Abstract
Vascular cognitive impairment and dementia (VCID) is a major public health concern because of the increased incidence of vascular disease in the aging population and the impact of vascular disease on Alzheimer’s disease. VCID is a heterogeneous group of diseases for which there are no proven treatments. Biomarkers can be used to select more homogeneous populations. Small vessel disease is the most prevalent form of VCID and is the optimal form for treatment trials because there is a progressive course with characteristic pathological changes. Subcortical ischemic vascular disease of the Binswanger type (SIVD-BD) has a characteristic set of features that can be used both to identify patients and to follow treatment. SIVD-BD patients have clinical, neuropsychological, CSF and imaging features that can be used as biomarkers. No one feature is diagnostic but a multimodal approach defines the SIVD-BD spectrum disorder. The most important features are large white matter lesions with axonal damage, blood-brain barrier disruption as shown by MRI and CSF, and neuropsychological evidence of executive dysfunction. We have used these features to create a Binswanger Disease Scale and a probability of SIVD-BD, using a machine-learning algorithms. The patients discussed in this review are derived from published studies. Biomarkers aid in early diagnosis before the disease process have progressed too far for treatment, but also can indicate response to treatment. Refining the use of biomarkers will allow dementia treatment to enter the era of precision medicine.
Graphical Abstract
Vascular cognitive impairment and dementia (VCID) is a major public health concern because of the increased incidence of vascular disease in the aging population and the impact of vascular disease on Alzheimer’s disease. Subcortical ischemic vascular disease of the Binswanger type (SIVD-BD) has a characteristic set of features that can be used both to identify patients and to follow treatment. We have used clinical features to create a Binswanger Disease Scale and a probability of SIVD-BD, using a machine-learning algorithms. Refining the use of biomarkers will allow dementia treatment to enter the era of precision medicine. The schematic shows the matrix metalloprotinase mechanism involved in blood-brain barrier opening in chronic vascular disease.
INTRODUCTION
Vascular cognitive impairment and dementia (VCID), the second leading cause of dementia, is undergoing a renaissance due to the improvement in diagnostic tools and the realization of the major impact of vascular disease on Alzheimer’s disease (AD).(Corriveau et al. 2016, Gorelick et al. 2011) This new interest has lead to research on biomarkers that could be used for more precise separation between the vascular and neurodegenerative processes involved in the dementias. Advances in neuroimaging and biochemical analysis of blood and CSF provide unique multimodal biomarkers to stratify VCID patients.(Rosenberg et al. 2014) Small vessel disease is recognized as the most common form of VCID, and is more amenable to treatment trials because the natural history involves a progressive growth of the lesions in the white matter, which can be followed over time with MRI methods. Using a combination of clinical information from the neurological examination and neuropsychological testing, identification of inflammatory markers in serum and CSF, and advanced DTI (Lawrence et al. 2014), a subpopulation of patients with inflammatory damage to the deep white matter, death of cells, and symptoms related to those pathological changes can be separated from the heterogeneous group of patients with both large and small vessel VCID. The advantage of the use of biomarkers in this personalized approach to diagnosis is that it is simultaneously identifies a unique population with a similar pathophysiology that can become part of a machine-learning approach and the biomarkers related to the underlying disease processes can be used to develop targeted treatments.
Biomarkers derived from MRI can be used to characterize white matter that is injured by small vessel disease. Proton magnetic resonance spectroscopy (1H-MRS) and DTI show axonal injury.(Sappey Marinier et al. 1992, Brooks et al. 1997, Gasparovic et al. 2013, Lawrence et al. 2014) Inflammatory blood-brain barrier (BBB) damage can be demonstrated with dynamic contrast-enhanced MRI (DCEMRI),(Taheri et al. 2011) and elevated albumin in the CSF,(Wallin et al. 1990) providing evidence of inflammation. Further diagnostic certainty is added by the observation of lacunar strokes on MRI and impaired performance on the executive parts of the neuropsychological examination. Using multimodal biomarkers, it is possible to stratify VCID patients, removing those with white matter hyperintensities (WMHs) due to aging, large vessel disease and neurodegenerative diseases, such as Alzheimer’s disease. In addition, studies of the CSF and blood can be used to eliminate patients with other causes such as multiple sclerosis, collagen vascular disease, and metabolic causes. There is a group of patients for which the etiology of the white matter hyperintensities (WMHs) remains obscure after a full work-up; these patients are best described by the non-specific term, leukoaraiosis.(Hachinski et al. 1987) More important, the white matter lesions with axonal damage tend to expand, allowing them to be used in clinical trials as surrogate markers, which can be combined with a fall in neuropsychological test scores to provide evidence of disease progression. Furthermore, the characteristic expanding lesions in the white matter and the increasing difficulty shown in the executive function testing combine to provide parameters to follow over reasonably short times in clinical trials.
Subcortical ischemic vascular disease of the Binswanger type (SIVD-BD) has a progressive course characterized by extensive damage to the white matter, executive dysfunction, disruption of the BBB, unstable gait and urinary incontinence.(Fisher 1989, Caplan 1995, Huisa & Rosenberg 2014) Chronic hypertension is the most common cause of SIVD-BD; the sustained hypertension causes the blood vessel lumen to narrow and the outer wall to develop fibrosis. Reduced cerebral blood flow leads to hypoxia. This fall in tissue oxygen causes an increase in hypoxia inducible factor-1α (HIF-1α), which triggers the infiltration of macrophages from the systemic circulation and activation of endogenous microglia. These inflammatory cells release proteases and free radicals that produce disruption of the blood-brain barrier (BBB) and breakdown of myelin, causing the characteristic pathology of SIVD-BD.
Identification of Neuroinflammation in VCID Patients
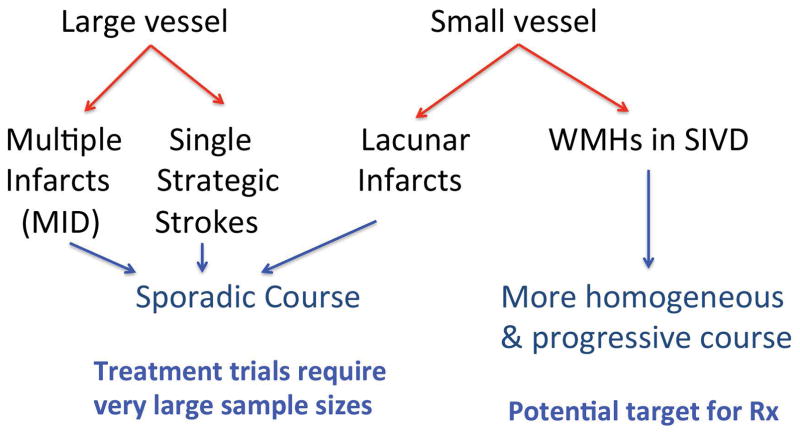
The increased interest in vascular causes of cognitive loss, particularly with the important association of vascular risk factors and AD, has resulted in an attempt to more accurately diagnose forms of VCID. This effort is driven by the heterogeneous nature of the diseases grouped together under the umbrella term of VCID (FIGURE 1). Understanding the natural history of a disease is critical to determine the efficacy of a treatment. Large vessel disease with a single strategic stroke or multiple strokes is too sporadic to allow for clinical trials except with very large sample sizes. Small vessel disease, on the other hand, often has a progressive course making it more amenable to clinical trails of shorter duration with smaller more homogeneous populations. Several terms have been used to describe patients with progressive white matter disease associated with neuroinflammation, including subcortical ischemic vascular disease (SIVD) and SIVD-BD. Diagnosis is based on demyelination in the deep white matter with damage to the axons shown by MRI using 1H-MRS or DTI. Cerebrospinal fluid and blood are important for confirmation of the presence of inflammatory biomarkers. Also important, but less specific for SIVD-BD are the clinical examination and neuropsychological testing.
Figure 1.
Vascular cognitive impairment dementia diagnoses. Large vessel disease leads to multiple infarcts or multi-infarct dementia (MID) and single strategic stroke. Small vessel disease can cause lacunar infarcts limited to the basal ganglia or to white matter hyperintensities (WMHs) The more homogeneous nature and the progressive course make the small vessel form of VCID optimal for treatment (Rx).
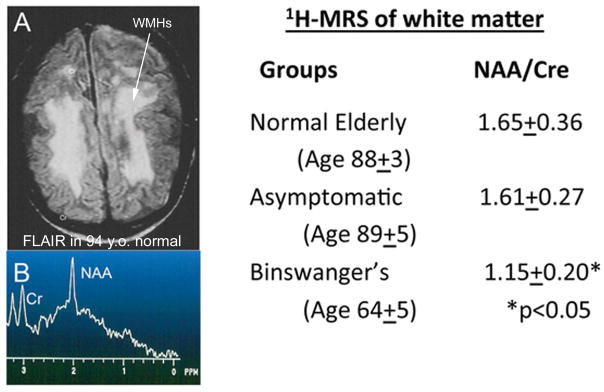
Large studies of normal people over the age of 65 showed that as many as 15 to 30% had white matter hypertensities (WMHs) unaccompanied by clinical findings, which resulted in radiologists including normal aging in MRI reports.(Awad et al. 1986b, Awad et al. 1986a, Hunt et al. 1989) Occasionally a normal elderly patient would be encountered that had extensive white matter changes that closely resembled those seen in SIVD-BD. In an earlier study done by our group in a healthy aging cohort all of whom were over the age of 65 at entry into the study, in a subgroup of 100 that underwent MRI, we identified 7 that had complete white matter changes that were consistent with the radiological diagnosis of SIVD-BD, but who were healthy people over the age of 80. A 94 year old healthy women with almost complete white matter changes is shown; note that the white matter spectroscopy measurements of an-actylaspartate (NAA) are normal indicating that the white matter is unaffected in spite of the diffuse changes in rhe FLAIR MRI (FIGURE 2). The results from normal elderly without WMHs, normal elderly with WMHs, and Binswanger patients are given in the Table in Figure 2. There is a large overlap of normal elderly with white matter changes and patients with pathological changes in the white matter, which has led to a major effort to identify more precise methods for the study of white matter disease.
Figure 2.
White matter hyperintensities (WMHs) are seen on FLAIR imaging in normal elderly, making them a nonspecific finding. Proton magnetic resonance spectroscopy (1H-MRS) can show axonal damage as indicated by a reduced N-acetylaspartate (NAA) signal. A) FLAIR MRI scan in healthy 94 year old women. All of the white matter has abnormal signal (WMHs) (arrow). B) 1H-MRS from the white matter in the person shown in A has normal peaks at N-acetylaspartate (NAA) and creatine (Cr), indicating that the white matter axons are normal. The x-axis reads from left to right and shows parts per million (PPM) in the spectra. There is no y-axis since the values are calculated by ratios. The table to the right shows the results of an earlier study of three groups of people: Normal elderly, asymptomatic with large WMHs as shown in the FLAIR MRI in Figure A, and a group of symptomatic patients with Binswanger’s disease. The ratios of NAA/Cr were normal in the normals and the asymptomatic people, but markedly reduced in the symptomatic Binswanger group, indicating the the axons are damaged in the latter group. Data derived from (Brooks et al. 1997).
Along with SIVD-BD are other diagnostic categories, including multiple strokes due to large vessel disease or small vessel strokes limited to the basal ganglia. When both AD and VCID are present, the diagnosis is mixed dementia, single strategic strokes are generally in the basal ganglia. When the white matter is abnormal on MRI FLAIR, but a firm diagnosis cannot be made, the term leukoaraiosis is appropriate. Table 1 shows the diagnostic categories.
TABLE 1.
Diagnostic categories of various types of VCID and AD
| Diagnostic Category | Abbreviation | Features |
|---|---|---|
| Subcortical ischemic vascular disease of the Binswanger type | SIVD-BD | Vascular risk factors; imbalance; Extensive white matter disease; BBB opening |
| Multiple infarcts | MI | Large vessel strokes |
| Single strategic strokes | SSS | Lacunar strokes in basal ganglia |
| Mixed | VCI/AD | Evidence of both vascular disease and Alzheimer’s disease |
| Leukoaraiosis | SIVD-LA | White matter changes of unknown etiology |
| Alzheimer’s disease | AD | Primary memory loss; posterior cortical atrophy; CSF phosphoTau elevated and Aβ decrease |
Diagnosis of SIVD-BD
SIVD-BD is a spectrum disorder
Multimodal biomarkers aid in the diagnosis, but no one biomarker is sufficient. Several reports have described the characteristic features of the SIVD-BD patients, which provide a basis for a heuristic approach to diagnosis based on multiple features.(Caplan & Schoene 1978, Rosenberg et al. 1979, De Reuck et al. 1980, Roman 1987b, Fisher 1989) The main clinical features in these reports were apathy, imbalance and asymmetric hyperreflexia. Patients had executive dysfunction on neuropsychological testing. Structural damage to the white matter was an essential feature in all of the reports with white matter changes identified at autopsy, on CT or MRI. More recently use of advanced MRI methods have shown disruption of the BBB in SIVD-BD.(Wardlaw et al. 2013, Huisa et al. 2015) Additional confirmatory biomarkers include evidence of inflammation in the CSF, such as elevated albumin and matrix metalloproteinases (MMPs). Demonstration of axon damage by 1H-MRS and DTI have further added to diagnostic certainty.
Vascular risk factor are commonly seen in SIVD-BD, including hypertension, diabetes mellitus, and hyperlipidemia. An approach to diagnosis of spectrum disorders involves selection of a combination of features rather than relying on a single one. In an earlier report, we described a Binswanger disease scale score (BDSS), which combines multiple features to form a composite score.(Rosenberg et al. 2015) The features used to construct the scale are shown in Table 2. While use of such a scale with a cut-point for making the diagnosis of SIVD-BD provides an approximation of diagnoses, it is limited by the inability to grow as new information is obtained. This can be overcome by the use of machine-learning and “big data”.
TABLE 2.
Components of the scoring system used to select patients most likely to have SIVD-BD
| Features used in scale scores | BDS | PCA/EFA |
|---|---|---|
| I. Clinical features (4 points if present)* | ||
| 1. Hypertension (HTN) | X | |
| 2. Diabetes mellitus (DM) | X | |
| 3. Hyper-reflexia (REF) | X | |
| 4. Imbalance (GAIT) | X | |
| II. Neuropsychological testing (1 point) | ||
| 5. Executive <45 (Exec T score)† | X | X |
| III. Metabolites in WM (1H-MRSI) (1 point) | ||
| 6. N-acetylaspartate (NAA)<1212 | X | X |
| 7. Choline (CHO) | X | |
| 8. Creatine and phosphocreatine (CR) | X | |
| IV. Inflammation and BBB (3 points) | ||
| 9. Albumin index >6.0 (albumin ratio) | X | X |
| 10. BBB permeability Ki >0.001813 | X | X |
| 11. MMP-2 index <0.0118 | X | X |
| 12. MMP-9 index | X | |
| V. Alzheimer’s biomarkers (1 point) | ||
| 13. Aβ42/log(P-τ181) >150 | X | X |
Two scales were shown: (1) a 10-point Binswanger Disease Score (BDS) with one point for each feature; and (2) the Principal Component Analysis/Exploratory Factor Analysis (PCA/EFA).
Points are used in calculation of the BDS with score of 6 or greater suggestive of BD.
Cut-off determined from control sample.
Aβ42, amyloid-β1–42; BBB, blood-brain barrier; BD, Binswanger disease; BDS, Binswanger disease score; EFA, exploratory factor analysis; 1H-MRSI, proton MR spectroscopic imaging; MMP, matrix metalloproteinase; P-τ181, phosphorylated-τ181; PCA, principal component analysis; WM, white matter. (See (Rosenberg et al. 2015) for discussion of use of BDS and EFA.)
Diagnosis of SIVD-BD is confounded by the overlap with neurodegenerative processes and normal aging (FIGURE 3). In the early stages of SIVD-BD, it may be difficult to separate it from Alzheimer’s disease (AD) when the vascular disease leads to slowly progressive symptoms or the changes in the white matter that are thought to suggest ischemic disease are actually due to aging. A significant proportion of patients with AD have white matter changes due to what is referred to as incomplete ischemia, but aging changes also are present.(Brun & Englund 1986) This can result in a long delay before there is clarity as to the correct diagnosis. During this time the damage to the white matter can progress from a potentially treatable form to irreversible damage. Biomarkers can be used to establish a diagnosis prior to a clinical diagnosis.
Figure 3.
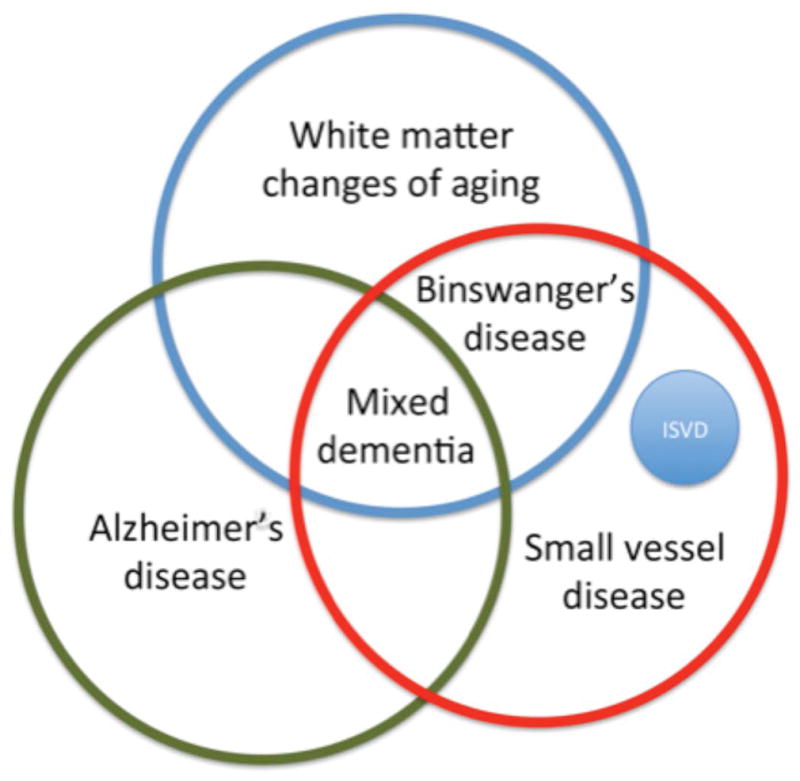
A Venn diagram to illustrate the ovelapping nature of the dementias, which shows the need for biomarkers that can separate the various diagnoses. The overlap in diagnoses in the early stage of a dementia illustrate the problems in establishing a diagnosis. The major causes of dementia are Alzheimer’s disease and small vessel disease. When the small vessel disease is extensive and contributes to the symptomatology the term Binswanger’s disease is used. Confounding all of the diagnostic categories is the white matter changes that are part of aging, and they can be present in both Alzheimer’s disease and small vessel disease. Mixed dementia is used to describe patients with both Alzheimer’s disease and small vessel disease. Inherited small vessel disease (ISVD) is a small group of small vessel disease patients. (Illustration from Rosenberg et al 2016; permission pending)
Biomarkers in Binswanger’s disease
Pathophysiology
Optimal biomarkers should be based on pathological changes in the brain. These can be identified by neurological and neuropsychological findings, changes on MRI and alterations in body fluids. All patients with SIVD-BD have by definition extensive damage to the white matter due to vascular risk factors. The most common vascular risk factor is hypertension, which can slowly damage white matter over many years. Within the vascular tree, the medium sized arteries, called arterioles, are the most vulnerable with the effects of chronic hypertension leading to arteriolosclerosis. As opposed to the lipid containing plaques that are found in atherosclerosis, the medium sized arterioles show a characteristic combination of narrowing of the vessel lumen and expansion of the vessel outer wall by fibrosis. Various terms have been used to describe these changes, depending on the stage of the damage. This is illustrated in the spontaneously hypertensive stroke prone rat.(Pires et al. 2011) The consequences of the narrowed lumen are reduced cerebral blood flow that can reach hypoxic levels. Fibrotic thickening of the vessel wall reduces the ability to dilate in times of increased metabolic need. This has been demonstrated in patients with WMHs given a challenge with carbon dioxide, a potent vasodilator, who failed to increase blood flow, leading to intermittent hypoxia.(Sam et al. 2016)
Consequences of hypoxia
Hypoxia leads to inflammation by the up regulation of HIF-1α, activating a cassette of genes that are involved in both injury and repair.(Semenza 2014) Macrophages are recruited and microglia activated secondary to the reduced oxygen. Proteases and free radicals are released by the inflammatory cells in the process of remodeling the damaged vessels. Free radicals and proteases break down the fibrotic basal lamina, disrupt tight junction proteins, claudin and occludin, and finally, attack myelinated fibers, releasing myelin basic protein and neurofilament light in a process that has been referred to a “by-stander demyelination”.(Bloom et al. 1978, Matyszak & Perry 1995) Thus, the proteases and free radicals, which activate other proteases, create the dual pathology that characterizes the inflammatory phase of small vessel disease, involving vasogenic edema due to opening of the blood-brain barrier, and release of proteases that cause demyelination. MRI reveals both the edema from the disrupted blood-brain barrier, and the demyelination. During this process, a series inflammatory molecules, including cytokines and matrix metalloproteinases (MMPs), can be used as biomarkers through their measurement in the CSF.
MRI Biomarkers
MRI is the basis for the diagnosis of SIVD-BD since all of the patients show injury to the white matter. However, the converse is not true, and not all patients with changes in the white matter have SIVD-BD. Fluid attenuated inversion recovery (FLAIR) is best for screening patients since it is highly sensitive to the presence of increased water either due to edema or loss of tissue. While it is highly sensitive, FLAIR lacks specificity since white matter changes occur in many neurological disorders, but more importantly it can confound the diagnosis of SIVD-BD, since from 30% of normal elderly over the age of 65 can have moderate white matter hyperintensiites (WMHs).(Hunt et al. 1989)
Damage to the white matter can be separated from changes of aging with either proton magnetic resonance spectroscopy (1H-MRS) or DTI; DTI is preferred since it is more universally available and requires less time to acquire. DTI has the added advantage that it can reveal intermediate tissue changes in white matter regions that appear normal on FLAIR. This ability to identify tissue at risk, and its ability to be run on any MRI scanner, make DTI very valuable in the early stages of the illness as well as a method to be used in treatment trials.
White matter hyperintensities increase in size over time in the elderly; the location of the growth in this group is periventricular, which can separate it from the more ominous changes that occur in the deep white matter and at the gray white matter junction.(Wolfson et al. 2013). The etiology of periventricular WMH growth in healthy elderly is unclear. The region around the large WMHs has been referred to as a “penumbral” region, and this is the site of gradual growth of the lesion size.(Maillard et al. 2014) Penumbral tissue has reduced cerebral blood flow.(Promjunyakul et al. 2016) Regions that appear normal on FLAIR can show abnormalities in the DTI with reduced fractional anisotropy (FA) and increased mean diffusivity (MD).(Benjamin et al. 2016) These areas with abnormal diffusion are thought to be at risk of becoming WMHs on FLAIR.(Maillard et al. 2013)
The white matter enigma
From the early days of MRI the dilemma of role of white matter changes as an indicator of vascular disease has been debated. In the early reports the white matter changes were used as a criteria for BD in spite of the lack of symptoms,(Kinkel et al. 1985) and radiologists often report these findings as indicative of cerebral ischemia or “small strokes”, however, the etiology of these findings in the absence of clinical finding remains challenging.
Longitudinal studies in large populations from European consortium show that the white matter is a predictor of cognitive impairment.(Poggesi et al. 2014, Inzitari et al. 2009, de Groot et al. 2013). There is a higher incidence of vascular risk factors, primarily hypertension, in those with the progressive dementia. While such studies are important to understand in general the consequence of WMHs, they are not useful in the individual patient, and cannot provide adequate information for patient studies except in very large groups of patients. While FLAIR has limitations in predicting those with more serious injury to the white matter since many with leukoaraiosis remain asymptomatic, the use of imaging modalities that are more sensitive to white matter fiber damage appear to be more predictive. To date few longitudinal studies have been done using 1H-MRS and DTI, and these will be on great interest, particularly if it can shown that they can detect patients at greatest risk of progression of the WMHs.
Biomarkers in the CSF and Blood
Another important source of biomarkers is the CSF, which reflects changes occurring in the brain more accurately than the blood. Biomarkers for SVD in the CSF include elevated albumin, which needs to be compared to albumin levels in the blood to calculate the albumin index. When the blood-brain barrier is opened, the albumin increases. Matrix metalloproteinases and cytokines are biomarkers of inflammation.
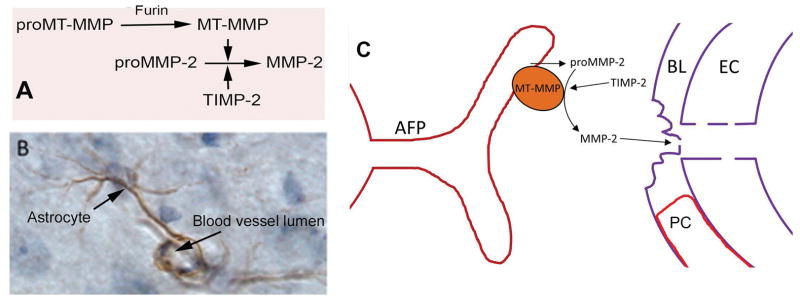
Matrix metalloproteinases are a family of 26 proteases that play major roles in the body. The MMPs in the brain form two groups; constitutively expressed enzymes that are present all the time and those that are expressed in injury and tissue remodeling. The two main constitutive enzymes are MMP-2 and membrane type MMP (MMP-14), which maintain normal extracellular matrix. Astrocytes normally contain MMP-2 and MMP-14, primarily in the end-feet where they have direct access to basal lamina and tight junction proteins (FIGURE 4). Inflammatory cells, including microglia/macrophages, express the inducible enzymes, MMP-3 and MMP-9, which are induced after injury in either acutely in the injury phase or delayed during tissue repair. These inducible MMPs are more destructive since they are released into the extracellular space rather than constrained to the region in the immediate vicinity of the vessel.
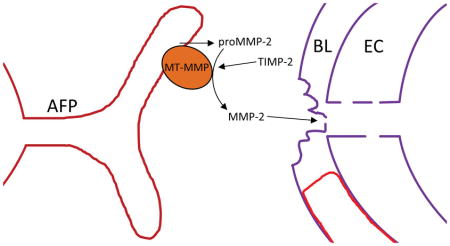
Figure 4.
Role of MMP-2 in the disruption of the blood-brain barrier. A) MMP-2 is normally present in the brain and CSF in a latent form (proMMP-2). Activation requires a trimolecular complex formed by membrane-type metalloproteinase (MT-MMP) and tissue inhibitor of metalloproteinases-2 (TIMP-2). Furin activates proMT-MMP initiating the cascade. B) An astrocytic process immunostained with MMP-2. The foot process is wraped around the capillary. (Image of astrocyte modified from figure in Rosenberg et. al. Brain Research 893: 104–114, 2001) C) A schematic drawing showing the location of the MT-MMP on the astrocyte foot process, which constrains the activation process to the region between the foot process and the basal lamina.
MMPs are maintained in the latent state to protect the brain from unwanted proteolytic injury. MMP-2 is further constrained by the need for the formation of a trimolecular complex comprised of latent MMP-2, MT-MMP and tissue inhibitor of metalloproteinases-2 (TIMP-2). The MT-MMP is bound to the astrocytic foot process, limiting the spread of the active MMP to the region close to the basement membrane. Inactive MMPs require activation, which can occur by the action of other proteases or free radicals. MMPs are released into the CSF where they can be detected, making lumbar puncture a critical test to determine their presence in brain. Both the latent and active MMPs can be measured in the CSF.
In order to measure the endogenous production of MMP by the brain it is necessary to measure the enzymes in the blood and CSF.(Liuzzi et al. 2002) When the CSF MMPs are derived from the systemic circulation by transport across a leaky blood-brain barrier, indexing the CSF and blood MMP values to the albumin in both compartments can be done similarly to the IgG index.(Candelario-Jalil et al. 2011) Using that method the endogenous production can be separated from that coming from the blood. MMP-9 is less useful in identifying SIVD-BD probably because the MMP-9 is mainly increased by acute ischemia.
Gelatinases (MMP-2 and MMP-9) can be measured by gelatin zymography. During the electrophoresis the enzymes are activated and appear as a white band (where the gelatin has been proteolytically removed by the gelatinase) against the blue-stained gels. A latent band is seen at a higher molecular weight and a lower molecular weight representing the active form. Both forms are seen because the enzymes are activated during the electrophoresis and then inactivated. An assay of the activity of MMP-2, MMP-3 and MMP-9 has been developed; the enzyme is immunocaptured and precipitated with an antibody that avoids the active end, activity is detected when a fluorescent peptide is cleaved in the presence of the active enzymes, emitting a signal.(Hawkins et al. 2013)
Biomarkers and Classification methods
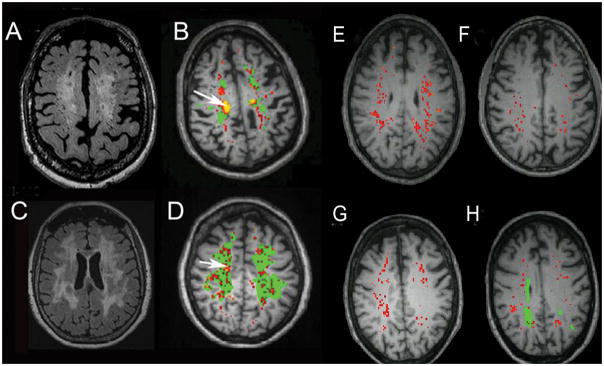
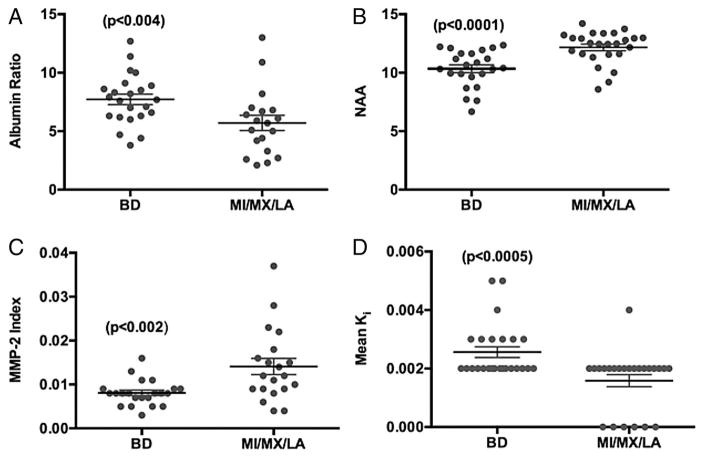
None of the biomarkers that have been described are diagnostic for SIVD-BD. However, combining multiple biomarkers increases the probability of the individual being in the SIVD-BD class. There are multiple ways to use biomarkers in classification. All of them require an expert to identify the major features involved. In an earlier report, we described a heuristic method of classification based on reports of SIVD-BD in the literature.(Rosenberg 1986, Babikian & Ropper 1987, Roman 1987a, Fisher 1989, Bennett et al. 1990, Caplan 1995) BDSS was constructed with various features used in making the diagnosis; higher scores indicated a higher probability of the patient having SIVD-BD (TABLE 2). The major components were structural damage to the white matter as indicated by a low NAA. Inflammatory factors were important including reduced MMP-2 index. Other indicators of inflammation were increased permeability with either DCEMRI or elevated albumin index (FIGURE 5). Several features had limited diagnostic potential due to the overlap of clinical findings and neuropsychological test results with other forms of VCID and AD, including hypertension, diabetes, and hyperreflexia, precluding their use as major indicators of SIVD-BD. In an earlier report, several of the features predicted the diagnosis of SIVD-BD (FIGURE 6).(Rosenberg 2015)
Figure 5.
FLAIR MRI (A and C) and corresponding dynamic contrast-enhanced MRI permeability map (B and D) . In A the large area of white matter injury, which can be seen to extend into the the subcortical white matter, which corresponds to the permeabilty map in B. Green regions are the WMHs and the red and yellow represent incresed permeability. The arrow indicates the region of highest permeabilty (yellow). Similarly C shows the extensive WMHs and D demonstrates the multiple areas of blood-brain barrier leakage. The arrow shows a region of high permeability (yellow). For comparison 4 representative permeability maps from healthy controls are shown (E–H). There are some regions of red indicating low level increases in permeability. One of the patients has some WMHs, which are defined as leukoaraiosis.
Figure 6.
Comparison of individual biomarkers between patients with Binswanger ‘s disease (BD) and non-BD, including multiple infarcts (MI), mixed Alzheimer’s disease/vascular cognitive impairment (MX), leucoaraiosis (LA). (A) Albumin index (ratio of cerebrospinal fluid albumin to serum albumin) shows significant elevation in BD, suggesting disruption of the blood-brain barrier. (B) N-acetylaspartate (NAA) measured in white matter by proton magnetic resonance spectroscopy (1H-MRS) was significantly lower in BD due to ischaemic injury to the white matter. (C) Matrix metalloproteinase-2 (MMP-2) index was significantly reduced in BD. (D) Mean blood-brain barrier permeability, Ki, measured by dynamic contrast-enhanced MRI was elevated in BD. Numbers in parentheses represent statistical significance.(Rosenberg et al. 2015)
As the number of features increases and the training set based on expert opinions grows, a machine-learning system can be helpful. Such approaches to diagnosis have been used in other medical fields for so-called “precision diagnosis”.(DeMarshall et al. 2016, Lebedev et al. 2014) Recently, we used several statistical methods to determine the probability of a diagnosis of SIVD-BD. Both logistical regression and the machine-learning algorithm, Random Forests, using an enlarged set of biomarkers derived from an expanded cohort of patients, made an accurate determination of SIVD-BD compared to other diagnoses. This encouraging result suggests that it will be possible to predict a diagnosis several years prior to the clinicians’ ability to separate overlapping diseases.(Erhardt et al. Manuscript submittted). As more patients are entered into the system and a sufficient amount of time for follow up is available to increase certainty of diagnosis, these patients will enlarge the training set and improve diagnostic accuracy.
Future Directions
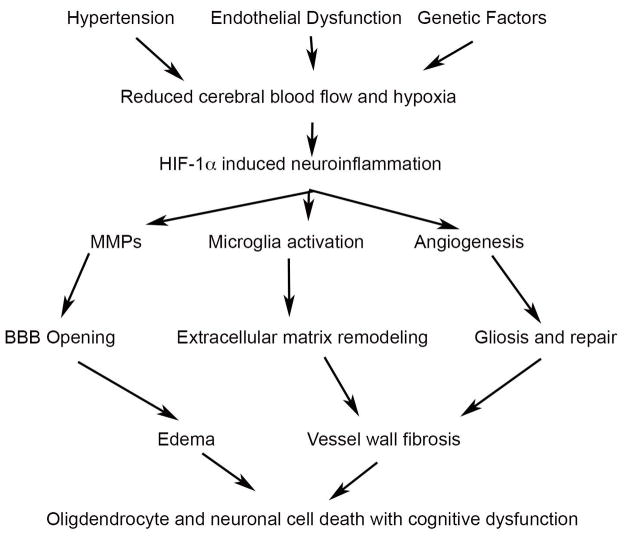
The number of patients with vascular causes of dementia is projected to greatly increase in the next 30 years, adversely impacting the health care system. Autopsy stu dies indicate that vascular disease hastens the course of Alzheimer’s disease. There is a growing realization that neuroinflammation is a potentially treatable target, making it critical that the transition into the inflammatory stage be understood. This is the benefit of developing a set of biomarkers that are linked to inflammation, and which can indicate when the inflammatory phase of the illness has begun. The biomarkers need to reflect the current concepts of mechanisms of tissue injury. At the present time, the dominant theories involve long-standing vascular injury generally through hypertension, reduced cerebral blood flow, intermittent hypoxia, leading to an inflammation response with opening of the blood-brain barrier, release of proteases and myelin damage. This scenario will most likely need to be revised over time, but it currently guides selection of the optimal set of biomarkers that conform to the proposed pathophysiology (FIGURE 7).
Figure 7.
Schematic diagram of hypthetical injury cascade. The early insult involves hypertension, endotheilial dysfunction and genetic factors. The damaged blood vessels reduce cerebral blood flow causing hypoxia, which triggers hypoxia inducibe factor-1α (HIF-1α). Matrix metalloproteinases (MMPs), microglia activation and angiogenesis are induced by HIF-1α. Tissue damage occurs from blood-brain barrier opening (BBB) with vasogenic edema. As the vessel wall is remodeled, gliosis and fibrosis result, leading to cell death. Ultimately cognitive function is impaired.
In addition to the critical role in classification, biomarkers will provide surrogates for treatment trials. While few longitudinal studies have been described, there are many cross-sectional ones. The early injury to the normal appearing white matter indicates the tissue at risk of conversion into an irreversible glial scar. DTI appears to be the optimal modality to identify the tissue at risk. In SIVD-BD there is generally a progressive course that can be established. Once this course is identified, it will become the target for treatment trials aimed at slowing the damage to the white matter.
Acknowledgments
There are no conflicts of interest to report. The studies were supported by grants from the NIH (RO1 NS 052305), the US-Israeli Binational Research Foundation, and the National Center for Research Resources and the National Center for Advancing Translational Sciences of the NIH through Grant Number 8UL1TR000041, UNM Clinical and Translational Science Center.
Table of Abbreviations
| Abbr | Full |
|---|---|
| 1H-MRS | proton magnetic resonance spectroscopy imaging |
| AD | Alzheimer’s disease |
| Aβ42 | amyloidβ1-42 |
| BBB | blood-brain barrier |
| BIC | Bayesian information criterion |
| CSF | cerebrospinal fluid |
| DCEMRI | dynamic contrast-enhanced MRI |
| EFA | exploratory factor analysis |
| FLAIR | Fluid-attenuated inversion recovery MRI |
| Gd-DTPA | gadolinium-diethylenetriaminepentaacetic acid |
| LA | leukoaraiosis |
| LR | logistic regression |
| LVD | large vessel disease |
| MI | multiple or single cerebral infarcts |
| MMP-2 | matrix metalloproteinase-2 |
| MRI | magnetic resonance imaging |
| NAA | N-acetyl-containing compounds and N-acetylglutamylaspartate |
| PRESS | point-resolved spectroscopy sequence |
| PTau | phosphorylated tau181 |
| RF | random forests |
| SIVD | subcortical ischemic vascular disease |
| SIVD-BD | subcortical ischemic vascular disease-Binswanger’s disease |
| VCID | vascular cognitive impairment and dementia |
| WM | white matter |
| WMH | WM hyperintensities |
References
- Awad IA, Johnson PC, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke; a journal of cerebral circulation. 1986a;17:1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- Awad IA, Spetzler RF, Hodak JA, Awad CA, Carey R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke; a journal of cerebral circulation. 1986b;17:1084–1089. doi: 10.1161/01.str.17.6.1084. [DOI] [PubMed] [Google Scholar]
- Babikian V, Ropper AH. Binswanger’s disease: a review. Stroke; a journal of cerebral circulation. 1987;18:2–12. doi: 10.1161/01.str.18.1.2. [DOI] [PubMed] [Google Scholar]
- Benjamin P, Zeestraten E, Lambert C, et al. Progression of MRI markers in cerebral small vessel disease: Sample size considerations for clinical trials. J Cereb Blood Flow Metab. 2016;36:228–240. doi: 10.1038/jcbfm.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Gilley DW, Fox JH. Clinical diagnosis of Binswanger’s disease [see comments] J Neurol Neurosurg Psychiatry. 1990;53:961–965. doi: 10.1136/jnnp.53.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom BR, Ju G, Brosnan C, Cammer W, Norton W. Notes on the pathogenesis of multiple sclerosis. Neurology. 1978;28:t293–t2101. doi: 10.1212/wnl.28.9_part_2.93. [DOI] [PubMed] [Google Scholar]
- Brooks WM, Wesley MH, Kodituwakku PW, Garry PJ, Rosenberg GA. 1H-MRS differentiates white matter hyperintensities in subcortical arteriosclerotic encephalopathy from those in normal elderly. Stroke; a journal of cerebral circulation. 1997;28:1940–1943. doi: 10.1161/01.str.28.10.1940. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Thompson J, Taheri S, Grossetete M, Adair JC, Edmonds E, Prestopnik J, Wills J, Rosenberg GA. Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke; a journal of cerebral circulation. 2011;42:1345–1350. doi: 10.1161/STROKEAHA.110.600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan LR. Binswanger’s disease--revisited. [Review] Neurology. 1995;45:626–633. doi: 10.1212/wnl.45.4.626. [DOI] [PubMed] [Google Scholar]
- Caplan LR, Schoene WC. Clinical features of subcortical arteriosclerotic encephalopathy (Binswanger disease) Neurology. 1978;28:1206–1215. doi: 10.1212/wnl.28.12.1206. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, Pahigiannis K, Waddy SP, Koroshetz W. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell Mol Neurobiol. 2016;36:281–288. doi: 10.1007/s10571-016-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot M, Verhaaren BF, de Boer R, Klein S, Hofman A, van der Lugt A, Ikram MA, Niessen WJ, Vernooij MW. Changes in normal-appearing white matter precede development of white matter lesions. Stroke; a journal of cerebral circulation. 2013;44:1037–1042. doi: 10.1161/STROKEAHA.112.680223. [DOI] [PubMed] [Google Scholar]
- De Reuck J, Crevits L, De Coster W, Sieben G, vander Eecken H. Pathogenesis of Binswanger chronic progressive subcortical encephalopathy. Neurology. 1980;30:920–928. doi: 10.1212/wnl.30.9.920. [DOI] [PubMed] [Google Scholar]
- DeMarshall CA, Nagele EP, Sarkar A, et al. Detection of Alzheimer’s disease at mild cognitive impairment and disease progression using autoantibodies as blood-based biomarkers. Alzheimer’s & dementia. 2016;3:51–62. doi: 10.1016/j.dadm.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CM. Binswanger’s encephalopathy: a review. J Neurol. 1989;236:65–79. doi: 10.1007/BF00314400. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Prestopnik J, Thompson J, Taheri S, Huisa B, Schrader R, Adair JC, Rosenberg GA. 1H-MR spectroscopy metabolite levels correlate with executive function in vascular cognitive impairment. Journal of neurology, neurosurgery, and psychiatry. 2013;84:715–721. doi: 10.1136/jnnp-2012-303878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, et al. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Archives of Neurology. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- Hawkins KE, DeMars KM, Yang C, Rosenberg GA, Candelario-Jalil E. Fluorometric immunocapture assay for the specific measurement of matrix metalloproteinase-9 activity in biological samples: application to brain and plasma from rats with ischemic stroke. Mol Brain. 2013;6:14. doi: 10.1186/1756-6606-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisa BN, Caprihan A, Thompson J, Prestopnik J, Qualls CR, Rosenberg GA. Long-Term Blood-Brain Barrier Permeability Changes in Binswanger Disease. Stroke; a journal of cerebral circulation. 2015;46:2413–2418. doi: 10.1161/STROKEAHA.115.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisa BN, Rosenberg GA. Binswanger’s disease: toward a diagnosis agreement and therapeutic approach. Expert Rev Neurother. 2014;14:1203–1213. doi: 10.1586/14737175.2014.956726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt AL, Orrison WW, Yeo RA, Haaland KY, Rhyne RL, Garry PJ, Rosenberg GA. Clinical significance of MRI white matter lesions in the elderly. Neurology. 1989;39:1470–1474. doi: 10.1212/wnl.39.11.1470. [DOI] [PubMed] [Google Scholar]
- Inzitari D, Pracucci G, Poggesi A, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009;339:b2477. doi: 10.1136/bmj.b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkel WR, Jacobs L, Polachini I, Bates V, Heffner RR. Subcortical arteriosclerotic encephalopathy (Binswanger’s disease). Computed tomographic, nuclear magnetic resonance, and clinical correlations. Arch Neurol. 1985;42:951–959. doi: 10.1001/archneur.1985.04060090033010. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Chung AW, Morris RG, Markus HS, Barrick TR. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology. 2014;83:304–311. doi: 10.1212/WNL.0000000000000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev AV, Westman E, Van Westen GJ, et al. Random Forest ensembles for detection and prediction of Alzheimer’s disease with a good between-cohort robustness. NeuroImage Clinical. 2014;6:115–125. doi: 10.1016/j.nicl.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi GM, Trojano M, Fanelli M, Avolio C, Fasano A, Livrea P, Riccio P. Intrathecal synthesis of matrix metalloproteinase-9 in patients with multiple sclerosis: implication for pathogenesis. Mult Scler. 2002;8:222–228. doi: 10.1191/1352458502ms800oa. [DOI] [PubMed] [Google Scholar]
- Maillard P, Carmichael O, Harvey D, Fletcher E, Reed B, Mungas D, DeCarli C. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. Ajnr. 2013;34:54–61. doi: 10.3174/ajnr.A3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P, Fletcher E, Lockhart SN, Roach AE, Reed B, Mungas D, DeCarli C, Carmichael OT. White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain. Stroke; a journal of cerebral circulation. 2014;45:1721–1726. doi: 10.1161/STROKEAHA.113.004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyszak MK, Perry VH. Demyelination in the central nervous system following a delayed-type hypersensitivity response to bacillus Calmette-Guerin. Neuroscience. 1995;64:967–977. doi: 10.1016/0306-4522(94)00448-e. [DOI] [PubMed] [Google Scholar]
- Pires PW, Rogers CT, McClain JL, Garver HS, Fink GD, Dorrance AM. Doxycycline, a matrix metalloprotease inhibitor, reduces vascular remodeling and damage after cerebral ischemia in stroke-prone spontaneously hypertensive rats. American journal of physiology. 2011;301:H87–97. doi: 10.1152/ajpheart.01206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggesi A, Gouw A, van der Flier W, et al. Neurological abnormalities predict disability: the LADIS (Leukoaraiosis And DISability) study. J Neurol. 2014;261:1160–1169. doi: 10.1007/s00415-014-7332-9. [DOI] [PubMed] [Google Scholar]
- Promjunyakul NO, Lahna DL, Kaye JA, Dodge HH, Erten-Lyons D, Rooney WD, Silbert LC. Comparison of cerebral blood flow and structural penumbras in relation to white matter hyperintensities: A multi-modal magnetic resonance imaging study. J Cereb Blood Flow Metab. 2016;36:1528–1536. doi: 10.1177/0271678X16651268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman GC. Senile dementia of the Binswanger type. A vascular form of dementia in the elderly. JAMA. 1987a;258:1782–1788. doi: 10.1001/jama.1987.03400130096040. [DOI] [PubMed] [Google Scholar]
- Roman GC. Senile dementia of the Binswanger type. A vascular form of dementia in the elderly. JAMA. 1987b;258:1782–1788. doi: 10.1001/jama.1987.03400130096040. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Binswanger’s disease. Arch Neurol. 1986;43:641–642. doi: 10.1001/archneur.1986.00520070003001. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Bjerke M, Wallin A. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke; a journal of cerebral circulation. 2014;45:1531–1538. doi: 10.1161/STROKEAHA.113.004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, Kornfeld M, Stovring J, Bicknell JM. Subcortical arteriosclerotic encephalopathy (Binswanger): computerized tomography. Neurology. 1979;29:1102–1106. doi: 10.1212/wnl.29.8.1102. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Prestopnik J, Adair JC, et al. Validation of biomarkers in subcortical ischaemic vascular disease of the Binswanger type: approach to targeted treatment trials. Journal of neurology, neurosurgery, and psychiatry. 2015;86:1324–1330. doi: 10.1136/jnnp-2014-309421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, Wallin A, Wardlaw JM, et al. Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab. 2016;36:6–25. doi: 10.1038/jcbfm.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam K, Crawley AP, Conklin J, et al. Development of White Matter Hyperintensity Is Preceded by Reduced Cerebrovascular Reactivity. Annals of neurology. 2016;80:277–285. doi: 10.1002/ana.24712. [DOI] [PubMed] [Google Scholar]
- Sappey Marinier D, Calabrese G, Hetherington HP, Fisher SN, Deicken R, Van Dyke C, Fein G, Weiner MW. Proton magnetic resonance spectroscopy of human brain: applications to normal white matter, chronic infarction, and MRI white matter signal hyperintensities. Magn Reson Med. 1992;26:313–327. doi: 10.1002/mrm.1910260211. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annual review of pathology. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- Taheri S, Gasparovic C, Huisa BN, et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke; a journal of cerebral circulation. 2011;42:2158–2163. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin A, Blennow K, Fredman P, Gottfries CG, Karlsson I, Svennerholm L. Blood brain barrier function in vascular dementia. Acta neurologica Scandinavica. 1990;81:318–322. doi: 10.1111/j.1600-0404.1990.tb01562.x. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Doubal FN, Valdes-Hernandez M, Wang X, Chappell FM, Shuler K, Armitage PA, Carpenter TC, Dennis MS. Blood-brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke; a journal of cerebral circulation. 2013;44:525–527. doi: 10.1161/STROKEAHA.112.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson L, Wakefield DB, Moscufo N, Kaplan RF, Hall CB, Schmidt JA, Guttmann CR, White WB. Rapid buildup of brain white matter hyperintensities over 4 years linked to ambulatory blood pressure, mobility, cognition, and depression in old persons. J Gerontol A Biol Sci Med Sci. 2013;68:1387–1394. doi: 10.1093/gerona/glt072. [DOI] [PMC free article] [PubMed] [Google Scholar]