CRT is used, evidence is in favor of the addition of a docetaxel to cisplatin/5-fluorouracil2. Due to high toxicity, it is not recommended to follow cisplatin-based induction with cisplatin-based CRT2,65,66. Recommended agents for CRT after induction include weekly carboplatin or cetuximab63,67,68.
What Are the Considerations for Patients Who Have Distant Metastatic Disease at Initial Presentation?
The standard treatment of patients with HNSCC who are determined to have distant metastases at first diagnosis should be largely dictated by the patient’s performance status, personal goals, co-morbidities, potential cost, and consideration of toxicities and prognosis. Treatment options for these patients include combination systemic chemotherapy, single agent chemotherapy, palliative RT or best supportive care2. Enrollment in clinical trials is preferred, if possible2. Many of the regimens employed to treat unresectable disease are similarly recommended for patients with distant metastasis, as many of the studies in support of these regimens included patients with unresectable and/or distant metastatic disease for inclusion.
Acceptable regimens include cisplatin or carboplatin in combination with 5-fluorouracil/cetuximab69,70, cisplatin or carboplatin/docetaxel (or paclitaxel)71,72, cisplatin/cetuximab73, cisplatin/5-fluorouracil72,74,75 or cisplatin or carboplatin in combination with docetaxel (or paclitaxel)/cetuximab76–78. Drug options for single agent therapy include cisplatin73,75, carboplatin79, paclitaxel80, docetaxel81,82, 5-fluorouracil75, methotrexate74,83 cetuximab84, afatinib85,86 or capecitabine87,88.
The multi-drug regimens included above generally improve response rates over single agents, but until the addition of cetuximab, have never been shown to improve survival over single agent approaches2. Response rates for single agent chemotherapeutics for recurrent or metastatic disease is about 10-20% with a modest increase in response (30-40%) when platinum-based combination regimens are selected89. Only a few studies have described differences between using multiple cytotoxic agents and single agents. One such study by Jacobs et al.75 reported a phase III study involving patients with recurrent or metastatic HNSCC randomized to cisplatin alone, 5-fluorouracil only, or the combination once every three weeks. The ORR improved with the combination; however, the median survival remained 5.7 months for all groups. The Southwest Oncology Group conducted a similar phase III trial74 treating patients with cisplatin and 5-fluorouracil, carboplatin and 5-fluorouracil, or methotrexate. The response rate of cisplatin and 5-fluorouracil was greater than methotrexate. The difference in response rate was not significant for the combination of carboplatin and 5-fluorouracil versus methotrexate. Further, the median OS was not significantly different across all three arms.
Based on literature comparing different combinational approaches, there is no straightforward consensus for choosing between various platinum regimens. A trial (E1395) by the Eastern Cooperative Oncology Group72 compared cisplatin and 5-fluorouracil to cisplatin and paclitaxel in patients with recurrent or metastatic HNSCC. At one year there was no difference in OS between the treatment groups. Nevertheless, taxane combinations with platinum agents offer an advantage in terms of ease of administration and schedule and obviates the need for an indwelling catheter or port.
The addition of cetuximab in first line treatment of metastatic disease in combination with chemotherapy and as a single agent in patients with platinum resistant disease has been well documented. The EXTREME trial by Vermoken et el.69 was the first phase III trial to show improvement in OS with the addition of cetuximab to previous standard agents. This study enrolled patients with untreated recurrent or metastatic HNSCC and randomized them to chemotherapy (with either cisplatin or carboplatin plus 5-fluorouracil every 3 weeks) with or without cetuximab. The addition of cetuximab significantly improved OS from 7.4 months in the chemotherapy alone arm (either cisplatin or carboplatin plus 5-fluorouracil) to 10.1 months for the chemotherapy plus cetuximab group. Progression free survival was also improved from 3.3 months to 5.6 months, respectively. The phase III study by Burtness et al.73 randomized patients with recurrent or metastatic HNSCC to receive cisplatin with either cetuximab or placebo. The addition of cetuximab to cisplatin improved the ORR from 10% to 26% over cisplatin/placebo but did not demonstrate a statistically significant improvement in either progression free survival or OS.
Recently developed drugs under investigation for use in metastatic HNSCC include capecitabine and afatinib. Afatinib is an irreversible inhibitor of EGFR, HER 2 and HER485. Capecitabine is a prodrug that is converted to its only active metabolite, fluorouracil, by thymidine phosphorylase87. Higher levels of this enzyme are found in several tumors and the liver, compared with normal healthy tissue. Both of these drugs have low level recommendations by the NCCN for use in recurrent or metastatic disease in patients who have had disease progression on platinum-based therapy2. Afatinib has more robust data with a published phase III study86, while use of capecitabine is advised based on evidence from phase II studies87,88. Research toward the utility of these agents in the setting of failure after more substantiated regiments is ongoing.
How Do the Guidelines Change for Recurrent/Persistent Disease? What if the Patient Has Had Prior Radiotherapy?
If disease-extent allows, patients with recurrent or persistent disease should have surgery, regardless of radiation history. High-risk pathological features after salvage surgery should be managed by PORT or POCRT as detailed in the high-risk disease section above. For patients with recurrent or persistent disease who had previous RT and have resectable disease, surgery with possible reirradiation (with or without concurrent chemotherapy) is indicated. Alternatively, if the disease is resectable but surgery is not performed, similar schedules of CRT that are used for treatment-naïve disease, as mentioned in a preceding section, are indicated2.
The rationale supporting salvage surgery for patients with resectable recurrent or persistent disease is a complex topic and exceeds the scope of this article; but, it is important to address a few aspects. Salvage surgery may offer a high chance of long-term disease control and a possible cure to select patients, yet morbidity from surgery in this setting can result in significant suffering and cost with marginal benefit in survival89. Salvage procedures often result in reduction or permanent loss of function of important head and neck structures (ie. the larynx, pharynx, oral cavity, etc.), leading to significant cosmetic detriments, high economic burden, and even death. One of the most difficult, but critical, questions is determining which patients will maximally benefit from salvage surgery while reducing potential harms as much as possible.
An extensive informed discussion must occur between the surgeon and patient, particularly assessing prognostic factors which may help guide expectations and choice of suitable surgical candidates. The patient’s co-morbidities, life expectancy, performance status, speech and swallowing function, nutritional status and severity of current symptoms need to be evaluated. Significant prognostic factors that should be considered to facilitate management decisions include recurrent stage90–92, recurrent site90,91, disease-free interval, initial treatment by chemoradiation, and locoregional versus limited local recurrence91. Improved outcomes following salvage surgery have been noted in patients with lower stage disease90–92, disease-free interval greater than 6 months91, initial treatment with radiation alone compared to CRT91, and laryngeal recurrence in respect to other subsites90,92.
If previous treatment with radiation was performed, and the disease is unresectable, management includes reirradiation with or without chemotherapy, chemotherapy alone or best supportive care2. Regimens for CRT or chemotherapy, are similar to metastatic disease, as noted above. Patients with this level of disease severity should be offered enrollment into clinical trials.
Reirradiation may also be considered in the post-operative setting. The efficacy of reirradiation may be negatively affected by local scarring and fibrosis from previous radiation, as well as, difficulty in achieving adequate dosage because of proximity to vital structures which are already near tissue tolerance. Additionally, recurrence implies a degree of radiation resistance if the initial therapy was properly administered. Reirradiation should be carefully considered because of an increase in acute and late toxicities2.
Janot et al.93 reported on 130 patients with HNSCC who previously underwent surgery for a recurrence or a second primary tumor in a previously irradiated area that were randomly assigned to full-dose reirradiation combined with chemotherapy or to observation. Patients in the reirradiation arm received 60 Gy over 11 weeks combined with concomitant fluorouracil and hydroxyurea. A significant difference in locoregional control was found between the two groups, in favor of the reirradiation arm. Disease-free survival was significantly improved in the chemotherapy plus reirradiation arm, but OS was not statistically different. At 24 months, 39% of patients in the RT arm and 10% in the observation arm experienced grade 3 or 4 late toxicity. The main grade 3 and 4 late toxicities were sclerosis, trismus, and osteoradionecrosis. The higher likelihood of increased toxicity should be strongly considered if treating with reirradiation (with or without concurrent chemotherapy) for the potential benefit to locoregional control and disease-free survival.
What are the Recommendations Regarding Follow-Up?
Despite aggressive treatment of advanced disease, locoregional recurrences develop in 30% to 40% of patients and distant metastases occur in 20% to 30% (91,92). These patients also have an elevated risk of developing a second primary cancer if they have pronounced smoking and/or alcohol history. These second primaries more commonly consist of lung or upper aerodigestive tract cancers, but also include head and neck malignancies94,95. Post-treatment surveillance assumes that early recurrence detection can improve outcomes among patients with advanced HNSCC. However, the literature regarding head and neck cancer suggests that regular follow-up is not significantly associated with improved survival outcomes96–99.
Nonetheless, proper follow up is essential for monitoring any recurrence or development of a second primary, assessing treatment results, managing acute and chronic treatment related side effects, providing tobacco education, monitoring for psychosocial well-being, meeting any needs for speech or swallow treatment and offering emotional and morale support. A follow-up schedule must be tailored to the individual patient based on stage of disease at diagnosis, extent of treatment received, the site of the tumor, and patient variables including age and comorbidities.
The NCCN guidelines recommend complete clinical exam and physical, including fiberoptic examination, every 1 to 3 months for the first year, every 2 to 6 months for the second year, every 4 to 8 months between years 3 and 5, and annually thereafter. Thyroid stimulating hormone should be ordered every 6-12 months if irradiation of the neck occurred. For patients 50 years or older with more than a 20 pack-year history of smoking, initial screening recommendations include a low-dose CT scan, followed by annual low-dose CT scans for 2 years2. Continued routine imaging may be indicated based on sign/symptoms or desire to assess areas inaccessible to clinical examination. A post-treatment baseline imaging using PET/CT is recommended for patients with T3/T4 or N2/N3 cancers of the oropharynx, hypopharynx, larynx, and nasopharynx. This PET/CT should be within 6 months of treatment, but is ideally performed approximately 12 weeks after the last treatment2.
A recent study by Mehanna et al.100 investigated the utility of PET/CT surveillance versus planned neck dissection in 564 patients with nodal stage N2 or N3 HNSCC without metastasis treated with primary chemoradiotherapy. The 2-year OS rate was 84.9% (95% CI 80.7 to 89.1) in the surveillance group and 81.5% (95% CI 76.9 to 86.3) in the planned-surgery group. The HR for death with surveillance as compared with planned surgery was 0.92 (95% CI 0.65 to 1.32). This study suggests that there may be a role for PET/CT in follow-up for patients with severe nodal disease to monitor for recurrence, sparing routine neck dissections for patients. More information on the question of routine surveillance imaging on asymptomatic patients and additional follow-up interventions may be found in the follow-up and surveillance entry in this series101.
What is the Emerging Role of Immunotherapy?
For patients with recurrent/persistent or metastatic HNSCC that have had disease progression despite primary platinum-containing therapy, new immunotherapy agents are now a standard of care option for second-line therapy. Two monoclonal antibodies directed against programmed death receptor-1 (PD-1), pembrolizumab and nivolumab, have received FDA approval for the treatment of patients with recurrent or metastatic HNSCC with disease progression on or after platinum-containing chemotherapy.
Immunotherapy engages and/or enhances immune system function leading to tumor eradication or long-term growth inhibition. Recent advancements in immunotherapy for HNSCC have focused on inhibition of PD-1/PD-L1. PD-1 is a cell membrane receptor expressed on antigen stimulated T cells, B cells, natural killer T cells and dendritic cells102–104. In normal tissues, activation of PD-1 results in inhibition and apoptosis of immune cells, largely utilized to enhance self-tolerance by T cells and prevents autoimmune reactions. Binding of PD-1 to its ligands, PD-L1 and PD-L2, suppresses the immune response by inhibiting T cell proliferation, cytokine release and cytotoxicity102,103,105. Tumor cells with abnormal PD-L1 expression activate the PD-1 receptor, suppressing cytotoxic T cells and avoiding recognition and elimination by the immune system. Anti PD-1 therapies represent a form of reactivating the immune system to attack the tumor by blocking interaction between PD-1 and its ligands. Multiple studies have examined the expression of the programmed death receptor ligand PD-L1 in HNSCC across multiple primary sites revealing expression levels between 46 to 100% depending on staining method, fixation and site105–110.
Pembrolizumab, was given accelerated approval by the FDA in August 2016111, based on the work by Seiwert and Chow et al. in the KEYNOTE-012 trial investigating activity of pembrolizumab for patients with recurrent or metastatic HNSCC, published in July 2016112. 104 patients were screened,81 (78%) were identified to have PD-L1 positive tumors and 60 were treated with pembrolizumab. The ORR by central imaging review (CT or MRI) was 18%. An expansion cohort, including patients with recurrent or metastatic disease, regardless of PD-L1 biomarker status, was published in September 2016113. 132 patients were enrolled and after a medial follow up of 9 months the ORR was 18%.
Similarly, an additional PD-1 monoclonal antibody, nivolumab, gained approval from the FDA in November 2016 for the same indication as pembrolizumab114. Ferris et al. investigated the use of nivolumab in HNSCC in a phase III trial115. In this study, 361 patients with recurrent or metastatic disease were enrolled comparing nivolumab to standard single agent therapy (either cetuximab, methotrexate, or docetaxel). In the nivolumab group, the median OS was improved versus the standard therapy group, 7.5 months and 5.1 months, respectively. Although recent top line data from the Keynote-040 phase III study, released in June 2017, demonstrated that pembrolizumab failed to meet its pre-specified primary specified endpoint of overall survival116, both nivolumab and pembrolizumab are under extensive investigation with multiple ongoing trials assessing their utility in combination with current therapy for HNSCC. Regimens combining immunotherapy with other modalities will likely further improve outcomes.
CONCLUSION
Treatment of advanced HNSCC remains a complex decision process, and thorough understanding of the current management recommendations is essential for providing high quality care to patients burdened by this often grave disease.
Figure 1.
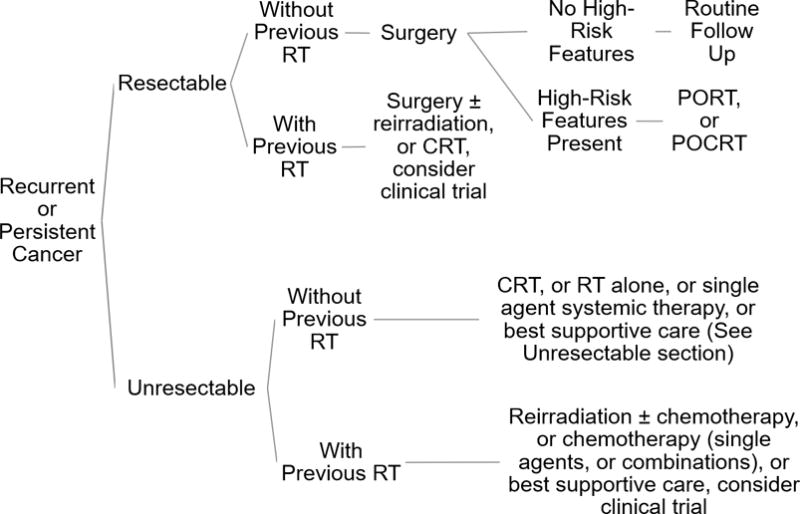
Treatment Schematic for Recurrent/Persistent Disease. High-risk features: positive surgical margins, extracapsular extension, perineural invasion, lymphovascular invasion. Abbreviation: RT, radiotherapy; PORT, post-operative radiotherapy; POCRT, post-operative chemoradiotherapy; CRT, chemoradiotherapy. Summarized from the NCCN guidelines2.
Acknowledgments
Dr. Thomas J. Ow’s contribution to this manuscript was supported by NIH-NCI grant 2K12 CA132783-06. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. Head and Neck Cancers, version 1. 2017 doi: 10.6004/jnccn.2015.0102. Available at: https://www.nccn.org/professionals/physician_gls/PDF/head-and-neck.pdf. [DOI] [PMC free article] [PubMed]
- 3.Marur S, D’souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen-Tan PF, Zhang Q, Ang KK. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(34):3858–66. doi: 10.1200/JCO.2014.55.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Posner MR, Lorch JH, Goloubeva O. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Annals of oncology: official journal of the European Society for Medical Oncology. 2011;22(5):1071–7. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rischin D, Young RJ, Fisher R. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(27):4142–8. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Sullivan B, Huang SH, Su J. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. The Lancet Oncology. 2016;17(4):440–51. doi: 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan B, Lydiatt WM, Haughey BH, et al. HPV-Mediated (p16+) Oropharyngeal Cancer. In: Amin MB, editor. AJCC Cancer Staging Manual. 8th. Springer; New York: 2017. p. 113. [Google Scholar]
- 9.Lydiatt WM, Ridge JA, Patel SG, et al. Oropharynx (p16-) and Hypopharynx. In: Amin MB, editor. AJCC Cancer Staging Manual. 8th. Springer; New York: 2017. p. 123. [Google Scholar]
- 10.Mirimanoff RO, Wang CC, Doppke KP. Combined surgery and postoperative radiation therapy for advanced laryngeal and hypopharyngeal carcinomas. Int J Radiat Oncol Biol Phys. 1985;11(3):499–504. doi: 10.1016/0360-3016(85)90180-4. [DOI] [PubMed] [Google Scholar]
- 11.Al-sarraf M, Pajak TF, Byhardt RW, Beitler JJ, Salter MM, Cooper JS. Postoperative radiotherapy with concurrent cisplatin appears to improve locoregional control of advanced, resectable head and neck cancers: RTOG 88-24. Int J Radiat Oncol Biol Phys. 1997;37(4):777–82. doi: 10.1016/s0360-3016(96)00614-1. [DOI] [PubMed] [Google Scholar]
- 12.Carter RL, Barr LC, O’brien CJ, Soo KC, Shaw HJ. Transcapsular spread of metastatic squamous cell carcinoma from cervical lymph nodes. Am J Surg. 1985;150(4):495–9. doi: 10.1016/0002-9610(85)90162-x. [DOI] [PubMed] [Google Scholar]
- 13.Olsen KD, Caruso M, Foote RL, et al. Primary head and neck cancer. Histopathologic predictors of recurrence after neck dissection in patients with lymph node involvement. Arch Otolaryngol Head Neck Surg. 1994;120(12):1370–4. doi: 10.1001/archotol.1994.01880360066012. [DOI] [PubMed] [Google Scholar]
- 14.Langendijk JA, Slotman BJ, Van der waal I, Doornaert P, Berkof J, Leemans CR. Risk-group definition by recursive partitioning analysis of patients with squamous cell head and neck carcinoma treated with surgery and postoperative radiotherapy. Cancer. 2005;104(7):1408–17. doi: 10.1002/cncr.21340. [DOI] [PubMed] [Google Scholar]
- 15.Carter RL, Tanner NS, Clifford P, Shaw HJ. Perineural spread in squamous cell carcinomas of the head and neck: a clinicopathological study. Clin Otolaryngol Allied Sci. 1979;4(4):271–81. doi: 10.1111/j.1365-2273.1979.tb01900.x. [DOI] [PubMed] [Google Scholar]
- 16.Brandwein-gensler M, Smith RV. Prognostic indicators in head and neck oncology including the new 7th edition of the AJCC staging system. Head Neck Pathol. 2010;4(1):53–61. doi: 10.1007/s12105-010-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 18.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–44. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 19.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27(10):843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 20.Cooper JS, Zhang Q, Pajak TF. Long-term follow-up of the RTOG (#9501)/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. International journal of radiation oncology, biology, physics. 2012;84(5):1198–205. doi: 10.1016/j.ijrobp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edge SB. AJCC Cancer Staging Manual. New York, NY: Springer; 2010. pp. 21–68. [Google Scholar]
- 22.Vermorken J, Specenier P. Cetuximab: its unique place in head and neck cancer treatment. BTT. 2013 doi: 10.2147/BTT.S43628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer research. 1993;53(15):3579–84. [PubMed] [Google Scholar]
- 24.Ang KK, Berkey BA, Tu X. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer research. 2002;62(24):7350–6. [PubMed] [Google Scholar]
- 25.Rubin Grandis J, Melhem MF, Gooding WE. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. Journal of the National Cancer Institute. 1998;90(11):824–32. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 26.Chung CH, Ely K, McGavran L. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. JCO. 2006;24(25):4170–6. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 27.Temam S, Kawaguchi H, El-Naggar AK, et al. Epidermal Growth Factor Receptor Copy Number Alterations Correlate With Poor Clinical Outcome in Patients With Head and Neck Squamous Cancer. JCO. 2007;25(16):2164–2170. doi: 10.1200/JCO.2006.06.6605. [DOI] [PubMed] [Google Scholar]
- 28.Harari PM, Harris J, Kies MS, et al. Postoperative Chemoradiotherapy and Cetuximab for High-Risk Squamous Cell Carcinoma of the Head and Neck: Radiation Therapy Oncology Group RTOG-0234. JCO. 2014;32(23):2486–2495. doi: 10.1200/JCO.2013.53.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 30.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. The Lancet Oncology. 2010;11(1):21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 31.Suntharalingam M, Kwok Y, Goloubeva O, et al. Phase II Study Evaluating the Addition of Cetuximab to the Concurrent Delivery of Weekly Carboplatin, Paclitaxel, and Daily Radiotherapy for Patients With Locally Advanced Squamous Cell Carcinomas of the Head and Neck. International Journal of Radiation Oncology Biology Physics. 2012;82(5):1845–1850. doi: 10.1016/j.ijrobp.2011.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kao J, Genden EM, Gupta V, et al. Phase 2 trial of concurrent 5-fluorouracil, hydroxyurea, cetuximab, and hyperfractionated intensity-modulated radiation therapy for locally advanced head and neck cancer. Cancer. 2011;117(2):318–326. doi: 10.1002/cncr.25374. [DOI] [PubMed] [Google Scholar]
- 33.Tong CC, Lau KH, Rivera M. Prognostic significance of p16 in locoregionally advanced head and neck cancer treated with concurrent 5-fluorouracil, hydroxyurea, cetuximab and intensity-modulated radiation therapy. Oncology reports. 2012;27(5):1580–6. doi: 10.3892/or.2012.1679. [DOI] [PubMed] [Google Scholar]
- 34.Merlano M, Russi E, Benasso M. Cisplatin-based chemoradiation plus cetuximab in locally advanced head and neck cancer: a phase II clinical study. Annals of oncology: official journal of the European Society for Medical Oncology. 2011;22(3):712–7. doi: 10.1093/annonc/mdq412. [DOI] [PubMed] [Google Scholar]
- 35.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin With or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. JCO. 2014;32(27):2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denis F, Garaud P, Bardet E, et al. Final Results of the 94–01 French Head and Neck Oncology and Radiotherapy Group Randomized Trial Comparing Radiotherapy Alone With Concomitant Radiochemotherapy in Advanced-Stage Oropharynx Carcinoma. JCO. 2004;22(1):69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Bourhis J, Sire C, Graff P, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. The Lancet Oncology. 2012;13(2):145–153. doi: 10.1016/S1470-2045(11)70346-1. [DOI] [PubMed] [Google Scholar]
- 38.Garden A, Harris J, Vokes E, et al. Preliminary Results of Radiation Therapy Oncology Group 97-03: A Randomized Phase II Trial of Concurrent Radiation and Chemotherapy for Advanced Squamous Cell Carcinomas of the Head and Neck. JCO. 2004;22(14):2856–2864. doi: 10.1200/JCO.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Taylor SG, Murthy AK, Vannetzel JM, et al. Randomized comparison of neoadjuvant cisplatin and fluorouracil infusion followed by radiation versus concomitant treatment in advanced head and neck cancer. JCO. 1994;12(2):385–395. doi: 10.1200/JCO.1994.12.2.385. [DOI] [PubMed] [Google Scholar]
- 40.Suntharalingam M, Haas ML, Conley BA, et al. The use of carboplatin and paclitaxel with daily radiotherapy in patients with locally advanced squamous cell carcinomas of the head and neck. International Journal of Radiation Oncology Biology Physics. 2000;47(1):49–56. doi: 10.1016/s0360-3016(00)00408-9. [DOI] [PubMed] [Google Scholar]
- 41.Browman GP, Cripps C, Hodson DI, Eapen L, Sathya J, Levine MN. Placebo-controlled randomized trial of infusional fluorouracil during standard radiotherapy in locally advanced head and neck cancer. JCO. 1994;12(12):2648–2653. doi: 10.1200/JCO.1994.12.12.2648. [DOI] [PubMed] [Google Scholar]
- 42.Merlano M, Benasso M, Corvò R. Five-year update of a randomized trial of alternating radiotherapy and chemotherapy compared with radiotherapy alone in treatment of unresectable squamous cell carcinoma of the head and neck. Journal of the National Cancer Institute. 1996;88(9):583–9. doi: 10.1093/jnci/88.9.583. [DOI] [PubMed] [Google Scholar]
- 43.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated Irradiation with or without Concurrent Chemotherapy for Locally Advanced Head and Neck Cancer. N Engl J Med. 1998;338(25):1798–1804. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 44.Wendt TG, Grabenbauer GG, Rödel CM, et al. Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: a randomized multicenter study. JCO. 1998;16(4):1318–1324. doi: 10.1200/JCO.1998.16.4.1318. [DOI] [PubMed] [Google Scholar]
- 45.Jeremic B, Shibamoto Y, Milicic B, et al. Hyperfractionated Radiation Therapy With or Without Concurrent Low-Dose Daily Cisplatin in Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Prospective Randomized Trial. JCO. 2000;18(7):1458–1464. doi: 10.1200/JCO.2000.18.7.1458. [DOI] [PubMed] [Google Scholar]
- 46.Adelstein DJ, Li Y, Adams GL, et al. An Intergroup Phase III Comparison of Standard Radiation Therapy and Two Schedules of Concurrent Chemoradiotherapy in Patients With Unresectable Squamous Cell Head and Neck Cancer. JCO. 2003;21(1):92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Munro AJ. An overview of randomised controlled trials of adjuvant chemotherapy in head and neck cancer. British journal of cancer. 1995;71(1):83–91. doi: 10.1038/bjc.1995.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Sayed S, Nelson N. Adjuvant and adjunctive chemotherapy in the management of squamous cell carcinoma of the head and neck region. A meta-analysis of prospective and randomized trials. JCO. 1996;14(3):838–847. doi: 10.1200/JCO.1996.14.3.838. [DOI] [PubMed] [Google Scholar]
- 49.Pignon J, Bourhis J, Domenge C, Designé L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. The Lancet. 2000;355(9208):949–955. [PubMed] [Google Scholar]
- 50.Pignon J, le Maître A, Bourhis J. Meta-Analyses of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update. International Journal of Radiation Oncology Biology Physics. 2007;69(2):S112–S114. doi: 10.1016/j.ijrobp.2007.04.088. [DOI] [PubMed] [Google Scholar]
- 51.Pignon JP, le Maître A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Blanchard P, Landais C, Petit C, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 100 randomized trials and 19,248 patients, on behalf of MACH-NC group. 2016;27 [Google Scholar]
- 53.Haigentz M, Cohen EE, Wolf GT, Strojan P, Eisbruch A, Ferlito A. The future of induction chemotherapy for head and neck squamous cell carcinoma. Oral oncology. 2012;48(11):1065–7. doi: 10.1016/j.oraloncology.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Induction Chemotherapy plus Radiation Compared with Surgery plus Radiation in Patients with Advanced Laryngeal Cancer. N Engl J Med. 1991;324(24):1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 55.Lefebvre J, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx Preservation in Pyriform Sinus Cancer: Preliminary Results of a European Organization for Research and Treatment of Cancer Phase III Trial. JNCI Journal of the National Cancer Institute. 1996;88(13):890–899. doi: 10.1093/jnci/88.13.890. [DOI] [PubMed] [Google Scholar]
- 56.Forastiere AA, Goepfert H, Maor M, et al. Concurrent Chemotherapy and Radiotherapy for Organ Preservation in Advanced Laryngeal Cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 57.Forastiere AA, Zhang Q, Weber RS, et al. Long-Term Results of RTOG 91-11: A Comparison of Three Nonsurgical Treatment Strategies to Preserve the Larynx in Patients With Locally Advanced Larynx Cancer. JCO. 2013;31(7):845–852. doi: 10.1200/JCO.2012.43.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hitt R, López-Pousa A, Martínez-Trufero J, et al. Phase III Study Comparing Cisplatin Plus Fluorouracil to Paclitaxel, Cisplatin, and Fluorouracil Induction Chemotherapy Followed by Chemoradiotherapy in Locally Advanced Head and Neck Cancer. JCO. 2005;23(34):8636–8645. doi: 10.1200/JCO.2004.00.1990. [DOI] [PubMed] [Google Scholar]
- 59.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and Fluorouracil Alone or with Docetaxel in Head and Neck Cancer. N Engl J Med. 2007;357(17):1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 60.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, Fluorouracil, and Docetaxel in Unresectable Head and Neck Cancer. N Engl J Med. 2007;357(17):1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 61.Pointreau Y, Garaud P, Chapet S, et al. Randomized Trial of Induction Chemotherapy With Cisplatin and 5-Fluorouracil With or Without Docetaxel for Larynx Preservation. JNCI Journal of the National Cancer Institute. 2009;101(7):498–506. doi: 10.1093/jnci/djp007. [DOI] [PubMed] [Google Scholar]
- 62.Hitt R, et al. Final results of a randomized phase III trial comparing induction chemotherapy with cisplatin/5-FU or docetaxel/cisplatin/5-FU follow by chemoradiotherapy (CRT) versus CRT alone as first-line treatment of unresectable locally advanced head and neck cancer (LAHNC) JCO. 2009;27:15s. suppl; abstr 6009. [Google Scholar]
- 63.Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. The Lancet Oncology. 2013;14(3):257–264. doi: 10.1016/S1470-2045(13)70011-1. [DOI] [PubMed] [Google Scholar]
- 64.Cohen EE, Karrison TG, Kocherginsky M, et al. Phase III Randomized Trial of Induction Chemotherapy in Patients With N2 or N3 Locally Advanced Head and Neck Cancer. JCO. 2014;32(25):2735–2743. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hitt R, Grau JJ, Lopez-Pousa A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Annals of Oncology. 2013;25(1):216–225. doi: 10.1093/annonc/mdt461. [DOI] [PubMed] [Google Scholar]
- 66.Lefebvre JL, Pointreau Y, Rolland F, et al. Induction Chemotherapy Followed by Either Chemoradiotherapy or Bioradiotherapy for Larynx Preservation: The TREMPLIN Randomized Phase II Study. JCO. 2013;31(7):853–859. doi: 10.1200/JCO.2012.42.3988. [DOI] [PubMed] [Google Scholar]
- 67.Paccagnella A, Ghi MG, Loreggian L, et al. Concomitant chemoradiotherapy versus induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by concomitant chemoradiotherapy in locally advanced head and neck cancer: a phase II randomized study. Annals of Oncology. 2009;21(7):1515–1522. doi: 10.1093/annonc/mdp573. [DOI] [PubMed] [Google Scholar]
- 68.Buiret G, Combe C, Favrel V, et al. A Retrospective, Multicenter Study of the Tolerance of Induction Chemotherapy With Docetaxel, Cisplatin, and 5-Fluorouracil Followed by Radiotherapy With Concomitant Cetuximab in 46 Cases of Squamous Cell Carcinoma of the Head and Neck. International Journal of Radiation Oncology*Biology*Physics. 2010;77(2):430–437. doi: 10.1016/j.ijrobp.2009.04.066. [DOI] [PubMed] [Google Scholar]
- 69.Vermorken JB, Mesia R, Rivera F, et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 70.Herbst RS, Arquette M, Shin DM, et al. Phase II Multicenter Study of the Epidermal Growth Factor Receptor Antibody Cetuximab and Cisplatin for Recurrent and Refractory Squamous Cell Carcinoma of the Head and Neck. JCO. 2005;23(24):5578–5587. doi: 10.1200/JCO.2005.07.120. [DOI] [PubMed] [Google Scholar]
- 71.Samlowski WE, Moon J, Kuebler JP, et al. Evaluation of the Combination of Docetaxel/Carboplatin in Patients with Metastatic or Recurrent Squamous Cell Carcinoma of the Head and Neck (SCCHN): A Southwest Oncology Group Phase II Study. Cancer Investigation. 2009;25(3):182–188. doi: 10.1080/07357900701209061. [DOI] [PubMed] [Google Scholar]
- 72.Gibson MK, Li Y, Murphy B, et al. Randomized Phase III Evaluation of Cisplatin Plus Fluorouracil Versus Cisplatin Plus Paclitaxel in Advanced Head and Neck Cancer (E1395): An Intergroup Trial of the Eastern Cooperative Oncology Group. JCO. 2005;23(15):3562–3567. doi: 10.1200/JCO.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 73.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III Randomized Trial of Cisplatin Plus Placebo Compared With Cisplatin Plus Cetuximab in Metastatic/Recurrent Head and Neck Cancer: An Eastern Cooperative Oncology Group Study. JCO. 2005;23(34):8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 74.Forastiere AA, Metch B, Schuller DE, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. JCO. 1992;10(8):1245–1251. doi: 10.1200/JCO.1992.10.8.1245. [DOI] [PubMed] [Google Scholar]
- 75.Jacobs C, Lyman G, Velez-García E, et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. JCO. 1992;10(2):257–263. doi: 10.1200/JCO.1992.10.2.257. [DOI] [PubMed] [Google Scholar]
- 76.Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Annals of Oncology. 2010;21(Supplement 7):vii252–vii261. doi: 10.1093/annonc/mdq453. [DOI] [PubMed] [Google Scholar]
- 77.Hitt R, Irigoyen A, Cortes-Funes H, Grau JJ, García-Sáenz JA, Cruz-Hernandez JJ. Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. 2012;23(4):1016–1022. doi: 10.1093/annonc/mdr367. [DOI] [PubMed] [Google Scholar]
- 78.Buentzel J, et al. Experience with cetuximab plus paclitaxel/carboplatinum in primary platinum-resistant recurrent head and neck cancer. JCO. 2007 Jun;25:6077–6077. no. 18_suppl. [Google Scholar]
- 79.Al-Sarraf M, Metch B, Kish J. Platinum analogs in recurrent and advanced head and neck cancer: a Southwest Oncology Group and Wayne State University Study. Cancer treatment reports. 1987;71(7–8):723–6. [PubMed] [Google Scholar]
- 80.Grau JJ, Caballero M, Verger E, Monzó M, Blanch JL. Weekly paclitaxel for platin-resistant stage IV head and neck cancer patients. Acta oto-laryngologica. 2009;129(11):1294–9. doi: 10.3109/00016480802590451. [DOI] [PubMed] [Google Scholar]
- 81.Catimel G, Verweij J, Mattijssen V. Docetaxel (Taxotere): an active drug for the treatment of patients with advanced squamous cell carcinoma of the head and neck. EORTC Early Clinical Trials Group. Annals of oncology: official journal of the European Society for Medical Oncology. 1994;5(6):533–7. doi: 10.1093/oxfordjournals.annonc.a058908. [DOI] [PubMed] [Google Scholar]
- 82.Guardiola E, Peyrade F, Chaigneau L. Results of a randomised phase II study comparing docetaxel with methotrexate in patients with recurrent head and neck cancer. European journal of cancer (Oxford, England: 1990) 2004;40(14):2071–6. doi: 10.1016/j.ejca.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 83.Stewart JS, Cohen EE, Licitra L. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected] Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(11):1864–71. doi: 10.1200/JCO.2008.17.0530. [DOI] [PubMed] [Google Scholar]
- 84.Vermorken JB, Trigo J, Hitt R, et al. Open-Label, Uncontrolled, Multicenter Phase II Study to Evaluate the Efficacy and Toxicity of Cetuximab As a Single Agent in Patients With Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck Who Failed to Respond to Platinum-Based Therapy. JCO. 2007;25(16):2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 85.Specenier P, Vermorken J. Afatinib in squamous cell carcinoma of the head and neck. Expert opinion on pharmacotherapy. 2016;17(9):1295–301. doi: 10.1080/14656566.2016.1183647. [DOI] [PubMed] [Google Scholar]
- 86.Machiels JP, Haddad RI, Fayette J. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. The Lancet. Oncology. 2015;16(5):583–94. doi: 10.1016/S1470-2045(15)70124-5. [DOI] [PubMed] [Google Scholar]
- 87.Iqbal H, Pan Q. Capecitabine for treating head and neck cancer. Expert opinion on investigational drugs. 2016;25(7):851–9. doi: 10.1080/13543784.2016.1181747. [DOI] [PubMed] [Google Scholar]
- 88.Martinez-Trufero J, Isla D, Adansa JC. Phase II study of capecitabine as palliative treatment for patients with recurrent and metastatic squamous head and neck cancer after previous platinum-based treatment. British journal of cancer. 2010;102(12):1687–91. doi: 10.1038/sj.bjc.6605697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haigentz M, Hartl DM, Silver CE. Distant metastases from head and neck squamous cell carcinoma. Part III. Treatment. Oral oncology. 2012;48(9):787–93. doi: 10.1016/j.oraloncology.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 90.Goodwin WJ. Salvage Surgery for Patients With Recurrent Squamous Cell Carcinoma of the Upper Aerodigestive Tract: When Do the Ends Justify the Means? The Laryngoscope. 2000;110(S93):1–18. doi: 10.1097/00005537-200003001-00001. [DOI] [PubMed] [Google Scholar]
- 91.Hamoir M, Holvoet E, Ambroise J, Lengelé B, Schmitz S. Salvage surgery in recurrent head and neck squamous cell carcinoma: Oncologic outcome and predictors of disease free survival. Oral Oncol. 2017;67:1–9. doi: 10.1016/j.oraloncology.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 92.Zafereo ME, Hanasono MM, Rosenthal DI, et al. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009;115(24):5723–5733. doi: 10.1002/cncr.24595. [DOI] [PubMed] [Google Scholar]
- 93.Janot F, de Raucourt D, Benhamou E, et al. Randomized Trial of Postoperative Reirradiation Combined With Chemotherapy After Salvage Surgery Compared With Salvage Surgery Alone in Head and Neck Carcinoma. JCO. 2008;26(34):5518–5523. doi: 10.1200/JCO.2007.15.0102. [DOI] [PubMed] [Google Scholar]
- 94.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 95.Priante AV, Castilho EC, Kowalski LP. Second primary tumors in patients with head and neck cancer. Curr Oncol Rep. 2011;13(2):132–7. doi: 10.1007/s11912-010-0147-7. [DOI] [PubMed] [Google Scholar]
- 96.Ritoe, et al. Value of routine follow-up for patients cured of laryngeal carcinoma. Cancer. 2004;101(6):1382–1389. doi: 10.1002/cncr.20536. [DOI] [PubMed] [Google Scholar]
- 97.Schwartz, et al. Postradiotherapy surveillance practice for head and neck squamous cell carcinoma—too much for too little? Head Neck. 2003;25(12):990–999. doi: 10.1002/hed.10314. [DOI] [PubMed] [Google Scholar]
- 98.Francis DO, Yueh B, Weymuller EA, Merati AL. Impact of surveillance on survival after laryngeal cancer in the medicare population. The Laryngoscope. 2009;119(12):2337–44. doi: 10.1002/lary.20576. [DOI] [PubMed] [Google Scholar]
- 99.Cooney TR, Poulsen MG. Is Routine Follow-up Useful After Combined-Modality Therapy for Advanced Head and Neck Cancer? Arch Otolaryngol Head Neck Surg. 1999;125(4):379–382. doi: 10.1001/archotol.125.4.379. [DOI] [PubMed] [Google Scholar]
- 100.Mehanna H, Wong WL, Mcconkey CC, et al. PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer. N Engl J Med. 2016;374(15):1444–54. doi: 10.1056/NEJMoa1514493. [DOI] [PubMed] [Google Scholar]
- 101.Roman BR, Goldenberg D, Givi B. AHNS Series-Do you know your guidelines? Guideline recommended follow-up and surveillance of head and neck cancer survivors. Head Neck. 2016;38(2):168–174. doi: 10.1002/hed.24100. [DOI] [PubMed] [Google Scholar]
- 102.Ascierto PA, Kalos M, Schaer DA, Callahan MK, Wolchok JD. Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(5):1009–20. doi: 10.1158/1078-0432.CCR-12-2982. [DOI] [PubMed] [Google Scholar]
- 103.Mamalis A, Garcha M, Jagdeo J. Targeting the PD-1 pathway: a promising future for the treatment of melanoma. Arch Dermatol Res. 2014;306(6):511–519. doi: 10.1007/s00403-014-1457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Current Opinion in Immunology. 2012;24(2):207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lyford-Pike S, Peng S, Young GD. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer research. 2013;73(6):1733–41. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strome SE, Dong H, Tamura H. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer research. 2003;63(19):6501–5. [PubMed] [Google Scholar]
- 107.Ukpo OC, Thorstad WL, Lewis JS. B7-H1 Expression Model for Immune Evasion in Human Papillomavirus-Related Oropharyngeal Squamous Cell Carcinoma. Head and Neck Pathol. 2012;7(2):113–121. doi: 10.1007/s12105-012-0406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou Y, Shi D, Miao J, et al. PD-L1 predicts poor prognosis for nasopharyngeal carcinoma irrespective of PD-1 and EBV-DNA load. Sci Rep. 2017;7:43627. doi: 10.1038/srep43627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hsu M, Hsiao J, Chang K, et al. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol. 2010;23(10):1393–1403. doi: 10.1038/modpathol.2010.130. [DOI] [PubMed] [Google Scholar]
- 110.Cho Y, Yoon H, Lee J, Hong S, Hong S. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncology. 2011;47(12):1148–1153. doi: 10.1016/j.oraloncology.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 111.U.S. Food and Drug Administration. pembrolizumab (KEYTRUDA) 2016 Aug 9; www.fda.gov.
- 112.Seiwert TY, Burtness B, Mehra R. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. The Lancet. Oncology. 2016;17(7):956–65. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 113.Chow LQ, Haddad R, Gupta S, et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. JCO. 2016;34(32):3838–3845. doi: 10.1200/JCO.2016.68.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.U.S. Food and Drug Administration. Nivolumab for SCCHN. 2016 Nov 10; www.fda.gov.
- 115.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Merck & Co., Inc. Merck Provides Update on Phase 3 Study of KEYTRUDA® (pembrolizumab) Monotherapy in Patients with Previously Treated Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (HNSCC) 2017 [Press release]. Retrieved from http://investors.merck.com/news/press-release-details/2017/Merck-Provides-Update-on-Phase-3-Study-of-KEYTRUDA-pembrolizumab-Monotherapy-in-Patients-with-Previously-Treated-Recurrent-or-Metastatic-Head-and-Neck-Squamous-Cell-Carcinoma-HNSCC/default.aspx.


