Abstract
Background/Objectives
To examine whether chronic kidney disease (CKD) at all stages is associated with fracture risk after adjusting for competing mortality, and determine whether age or race modifies the relationship between CKD and fracture risk.
Design
Prospective cohort study.
Setting
Department of Veterans Affairs (VA) national healthcare system.
Participants
Men receiving VA primary care aged ≥ 65 years with no history of fracture or osteoporosis therapy (n=712, 918).
Measurements
We determined CKD stage from baseline estimated glomerular filtration rate (eGFR) values. Participants were followed up to 10 years for occurrence of any fracture or death. We ascertained fractures and covariates from VA medical records and Medicare claims.
Results
Of the 356,459 older Veterans with CKD (defined as eGFR < 60ml/min/1.73m2), 15.7% (n=56,032) experienced a fracture and 43.0% (n=153,438) died over a median time at risk of 5.2 years. Compared to veterans without CKD, those with CKD Stages 3-5 had an elevated risk of death, which biased estimates from traditional survival models. Competing risk models showed that Stage 3 CKD was associated with increased hazard [adjusted subdistribution hazard ratio (sdHR), 1.07, 95% CI 1.02-1.11] of fracture (compared to those without CKD), and a trend towards increased hazards for Stage 4 (sdHR,1.07, 95% CI 0.94-1.22) and Stage 5 (sdHR,1.31, 95% CI 0.97-1.77) CKD. Age, race, and bone mineral density did not modify the relationship between CKD and fracture risk.
Conclusions
Among older male Veterans, CKD, including stage 3, is associated with a moderately increased fracture risk irrespective of age, race, or bone mineral density.
Keywords: geriatric nephrology, osteoporosis, competing risks
Introduction
Osteoporotic fractures are a major source of morbidity, mortality, and cost in older adults. Because fracture risk increases exponentially with age, multi-morbidity is the norm for most patients and can complicate assessment of the risks and benefits of osteoporosis screening and treatment. One of the most common but understudied such co-morbidities is chronic kidney disease (CKD).
Compared to those without CKD, older adults with CKD have a greater risk of death at worsening levels of kidney function.1 Because impaired kidney function negatively impacts bone quality and increases risk for falls, CKD also has been associated with fractures.2–7 However, these studies didn’t take into account the competing mortality risk, which can bias the fracture risk prediction. Furthermore, it remains unknown if CKD in an older adult with the mild, age-related decline in kidney function independently increases the risk of fracture or if the presence of CKD is merely a marker of other chronic illnesses associated with fracture. Precise estimates of the risk of fracture at all levels of CKD, accounting for competing mortality risk and other risk factors, are therefore needed. Finally, we have insufficient evidence to understand if race/ethnicity modifies fracture risk associated with CKD. This is especially important because CKD disproportionately affects African Americans who generally have a lower fracture risk than Caucasians.8
Despite the increasing prevalence of CKD among older adults, current fracture prediction tools, such as FRAX, Garvan, and the Canadian Association of Radiologists and Osteoporosis Canada (CAROC) system,9–12 do not include a measure of kidney function, or level of CKD, as a risk factor. It is important for clinicians to understand the impact of varying levels of CKD on fracture risk when making treatment decisions. Therefore, we examined the extent to which varying levels of kidney function are associated with fracture risk when accounting for competing mortality risk.
Methods
Study Design
We conducted a retrospective cohort study of male Veterans aged ≥ 65 years receiving primary care within the Veterans Affairs (VA) health system between January 1, 2000 and December 31, 2010. We used merged data from Veterans Affairs’ medical records (including inpatient files, outpatient files, laboratory, and pharmacy data) and Medicare claims (Part A and Part B) to ascertain clinical characteristics, fractures, and deaths. This study was approved by the Durham VA Institutional Review Board.
Study Population
This cohort is a subgroup of a larger population-based retrospective cohort study designed to evaluate osteoporosis screening in male Veterans. In the parent study, the analytic sample included men aged ≥ 65 years who received primary care at a VAMC equipped to conduct Dual X-ray Absorptiometry (DXA) screening between 2000 and 2010. Veterans with prior diagnoses of osteoporosis, any fractures (defined as presence of any fracture ICD9 or CPT codes, excluding skull, facial, digital, and pathological fractures), or evidence of bisphosphonate use during a 3 year look-back period were excluded. For this analysis, we also excluded Veterans with missing creatinine values during a 3 year look-back period. With this selection criteria, we identified 356,459 older Veterans with CKD, defined as estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73m2 on at least 2 measurements at least 3 months apart (Figure 1). We selected this cohort of veterans with prevalent CKD to represent what clinicians would likely encounter in clinical practice. Compared to incident CKD, prevalent CKD is more accurately identifiable in administrative data and allows time for CKD-related bone abnormalities to manifest.13 For analytic efficiency, we selected a random sample of equal size of subjects without CKD to include in our cohort.
Figure 1. Consort Diagram.
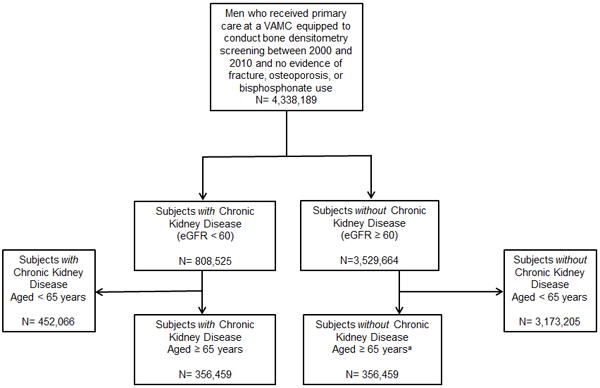
VAMC = Veterans Affairs Medical Center
aAn equal random sample of older adults aged ≥ 65 years who did not have chronic kidney disease was selected for the analyses.
Variables
We assessed for the occurrence of fractures or death from the start of the observation period, defined as the date of a subject’s first primary care visit after DXA became available in their facility, through December 31, 2010. Fracture events and types of fractures were determined from ICD9 and CPT codes from inpatient and outpatient encounters in our merged dataset. We assessed death from the National Death Index and the VA Benefits Identification Records Locator Subsystem death file.
Our primary exposure variable was kidney function as measured by eGFR. We calculated eGFR from the Modification of Diet in Renal Disease equation using VA laboratory data between three years before and after a subject’s start date. In the absence of other markers of kidney damage (e.g., abnormal urine composition or kidney imaging test), we used eGFR alone to classify the cohort into four clinically relevant categories: 1) no/mild CKD (eGFR≥60 ml/min/1.73m2), 2) Stage 3 CKD (eGFR≥30ml/min/1.73m2 and <60 ml/min/1.73m2), 3) Stage 4 (eGFR≥15ml/min/1.73m2 and <30 ml/min/1.73m2), and 4) Stage 5 (eGFR<15 ml/min/1.73m2).
We identified covariates associated with fracture and/or death from clinical data assessed at baseline. These variables included patient demographics (age, race), region of residence, body mass index (BMI), and additional fracture risk factors listed below. We used International Classification of Disease-Ninth Revision (ICD-9) and Current Procedural Terminology (CPT) codes from inpatient and outpatient VA and Medicare claims, supplemented with prescription medication use or laboratory values as appropriate, to identify the presence of specific fracture risk factors. To enhance accuracy in identification of each risk factor, these codes had to be identified at two or more visits (inpatient or outpatient) or at one visit accompanied by a relevant prescription for treatment of the risk factor (when applicable). The fracture risk factors include smoking, chronic glucocorticoid use, androgen deprivation therapy, chronic alcohol abuse, rheumatoid arthritis, hyperthyroidism, diabetes mellitus, chronic obstructive pulmonary disease (COPD), chronic liver disease, and malabsorption disorder. Additional details regarding operational definitions for these fracture risk factors are available upon request.
Statistical Analysis
We used the FRAX tool with BMI to calculate each subject’s ten-year probability of major fracture.10 In the subset of patients in which Bone Mineral Density was assessed during routine care, results were extracted using a validated natural language processing tool,14 and FRAX with BMD was also calculated. FRAX scores, along with all other study variables, were reported as proportions for categorical variables and means with standard deviations (SD) or medians with interquartile ranges (IQR) for continuous variables. For descriptive purposes, we conducted bivariate analyses between a 4-level ordinal CKD group definition based on eGFR (described above), with the reference group being no CKD (i.e., eGFR ≥ 60), and each covariate using ANOVA for continuous variables and Chi-square tests for categorical variables with appropriate contrasts to compare each CKD level to the reference group. To evaluate the hazards of fracture attributable to each CKD group, we performed Cox Proportional Hazards regression models adjusting for demographics (except for age), region, BMI, and fracture risk factors (listed above). Because cohort members enter the observation period at different ages, data was left-truncated and age served as the time-to-event scale.15 Using the Cox models, we performed tests for interaction between eGFR subgroups and baseline age groups (65-74, 75-84, and ≥ 85 years) and race [Caucasian, African-American, and other (i.e., Asian or Hispanic ethnicity)]. To account for the competing risk of death, we used the Fine and Gray method to estimate unadjusted and multivariable adjusted sub-distribution relative hazards of fracture.16 After confirming the proportional hazards assumption, we performed an additional competing risk analysis adjusting for bone mineral density (BMD) among cohort members with available BMD data. All statistical analyses were performed with SAS 9.4.
Results
Cohort Characteristics
As shown in Figure 1, our analytic sample included 712,918 male Veterans. Of the 356,459 subjects with CKD, 95.2% (n=339,278), 4.3% (n=15,167), 0.5% (n=2014) of cohort members had Stage 3, Stage 4, and Stage 5 CKD respectively. Compared to all three CKD groups, cohort members without CKD were younger, were less likely to have chronic alcohol use, hyperthyroidism, diabetes, COPD, chronic liver disease, malabsorption disorders, and had lower FRAX-BMI scores (Table 1).
Table 1.
Cohort Characteristics by Chronic Kidney Disease Stage
| Characteristic | No CKD n= 356458 |
Stage 3 CKD n=339278 |
Stage 4 CKD n=15167 |
Stage 5 CKD n=2014 |
|---|---|---|---|---|
| Age, mean (SD) | 70.9 (6.3) | 75.1 (6.4)** | 75.6 (6.8)** | 73.5 (6.4)** |
| Black Race, n (%) | 33299 (9.3%) | 18371 (5.4%)** | 1507 (9.9%)* | 423 (21.0%)** |
| Body Mass Index, mean (SD) | 28.7 (5.1) | 28.7 (4.8) | 28.3 (5.1)** | 27.5 (4.9)** |
| Current Smoking, n (%) | 80112 (22.5%) | 64687 (19.1%)** | 3085 (20.3%)** | 481 (23.9%)** |
| Chronic Alcohol Use, n (%) | 65185 (18.3%) | 56457 (16.6%)** | 2507 (16.5%)** | 333 (16.5%)* |
| Chronic Steroid use, n (%) | 5936 (1.7%) | 5664 (1.7%) | 305 (2.0%)* | 45 (2.2%) |
| Rheumatoid Arthritis, n (%) | 2315 (0.7%) | 1750 (0.5%)** | 61 (0.4%)* | 6 (0.3%) |
| Hyperthyroidism, n (%) | 4060 (1.1%) | 4794 (1.4%)** | 245 (1.6%)** | 33 (1.6%)* |
| Diabetes, n (%) | 108647 (30.5%) | 124345 (36.7%)** | 7855 (51.8%)** | 1140 (56.6%)** |
| Chronic Lung Disease, n (%) | 85937 (24.1%) | 90906 (26.8%)** | 4514 (29.8%)** | 585 (29.1%)** |
| Chronic Liver disease, n (%) | 27378 (7.7%) | 25229 (7.4%)** | 1673 (11.0%)** | 401 (19.9%)** |
| Malabsorption, n (%) | 1178 (0.3%) | 1453 (0.4%)** | 120 (0.8%)** | 67 (3.3%)** |
| 10-Yr Probability of Major Fracture (FRAX), mean (SD) | 5.5 (2.7) | 6.6 (3.0)** | 6.8 (3.3)** | 6.3 (3.5)** |
P value refers to comparison of each CKD Stage to the reference group (no CKD which is defined as estimated glomerular filtration rate ≥ 60ml/min/1.73m2).
P < .05.
P ≤ .0001.
Fracture and Death Events
Over a median follow-up period of 5.2 years, 14.1% (n=100,254) of cohort members experienced fractures; 12.4% (n=44,223) without CKD and 15.7% (n=56,031) with CKD. The three most common sites of fracture among those with CKD were hip/femur (25.8%, n=14,452), rib/clavicular (22.6%, n=12,640), and vertebral (16.9%, n= 9489) (Supplementary Table S1). Overall mortality over the follow-up period was high at 35.5% (n=253,415). In adjusted analyses, relative to no CKD, Stage 3 CKD was associated with small increased hazards of death [hazard ratio (HR), 1.05, 95% confidence interval (CI), 1.03 to 1.08]. There was a stepwise increase in hazards of death for Stage 4 CKD (HR, 2.07, 95% CI, 1.94 to 2.20) and Stage 5 CKD (HR, 2.48, 95% CI, 2.12 to 2.91).
Association of CKD with Fracture Risk with and without Competing Mortality
Survival curves demonstrate that the rate of fracture events increased with worsening kidney function (Figure 2 – dotted lines). Tests for interaction showed that older age and race did not modify that CKD-fracture association. In analyses adjusting for fracture risk factors and censoring for death, cohort members with Stage 3 CKD had a significantly lower risk of fracture [hazard ratio (HR), 0.95, 95% confidence interval (CI) 0.92-0.99] at any point in the observation period compared to those without CKD (Table 2). In contrast, fracture risk increased for those with Stage 4 (HR, 1.32, 95% CI 1.16-1.49) or Stage 5 CKD (HR, 1.91, 95% CI 1.45-2.50).
Figure 2. A Comparison of Fracture-free Survival Curve by Kaplan-Meier and Competing Risk Methods Across Levels of Kidney Function.
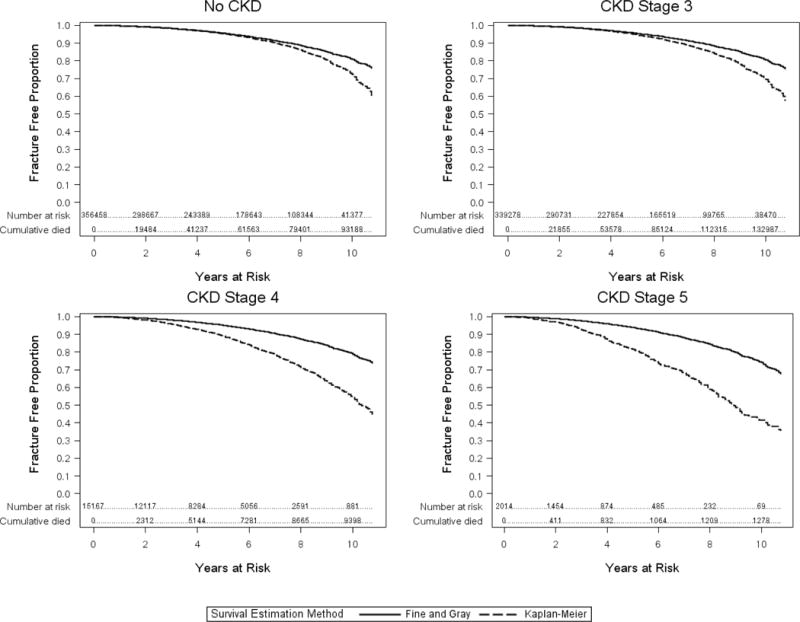
CKD=Chronic Kidney Disease
For each CKD stage, there is a panel that demonstrates the Kaplan-Meier Survival Curve for fracture (dotted line) and a plot of individuals who remain in the risk set (subjects without fracture event and those with death events) of the Fine and Gray analysis (solid line). At higher CKD stages, the Fine and Gray analysis shows smaller proportion of fractures than the survival curve because individuals who die remain in the risk set of this analysis.
Table 2.
Adjusted Hazard Ratios for Fracture by Chronic Kidney Disease Stage
| Level of Kidney Function | Cox Proportional Hazards Adjusted HR (95% CI) |
Fine and Gray Adjusted sdHR (95% CI) |
|---|---|---|
| No CKD | Reference | Reference |
| CKD Stage 3 | 0.95 (0.91, 0.99) | 1.07 (1.02, 1.11) |
| CKD Stage 4 | 1.32 (1.16, 1.49) | 1.07 (0.94, 1.22) |
| CKD Stage 5 | 1.91 (1.45, 2.50) | 1.31 (0.97, 1.77) |
CKD= chronic kidney disease, HR=hazard ratio, CI= confidence interval, sdHR=subdistribution hazard ratio
Data expressed as HR for Cox Proportional Hazards Model [or sdHR for competing risk model]. Models were adjusted for baseline demographics (except for age), region, body mass index, smoking history, chronic steroid use, chronic alcohol use, rheumatoid arthritis, hyperthyroidism, diabetes, chronic obstructive pulmonary disease, chronic liver disease, and malabsorption.
Accounting for the competing risk of death changed the risk estimates for all levels of kidney function (Figure 2). Specifically, Stage 3 CKD was associated with increased hazards [adjusted subdistribution hazard ratio (sdHR), 1.07, 95% CI 1.02-1.11] of fracture compared to those without CKD. Stage 4 CKD was associated with similar increased hazards [adjusted subdistribution hazard ratio (sdHR), 1.07, 95% CI 0.94-1.22] of fracture compared to those without CKD. Accounting for the high competing risk of death in stage 4 or 5 CKD (60% died during the observation period) attenuated the association seen in the Cox regression models but still demonstrated a trend towards increased fracture risk (Table 2). When FRAX-BMD was added to a competing risk model with eGFR alone for the subset of patients in whom it was available (n= 36,563), the strength of association of eGFR with fracture was relatively unchanged, indicating that the association between eGFR and fracture is not mediated by the addition of bone mineral density to the model.
Discussion
It is estimated that 20-33% of older adults worldwide will develop osteoporotic fractures,17,18 and at least 25% of older adults will also develop chronic kidney disease in their lifetime.19 With the high prevalence of both osteoporosis and CKD in older adults, it is important for clinicians and researchers to understand how CKD at various stages modifies the risk of fracture. We demonstrated that even moderate (or Stage 3) CKD remains associated with fracture risk even in the face of competing mortality.
Our findings demonstrate the importance of considering the competing risk of mortality when estimating the impact of CKD on fracture risk. Most existing observational studies that show an association of CKD with fracture risk did not account for competing mortality and limited analyses to hip fractures.2,4 In one population-based study that did perform competing risk analyses, Perez-Saez et al identified an 83% greater risk of death and 16% greater hip fracture risk in patients with CKD diagnoses, and the risk of hip fracture was mildly attenuated to 14% in competing risk models.7 Adding to this evidence, we examined the association of each CKD stage with risk of any fracture in the face of competing risk of mortality. Although we had smaller sample of patients with Stage 4 CKD, the effect size was identical to that for Stage 3 CKD (HR 1.07). Thus, our study provides further evidence that high mortality seen in CKD does not preclude strategic fracture risk prediction and management in these patients, including those with Stage 3 CKD.
Our findings have implications for osteoporosis screening. Current U.S. practice guidelines recommend DXA screening in men over age 65 who have one or more fracture risk factors, but CKD is not commonly considered a fracture risk factor by the American College of Physicians Male Osteoporosis Screening Guideline,20 the Endocrine Society Osteoporosis in Men Guideline,21 or the Veterans Affairs Male Osteoporosis Screening Algorithm.22 Similarly, in the UK, guidelines recommend decisions to pursue DXA screening are determined from FRAX (with BMI) estimates of fracture risk, but the FRAX tool doesn’t include a measure of kidney function.23 The magnitude of the increased risk we observed, especially for stage 4 and 5 CKD, is comparable to that of well-accepted risk factors including smoking, chronic lung disease, and chronic liver disease.24,25 Notably, we identified this increased risk of fracture attributable to CKD, including Stage 3 CKD, despite the inherent limitations of the MDRD estimating equation to overestimate eGFR in older adults.26,27 This would tend to bias our analysis against finding a significant effect of stage 3 CKD, making our positive findings even more compelling. Since many patients with stage 3 CKD will progress to later stages where treatment options are more limited, and our results suggests that even moderately reduced kidney function is associated with elevated fracture risk after controlling for traditional fracture risk factors, such men should be considered for DXA screening. While men with advanced CKD may not be screened due to concerns about competing mortality risk yielding insufficient time to benefit, our results support screening selected men with advanced CKD given that their fracture risk remains elevated after considering the competing risk of mortality. Further research is needed to develop a clinical prediction tool to help clinicians identify men with advanced CKD who may benefit from DXA screening.
Our findings also have implication for osteoporosis treatment in older adults with CKD. Current guidelines recommend osteoporosis treatment for men and women who have a 10 year probability of hip fracture ≥ 3% or major osteoporosis-related fracture ≥ 20%.28 This fracture risk assessment does not currently consider level of kidney function. We found that level of kidney function, as measured by eGFR, is associated with increased fracture risk independent of BMD. This finding may be explained in part by the fact that altered vitamin D metabolism resulting in renal osteodystrophy has a greater effect on bone quality, which is not measured by DXA, than bone density. The independent risk of fracture attributable to eGFR could modify treatment decisions. For example, the FRAX tool calculates that a 79 year old male smoker with a parental history of hip fracture and BMI of 22.8 has a ten year risk of major osteoporotic fracture (MOF) of 14%. From our analyses, his Stage 3 CKD introduces an additional instantaneous risk of fracture of 7%. By accounting for risk attributable to CKD, this patient’s actual risk for fracture in any given timeframe is potentially higher, which would influence a clinician’s decision to initiate osteoporosis treatment.29 While many osteoporosis treatments are not approved for use in advanced CKD, newer agents such as denosumab are options for these patients. Denosumab, teriparatide, alendronate, and raloxifene all have some evidence for benefit in BMD or fractures in Stage 3 CKD.30–33 However, additional data regarding benefits and side effects is needed to inform the treatment of older adults with Stage 3 or more advanced CKD.34
This study’s strength derives from its large population-based cohort of male Veterans. However, administrative data lacks important information and introduces study limitations. First, we did not optimally classify patients by level of kidney function because we did not have urine albumin data or other evidence of kidney damage (i.e., renal imaging or clinical notes). As a result, there may have been misclassification of some subjects with Stage 3 as subjects without CKD. However, this misclassification error would likely bias our findings towards no significant difference between these groups. Second, we used clinically diagnosed fractures as our endpoint which is accurate for most fracture types, but misses up to two-thirds of vertebral fractures. The under-diagnosis of vertebral fractures may be more likely in non-white men who tend to access less healthcare services, and therefore have less opportunity for vertebral fractures to be diagnosed incidentally during other imaging studies. Third, our models did not capture important unmeasured confounders, such as falls, frailty, comorbidity burden, Vitamin D status, or markers associated with secondary hyperparathyroidism (i.e., serum parathyroid hormone level, calcium, FGF23, or phosphorus levels). Last, associations based on our cohort of male veterans who receive primary care in the VA may not be fully generalizable to the larger population of older men and women with chronic kidney disease or patients younger than 65 years.
Compared to older adults without CKD, older adults with Stage 3 CKD have a greater risk of any fracture when accounting for competing risk of mortality. Attention to moderate CKD as an independent fracture risk factor could improve osteoporosis screening and treatment decisions.
Supplementary Material
Supplementary Table S1. Fracture Site by Chronic Kidney Disease Stage.
Impact Statement.
The potential impact of this research on clinical care or health policy includes the following: mild chronic kidney disease remains associated with an increase in fracture risk after accounting for competing mortality risk suggesting potential value in adding eGFR to fracture risk prediction for older adults with chronic kidney disease.
Acknowledgments
Funding Sources: This study is supported by the Department of Defense (W81XWH-12-2-0093), the National Institutes of Health: P30 AG028716, R03 AG050834, K24 AG049077-01A1, and KL2 TR001115, T. Franklin Williams Scholarship Award (funding provided by Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Alliance for Academic Internal Medicine-Association of Specialty Professors, and the American Society of Nephrology Foundation).
Sponsor’s Role: The NIH (and other sponsors) did not have a role in study design, data collection, analysis, or interpretation, or manuscript preparation.
Footnotes
DR. RASHEEDA HALL (Orcid ID : 0000-0002-3057-4828)
Conflicts of Interest:
|
| ||||||||||||||
| Elements of Financial/Personal Conflicts | RH | RS | CP | CV | JL | RA | CCE | |||||||
|
| ||||||||||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
|
| ||||||||||||||
| Employment or Affiliation | x | x | x | x | x | x | x | |||||||
|
| ||||||||||||||
| Grants/Funds | x | x | x | x | x | x | x | |||||||
|
| ||||||||||||||
| Honoraria | x | x | x | x | x | x | x | |||||||
|
| ||||||||||||||
| Speaker Forum | x | x | x | x | x | x | x | |||||||
|
| ||||||||||||||
| Consultant | x | x | x | x | x | x | x | |||||||
|
| ||||||||||||||
| Stocks | x | x | x | x | x | x | x | |||||||
|
| ||||||||||||||
| Royalties | x | x | x | x | x | x | x | |||||||
|
| ||||||||||||||
| Expert Testimony | x | x | x | x | x | x | x | |||||||
|
| ||||||||||||||
| Board Member | x | x | x | x | x | x | x | |||||||
|
| ||||||||||||||
| Patents | x | x | x | x | x | x | x | |||||||
|
| ||||||||||||||
| Personal Relationship | x | x | x | x | x | x | x | |||||||
|
| ||||||||||||||
Author Contributions:
|
| ||||
| Study concept and design | Acquisition of subjects and/or data | Analysis and interpretation of data | Preparation of manuscript | |
|
| ||||
| Rasheeda K. Hall | x | x | x | |
|
| ||||
| Richard Sloane | x | x | x | x |
|
| ||||
| Carl Pieper | x | x | x | x |
|
| ||||
| Courtney VanHoutven | x | x | ||
|
| ||||
| Joanne LaFleur | x | x | ||
|
| ||||
| Robert Adler | x | x | ||
|
| ||||
| Cathleen Colón-Emeric | x | x | x | x |
|
| ||||
References
- 1.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Dooley AC, Weiss NS, Kestenbaum B. Increased risk of hip fracture among men with CKD. Am J Kidney Dis. 2008;51(1):38–44. doi: 10.1053/j.ajkd.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Ensrud KE, Lui LY, Taylor BC, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167(2):133–139. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 4.Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18(1):282–286. doi: 10.1681/ASN.2006050546. [DOI] [PubMed] [Google Scholar]
- 5.Jamal SA, West SL, Miller PD. Fracture risk assessment in patients with chronic kidney disease. Osteoporos Int. 2012;23(4):1191–1198. doi: 10.1007/s00198-011-1781-0. [DOI] [PubMed] [Google Scholar]
- 6.Naylor KL, McArthur E, Leslie WD, et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. 2014;86(4):810–818. doi: 10.1038/ki.2013.547. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Saez MJ, Prieto-Alhambra D, Barrios C, et al. Increased hip fracture and mortality in chronic kidney disease individuals: The importance of competing risks. Bone. 2014;73C:154–159. doi: 10.1016/j.bone.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 8.George A, Tracy JK, Meyer WA, et al. Racial differences in bone mineral density in older men. J Bone Miner Res. 2003;18(12):2238–2244. doi: 10.1359/jbmr.2003.18.12.2238. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16(6):581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 10.Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leslie WD, Berger C, Langsetmo L, et al. Construction and validation of a simplified fracture risk assessment tool for Canadian women and men: results from the CaMos and Manitoba cohorts. Osteoporos Int. 2011;22(6):1873–1883. doi: 10.1007/s00198-010-1445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen ND, Frost SA, Center JR, et al. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int. 2008;19(10):1431–1444. doi: 10.1007/s00198-008-0588-0. [DOI] [PubMed] [Google Scholar]
- 13.Grams ME, Rebholz CM, McMahon B, et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis. 2014;64(2):214–221. doi: 10.1053/j.ajkd.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaFleur J, Duvall S, Ginter T. Natural language processing (NLP) technology for extracting bone mineral density (BMD) results from radiology reports in the Veterans Affairs (VA) healthcare system. American Society for Bone and Mineral Research; San Diego, CA: 2011. [Google Scholar]
- 15.Lamarca R, Alonso J, Gomez G, et al. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci. 1998;53(5):M337–343. doi: 10.1093/gerona/53a.5.m337. [DOI] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 17.Melton LJ, 3rd, Atkinson EJ, O’Connor MK, et al. Bone density and fracture risk in men. J Bone Miner Res. 1998;13(12):1915–1923. doi: 10.1359/jbmr.1998.13.12.1915. [DOI] [PubMed] [Google Scholar]
- 18.Melton LJ., 3rd How many women have osteoporosis now? J Bone Miner Res. 1995;10(2):175–177. doi: 10.1002/jbmr.5650100202. [DOI] [PubMed] [Google Scholar]
- 19.Tonelli M, Riella M. Chronic kidney disease and the aging population. Am J Physiol Renal Physiol. 2014;306(5):F469–472. doi: 10.1152/ajprenal.00063.2014. [DOI] [PubMed] [Google Scholar]
- 20.Qaseem A, Snow V, Shekelle P, et al. Screening for osteoporosis in men: A clinical practice guideline from the american college of physicians. Annals of Internal Medicine. 2008;148(9):680–684. doi: 10.7326/0003-4819-148-9-200805060-00008. [DOI] [PubMed] [Google Scholar]
- 21.Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802–1822. doi: 10.1210/jc.2011-3045. [DOI] [PubMed] [Google Scholar]
- 22.Adler RA, Semla T, Cunningham F, et al. The VHA Male Osteoporosis Program: a national model for bone health. Fed Practitioner. 2012;29(2):33–38. [Google Scholar]
- 23.Compston J, Cooper A, Cooper C, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12(1):43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyes C, Estrada P, Nogues X, et al. The impact of common co-morbidities (as measured using the Charlson index) on hip fracture risk in elderly men: a population-based cohort study. Osteoporos Int. 2014;25(6):1751–1758. doi: 10.1007/s00198-014-2682-9. [DOI] [PubMed] [Google Scholar]
- 25.Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16(2):155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 26.Dharmarajan TS, Yoo J, Russell RO, et al. Chronic kidney disease staging in nursing home and community older adults: does the choice of cockcroft-gault, modification of diet in renal disease study, or the chronic kidney disease epidemiology collaboration initiative equations matter? J Am Med Dir Assoc. 2012;13(2):151–155. doi: 10.1016/j.jamda.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Drenth-van Maanen AC, Jansen PA, Proost JH, et al. Renal function assessment in older adults. Br J Clin Pharmacol. 2013;76(4):616–623. doi: 10.1111/bcp.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gadam RK, Schlauch K, Izuora KE. Frax prediction without BMD for assessment of osteoporotic fracture risk. Endocr Pract. 2013;19(5):780–784. doi: 10.4158/EP12416.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishani A, Blackwell T, Jamal SA, et al. The effect of raloxifene treatment in postmenopausal women with CKD. J Am Soc Nephrol. 2008;19(7):1430–1438. doi: 10.1681/ASN.2007050555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamal SA, Bauer DC, Ensrud KE, et al. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the fracture intervention trial. J Bone Miner Res. 2007;22(4):503–508. doi: 10.1359/jbmr.070112. [DOI] [PubMed] [Google Scholar]
- 32.Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res. 2011;26(8):1829–1835. doi: 10.1002/jbmr.403. [DOI] [PubMed] [Google Scholar]
- 33.Miller PD, Schwartz EN, Chen P, et al. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int. 2007;18(1):59–68. doi: 10.1007/s00198-006-0189-8. [DOI] [PubMed] [Google Scholar]
- 34.Wilson LM, Rebholz CM, Jirru E, et al. Benefits and Harms of Osteoporosis Medications in Patients With Chronic Kidney Disease: A Systematic Review and Meta-analysis. Ann Intern Med. 2017;166(9):649–658. doi: 10.7326/M16-2752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Fracture Site by Chronic Kidney Disease Stage.


