Abstract
Dopamine neuron excitability is regulated by inhibitory GABAergic synaptic transmission and modulated by nicotinic acetylcholine receptors (nAChRs). The aim of this study was to evaluate the role of α6 subunit-containing nAChRs (α6*-nAChRs) in acute ethanol effects on ventral tegmental area (VTA) GABA and dopamine (DA) neurons. α6*-nAChRs were visualized on GABA terminals on VTA GABA neurons and α6*-nAChR transcripts were expressed in most DA neurons, but only a minority of VTA GABA neurons from GAD67 GFP mice. Low concentrations of ethanol (1–10 mM) enhanced GABAA receptor (GABAAR)-mediated spontaneous and evoked inhibition with blockade by selective α6*-nAChR antagonist α-conotoxins (α-Ctxs) and lowered sensitivity in α6 knock-out (KO) mice. Ethanol suppression of VTA GABA neuron firing rate in wild-type (WT) mice in vivo was significantly reduced in α6 KO mice. Ethanol (5–100 mM) had no effect on optically-evoked GABAAR-mediated inhibition of DA neurons and ethanol enhancement of VTA DA neuron firing rate at high concentrations was not affected by α-Ctxs. Ethanol conditioned place preference was reduced in α6 KO mice compared to WT controls. Taken together, these studies indicate that relatively low concentrations of ethanol act through α6*-nAChRs on GABA terminals to enhance GABA release onto VTA GABA neurons, in turn to reduce GABA neuron firing, which may lead to VTA DA neuron disinhibition, suggesting a possible mechanism of action of alcohol and nicotine co-abuse.
Introduction
Nicotine (NIC) has high affinity for nicotinic acetylcholine receptors (nAChRs). Activation and desensitization of nAChRs in the mesolimbic dopamine (DA) reward system may be crucial factors underlying the effects of NIC on DA neurons in the midbrain ventral tegmental area [VTA; (Mansvelder & McGehee, 2000; Mansvelder et al., 2002)] that projects to limbic structures such as the nucleus accumbens (NAc) and prefrontal cortex [PFC; (de Rover et al., 2002)]. Neurons within the VTA express a wide variety of nAChRs (Wooltorton et al., 2003), and NIC can activate both DA and γ-aminobutyric acid (GABA) neurons (Yin & French, 2000; Mansvelder et al., 2002) via an increase in activation of specific nAChRs, although the majority of endogenous cholinergic inputs into the VTA appear to contact GABA rather than DA neurons (Garzon et al., 1999; Fiorillo & Williams, 2000). In addition to α4β2 and homomeric α7-nAChRs, there is considerable expression of heteromeric α6-containing nAChRs (α6*-nAChRs; * denotes α6 subunits combined with other nAChR subunits) in the VTA. Alpha6*-nAChRs have been implicated in DA transmission and NIC dependence (Drenan et al., 2008; Exley et al., 2008; Pons et al., 2008; Jackson et al., 2009; Brunzell et al., 2010; Drenan et al., 2010; Gotti et al., 2010; Sanjakdar et al., 2015). We have recently shown that α6*-nAChRs are located on GABA terminals on VTA DA neurons and that their activation by acetylcholine (ACh) enhances GABAergic synaptic inhibition to VTA DA neurons (Yang et al., 2011).
There are known interactions between ethanol, NIC and nAChRs [for review see (Davis & de Fiebre, 2006)]. Superfusion of NIC and ethanol to brain slices produces synergistic effects on DA neuron firing rate, depending upon the dose level of each drug (Clark & Little, 2004). Synergistic effects are also seen on DA release in the NAc when low-dose ethanol and NIC are injected into the VTA (Tizabi et al., 2002). Both NIC (David et al., 2006) and ethanol (Gatto et al., 1994; Nowak et al., 1998) are self-administered when infused into the VTA. Ethanol and NIC self-administration can be modulated by interfering with GABA neurotransmission into the VTA, as microinfusion of GABAAR agonists into the VTA reduces NIC self-administration (Corrigall et al., 2000), while microinjection of GABAAR antagonists into the VTA reduces ethanol self-administration (Gatto et al., 1994; Nowak et al., 1998). A possible target for interactive effects of NIC and ethanol is VTA GABA neurons. We have shown in multiple publications that VTA GABA neurons are inhibited by low to high doses (0.25 – 4.0 g/kg) of ethanol in vivo (Diana et al., 2003; Stobbs et al., 2004; Ludlow et al., 2009; Steffensen et al., 2009; Steffensen et al., 2011), and we have demonstrated more recently the sensitivity of these neurons to NIC (Taylor et al., 2013). Here, we show that low-dose ethanol effects on GABA neurons in the VTA are mediated by α6*-nAChRs on GABA terminals.
Materials and Methods
Animal Subjects
Male wild-type (WT) C57BL/6 mice, α6 knock-out (KO) mice on the C57BL/6J background, CD-1 glutamate-decarboxylase-67 (GAD-67)-green fluorescent protein (GAD-67 GFP) knock-in mice (Tamamaki et al., 2003), and mice hemizygous for vesicular GABA transporter (VGAT) channel rhodopsin-2 (ChR2) yellow fluorescent protein (YFP) bacterial artificial chromosome (BAC) transgene on the C57BL/6 background (Jackson Labs) were bred and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experiments were performed between P28–P45 except for the dissociated neuron studies which were performed between 21–25 days. Mice were treated in strict accordance with the Brigham Young University, Barrow Research Institute and Arizona State University Animal Research Committee (IACUC) guidelines, which incorporate and exceed current NIH guidelines. Once weaned at PND 21, all mice were housed 4–5/cage and given ad libitum access to solid food and water and placed on a reverse light/dark cycle with lights ON from 0800 to 2000 hrs.
Preparation of Brain Slices and Characterization of VTA Neurons
All brain slice preparations were performed in P28–45 day old C57BL/6J, GAD-GFP knock-in mice or VGAT transgenic mice. Brains were extracted under isoflurane (5%) anesthesia. Brains were sectioned in ice-cold cutting solution (in mM: 220 Sucrose, 3 KCl, 1.25 NaH2PO4, 25 NaH2CO3, 12 MgSO4, 10 Glucose, 0.2 CaCl2, and 0.4 Ketamine) infused with 95% O2 / 5% CO2. Horizontal slices (targeting the VTA) were sectioned 210 μM thick and were placed in an incubation chamber containing artificial cerebral spinal fluid (ACSF; in mM: 124 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 12 glucose, 1.5 MgSO4, 2 CaCl2) infused with 95 % O2 / 5 % CO2 for at least 30 min. Brain slices were placed in a recording tissue chamber with ACSF continuously flowing at 36 °C.
GABA neurons in the VTA of brain slices were studied by visualizing GAD-67+ neurons in GAD-67 GFP mice as reported previously (Allison et al., 2011b; Steffensen et al., 2011). VTA neurons were further characterized using a GABA spike command waveform (spikes at 200 Hz for 500 msec), as GABA neurons will follow the spike command waveform with fidelity, while DA neurons will not (Steffensen et al., 2008). Neurons that did not fluoresce in GAD-67 GFP mice and/or exhibit a non-cation specific inward rectifying current (Ih) with low input resistance in C57BL/6 mice (e.g., VGAT::ChR2 mice), and did not follow the spike command waveform were assumed to be non-GABA putative DA neurons (Johnson & North, 1992; Allison et al., 2006; Margolis et al., 2006; Allison et al., 2011a; Steffensen et al., 2011). Light stimulation in VGAT::ChR2 transgenic mice was accomplished by focusing blue light from a LED illuminator (TLED; Sutter Instruments; 1–200 msec pulse widths) on the slice through a 40X objective in Nikon microscopes. Optically-evoked IPSCs (oIPSCs) were induced in putative VTA DA neurons and the stimulus intensity of the light was adjusted to obtain 50% maximum amplitude before initiating pharmacological experiments.
Cells were acutely dissociated from the VTA of GAD-67 GFP mice following procedures as previously described (Wu et al., 2004; Yang et al., 2009). Briefly, mice were anesthetized with isoflurane, and brain tissue was rapidly removed and immersed in cold (2 – 4 °C) ACSF. From each mouse, two 400 μm coronal slices containing the VTA were cut using a vibratome (Vibratome 1000 Plus, St. Louis, MO). After cutting, the slices were continuously bubbled with 95 % O2 – 5 % CO2 at room temperature (22 ± 1° C) for at least 1 hr in ACSF. Thereafter, the slices were treated with papain (6 mg/mL, Sigma-Aldrich) at 33 °C for 60 min in ACSF. After enzyme treatment, the slices were washed and transferred back to well-oxygenated ACSF. The VTA was first identified using a stereomicroscope and then micro-punched out from the slices using a well-polished needle (0.5 mm diameter). Each punched piece was then separately transferred to a 35-mm culture dish filled with well-oxygenated standard extracellular solution, which contained (in mM): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES; pH 7.4 (with Tris-base). Each punched piece was then dissociated mechanically using a fire-polished micro-Pasteur pipette visualized under an inverted microscope (Olympus IX-70, Lake Success, NY). The separated cells adhered to the bottom of the culture dish within 30 min. We recorded only VTA neurons that were GFP+.
Voltage-clamp Recordings
For whole-cell recordings in brain slices, electrodes were pulled from borosilicate glass capillary tubes (1.5 mm o.d.; A-M Systems, Sequim, WA) and filled with KCl pipette solutions (in mM: 128 KCl, 20 NaCl, 0.3 CaCl2, 1.2 MgCl2, 10 HEPES, 1 EGTA, 2 Mg-ATP, 0.25 Na-GTP and 5 QX-314; pH 7.3) for electrically-evoked IPSC (eIPSC) and oIPSC studies. Pipettes having tip resistances of 2.5 – 5 MΩ, and series resistances typically ranging from 7 – 15 MΩ were used. Voltage clamp recordings were filtered at 2 kHz with an Axon Instruments Multiclamp 700B amplifier and digitized at 5 kHz using Axon 1440A digitizers. Axon Instruments pClamp ver10, Mini Analysis (Synaptsoft: Decatur, GA), and Igor Pro (Wavemetrics: Oswego, OR) software packages were utilized for data collection and analysis. Evoked IPSCs were recorded in the presence of 50 μM APV and 30 μM CNQX or 3 mM kynurenic acid to block NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) excitatory GLU-mediated synaptic currents and isolate GABAAR-mediated synaptic currents. For perforated patch-clamp whole-cell recordings in dissociated neurons, a previously-reported method was employed (Wu et al., 2004; Yang et al., 2009b). GFP-producing cells and adherent VGAT-positive boutons (blue and yellow arrows) were mechanically dissociated from the VTA of embryonic mice (Deng et al., 2009; Xiao et al., 2009; Yang et al., 2011) expressing GAD-67 GFP. Rapid application of drugs was performed using a computer-controlled “U-tube” system that allowed for complete exchange of solution surrounding the recorded cell within 30 msec.
Single-cell Quantitative RT-PCR
To examine nAChR subunits, GFP+ neurons from the VTA, NAc and rostro-medial tegmental nucleus (RMTg) and GFP-negative putative DA neurons from the VTA were aspirated under visual observation by application of suction using a 10 mL syringe attached to the recording pipette, and were then added to a reverse transcription (RT) reaction mixture as described previously (Merrill et al., 2012). Real-Time quantitative PCR (qRTPCR) using gene specific primers with FAM-TAMRA TaqMan® probes (Applied Biosystems) for the nAChR subunits α4, β2, and α6, as well as TH and 18s rRNA was performed using the iQ Supermix (Bio-Rad) with a CFX-96 Real-Time System (Bio-Rad). Primers sequences for each target were as follows (forward primer, reverse primer and probe): α4, TGGTCCTTGTCCGCTTTG, CGTCATCATCTGGTTTTTCTCATC, CTTGTCGATTGCTCAGCTCATTGATGT; β2, GGAAGCTGTGGACGGTGTAC, CCCTCACACTCTGGTCATCATC, TCATTGCGGACCATATGCGAAGTG; α6, TCACGGTGCATTTTGAATTG, GGTCTCCATAATCTGGTTGACTTC, CAATCACGCAACTGGCCAATGTG; TH, GGACAAGCTCAGGAACTATGC, GGTGTACGGGTCAAACTTCAC, TCTCGTATCCAGCGCCCATTCTC; and 18s GTGCATGGCCGTTCTTAGTTG, GCCACTTGTCCCTCTAAGAAGTTG, TGGAGCGATTTGTCTGGTTAATTCCGATAAC. Samples were amplified in triplicate, together with a negative control for each subunit (an ACSF-only aspiration was taken from the brain slice recording chamber). The amplification protocol was 95 °C for 3 min, then 50 cycles of 95 °C for 15 sec, 57 °C for 20 sec, and 72 °C for 25 sec. Cycle threshold (Ct) values were calculated using CFX Manager software (BioRad). The 18S was a standard housekeeping control gene, which in this case ensured successful harvesting of the cell, as we used it to determine presence or absence of nAChR subtypes rather than their relative expresson. 18S from cells was also compared to ACSF controls to ensure background levels of nAChR targets were significantly greater within cells than background to avoid any false positives. Any false positives were excluded. Amplification products from each sample were verified by gel electrophoresis using 4% agarose gels.
Immunofluorescence and Alpha Conotoxin MII Binding
The immunohistochemistry and fluorescence imaging of GABAergic synaptic buotons on dissociated GABA neurons was similar to what we reported previously in DA neurons (Yang et al., 2011). Cells were probed overnight with 5 μg/ml rabbit VGAT (Millipore, Darmstadt, Germany) antibody in 1% normal goat serum in 1× PBS at 4° C. Control cells were incubated in 1% normal goat serum containing no primary antibody in 1× PBS. The next day, cells were rinsed once briefly, then twice for 5 min in wash buffer (0.15 M NaCl, 0.1 M Tris-HCl, 0.05% Tween-20, pH 7.5) followed by application of goat, anti-rabbit secondary antibody conjugated to Alexa Fluor® 405 (Thermo Fisher, Waltham, MA) diluted 1:250 in 1× PBS for 1 hour at room temperature. Cells were washed in buffer for 5 min. Biotinylated α-conotoxin (α-Ctx) MII binding protocol was adapted from Whiteaker et al. (Whiteaker et al., 2000). Cells were incubated in 1× binding buffer (144 mM NaCl, 1.5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 20 mM HEPES, 0.1% BSA and 1 mM phenylmethylsulfonyl fluoride, pH 7.5) briefly, and a second time for 5 min. Cells were then incubated in 0.5 nM biotinylated α-Ctx MII in binding buffer supplemented with 5 mM EDTA, 5 mM EGTA, and ULTRA EDTA-free protease-inhibitor cocktail (Roche, Indianapolis IN) for 2 hours. For control conditions, 50 nM non-biotinylated α-Ctx MII was included to compete for α6-containing nAChR binding sites. Cells were subsequently washed with 1 × binding buffer for 30 seconds, washed again in 1 × binding buffer for 30 seconds at 0° C and two more times in 0.1 × binding buffer briefly at 0° C. To quench endogenous peroxidases, cells were incubated in 0.3% H2O2 in PBS for 10 min, followed by one brief and one 5 min wash in 1× PBS. A solution of “ready-to-use” horseradish peroxidase conjugated to streptavidin (SA:HRP; Vector Laboratories, Burlingame, CA) was applied to cells for 30 min. Unbound SA:HRP was removed by washing once briefly and two more times for 5 min with wash buffer. Then cells were incubated in a solution of tyramide-conjugated Cy5 (PerkinElmer, Waltham, MA) diluted 1:50 in Amplification Diluent (provided by PerkinElmer) for 12 min, washed briefly and then washed two more times for 5 min with wash buffer. After a brief 1 × PBS wash, coverslips were mounted onto glass microscope slides (VWR, Randor, PA) using Prolong Diamond mounting media (Life Technologies, Grand Island, NY). At least eight cells from three different mice were visualized and images captured using a LEICA DMI SPEII Confocal System (Leica Microsystems, Buffalo Grove, IL) in three independent experiments. Alexa Fluor® 405, GFP and Cy5 fluorophores were excited with 405, 488 nm and 635 nm lasers, respectively, and images were captured using a 63X oil objective and a zoom factor of 1.5. Laser intensity, gain, pinhole size and offset remained constant for each color channel in order to confirm primary antibody labeling specificity and blockade of biotinylated α-Ctx MII binding by excess non-biotinylated α-Ctx MII.
Conditioned Place Preference
The conditioned place preference (CPP) apparatus (Med Associates, St. Albans, VT) consisted of two adjacent conditioning compartments (20 × 16 × 21 cm) separated by a manual guillotine-type door. One of the compartments was equipped with vertical striped acrylic walls and a steel mesh floor; the other was equipped with plain acrylic walls and a wire rod floor. Infrared photobeams monitored the animal’s position in the apparatus and provided a measure of motor activity. First, animals were habituated to the testing apparatus during a single 20 min session with free access to both conditioning compartments. Animals were then subjected to two 20 min pre-conditioning tests in order to determine any initial preference for one of the conditioning compartments. Each animal was then assigned ethanol (0.1 – 2.0 g/kg IP) in the non-preferred compartment, and an equivalent volume of saline in the preferred compartment. The animals underwent 20 min conditioning sessions twice-daily. Saline conditioning sessions were conducted in the morning and ethanol conditioning sessions were conducted in the afternoon. Following 4 sequential conditioning days, animals were tested for place preference the next morning by allowing free access to both conditioning compartments for 20 min.
Drug Preparation and Administration
Conotoxins were synthesized as previously described (Dowell et al., 2003; McIntosh et al., 2004). CNQX, DL-2-amino-5-phosphonopentanoic acid (APV) and CGP55845 were obtained from Abcam and kynurenic acid, atropine and eticlopride were obtained from Sigma-Aldrich and dissolved in water or ACSF. Six concentrations of ethanol were studied (1, 5, 10, 30, 50 and 100 mM). In most experiments, only one concentration of ethanol per slice was applied. In some experiments, CGP55845, atropine, and eticlopride was added to kynurenic acid/APV+CNQX to rule out GABABR, D2R, and cholinergic muscarinic effects.
Statistical Analyses
All results were presented as raw mean values and percent control ± SEM. Parametric measures within groups (e.g., drug effects) were compared using a two-tailed t test and results between groups with ANOVA with Newman-Keuls or t-test post-hoc analyses to compare pharmacological treatments. Behavioral experiments used a post-hoc Tukey’s test to compare groups. Non-parametric measures (e.g., coefficient of variance) were compared using Wilcoxon signed-rank test. Statistical significance required ≥ 95% level of confidence (P≤0.05). Analysis software included Microsoft Excel, Igor Pro (Wavemetrics, Oswego, OR) and STATA. Significance levels were indicated on graphs with asterisks *,**,*** and correspond to significance levels P<0.05, 0.01 and 0.001, respectively. Figures were constructed with Igor Pro software.
Results
α6*-nAChRs are Expressed on GABAergic Boutons on GABAergic Neurons
We have previously demonstrated that GABA terminals on DA neurons in the VTA express α6*-nAChRs (Yang et al., 2011). In order to characterize the subtypes of nAChRs that modulate GABA release onto GABAergic VTA neurons, binding experiments were performed on dissociated GFP-producing neurons from GAD-67 GFP mice using biotinylated α-Ctx MII, an antagonist selective for α6- and α3β2- containing nAChRs (Fig. 1A). Cy5 (red) fluorescence indicated binding of α-Ctx MII on VGAT-positive boutons (red and yellow arrows), implying that GABA release onto GABAergic cells may be affected by α6-containing nAChR modulation. Nineteen cells were imaged from three GAD-67 GFP mice. All nineteen GAD-67+ neurons showed biotinylated MII+ labeling on VGAT+ terminals. To determine the molecular signature of nAChRs in VTA neurons, we evaluated the expression of select nAChR subunits using single-cell qRT-PCR. VTA GABA neurons in GAD-67 GFP mice were positively identified by their expression of GFP and positive response to a GABA spike voltage command waveform [spikes at 200 Hz for 500 msec; (Steffensen et al., 2008)]. VTA GABA neurons followed the spike command waveform with fidelity while DA neurons did not. VTA DA neurons were also identified by their lack of GFP expression. We evaluated the expression of α4, β2, and α6 nAChR mRNA transcripts as well as the housekeeping gene 18S and tyrosine hydroxylase (TH) in 63 VTA neurons from 9 GAD-67 GFP mice. All GABA neurons in the VTA were TH-negative and all putative DA neurons included were TH+. α4 and β2 subunits were expressed in approximately 50–65% of VTA GABA (Fig. 1B) and DA (Fig. 1C) neurons. About two-thirds of DA neurons expressed α6*-nAChRs while only 10% of GABA neurons expressed α6*-nAChRs (Fig. 1D; expressed at % of total cells of each type). In order to determine other GABA neurons that might be providing GABA input to VTA GABA neurons (Fig. 1A) we evaluated the expression of α6*-nAChRs in NAc and RMTg GABA neurons in GAD GFP mice because of their well-known GABA projections to the VTA. Only 7% of NAc neurons and 10 % of RMTg neurons expressed α6*-nAChR transcripts (data not shown). Thus, at least a subpopulation of GABA cells, and most DA neurons in the VTA, express α6*-nAChRs, and either local circuit GABA neurons in the VTA, or projections neurons from the NAc or RMTg, could provide the α6*-nAChRs at GABA terminals on VTA GABA neurons (Fig. 1A).
Figure 1.
GAD-67 and α-conotoxin (Ctx) MII-labeled α6*-nAChRs are co-expressed on presynaptic terminals attached to GAD-67-producing cells in the mouse VTA. (A) The four confocal images show two representative dissociated VTA GABA neurons from a GAD-67 GFP mouse evincing diffuse GFP labeling (green), as well as dense GFP labeling at putative boutons. Biotin α-Ctx MII labeling (red; Cy5) in the same neurons shows α6*nAChRs co-expressed on presynaptic terminals attached to GAD-67-producing cells. VGAT labeling (blue; Alexa Fluor® 405) on the same neurons shows punctate boutons on VTA GABA neurons. The merge shows α6*-nAChRs on GABA boutons attached to VTA GABA neurons. Scale bar represents 10 μm. (B) Single-cell, quantitative real-time PCR (qRT-PCR) was used to examine nAChR subunit expression in VTA GABA neurons (GFP+, TH−). Relative fluorescence acquired using a FAM-TAMRA hydrolysis probe is a measure of cDNA quantity for each nAChR target relative to PCR cycle number. 18S is used as a control housekeeping gene. Inset: This agarose gel illustrates the expected cDNA amplicon size for α6 (71 base pairs) taken from the PCR reaction, compared to the 50 base pair ladder (left lane). This representative VTA GABA neuron from a GAD-67 GFP mouse expressed α6, α4, and β2 nAChR subunit mRNA. (C) This representative VTA DA neuron from a GAD-67 GFP mouse expressed α6 and β2, but not α4 subunit mRNA. It is important to point out that as single cell PCR has some level of false negatives, actual expression levels are likely higher than reported. Therefore, ~45% expression in GABA cells and ~55–65% in DA cells suggests that α4, and β2 are likely present in the vast majority of DA cells and most of the GABA cells, even though it may not be detected in every neuron. (D) Summary of nAChR mRNA expression in VTA GABA and DA neurons evaluated for single-cell qRT-PCR. α6*-nAChR subunit mRNA was expressed in ~10% of GABA neurons and 45% of DA neurons, while α4 and β2 mRNA were expressed in ~50% of VTA neurons. These data support the expression of α6*-nAChRs in a subpopulation of midbrain GABA neurons.
Electrophysiological studies on the Role of α6*-nAChRs in Acute Ethanol Effects on GABA Neurons in the VTA
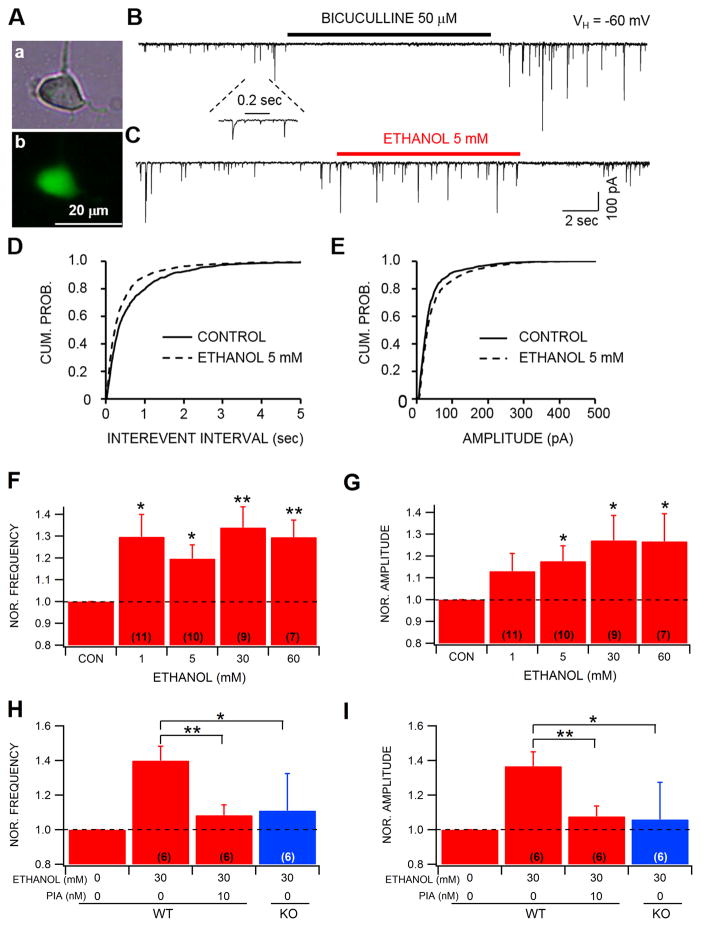
We evaluated the effects of α6*-nAChR antagonist α-conotoxins on ethanol effects on GABAAR-mediated inhibitory synaptic responses in dissociated GAD-67+ neurons of the VTA (Fig. 2A). In order to isolate GABAAR-mediated response and nAChR involvement, the GABABR antagonist CGP55845 (10 μM) and the muscarinic ACh antagonist atropine (10 μM) were included in the superfusate. We also superfused the GABAAR antagonist bicuculline (10 μM) at the end of most experiments to confirm that the mIPSCs were mediated by GABA (Fig. 2B). Similar to what has been reported previously in dissociated DA neurons (Yang et al., 2011), dissociated VTA GABA neurons from GAD-67 GFP mice evinced mIPSCs, a result of functional spontaneous input from synaptic boutons preserved in the mechanical dissociation. Ethanol significantly enhanced the frequency of GABA mIPSCs at all ethanol concentration levels (Fig. 2C,D,F; 1 mM: t(1,10)=2.74, p=0.02; 5 mM: t(1,9)=2.9, p=0.02; 30 mM: t(1,7)=3.6, p=0.008; and 60 mM: t(1,6)=3.8, p=0.008) and amplitude at the 5, 30 and 60 mM ethanol levels (Fig. 2C,E,G; 5 mM: t(1,9)=2.5, p=0.03; 30 mM: t(1,7)=2.6, p=0.04; and 60 mM: t(1,6)=2.9, p=0.03) compared to baseline. In a separate experiment, there was an overall involvement of α6*-nAChRs in ethanol effects on mIPSC frequency (F(1,30)=4.2, p=0.01) and amplitude (F(1,30)=3.1, p=0.03) in VTA GABA neurons at the 30 mM ethanol dose level (Fig. 2H,I). Post hoc tests revealed that there was a significant difference between ethanol alone and ethanol in the presence of the α-Ctx PIA for both mIPSC frequency (q=5.48; Fig. 2H) and amplitude (q=4.3; Fig. 2I), as well as between ethanol in WT mice vs ethanol in α6 KO mice for both mIPSC frequency (q=3.5; Fig. 2H) and amplitude (q=3.4; Fig. 2I).
Figure 2.
Ethanol enhances mIPSC frequency and amplitude in VTA GABA neurons. (A) Images show an acutely-dissociated VTA neuron from a GAD-67 GFP mouse using phase-contrast (Aa) and GPF-filtered (Ab) microscopic modes of illumination. (B) A typical trace shows spontaneous mIPSCs (in the presence of 0.5 μM TTX). They were sensitive to a selective GABA(A)R blocker bicuculline indicating that they were mediated by GABA. (C) Bath exposure of 5 mM ethanol increased the frequency of GABA mIPSCs. Further analysis revealed that 5 mM ethanol increases the frequency (D) and the amplitude (E) of spontaneous mIPSCs. (F,G) Ethanol enhanced GABA mIPSC frequency to VTA GABA neurons at all concentrations tested and amplitude at 5, 30 and 60 mM ethanol. Pre-treatment with the α-Ctx P1A (10 nM) prevented the ethanol (30 mM)-induced increase in mIPSC frequency (H) and amplitude (I) in WT mice. Similarly, 30 mM ethanol did not affect mIPSC frequency and amplitude in α6 KO mice. Vertical bars represent means ± SEM. Asterisks *,** indicate significance levels P<0.05 and 0.01, respectively. Values in parentheses represent n values.
In order to further evaluate ethanol’s effects on GABAAR-mediated synaptic responses and the role of α6*-nAChRs, we performed electrically-evoked eIPSC studies in brain slices. At the 50% maximum stimulus level eIPSCs were 357.9 ± 47.2 pA (n=38). Ethanol (1–100 mM) had concentration-dependent effects on eIPSCs (Fig. 3). Low-concentrations of ethanol (1 and 5 mM) consistently enhanced VTA GABA neuron eIPSC amplitudes (Fig. 3A,C; 1 mM: t(1,10)=6.65, p=0.02, n=7; 5 mM: F(1,31)=17.2, p=0.0002, n=16), while higher concentrations (50 and 100 mM) significantly decreased eIPSC amplitudes (Fig. 3C; 50 mM: F(1,11)=7.95, p=0.02, n=6; and 100 mM: F(1,11)=8.04, p=0.02, n=6). Superfusion of 100 nM of the α-Ctx MII slightly (15.8 ± 4.9 %), but not significantly (F(1,25)=8.95, p=0.32, n=13), decreased VTA GABA neuron eIPSC amplitudes, but blocked eIPSC enhancement by ethanol at lower concentrations (Fig. 3B,C), as well as eIPSC reduction by ethanol at higher concentrations (Fig. 3C; 1 mM: F(1,11)=0.16, p=0.691, n=6; 5 mM; F(1,25)=0.04, p=0.84, n=13; and 100 mM; F(1,9)=0.90, p=0.379, n=5). Evoked IPSCs in VTA GABA neurons were not consistently modulated by conditioned stimulation at any interstimulus interval (Fig. 3A,B; mean paired pulse ratio = 0.99 ± 0.04 at 50 msec). Ethanol did not significantly alter eIPSC paired-pulse ratio at any concentration (e.g., 5 mM: F(1,31)=1.06, p=0.32, n=16). To further evaluate the locus of ethanol and MII effects on eIPSCs, a coefficient of variance (CV) analysis was performed on eIPSCs in VTA GABA neurons at the 5 mM ethanol level, the most significant level for ethanol effects (with block by MII). There was no significant effect of ethanol on eIPSC CV (Baseline = 0.34 ±0.1 vs Ethanol = 0.36 ± 0.11; z = 0.46, p = 0.65; data not shown) or ethanol vs MII+ethanol (z = −0.86, p = 0.39). However, there was a significant increase in CV between baseline and MII (z = −2.667, p = 0.008), providing additional support to the mIPSC data for a presynaptic locus for MII actions at α6*-nAChRs.
Figure 3.
Effects of ethanol and α-Ctxs on evoked IPSCs (eIPSCs) in VTA GABA Neurons. Evoked IPSCs were recorded in VTA GABA neurons in the horizontal slice of GAD-67 GFP mice in the presence of APV/CNQX (or kynurenic acid) to block GLUR-mediated synaptic transmission, atropine to block muscarinic cholinergic effects and CGP55845 to block GABAB receptors. (A) Only one neuron/slice was tested for each of the concentrations of ethanol. (A) Inset shows representative superimposed recordings of IPSCs evoked in VTA GABA neurons at a paired-pulse interval of 50 msec before and after superfusion of 5 mM ethanol. Ethanol enhanced eIPSC amplitudes. The graph plots the time course of eIPSC amplitudes (integrated over 1 min intervals) as well as the paired-pulse ratio (PPR) for all cells studied with ethanol at 5 mM. Note the enhancement of eIPSC amplitude at this low concentration. There was no obvious effect of ethanol on PPR. (B) Insets show representative superimposed recordings of eIPSCs during superfusion ofthe α-Ctx MII and after ethanol + MII. In the presence of MII, ethanol had little effect on eIPSC amplitudes in this representative VTA GABA neuron. (C) Summary of the effects of ethanol (1–100 mM) on eIPSCs with block at lower and higher concentrations by MII. Vertical bars represent means ± SEM. Asterisks **,*** indicate significance levels P<0.01 and 0.001, respectively.
We have previously demonstrated in multiple reports that ethanol inhibits the firing rate of VTA GABA neurons in rats (IC50 = 1.0 g/kg) and mice (IC50 = 0.25 g/kg) at physiologically-relevant (e.g., intoxicating and rewarding) levels in vivo (Diana et al., 2003; Stobbs et al., 2004; Ludlow et al., 2009; Steffensen et al., 2009; Steffensen et al., 2011). We evaluated the role of α6*-nAChRs in ethanol inhibition of VTA GABA neuron firing rate. In WT mice, intraperitoneal (IP) administration of 1.5 g/kg ethanol inhibited the firing rate of VTA GABA neurons 81.1 ± 5.0 % within 10 – 30 min (Fig. 4A,D), as we have previously reported in mice (Steffensen et al., 2011). A dose of 1.5 g/kg ethanol was chosen based on IC50 dose-response studies previously obtained in WT mice (Steffensen et al., 2011). Alpha-6 KO mice exhibited significantly slower baseline firing rates (8.9 ± 1.4 Hz vs 31.2 ± 6.3 Hz; t(1,11)=3.7, p=0.004; n=10 each) than WT mice (Fig. 4C) and with less sensitivity to ethanol (Fig. 4B,D). There was a significant difference in ethanol effects between α6 KO mice vs WT controls (27.7 ± 43.5 % increase vs 81.1 ± 5.0 % decrease; F(1,21)=6.2, p=0.02; n=10 each; Fig. 4D).
Figure 4.
A role for α6*-nAChRs in ethanol effects on VTA GABA neuron firing rate in vivo and in the slice preparation ex vivo. For in vivo studies, VTA GABA neurons were identified by stereotaxic coordinates and by spontaneous electrophysiological criteria under isoflurane anesthesia (Steffensen et al., 1998). These included in mice: Relatively fast firing rate (>10Hz); ON-OFF phasic non-bursting activity under isoflurane anesthesia; spike duration less than 200 μsec; and activation by generalized sensory stimulation (Ludlow et al., 2009; Steffensen et al., 2011). In some experiments, multiple spike discharges were evoked in putative GABA neurons by electrical stimulation of the internal capsule, as previously reported (Steffensen et al., 1998). (A) This ratemeter record shows that intraperitoneal administration of 1.5 g/kg ethanol inhibited the firing rate of this representative VTA GABA neuron in an isoflurane-anesthetized WT mouse. The baseline firing rate of this neuron was approximately 5 Hz. (B) This ratemeter record shows that intraperitoneal administration of 1.5 g/kg ethanol increased the firing rate of this representative VTA GABA neuron recorded in a α6 KO mouse. The baseline firing rate for this neuron was 4 Hz. This example was chosen to match the firing rate of the example in (A). (C) However, on average, VTA GABA neurons were characterized by significantly faster firing rates than those recorded in α6 KO mice. Horizontal bar denotes mean. (D) Knock-out mice were resistant to the typical inhibition of firing rate in WTs by this dose of ethanol. In fact, they were slightly excited on average. Vertical bars represent means ± SEM. Asterisk * indicates significance level P<0.05. Values in parentheses represent n values.
Electrophysiological studies on the Role of α6*-nAChRs in Acute Ethanol Effects on Dopamine Neurons in the VTA
Using VGAT::ChR2 transgenic mice on a C57BL/6 background we were able to selectively activate GABA IPSCs in VTA DA neurons without using pharmacological blockers (Fig. 5). In cell attached, voltage-clamp mode VTA GABA neuron spikes in VGAT::ChR2 mice were activated by optical stimulation (200 msec duration; Fig. 5A) while VTA DA neuron spikes were inhibited (Fig. 5B). In whole cell, voltage-clamp mode VTA GABA neurons in VGAT::ChR2 mice were characterized by large, fast depolarizing currents with optical stimulation, while DA neurons were characterized by oIPSCs (Fig. 5C). The threshold for evoking oIPSCs in VTA DA neurons was 1.0 msec duration at maximum stimulation intensity, while 5.0 msec duration evoked oIPSCs with a good dynamic light intensity range focused through the 40X objective. The mean amplitude (50% maximum stimulus level) of oIPSCs in DA neurons in VGAT::ChR2 mice was 611.3 ± 76.0 pA (n=38). Light stimulation was without effect in VGAT::ChR2-negative WT littermates from heterozygous crosses, even across stimulation durations 0.5–500 msec (n=8; data not shown), indicating that blue light had no effect on DA neurons. Optically-evoked IPSCs in DA neurons were markedly reduced, if not abolished, in most DA neurons by the GABAAR antagonist bicuculline (50 μM; Fig. 5D; t(1,17)=147, p=7.5E–28). However, there was a residual oIPSC in some DA neurons. As DA neurons are known to exhibit D2R-mediated IPSCs, and DA is now known to be co-released with GABA in the NAc (Tritsch et al., 2014; Berrios et al., 2016; Tritsch et al., 2016), we evaluated the effects of the D2R antagonist eticlopride (0.01 – 0.1 μM) on oIPSCs and found that they were significantly reduced in DA neurons at the 0.1 μM level (Fig. 5D; t(1,11)=4.2, p=0.01). Thus, all experiments with ethanol on oIPSCs in DA neurons were performed in its presence, and combined eticlopride and bicuculline superfusion always abolished oIPSCs. Ethanol (10 – 100 mM) had no overall effect on DA neuron GABAAR-mediated oIPSC amplitudes (F(3,62)=0.485, p=0.69; Fig. 5E) or area under the curve (AUC; F(3,29)=0.56, p=0.58; Fig. 5E) or significant effects at any ethanol concentration.
Figure 5.
Lack of effects of ethanol on GABAAR-mediated inhibition to DA neurons in the slice preparation of VGAT ChR2 transgenic mice. VTA GABA and DA neuron spikes were recorded in cell-attached, voltage clamp mode. Dopamine neurons were characterized by the expression of TH transcripts and response to the GABA spike command waveform (in cells where whole cell recordings were made). In VGAT::ChR2 mice, blue light stimulation was used to evaluate ethanol effects on optically-evoked IPSCs (oIPSCs). (A) Inset shows a representative recording of a VTA GABA neuron that is activated by a 200 msec light pulse (denoted by blue bar), evincing multiple spikes during the stimulation. The peri-stimulus interval spike histogram shows this spike’s cumulated activity over 12 stimulation epochs, demonstrating the marked activation and subsequent inhibition of spontaneous GABA neuron spikes after the optical stimulation. (B) Inset shows a representative recording of a VTA DA neuron whose spontaneous activity is inhibited by a 200 msec light pulse, evincing inhibition of DA neuron spontaneous spiking. The peri-stimulus interval spike histogram shows this spike’s cumulated activity over 12 stimulation epochs, demonstrating the marked inhibition of spontaneous DA spikes. (C) In whole cell mode, optical stimulation (5.0 msec) evoked fast, large inward sodium currents in GABA neurons and relatively slow, small Cl− currents in DA neurons. (D) oIPSCs were markedly reduced by bicuculline and moderately reduced by eticlopride. (E) In the presence of eticlopride, ethanol (10 – 100 mM) had no effect on oIPSC amplitudes or area under the curve in DA neurons. Vertical bars represent means ± SEM. Asterisks **,*** indicate significance levels P<0.01 and P<0.001, respectively. Values in parentheses represent n values.
In order to further link the changes we found in VTA GABA neurons to DA neurons, we evaluated the effects of ethanol on VTA DA neuron activity in the slice preparation, and examined the role of α6*-nAChRs. We performed cell-attached, voltage-clamp firing rate studies on VTA DA neurons in the slice preparation from GAD-67 GFP mice in the presence of eticlopride for reasons described above. Only high concentrations (50 mM: F(1,15)=4.91, p=0.04, n=11; and 100 mM: F(1,7)=9.98, p=0.02, n=11) increased DA neuron firing rate (Fig. 6A,C), as reported by others (Brodie et al., 1990; Brodie et al., 1999; Xiao et al., 2007). Superfusion of α-Ctx MII did not prevent the increase in firing rate by ethanol (50 mM: F(1,7)=6.39, p=0.04, n=5; and 100 mM: F(1,8)=5.84, p=0.04, n=5; Fig. 6B,C).
Figure 6.
Lack of a role for α6*-nAChRs in ethanol enhancement of VTA DA neuron firing rate in the slice preparation. Using cell-attached mode, spontaneous spike activity was measured in horizontal slices of GAD-67 GFP mice in the presence of eticlopride. Only one neuron/slice was tested for each of the concentrations of ethanol unless indicated. Insets a,b are representative 5 sec recordings of separate VTA DA neuron spikes recorded in GAD-67 GFP mice at the times indicated on each of the ratemeter graphs. (A) Superfusion of 100 mM ethanol enhanced the firing rate of this representative VTA DA neuron recorded in a GAD-67 GFP mouse. (B) In a neuron from a separate slice, superfusion of 100 nM α-Ctx MII had no effect on 100 mM ethanol enhancement of the firing rate of this representative DA neuron. (C) Summary of the effects of MII on ethanol effects across ethanol concentrations 5–100 mM. MII had no significant effects on ethanol enhancement of VTA DA neuron firing rate.
Behavioral Studies on the role of α6*-nAChRs in Acute Ethanol Effects: Conditioned Place Preference and Locomotor Activity
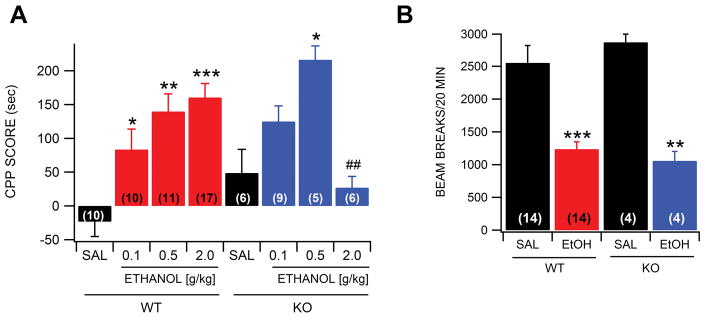
We evaluated WT and α6 KO mice in the CPP procedure to determine the role of α6*-nAChR in ethanol reward. We determined the difference in time spent on the ethanol paired side in the CPP procedure before and after conditioning. We first compared difference scores between saline vs ethanol in WT mice at three separate IP doses: 0.1, 0.5 and 2.0 g/kg (Fig. 7A). A significant ethanol CPP was found in WT mice at 0.1 g/kg (F(1,19)=6.6, p=0.02; n=10), 0.5 g/kg (F(1,20)=18.3, p=0.0004; n=11) and 2.0 g/kg dose levels (F(1,26)=28.8, p=1.46E-05; n=17) compared to saline, as we have previously reported (McGeehan & Olive, 2003a; b). ANOVA analysis with Tukey’s post-hoc test revealed that WT mice tested at the 2.0 g/kg ethanol dose evinced a higher preference for the ethanol-paired compartment than α6 KO mice at this dose level (F(1,22)=11.7, p=0.003; n=6), but not at the 0.1 or 0.5 g/kg dose levels. ANOVA analysis with Tukey’s post-hoc test also revealed a significant difference in locomotor activity for WT vs KO mice during the 20 min conditioning sessions at the 2 g/kg dose level (F(3,30)=13.41, p<0.0001). Locomotor activity was significantly reduced by ethanol during the 20 min conditioning sessions at the 2 g/kg ethanol dose level in both WTs (p<0.001; Fig. 7B) and KOs (p<0.01; Fig. 7B) compared to saline conditioning. However, there was no significant difference in locomotor activity for WT vs KO mice during conditioning sessions with saline or ethanol (p>0.05).
Figure 7.
α6*-nAChRs mediate ethanol reward. (A) Results show WT mice have a preference for the ethanol paired chamber at 0.1, 0.5 and 2.0 g/kg ethanol, but was significantly reduced in the α6 KO mice at the 2.0 g/kg level. (B) Locomotor activity in WT and KO mice was inhibited similarly by ethanol during the 20 min conditioning sessions compared to saline at the 2.0 g/kg dose level. Asterisks *,**,*** indicate significance levels P<0.05, P<0.01 and P<0.001, respectively compared to saline and ## indicate significance level P<0.01 between WT and KO mice at the 2.0 g/kg level. Values in parentheses represent n values.
Discussion
While α6*-nAChRs have relatively limited expression in the brain, they are markedly expressed in the VTA. In fact, they are expressed 16X more than any other nAChR subunit in the VTA (Yang et al., 2009a). One purpose of our study was to evaluate the role of α6*-nAChRs in ethanol modulation of VTA neuron excitability and reward. Our study differed significantly from previous studies. Mainly, the primary focus of this study was VTA GABA neurons, which are believed to be local circuit regulators of DA neuron excitability, as well as DA release at terminals in the NAc (van Zessen et al., 2012). Thus, modulation of GABA inhibition of GABA neurons by α6*-nAChRs may be an important regulator of GABA neuron excitability, which ultimately will govern DA neurotransmission.
Our immunohistochemical and mRNA expression experiments demonstrate that α6*-nAChRs are expressed in a subpopulation of GABA neurons from regions known to innervate both VTA GABA and DA neurons (i.e., NAc and RMTg) including VTA GABA neurons themselves, and are expressed in most DA neurons and on GABA terminals to VTA GABA neurons, as well as DA neurons, as we have reported previously (Yang et al., 2011). Regarding nAChR subunit expression, while it has been reported that α4 and β2 are widely expressed in VTA DA cells and among some populations of VTA GABA cells (Charpantier et al., 1998; Klink et al., 2001; Azam et al., 2002), data regarding α6*-nAChRs is less clear. Two reports suggest α6*-nAChRs are absent in VTA GABA neurons (Drenan et al., 2008; Mackey et al., 2012). However in one of these studies, immunohistochemistry was not quantitatively analyzed nor illustrated at the cell level, though occasional TH-negative/α6*-nAChRs appear to be present in a low magnification image (Drenan et al., 2008; Mackey et al., 2012). Importantly, Drenan et al. noted α6-induced electrophysiological responses in 1 of 5 VTA GABA cells recorded, suggesting α6*-nAChRs are only expressed in a subset of VTA GABA cells, a similar proportion noted in this study supporting our findings.
Activation of α6*-nAChRs on GABA terminals enhances release of GABA, similar to how activation of α7*-nAChRs enhances the release of GLU (Taylor et al., 2013). Given our histochemical and molecular findings that α6*-nAChRs were localized to GABA terminals on VTA GABA neurons we evaluated the involvement of α6*-nAChRs in mediating ethanol effects on GABAergic synaptic transmission to dissociated VTA neurons and VTA neurons in the slice preparation. Spontaneous mIPSC frequency and amplitude in dissociated VTA GABA neurons were enhanced by extraordinarily low concentrations of ethanol (1 – 10 mM). The increase in mIPSC frequency by ethanol was blocked by α6*-nAChR antagonists in WT mice, and was absent in α6 KO mice. An enhancement in mIPSC amplitude indicated that ethanol also had a postsynaptic effect on VTA GABA neurons. Future studies will address the postsynaptic effect. Regardless, ethanol appears to be enhancing inhibition to VTA GABA neurons by enhancing presynaptic GABA release to VTA GABA neurons via α6*-nAChRs. The enhancement in GABAAR-mediated inhibition by ethanol is sufficient to reduce VTA GABA neuron firing rate.
While studies in dissociated neurons support a link between α6*-nAChRs and ethanol in the VTA, in order to strengthen the physiological relevancy we studied the effects of ethanol on GABAAR-mediated synaptic transmission in VTA neurons in the slice preparation. Consistent with studies in dissociated neurons, we found that low concentrations of ethanol (1 and 5 mM) enhanced, and high concentrations (50 and 100 mM) decreased, eIPSC amplitudes recorded in VTA GABA neurons. Both enhancement and reduction of eIPSCs was blocked by α-Ctxs, providing further support that presynaptic α6*-nAChRs on GABA terminals modulate GABA release, which is sensitive to ethanol. A molecular target for direct ethanol actions on the α6*-nAChR has not been elucidated. However, using site-directed mutagenesis and recombinant expression systems we are currently evaluating this possibility. Ethanol enhancement of eIPSCs at low dose levels is consistent with our previous reports that ethanol reduces the firing rate of VTA GABA neurons. In order to increase our understanding of the role α6*-nAChRs might play in acute ethanol effects on the mesolimbic DA reward system, we evaluated its effects on the firing rate of VTA GABA neurons in vivo and ex vivo. Interestingly, the baseline firing rate of VTA GABA neurons in α6 KO mice was only one-third as fast as those in WT mice, suggesting that α6*-nAChRs influence excitatory tone to VTA GABA neurons. α6 KO mice were relatively insensitive to ethanol inhibition of their firing rate, suggesting the involvement of α6*-nAChRs.
In order to link the changes we found in VTA GABA neurons to DA neurons, we evaluated the effects of ethanol on VTA DA neuron GABAAR-mediated inhibitory synaptic responses and firing rate in the slice preparation. Ethanol had no effect on GABAAR-mediated IPSC amplitudes or area under the curve recorded in DA neurons that were selectively activated by optical stimulation in VGAT::ChR2 mice, providing further support to our hypothesis that VTA GABA neurons are the most sensitive substrate for ethanol, especially at low concentrations. Others have shown that ethanol enhances VTA DA neuron firing rate at high concentrations in the slice preparation (Brodie et al., 1990; Brodie et al., 1999; Xiao et al., 2007), which we confirmed. However, ethanol enhancement of DA neuron activity was unaffected by MII Ctxs, suggesting the lack of involvement of α6*-nAChRs in high-dose ethanol effects on DA neurons. These findings in VTA neurons provide compelling evidence that enhancement in GABA release by low-dose ethanol reduces VTA GABA neuron firing rate, and that ethanol enhancement of inhibition is mediated presynaptically via α6*-nAChRs on GABA terminals to VTA GABA neurons, and not DA neurons. It has been demonstrated by us (Allison et al., 2011b) and others (Tan et al., 2010) that the local circuit inhibitory drive to GABA neurons exceeds that of DA neurons. Ethanol inhibition of VTA GABA neurons appears to be mediated through local circuit GABA neurons, perhaps due to the stronger inhibitory synaptic drive from other GABA neurons in the VTA. Although we have shown previously that α6*-nAChRs are located on GABA terminals to putative VTA DA neurons (Yang et al., 2011), we speculate that only α6*-nAChRs on GABA terminals to VTA GABA neurons are sensitive to ethanol, resulting in enhanced GABAergic tone to VTA GABA neurons, which explains why VTA GABA neuron firing rate is lowered in vivo and ex vivo by low-dose ethanol. Future studies will address differences in α6*-nAChRs on VTA GABA vs DA neurons and their inhibitory inputs that express α6*-nAChRs. Regardless, lowering of VTA GABA neuron activity would ultimately disinhibit DA neuron activity and/or release at terminals, which is consistent with the dogma that ethanol’s rewarding effects are mediated through enhancement of DA transmission. However, ethanol was without affect on GABAAR-mediated inhibition of VTA DA neurons and firing rate at the low doses that enhanced GABAAR-mediated inhibition to VTA GABA neurons and lowered their firing rate. We can only speculate that other GABA inputs from the RMTg, NAc or ventral pallidum are implicated, especially in the intact system where there afferent fibers are not severed.
Several studies have addressed the role of α6*-nAChRs in ethanol consumption and reward. Kamens et al. reported that α6 KO mice did not differ from WTs in two bottle choice ethanol consumption (Kamens et al., 2012). Powers et al. have assessed the role of α6*-nAChRs in modulating alcohol reward using transgenic mice expressing mutant, hypersensitive α6*-nAChR subunits (Powers et al., 2013). Transgenic α6 mice showed significantly higher alcohol intake at low concentrations of alcohol (3% and 6%) in the two bottle choice procedure, drank more in the drink-in-the-dark procedure, and exhibited preference to low doses of alcohol (0.5 g/kg, data not shown) in the CPP paradigm. Our behavioral CPP studies showed that WT and KO mice did not differ in preference at lower doses of ethanol (0.1 and 0.5 g/kg), but differed significantly at the 2.0 g/kg level, suggesting that α6*-nAChRs mediate ethanol reward in mice, at least at moderately intoxicating levels of ethanol, wherein similar ethanol-induced locomotor effects were found. This is vital, as it shows that α6*-nAChRs are involved in the process of dependence and its accompanying behaviors, rather than the many physiological influences, such as ataxia, that ethanol can have on the brain other than addiction. Contrary to our findings, Guildford et al. (Guildford et al., 2016) recently reported that α6 KO mice demonstrate an ethanol CPP (2 g/kg IP) that is similar in magnitude to that of WTs. However, at a conditioning dose of 3 g/kg, CPP was absent in α6 KO mice, as was observed with the 2 g/kg dose in our study. While both the present study and that by Guildford and colleagues used KO mice on a C57BL/6J background in a biased CPP design, there are several procedural differences that may account for the differences. For example, in the present study, we utilized a two-chamber apparatus and conditioning sessions that were 20 min duration, while Guildford et al. utilized a three-chambered compartment with a neutral central compartment, and conditioning session durations were 5 min. It is possible that the longer conditioning session duration used in the present study allowed for increased associations between the effects of ethanol and the environmental context, and increased doses of ethanol (e.g., 3 g/kg) were necessary to produce genotypic differences in the shorter conditioning sessions in the Guildford et al study. We show clear ambulatory reductions by ethanol during the 20 min conditioning sessions that were were not experienced by the animals in the 5 min conditioning sessions in the Guildford et al study. Thus, there were profound contextual differences in the two studies.
In conclusion, α6*-nAChRs appear to be sensitive substrates for low-dose ethanol effects on VTA GABA neurons and reward. Owing to their sensitivity to ethanol, electrical network connectivity, widespread projection to the limbic system, and wide bandwidth, VTA GABA neurons are in a critical position to modulate DA psychomotor output as integrators of convergent information from sensory, cortical and limbic areas subserving reinforcing behaviors. Studies are ongoing in recombinant nAChR expression systems to address if α6*-nAChRs are a direct molecular target for ethanol.
Acknowledgments
This work was supported by USPHS NIH grants AA020919 and DA035958 to SCS, AA013852 to MFO, NS078645 to JGE, and GM103801 and GM48677 to JMM. The authors wish to thank Dr. C. Fernando Valenzuela for reading and commenting on the manuscript. The authors wish to extend our condolences to the family of Samuel I. Shin (co-author), who performed many of the experiments in this study in connection with his thesis project, but died tragically in a climbing accident during the preparation of this manuscript. All authors have no financial conflicts to disclose.
References
- Allison DW, Ohran AJ, Stobbs SH, Mameli M, Valenzuela CF, Sudweeks SN, Ray AP, Henriksen SJ, Steffensen SC. Connexin-36 gap junctions mediate electrical coupling between ventral tegmental area GABA neurons. Synapse. 2006;60:20–31. doi: 10.1002/syn.20272. [DOI] [PubMed] [Google Scholar]
- Allison DW, Wilcox RS, Ellefsen KL, Askew CE, Hansen DM, Wilcox JD, Sandoval SS, Eggett DL, Yanagawa Y, Steffensen SC. Mefloquine effects on ventral tegmental area dopamine and GABA neuron inhibition: a physiologic role for connexin-36 GAP junctions. Synapse. 2011a;65:804–813. doi: 10.1002/syn.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DW, Wilcox RS, Ellefsen KL, Askew CE, Hansen DM, Wilcox JD, Sandoval SS, Eggett DL, Yanagawa Y, Steffensen SC. Mefloquine effects on ventral tegmental area dopamine and GABA neuron inhibition: A physiologic role for connexin-36 gap junctions. Synapse. 2011b doi: 10.1002/syn.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Berrios J, Stamatakis AM, Kantak PA, McElligott ZA, Judson MC, Aita M, Rougie M, Stuber GD, Philpot BD. Loss of UBE3A from TH-expressing neurons suppresses GABA co-release and enhances VTA-NAc optical self-stimulation. Nature communications. 2016;7:10702. doi: 10.1038/ncomms10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2010;35:665–673. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpantier E, Barneoud P, Moser P, Besnard F, Sgard F. Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport. 1998;9:3097–3101. doi: 10.1097/00001756-199809140-00033. [DOI] [PubMed] [Google Scholar]
- Clark A, Little HJ. Interactions between low concentrations of ethanol and nicotine on firing rate of ventral tegmental dopamine neurones. Drug and alcohol dependence. 2004;75:199–206. doi: 10.1016/j.drugalcdep.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J. Response of nicotine self-administration in the rat to manipulations of mu-opioid and gamma-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacology (Berl) 2000;149:107–114. doi: 10.1007/s002139900355. [DOI] [PubMed] [Google Scholar]
- David V, Besson M, Changeux JP, Granon S, Cazala P. Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: dependence on cholinergic nicotinic and dopaminergic D1 receptors. Neuropharmacology. 2006;50:1030–1040. doi: 10.1016/j.neuropharm.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- de Rover M, Lodder JC, Kits KS, Schoffelmeer AN, Brussaard AB. Cholinergic modulation of nucleus accumbens medium spiny neurons. Eur J Neurosci. 2002;16:2279–2290. doi: 10.1046/j.1460-9568.2002.02289.x. [DOI] [PubMed] [Google Scholar]
- Deng C, Li KY, Zhou C, Ye JH. Ethanol enhances glutamate transmission by retrograde dopamine signaling in a postsynaptic neuron/synaptic bouton preparation from the ventral tegmental area. Neuropsychopharmacology. 2009;34:1233–1244. doi: 10.1038/npp.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Brodie M, Muntoni A, Puddu MC, Pillolla G, Steffensen S, Spiga S, Little HJ. Enduring effects of chronic ethanol in the CNS: basis for alcoholism. Alcohol Clin Exp Res. 2003;27:354–361. doi: 10.1097/01.ALC.0000057121.36127.19. [DOI] [PubMed] [Google Scholar]
- Dowell C, Olivera BM, Garrett JE, Staheli ST, Watkins M, Kuryatov A, Yoshikami D, Lindstrom JM, McIntosh JM. Alpha-conotoxin PIA is selective for alpha6 subunit-containing nicotinic acetylcholine receptors. J Neurosci. 2003;23:8445–8452. doi: 10.1523/JNEUROSCI.23-24-08445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Steele AD, McKinney S, Patzlaff NE, McIntosh JM, Marks MJ, Miwa JM, Lester HA. Cholinergic modulation of locomotion and striatal dopamine release is mediated by alpha6alpha4* nicotinic acetylcholine receptors. J Neurosci. 2010;30:9877–9889. doi: 10.1523/JNEUROSCI.2056-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, Bupp S, Heintz N, McIntosh JM, Bencherif M, Marks MJ, Lester HA. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha 6 nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Cholinergic inhibition of ventral midbrain dopamine neurons. J Neurosci. 2000;20:7855–7860. doi: 10.1523/JNEUROSCI.20-20-07855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon M, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. Cholinergic axon terminals in the ventral tegmental area target a subpopulation of neurons expressing low levels of the dopamine transporter. J Comp Neurol. 1999;410:197–210. doi: 10.1002/(sici)1096-9861(19990726)410:2<197::aid-cne3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring (P) rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guildford MJ, Sacino AV, Tapper AR. Modulation of ethanol reward sensitivity by nicotinic acetylcholine receptors containing the alpha6 subunit. Alcohol. 2016;57:65–70. doi: 10.1016/j.alcohol.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther. 2009;331:547–554. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Hoft NR, Cox RJ, Miyamoto JH, Ehringer MA. The alpha6 nicotinic acetylcholine receptor subunit influences ethanol-induced sedation. Alcohol. 2012;46:463–471. doi: 10.1016/j.alcohol.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow KH, Bradley KD, Allison DW, Taylor SR, Yorgason JT, Hansen DM, Walton CH, Sudweeks SN, Steffensen SC. Acute and chronic ethanol modulate dopamine D2-subtype receptor responses in ventral tegmental area GABA neurons. Alcohol Clin Exp Res. 2009;33:804–811. doi: 10.1111/j.1530-0277.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey ED, Engle SE, Kim MR, O’Neill HC, Wageman CR, Patzlaff NE, Wang Y, Grady SR, McIntosh JM, Marks MJ, Lester HA, Drenan RM. alpha6* nicotinic acetylcholine receptor expression and function in a visual salience circuit. J Neurosci. 2012;32:10226–10237. doi: 10.1523/JNEUROSCI.0007-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The anti-relapse compound acamprosate inhibits the development of a conditioned place preference to ethanol and cocaine but not morphine. Br J Pharmacol. 2003a;138:9–12. doi: 10.1038/sj.bjp.0705059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003b;47:240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, Garrett JE, Marks MJ, Whiteaker P. Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Molecular pharmacology. 2004;65:944–952. doi: 10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- Merrill CB, McNeil M, Williamson RC, Poole BR, Nelson B, Sudweeks S, Edwards JG. Identification of mRNA for endocannabinoid biosynthetic enzymes within hippocampal pyramidal cells and CA1 stratum radiatum interneuron subtypes using quantitative real-time polymerase chain reaction. Neuroscience. 2012;218:89–99. doi: 10.1016/j.neuroscience.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak KL, McBride WJ, Lumeng L, Li TK, Murphy JM. Blocking GABA(A) receptors in the anterior ventral tegmental area attenuates ethanol intake of the alcohol-preferring P rat. Psychopharmacology (Berl) 1998;139:108–116. doi: 10.1007/s002130050695. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MS, Broderick HJ, Drenan RM, Chester JA. Nicotinic acetylcholine receptors containing alpha6 subunits contribute to alcohol reward-related behaviours. Genes, brain, and behavior. 2013;12:543–553. doi: 10.1111/gbb.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjakdar SS, Maldoon PP, Marks MJ, Brunzell DH, Maskos U, McIntosh JM, Bowers MS, Damaj MI. Differential roles of alpha6beta2* and alpha4beta2* neuronal nicotinic receptors in nicotine- and cocaine-conditioned reward in mice. Neuropsychopharmacology. 2015;40:350–360. doi: 10.1038/npp.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Bradley KD, Hansen DM, Wilcox JD, Wilcox RS, Allison DW, Merrill CB, Edwards JG. The role of connexin-36 gap junctions in alcohol intoxication and consumption. Synapse. 2011;65:695–707. doi: 10.1002/syn.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Taylor SR, Horton ML, Barber EN, Lyle LT, Stobbs SH, Allison DW. Cocaine disinhibits dopamine neurons in the ventral tegmental area via use-dependent blockade of GABA neuron voltage-sensitive sodium channels. The European journal of neuroscience. 2008;28:2028–2040. doi: 10.1111/j.1460-9568.2008.06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Walton CH, Hansen DM, Yorgason JT, Gallegos RA, Criado JR. Contingent and non-contingent effects of low-dose ethanol on GABA neuron activity in the ventral tegmental area. Pharmacology, biochemistry, and behavior. 2009;92:68–75. doi: 10.1016/j.pbb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Luscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DH, Burman PN, Hansen DM, Wilcox JD, Larsen BR, Blanchard JK, Merrill CB, Edwards JG, Sudweeks SN, Wu J, Arias HR, Steffensen SC. Nicotine Enhances the Excitability of Gaba Neurons in the Ventral Tegmental Area via Activation of Alpha 7 Nicotinic Receptors on Glutamate Terminals. Biochem & Pharm: Open Access. 2013:1. [Google Scholar]
- Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- Tritsch NX, Granger AJ, Sabatini BL. Mechanisms and functions of GABA co-release. Nature reviews Neuroscience. 2016;17:139–145. doi: 10.1038/nrn.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Oh WJ, Gu C, Sabatini BL. Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis. Elife. 2014;3:e01936. doi: 10.7554/eLife.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker P, McIntosh JM, Luo S, Collins AC, Marks MJ. 125I-alpha-conotoxin MII identifies a novel nicotinic acetylcholine receptor population in mouse brain. Molecular pharmacology. 2000;57:913–925. [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, George AA, Schroeder KM, Xu L, Marxer-Miller S, Lucero L, Lukas RJ. Electrophysiological, pharmacological, and molecular evidence for alpha7-nicotinic acetylcholine receptors in rat midbrain dopamine neurons. J Pharmacol Exp Ther. 2004;311:80–91. doi: 10.1124/jpet.104.070417. [DOI] [PubMed] [Google Scholar]
- Xiao C, Yang KC, Zhou CY, Jin GZ, Wu J, Ye JH. Nicotine modulates GABAergic transmission to dopaminergic neurons in substantia nigra pars compacta. Acta pharmacologica Sinica. 2009;30:851–858. doi: 10.1038/aps.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhang J, Krnjevic K, Ye JH. Effects of ethanol on midbrain neurons: role of opioid receptors. Alcohol Clin Exp Res. 2007;31:1106–1113. doi: 10.1111/j.1530-0277.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- Yang K, Buhlman L, Khan GM, Nichols RA, Jin G, McIntosh JM, Whiteaker P, Lukas RJ, Wu J. Functional nicotinic acetylcholine receptors containing alpha6 subunits are on GABAergic neuronal boutons adherent to ventral tegmental area dopamine neurons. J Neurosci. 2011;31:2537–2548. doi: 10.1523/JNEUROSCI.3003-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Hu J, Lucero L, Liu Q, Zheng C, Zhen X, Jin G, Lukas RJ, Wu J. Distinctive nicotinic acetylcholine receptor functional phenotypes of rat ventral tegmental area dopaminergic neurons. J Physiol. 2009a;587:345–361. doi: 10.1113/jphysiol.2008.162743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang KC, Jin GZ, Wu J. Mysterious alpha6-containing nAChRs: function, pharmacology, and pathophysiology. Acta pharmacologica Sinica. 2009b;30:740–751. doi: 10.1038/aps.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin R, French ED. A comparison of the effects of nicotine on dopamine and non-dopamine neurons in the rat ventral tegmental area: an in vitro electrophysiological study. Brain research bulletin. 2000;51:507–514. doi: 10.1016/s0361-9230(00)00237-9. [DOI] [PubMed] [Google Scholar]