Abstract
The NLRP3 inflammasome, a caspase-1 activation platform plays a key role in the modulation of liver inflammation and fibrosis. Here, we tested the hypothesis that interleukin 17 (IL-17) and tumor necrosis factor (TNF) are key cytokines involved in amplifying and perpetuating the liver damage and fibrosis resulting from NLRP3 activation. To address this hypothesis gain of function Nlrp3A350V knock-in mice were bred onto il17a and Tnf knockout backgrounds allowing for constitutive Nlrp3 activation in myeloid derived cells in mice deficient in IL-17 or TNF. Livers of Nlrp3A350V knock-in mice exhibited severe liver inflammatory changes characterized by infiltration with neutrophils, increased expression of CXCL1 and CXCL2 chemokines, activated inflammatory macrophages and elevated levels of IL-17 and TNF. Mutants with ablation of il17a signal showed fewer neutrophils when compared to intact Nlrp3A350V mutants, but still significant inflammatory changes when compared to the non-mutant il17a knockout littermates. The severe inflammatory changes associated with mutant Nlrp3 were almost completely rescued by Tnf knockout in association with a marked decrease in circulating IL-1β levels. Intact Nlrp3A350V mutants showed changes of liver fibrosis, as evidenced by morphometric quantitation of Sirius red staining and increased mRNA levels of profibrotic genes including CTGF and TIMP-1. Il17a lacking mutants exhibited amelioration of the aforementioned fibrosis, while Tnf deficient mutants showed no signs of fibrosis when compared to littermate controls.
Conclusion
Our study uncovers key roles for TNF, and to a lesser extent IL-17, as mediators of liver inflammation and fibrosis induced by constitutive NLRP3 inflammasome activation in myeloid derived cells. These findings may lead to novel therapeutic strategies aimed at halting the progression of liver injury and fibrogenesis in various liver pathogenesis driven by NLRP3 activation.
Keywords: NLRP3, inflammation, liver fibrosis, IL17, TNF
Introduction
Inflammation in the absence of infection (sterile inflammation) (SI) has evolved as an important mechanism of liver injury in various acute and chronic liver disorders. (1, 2) SI in the liver is often initiated when damaged hepatocytes, undergoing cell death, expose intracellular molecules that can be recognized by cells of the innate immune system. In particular, resident macrophages or Kupffer cells become activated and trigger an inflammatory response through common pathways involving the NLRP3 inflammasome and interleukin 1 beta (IL-1β). (3, 4) Activation of the NLRP3 inflammasome results in a wide range of immune responses including production of pro-inflammatory cytokines, chemokines with the subsequent recruitment of neutrophils and other immune cells, and cell death.(5, 6) Growing evidence has linked inflammasome-driven inflammation to tissue damage and liver fibrosis in conditions such as drug-induced liver injury, alcoholic steatohepatitis (ASH), and nonalcoholic steatohepatitis (NASH).(7–9)
Recent studies from our lab using constitutively activated Nlrp3 mutant mice demonstrated that NLRP3 activation is not only required for hepatic inflammation and fibrosis, but is also sufficient to induce programmed inflammatory cell death termed pyroptosis.(10) The present study was conducted to dissect the downstream signals involved in this process and in particular to test the hypothesis that two pathways with growing importance in liver pathology, interleukin 17 (IL-17)(11–17) and tumor necrosis factor (TNF) (18–20), are key in amplifying and perpetuating the liver damage and fibrosis resulting from NLRP3 activation. We approached this hypothesis by breeding unique mouse models that express mutant hyperactive Nlrp3 in myeloid derived cells in mice that are deficient in IL-17 or TNF and investigate liver inflammation and fibrogenesis in these mice.
Materials and Methods
Mouse Strains
Nlrp3A350VneoR mice were generated as previously described, with an alanine 350 to valine (A350V) substitution and the presence of an intronic floxed neomycin resistance cassette, in which expression of the mutation does not occur unless the Nlrp3 mutants are first bred with mice expressing Cre recombinase.(21) Nlrp3A350VneoR mice were then bred onto il17a knockout mice (a gift from Y. Iwakura (University of Tokyo, Tokyo, Japan) and E. Raz (University of California San Diego, La Jolla, CA, US)) - or Tnf knockout mice – B6;129S-Tnftm1Gkl/J (TNF−/−) mice purchased from Jackson Laboratory (Bar Harbor, ME, US)- background. The resultant mice were further crossed with il17a or Tnf knockout mice expressing a Cre recombinase under the Lysozyme promoter allowing for mutant Nlrp3 expression in myeloid derived cells in mice deficient in il17a or Tnf.(22) Hemozygous Nlrp3 knockout littermates lacking the Cre recombinase were employed as control animals. The experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of California, San Diego. All efforts were made to minimize pain and distress during animal husbandry and experimental assessments.
In vivo LPS Induction
Nlrp3A350V, Nlrp3A350VIl17−/− and Nlrp3A350VTnf−/− mice were given a single intraperitoneal injection of lipopolysaccharides from Escherichia coli 0127:B8 (Sigma-Aldrich, St. Louis, MO, USA). LPS was injected at a concentration of 25 μg/kg and mice were sacrificed and liver was collected four hours post-injection.
Liver sample preparation
At an age of seven to nine days pups were sacrificed, the peritoneal and thoracic cavities opened, and blood samples were taken via cardiac puncture. Next, using phosphate-buffered saline (PBS), blood was flushed out of the liver via a catheter in the left main cardiac chamber. The livers were harvested and tissue was divided: (i) a representative section was fixed in 10% formalin for 24h and embedded in paraffin, (ii) samples of 50 mg were placed in 500µl of RNAlater® Solution (Life technologies, Carlsbad, CA, USA), (iii) Remaining liver tissue was quickly frozen in liquid nitrogen and stored in −80°C.
Histology, immunohistochemistry and Sirius Red staining
Liver tissues were fixed, embedded in paraffin, and processed on slides for hematoxylin-eosin (H&E) staining. Inflammation was quantified on the basis of the NAFLD activity score (NAS) by an experienced pathologist (Dr. Bettina Papouchado) in routinely stained tissue sections.(23) Liver fibrosis was assessed using Sirius Red, whereby liver sections were incubated for 2 h at room temperature with an aqueous solution of saturated picric acid containing 0.1% Fast Green FCF and 0.1% Direct Red.
Primary monoclonal antibodies used to perform immunostaining were: anti-F4/80 (a murine pan-macrophage marker) (AbDSerotec, Hercules, CA, USA), anti-myeloperoxidase (MPO) (Thermo Scientific, Waltham, MA, USA), anti-Ly6C (Abcam, Cambridge, UK), negative controls has primary antibodies omitted. The specimens were deparaffinized and hydrated in ethanol, the antigens were retrieved in citrate buffer pH 6.0 for 30 minutes at 95°C or treated with 2% BSA 1x Triton in TBS-T for 30 min at room temperature. Following overnight incubation with primary antibodies, horseradish peroxidase (HRP) or Alexa-Fluor 488 conjugated second antibody were applied. For color reaction of HRP, Streptavidin-peroxidase complex 3, 3-diaminobenzidine tetrahydroxychloride was used as chromogen and the slides were counterstained with hematoxylin. All photos were taken by NanoZoomer 2.0HT Slide Scanning System (Hamamatsu, Japan). A total of three fields per sample were examined. The number of MPO-positive cells as well as the F4/80 stained-area per field was determined using ImageJ software (National Institute of Health, USA) (Supplemental Figure 4).
Fluorescence in situ hybridization (FISH)
After deparaffinization and rehydration, the specimens were permeabilized with PBS-0, 3% Triton for 30 min and then, incubated at 72°C for 2 min. Following incubation overnight at 62°C with either TNF or IL17A probe, the slides were washed with hybridization buffer and mounted.
Real-time PCR
Total RNA was isolated from liver tissue and analyzed as previously described.(10) The sequences of the primers used for quantitative PCR are given in supplemental table 1.
Immunoblot analysis and ELISA
Immunoblot analysis was performed as previously described.(24) Anti- IL-17A antibody (Cell Signaling, Danvers, MA, US), anti- TNF-α (abcam, Cambridge, MA, US), and anti-α-SMA (GeneTex, Irvine, CA, USA) antibodies were used in combination with appropriate peroxidase-conjugated secondary antibodies. Protein load was verified with GAPDH antibody (Genetex, Irvine, CA) or α-tubulin antibody (dilution 1:10,000) (Hybridomabank, University of Iowa) (kindly provided by M. Kaulich, University of California San Diego, La Jolla, CA, US). Bands were visualized with the enhanced chemiluminescence reagent and digitized using a CCD camera (ChemiDoc®, Biorad, Hercules, CA, USA). Expression intensity was quantified by ImageLab (Biorad). Quantification of serum IL-1β levels were performed according to the manufactures’ instruction (R&D Systems, Minneapolis, MN, USA).
Unless stated otherwise, all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
FACS analysis
Whole livers were force filtered through a 70µm nylon cell strainer (BD) with PBS. The solution was centrifuged for 1min at 8°C with 500rpm producing a pellet of parenchymal cells and a supernatant incorporating the infiltrating cells. Infiltrating cells were classified by multicolour analysis after incubation with CD11b (eBioscience, San Diego, CA, USA), F4/80 (AbD serotec, Kidlington, UK) and Ly6G (eBioscience) for 30min on ice and washed with 3% FBS-PBS. For analysis of intracellular production of TNF and IL17A, cells were further permeabilized and incubated with TNF (eBioscience) or IL17 (eBiosicence) antibodies. Cells were analyzed by flow cytometry (BD LRS II).
Statistical analyses
Analyses were performed with Graph Pad (version 5.03; Graph Pad, Graph Pad Software Inc., CA, USA). The significance level was set at α = 5% for all comparisons. Data were evaluated using Kruskall-Wallis test and Dunn's Multiple Comparison Test. For group comparisons, we limit this to three comparisons that are of particular interest: Control vs. Nlrp3A350V, Nlrp3A350V vs.Nlrp3A350V/ il17a−/−, and Nlrp3A350V vs.Nlrp3A350V/ Tnf−/−. Unless otherwise stated, data are expressed as mean +/− SEM or as absolute number or percentage for categorical variables.
Results
Liver inflammation in Nlrp3A350V mice is associated with increase in TNF and IL-17
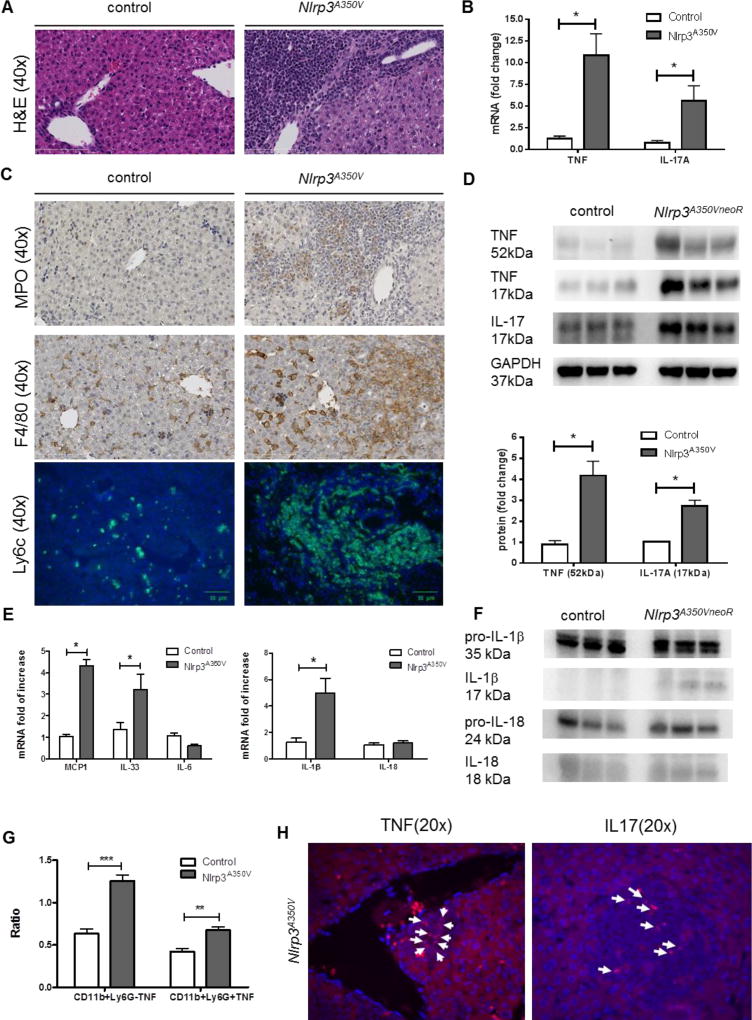
Mice expressing the Nlrp3A350V mutation under the control of the lysozyme promoter exhibited severe liver inflammation (Fig. 1A). Further histological analysis and immunohistochemical staining revealed an increase in neutrophils staining positive for MPO (Fig. 1C). In addition, livers of Nlrp3A350V mice displayed elevated numbers of macrophages with activated inflammatory macrophages being the dominant additional cell type, evidenced by high expression of F4/80 and Ly6C (Fig. 1C). As TNF and IL-17 are both known to be increased in several models of liver injury and have been increasingly linked to liver inflammation and fibrogenesis(11, 20), we next assessed their levels in the livers of Nlrp3A350V mice. Interestingly, mRNA levels of Tnf in the liver of Nlrp3A350V mice were increased 10 fold when compared to non-mutant littermates. Hepatic mRNA levels of Il17a were also significantly increased (5-fold, p<0.05) in Nlrp3A350V mice when compared to control animals (Fig. 1B). In line with these changes, protein levels of TNF, the non-biologically active monomerized form (17kDa) as well as the biologically active homotrimer (52kDa),(25) were significantly increased in livers of Nlrp3 mutant mice (4-fold, p<0.05) (Fig. 1D). Protein levels of IL-17A were also found to be increased in Nlrp3A350V mice when compared to controls (3-fold, p<0.05) (Fig 1D). FISH staining showed a strong expression of TNF in portal track infiltration sites and IL17A in inflammatory foci (Fig 1H), indicating that infiltrating cells are the main source responsible for the production of these cytokines. Flow cytometry analysis revealed that Nlrp3 A350V mice have increase TNF-producing CD11b+Ly6G− cells (2-fold, p<0.01) as well as TNF-producing CD11b+Ly6G+ cells (1.5-fold, p<0.05) compared to controls (Fig 1G). We also investigated whether other proinflammatory and profibrotic cytokines or chemokines were also upregulated in Nlrp3A350V mice. Increased hepatic mRNA levels of IL-1β (5-fold, p<0.05), IL-33 (3-fold, p<0.05) as well as MCP-1 (4-fold, p<0.05) were observed in mutant mice when compared to controls, while no change was found in mRNA levels of IL-6 and IL-18 (Fig. 1E). Nlrp3A350V mice showed increased protein levels of mature IL-1β and IL18 as measure by western blot (Fig. 1F).
Figure 1. Evidence of IL17 and TNF in Nlrp3 mutant mice.
Nlrp3A350V mice exhibited severe liver inflammation (A). mRNA levels of TNF and IL-17 were increased in Nlrp3A350V mice when compared to control mice (B) Detection of myeloperoxidase (MPO, neutrophils), F4/80 (murine pan-macrophage marker) as well as lymphocyte antigen 6 complex (Ly6C) (activated macrophages) positive cells in liver of Nlrp3A350V mice (C). Protein levels of TNF monomer (17kDa), trimeric TNF (52kDa), and IL-17A were increased in liver samples of Nlrp3A350V mice when compared to control mice (D). MCP-1, IL-33 as well as IL-1β mRNA levels were upregulated in Nlrp3A350V mice when compared to control mice, while IL-6 and IL-18 mRNA levels were similar between both groups (E) Mature IL-1β (17kDa) as well as mature IL-18 (18kDa) but not pro-IL1β (35kDa) or pro-IL18 (24kDa) protein levels were increased in liver samples of Nlrp3A350V mice when compared to control mice (F). Flow cytometry analysis showed that Nlrp3A350V mice have increase TNF-producing CD11b+Ly6G− cells as well as TNF-producing CD11b+Ly6G+ cells compared to controls (G). FISH staining showed a strong expression of TNF in portal track infiltration sites and IL17A in inflammatory foci (H). (* = p<0.05, ** = p<0.01, = p<0.001)
TNF but not IL-17 ameliorates Nlrp3 dependent liver inflammation
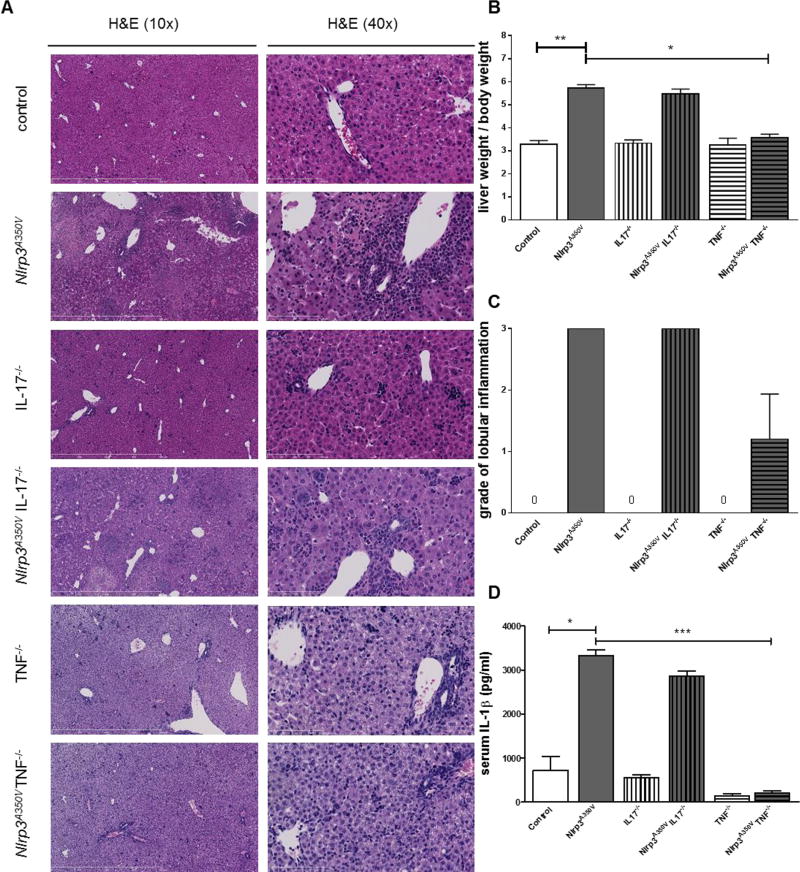
Based on the increase levels of TNF and IL-17A in the livers of Nlrp3A350V mice, we next sought to investigate their role on the liver phenotype resulting from NLRP3 activation. Therefore, we crossed our Nlrp3A350V mice with mice globally deficient for Tnf or Il17a generating mice that exhibit mutant Nlrp3 expression in myeloid derived cells, but were globally deficient in Il17a or Tnf. We included seven animals per group and sacrificed them at day nine of age (Supplemental table 2). Control mice exhibited an average body weight of 4.4g (range 3.4g to 5.2g), while Nlrp3A350V mice were significantly smaller (1.8g, range 1.6g to 2.2g). Nlrp3 mutant mice deficient for il17a also showed a reduced body weight when compared to il17a knockout mice, while Nlrp3 mutant mice deficient for Tnf were indistinguishable from Tnf knockout mice (Supplemental table 2). Analysis of liver weight revealed a significant increase in liver weight per body weight in Nlrp3A350V mice when compared to control mice (Supplemental table 2, Fig. 2B). This increase was also documented in Nlrp3A350VIl17a−/− mice when compared to Il17a−/− mice, while liver to body weight ratio was similar in Nlrp3A350VTnf−/− and Tnf−/− mice (Supplemental table 2, Fig. 2B). Examination of liver histology revealed strong inflammation in Nlrp3A350V mice (grade 3) and Nlrp3A350VIl17a−/− mice (grade 3), while Nlrp3A350VTnf−/− mice only showed mild inflammatory changes (grade 1) (Fig. 2A, C). Analysis of serum IL-1β levels revealed a significant increase in in Nlrp3A350V mice when compared to control mice (Fig. 2D). Increased serum IL-1β levels were also present in Nlrp3A350VIl17a−/− mice when compared to Il17a−/− mice, while levels in Nlrp3A350VTnf−/− and Tnf−/−mice were significantly reduced when compared to Nlrp3A350V mice (Fig. 2D).
Figure 2. Histology and weight comparison.
Representative pictures of Hematoxylin & Eosin (H&E) stained liver sections of animals from the six different groups (A). At seven to nine days of age control mice weighed an average of 4.4g, while Nlrp3A350V mice were significantly smaller. IL-17−/− mice were larger than Nlrp3A350V IL-17−/− mice, while Nlrp3A350V TNF−/− and TNF−/− mice were indistinguishable. Percent liver weight was significantly increased in Nlrp3A350V mice when compared to control mice, the same pattern was observed in Nlrp3A350V and IL-17−/− mice, while Nlrp3A350V TNF−/− and TNF−/− showed a similar liver/body weight percentage (B). Severe liver inflammation – grade 3 - was present in Nlrp3A350V mice and Nlrp3A350V IL-17−/− mice, while Nlrp3A350V TNF−/− mice only showed mild inflammation (grade 1). (C) (* = p<0.05). Levels of IL1 β were increased in Nlrp3A350V and Nlrp3A350V IL-17−/− mice, but remained unchanged in Nlrp3A350V TNF−/− compared to controls (D).
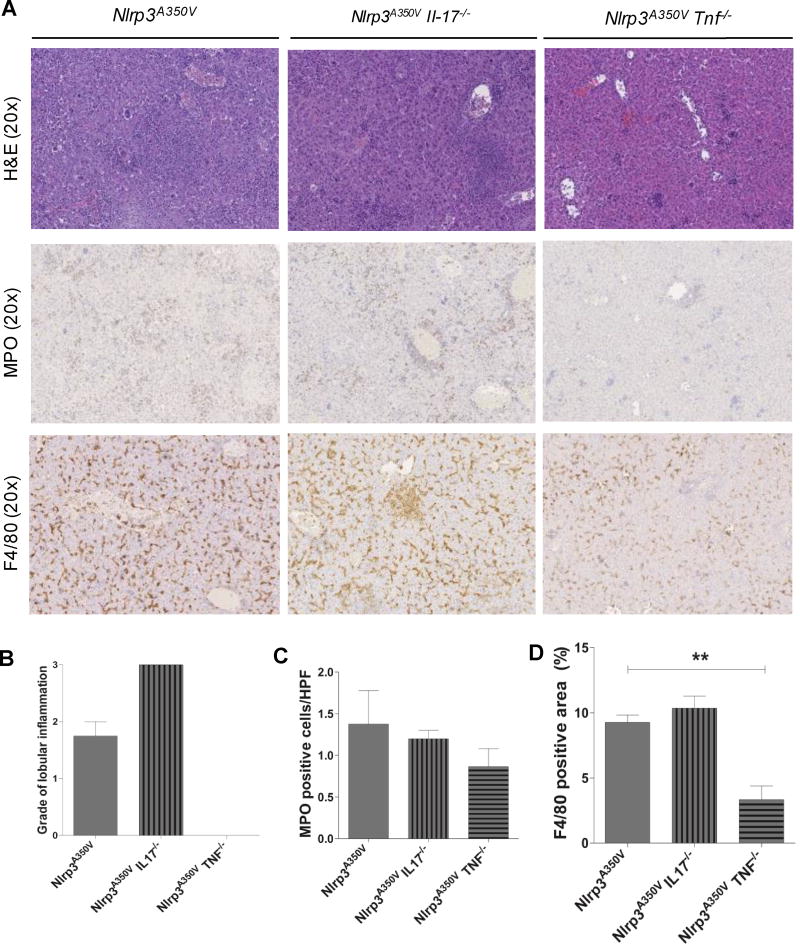
Infiltration with neutrophils and inflammatory macrophages in Nlrp3 mutant mice depends on TNF signaling
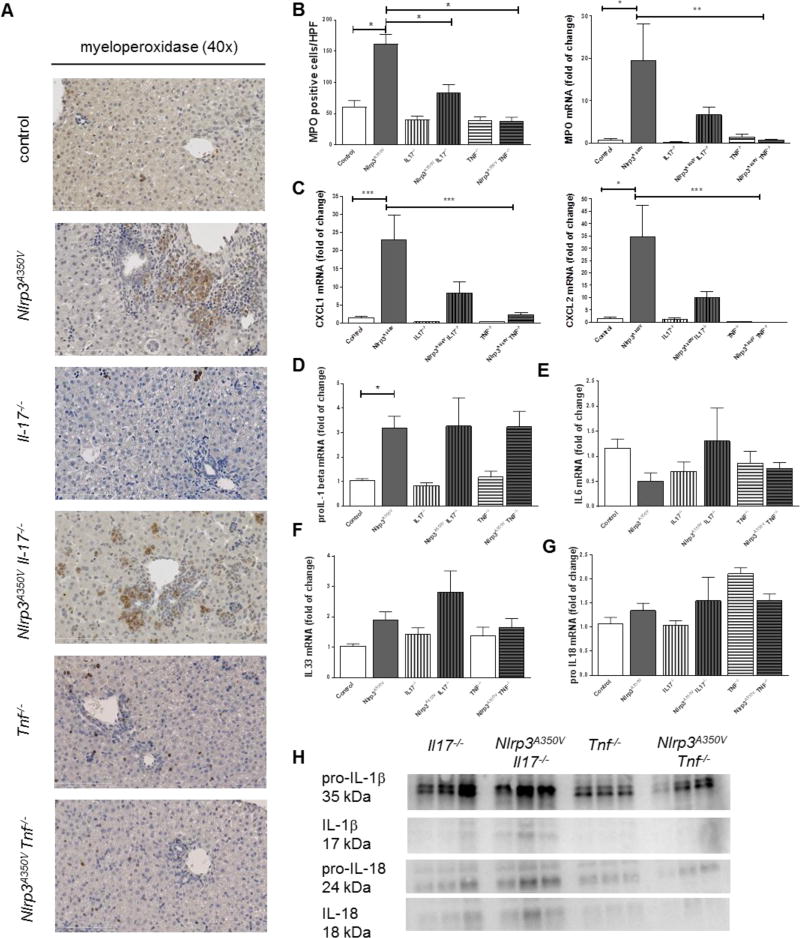
As hepatic infiltration with neutrophils and inflammatory macrophages are prominent in Nlrp3A350V mice we next investigated these events in the combined knock-in knockout mice. Immunohistochemistry of liver sections showed higher numbers of MPO positive cells in Nlrp3A350V when compared to control mice. Nlrp3A350VIl17a−/− livers exhibit significant fewer amount of MPO cells while the number of positive cells in Nlrp3A350V TNF−/− mice was not different from TNF−/− mice (Fig. 3A, B). In line with this, mRNA levels of MPO were increased by 20-fold in Nlrp3A350V mice when compared to control mice; levels in Nlrp3A350VIl17a−/−mice were still increased 5-fold, and levels in Nlrp3A350VTnf−/−mice were not increased (Fig. 3B). As neutrophilic infiltration as a consequence of NLRP3 activation is mediated in part via a chemokine gradient,(26) we next quantified mRNA levels of CXCL1 and CXCL2, two key chemokines involved in this process in livers of mice from the various experimental groups. We found a marked increase in Nlrp3A350V mice (20-fold for CXCL1 and 30-fold for CXCL2) when compared to control animals (Fig. 4C). This increase was also present in Nlrp3A350VIl17a−/−mice (10-fold for CXCL1 and CXCL2). A significant decrease of CXCL1 and CXCL2 expression was observed in Nlrp3A350VTnf−/− mice when compared to Nlrp3A350V mice (Fig. 4C). Interestingly, pro-IL-1beta mRNA levels were still upregulated in both Nlrp3A350VTnf−/− mice (3-fold) and Nlrp3A350VIl17a−/− mice (3-fold) compared to control mice (Fig 3D), while all groups of mice displayed similar IL-6 (Fig 3E), IL-33 (Fig 3F) and pro-IL18 (Fig 3G) mRNA levels. However, protein levels of pro-IL1β and pro-IL18 as well as mature IL-1β and IL-18 were higher only in Nlrp3A350VIl17a−/− but not in Nlrp3A350VTnf−/− mice (Fig. 3H).
Figure 3. Regulation of neutrophilic infiltration, and proinflammatory cytokines.
Immunohistochemistry for myeloperoxidase (MPO) showed increased staining in Nlrp3A350V and Nlrp3A350V IL-17−/−, while the staining in livers of Nlrp3A350V TNF−/− mice was not different from TNF−/− mice (A, B). mRNA levels of MPO were increased Nlrp3A350V and Nlrp3A350V IL-17−/− mice but not elevated in Nlrp3A350V TNF−/− mice (B). As neutrophilic migration in mediated via chemokine ligands we quantified mRNA levels of CXCL1 and CXCL2 in livers. We found a marked increase of these chemokines in Nlrp3A350V mice (20-fold for CXCL1 and 30-fold for CXCL2) when compared to control mice (C). This increase was also present in Nlrp3A350V IL-17−/− mice (10-fold for CXCL1 and CXCL2) (C). pro-IL-1beta mRNA levels were upregulated in both Nlrp3A350V TNF−/− mice and Nlrp3A350V IL-17−/− mice compared to control mice (D), while all groups of mice displayed similar IL-6 (E), IL-33 (F) and pro-IL18 mRNA levels (G). However, protein levels of pro-IL1β and pro-IL18 as well as mature IL-1β and IL-18 were higher only in Nlrp3A350V IL-17−/− but not in Nlrp3A350V TNF−/− mice (H). (* = p<0.05; ** = p<0.01; *** = p<0.001).
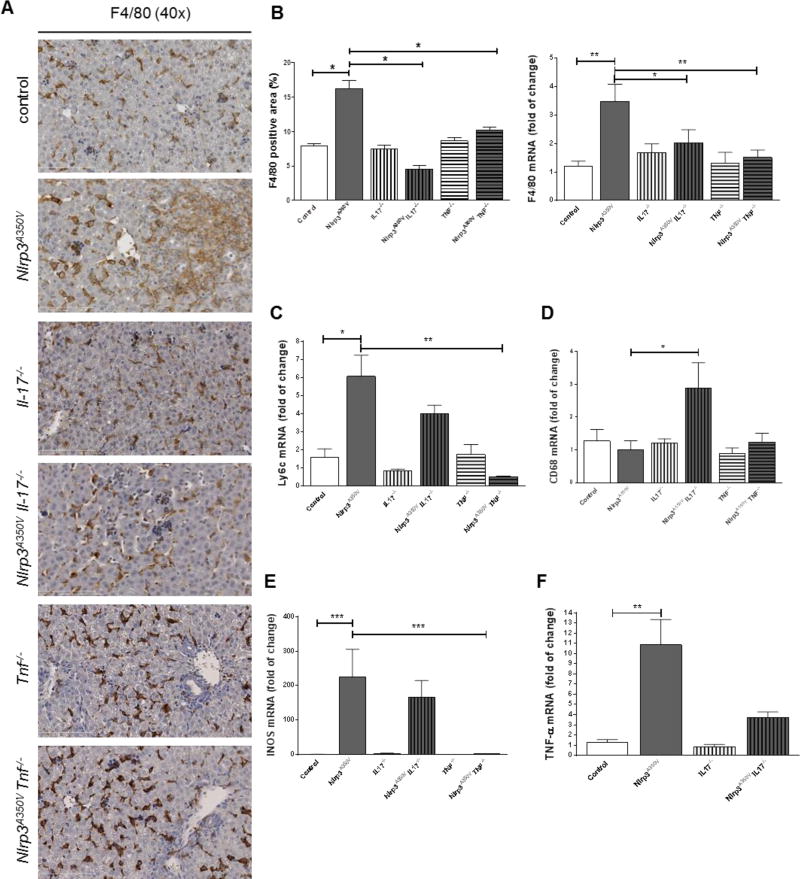
Figure 4. Macrophages infiltration and polarization in combined Nlrp3A350V knockin and IL-17, TNF− knockout mice.
Hepatic macrophages when assessed via immunohistochemistry or mRNA levels for F4/80 were increased in Nlrp3A350V mice and unchanged in all other groups of mice (A, B). Ly6-c mRNA levels were increased in Nlrp3A350V mice and Nlrp3A350V IL-17−/− mice but not in Nlrp3A350V TNF−/− mice when compared to control mice (C). Surprisingly, CD68 mRNA levels were increased in Nlrp3A350V IL-17−/− mice compared to Nlrp3A350V, even though similar levels were found between mutant and control mice (D) The inflammatory phenotype of infiltrating macrophages in Nlrp3A350V mice and Nlrp3A350V IL-17−/− mice as measured by mRNA levels of iNOS (inducible form of nitric oxide synthase) and TNF were found to be increased (E, F). (* = p<0.05; ** = p<0.01).
Analysis of hepatic macrophages by immunohistochemistry revealed increased areas of F4/80 and levels of F4/80 mRNA in Nlrp3A350V mice (6-fold, p<0.05) when compared to control animals and unchanged levels in all other groups of mice (Fig. 4A, B). However, further investigations in macrophage phenotype uncovered a significant increase in inflammatory macrophages as demonstrated by lymphocyte antigen 6 complex (Ly6-c) positive quantification in Nlrp3A350V mice and Nlrp3A350VIl17a−/−mice with no detectable increase in Nlrp3A350VTnf−/− mice when compared to control mice (Fig. 4C). Surprisingly, CD68 mRNA levels were upregulated only in Nlrp3A350VIl17a−/− mice but not in Nlrp3A350V or Nlrp3A350VTnf−/− mice. (Fig. 4D) Further supporting the pro-inflammatory phenotype of macrophages in the livers of Nlrp3A350V mice and Nlrp3A350VIl17a−/−mice, mRNA levels of iNOS (inducible form of nitric oxide synthase) and TNF were found to be increased in both groups. iNOS mRNA levels were found unchanged in Nlrp3A350V TNF−/− mice (Fig. 4E, F).
TNF but not IL-17 prevents hepatic stellate cell activation in Nlrp3 mutant mice
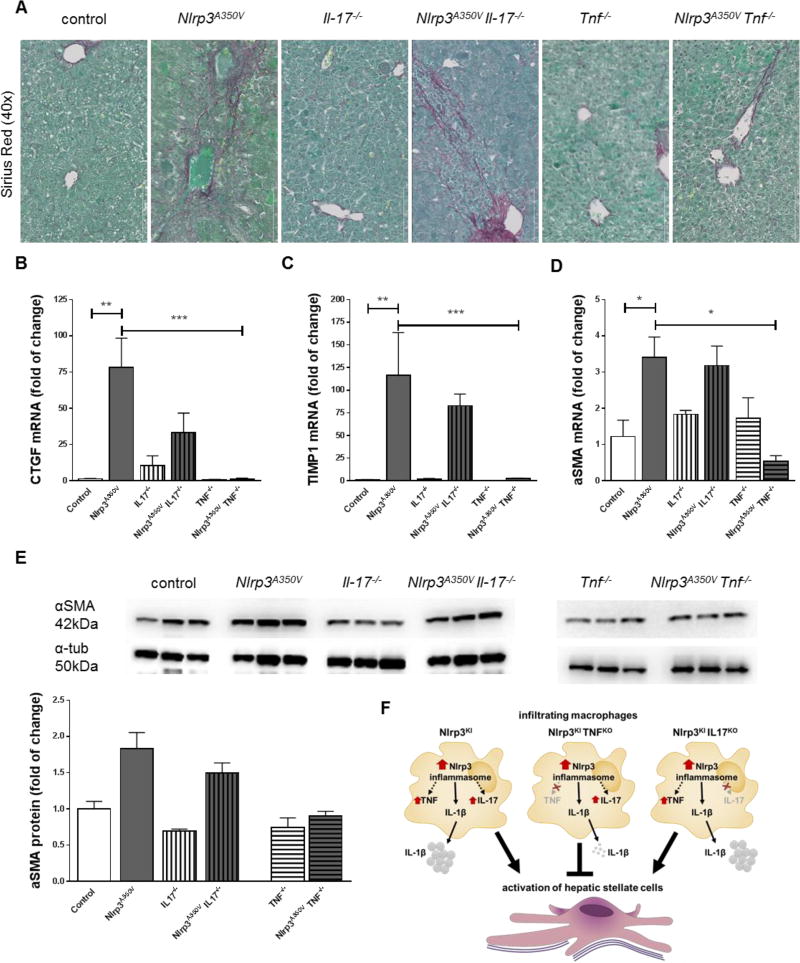
Liver inflammation and chronic infiltration with inflammatory macrophages is closely correlated to the activation of hepatic stellate cells (HSCs) and the development of liver fibrosis.(27) Liver sections were therefore stained with Sirius red to assess collagen deposition (Fig. 5A). As expected, positive Sirius red staining was detectable in liver sections of control mice, Il17a−/− mice, and Tnf−/− mice only in close proximity of the portal field (Fig. 5A). Liver sections of Nlrp3A350V and Nlrp3A350VIl17a−/− mice exhibited bridging fibrosis, while liver sections of Nlrp3A350VTnf−/− mice did not show increased collagen deposition. The mRNA levels of connective tissue growth factor (ctgf) (Fig. 5B), tissue inhibitor of matrix metalloproteinase 1 (timp1) (Fig. 5C), and alpha smooth muscle actin (α-SMA) (Fig. 5D) were increased in livers of Nlrp3A350V and Nlrp3A350VIl17a−/− mice, while levels in Nlrp3A350VTnf−/− mice did not differ from controls. In line with this, protein levels of α-SMA were also increased in Nlrp3A350V and Nlrp3A350VIl17a−/−mice when compared to their corresponding control animals with no difference in Nlrp3A350VTnf−/− and Tnf−/− mice (Fig. 5E).
Figure 5. Collagen deposition and hepatic stellate cell activation in combined Nlrp3A350V knockin and IL-17, TNF− knockout mice.
Sirius red staining uncovered bridging fibrosis in Nlrp3A350V and Nlrp3A350V IL-17−/−, while control mice, IL-17−/−, TNF−/−, and Nlrp3A350V TNF−/− only presented collagen deposition in close proximity to the portal fields (A). Markers for hepatic stellate cell (HSC) activation - connective tissue growth factor (CTGF), tissue inhibitor of matrix metalloproteinase 1 (TIMP1), and alpha smooth muscle actin (α-SMA) – were increased in Nlrp3A350V and Nlrp3A350V IL-17−/− mice when compared to control mice, while aforementioned markers were not increased in Nlrp3A350V TNF−/− mice when compared to TNF−/− mice (B-D). Protein levels of α-SMA were also increased in Nlrp3A350V and Nlrp3A350V IL-17−/− mice when compared to their corresponding control animals with no difference in Nlrp3A350V TNF−/− and TNF−/− mice (E). Summary of the role of TNF and IL17 pathways in Nlrp3-driven liver damage (F) (* = p<0.05; ** = p<0.01; *** = p<0.001).
LPS-driven liver damage is ameliorated by TNF but not IL17 in Nlrp3 mutant mice
In order to further evaluate the involvement of TNF and IL17 in NLRP3 mediated liver damage, we challenged the different group of mice with an inflammatory stimulus. We injected LPS intraperitoneally and observed the liver damage after 4 hours. Liver histology revealed strong inflammation in Nlrp3A350V mice (grade 2) as well as Nlrp3A350VIl17a−/− mice (grade 3), while Nlrp3A350VTnf−/− mice showed no signs of inflammatory changes (grade 0) (Fig. 6A, B). Immunohistochemistry of liver sections displayed extensive MPO positive cells infiltration (Fig. 6A, C) along with the presence of high number of F4/80 macrophages (Fig 6A, D) in Nlrp3A350V and Nlrp3A350VIl17a−/−. Liver sections of Nlrp3A350VTn f−/− mice displayed fewer number of MPO infiltrating positive cells (Fig 6A, C) as well as decreased amount of F4/80 positive cells (Fig 6A, D).
Figure 6. LPS-driven liver damage in combined Nlrp3A350V knockin and IL-17, TNF− knockout mice.
Representative pictures of H&E, MPO, and F4/80 stained liver sections of animals from the three different groups after 4hr 25 µg/kg LPS injection (A). Nlrp3A350V as well as Nlrp3A350V Il17−/− mice exhibited severe liver inflammation (B) along with MPO positive cells infiltration (B) and the presence of high amount of F4/80 macrophages (D). Nlrp3A350V Tnf−/− mice displayed a significant abrogation of liver inflammation as well as decreased numbers of MPO infiltrating cells and diminished amount of F4/80 positive cells (** = p<0.01).
Discussion
The key findings of the present study provide new information about the downstream pathways involved in the development of liver inflammation and fibrogenesis associated with myeloid cell specific NLRP3 activation. Our results demonstrate that the levels of both TNF and IL-17, two key cytokines linked to liver pathology, are increased in the livers of mice with constitutively activated Nlrp3 in myeloid derived cells. TNF signaling but not IL-17 appears to be crucial in triggering liver inflammation, neutrophils and pro-inflammatory macrophage recruitment, as well as in activation of fibrogenic pathways that are central to the development of liver fibrosis.
Emerging evidence supports a central role of NLRP3 Inflammasome in the pathogenesis of many liver diseases including alcoholic and nonalcoholic fatty liver disease (NAFLD) as well as liver fibrosis.(24, 28–31) Indeed, studies using global knockout mice of different inflammasome components, or pharmacological inhibitors of IL-1β signaling, have suggested protective effects of NLRP3 signaling suppression in liver injury and fibrotic changes associated with these conditions.(28) IL-1β signaling appears to be an important contributor for the liver changes resulting from NLRP3 activation. However, our previous results indicate that IL-1-independent pathways also play an important role as treatment with an interleukin-1 receptor antagonist (anakinra) only partially attenuated liver inflammation, whereas fibrogenesis was unchanged.(10) Additionally, deletion of the Interleukin 18 (IL-18) receptor in Nlrp3 knockin mice resulted in partial phenotypic rescue that abolished skin and visceral disease in young mice and normalized serum cytokines to a greater extent than breeding to Interleukin 1 receptor knockout mice.(32) Significant systemic inflammation developed in aging Nlrp3 mutant IL-18 receptor knockout mice, suggesting that IL-1β and IL-18 drive pathology at different stages of the disease process.(32) The current study was conducted to further dissect the downstream events that may amplify and perpetuate the inflammatory responses and contribute to activation of HSCs, a central event in liver fibrosis.(33) We focused on IL-17 and TNF, two pathways that have been increasingly linked to liver injury, particular in the context of NAFLD and alcoholic liver disease. While IL-17 production can be regulated via NLRP3 activation,(34–36) TNF production is mainly thought to occur via signals that lead to activation of NFkB. (37, 38) NLRP3 activation may amplify this production in an autocrine or paracrine fashion by inducing the maturation and release of IL-1β and IL-18, which then interact with their own membrane receptors.(39, 40) Furthermore, both cytokines have been involved in creating an autoinflammatory loop that characterizes these disorders,(41) while IL-17 is an important contributor for tissue recruitment of neutrophils, a central feature found in the livers of mice with constitutive active NLRP3.
Our results demonstrate that NLRP3 inflammasome induced liver inflammation and liver fibrogenesis are influenced by IL-17 and TNF, with TNF having more prominent effect (Fig. 5F). The expression of both cytokines was increased in the livers of Nlrp3 mutant mice. Studies using FISH and FACS analysis demonstrated the infiltrating inflammatory cells are the main source for these cytokines. The unexpected significance of TNF ablation in Nlrp3 mutant mice could be explained by a feedback loop with IL-1β as reported by Tangi et al. The authors showed an increase in IL-1β mRNA and protein levels in cell culture supernatants of aortic smooth muscle cells upon stimulation with TNF that was dependent on NLRP3 signaling. (42). Consistent with this, our current data demonstrate a significant reduction of IL-1β levels in Nlrp3 mutants in the absence of TNF. Due to the important role for IL-17 in recruitment of neutrophils to the liver and its role in the context of liver fibrosis and activation of HSCs, we expected a major beneficial effect of IL-17 ablation on liver disease in our Nlrp3 gain-of-function model.(14, 16). Surprisingly, we observed only a slight reduction in neutrophilic infiltration by IL-17 ablation. These finding suggest that the inflammatory environment generated by NLRP3 activation and the recruitment of circulating neutrophils occur by parallel redundant pathways, which may explain the only minimal protective effects on the development of liver fibrosis observed in our mutant mice.
Although the role of TNF and its inhibition in animal models of liver injury offered encouraging therapeutic perspectives, targeting this cytokine for the treatment of human liver diseases remains uncertain.(43) Indeed, extensive evidence from animal models on various liver disorders such nonalcoholic and alcoholic liver disease have demonstrated a central role for TNF in development of liver injury and modulation of disease severity. Treatment of leptin deficient ob/ob mice with anti-TNF antibodies improves NASH and hepatic insulin resistance.(44) Mice genetically deficient in TNF type 1 receptor (TNFR1) are resistant to steatosis and liver injury induced by high caloric diets as well as alcohol exposure.(45–47) Our current results also suggest the potential beneficial effects of modulating TNF signaling in the context of NLRP3 driven liver pathology. In humans, various molecules that target TNF such as pentoxifylline, etanercept, (a fusion protein that acts as a “decoy receptor” for TNF) as well as the various anti-TNF monoclonal antibodies currently available for clinical use, have been proposed as potential therapy for the treatment of liver disease. Early studies using pentoxifylline, a weak TNF inhibitor, have shown positive results with diminished serum levels of aminotransferases and improved inflammation and fibrosis in patients with NASH.(48) However, caution must be taken as a randomized placebo-controlled trial using etanercept was associated with a significantly higher mortality rate in patients with moderate-to-severe acute alcoholic hepatitis, an effect that was mainly attributed to an increased rate of severe infections.(49)
In summary, our current study reveals key roles for TNF, and to a lesser extent IL-17, as mediators of liver inflammation and fibrosis induced by constitutive NLRP3 inflammasome activation in myeloid derived cells. These findings may lead to novel therapeutic strategies aimed at halting the progression of liver injury and fibrogenesis in various liver pathologies driven by NLRP3 activation.
Supplementary Material
Acknowledgments
This work used the facilities of the UCSD Microscopy Core supported by the UCSD Neuroscience Microscopy Shared Facility grant (P30 NS047101). We thank Dr. Lori Broderick for editorial insights.
Funding
The work was funded by NIH (DK076852 and DK082451 to AEF and AI52430 to HMH), German Research Foundation (DFG-grant WR173/2-1 and SFB/TRR 57 to AW), and START-Program of the Faculty of Medicine, RWTH Aachen to AW).
Abbreviations
- α-SMA
alpha smooth muscle actin
- ASC
apoptosis-associated speck-like protein containing a caspase recruitment domain
- CARD
caspase recruitment domain-containing protein 8
- CLD
chronic liver disease
- CTGF
connective tissue growth factor
- CXCL
chemokine (C-X-C motif) ligand
- F4/80
murine macrophage marker
- HSC
hepatic stellate cell
- IL
interleukin
- iNOS
inducible form of nitric oxide synthase
- MPO
myeloperoxidase
- NAS
nonalcoholic fatty liver disease activity score
- NLRs
nucleotide-binding oligomerization domain (NOD) leucine-rich repeat containing receptors
- SI
sterile inflammation
- TIMP1
tissue inhibitor of matrix metalloproteinase 1
- TNF
tumor necrosis factor.
Footnotes
Disclosures
HMH is a consultant and speaker for Novartis and SOBI. All other authors state that they have nothing to disclose.
References
- 1.Hoque R, Farooq A, Mehal WZ. Sterile inflammation in the liver and pancreas. J Gastroenterol Hepatol. 2013;(28 Suppl 1):61–67. doi: 10.1111/jgh.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 4.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627–636. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 6.Broderick L, De Nardo D, Franklin BS, Hoffman HM, Latz E. The Inflammasome and Autoinflammatory Syndromes. Annu Rev Pathol. 2014 doi: 10.1146/annurev-pathol-012414-040431. [DOI] [PubMed] [Google Scholar]
- 7.Maher JJ. DAMPs ramp up drug toxicity. J Clin Invest. 2009;119:246–249. doi: 10.1172/JCI38178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, Nagy L, Szabo G, et al. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516–525. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner DA, Seki E, Taura K, Kisseleva T, Deminicis S, Iwaisako K, Inokuchi S, et al. Non-alcoholic steatohepatitis-induced fibrosis: Toll-like receptors, reactive oxygen species and Jun N-terminal kinase. Hepatol Res. 2011;41:683–686. doi: 10.1111/j.1872-034X.2011.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, Hoffman HM, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation and fibrosis. Hepatology. 2013 doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol. 2011;2011:345803. doi: 10.1155/2011/345803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Lemmers A, Moreno C, Gustot T, Marechal R, Degre D, Demetter P, de Nadai P, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–657. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 14.Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, Cong M, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765–776. e761–763. doi: 10.1053/j.gastro.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN, Jin L, Fu JL, et al. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51:81–91. doi: 10.1002/hep.23273. [DOI] [PubMed] [Google Scholar]
- 16.Hara M, Kono H, Furuya S, Hirayama K, Tsuchiya M, Fujii H. Interleukin-17A plays a pivotal role in cholestatic liver fibrosis in mice. J Surg Res. 2013;183:574–582. doi: 10.1016/j.jss.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Yasumi Y, Takikawa Y, Endo R, Suzuki K. Interleukin-17 as a new marker of severity of acute hepatic injury. Hepatol Res. 2007;37:248–254. doi: 10.1111/j.1872-034X.2007.00040.x. [DOI] [PubMed] [Google Scholar]
- 18.Bieghs V, Trautwein C. The innate immune response during liver inflammation and metabolic disease. Trends Immunol. 2013;34:446–452. doi: 10.1016/j.it.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 20.Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clin Rev Allergy Immunol. 2009;36:4–12. doi: 10.1007/s12016-008-8091-0. [DOI] [PubMed] [Google Scholar]
- 21.Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, Putnam CD, et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30:875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic research. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24.Wree A, McGeough MD, Pena CA, Schlattjan M, Li H, Inzaugarat ME, Messer K, et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl) 2014 doi: 10.1007/s00109-014-1170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith RA, Baglioni C. The active form of tumor necrosis factor is a trimer. J Biol Chem. 1987;262:6951–6954. [PubMed] [Google Scholar]
- 26.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 27.Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473–1492. doi: 10.1002/cphy.c120035. [DOI] [PubMed] [Google Scholar]
- 28.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon LJ, Flask CA, Papouchado BG, Feldstein AE, Nagy LE. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS One. 2013;8:e56100. doi: 10.1371/journal.pone.0056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon LJ, Berk M, Thapaliya S, Papouchado BG, Feldstein AE. Caspase-1-mediated regulation of fibrogenesis in diet-induced steatohepatitis. Lab Invest. 2012;92:713–723. doi: 10.1038/labinvest.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szabo G, Csak T. Inflammasomes in liver diseases. Journal of hepatology. 2012;57:642–654. doi: 10.1016/j.jhep.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 32.Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest. 2013 doi: 10.1172/JCI71543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maher BM, Mulcahy ME, Murphy AG, Wilk M, O'Keeffe KM, Geoghegan JA, Lavelle EC, et al. Nlrp-3 driven IL-17 production by gammadelta T-cells controls infection outcomes during Staphylococcus aureus surgical site infection. Infect Immun. 2013 doi: 10.1128/IAI.01026-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 36.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 38.Bohuslav J, Kravchenko VV, Parry GC, Erlich JH, Gerondakis S, Mackman N, Ulevitch RJ. Regulation of an essential innate immune response by the p50 subunit of NF-kappaB. J Clin Invest. 1998;102:1645–1652. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paramel GV, Sirsjo A, Fransen K. Role of genetic alterations in the NLRP3 and CARD8 genes in health and disease. Mediators Inflamm. 2015;2015:846782. doi: 10.1155/2015/846782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grine L, Dejager L, Libert C, Vandenbroucke RE. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015;26:25–33. doi: 10.1016/j.cytogfr.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Tangi TN, Elmabsout AA, Bengtsson T, Sirsjo A, Fransen K. Role of NLRP3 and CARD8 in the regulation of TNF-alpha induced IL-1beta release in vascular smooth muscle cells. Int J Mol Med. 2012;30:697–702. doi: 10.3892/ijmm.2012.1026. [DOI] [PubMed] [Google Scholar]
- 43.Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18:727–735. doi: 10.3748/wjg.v18.i8.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 45.Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 46.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 48.Lee YM, Sutedja DS, Wai CT, Dan YY, Aung MO, Zhou L, Cheng CL, et al. A randomized controlled pilot study of Pentoxifylline in patients with non-alcoholic steatohepatitis (NASH) Hepatol Int. 2008;2:196–201. doi: 10.1007/s12072-008-9058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–1960. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.